Translate this page into:
UPLC-Q/TOF-MS coupled with multivariate analysis for comparative analysis of metabolomic in Dendrobium nobile from different growth altitudes
⁎Corresponding author at: Guizhou Engineering Research Center of Industrial Key-technology for Dendrobium Nobile, Zunyi Medical University, Zunyi, Guizhou 563000, China; Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, Guizhou 563000, China yqhe.pharm@foxmail.com (Yu-qi He)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Dendrobium nobile alkaloids (DNLA) and glycosides are the main active components extracted from Dendrobium nobile Lindl. (D. nobile) used for thousands of years in China. The pharmacological effects of the above chemical components are significantly different. D. nobile is mainly grown at an altitude ranging from 230 to 800 m in Chishui City, Northwest Guizhou Province. However, it is unclear whether the metabolite in D. nobile is influenced by the planting altitude. Hence, to reveal the different metabolite in D. nobile cultivated at the altitude of 336 m, 528 m, and 692 m, ultra-high performance liquid chromatography with Q/TOF-MS couple with multivariate analysis were developed. Using the orthogonal partial least squares-discriminant analysis, 19 different metabolites were discovered and then tentatively assigned their structures as alkaloids and glycosides by comparing mass spectrometry data with in-house database and literature. Moreover, the result of semiquantitative analysis showed the content of dendrobine that was belonged to alkaloids significantly increased at the altitude of 692 m, whereas the content of glycosides demonstrated an accumulation trend at the altitude of 528 m. The results could provide valuable information for the optimal clinical drug therapeutics and provide a reference for quality control.
Keywords
Dendrobium nobile. Lindl
Alkaloids
Glycosides
UPLC-Q-TOF/MS
Altitude
- BPC
-
Base Peak Chromatograms
- ChP
-
the Pharmacopoeia of the People’s Republic of China
- D. nobile
-
Dendrobium Nobile Lindl.
- DNLA
-
Dendrobium Nobile Lindl. Alkaloids
- ESI
-
Electrospray Ionization
- PCA
-
Principal Component Analysis
- OPLS-DA
-
Orthogonal Partial Least Squares Discrimination Analysis
- RT
-
Retention Time
- TCM
-
Traditional Chinese Medicine
- UPLC-Q/TOF-MS
-
Ultra Performance Liquid Chromatography Coupled with Triple-Quadrupole Mass Spectrometry
- VIP
-
Variable Importance of Projection
Abbreviations
1 Introduction
The genus Dendrobium, one of the largest genera in the family Orchidacea, contains approximately 2000 species mainly distributed in the tropics and subtropics in South Asia, Oceanica (Zheng et al. 2018). Of these, 74 Dendrobium species and two varieties were reported in China (Feng et al., 2015). The fresh and dried stems of many Dendrobium species, used in Traditional Chinese Medicine (TCM), were documented as a “superior grade” herbal medicine in the ancient book named as “Shen Nong’s Classic of Materia Medica” (Xu et al. 2013).
D. nobile, also called as “Jin Chai Shi Hu”, is one of the primary plant sources of Dendrobii Caulis specified in the Chinese Pharmacopeia. In term of pharmacological effects, D. nobile exhibits effects of regulating lipid metabolism, protecting the nervous system, anti-immune activity, antitumor, antifibrosis, antioxidant activity, and others. Among those effects, protecting the nervous system, regulating lipid metabolism has been closely related to alkaloids (Huang et al. 2019, Lv et al. 2020). However, for the effect of anti-immune activity, glycosides were found to stimulate the proliferation of B cells in vitro (Zhao et al., 2001; Zhao et al., 2003). Due to its broad applications, overexploitation, low natural reproduction rate, and ecological environmental destruction, wild D. nobile resource was listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (Cheng et al. 2019).
To meet the market demands, a wild imitation cultivation method of D. nobile has been greatly successful at Chishui City, Guizhou Province, China. D. nobile from Chishui obtained the designation of the national geographical indication product. At present, the yield of D. nobile from Chishui has accounted for >90% of the country (Zhang et al., 2020). As the main producing area of D. nobile, Chishui City has a unique geographical environment of Plateau. D. nobile is grown at the altitude range of 230 to 800 m. The active constituents of D. nobile are primarily alkaloids, sesquiterpenes glycosides, phenanthrene, bibenzyl. The occurrence of D. nobile over a wide range of altitudinal zones makes it necessary to study the variation of its metabolic profiles under different altitude.
Due to the superiorities of high resolution, good selectivity, and shorter analysis time of ultra-high performance liquid chromatography with Q/TOF-MS (UPLC–Q/TOF-MS), the method has been widely used as a powerful tool for qualitative analysis of unknown compounds in TCM (Feng et al., 2020). In this study, UPLC-Q/TOF-MS technology was applied to obtain the metabolome data of D. nobile. The PCA was used to describe the profile of the metabolome. The OPLS-DA model was used to probe the distinct components of D. nobile in different altitude.
2 Materials and methods
2.1 Chemicals and plant samples
Acetonitrile (LC-MS grade), methanol (LC-MS grade), formic acid (LC-MS grade), and distilled water, used as mobile phase in LC-MS analysis, were purchased from Merck (Darmstadt, Germany), CNW Technologies GmbH (Duesseldorf, Germany) and Watsons (Hong Kong, China), respectively. Dendrobine (purity > 99%) was purchased from the National Institutes for Food and Drug Control (Beijing, China). Naphthalene (purity > 99%), used as internal standard, was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Under the premise of ensuring sustainable utilization and representativeness, thirty-nine batches of fresh stems of D. nobile were collected from the Good Agricultural Practices (GAP) bases located in Chishui City, Guizhou Province. Of these, twelve batches of fresh stem were collected from an altitude of 336 m. Thirteen batches were from an altitude of 528 m. The other samples were from an altitude of 692 m. The information in detail is listed in Table 1. The fresh stems were dried, ground into powder, passed through a sieve with 300 mesh, and stored at −80 °C. All D. nobile samples were identified by Dr. Daopeng Tan (School of Pharmacy, Zunyi Medical University, Zunyi, Guizhou).
Planting Place
Harvest Time
Latitude and Longitude
Altitude (m)
Number of Samples
Chishui, Guizhou
July 2019
E105°53′48″; N28°30′3″
336 m
12
E105°47′2″; N28°26′48″
528 m
13
E105°58′54″; N28°44′25″
692 m
14
2.2 Metabolites profiling in D. nobile
The pulverized powder samples (75 mg) were accurately weighed then extracted ultrasonically (400 W, 50 kHz) with 1 mL of solvent (methanol: water = 70:30, V/V) for 30 min. The extracted solution was centrifuged at a rotating speed of 12000 rpm for 5 min. Finally, the resultant supernatant was used for the UPLC-Q/TOF-MS analysis.
An aliquot of 0.1 mL of multiple batches of sample solutions were added into a 5 mL centrifuge tube and then mixed. The mixture was centrifuged at 12000 rpm for 5 min. The supernatant was used as a quality control (QC) sample.
The prepared sample extraction was analyzed by a UPLC–Q/TOF-MS system that included a 1290 Infinity II UPLC system (Agilent, MA, USA) and an Agilent 6545 Q/TOF-MS system (Agilent, MA, USA). UPLC system was equipped with a binary pump, an online degasser, an autosampler, and a thermostatically controlled column compartment. The separation of an aliquot of 0.1 μL sample solutions was performed on a Waters CORTECS UPLC C18 (100 mm × 2.1 mm, 1.6 μm), and the temperature of the column oven was maintained at 40 °C. A binary mobile phase consisted of solvent A (0.1% formic acid in water, v/v) and solvent B (0.1% formic acid in acetonitrile, v/v). Chromatographic separation was conducted at a flow rate of 0.4 mL∙min−1 following a gradient elution program: 0–0.5 min, 5% B; 0.5–4 min, 5% − 40% B; 4–5 min, 40%–75% B; 5–5.1 min, 75%–95% B; 5.1–6.5 min, 95% B; 6.5–6.6 min, 95%–5% B; 6.6–10 min, 5% B.
Mass-spectrometry detection was performed on an Agilent 6545 Q/TOF-MS system equipped with an electrospray ionization (ESI) source in both positive and negative ion modes. The optimized conditions of ESI source were as follows: gas temperature, 350 °C; drying gas flow rate, 10 L∙min−1; nebulizer, 45 psi; shealth gas temperature, 350 °C; shealth gas flow rate, 11 L∙min−1; vcap voltage, 4000 V; nozzle voltage, 1000 V; fragmentor, 175 V; skimmer voltage, 65 V. The collision energy at 20 V, 30 V, 40 V were used to obtain sufficient product ion. The scanned ions were range from 50 to 1200 m/z.
2.3 Quantification of dendrobine in D. nobile
Quantification detection of dendrobine in D. nobile followed the guidance of China Pharmacopeia (Li et al., 2017b). The specific experimental procedure was as follows: The powdered sample (0.25 g) was extracted with 25 mL of extraction solvent (0.05% formic acid (v/v) in methanol) at 80°C in the flask coupled with a condensation reflux for 3 h. The extracted solution was cooled, and then compensated the lost weight to primary volume by adding the extraction solvent. 2 mL of filtrate was diluted to 5 mL. Finally, the diluted solution that was filtered through a 0.22 μm membrane filter was used for GC analysis.
Quantification of dendrobine in D. nobile also followed the guidance of China Pharmacopeia. The content of dendrobine was analyzed using an Agilent 7820 gas chromatograph (Agilent, MA, USA) equipped with a DB-1 column (30 m X 0.25 mm X 0.25 μm, Agilent Technologies). The initial temperature of column oven was 80 °C, and then increased to 250 °C at a ramping rate of 10 °C per minute, which was held for 5 min. With a split ratio of 1:1, each 1 µL aliquot of samples was analyzed. The main parameters were as follow: the detector temperature was 250 °C, the flow rate of air, hydrogen and makeup gas was 300 mL∙min−1, 30 mL∙min−1, and 25 mL∙min−1, respectively. High purity helium was used as the carrier gas at a constant flow rate of 1 mL∙min−1.
2.4 Data pre-processing
The raw MS data were processed with Agilent Mass Hunter Profinder10.0. Briefly, the raw data were imported into the software and then subjected to peak matching, peak alignment, ion fusion, and deconvolution processing. Finally, a data matrix consisting of metabolic features with m/z value, retention time, intensity, and sample number was exported as CSV files. A total of 647, 476 features were detected with positive and negative ion modes, respectively.
2.5 Establishment of the in-house database for D. nobile
To improve the confidence of the metabolites annotation, an in-house database of Dendrobium was constructed by retrieving several databases, including Scifinder, Web of Science, ChemSpider, PubChem and some other available databases. The detailed information of 430 compounds was collected and list in an Excel document, including compound names, molecular formulaes, molecular weights, CAS numbers, and chemical structures in MOL files. These compounds that matched the determined molecular formulae were considered as potential candidates. The chemical structures of these compounds were identified by further matching with the experimental MS/MS data.
2.6 Data analysis
An unsupervised PCA and supervised OPLS-DA were performed using the SIMCA-P 14.0 software (Umetrics AB, Umea, Sweden). Other visualizations were performed in the R program (version 4.1.1) using the packages of ggplot2, pheatmap, and others. The multiple comparisons were performed using one-way ANOVA in the SPSS Statistics 18.0 (IBM, Chicago, USA). p values <0.05 was considered statistically significant.
3 Results and discussion
3.1 Profiling of metabolome in D. nobile stems with different altitude
In this study, the non-targeted metabolomics technique was used to detect the metabolic information of D. nobile cultivated at the altitude of 336 m, 528 m, and 692 m. The base peak chromatogram (BPC) profiles of QC sample and typical D. nobile samples cultivated at the altitude of 336 m, 528 m, and 692 m are displayed in Fig. 1. The results showed that the chemical compounds were mainly eluted from one to seven minutes (Fig. 1A, C). In the BPC of the positive mode, peaks of D. nobile from different altitudes showed inapparent discrepancy (Fig. 1B). However, in the BPC of the negative mode, several peaks showed increased trends in the planting altitude of 528 m (Fig. 1D).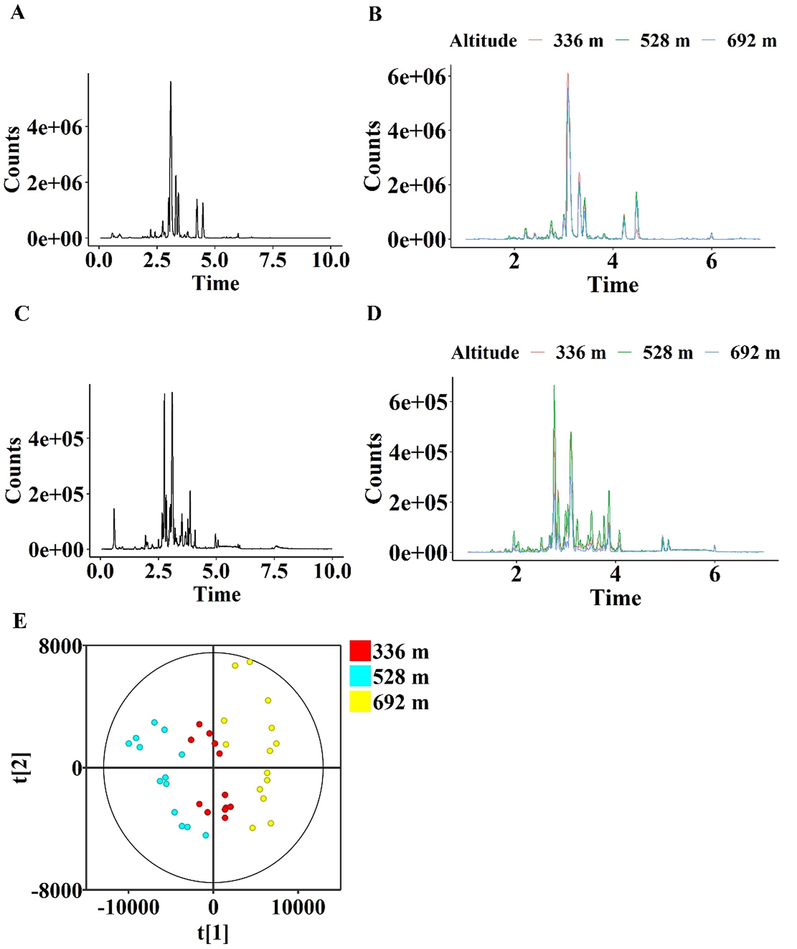
Effect of growth altitude on the profile of metabolome of D. nobile stems. (A) The BPC of positive modes of the QC sample. (B) The BPC of positive modes of the stem of typical D. nobile samples cultivated at altitude of 336 m, 528 m, and 692 m. (C) The BPC of negative modes of the QC sample. (D) The BPC of negative modes of the stem of typical D. nobile samples cultivated at altitude of 336 m, 528 m, and 692 m. (E) Score plots of PCA on metabolome profiles. Each spot represents a D. nobile sample.
All ions detected in both positive and negative modes were used for the PCA. The constructed PCA model was generated from four components and explained 52.2% of the total variance as first principal component (PC1) and second principal component (PC2) accounted for 39.0% and 13.2%, respectively. In score plots of PCA, the general profile of D. nobile grown at the altitude of 336 m, 528 m, and 692 m were observed (Fig. 1E). The samples from the three altitudes were separated in the direction of t [1], indicating that the constituents with larger loading values in component 1 were affected by the altitude factor deeper. Thus, based on the profile results of the BPC and the PCA, the relationship of metabolome in D. nobile stems with altitude was considered as an altitude-dependent style.
3.2 Discovery of the differential metabolites with an altitude-dependent in D. nobile stems
PCA was an effectual method used to describe the profile of metabolites in D. nobile from the three altitudes, but it is not sensitive enough to screen the variables with the largest correlations. However, OPLS-DA can achieve this. To further discover different chemical composition of D. nobile collected from the three altitudes. The metabolites of 39 D. nobile samples were analyzed using OPLS-DA, a multivariate statistical analysis method. The score plots of OPLS-DA are shown in Fig. 2A-C. The established three OPLS-DA model (336 m vs 528 m, 336 m vs 692 m, 528 m vs 692 m) showed good fitness (R2X = 0.418, 0.458, 0.581; R2Y = 0.843, 0.891, 0.942) and predictability (Q2 = 0.666, 0.798, 0.893). The score plots of OPLS-DA displayed samples from the same altitude were tightly clustered together, and different altitude groups were well-segregated, indicating the remarkable differences in metabolites among these three altitudes.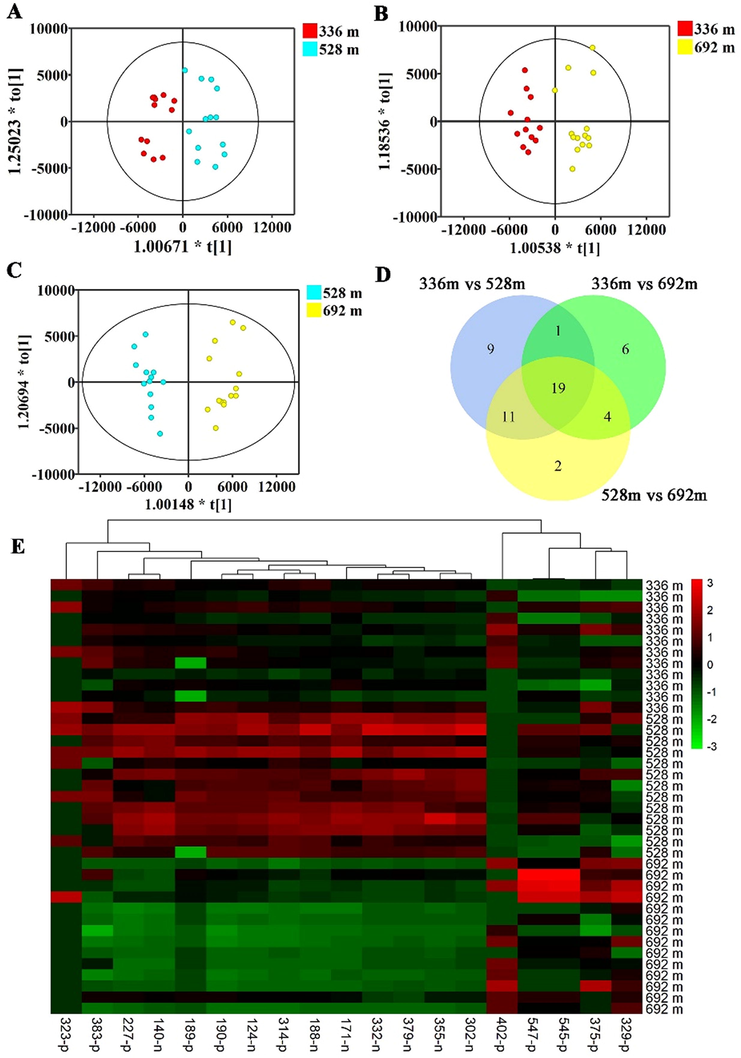
Discovery of the constituents expressed in D. nobile stems with an altitude-dependent style. (A) Sore plots of OPLS-DA of metabolites in D. nobile cultivated at altitude of 336 m and 528 m; (B) Sore plots of OPLS-DA of metabolites in D. nobile cultivated at altitude of 336 m and 692 m; (C) Sore plots of OPLS-DA of metabolites in D. nobile cultivated at altitude of 528 m and 692 m; (D) Venn diagram of differential compounds detected in the D. nobile from the altitude of 336 m, 528 m, and 692 m; (E) The one-way hierarchical clustering analysis heatmap of 19 differential compounds in all samples cultivated at altitude of 336 m, 528 m, and 692 m. p indicates positive ion mode. n indicates negative ion mode.
Furthermore, to better evaluate the changes in metabolite abundance among altitudes of 336 m, 528 m, and 692 m. The variable importance in the projection (VIP) plot was used to find the differential compounds. In this study, VIP value was set at 2.0, and 41, 31, and 37 differential metabolites were obtained in the three established OPLS-DA models, respectively. Finally, 19 common differential compounds of D. nobile between 336 m, 528 m, and 692 m were obtained (Fig. 2D). A heatmap with a one-way hierarchical clustering analysis was used to reveal the profile of 19 differential compounds in D. nobile among three altitude groups (Fig. 2E). These constituents were clustered into two clusters based on the expression pattern and the planting altitude. The content of 14 out of 19 constituents in D. nobile planted at the altitude of 528 m was higher than the other altitudes, while the content of 5 out of 19 constitutes in D. nobile planted at the altitude of 692 m was higher than the other altitudes. Thus, the above results confirmed that altitude is one of the main factors affecting secondary metabolites in D. nobile.
3.3 Qualitative analysis of the differential metabolites with an altitude-dependent in D. nobile stems
To identify the structures or structural features of the 19 differential compounds with an altitude-dependent expression style in D. nobile stems, the in-house database and literatures were used for metabolite annotation of the unknown features by comparing accurate mass and fragments. The detailed information of 19 compounds, including retention time, adducts, experimental m/z, theoretical m/z, molecular formula, identification, and MS/MS data, is displayed in the Table 1. These metabolites were classified according to their chemical structure: 11 glycosides, 6 alkaloids, and 2 unknowns. Figs. 3–5 shows the cleavage pathway and main fragments of the structure-confirmed compounds in the D. nobile.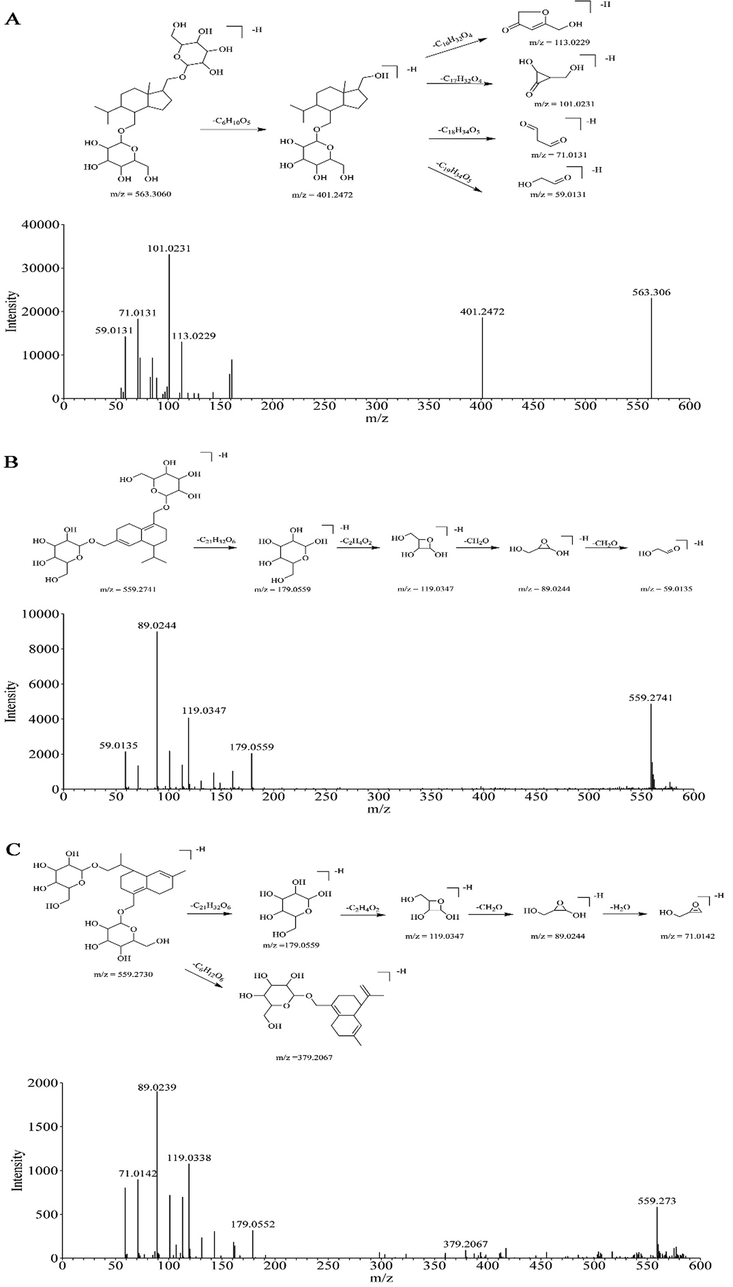
The proposed cleavage pathway and spectra of ion fragments in MS/MS analysis of Dendronobiloside A (A), Dendronobiloside C (B), and Dendronobiloside D (C).
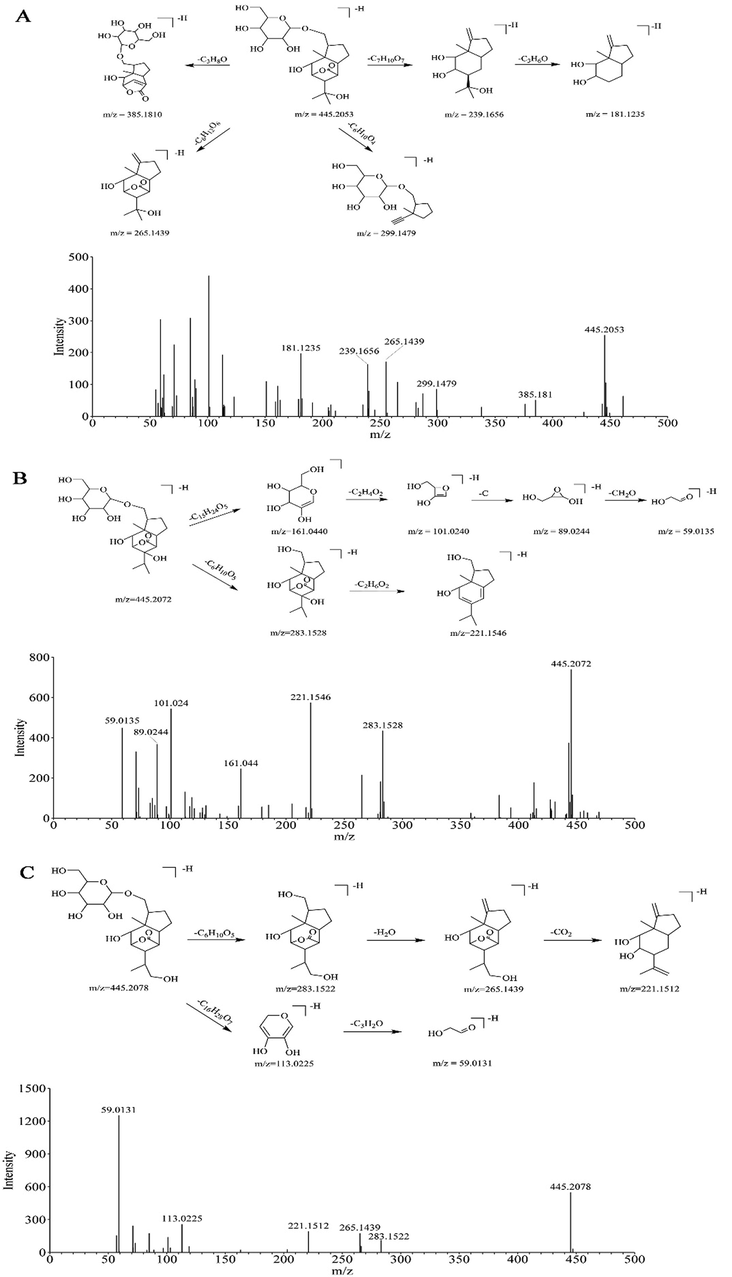
The proposed cleavage pathway and spectra of ion fragments in MS/MS analysis of Dendromoniliside D (A), Dendroside G (B), and New Compound (C).
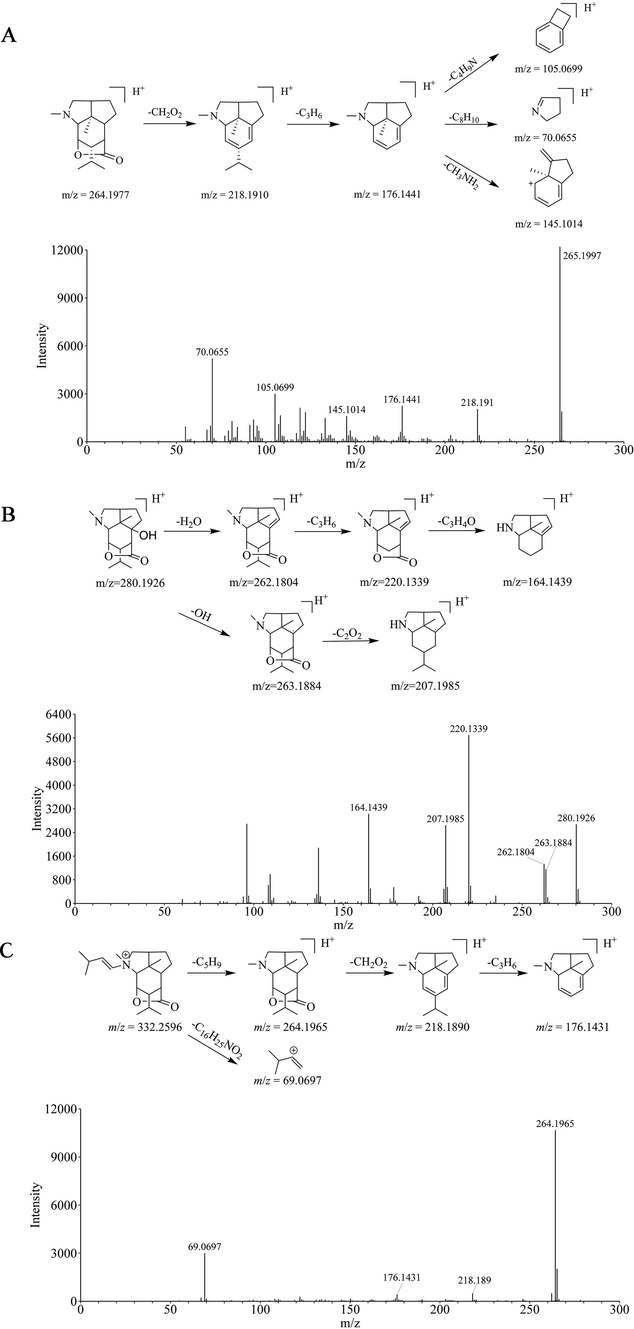
The proposed cleavage pathway and spectra of ion fragments in MS/MS analysis of Dendrobine (A), Dendramine (B), and N-isopentenyl-dendrobinium (C).
Compound 379-n showed a [M−H]− peak with an m/z value of 563.3060, with the chemical formula C27H48O12. The MS/MS spectrum of negative ion mode showed fragment ions at m/z 401.2472 [M−H−C6H10O5]−, 113.0229 [M−H−C6H10O5−C16H32O4]−, 101.0231 [M−H−C6H10O5−C17H32O4]−, 71.0131 [M−H−C6H10O5−C18H34O5]−, and 59.0131 [M−H−C6H10O5−C19H34O5]−. Compound 379-n was tentatively identified as Dendronobiloside A compared with literature (Yang et al., 2020) (Fig. 3A).
Compound 355-n displayed pseudo-molecular ions of m/z 559.2741 [M−H]−, with the molecular formula C27H44O12. In the negative ion mode, the fragment ions at m/z 179.0559 [M−H−C21H32O6]−, 119.0347 [M−H−C21H32O6−C2H4O2]−, 89.0244 [M−H−C21H32O6−C2H4O2−CH2O]−, and 59.0135 [M−H−C21H32O6−C2H4O2−CH2O−CH2O]− were observed. According to the mass spectra data obtained in this study and the previous study (Ye and Zhao 2002), compound 355-n was tentatively identified as Dendronobiloside C (Fig. 3B). Compound 302-n had the same [M−H]− ions and similar fragments ions (m/z 179.0559, m/z 119 0.0347, m/z 89.0244) as the compound 355-n. Moreover, it also showed the fragments ions at m/z 71.0142 [M−H−C21H32O6−C2H4O2−CH2O– H2O]−, and 379.2067 [M−H−C6H12O6]− (Fig. 3C). The above cleavage pathway of compounds showed the two compounds have the same parent nucleus structure. Finally, compound 302-n was tentatively identified as Dendronobiloside D (Ye and Zhao 2002).
Compound 124-n (tR = 2.75 min) showed [M−H]− ion peak of 445.2053 in the negative ion mode, with the chemical formula C21H34O10. In the MS/MS spectrum, it generated fragment ions at m/z 385.1810 [M−H−C3H8O]−, 299.1479 [M−H−C6H10O4]−, 265.1439 [M−H−C6H12O6]−, 239.1656 [M−H−C7H10O7]−, and 181.1235 [M−H−C7H10O7−C3H6O]− (Fig. 4A). Comparing the MS/MS data with the literature (Zhao et al., 2003, Yoo et al. 2021), it was tentatively identified as Dendromoniliside D. In the positive ion mode, compound 189-p displayed [M+Na]+ with an m/z value of 469.2044. The retention time (tR = 2.74) of compound 189-p was as same as compound 124-n. Thus, compound 189-p was also tentatively identified by an in-house database as Dendromoniliside D.
Compound 188-n (tR = 3.02 min) showed a [M−H]− peak with an m/z value of 445.2072, with the molecular formula C21H34O10. The MS/MS spectrum showed fragments ions at m/z 283.1528 [M−H−C6H10O5]−, 221.1546 [M−H−C6H10O5−C2H6O2]−, 161.0440 [M−H−C15H24O5]−, 101.0240 [M−H−C15H24O5−C2H4O2]−, 89.0244 [M−H−C15H24O5−C2H4O2−C]−, 59.0135 [M−H−C15H24O5−C2H4O2−C−CH2O]− (Fig. 4B). Thus, compound 188-n was tentatively identified as Dendroside G compared to the literature (Ye et al. 2002). In the positive ion mode, compound 314-p displayed [M+Na]+ with an m/z value of 469.2037 at the retention time (tR = 3.02) of compound 188-n. Therefore, compound 314-p was tentatively identified by an in-house database as Dendroside G.
Compound 140-n (tR = 2.83 min) may be a new sesquiterpene glycoside compared with the in-house database. The m/z value of the new compound is 445.2078 [M−H]−. Compound 140-n shared the same molecular formula and m/z value with 124-n, and 188-n. The MS/MS spectrum of compound 140-n showed fragment ion with m/z values of 283.1522 [M−H−C6H10O5]−, 265.1439 [M−H−C6H10O5−H2O]−, 221.1512 [M−H−C6H10O5−H2O−CO2]−, 113.0225 [M−H−C16H28O7]−, 59.0131 [M-H-C16H28O7-C3H2O]−. Moreover, based on the above fragments of compound 140-n, two fragments ion at m/z values of 283.1528, 59.0131 were as same as compound 188-n. We assume the parent nucleus structure of compound 140-n was similar to compound 188-n. The detail cleavage pathway of new compound 1 was displayed in Fig. 4C. In the positive ion mode, compound 227-p displayed the [M+Na]+ ion at m/z 469.2055 at retention time (tR = 2.82 min) of compound 140-n. Thus, compound 227-p may be a new compound.
Compound 171-n showed the [M−H]− ion at m/z 413.2173. In the negative ion mode, the six fragment ions (m/z 350.1051, m/z 293.1501, m/z 265.1447, m/z 216.1370, m/z 101.0241, m/z 59.0132) were detected in the MS/MS spectrum. According to an in-house database, compound 171-n may be Dendronobiloside E or Dendromoniliside A. Since they have the same molecular weight and fragment ions, the accurate structure needs to be further identified (Table 2). Compound 332-n displayed the [M−H]− ion at m/z 561.2917. The three fragment ions (m/z 413.2159, m/z 399.2380, m/z 119.0352) in the negative ion mode were detected in the MS/MS spectrum. However, due to a lack of reference standards, compound 332-n was tentatively identified as atractyloside D or isomer.
ID
RT
Adducts
Experimental
Theoretical
Molecular formula
Identification
MS/MS
379-n
3.91
[M−H]-
563.3060
563.3073
C27H48O12
Dendronobiloside A
401.2472;113.0229;101.0231;71.0131;59.0131.
355-n
3.77
[M−H]-
559.2741
559.2760
C27H44O12
Dendronobiloside C
179.0559;119.0347;89.0244;59.0135.
302-n
3.51
[M−H]-
559.2730
559.2760
C27H44O12
Dendronobiloside D
379.2067;179.0552;119.0338;89.0239;71.0142.
124-n
2.75
[M−H]-
445.2053
445.2079
C21H34O10
Dendromoniliside D
385.1810;299.1479;265.1439;239.1656;181.1235.
189-p
2.74
[M+Na]+
469.2044
469.2052
C21H34O10
Dendromoniliside D
309.1248;165.1274.
188-n
3.02
[M−H]-
445.2072
445.2079
C21H34O10
Dendroside G
283.1528;221.1546;161.0440;101.0240;89.0244;59.0135.
314-p
3.02
[M+Na]+
469.2037
469.2044
C21H34O10
Dendroside G
469.2044;407.2023;305.1038;247.1278.
140-n
2.83
[M−H]-
445.2078
445.2079
C21H34O10
New compound
283.1522;265.1439;221.1512;113.0225;59.0131.
227-p
2.82
[M+Na]+
469.2055
469.2044
C21H34O10
New compound
NA
171-n
2.98
[M−H]-
413.2173
413.2181
C21H34O8
Dendronobiloside E/ Dendromoniliside A
350.1051;293.1501;265.1447;216.1370;101.0241;59.0132.
332-n
3.66
[M−H]-
561.2917
561.2890
C27H46O12
Atractyloside D or isomer
413.2159;399.2380;119.0352.
329-p
3.09
[M+H]+
264.1977
264.1958
C16H25NO2
Dendrobine
218.1910; 176.1441; 145.1014;105.0699; 70.0655.
375-p
3.32
[M+H]+
280.1926
280.1907
C16H25NO3
Dendramine
263.1884;262.1804;220.1339;207.1985;164.1439.
323-p
3.06
[M+H]+
280.1905
280.1907
C16H25NO3
Isomer of Dendramine
NA
547-p
4.22
[M]+
332.2596
332.2584
C21H34NO2+
N-isopentenyl-dendrobinium
264.1965;218.1890;176.1431;69.0697.
545-p
4.22
[M+H]+
264.1958
264.1970
C16H25NO2
Fragment of N-isopentenyl-dendrobinium
176.1434;122.0970;108.0810.
383-p
3.42
[M+H]+
306.2073
306.2064
C18H27NO3
40142-19-6 or isomer
292.1860;263.1867;233.0591;175.1476;133.1012;107.0852.
190-p
2.74
[M+H]+
471.1812
471.1802
C29H26O6
Unknown
NA
402-p
3.50
[M+H]+
263.1267
263.1278
C15H18O4
Unknown
NA
Compound 329-p was identified as dendrobine, which belongs to dendrobine-type alkaloids, compared with the standard. Dendrobine showed a [M+H]+ parent ion peak with an m/z value of 264.1977 (C16H25NO2). In the MS/MS spectrum of the positive ion mode, it generated fragment ions at m/z 218.1910 [M+H-CH2O2]+, 176.1441 [M+H-CH2O2-C3H6]+, 145.1014 [M+H-CH2O2-C3H6-CH3NH2]+, 105.0699 [M+H-CH2O2-C3H6- C4H9N]+, 70.0655 [M+H-CH2O2-C3H6- C8H10]+ (Fig. 5A).
Compound 375-p (tR = 3.32 min) showed [M+H]+ ions with an m/z value of 280.1926, with a molecular formula of C16H25NO3. A fragment ion at m/z 262.1804 in the positive ion mode was obtained by losing a H2O. Another fragment ion at m/z 263.1884 was obtained by losing a hydroxyl. Other predominant fragment ions appeared at m/z 220.1339 [M+H-H2O-C3H6]+, 164.1439 [M+H-H2O-C3H6-C3H4O]+, 207.1985 [M+H-OH-C2O2]+ (Fig. 5B). Compound 375-p was identified using an in-house database and literatures as Dendramine (Inubushi et al. 1966, Okamoto et al. 1966). Compound 323-p displayed [M+H]+ ions with an m/z value of 280.1905, with the same molecular formula as compound 375-p. Moreover, the retention times (tR = 3.06 min) of compound 323-p were closed to compound 375-p. Thus, compound 323-p was tentatively deduced as an isomer of Dendramine using an in-house database.
Compound 547-p (tR = 4.22 min) produced an [M]+ ion at m/z 332.2596 (C21H34NO2+), and the characteristic fragment ions at m/z 264.1965 by losing side chain (C5H9) on nitrogen were obtained. Interestingly, the ion was found in agreement with traits of the dendrobine [M+H]+ ion. Moreover, fragment ions at m/z 218.1890, and 176.1431 were also in accord with the fragment ions of dendrobine. Thus, compound 547-p was tentatively deduced as N-isopentenyl-dendrobinium comparing with the literature (Wang et al., 2016). Compound 545-p (tR = 4.22 min) produced an ion with m/z 264.1958 [M+H]+ that was the same as the with fragment ion of compound 547-p. Due to the same retention time as compound 547-p, compound 545-p was tentatively characterized as a fragment of N-isopentenyl-dendrobinium.
Compounds 383-p display the [M+H]+ ion at m/z 306.2073. The six fragment ions (m/z 292.1860, m/z 263.1867, m/z 233.0591, m/z 175.1476, m/z 133.1012, m/z 107.0852.) were detected in the MS/MS spectrum of the positive ion mode. Since the above fragment ions didn’t belong to the characteristic fragment ions of 1H-Cyclopent[cd]indole-5-carboxylic acid, decahydro-1,7b-dimethyl-7-methylene-6-(1-methylethyl)-2-oxo-, methyl ester, (2aa,4aa,5b,6a,7aa,7ba) - (9CI) (CAS Registry Number: 40142-19-6). Hence, compound 383-p was tentatively identified as 40142-19-6 or isomer. Meanwhile, compounds 190-p, 402-p showed the [M+H]+ ion at m/z 471.1812 and 263.1267, respectively. The molecular formula of the two compounds was calculated as C29H26O6 and C15H18O4 using Qualitative Analysis B.07.00 software. However, fragment ions of compounds 190-p, 402-p were not detected due to the weak intensity. Therefore, the structures of the above compounds were still not clear.
3.4 Semi-quantitative analysis of the 12 structure-confirmed altitude-dependent constituents and quantitative analysis of dendrobine
The structures of a total of 12 compounds were confirmed by combining the MS/MS spectrum data. They were classified into alkaloids and sesquiterpene glycosides. Data of peak area were performed a student's t test that was employed to understand that the altitude-dependent style of those structure-confirmed constituents was an apparent trend and statistically significant (Fig. 6). Among all structure-confirmed alkaloids, the content of dendrobine (compound 329-p) of D. nobile cultivated at the altitude of 692 m was the significantly highest compared to 528 m (p < 0.05). However, Dendramine (compound 375-p) and N-isopentenyl-dendrobinium (compound 547-p) failed to pass the test between the two groups. Moreover, the content of sesquiterpene glycosides, including Dendronobiloside A (compound 379-n), Dendronobiloside C (compound 355-n), Dendronobiloside D (compound 302-n), Dendromoniliside D (compound 124-n, 189-p), new compounds (compound 140-n, 227-p), and Dendroside G (compound 188-n, 314-p) of D. nobile cultivated at the altitude of 528 m were the highest (p < 0.05) and passed the test between the two groups.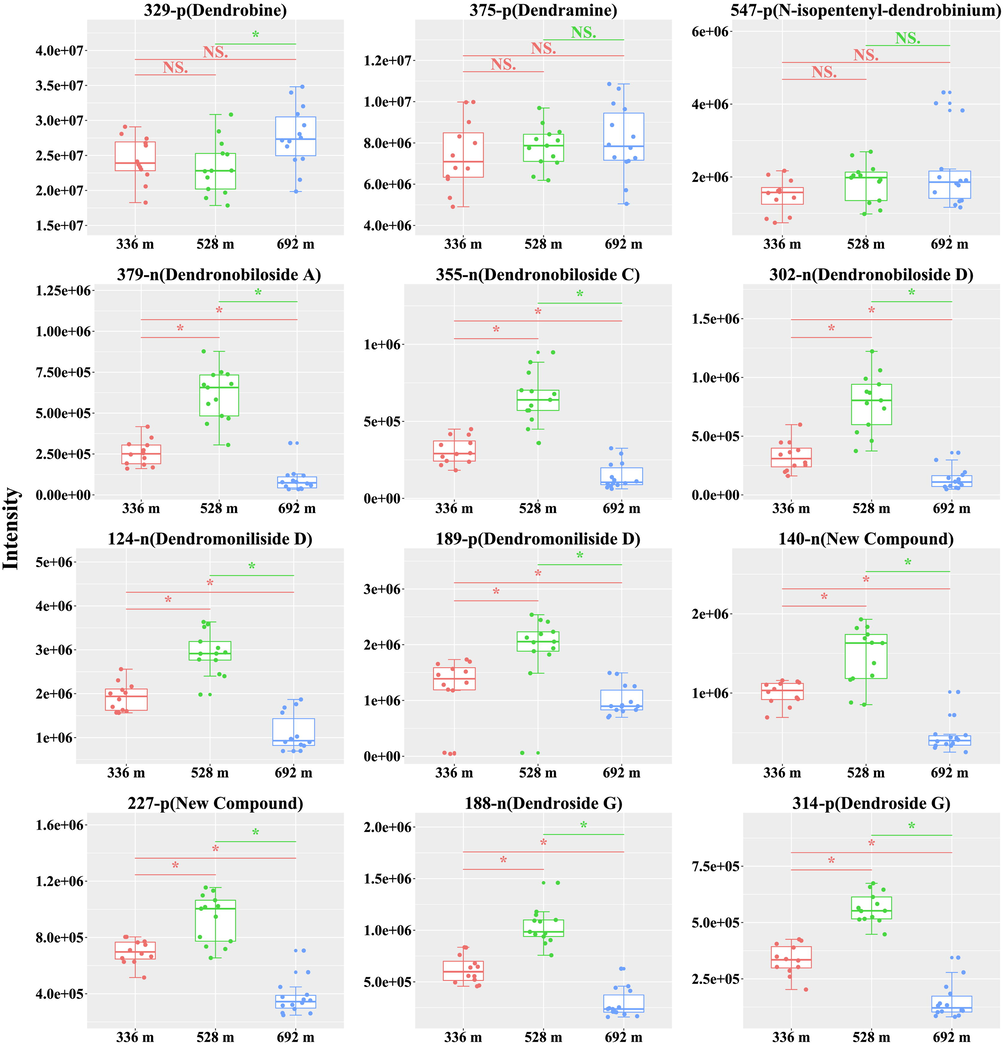
Comparing intensity of 12 identified compounds in the stems of D. nobile from different altitude. The asterisk indicates significant differences (*P < 0.05) and the letters “ns” indicate no significant differences.
Dendrobine is used to be considered as a represented constituent of D. nobile and used as an index to control quality of D. nobile in some countries or regions like China, we conducted an absolute quantification detection of dendrobine in D. nobile using gas chromatography. The absolute quantification of dendrobine was used to perform the statistic results using percentage content. The same as the data from mass spectrometry, data from the GC also reassured that dendrobine content in D. nobile showed a altitude-dependent style. The difference of dendrobine content between the altitude of 336 m and 528 m was not passed the statistical examination. Moreover, the difference of dendrobine content between the altitude of 336 m and 692 m, 528 m and 692 m also passed the statistical examination (p < 0.05, Fig. 7). Thus, the study showed the altitude-dependent expression pattern of metabolites in D. nobile based on the visualized data and the statistically significant level. The above compounds could be able to use as the marker to identify D. nobile from different planting altitude.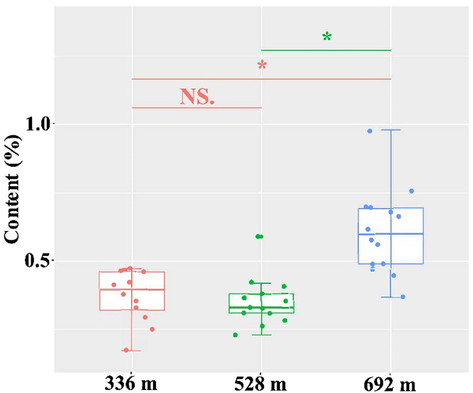
Comparing percentage content of dendrobine in the stems of D. nobile from different altitude. The asterisk indicates significant differences (*P < 0.05) and the letters “ns” indicate no significant differences.
In most cases, the content of alkaloids, a class of natural-existing nitrogen-containing compounds in plants, increased along with the altitude. It has been shown that total alkaloids were expressed in an altitude-dependent style, at least in some famous medicinal plants such as Meconopsis quintuplinervia, Coptis chinensis (Yang et al., 2006; Liu et al. 2016). Moreover, to data, the content of some alkaloids, including isomitraphylline, berberine and palmatine isolated from Uncaria tomentosa, and Coptis teeta were increased while the plants were cultivated at high altitudes (Zhang et al., 2008, Honório et al. 2017). Thus, it was not surprising that the content of alkaloids such as dendrobine was highest at an altitude of 692 m in the present study.
Glycosides were also broadly expressed in lots of plants. In most cases, the contents of flavonoid glycosides are isolated from the medical herbs such as Hippophae rhamnoides, Ginkgo biloba, Indocalamus latifolius, and Calluna vulgaris were the positive altitude-dependent (Rieger et al. 2008, Kaur et al. 2012, Ni et al. 2013, Ma et al. 2016). Meanwhile, the anthraquinone glycoside content in rhubarb and saikosaponins of Bupleuri Radix showed the same trend as flavonoid glycosides (Wang et al., 2013, Li et al., 2017a). However, the expression level of sesquiterpene-type glycosides such as Dendronobiloside A, Dendronobiloside C, Dendronobiloside D, Dendromoniliside D, New compound, Dendroside G in D. nobile cultivated at the altitude of 528 m was increased. The altitude-dependent expressions of the sesquiterpene-type glycosides were reported for the first time.
4 Conclusion
In conclusion, alkaloids and sesquiterpene glycosides are the main compound affected by altitude. This result was shown as follows: the contents of alkaloids in stems of D. nobile were found to increase at an altitude of 692 m, whereas the content of sesquiterpene glycosides was increased at an altitude of 528 m. Combining the different pharmacological effects of alkaloids and sesquiterpene glycosides, we conclude the following recommendations for clinical treatment: D. nobile cultivated at the altitude of 692 m is more suitable for treating neurological diseases, while D. nobile cultivated at the altitude of 528 m is more suitable for improving immunity. Thus, in addition to dendrobine, it is worth considering that sesquiterpene glycosides might be another kind of quality-related constituent and could also use as a quality marker in D. nobile.
Funding
The study was supported by the Department of Science and Technology of Guizhou Province (nos. QKHZC [2019]2961, QKHZC [2020]4Y072, QKHZC[2021]420), Zunyi City of China (ZSKHHZ [2021]188), the Science and Technology Innovation Action Plan of Domestic Science and Technology Cooperation Projects in Shanghai (20025800400), Department of Education of Guizhou Province (QJHKY [2021]049), and Guizhou Engineering Research Center of Industrial Key-technology for Dendrobium Nobile (QJJ[2022]048 and QJJ [2022]006).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper..
References
- An assessment of the Chinese medicinal Dendrobium industry: Supply, demand and sustainability. J. Ethnopharmacol.. 2019;229:81-88.
- [Google Scholar]
- An integrated strategy for discovering effective components of Shaoyao Gancao decoction for treating neuropathic pain by the combination of partial least-squares regression and multi-index comprehensive method. J. Ethnopharmacol.. 2020;260:113050.
- [Google Scholar]
- Start codon targeted (SCoT) and target region amplification polymorphism (TRAP) for evaluating the genetic relationship of Dendrobium species. Gene. 2015;567(2):182-188.
- [Google Scholar]
- Genetic and chemical diversity of Uncaria tomentosa (Willd. ex. Schult.) DC. in the Brazilian Amazon. PLoS One. 2017;12(5):e0177103.
- [Google Scholar]
- Alkaloids of dendrobium nobile lindl. Altered hepatic lipid homeostasis via regulation of bile acids. J. Ethnopharmacol.. 2019;241:111976
- [Google Scholar]
- Spatial and temporal variation of secondary metabolite profiles in Ginkgo biloba leaves. Chem. Biodivers.. 2012;9(2):409-417.
- [Google Scholar]
- Research on topographic factors of ecology suitability regionalization of Bupleuri Radix in Hebei province. Zhongguo Zhong Yao Za Zhi. 2017;42(22):4402-4407.
- [Google Scholar]
- Influence of light intensity and water content of medium on total dendrobine of Dendrobium nobile Lindl. Asian Pac. J. Trop. Med.. 2017;10(11):1095-1100.
- [Google Scholar]
- Cultural regionalization for Coptis chinensis based on 3S technology platform Ⅰ. Study on growth suitability for Coptis chinensis based on ecological factors analysis by Maxent and ArcGIS model. Zhongguo Zhong Yao Za Zhi. 2016;41(17):3186-3193.
- [Google Scholar]
- Dendrobium nobile Lindl. Alkaloids Ameliorate Cognitive Dysfunction in Senescence Accelerated SAMP8 Mice by Decreasing Amyloid-β Aggregation and Enhancing Autophagy Activity. J. Alzheimers Dis.. 2020;76(2):657-669.
- [Google Scholar]
- Flavonol glycosides in berries of two major subspecies of sea buckthorn (Hippophaë rhamnoides L.) and influence of growth sites. Food Chem.. 2016;200:189-198.
- [Google Scholar]
- Altitudinal variation of antioxidant components and capability in Indocalamus latifolius (Keng) McClure leaf. J. Nutr. Sci. Vitaminol. (Tokyo). 2013;59(4):336-342.
- [Google Scholar]
- The structure of dendramine(6-oxydendrobine) and 6-oxydendroxine. The fourth and fifth alkaloid from Dendrobium nobile. Chem. Pharm. Bull. (Tokyo). 1966;14(6):676-680.
- [Google Scholar]
- Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem.. 2008;56(19):9080-9086.
- [Google Scholar]
- Tandem Mass Spectrometry for Structural Identification of Sesquiterpene Alkaloids from the Stems of Dendrobium nobile Using LC-QToF. Planta Med.. 2016;82(7):662-670.
- [Google Scholar]
- Evaluation of the content variation of anthraquinone glycosides in rhubarb by UPLC-PDA. Chem. Cent. J.. 2013;7(1):170.
- [Google Scholar]
- Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev.. 2013;12(2):341-367.
- [Google Scholar]
- Comparison of metabolomics of Dendrobium officinale in different habitats by UPLC-Q-TOF-MS. Biochem. Syst. Ecol.. 2020;89:104007-104012.
- [Google Scholar]
- Analysis of total alkaloids in Meconopsis quintuplinervia from different localtites of Qinghai. Zhong Yao Cai. 2006;29(5):430-432.
- [Google Scholar]
- Immunomodulatory sesquiterpene glycosides from Dendrobium nobile. Phytochemistry. 2002;61(8):885-890.
- [Google Scholar]
- New alloaromadendrane, cadinene and cyclocopacamphane type sesquiterpene derivatives and bibenzyls from Dendrobium nobile. Planta Med.. 2002;68(8):723-729.
- [Google Scholar]
- UPLC-ESI-Q-TOF-MS-Based Metabolite Profiling, Antioxidant and Anti-Inflammatory Properties of Different Organ Extracts of Abeliophyllum distichum. Antioxidants (Basel). 2021;10(1):70-84.
- [Google Scholar]
- Variation patterns of Coptis teeta biomass and its major active compounds along an altitude gradient. Ying Yong Sheng Tai Xue Bao. 2008;19(7):1455-1461.
- [Google Scholar]
- Technical evaluation and principle analysis of simulative habitat cultivation of Dendrobium nobile. Zhongguo Zhong Yao Za Zhi. 2020;45(9):2042-2045.
- [Google Scholar]
- Copacamphane, picrotoxane, and alloaromadendrane sesquiterpene glycosides and phenolic glycosides from Dendrobium moniliforme. J. Nat. Prod.. 2003;66(8):1140-1143.
- [Google Scholar]
- Three new sesquiterpene glycosides from Dendrobium nobile with immunomodulatory activity. J. Nat. Prod.. 2001;64(9):1196-1200.
- [Google Scholar]
- Genome-wide researches and applications on Dendrobium. Planta. 2018;248(4):769-784.
- [Google Scholar]







