Translate this page into:
Valorization of walnut husks as a natural coagulant for optimized water decolorization
⁎Corresponding author. zourifali@gmail.com (Ali Zourif),
⁎⁎Corresponding author. a.benbiyi@gmail.com (Asmaa Benbiyi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Coagulation-flocculation is an essential wastewater treatment and decolorization process. This study investigates walnut husks, an abundant agricultural waste, as a biocoagulant. The aim is to evaluate the coagulation efficacy of walnut husk powder (WHP) for removing methylene blue (MB) and turbidity (TUR) from water. WHP was prepared and characterized using Fourier transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM-EDX), and X-ray fluorescence spectrometry (XRF) techniques. Jar tests were conducted in varying doses, granulation, and the initial pH. Box Behnken Design based on the response surface method (BBD-RSM). Predicted MB and TUR eliminations matched experimental values. Under optimal conditions at pH 9, 89.48 % MB and 96.59 % TUR removal were attained at 900 mg. L-1 and 1000 mg. L-1 doses. Results prove that WHP shows promise as an efficient biocoagulant for the removal of dyes and turbidity from wastewater.
Keywords
Walnut husks
Coagulation-Flocculation
Box-Behnken
Response Surface Method Methylene Blue
Turbidity
1 Introduction
Each year, more than seven million tons of dye are produced worldwide. Various industries use these dyes, including paper, plastics, food, and cosmetics; the most important is the textile industry (Afkhami and Moosavi, 2010). Color is the primary contaminant recognized in wastewater. A quantity contaminated with these dyes, even at low concentrations, leads to a potential risk to human health and the ecosystem, as the colored effluents would interfere with photosynthesis (Gita et al., 2017). The byproducts of the degradation of these dyes also have a dangerous impact on the environment as they contain toxic aromatic amino compounds (Konstantinou and Albanis, 2004). Among these dyes, methylene blue (MB) is the industry's most classical and widely used dye (Domga et al., 2022). It is a basic dye used in mouthwash to reduce the viral load in the mouth and is a component of sunscreens (Arakeri and Rao US, 2021). It is also applied to colored textile products such as silk, linen, cotton, wool, and jute (Khan et al., 2022). MB is highly toxic and poses several health risks to humans upon exposure, including nausea, vomiting, eye injuries, and the development of methemoglobinemia (Khomri et al., 2022).
Therefore, it is important to develop innovative, low-cost processes by which dye molecules treated. Many techniques have recently been employed to remove hazardous dyes from industrial effluents to mitigate damage to the aquatic environment and improve the quality of discharged water, such as electrochemical and biological treatments (Qu and Liang, 2022) (Khalil and Liu, 2021), solvent extraction (Zhang et al., 2022), oxidation (Hodges et al., 2018), adsorption (Rashid et al., 2021), and coagulation-flocculation (Zhao et al., 2021; Benbiyi et al., 2022). However, the coagulation-flocculation technique is the most preferred method of wastewater treatment (Teh et al., 2016), due to its simplicity, rapidity, high efficiency, and availability of coagulants-flocculants (Zhang et al., 2018a, 2018b). The most common coagulants-flocculants used in the treatment of industrial effluents are aluminum sulfate (Zhou et al., 2008), ferric chloride (Farajnezhad and Gharbani, 2012), polyacrylamide (PAM) (Huang et al., 2016), and poly aluminum chloride (PAC) (Zhang et al., 2018a, 2018b). These coagulant-flocculants give a very interesting yield that can remove up to 100 % of a pollutant.
Despite the advantages of these last compounds, they present several disadvantages that limit their use such as toxicity, high costs, and the fact that they generate a lot of sludge (Verma et al., 2012). Biocoagulants-flocculants represent a significant advance in wastewater treatment, offering innovative solutions to the drawbacks associated with traditional chemical coagulants and flocculants. These substances are mainly derived from agricultural waste. Agricultural waste used as biocoagulants and flocculants often comes from renewable natural sources, such as fruit, vegetables, and plants. In this context, materials such as banana peels (Priyatharishini et al., 2019), Moringa oleifera pits (Landázuri et al., 2018), cassava peels (Mohd-Asharuddin et al. 2018), and Aloe vera (Benalia et al., 2022) are particularly valuable. These materials are generally considered to be by-products or waste products of the agricultural industry, which makes them abundantly available and inexpensive.
The efficiency of pollutant removal is strongly influenced by the active components of these biocoagulants. These active substances are responsible for coagulation, for example, cellulose, lignin, proteins, tannins, polyphenols, polysaccharides, and starches (Kurniawan et al., 2022). It is worth noting that these biocoagulants can be used directly in their natural (Mohd-Asharuddin et al. 2018) form or through the extraction of their active compounds for example, extraction of tannins from Acacia catechu bark (Azreen et al., 2021).
Currently, Morocco ranks twentieth in the world for the production of walnuts (Juglans regia L), with 7702 ha and more than 12,467 tons of nuts annually (Houmanat et al., 2021). Walnut husks are fruit wastes that disposed of after consumption in uncontrolled landfills. Nevertheless, they have proven an excellent capacity in the removal of heavy metal ions (Pehlivan and Altun, 2008), organic compounds (Gallo-Cordova et al., 2017), and dyes (Miyah et al., 2018) in the treatment of polluted water.
While walnut husks have demonstrated promising adsorption capacity for removing various pollutants, their potential as a coagulating agent has not yet been explored to date. However, given their composition rich in tannins and polyphenols (Kabiri et al. 2019), walnut husks are likely to exhibit similar coagulation properties as other natural biomaterials such as Moringa oleifera. The functional groups present on their surface could destabilize charged colloids through charge neutralization and inter-particle bridging. Moreover, walnut husks represent an abundant bio-sourced waste material in Morocco (Houmanat et al., 2021). Their valorization as a natural, eco-responsible coagulant for water treatment would enable more sustainable management of this bioresource. For these reasons, we have undertaken to evaluate for the first time the coagulation potential of walnut husks, thereby opening up new possibilities for this promising agent.
The main objective of this research project is to investigate the effectiveness of WHP in removing pollutants from contaminated water using the coagulation-flocculation process. To accomplish this goal, WHP powder was prepared and characterized using various analytical techniques, including FTIR, XRD, and XRF. In order to determine the optimal conditions for pollutant removal, the study employed the Box-Behnken design and Response Surface Methodology (BBD-RSM). Specifically, the effects of several parameters (dose, granulation, and initial pH) and their interactions examined concerning the removal of two specific pollutants, namely MB and TUR. The results of this study could provide valuable insights into the use of WHP as a coagulant or flocculant in the treatment of polluted water. By identifying the optimal conditions for pollutant removal, this research could help to improve the efficiency of the coagulation-flocculation process and contribute to the development of more effective, sustainable, and cost-effective solutions for the treatment of polluted waters.
2 Materials and methods
2.1 Reagents
MB is also known as base blue 9 (CAS number 61-73-4, chemical formula C16H18ClN3S, molecular weight 319.9 g. mol−1 and λmax = 662 nm) was used as a dye. Hydrochloric acid (HCl) (Sigma-Aldrich 37 %, CAS number 7647-01-0) and Sodium hydroxide (NaOH) (Solvachim 99 %, CAS number 1310-73-2) are used to adjust the initial pH of the pollutant solution.
2.2 Preparation and characterization of biocoagulant WHP
Walnut husks were collected from a local region near Ouarzazate (Skoura 31° 4′ 2″ N, 6° 33′ 55″ W), Morocco. They were selected as a natural coagulant due to their demonstrated effectiveness, economic feasibility, and abundant availability. The Walnut husks were thoroughly washed with distilled water to eliminate dirt, dust, and other surface contaminants. After cleaning, the Walnut husks were crushed using a mill, the powder was washed with distilled water and dried at 105 °C (Arulmathi et al., 2019). The powder obtained was then sieved through sieves with fractions between 112 and 63 µm. The obtained powder was stored in a dry place to avoid any possible alteration.
Different techniques were used to evaluate the WHP before and after the coagulation process. The mineralogy of WHP was undertaken by a Burker D8 ADVANCE X-ray diffractometer with a Cu λ(CuKα) = 1.5418 Å anticathode, the data were recorded by Xpert software. FT-IR spectra were obtained using a Bruker Tensor 27 FTIR spectrometer operating in the 4000–400 cm−1 range. Scanning electron microscopy coupled with energy dispersive X-ray spectroscopy (SEM-EDX) analysis of WHP was performed by Hirox microscopy to determine the morphology and elements present in the powder before and after coagulation. The chemical composition of WHP was determined by XRF using an epsilon 4 X-ray fluorescence spectrometer (Malvern Panalytical Company).
2.3 Experimental procedure
A precisely dosed amount of dye was dissolved in distilled water to prepare the mother solution. The solution (50 mg. L-1) was prepared by diluting the mother solution with distilled water. A turbid water solution at a concentration of 2.5 g. L-1 was prepared by stirring kaolin in distilled water at 50 Rpm for 2 h. After stirring, the solution was allowed to stand for 24 h for complete hydration of the kaolin.
WHP was added to colored or turbid water samples in doses of 300, 700, and 1000 mg. L-1 defined by the experimental design. The initial pH of each solution was adjusted using 0.1 M HCl and NaOH before testing. The solutions underwent rapid mixing by jar test (VELP Scientifica JLT 4 Flocculator) at 300 Rpm for 3 min, followed by slow agitation at 60 Rpm for 20 min. Flocculation was then allowed by sedimentation for one hour. The clarified supernatant was collected and analyzed. Settled particles (sludge) were also recovered by filtration for analysis.
2.4 Pollutants reduction percentage calculation
The percentage removal of MB after coagulant treatment was calculated using Equation (Eq.1) using the UV- 6300 PC and the percentage removal of TUR is determined by Equation (Eq.2) using a VELP turbidimeter:
Ci: Initial reading of parameter before treatment.
Cf: Final reading of parameter after treatment.
To: Initial turbidity value.
Tf: Final turbidity value at each measurement point.
2.5 Box-Benhken design (BBD)
The traditional method uses single-factor experiments by changing one variable at a time. However, this method makes it difficult to study the optimization effect between variables (Ölmez, 2009). Therefore, the BBD-RSM was used to improve the study. RSM is a set of techniques that relate experimental factors to responses according to one or more criteria (Amini et al., 2008).
The advantages of the Box Behnken planes include that they are all spherical and only require the use of three factors. These planes are also rotatable and provide orthogonal locking (Watson et al., 2015). This design is not fully functional but provides minimal effort and accurate results. This optimization process consists of three steps. First is performing an ANOVA, which is a statistical technique that decomposes the total variance of a data set into fractions associated with specific sources of variance to test hypotheses about the model parameters. The second is estimating the coefficient of the mathematical model. The third is predicting the response and verification of the model fit (Ferreira et al., 2007).
The performance of the coagulation-flocculation process can depend on several parameters. The factors at three levels were varied to study the optimization of biocoagulation-flocculation parameters, such as dose (X1), initial pH (X2), and granulation (X3). The choice of these factors is not arbitrary and depends on the specific application and the type of pollutants present in the water. Two types of pollutants are MB and TUR, and they are influenced by the dose of the coagulant, the initial pH, and the granulation of the coagulant.
It is important to note that there is a range of optimal doses for each coagulant employed. If the dose is too low, particles will not be removed effectively, while if it is too high, the water may become excessively cloudy and may also be toxic. Therefore, the dose optimal of the coagulant used plays a critical role in the effectiveness of the process. The charge and structure of different polymeric coagulants change as a function of the pH because their functional groups accept protons or dissociate, depending on the pH value, such as organic coagulants (chitosan, banana peels, Moringa oleifera) or synthetic coagulants based on polyacrylamide. Therefore, the initial pH is a very influential factor in the coagulation-flocculation process (Naceradska et al., 2019). The granulation or size distribution of coagulants had a distinct influence on the mechanism of the coagulation-flocculation process. Nanoparticles cause self-aggregation due to Brownian motion, while microparticles are affected mainly by two physical processes, interception and sedimentation, because they are more stable and disperse easily (Sun et al., 2019).
The quadratic equation (Eq.3) explains the performance of the system:
Y is the process response, k is the number of patterns, i and j are the index numbers for the pattern, βo is the free term, x1, x2…, xk are the independent variables, βi is the first order effect, βii is the quadratic effect, βij is the interaction effect, and ε is the random error between the predicted and experimental values.
3 Results and discussion
3.1 Characterization of biocoagulant
The XRD pattern of the WHP shows a semi-crystalline structure in Fig. 1. The amorphous region can be attributed to the lignin content of the biomass, and the crystalline peaks can be attributed to the cellulose fraction (Okolo et al., 2021). Moreover, it indicates that there is no significant change in the semi-crystalline structure of the WHP after coagulation of the MB dye. Therefore, the crystallinity is not affected by the dye molecule (Basu et al., 2017). The following bands are observed in the FTIR spectrum (Fig. 2), a strong band at 3400 cm−1 which is attributed to the O—H group; a clear band at 2935 cm−1 is attributed to the C—H stretching (Mashkoor et al., 2018); two bands at 1737 cm−1 and 1615 cm−1 correspond to the carbonyl groups (—COOH, —COOCH3) of the carboxylic acid, a small band at 1396 cm−1 is assigned to OH bending; 1046 cm−1 is attributed to the C—O stretching of cellulose; the significant band at 609 cm−1 belongs to the yellow pigment of the nut husks (Uddin and Nasar, 2020).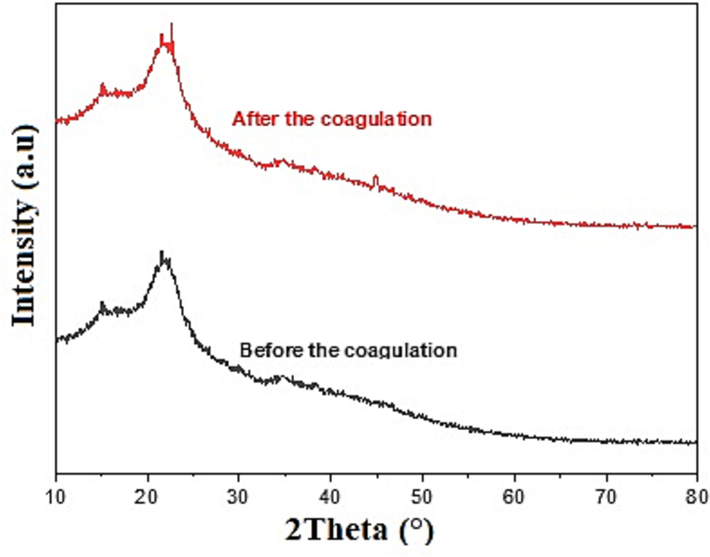
XRD pattern of WHP before and after the coagulation.
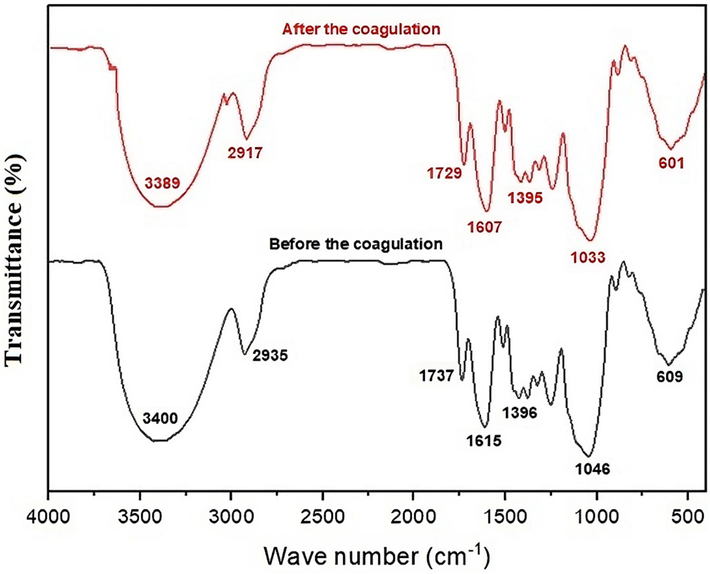
FTIR of WHP before and after the coagulation.
During the flocculation coagulation process, different functional groups are involved, which is demonstrated by the changes in intensity and position of the peaks in the WHP after the process. Fig. 2 shows a shift in peaks after flocculation coagulation, indicating the involvement of different functional groups in the process. Specifically, the involvement of alcoholic and carboxylic groups that have negative centers is evident in the coagulation-flocculation of MB by WHP. These functional groups have strong attractive forces with the positive centers of the MB dye, which are cationic. Therefore, they play an active role in the process, resulting in a shift in the peaks observed in WHP. Overall, the changes in the WHP peaks provide valuable insights into the involvement of different functional groups during flocculation coagulation, which can help optimize the process for the efficient removal of cationic dyes from wastewater.
SEM showed that the powder consists of small particles with sizes of about 20 μm and a homogeneous texture. The particles have different shapes and are distant from each other (Fig. 3). EDX analysis of the WHP before and after coagulation-flocculation was performed to judge the adhesion of MB molecules to the coagulant. The EDX results presented in Table 1 show that the percentage of Ca, Fe, Al, and Si elements present in the WHP decreases after coagulation-flocculation therefore, it is suggested that these elements have the power to destabilize the polluted water. Changes in the weight of C and O atoms were also observed, and clearly due to the adsorption of the dye molecule (Mashkoor et al., 2018). Chemical analysis by XRF showed that the WHP contains mainly organic matter (88.348 wt%), with a high content of CaO (5.653 wt%) and Fe2O3 (2.523 wt%) and the other minerals are trace elements (Table 2). This analysis confirms and coheres well with the EDX analysis.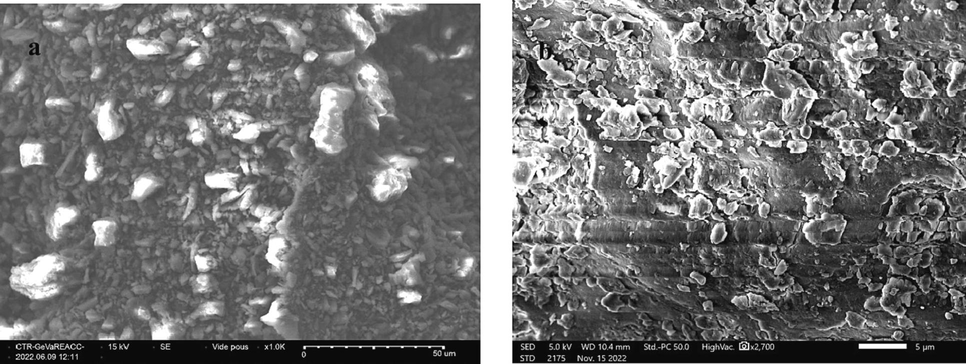
SEM of WHP before (a) and after (b) the coagulation.
Element
Weight (%) before
Weight (%) After
C
66.15
68.85
O
27.63
28.03
Ca
2.65
1.44
Fe
1.54
0.58
Al
0.93
0.31
Si
0.48
0.30
K
0.42
0.27
Mg
0.10
0.06
S
0.10
0.06
Sample
MgO
Al2O3
SiO2
Fe2O3
P2O5
CaO
K2O
SO3
Cr2O3
Traces
Before
0.240
0.176
0.576
2.523
0.390
5.653
1.332
0.139
0.186
0.438
After
0.015
0.094
0.277
0.894
0.357
2.789
1.007
0.084
0.180
0.431
3.2 BBD-RSM statistical analysis
WHP was used as a coagulant for MB and TUR polluted water. Each experiment was repeated three times to observe reproducibility, and the result was subjected to a three-step process. Table 3 gives the experimental range and levels of the independent variables in this study. The independent variables were coded as −1 (low), 0 (medium), and +1 (high).
Independent variables
Factor Code
Range and levels
−1
0
+1
Dose (mg. L-1)
X1
300
700
1000
initial pH
X2
3
6
9
Granulation (µm)
X3
63
80
112
Table 4 shows the experimental results of the two responses and the predicted results by the JMP Pro 16 software using the BBD-RSM.
N°
Factors
Experimental values
Predicted values
X1
X2
X3
MB removal % (Y1)
TUR removal % (Y2)
MB removal % (Y’1)
TUR removal % (Y’2)
1
300
3
80
83.17
85.08
79.92
85.52
2
300
9
80
76.06
86.07
75.92
86.72
3
1000
3
80
76.93
90.96
76.54
90.27
4
1000
9
80
95.88
92.36
99.65
91.94
5
300
6
63
76.07
89.13
77.42
88.87
6
300
6
112
75.46
86.12
77.72
86.61
7
1000
6
63
92.05
92.83
88.66
92.25
8
1000
6
112
89.93
84.16
89.68
84.49
9
700
3
63
74.73
85.87
77.80
85.49
10
700
3
112
87.01
92.29
85.95
93.48
11
700
9
63
74.83
86.18
75.13
85.44
12
700
9
112
91.46
84.84
89.12
84.75
13
700
6
80
74.56
89.31
75.01
89.39
14
700
6
80
74.70
89.47
75.01
89.39
15
700
6
80
75.80
89.42
75.01
89.39
The relationship between the observed values of MB and TUR removal and the values predicted by the fitted regression models (Fig. 4) shows that the clustering of points around the diagonal line indicates a good correlation between the values obtained in the experiment and the values predicted by the models (Sharma and Simsek, 2020).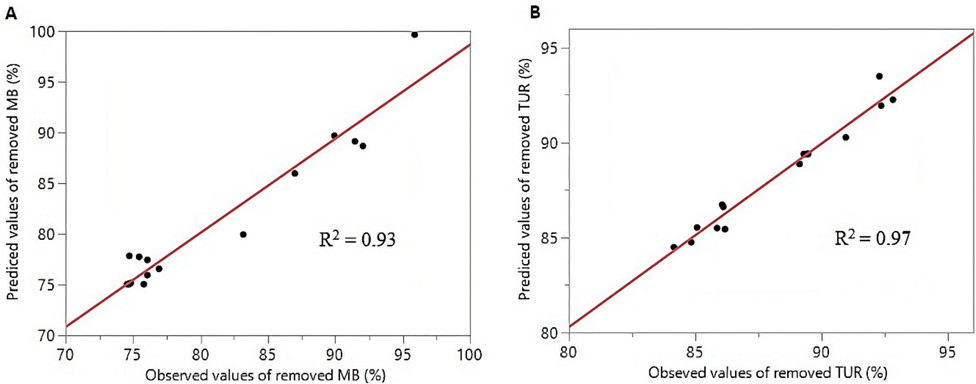
MB and TUR values removed obtained experimentally versus predicted values by the models.
3.2.1 Analysis of variance (ANOVA)
The results of the ANOVA for each MB and TUR removal are presented in Table 5. A large F-value indicates that most of the response changes can be explained by the regression equation. The associated probability value (P-value) is used to estimate whether the F-value is large enough to indicate statistical significance. A P-value > F-value, less than 0.05 indicates that the model is considered statistically significant (Kumar et al., 2007). This means that at least one term in the regression equation is closely related to the response variable. The model chosen to explain the relationship between the factors and the responses is valid (Ravikumar et al., 2006).
Source
MB removal
TUR removal
F-value
P-value
F-value
P-value
Model
7.2922
0.0207*
15.9609
0.0036*
X1
2.1550
0.0070*
2.9720
0.0026*
X2
1.9500
0.0123*
0.3680
0.4112
X3
0.0340
0.9242
2.9720
0.0010*
X1X2
1.9500
0.0112*
0.0940
0.8051
X1X3
0.3700
0.4267
2.3430
0.0454*
X2X3
0.0360
0.9200
1.5630
0,0273
X12
1.1220
0.0754
0.4250
0.3758
X22
1.3000
0.0501
0.0270
0.9395
X32
0.9640
0.1087
0.5790
0.2634
Lack of fit
43.0178
0.0228*
177.5109
0.0056*
R2
0.9292
---------
0.9663
---------
Adjusted R2
0.8178
---------
0.9058
---------
The ANOVA showed a linear relationship between the main effects, quadratic effects, and interaction effects of inputs on the removal of MB (Y1; Eq.4) and TUR (Y2; Eq.5):
ANOVA was used to quantify the significance of the developed model based on the P-value and corresponding F-value. When WHP was used as a coagulant, the F-value statistical values for MB and TUR were 7.2922 and 15.9609 in Table 5, respectively. The low probability value (P model < 0.05) proves this model is significant. The high F-value and lack of significant fit indicate that the experimental data obtained have a good fit with the model. The R2 values obtained for MB and TUR were 0.9292 and 0.9663, respectively. The correlation coefficient (R2) value obtained was very high and much closer to 1, indicating a good fit for the statistical model (Henseler and Sarstedt, 2013).
3.2.2 Response surface plots
Fig. 5 shows the three-dimensional response surface plot of MB removal with the interactive effect of X1 coagulant dose, X2 pH of the initial solution, and X3 coagulant granulation set to the central code (0) 80 µm. While the TUR with the interactive effect of X1 the coagulant dose, X3 the coagulant granulation (µm), and X2 pH of the initial solution that was set to the central code (0) 6. The elliptical shape of the three-dimensional surface curves shows a good interaction between the two variables. The three-dimensional surface curves provide an accurate geometric representation and give the appropriate statistics, such as the optimal range for different values of the test variables within the experimental design (Nair et al., 2014).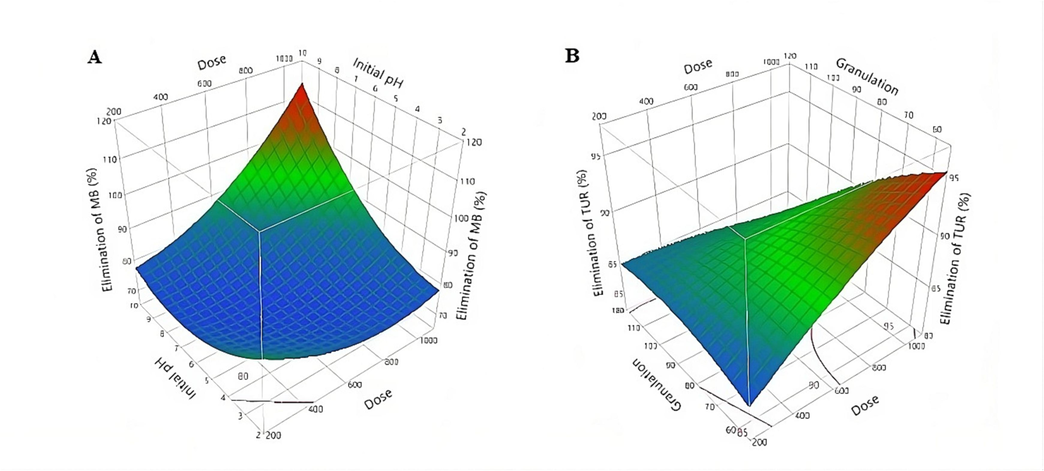
Three-dimensional response surface plot of MB (A), and TUR (B) removal.
WHP showed a maximum MB removal of 99.59 % under the basic condition of initial pH 9 with a dose of 900 mg. L-1 and granulation of 112 µm. Fig. 6 shows that pH has a critical role in color removal as it strongly influences the solubility and hydrolysis of dyes (Dao et al., 2016).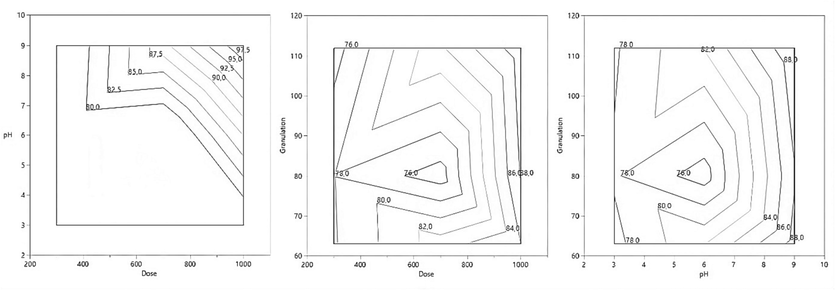
The interaction between dose, initial pH, and granulation in the elimination of MB.
The combined effect of the dose and initial pH of the solution of the coagulation process on MB removal is shown in the same figure. It was observed that the percentage of dye removal increases with the dose but decreases with the increase in the initial pH of the solution. This means that higher values of dye removal can be obtained by simultaneously increasing the dose and keeping the pH of the initial solution in the range of 7–9. This is due to the decrease in charge density of the solution that changes with the initial solution pH (Arulmathi et al., 2019). It can be suggested that the mechanism of coagulation between MB and WHP is adsorption and charge neutralization. The binding between the adsorption sites of WHP and methylene blue (cationic) is chemical in nature, specifically an electrostatic interaction between the opposite charges. The adsorption sites of WHP are negatively charged because it is rich in alcoholic and carboxylic groups, while methylene blue is positively charged due to the presence of nitrogen atoms that have gained an electron. This electrostatic interaction attracts the methylene blue to the adsorption sites and keeps it bound to the WHP surface.
Particle destabilization and charge neutralization occur in coagulation due to the addition of positively charged ions of metal salt or polyelectrolyte. Fig. 7 shows the effects of the mutual interaction between pH and coagulant dose on TUR removal. After coagulation-flocculation, dissolved/suspended particulate matter can be precipitated and removed by gravity, resulting in the formation of a clear supernatant liquid (due to the removal of dissolved matter, there will be considerable TUR removal) (Verma et al., 2010). From the experimental results, it is suggested that the elimination of TUR occurred by the neutralization of the particle load in the solution and that the mechanism of entrapment floc formation occurred when the coagulant was neutralized by the metal salts based mainly on iron and aluminum, as they showed their presence in EDX and XRF (Fig. 8). The WHP showed a maximum TUR removal of 95.31 % in the basic condition of pH 9, a dose of 1000 mg of coagulant, and granulation of 63 µm.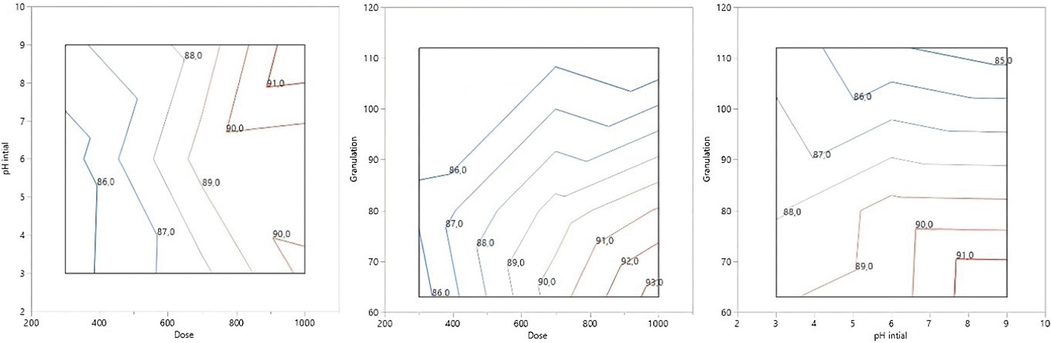
The interaction between dose, initial pH, and granulation in the elimination of TUR.
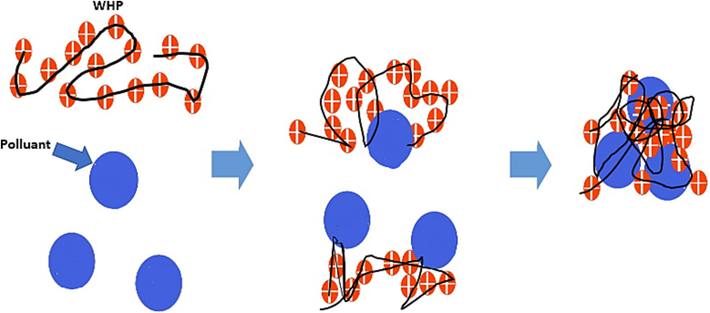
Suggested mechanism during the coagulation-flocculation process.
3.3 Comparison with previous works
The WHP biocaogulant achieved 99.59 % MB dye removal under optimal pellet conditions of 112 µm, initial pH of 9, and 1 g.L-1 using the BBD-RSM. The experiments under the optimum conditions were replicated three times to ensure data reliability (Table 6).
Response
Optimized condition
Removal (%)
Error
Dose
Initial pH
Granulation
Predicted
Experiment
MB
1000
9
112
99.59 %
98.47 %
1.12 %
TUR
1000
9
63
95.31 %
94.06 %
1.25 %
Biocoagulation-flocculation and bioadsorption are wastewater treatment processes that use waste to remove pollutants. Biocoagulation-flocculation consists of using waste (in its native form) to coagulate and flocculate particles in suspension in water, forming agglomerates that can be more easily separated. Bioadsorption uses waste (mainly transformed into carbon) to adsorb pollutants on its surface, thus retaining them in the treatment system. This results in a higher cost for the preparation of the adsorbents (Verma et al., 2012).
Table 7 summarizes some previous studies on MB removal with the processes of biocoagulation-flocculation and bioadsorption Various biomasses have been tested as bio-coagulants or bio-adsorbents for this dye. High removal efficiencies were achieved, for instance with laterite soils (99.61 %) or bentonite combined with Opuntia ficus-indica (98.25 %) in coagulation, and with cashew nut shells (99.97 %) or bamboo (99.67 %) in adsorption. Additionally, it can be noted that optimal MB removal via adsorption generally occurs at more acidic pH conditions, whereas alkaline conditions favor removal by coagulation-flocculation. This highlights the different mechanisms involved in these two processes.
Coagulant / adsorbant
Process
experimental design
Dose
(mg. L-1)Initial pH
Efficiency (%)
Reference
Laterite Soil
Coagulation
--------
2500
2
99.61
(Lau et al., 2015)
Bentonite and Opuntia ficus indica
Coagulation
--------
400
+
9006–7
98.25
(Ihaddaden et al., 2022)
Cashew NUT shell
Adsorption
Factorial central composite design / MSR
2184
10
99.97
(Subramaniam and Kumar, 2015)
Bamboo
Adsorption
------------
1000
3–5
99.67
(Guo et al., 2014)
Onion skins
Adsorption
------------
1000
10
95.54
(Saka and Sahin, 2011)
WHP
Coagulation
BBD-RSM
900
9
99.59
This work
Most of the studies did not use an experimental design in their work. However, the adoption of an experimental design allows for optimizing the process parameters, avoiding experimental errors, and determining the optimal conditions, which allows for maximizing the efficiency and profitability of the process. By comparing this result with other studies, we can deduce that WHP is very effective and competitive with existing natural adsorbents and coagulants.
4 Reuse of WHP
The reuse of WHP coagulant is of crucial importance in coagulation-flocculation processes because of its major role in wastewater clarification (Garvasis et al. 2020). Efforts to evaluate and improve the reuse of this coagulant are of paramount importance from the point of view of environmental sustainability (El Messaoudi et al. 2022). Studies have shown that WHP, as a coagulant, has a certain capacity to be recovered and reused in subsequent applications, thereby minimizing waste and the associated costs. The reuse of WHP is mainly dependent on factors such as the residual coagulant concentration in the treated effluent, one of the methods used to recover WHP involves simple filtration of the sludge after the coagulation-flocculation process, followed by a drying phase at 105 °C to facilitate its reuse under optimum conditions. This approach guarantees the recovery of the WHP, promoting resource efficiency without the use of chemicals. A visual representation, as shown in Fig. 9, illustrates the assessment of the WHP's suitability for reuse after undergoing five cycles. In particular, the results show that WHP can achieve commendable performance, with removal efficiencies of 35 % for MB and 56 % for TUR. It is imperative to recognize that the observed reduction in performance can be attributed to a variety of factors and merits further investigation.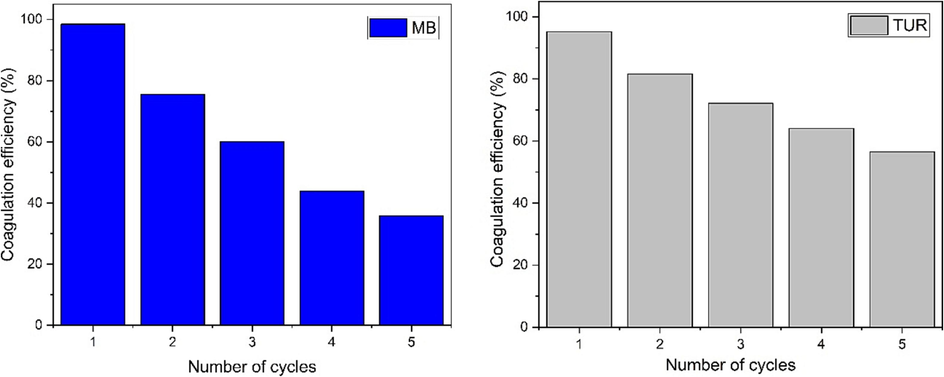
Coagulation efficiency of MB and TUR with recycled WHP.
5 Conclusion
This study explored the valorization of WHP as a biocoagulant via a coagulation-flocculation process. Optimal conditions for removing MB and TUR were determined using a BBD-RSM. The results demonstrated the effective elimination of both pollutants. Optimization revealed that MB removal efficiency depends primarily on dose (X1) and initial pH (X2), while TUR removal depends on dose (X1) and granulation (X3). Predicted optimal conditions were 99.59 % MB removal with X1 at 900 mg. L-1, X2 at 9, and X3 at 112 μm, and 95.12 % TUR removal with X1 at 1 g.L-1, X2 at 9, and X3 at 63 μm. IR, SEM, and XRF analyses suggested removal via adsorption and charge neutralization mechanisms. This study demonstrated the strong innovative potential of WHP as a natural biocoagulant, achieving markedly higher decolorization and turbidity removal than conventional biosorbents. Systematic optimization identified optimal conditions to maximize treatment performance. These findings highlight the promising potential for sustainably upcycling this agricultural waste into eco-friendly water remediation applications.
CRediT authorship contribution statement
Ali Zourif: Writing – original draft. Asmaa Benbiyi: Writing – review & editing, Supervision, Validation. Salma Kouniba: Experimental data & Modeling. Mohamed EL Guendouzi: Writing – review & editing, Supervision, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adsorptive removal of Congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater.. 2010;174:398-403.
- [CrossRef] [Google Scholar]
- Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J. Hazard. Mater.. 2008;154:694-702.
- [CrossRef] [Google Scholar]
- Methylene blue as an anti-COVID-19 mouthwash in dental practice. British Journal of Oral and Maxillofacial Surgery Br. J. Oral Maxillofac. Surg.. 2021;59:135-136.
- [CrossRef] [Google Scholar]
- Treatment of textile wastewater by coagulation-flocculation process using Gossypium herbaceum and Polyaniline Coagulants. Clean – Soil, Air Water. 2019;47:1800464.
- [CrossRef] [Google Scholar]
- Synthesising tannin-based coagulants for water and wastewater application: a review. J. Environ. Chem. Eng.. 2021;9:105007
- [CrossRef] [Google Scholar]
- The use of as natural coagulant in algerian drinking water treatment plant. J. Renew. Mater.. 2022;10:625-637.
- [Google Scholar]
- Response surface modeling for malachite green removal using the Box-Behnken experimental design. Environ. Ecol. Res.. 2022;10:830-838.
- [CrossRef] [Google Scholar]
- Effectiveness on color and COD of textile wastewater removing by biological material obtained from Cassia fistula seed. J. Viet. Env.. 2016;8:121-128.
- [CrossRef] [Google Scholar]
- Preparation and characterization of an activated carbon from the bones of Gudali (Bos Indicus) cattle from Adamawa. GSJ. 2022;10:1. Online: ISSN 2320-9186
- [Google Scholar]
- Optimization based on response surface methodology of anionic dye desorption from two agricultural solid wastes. Chem. Afr.. 2022;5:1083-1095.
- [CrossRef] [Google Scholar]
- Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: a review. Biomass Conv. Bioref. 2022
- [CrossRef] [Google Scholar]
- Coagulation treatment of wastewater in petroleum industry using poly aluminum chloride and ferric chloride. Int. J. Recent Res. Appl.. 2012;13
- [Google Scholar]
- Statistical designs and response surface techniques for the optimization of chromatographic systems. J. Chromatogr. A. 2007;1158:2-14.
- [CrossRef] [Google Scholar]
- Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Environ. Chem. Eng.. 2017;5:4041-4050.
- [CrossRef] [Google Scholar]
- Efficient removal of Congo red from aqueous solutions using phytogenic aluminum sulfate nano coagulant. Mater. Chem. Phys.. 2020;251:123040
- [CrossRef] [Google Scholar]
- Impact of textile dyes waste on aquatic environments and its treatment. Ecol. Environ.. 2017;35(3C):2349-2353.
- [Google Scholar]
- Removal of methylene blue from aqueous solutions by chemically modified bamboo. Chemosphere. 2014;111:225-231.
- [CrossRef] [Google Scholar]
- Goodness-of-fit indices for partial least squares path modeling. Comput. Stat.. 2013;28:565-580.
- [CrossRef] [Google Scholar]
- Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol.. 2018;13:642-650.
- [CrossRef] [Google Scholar]
- Molecular Diversity of Walnut (Juglans regia L.) Among Two Major Areas in Morocco in Contrast with Foreign Varieties. Int. J. Fruit Sci.. 2021;21:180-192.
- [CrossRef] [Google Scholar]
- Polyacrylamide as coagulant aid with polytitanium sulfate in humic acid-kaolin water treatment: Effect of dosage and dose method. J. Taiwan Inst. Chem. Eng.. 2016;64:173-179.
- [CrossRef] [Google Scholar]
- Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica) JWPE J. Water Process. Eng.. 2022;49:102952
- [CrossRef] [Google Scholar]
- Biochemical characterization and antioxidant activity of walnut kernel (Juglans regia L.) of accessions from Middle and High Atlas in Morocco. Acta Sci. Biol. Sci.. 2019;41:1-12.
- [Google Scholar]
- Greywater biodegradability and biological treatment technologies: A critical review. Int. Biodeter. Biodegr.. 2021;161:105211
- [CrossRef] [Google Scholar]
- Review on Methylene Blue: Its Properties, Uses. Toxicity and Photodegradation. Water. 2022;14:242.
- [CrossRef] [Google Scholar]
- TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations. Appl Catal B. 2004;49:1-14.
- [CrossRef] [Google Scholar]
- Process parametric study for ethene carboxylic acid removal onto powder activated carbon using Box-Behnken design. Chem. Eng. Technol.. 2007;30:932-937.
- [CrossRef] [Google Scholar]
- What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci. Total Environ.. 2022;806:150902
- [CrossRef] [Google Scholar]
- Experimental evaluation of crushed Moringa oleifera Lam. seeds and powder waste during coagulation-flocculation processes. J. Environ. Chem. Eng.. 2018;6:5443-5451.
- [CrossRef] [Google Scholar]
- Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv.. 2015;5:34206-34215.
- [CrossRef] [Google Scholar]
- Exploring the reusability of synthetically contaminated wastewater containing crystal violet dye using tectona grandis sawdust as a very low-cost adsorbent. Sci. Rep.. 2018;8:8314.
- [CrossRef] [Google Scholar]
- Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interfaces. 2018;11:74-81.
- [CrossRef] [Google Scholar]
- Performance Assessment of Cassava Peel Starch and Alum as Dual Coagulant for Turbidity Removal in Dam Water. Int. J. Integr. Eng.. 2018;10(4):185-192.
- [CrossRef] [Google Scholar]
- On the importance of pH value in coagulation. J. Water Supply: Res. Technol. - AQUA. 2019;68:222-230.
- [CrossRef] [Google Scholar]
- The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: a review. Water Sci. Technol.. 2014;69:464-478.
- [CrossRef] [Google Scholar]
- Coagulation kinetic study and optimization using response surface methodology for effective removal of turbidity from paint wastewater using natural coagulants. Sci. Afr.. 2021;14:e00959.
- [Google Scholar]
- The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J. Hazard. Mater.. 2009;162:1371-1378.
- [CrossRef] [Google Scholar]
- Biosorption of chromium (VI) ion from aqueous solutions using walnut, hazelnut and almond shell. J. Hazard. Mater.. 2008;155:378-384.
- [CrossRef] [Google Scholar]
- Study on the effectiveness of banana peel coagulant in turbidity reduction of synthetic wastewater. IJEST 2019
- [CrossRef] [Google Scholar]
- Novel electrochemical advanced oxidation processes with H2O2 generation cathode for water treatment: a review. J. Environ. Chem. Eng.. 2022;10:107896
- [CrossRef] [Google Scholar]
- A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. ESPR. 2021;28:9050-9066.
- [CrossRef] [Google Scholar]
- Application of response surface methodology to optimize the process variables for Reactive Red and Acid Brown dye removal using a novel adsorbent. Dyes Pigm.. 2006;70:18-26.
- [CrossRef] [Google Scholar]
- Removal of methylene blue from aqueous solutions by using cold plasma- and formaldehyde-treated onion skins. Color. Technol.. 2011;127:246-255.
- [CrossRef] [Google Scholar]
- Sugar beet industry process wastewater treatment using electrochemical methods and optimization of parameters using response surface methodology. Chemosphere. 2020;238:124669
- [CrossRef] [Google Scholar]
- Novel adsorbent from agricultural waste (cashew NUT shell) for methylene blue dye removal: optimization by response surface methodology. Water Resour. Ind.. 2015;11:64-70.
- [CrossRef] [Google Scholar]
- The influence of particle size and concentration combined with pH on coagulation mechanisms. J. Environ. Sci.. 2019;82:39-46.
- [CrossRef] [Google Scholar]
- Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res.. 2016;55:4363-4389.
- [CrossRef] [Google Scholar]
- Walnut shell powder as a low-cost adsorbent for methylene blue dye: isotherm, kinetics, thermodynamic, desorption and response surface methodology examinations. Sci. Rep.. 2020;10:7983.
- [CrossRef] [Google Scholar]
- A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage.. 2012;93:154-168.
- [CrossRef] [Google Scholar]
- Pretreatment of petrochemical wastewater by coagulation and flocculation and the sludge characteristics. J. Hazard. Mater.. 2010;178:1055-1064.
- [CrossRef] [Google Scholar]
- Investigation of wrinkling failure mechanics in metal spinning by Box-Behnken design of experiments using finite element method. J. Adv. Manuf. Technol.. 2015;78:981-995.
- [CrossRef] [Google Scholar]
- Flocculation performance of hyperbranched polyethyleneimine-grafted-cellulose in wastewater treatment. ACS Sustainable Chem. Ing. 2018;6(2):1592-1601.
- [CrossRef] [Google Scholar]
- Coagulation of low temperature and low turbidity water: adjusting basicity of polyaluminum chloride (PAC) and using chitosan as coagulant aid. Sep. Purif. Technol.. 2018;206:131-139.
- [CrossRef] [Google Scholar]
- Non-evaporative solvent extraction technology applied to water and heat recovery from low-temperature flue gas: Parametric analysis and feasibility evaluation. Energy. 2022;244:123062
- [CrossRef] [Google Scholar]
- Application of coagulation/flocculation in oily wastewater treatment: a review. Sci. Total Environ.. 2021;765:142795
- [CrossRef] [Google Scholar]
- Decolorization and COD removal of secondary yeast wastewater effluents by coagulation using aluminum sulfate. Desalination. 2008;225:301-311.
- [CrossRef] [Google Scholar]







