Translate this page into:
Variability in the volatile constituents and biological activities of Achillea millefolium L. essential oils obtained from different plant parts and by different solvents
⁎Corresponding author at: College of Pharmacy, Qassim University, Buraydah, Saudi Arabia. ham.mohammed@qu.edu.sa (Hamdoon A. Mohammed),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Achillea millefolium Linn. is an aromatic edible plant used traditionally for the remediation of wounds, liver disorders, and as an herbal tea for the treatment of various gastrointestinal disorders. The plant's volatile oils have economical value in cosmetic preparation and as an insect repellant. The essential oils obtained from different plant parts, i.e., leaves, stems, and aerial portions, by hydrodistillation and by non-polar solvents, i.e., n-hexane and diethyl ether, were phytochemically analyzed and biologically investigated from the plant species cultivated in Saudi Arabia for their potential antioxidant and anticancer activities. The GC-analysis revealed substantial variations among the major constituents and classes of volatile oils in all batches of the plant, characterized by the significant high percentages of monoterpenes, e.g., myrcene, 1,8 cineole, camphor, α-thujone, and β-thujone, in the distilled oils and sesquiterpenes in the non-polar extracts of the plant. Furthermore, the ketonic monoterpenes α-thujone and β-thujone were found to be the most representative compounds in the distilled and extracted batches of the plant. Variable antioxidant activity of different batches of the plant was recognized; however, distilled batches of leaves, stems, and aerial parts exhibited better total antioxidant activity (TAA) compared to the plant’s non-polar extracts. With the little cytotoxic activity of distilled batches, the non-polar extracts exhibited remarkable cytotoxic effects against Panc-1 and MCF-7-Adr (35 and 43.3 μg/mL and 41.3 and 57.1, respectively, in the n-hexane and diethyl ether extracts), which were attributed to their comparatively higher contents of germacrene D, viridiflorol, and caryophyllene oxide. The n-hexane extract induced a concentration- and time-dependent apoptotic effect in Panc-1 and MCF-7 Adr cells, as observed by staining with FITC/PI and flow cytometry analysis. The docking simulation of the major n-hexane extract constituents indicated the prospective mechanism behind the ability of the extract to inhibit MCF-7-Adr cells by inhibiting P-glycoprotein/multidrug resistance 1/adenosine triphosphate-binding cassette.
Keywords
Achillea millefolium L.
Yarrow
Essential Oils
GC-Analysis
Cytotoxicity
Antioxidants
Breast cancer
1 Introduction
The genus Achillea (Asteraceae; Daisy family) has a temperate and subtropical native distribution in the northern hemisphere. There are a total of 252 species, including 183 species, 64 subspecies, and 4 varieties, with 134 accepted species (POWO, 2023; WFO, 2022). Achillea millefolium Linn., or yarrow, is an aromatic plant that belongs to the family Asteraceae. The plant grows wildly in the Mediterranean region (Čulum et al., 2021; Falconieri et al., 2011). The plant is also widely cultivated due to its food and medical applications, besides its economic value in the pharmaceutical industry, cosmetic production, perfumery, and insect repellant formulations (Becker et al., 2016; Farhadi et al., 2020; Rohloff et al., 2000). The plant was named after the hero Achilles, son of Peleus, as the plant was used during the Trojan War for the treatment of wounds (Benedek and Kopp, 2007). The applications of A. millefolium in traditional medicine are prevalent in the treatment of common gastrointestinal disorders, e.g., GIT spasms, flatulence, and dyspepsia. The plant is also used as an appetizer, a menstrual regulator, a contraceptive agent, and for the treatment of liver complaints (Benedek and Kopp, 2007; Candan et al., 2003; Montanari et al., 1998; Vitalini et al., 2011). A. millefolium has also been reported to be an abortifacient and an emmenagogue (Montanari et al., 1998). Furthermore, the plant is traditionally used as a remedy for wounds, hemorrhoids, and inflammatory disorders (Cavalcanti et al., 2006; Teixeira et al., 2003). The plant is also edible and consumed as an herbal tea in the form of an infusion for the treatment of previous disorders. The medicinal usage of A. millefolium is attributed to its phytoconstituents of phenolic acids, flavonoids, and volatile oils (El-Kalamouni et al., 2017; Farhadi et al., 2020; Iguchi et al., 2019; Vitalini et al., 2011). Flavonoids, flavonoid glycosides, phenolic acids, quinic acid, and sesquiterpene derivatives are the commonly reported non-volatile constituents of A. millefolium (Iguchi et al., 2019; Vitalini et al., 2011). Among the volatile compositions of the plant, 1,8-cineole and camphor were reported as the main chemotypes. Also, terpinen-4-ol, borneol, germacrene D, and caryophyllene oxide were reported in relatively high concentrations in the volatile oil of the plant growing in different areas (Candan et al., 2003; El-Kalamouni et al., 2017; Farhadi et al., 2020; Özek et al., 2018). The moisture contents of the plant's sections, such as leaves, roots, and stems, have been reported to affect the volatile oil separation from A. millefolium, and the lower moisture contents of the stems compared to the leaves and roots of the plant have been reported as a reason for the higher recovery of the volatile oil from the stems of the plant (Costescu et al., 2014). Several biological activities have been reported for the plant essential oils, e.g., anti-ulcerative (Alomair et al., 2022), antimicrobial (Mazandarani et al., 2013; Sevindik et al., 2016), antioxidant, anti-inflammatory, and hepatoprotective (Al-Said et al., 2016).
The variability in the essential oil constituents of aromatic plants in response to variations in the environmental conditions and methods of preparation of the plants, including drying procedures and extraction methods, has been established in the literature (Fahmy et al., 2023; Mohammed et al., 2021a, 2020). Additionally, the literature review revealed considerable variations in the components of A. millefolium's essential oil as a result of the plant's geographic distribution (Table 1), reflecting the plant's sensitivity to its surrounding environment.
Location
Major volatile constituents
Ref.
Saudi Arabia, Eastern Province
Sabinene, 14.28%; β-pinene, 11.40%; 1,8-cineole, 1.87%; borneol, 5.26%; β-caryophyllene, 10.35%; germacrene D, 26.15%; chamazulene, 10.04%.
Mohamed et al., 2021
Italy
α-Pinene, 17.2%; sabinene, 3.9%; β-pinene, 2.1%; (E)-methyl isoeugenol, 8.8%; β-bisabolene, 16.6%.
Falconieri et al., 2011
Iran
cis-Sabinol, 2.5%; trans-carveol 3.7%; cis-carveo1, 5%; spathulenol, 12.4%; bisabolol, 22.9%; trans-farnesol, 4.0%.
Afsharypuor et al., 1996
France
Camphor, 12.8%; sabinene, 6.7%; (E)-p-mentha-2,8-dien-1-ol, 4.5%; germacrene-D, 12%; (E)-nerolidol, 7.3%; 1,8-cineole, 4%.
El-Kalamouni et al., 2017
India
Sabinene, 17.58%; bornyl acetate, 7.98%; α-pinene, 6.28%; 1,8-cineole, 13.04%; borneol, 12.41%; β-pinene, 6.26%; terpinine-4-ol, 6.17%; and chamazulene, 5.28%.
Nadim et al., 2011
Chile
α-Thujone, 1.2%; β-thujone, 96.2%.
Tampe et al., 2015
Lithuania
Borneol, 11.5–13.2%; trans-nerolidol, 5.5–13.5%; camphor, 7.2–13.1%; chamazulene, 9.8–23.2%; β-pinene, 5.5–31.0%; 1,8-cineole, 3.1–17.0%.
Mockute and Judzentiene, 2003
Bulgaria
1,8-Cineole, 3.76–9.36%; artemisia ketone, 5.73–14.38%; camphor, 8.18–12.62%; borneol, 9.30–12.46%; caryophyllene oxide, 2.81–5.40%.
Villanueva-Bermejo et al., 2017
Turkey
α-Pinene, 7.41%; β-pinene, 5.67%; 1,8-cineole, 22.83%; camphor, 3.38%; borneol, 1.57%; terpinen-4-ol, 4.50%; caryophyllene oxide, 1.50%; β-caryophyllene, 2.59%.
Sevindik et al., 2016
The relationship between anticancer activities, cytotoxicity, and apoptotic activity is an important aspect of cancer research and the development of effective cancer treatments. Understanding the mechanisms by which anticancer agents induce cell death, specifically through cytotoxic and apoptotic pathways, is crucial for developing targeted therapies and improving patient outcomes (Farghadani et al., 2016). The link between cytotoxicity and apoptotic activity is close yet different. While cytotoxicity generally discusses the capability to cause cell death, apoptotic activity deals with the production of intentional cell death through apoptotic pathways and is considered a particular type of cell death called apoptosis, sometimes known as “programmed cell death,” which is essential for maintaining the health of multicellular organisms (Dey et al., 2013). Therefore, not all cytotoxic medications produce apoptosis; some may promote necrosis or autophagy, two other kinds of cell death.
The importance of the current work lies in the phytochemical investigation of the essential oils obtained from A. millefolium species that have an economic value as a medicinal plant in Saudi Arabia for the treatment of gastrointestinal complaints, including maldigestion and cramps, besides the wound-healing and antibacterial abilities of the plant; therefore, the plant is widely cultivated in the region for those purposes (Mohamed et al., 2021). The plant was collected during the spring, when the temperature and humidity in the growing region were 20–35 °C and 10–30%, respectively. Therefore, variations in the phytochemical ingredients of A. millefolium, especially in its essential oil constituents, were expected. Although there are many non-conventional extraction methods, such as ultrasonic-assisted extraction, microwave-assisted extraction, and supercritical fluid extraction, which are effective techniques for the isolation of volatile oils in high yield (Gala et al., 2020; Kusuma et al., 2016), the distillation and extraction processes are considered the easiest and cheapest methods to obtain volatile oils from aromatic plants. The distillation process has the advantage over extraction in that the product of the process is only the volatile constituent of the plant; however, extraction under normal room temperature could preserve the nature of the volatile oil constituents that might be affected by the high temperature of the distillation process (De Castro et al., 1999; Stratakos and Koidis, 2016). In addition, using non-polar solvents for the extraction of the volatile oils could help avoid the extraction of other relatively more polar secondary metabolites from the plant, such as polyphenols (phenolic acids and flavonoids). The study provides valuable information on the best methods that could be used for the isolation of the volatile oils from the plant in terms of their chemical composition and biological activities. Therefore, the study includes a phytochemical comparison between batches of volatile oils obtained from different parts of the plant, i.e., stems, leaves, and aerial parts, by steam distillation and batches of volatile oils obtained from A. millefolium herb by non-polar solvents, i.e., n-hexane and diethyl ether. Besides the phytochemical analysis and elucidation of the volatile components of the plant in different batches, comparative biological investigations, including in vitro antioxidant and anticancer activities, were also conducted as part of the current work.
2 Materials and method
2.1 Chemicals and reagents
All the chemicals were of analytical grade. Anhydrous sodium sulfate, DPPH ((2,2-diphenyl-1-picrylhydrazyl), ammonium molybdate, TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine, ferric chloride, ferrous chloride, ferrozine, and EDTA (ethylenediaminetetraacetic acid), were purchased from Sigma Aldrich, Germany. The V-FITC apoptosis kit was obtained from Abcam Inc. in Cambridge Science Park, Cambridge, UK.
2.2 Instruments
Clevenger apparatus was obtained from Standard Scientific Glass Industries, Mumbai, India, and was used for the volatile oil distillation; a vacuum pump (Büchi Rotavapor R-114, Büchi, Switzerland) was used for the evaporation of the solvents; a Perkin Elmer Auto System XL (Waltham, MA, USA) was used for the analysis of the volatile oil batches; and a microplate reader (BMGLABTECH®-FLUO Star Omega, Ortenberg, Germany) was used to measure the absorbances of the quantitative antioxidant assays and the protein-bound SRB stain.
2.3 Plant materials
The plant materials were collected from local farms in the northern area of the Qassim government, Saudi Arabia, during spring 2020 (March–April). A representative sample of more than 100 individual plants was gathered, which was sufficient for the current experiment. The plant was identified as Achillea millefolium L. by the taxonomists at the Department of Plant Production and Protection, College of Agriculture, Qassim University. A voucher specimen of the plant has been stored at the College of Pharmacy, Qassim University.
2.4 Distillation of the volatile oil batches
About 500 g of the fresh plant materials, i.e., leaves, stems, or aerial parts, were divided into three equal parts and separately packed into the 2 L stoppered conical flask and mixed with 700 mL of the distilled water. The flask was then connected to the Clevenger apparatus (capacity of 2000 mL, Standard Scientific Glass Industries, Mumbai, India) and fixed to the heating mantel. The distillation processes were extended to 5 h, and the distilled oil was separated by a separating funnel and collected over anhydrous sodium sulfate to remove any moisture remaining from the distillation process. The produced volatile oil batches were stored at −20 °C in a freezer for further biological and phytochemical evaluations. The distillation procedure has been conducted in triplicate. The volatile oil yield percentages were found to be 0.33, 0.65, and 0.61% from leaves, stems, and aerial portions, respectively.
2.5 Extraction of the plant materials
About 200 g of the fresh plant materials as a whole herb including the stems, aerial parts, and leaves were reduced into small pieces and divided into two portions; one portion was macerated in n-hexane and the other in diethyl ether for 24 h. The extracts were filtrated and subjected to a vacuum pump (Büchi Rotavapor R-114, Büchi, Switzerland) under reduced pressure to remove the solvents. The semi-solid, sticky extracts were kept in the −20 C freezer, awaiting further phytochemical and biological analysis. The extraction procedure has been conducted in triplicate. The volatile oil yield percentages were found to be 1.34% and 1.11% for the n-hexane and diethyl ether extracts, respectively.
2.6 Phytochemical analysis of the plant essential oils and extracts
All the volatile oil batches and extracts obtained from the plant were analyzed by the GC-FID and GC–MS spectral analysis using the Perkin Elmer Auto System XL (Waltham, MA, USA) equipped flame ionization detector in the GC-FID and the Agilent 8890 GC system attached to a PAL RTC 120 auto-sampler and equipped with a mass detector in the GC–MS. The analysis conditions in both methods exactly followed our previous literature (Mohammed, 2022; Mohammed et al., 2022c, 2021).
2.7 Antioxidant activity of the plant essential oils and extracts
The antioxidant activity of all batches (distilled oils obtained from leaves, stems, and aerial parts, and extracts obtained by n-hexane and diethyl ether) was measured using four different in vitro assays. These assays include total antioxidant activity (TAA), DPPH scavenging activity (DPPH-SA), ferric reducing antioxidant power (FRAP), and metal chelating activity (MCA) assays, which were conducted in triplicate. The TAA, DPPH-SA, and FRAP activities of all batches were calculated as a Trolox equivalent using standard calibration curves of Trolox with the molybdate reagent, DPPH, and FRAP reagent, respectively.
In the TAA, freshly prepared molybdate reagent (3.6 mL) (Aroua et al., 2021) was mixed with 400 µL of the samples (containing 200 µg of the crude oils or extracts) in glass test tubes and heated over a water bath (90 °C) for 30 min. The color of the reaction mixtures was measured at 695 nm (Aroua et al., 2021). In the DPPH-SA, a mixture of 1 mL of the samples (containing 200 µg of the crude oils or extracts) and a freshly prepared DPPH solution (12 mg of the DPPH in 100 mL of the methanol) were prepared and kept for 30 min. The DPPH color reduction was measured at 517 nm (Sulaiman et al., 2013; Mohammed et al., 2021). For the FRAP, 2 mL of the FRAP reagent (freshly prepared as mentioned in the literature (Benzie and Strain, 1996)) was mixed with 0.1 mL of the sample solution (which contained 200 µg of the crude oils or extracts). The reaction mixtures were measured at 593 nm (Aroua et al., 2023). The MCA of all batches was calculated as EDTA equivalents using the procedures of Zengin et al. (Zengin et al., 2016). The mixtures of samples (2 mL contain 200 µg of the crude oils or extracts), 2 mM FeCl2 (25 µL), and 100 µL of ferrozine were measured at 562 nm against a blank (which contained all the mixture's ingredients except ferrozine).
2.8 Cytotoxic activity
2.8.1 Cell cultures
Cell lines (HSF: human skin fibroblasts; MCF-7: breast adenocarcinoma; MCF-7-Adr: doxorubicin-resistant breast cancer; A-431: human epidermoid skin carcinoma; Panc-1: pancreatic cancer) were of ATCC origin and obtained from Nawah Scientific Inc. (Mokatam, Cairo, Egypt). DMEM medium supplemented with 100 mg/mL streptomycin, 100 units/mL penicillin, and 10% heat-inactivated fetal bovine serum was used to sustain cells at 37 °C in a humidified, 5% (v/v) CO2 environment.
2.8.2 Cytotoxic assay
As per the literature, cell viability was measured using the sulforhodamine B (SRB) test (Elshibani et al., 2023). The cell suspension aliquot (100 µL) containing 5 × 103 cells was inserted into the plates of 96 wells, which were incubated for 24 h. The cells were treated with another 100 µL aliquot of media containing the samples (distilled oil batches and extracts) at various concentrations (0.01–100 µg/mL) and incubated for 72 h. Following drug exposure, the cells were fixed with 150 µL of trichloroacetic acid (TCA 10%) before being incubated at 4 °C for an hour. After the TCA solution was removed, the cells underwent five rounds of distilled water washing. After that, each well received 70 µL of a 0.4% w/v SRB solution, which was then incubated for 10 min at room temperature in the dark. The plates were given three 1% acetic acid washings before being left to air dry overnight. In order to dissolve the protein-bound SRB stain, 150 µL of TRIS (10 mM) was then added. A microplate reader (BMGLABTECH®-FLUO Star Omega, Ortenberg, Germany) was then used to detect the absorbance at 540 nm (Elshibani et al., 2023). The cytotoxic experiment against all cell lines has been conducted in triplicate.
2.9 Apoptosis assay
The most active batch, n-hexane, was used for the apoptotic test. Using a 2-fluorescent channel flow cytometer in conjunction with an Annexin V-FITC apoptosis detection kit from Abcam Inc. in Cambridge Science Park, Cambridge, UK, it was possible to determine the populations of cells that were in apoptosis and necrosis (Sulaiman, 2015). After treatment with n-hexane extract for 24/48 or 72 h, Panc-1 and MCF-7 Adr. cells (105cells) were collected by trypsinization and washed twice with ice-cold PBS (pH 7.4). Then, in accordance with the manufacturer's instructions, Panc-1 and MCF-7 Adr. cells were incubated in the dark for 30 min at room temperature with 0.5 mL of Annexin V-FITC/PI solution. After staining, Panc-1 and MCF-7 Adr. cells were inoculated using an ACEA Novocyte™ flow cytometer (ACEA Biosciences Inc., San Diego, CA, USA). FITC and PI fluorescent signals were then evaluated using FL1 and FL2 signal detectors, respectively (ex/em 488/530 nm for FITC and ex/em 535/617 nm for PI). 12,000 events were collected for each sample of n-hexane extract, and the numbers of positive FITC and/or PI cells were computed using ACEA NovoExpressTM software (ACEA Biosciences Inc., San Diego, CA, USA) and quadrant analysis.
2.10 Docking method
The three main components of the active anticancer extracts, n-hexane and diethyl ethers (germacrene D, viridiflorol, and caryophyllene oxide), were docked using AutoDock Vina v1.1.2. The X-ray crystal structure of ABCB1 was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB), protein data bank (available online at https://www.rcsb.org/structure/) (Alam et al., 2019; Mohammed et al., 2022a). The AutoDock Tool (ADT), included with the MGLTools package (version 1.5.6), was used to prepare and optimize proteins and ligands for the AutoDock Vina (Morris et al., 2009). Water molecules and the co-crystallized ligands were eliminated. After making any necessary adjustments for missing residues and close interactions, polar hydrogen atoms were then added to the protein molecules. During the preparation of the protein, charges were involved. The structures of Achillea millefolium compounds were retrieved from the PubChem database (Kim et al., 2019). The grids were generated with the autogrid4 program distributed with AutoDock, v1.5.6 (Mohammed et al., 2022b). The grid centers were selected to be the proteins' active binding locations. All the atoms in the ligand set fit inside the size of the central grid box (Kalinowsky et al., 2018). Using the Discovery Studio 2017 Client, several protein–ligand interactions, including hydrogen bonds and other hydrophobic interactions, were examined. The resulting docking scores were characterized as affinity binding (Kcal/mol) (Biovia, 2016).
2.11 Statistical analysis
The mean ± standard error of the mean/standard deviation was used to express the results. Tukey's multigroup comparison in GraphPad Prism 8.0.2 was conducted as a post hoc test, with a significance value set at p < 0.05.
3 Results and discussion
3.1 Phytochemical analysis
A. millefolium is a medicinal plant used in traditional medicine for several purposes, including wound healing, gastrointestinal disorders, reducing fever, epilepsy, hemorrhage, and microbial infections (Rohloff et al., 2000). The plant's volatile oil contents, which have been discovered to vary depending on the plant's growth environment, as shown in Table 1, are largely responsible for the plant's use in traditional medicine, making the economic worth of these oils an important consideration. The results revealed lower volatile oil percentages of yields that were obtained from the plant by distillation (volatile oil yield percentages were found to be 0.33, 0.65, and 0.61% from leaves, stems, and aerial portions, respectively) compared to the yields obtained by the extraction method (extract yield percentages were found to be 1.34 and 1.11% for n-hexane and diethyl ether extracts, respectively), an effect that could be related to the ability of non-polar solvents to extract other non-volatile constituents of the plant, such as sterols and fats. The current percentages of yield have been found to be high when compared to the literature. For example, the volatile oil yield of A. millefolium growing in Estonia has been reported as 0.13–0.14% from leaves and 0.02% from stems of the plant (Orav et al., 2001). However, our result for the aerial parts volatile oil yield was nearly similar to the yield obtained from the same part of the plant growing in Turkey (Candan et al., 2003). Also, the plant leaves growing in Lithuania have offered a similar volatile oil percentage (0.1–0.4%) of yield as the yield recorded for the leaves in the current study (Mockute and Judzentiene, 2003). The supercritical CO2 extraction of A. millefolium leaf essential oils is better than the hydrodistillation process, as the supercritical CO2 extraction of plant species growing in the Republic of Macedonia has offered a 0.65% volatile oil yield compared to the 0.33% obtained by the hydrodistillation in the current study (Bocevska and Sovová, 2007). The volatile constituents of the A. millefolium essential oils in the distillate batches from leaves, stems, and aerial parts, as well as the extracted batches by n-hexane and diethyl ether, were analyzed by gas chromatography (GC) spectroscopic techniques. The results shown in Table 2 indicated significant phytochemical variations between all the batches, which were reflected in the representation and quantification of the volatile constituents. The analysis revealed the presence of 21, 17, 15, and 24 identified compounds in the total chromatogram in the GC-FID spectra of n-hexane, diethyl ether, stem, and aerial parts, respectively. However, the highest number of the identified compounds was found in the volatile oil distillate of the leaves of the plant (33 compounds, representing 99.39% of the total peaks in the GC chromatogram). The analysis also indicated variations in the major classes of volatile oils among all batches of the plant. For example, the percentages of the monoterpene’s hydrocarbons (non-oxygenated monoterpenes) were found at the highest levels in the distillate volatile oil batches (obtained from leaves, stems, and aerial parts) compared to the batches extracted by the n-hexane and diethyl ether solvents (Table 2, Fig. 1), which might be an indication that these classes of compounds, e.g., α-pinene and myrcene, were better obtained from the plant by hydrodistillation. Furthermore, the highest class of the compounds were found as oxygenated monoterpenes; however, their representations in the plant batches were significantly varied as 68.58%, 75.34%, 60.42%, 38.91%, and 38.85% in the leaves, stem, aerial parts, n-hexane, and diethyl ether batches, respectively (Fig. 1). Variations were also found in the percentages of the oxygenated- and non-oxygenated sesquiterpenes in all batches; however, their representation in the extract batches (n-hexane and diethyl ethers) was comparatively higher than the distillated oil batches (leaves, stems, and aerial parts volatile oil batches) of the plant (Fig. 1, Table 2). The percentages of monoterpenoids and sesquiterpenoids in different batches also reflect the limited ability of non-polar solvents to extract the monoterpenes, including the oxygenated and non-oxygenated compounds. However, sesquiterpene compounds have a higher liability of being extracted by non-polar solvents, i.e., n-hexane and diethyl ether.
Compound No.
RT
Kovat index
Predicted compound
n-Hexane extract
Diethyl ether extract
Stem oil
Leaves oil
Aerial parts oil
1
8.71
734
Camphene
0.23 ± 01
2
12.24
849
3.Metilpentanol
0.71 ± 0.06
0.44 ± 0.04
3
14.39
907
Santolina triene
0.88 ± 0.08
1.45
4
15.20
925
α-Thujene
0.08 ± 0.01
5
15.72
938
α-Pinene
0.12 ± 0.01
6
18.43
1001
Myrcene
0.2 ± 0.01
1.26 ± 0.11
1.72 ± 0.16
1.4 ± 0.11
7
19.01
1011
α-Terpinene
0.12 ± 0.01
8
19.79
1029
P- Cymene
0.78 ± 0.03
0.3 ± 0.1
9
20.19
1036
Limonene
1.44 ± 0.01
10
20.30
1042
1,8 Cineole
4.37 ± 0.29
4.51 ± 0.19
14.19 ± 1.02
13.9 ± 0.13
8.29 ± 0.48
11
21.40
1064
Trans-Sabinene hydrate
4.92 ± 0.30
4.41 ± 0.12
3.70 ± 0.17
8.29 ± 0.37
7.37 ± 0.36
12
22.67
1091
Terpinolene
0.12
0.46 ± 0.08
0.18
13
23.49
1101
Linalool
0.26
0.3
0.6
0.16 ± 0.01
14
23.83
1117
α-Thujone
13.28 ± 0.83
13.35 ± 0.09
29.54 ± 0.11
37.02 ± 0.89
23.59 ± 0.32
15
24.35
1130
β-Thujone
8.93 ± 0.54
8.52 ± 0.12
18.05 ± 0.27
21.58 ± 0.29
14.70 ± 0.16
16
25.44
1149
Camphor
0.53 ± 0.04
0.54±
1.80 ± 0.12
1.04 ± 0.06
1.04 ± 0.04
17
26.46
1171
Borneol
0.91 ± 0.04
0.94 ± 0.03
0.78 ± 0.01
0.29 ± 0.01
0.41 ± 0.02
18
27.60
1187
Terpinolene-4-ol
0.14 ± 0.01
0.15 ± 0.01
19
28.14
1204
α-Terpineol
0.18
0.19 ± 0.01
20
30.05
1249
Carvone
0.99
1.48 ± 1.62
0.19 ± 0.02
0.28 ± 0.01
21
31.91
1291
Bornyl acetate
0.1 ± 0.0.01
22
32.17
1297
Thymol
4.15 ± 0.28
4.28 ± 0.01
6.68 ± 0.38
3.51 ± 0.24
4.15 ± 0.28
23
32.42
1303
Carvacrol
0.39 ± 0.01
0.52±
0.17 ± 0.03
0.44 ± 0.05
24
34.29
1348
Aromadendrene
0.33
0.08
0.28 ± 0.01
25
36.91
1407
Chamazulene
0.2 ± 0.02
0.19 ± 0.01
26
37.93
1432
β -caryophyllene
0.53 ± 0.07
0.31±
0.12 ± 0.01
0.33 ± 0.06
27
40.58
1497
Germacrene D
19.80 ± 1.66
12.32 ± 0.43
1.51 ± 0.10
3.31 ± 0.30
14.73 ± 0.46
28
41.14
1511
α-Farnesene
1.27 ± 0.20
0.57 ± 0.04
1.38 ± 0.03
29
41.62
1527
β-Sesquiphellandrene
0.43 ± 0.01
0.94 ± 0.07
0.07 ± 0.01
0.21 ± 0.01
30
41.98
1533
Viridiflorol
15.30 ± 1.53
16.17 ± 0.44
0.99 ± 0.34
1.25 ± 0.11
10.62 ± 0.44
31
44.43
1595
Caryophyllene oxide
1.99 ± 0.23
3.53 ± 0.06
0.44
0.30 ± 0.05
2.31 ± 44.42
32
46.53
1657
Cis-Methyl dihydrojasmonate
0.29 ± 0.03
0.53 ± 0.04
0.56 ± 0.02
0.31 ± 0.01
0.43 ± 0.03
33
50.14
1701
Heptadecane
11.93 ± 0.76
0.71
2.90 ± 0.59
0.06
Number of compounds
21
17
15
33
24
Total %
90.67
72.85
83.94
99.39
94.67
Non-oxygenated monoterpenes
0.32
0
1.26
6.54
3.77
Oxygenated monoterpenes
38.91
38.85
75.34
86.58
60.42
Non-oxygenated sesquiterpenes
21.93
13.06
2.45
4.35
17.12
Oxygenated sesquiterpenes
17.29
19.7
1.43
1.55
12.93
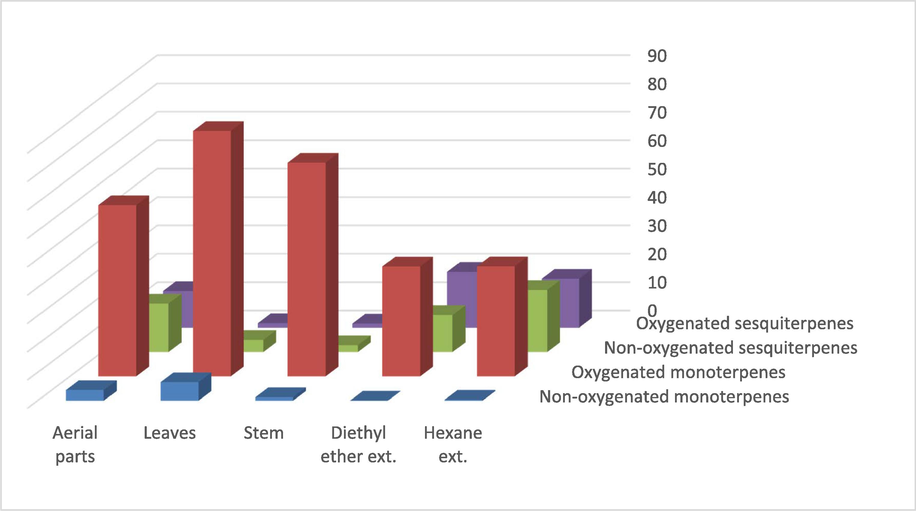
A cumulative histogram of the major classes of volatile oils in A. millefolium batches.
The results in Table 2 also indicated significant variations among the major volatile constituents of the plant. The componential analysis of the major constituents, e.g., myrcene, 1,8 cineole, trans-sabinene hydrate, α-thujone, β-thujone, thymol, germacrene D, and viridiflorol, revealed distinct up down flocculation in all batched of the plant. The ketonic monoterpenes α-thujone and β-thujone were found to be the most representative compounds in the volatile oils and extract batches of the plant. They together represented 58.60%, 47.59% and 38.29% in the leaves, stems, and aerial parts volatile oil batches, respectively. They also represented 22.21% and 21.87% of the n-hexane and diethyl ether extracts of the plant, respectively. On the other hand, the sesquiterpene hydrocarbon, germacrene D and sesquiterpene alcohol, viridiflorol, were measured at significantly higher levels in the plant extracts, i.e., n-hexane and diethyl ether, compared to the plant volatile oil distillates from leaves, stems, and aerial parts.
3.2 Antioxidant activity of A. millefolium essential oil
To assess the antioxidant potency of A. millefolium essential oil, four different in vitro assays were performed as outlined in the literature (Mohammed, 2022). These assays evaluated the batches reducing ability to the metals through measuring TAA and FRAP, the scavenging ability through the DPPH assay, and the metal-chelating activity through the MCA assay. The measurements were carried out in a manner comparable to that of standard antioxidant compound, Trolox, in the TAA, DPPH, and FRAP assays, and the standard metal-chelating agent, EDTA, in the MCA assay. The results of Fig. 2 display the comprehensive findings of the study, which demonstrate the remarkable antioxidant capabilities of the volatile oils and non-polar extracts derived from A. millefolium. The results also indicated variability in the antioxidant activity of different volatile oil batches and extracts of the plant. For example, all batches of the plant were comparable in inducing MCA, FRAP, and DPPH scavenging effects; however, volatile oil batches obtained from leaves, stems, and aerial parts of the plant exhibited substantially better TAA compared to the non-polar extracts of the plant. The better reducing activity of the volatile oil batches compared to the non-polar extracts might be attributed to the presence of myrcene, 1,8 cineole, camphor, α-thujone, and β-thujone, which have been reported for their antioxidant activity (Mohammed, 2022; Mohammed et al., 2021a, 2020; Mot et al., 2022), in higher concentrations in the volatile oil batches compared to their presence in the non-polar extracts, n-hexane, and diethyl ether extracts (Fig. 2).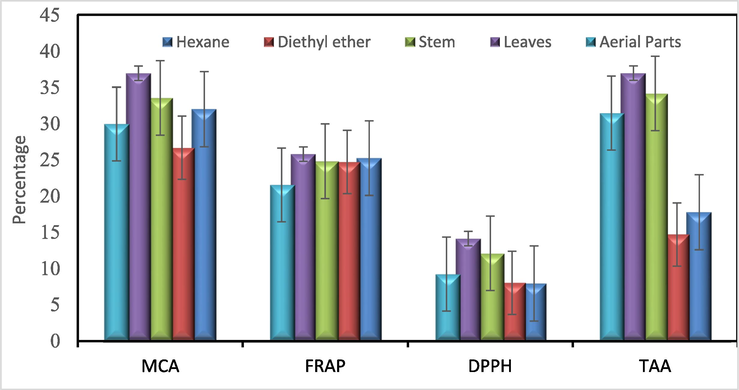
Histogram of antioxidant activity in the TAA, DPPH, FRAP and MCA tests of A. millefolium volatile oils in the distillate batches from leaves, stems, and aerial parts, as well as the extracted batches by n-hexane and diethyl ether, compared to reference standard antioxidant compound, Trolox, and standard metal-chelating agent, EDTA.
The results highlighted the environmental conditions effects on the biological activities of A. millefolium essential oils. The antioxidant activity of isolated essential oils was previously determined from A. millefolium on the mountain Jahorina (Bosnia and Herzegovina) using different antioxidant methods (ABTS, DPPH, and FRAP) with the same parameters used in our experiment. By comparing the chemical profiles of the oils of Achillea species from different locations, it showed significant differences in oil composition and in their antioxidant activity. In our species, it showed a higher record in the DPPH antioxidant method than in other countries. It is assumed to be related to the different geographical origins, which have a significant impact on the composition of volatile oils and their antioxidant activity (Čulum et al., 2021).
3.3 Cytotoxic and apoptotic activity of Achillea millefolium essential oil
It is worth noting that the susceptibilities of the cell lines examined to the n-hexane and diethyl ether extracts of A. millefolium were greater compared to those of the distilled oil batches at all concentrations (Fig. 3).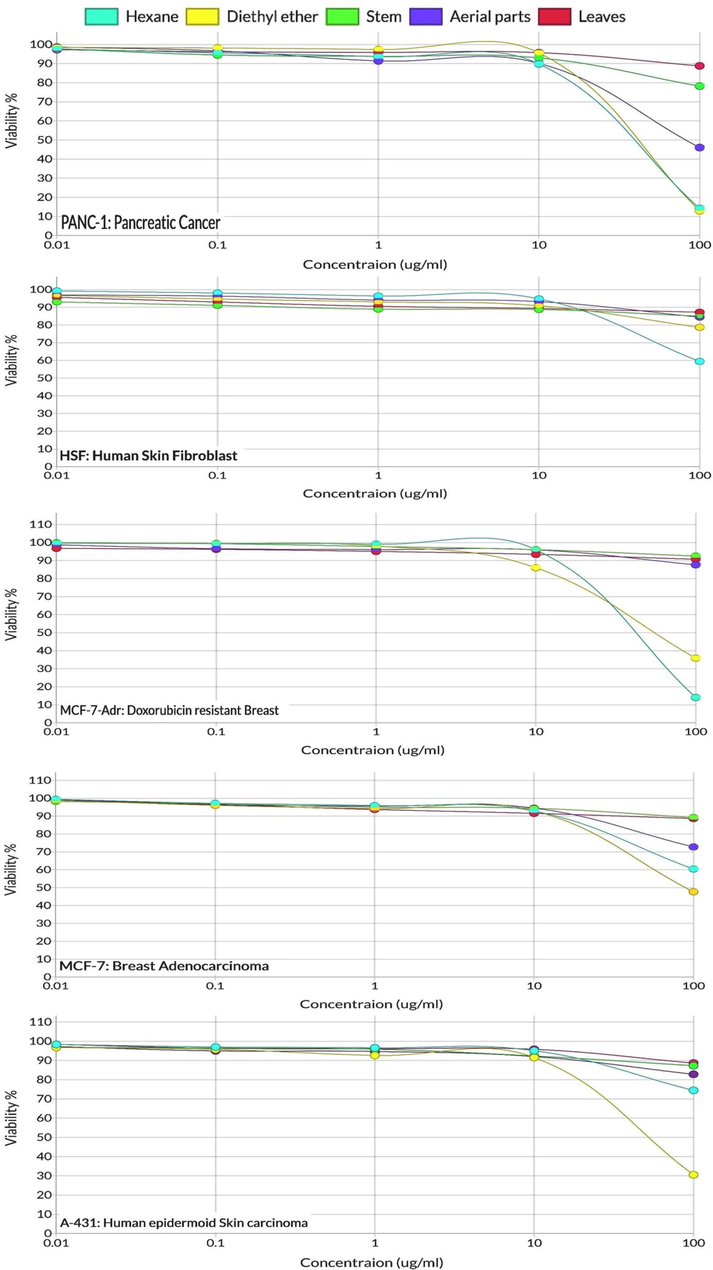
Cytotoxic activity of Achillea millefolium essential oil against different cell lines: (a) A-431: human epidermoid skin carcinoma cell lines; (b) HSF: human skin fibroblast cell lines; (c) MCF-7-Adr: doxorubicin-resistant breast cell lines; (d) MCF-7: breast adenocarcinoma cell lines; (e) PANC-1: pancreatic cancer cell lines.
The results also indicated that Panc-1 and MCF-7-Adr cell lines were more susceptible to the n-hexane and diethyl ether extracts, compared to the other cancer cell lines. This suitability of the cells was recognized by comparing the calculated IC50s displayed in Table 3, which indicated lower IC50 values for the Panc-1 and MCF-7-Adr (35 and 43.3 μg/mL, respectively, in the n-hexane extract and 41.3 and 57.1, respectively, in diethyl ether extract), compared to other cell lines, i.e., MCF-7, A-431, and HSF cells. In addition, the IC50 values represented in Table 3 indicated the superior cytotoxic effect of n-hexane extract over diethyl ether extract and all the volatile oil distilled batches of the plant. The dose dependent cytotoxic effects of all batches are represented in Fig. 3 and indicate the remarkable higher activity of non-polar extracts, n-hexane and diethyl ether extracts, compared to the oil distilled batches obtained from leaves, stems, and aerial parts, especially at the higher doses, i.e., 10 and 100 μg/mL. The overall higher cytotoxicity activity of non-polar extracts can be attributed to the existence of higher levels of germacrene D, viridiflorol, and caryophyllene oxide, which were previously recognized as cytotoxic sesquiterpenes (Karakaya et al., 2020; Akiel et al., 2022), respectively.
Sample
IC50 (μg/mL)
MCF-7
MCF-7 Adr.
Panc-1
A-431
HSF
n-Hexane
˃100
43.3
35
˃100
˃100
Diethyl ether
92.7
57.1
41.3
54.6
˃100
Stem VO
˃100
˃100
˃100
˃100
˃100
Leaves VO
˃100
˃100
˃100
˃100
˃100
Aerial Parts VO
˃100
˃100
86
˃100
˃100
The present study tested whether apoptosis induction may contribute to the growth inhibitory function of n-hexane extract in Panc-1 cells and MCF-7 Adr cells (Figs. 4 and 5). The ability of n-hexane extract to induce apoptosis in both cell lines was thus evaluated by treating cells with two concentrations of IC50 for 24, 48, and 72 h. The cells were subsequently subjected to staining with FITC/PI and flow cytometry analysis, in which only annexin V-stained cells were considered apoptotic cells. The treatment with n-hexane extract at IC50 35 µg ml−1 or double IC50 70 µg ml−1 caused a considerable apoptotic effect in Panc-1 cells in comparison with the negative control, and double IC50 70 µg ml−1 was the best in this regard. The numbers of late apoptotic and necrotic cells increased in a concentration- and time-dependent manner (Fig. 4). With respect to MCF-7 Adr. cells, treatment with IC50 43.3 µg ml−1 at three time periods (24, 48, and 72 h) induced mild apoptotic changes, but they were mostly observed at the later stages (Fig. 5). The necrotic changes were still less frequent when compared with the same period recorded in Panc-1 cells, and treatment with double IC50 86.6 µg ml−1 was more potent in this regard. In both cell lines, the concentrations employed in n-hexane extract were clearly more effective in inducing apoptosis or necrosis in Panc-1 cells than in MCF-7 Adr cells. According to GC analysis, the main identified chemical constituents were α-thujone, germacrene D, and heptadecane, which have potential effectiveness against cancer cell viability. Among Achillea species, the cytotoxic effects of A. alexandriregis, A. lavennae, and A. millefolium extracts have been tested on various tumor cell lines. Additionally, three human tumor cell lines were used to test the anti-tumoral effects of n-hexane, chloroform, aqueous methanol, and aqueous extracts of A. millefolium's aerial parts. Other studies in various human tumor cell lines demonstrated the anticancer potential of A. millefolium, where the chloroform extract had a mild impact on A431 cells and anticancer activity on HeLa and MCF-7 cells (Csupor-Löffler et al., 2009). Additionally, prior research has demonstrated that the methanolic fraction of A. millefolium decreased the cell proliferation of five various cancer cell lines (Abou Baker, 2020). The infusion of the plant herb has also been discovered by Dias et al. to be the most effective one in MCF-7 (Dias et al., 2013). Consequently, as shown in numerous studies, the makeup of various extracts may suggest anticancer potential (Kintzios et al., 2010; Pereira et al., 2018; Vitalini et al., 2011). Similar to this, certain sesquiterpenes from A. millefolium were isolated and demonstrated effectiveness against mouse P-388 leukemia cancer cells (TOzYO et al., 1994). HeLa cells may undergo apoptosis when exposed to A. millefolium essential oil, and pancreatic cancer cells may experience cell growth inhibition. It is found to downregulate SREBF1 and downstream molecular targets of this transcription factor, such as fatty acid synthase (FASN) and stearoyl-CoA desaturase (SCD). Previous studies on 1,8 cineole, or on its containing essential oils, reported apoptotic effects on a variety of cancer cell lines, including: human lung carcinoma (A549); human melanoma (SK-MEL-28); human Caucasian colon adenocarcinoma (Colo-205); human cervical carcinoma (SiHa); hepatocellular carcinoma (Hep-G2); breast adenocarcinoma MCF-7, T47D, MDA-MB-231; human colon carcinoma (RKO); human Caucasian colon adenocarcinoma (Caco-2); squamous cell carcinoma (A431); osteosarcoma (MG-63); and P815 (murine mastocytoma) (Abdalla et al., 2020; Akiel et al., 2022; Murata et al., 2013; Oliveira et al., 2015).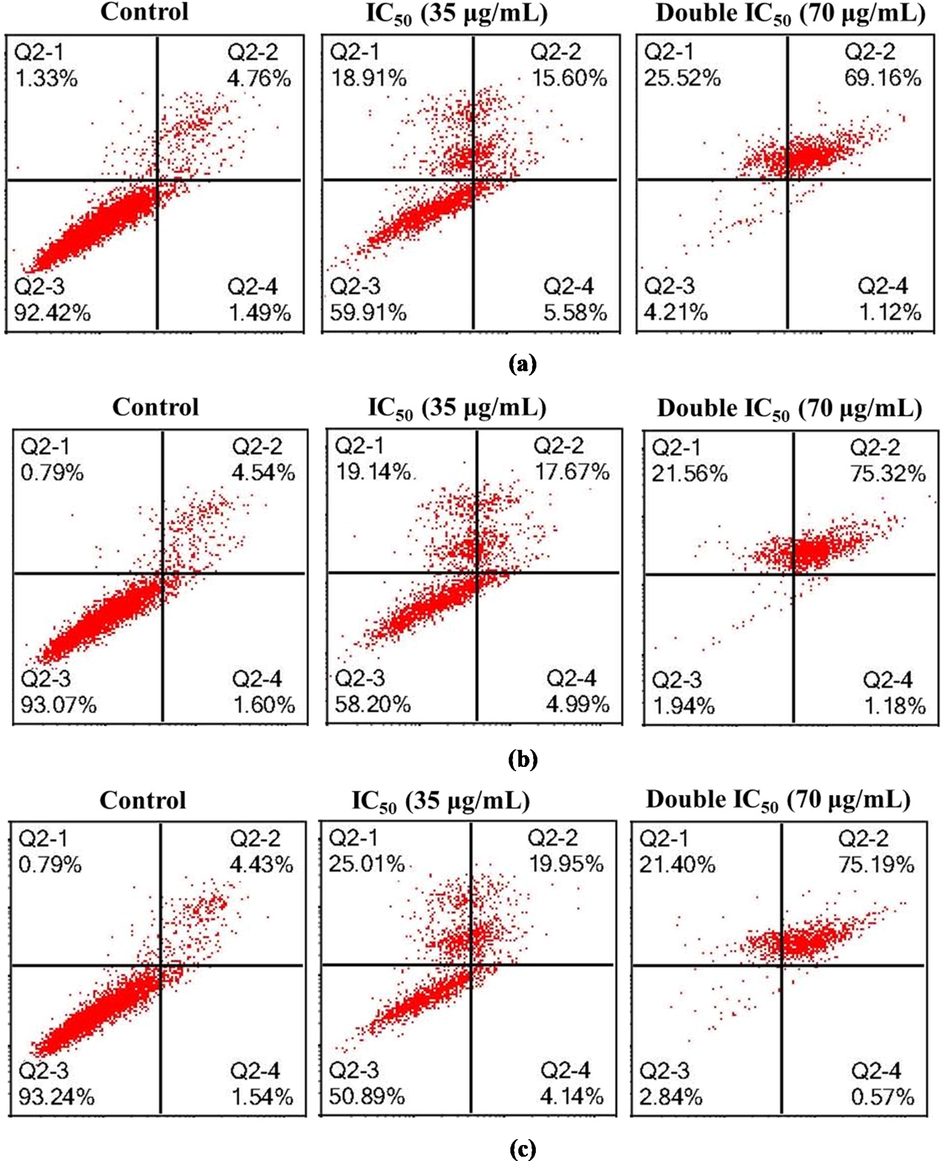
Representative cytogram of PANC-1 cells: PANC-1 cells were exposed to untreated (culture medium) and Hexane extract for (a) 24 h, (b) 48 h and (c) 72 h. Quadrant + YUI9L0\location for the representative dot plots: Q2-1: upper left—FITC-/PI+ (necrotic cells); Q2-2: upper right— FITC+/PI+ (late apoptotic cells); Q2-3: lower left— FITC-/PI- (viable cells) and Q2-4: lower right— FITC+/PI- (early apoptotic cells).
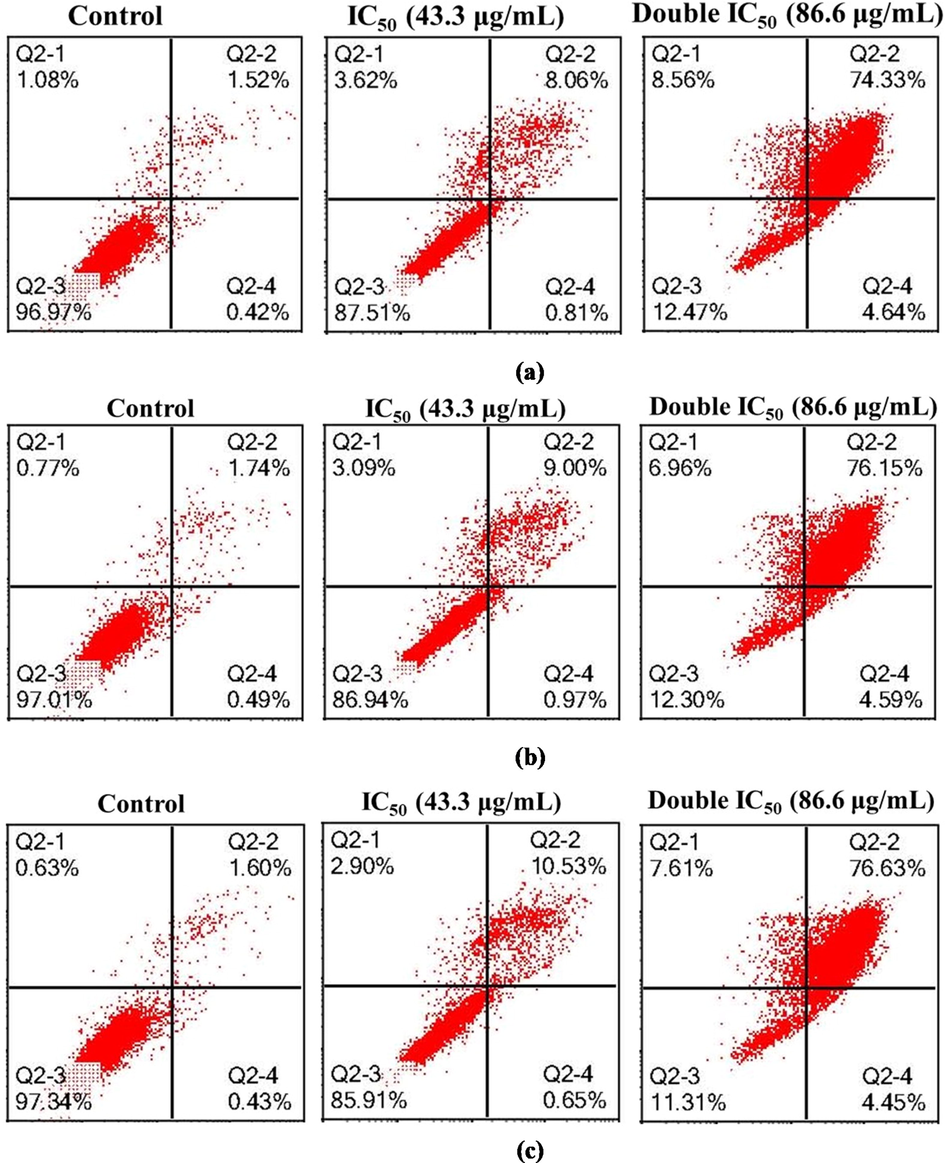
Representative cytogram of MCF-7 Adr. cells: MCF-7 Adr. cells were exposed to untreated (culture medium) and Hexane extract for (a) 24 h, (b) 48 h and (c) 72 h. Quadrant location for the representative dot plots: Q2-1: upper left—FITC-/PI+ (necrotic cells); Q2-2: upper right— FITC+/PI+ (late apoptotic cells); Q2-3: lower left— FITC-/PI- (viable cells) and Q2-4: lower right— FITC+/PI- (early apoptotic cells).
3.4 Docking results
Several chemotherapies, including paclitaxel, doxorubicin, and vincristine, have limited efficacy due to the resistance of tumor cells through P-glycoprotein/multidrug resistance 1/adenosine triphosphate-binding cassette (ABC) B1 (P-gp/MDR1/ABCB1), which promotes drug efflux and lowers their toxic effects (Adorni et al., 2023). ABCB1 has been shown to be an overexpressed transporter in numerous drug-resistant tumors (Wang et al., 2021). Molecular docking studies were carried out using the Autodock Vina software to evaluate and understand the potential of the three main constituents (germacrene D, viridiflorol, and caryophyllene oxide) of the active cytotoxic extracts, n-hexane and diethyl ethers, to sensitize MCF-7/ADR (DOX-resistant cells). In addition to the molecular dockings of the compounds of A. millefolium, DOX and the cocrystal ligand (zosuquidar) were also docked into the substrate binding sites of ABCB1. The docking score for DOX was −8.4 kcal/mol, while the docking score for zosuquidar was −9.5 kcal/mol. Germacrene D, viridiflorol, and caryophellene oxide identified from Achillea millefolium showed good binding potentials against ABCB1, as demonstrated by the lower the docking scores, the higher the binding affinity (Sabbah et al., 2010). The docking scores for Germacrene D, viridiflorol, and caryophyllene oxide were −7.3, −6.8, and −7.6 Kcal/mol, respectively (Table 4) and were involved in hydrophobic interactions, such as, alkyl - alkyl, and π – alkyl interactions (Fig. 6). The major residues involved in hydrophobic interactions in 1FN1 with Germacrene D, viridiflorol, and caryophyllene oxide were Phe302, Ala986, Val990, Phe993, and Ala994. The oxygen atom of viridiflorol and caryophyllene oxide can generate H-bonds with Gln837. These docking results showed that germacrene D, viridiflorol, and caryophyllene oxide have high binding affinity against the ABCB1 transporter, and they can be considered to be the likely ABCB1 inhibitors. They also appeared to be involved in improving the sensitivity.
Compound
Docking Scores (Kcal/mol)
Interacting Residues
Germacrene D
−7.3
Phe302, Ala986
Viridiflorol
−6.8
Gln837, Val990, Phe993, Ala994
Caryophyllene oxide
−7.6
Phe302, Gln837, Ala986
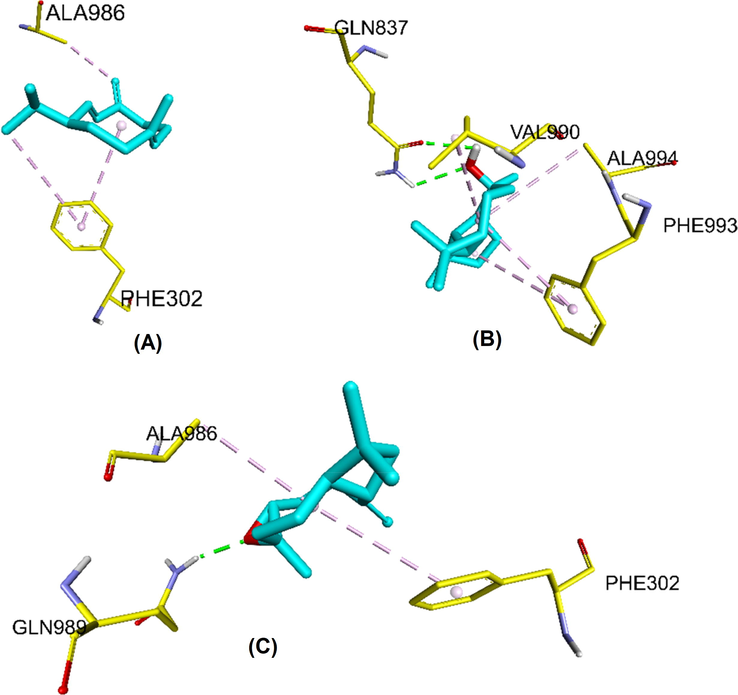
The docked poses of A) Germacrene D, B) viridiflorol, and C) caryophyllene oxide in the substrate binding sites of ABCB1 6FN1.
4 Conclusion
The volatile oil constituents of A. millefolium, a plant species cultivated in Saudi Arabia, were distilled from different plant parts, i.e., leaves, stems, and aerial parts, by the Clevenger apparatus using simple and low-cost water distillation methods. The volatile oil of the plant from the whole herb was also extracted by the non-polar solvents, i.e., n-hexane and diethyl ether, in order to make a phytochemical and biological comparison with the distilled oil batches of the plant. All the volatile oil batches were analyzed using GC-spectrometric methods. The higher percentages of the monoterpenes (oxygenated and non-oxygenated), as well as their low representation in the distilled batches and extracted batches of the plant, respectively, showed the variety in the volatile oil components in all batches of the plant. Additionally, compared to the distilled batches, the extracted batches of the plant had noticeably higher percentages of sesquiterpenes. The study indicated the higher ability of non-polar solvents to extract the relatively higher molecular weight volatile compounds, sesquiterpenes, more than monoterpenes, which were represented more in the distilled volatile oils. These phytochemical variations between the volatile oil batches were associated with significant differences in the biological activities, as demonstrated in their antioxidant and anticancer effects. Furthermore, relatively higher antioxidant and lower anticancer effects were found in distilled volatile oil batches compared to the extracted batches of the plant, which indicated the importance of preparation methods for volatile oils to target specific biological effects. As the plant, A. millefolium, is widely used in traditional medicine owing to its volatile oil constituents, and from an economic point of view, the current work highlighted the volatile constituents of the plant parts, stems, leaves, and aerial parts, indicating that the antioxidant activity of the plant is mostly associated with the monoterpene constituents, which can be obtained in higher concentrations by the distillation method. The work also indicated the potential use of the plant non-polar extract as an anticancer agent, the effect of which was evidenced by the in vitro cytotoxic and apoptotic assays, and the in silico simulation of the major constituents of the plant extracts, i.e., germacrene D, viridiflorol, and caryophyllene oxide. The in vivo anticancer experiments could be our plan for the continuation of the current study.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia under the project number (QU-IF-1-2-2).
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education and, Saudi Arabia for funding this research work through the project number (QU-IF-1-2-2). The authors are also thank the technical support of Qassim University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1, 8-cineole against A2780 ovarian cancer cells. Molecules. 2020;25:1582.
- [Google Scholar]
- Achillea millefolium L. ethyl acetate fraction induces apoptosis and cell cycle arrest in human cervical cancer (HeLa) cells. Ann. Agric. Sci.. 2020;65:42-48.
- [Google Scholar]
- A New ABCB1 Inhibitor Enhances the Anticancer Effect of Doxorubicin in Both In Vitro and In Vivo Models of NSCLC. Int. J. Mol. Sci.. 2023;24:989.
- [Google Scholar]
- Volatile constituents of Achillea millefolium L. ssp. millefolium from Iran. Flavour Fragr. J.. 1996;11:265-267.
- [Google Scholar]
- Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J. Biol. Sci.. 2022;29:816-821.
- [Google Scholar]
- Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science (80-.). 2019;363:753-756.
- [Google Scholar]
- Achillea millefolium Essential Oil Mitigates Peptic Ulcer in Rats through Nrf2/HO-1 Pathway. Molecules. 2022;27:7908.
- [Google Scholar]
- GC-MS analysis: in vivo hepatoprotective and antioxidant activities of the essential oil of Achillea biebersteinii afan. Growing in Saudi Arabia. Evid.-Based Complement. Altern. Med. 2016:2016.
- [Google Scholar]
- A facile approach synthesis of benzoylaryl benzimidazole as potential α-amylase and α-glucosidase inhibitor with antioxidant activity. Bioorg. Chem.. 2021;114:105073
- [Google Scholar]
- Synthesis, Molecular Docking, and Bioactivity Study of Novel Hybrid Benzimidazole Urea Derivatives: A Promising α-Amylase and α-Glucosidase Inhibitor Candidate with Antioxidant Activity. Pharmaceutics. 2023;15:457.
- [Google Scholar]
- Safety assessment of Achillea millefolium as used in cosmetics. Int. J. Toxicol.. 2016;35:5S-15S.
- [Google Scholar]
- Achillea millefolium L. sl revisited: recent findings confirm the traditional use. Wiener Medizinische Wochenschrift. 2007;157:312-314.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Biovia, D.S., 2016. Discovery studio modeling environment, release 2017 Dassault Systèmes. San Diego, CA.
- Supercritical CO2 extraction of essential oil from yarrow. J. Supercrit. Fluids. 2007;40:360-367.
- [Google Scholar]
- Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae) J. Ethnopharmacol.. 2003;87:215-220.
- [Google Scholar]
- Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J. Ethnopharmacol.. 2006;107:277-284.
- [Google Scholar]
- Obtaining and characterization of Achillea millefolium L. extracts. J. Agroaliment. Process. Technol.. 2014;20:142-149.
- [Google Scholar]
- Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium sl on cultured human tumour cell lines. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv.. 2009;23:672-676.
- [Google Scholar]
- Essential Oil Composition and Antioxidant Activity of Endemic Achillea lingulata Waldst. & Kit. Compared to Common A. millefolium L. Rec. Nat. Prod. 2021:1-11.
- [Google Scholar]
- Towards more rational techniques for the isolation of valuable essential oils from plants. TrAC Trends Anal. Chem.. 1999;18:708-716.
- [Google Scholar]
- Cytotoxic Activity and Apoptosis-Inducing Potential of Di-spiropyrrolidino and Di-spiropyrrolizidino Oxindole Andrographolide Derivatives. PLoS One. 2013;8:e58055.
- [Google Scholar]
- A comparative study of bioactive properties of wild and commercial Achillea millefolium L. In: 1st Symposium on Medicinal Chemistry of University of Minho. Universidade do Minho; 2013.
- [Google Scholar]
- Antioxidant and antimicrobial activities of the essential oil of Achillea millefolium L. grown in France. Medicines. 2017;4:30.
- [Google Scholar]
- Phytochemical and biological activity profiles of Thymbra linearifolia: An exclusively native species of Libyan Green Mountains. Arab. J. Chem. 2023:104775.
- [Google Scholar]
- Comparative GC-MS Analysis of Fresh and Dried Curcuma Essential Oils with Insights into Their Antioxidant and Enzyme Inhibitory Activities. Plants 2023
- [CrossRef] [Google Scholar]
- Chemical composition and biological activity of the volatile extracts of Achillea millefolium. Nat. Prod. Commun.. 2011;6
- [Google Scholar]
- 35Year Research History of Cytotoxicity and Cancer: a Quantitative and Qualitative Analysis. Asian Pac. J. Cancer Prev.. 2016;17:3139-3145.
- [Google Scholar]
- Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crops Prod. 2020:112570.
- [Google Scholar]
- Comparison of microwave and conventional extraction methods for natural dyes in wood waste of mahogany (Swietenia mahagoni) J. Appl. Eng. Sci.. 2020;18:618-623.
- [Google Scholar]
- Chemical Constituents from the Aerial Parts of Achillea millefolium and Their Aldose Reductase Inhibitory Activity. Shoyakugaku Zasshi. 2019;73:91-92.
- [Google Scholar]
- A Diverse Benchmark Based on 3D Matched Molecular Pairs for Validating Scoring Functions. ACS Omega. 2018;3:5704-5714.
- [Google Scholar]
- A Caryophyllene Oxide and Other Potential Anticholinesterase and Anticancer Agent in Salvia Verticillata Subsp. Amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae) J. Essent. Oil Res.. 2020;32:512-525.
- [Google Scholar]
- PubChem 2019 update: improved access to chemical data. Nucleic Acids Res.. 2019;47:D1102-D1109.
- [Google Scholar]
- Evaluation of the antioxidants activities of four Slovene medicinal plant species by traditional and novel biosensory assays. J. Pharm. Biomed. Anal.. 2010;53:773-776.
- [Google Scholar]
- Solvent-free Microwave Extraction as the Useful Tool for Extraction of Edible Essential Oils. Chem. Chem. Technol.. 2016;10:213-218.
- [CrossRef] [Google Scholar]
- Essential oil composition and antibacterial activity of Achillea millefolium L. from different regions in North east of Iran. J. Med. Plants Res.. 2013;7:1063-1069.
- [Google Scholar]
- Variability of the essential oils composition of Achillea millefolium ssp. millefolium growing wild in Lithuania. Biochem. Syst. Ecol.. 2003;31:1033-1045.
- [Google Scholar]
- Yarrow oil ameliorates ulcerative colitis in mice model via regulating the NF-κB and PPAR-γ pathways. Intest. Res.. 2021;19:194.
- [Google Scholar]
- Phytochemical Analysis, Antioxidant Potential, and Cytotoxicity Evaluation of Traditionally Used Artemisia absinthium L. (Wormwood) Growing in the Central Region of Saudi Arabia. Plants. 2022;11:1028.
- [Google Scholar]
- Drying Induced Impact on Composition and Oil Quality of Rosemary Herb, Rosmarinus Officinalis Linn. Molecules. 2020;25:2830.
- [Google Scholar]
- Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules. 2021;26:5757.
- [Google Scholar]
- Quercetin against MCF7 and CAL51 breast cancer cell lines: Apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine. 2021;16(22):1937-1961.
- [Google Scholar]
- Comparative Anticancer Potentials of Taxifolin and Quercetin Methylated Derivatives against {HCT}-116 Cell Lines: Effects of O-Methylation on Taxifolin and Quercetin as Preliminary Natural Leads. ACS Omega. 2022;7:46629-46639.
- [CrossRef] [Google Scholar]
- Phytochemical profiling, molecular docking, and in vitro anti-hepatocellular carcinoid bioactivity of Suaeda vermiculata extracts. Arab. J. Chem.. 2022;15:103950
- [CrossRef] [Google Scholar]
- Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine: Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants. 2022;11:332.
- [CrossRef] [Google Scholar]
- Antispermatogenic effect of Achillea millefolium L. in mice. Contraception. 1998;58:309-313.
- [Google Scholar]
- AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30:2785-2791.
- [Google Scholar]
- Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants. 2022;11:808.
- [Google Scholar]
- Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep.. 2013;30:2647-2652.
- [Google Scholar]
- The essential oil composition of Achillea millefolium L. cultivated under tropical condition in India. World J. Agric. Sci.. 2011;7:561-565.
- [Google Scholar]
- Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn.. 2015;25:183-188.
- [Google Scholar]
- Composition of the essential oil from Achillea millefolium L. from Estonia. J. Essent. Oil Res.. 2001;13:290-294.
- [Google Scholar]
- Chemical compositions of Achillea sivasica: Different plant part volatiles, enantiomers and fatty acids. Rec. Nat. Prod.. 2018;12:142.
- [Google Scholar]
- Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cell lines by interfering with cell cycle and inducing apoptosis. Food Chem. Toxicol.. 2018;118:635-644.
- [Google Scholar]
- POWO, 2023. The International Plant Names Index and World Checklist of Vascular Plants [WWW Document].
- Production of yarrow (Achillea millefolium L.) in Norway: essential oil content and quality. J. Agric. Food Chem.. 2000;48:6205-6209.
- [Google Scholar]
- Docking studies on isoform-specific inhibition of phosphoinositide-3-kinases. J. Chem. Inf. Model.. 2010;50:1887-1898.
- [Google Scholar]
- Determination of the chemical composition and antimicrobial activity of the essential oils of Teucrium polium and Achillea millefolium grown under North Anatolian ecological conditions. Biotechnol. Biotechnol. Equip.. 2016;30:375-380.
- [Google Scholar]
- Stratakos, A.C., Koidis, A., 2016. Methods for extracting essential oils. In: Essential Oils in Food Preservation, Flavor and Safety. Elsevier, pp. 31–38.
- In vitro study of molecular structure and cytotoxicity effect of luteolin in the human colon carcinoma cells. European Food Res. Techn.. 2015;241(1):83-90.
- [Google Scholar]
- Chemical composition, antimicrobial, antioxidant and cytotoxic activities of Eucalyptus chapmaniana grown in Iraq. Am. J. Agri. Biological Sci.. 2013;9(1):78-88.
- [Google Scholar]
- Repellent effect and metabolite volatile profile of the essential oil of Achillea millefolium against Aegorhinus nodipennis (Hope)(Coleoptera: Curculionidae) Neotrop. Entomol.. 2015;44:279-285.
- [Google Scholar]
- Assessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L., in in vitro and in vivo assays. Genet. Mol. Biol.. 2003;26:551-555.
- [Google Scholar]
- Novel antitumor sesquiterpenoids in Achillea millefolium. Chem. Pharm. Bull.. 1994;42:1096-1100.
- [Google Scholar]
- Supercritical fluid extraction of Bulgarian Achillea millefolium. J. Supercrit. Fluids. 2017;119:283-288.
- [Google Scholar]
- Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol.. 2011;58
- [Google Scholar]
- The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin. Drug Metab. Toxicol.. 2021;17:291-306.
- [Google Scholar]
- WFO, 2022. Asteraceae. The daisy family. World Flora Online Data [WWW Document]. URL http://worldfloraonline.org/organisation/Asteraceae (accessed 6.16.23).
- Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med.. 2016;8:286-292.
- [Google Scholar]







