Translate this page into:
Visnagin inhibits cervical cancer cells proliferation through the induction of apoptosis and modulation of PI3K/AKT/mTOR and MAPK signaling pathway
⁎Corresponding author. doctorwlh@126.com (Liehong Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cervical cancer, a silent killer is a second most common type of malignant tumor detected in women’s world wide. In modern medicine the usage of phytochemicals to develop drugs for treating various chronic diseases is rapidly increasing. One such phytochemical is visnagin, a furanochrome present in fruits of Ammi visnaga. We investigated the anticarcinogenic potency of visnagin against human cervical carcinoma cells. The antioxidant potency of visnagin was analyzed with FRAP assay, DPPH assay, Chemiluminscence assay and ORAC assay. The cytotoxic effect of visnagin on normal epithelial Vero cells and human cervical cancer HeLa cells were analyzed using MTT assay. The effect of visnagin on antioxidant system was examined by measuring the levels of TBARS, SOD and GSH using the colorimetric assay techniques. DCFH-DA staining, AO/EtBr staining, propidium iodide staining was performed to assess the apoptotic induction potency of visnagin against cervical cancer cells. The ability of visnagin to inhibit cancer cell migration was examined with scratch wound assay. The anticarcinogenic property of visnagin was confirmed by analyzing the gene expression of PI3K/AKT/mTOR signaling proteins and MAPK signaling proteins using qPCR analysis. Visnagin exhibited increased Trolox equivalent value in all the four antioxidant potency estimating experiments. Visnagin induced cytotoxic effect only on carcinoma cells, decreased the antioxidants and increased the generation of ROS. It also induced apoptosis and inhibited the cancer cell migration. The qPCR analysis confirms visnagin decreases the gene expression cell cycle regulating protein of both PI3K/AKT/mTOR and MAPK pathway. Overall our results authentically prove visnagin inhibits the progression of cervical cancer in vitro. Therefore it can be an ideal drug of choice which can subject to further investigation for treating cervical cancer.
Keywords
Cervical cancer
In vitro
HeLa cells
Treatment
Phytochemical
Visnagin
1 Introduction
A major global threat to women population of reproductive age is cervical cancer which ranks the fourth most commonly occurring malignancy in females (Bray et al., 2018). Approximately every year about 5million women were diagnosed with cervical cancer and 2.75 million deaths were reported. The cervical cancer induced mortality rates differ epidemiologically low and middle income contributes 90% of cervical cancer induced mortality. The exact causative agent of cervical cancer was not yet elucidated whereas the infection with human papilloma virus (HPV) was considered to be a major causative agent. Early coitus before the age of 20 years, women with multiple sexual partners were more prone to cervical cancer. The incidence rate of cervical cancer was reported more in middle aged women than in younger and older women population (Bhatla et al., 2018; Olusola et al., 2019).
Cervical cancer is a less progressive cancer and it can be easily cured if diagnosed at early stage. The lack of awareness of cervical cancer and lack of diagnosis leads to the increased mortality in lower-middle income countries which is 18% higher than the high income countries (Zhang et al., 2020). Surgery along with radiation therapy is the common treatment prescribed to treat cervical cancer at early stage whereas the progressive stage was treated with radiation therapy accompanied with chemotherapy. DNA interactive agents, hormones, antimetabolites, anti-tublin agents are the currently available chemodrugs. The chemodrugs are very expensive, non target specific, easily get resistant to cancer cells and recurrence of cancer occurs often. (Nussbaumer et al., 2011; Cohen et al., 2019). Hence it is need of today to discover a cost effective potent anticancer drug which cures cervical cancer.
Phytochemicals which are the guardians of plants were utilized long ago in traditional medicine to treat diverse diseases. The lack of scientific proven data for pharmaceutical property of phytochemicals were suppressed their usage in modern medicine. Specifically the antioxidant property of phytochemical exemplifies it as novel candidate to treat cancer ((Lee et al., 2013).
Visnagin (4-methoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one) is one such phytochemical which is derived from the fruits of Ammi visnaga L plant species. Ammi visnaga L is biennial herb indigenous to Asia, Europe and Mediterranean regions of North Africa (Chevallier 1996; Batanouny, 2001). It exhibits vasodialating effect decreases blood pressure (Duarte et al., 2000), prevents renal epithelial cells from oxalate induced cell damage (Vanachayangkul et al., 2010), act as anti-inflammatory agent (Lee et al., 2010), acts as diuretic infusion against kidney and bladder stone (Rauwald et al., 1994). The anticancer effect of visnagin was investigated in the present study with human cervical carcinoma HeLa cell in vitro.
2 Materials & method
2.1 Culturing of HELA and Vero cell lines
HeLa (Human cervical cancer cell line) and Vero (Animal kidney cell line) were procured from ATCC, Manassas, USA. Both the HeLa and Vero cells were cultured in EMEM with 10% FBS in 5% CO2 humidified atmosphere at 37 °C. Cells were replaced with new culture medium for every 48 h and the cells were subcultured upon attaining 80% confluence using 0.25% trypsin-EDTA solution.
2.2 Ferric reducing antioxidant potential assay
FRAP assay was performed according to the protocol of Benzie & Strain, 1996 to quantify the antioxidant potency of visnagin. FRAP reagent was freshly prepared with 300 mM acetate buffer, pH 3.6, 10 mM TPTZ in 40 mM hydrochloric acid and 20 mM ferric chloride in the ratio 10:1:1 respectively. 10 µl of different concentrations of visnagin samples ranging from 1 µM − 100 µM were mixed with 360 µl of FRAP reagent and incubated at 37 °C for 10 min. After incubation the absorbance of the samples were measured at 593 nm using spectrophotometer. Distilled water is used as blank sample and the standard curve is prepared using various concentrations of Trolox ranging from 0.2 to 0.8. The experiments were performed in triplicates. The FRAP value can be calculated as
where c – Concentration of Trolox in µmol/ml, V – sample volume in ml, t - dilution factor, and m – dry weight of sample in g.
2.3 DPPH assay
Free radical scavenging property of visnagin was assessed with DPPH radicals using the method of Miliauskas et al. (2004). Visnagin stock solution was prepared with methanol.
Fresh DPPH was prepared by dissolving 7.89 mg of DPPH with 100 ml of 99.5% methanol solution. Before utilizing for the experiment the DPPH solution was kept in dark for 2 h. To a test tube 1000 µl of DPPH solution and 800 µl of Tris-HCl buffer (pH 7.4) were added. To this mixture 200 µl of different concentrations of visnagin ranging from 1 µM − 100 µM was added and kept at 37 °C for 30 min. The OD values of the mixture were measured at 515 nm. Methanol was used as blank. Inhibition ratio percentage was calculated using formula
A1 - absorbance of blank, A2 - absorbance of test samples.
IC50 was calculated by using interpolation method by selecting two points enclosing 50% inhibition ratio and a regression line of Y = AX + B was drawn. The sample concentration was calculated by substituting 50 in Y value of the above regression equation
Trolox equivalent antioxidant capacity of the samples were calculated using the formula
2.4 Chemiluminescence assay
Different concentrations of visnagin solution were prepared with 0.1 M phosphate buffer, pH7.4 for the chemiluminescence assay. 0.1 M phosphate buffer was used as blank solution. To10µl test sample or blank, luminol solution was added (1.13x10-4 M concentration) and then hydrogen peroxide solution was added to make up the final concentration to 5x10-5 M. 0.2 IU/ml of HRP reagent was added and the chemiluminescence was measured for 10 min at 25 °C. The TEAC of the sample was calculated same as done for the DPPH assay (Krol et al., 1994).
3 Oxygen radical absorbance capacity (ORAC) assay
ORAC assay was done according to the method of Thaipong et al. (2006). 153 mmol/l of AAPH solution was prepared in 75 mmol/l of phosphate buffer pH 7.4. Fluorescein was prepared by dissolving 20 mg fluorescein in 75 mmol/l phosphate buffer and the sample is diluted to 25 ml. 50 µl of this mixture was further diluted to 25 ml with phosphate buffer and it was used as diluent for fluorescein working solution. 1 mmol/l Trolox was prepared and it was diluted for various concentration of working solution ranging from 0.10 to 0.02 mmol/l. To a 96 well plate 100 µl of fluorescein working solution was added and the fluorescence value A0 was measured immediately at excitation wavelength of 490 and emission wavelength of 514 nm. 50 µl of different concentrations of test samples and the standard trolox working solution were added and kept in shaker for 3 min, then incubated t 37 °C for 10 min. 50 µl of Inducer APPH working solution was added and the fluorescence value Fn was measured for every 90 s. The measuring of fluorescence was stopped once the fluorescence value decayed to a straight line. ORAC value was calculated using the formula
3.1 Cell viability assay
6x104 cells/well of HeLa and Vero cell lines were seeded on to 96 well cell culture plate with DMEM culture medium and grown in 5% CO2 for overnight. Then cells were treated with visnagin (1 µM-100 µM concentration) and subjected to 24 h incubation. After incubation period 50 µl of MTT solution was added to the wells and incubated for 3 h in 5% CO2 incubator. The formazan crystals formed due to MTT reaction was dissolved with 100 µl of DMSO and absorbance of solution was measured at 540 nm.
3.2 TBARS & antioxidant assay
The TBARS, SOD and GSH assay were done using the commercially available colorimetric kit procured from Abcam, USA. HeLa cells were treated with 10 µM and 25 µM, incubated at 37 °C in 5% CO2 for 24 h. After incubation the cells were collected, sonicated and utilized for the current experiment. The experiments were performed according to the standard manufacturer’s protocol provided in the kit. The absorbance of the samples was measured using ELISA spectrophotometer. The standard graph was drawn using the absorbance of known concentration. The experiments were performed in triplicates.
3.3 ROS staining
The levels of ROS generated by visnagin against HeLa carcinoma cells were detected using 2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA) staining. After 24 h incubation fo HeLa cells with 10 µM, 25 µM the cells were subjected to DCFH-DA staining. The cells were stained with 10 µM DCFH-DA stain and incubated in dark for 30 min. After incubation the cells were washed with PBS twice and viewed under fluorescent microscope (Olympus).
3.4 AO/ETBr staining
The induction of apoptosis by visnagin on HeLA cells were examined with AO/EtBr staining technique. Vianagin treated cells were stained with mixture of acridine orange and ethidium bromide which was prepared in equal ratio and kept in dark for a period. The stained cells were rinsed with PBS and then viewed under fluorescent microscope.
3.5 PI staining
HeLa cells were treated with 10 µM and 25 µM, incubated at 37 °C in 5% CO2 for 24 h. The cells were then stained with Propidium Iodide stain for 20 min incubated in dark. The stained cells were rinsed with PBS and then viewed under fluorescent microscope.
3.6 Scratch wound assay
HeLa cell were seeded on to 6 well culture plates and incubated for 24 h, upon attaining 90% confluency the wells were scratched with a sterile 1 ml micropipette tip. The uniformity of scratch was maintained among all the wells. The cells were rinsed with DMEM medium to remove the scratched cells and treated with 10 µM and 25 µM, incubated at 37 °C in 5% CO2 for 24 h. After incubation the wound closure was viewed microscopically and photographed for further analysis (Liang et al., 2007).
3.7 qPCR analysis
Visnagin treated HeLa cell lines were subjected to qPCR analysis. Total RNA was isolated with TRI reagent procured from Sigma Aldrich, USA. 1 ml of TRI reagent was added to the cells and the cells were scrapped gently with the pipette to obtain a homogenous cell lysate. The cells lysate was sonicated and then 0.2 ml of chloroform was added. The mixture was subjected to 12000rmp for 10 min at 4 °C for phase separation. Aqueous phase was then collected and to that equal volume of ice cold propanol was added, centrifuged at 12000rmp for 10 min at 4 °C for precipitation of RNA. RNA pellet was collected and washed twice with 70% ethanol. RNA pellet was air dried and dissolved in 20 µl milliQ water. RNA purity was examined with NanoDrop spectrophotometer and subjected to complementary DNA synthesis with HiScript II Q RT SuperMix kit.
PI3K, Akt, mTOR, ERK1/2, p38, JNK1/2 gene expression were quantified by amplifying the above genes using SYBR green master mix. The results were analyzed using comparative CT method (2−ΔΔCt) and the fold change of samples was calculated using CFX Manager Version 2.1 (Bio Rad, USA).
3.8 Statistical analyses
All the experiments were performed in triplicates and the data were analyzed statistically using statistics software GraphPad Prism. The data were analyzed with One Way ANOVA followed by the Student Newman Keuls test. The results were expressed as mean ± SEM, and p < 0.05 was considered to statistically significant.
4 Results
4.1 Antioxidant potency of furanochromone visnagin
In present study the antioxidant potency of furanochrome visnagin was assessed with four different assays and trolox equivalent (TE) values were shown. Fig. 1A depicts the results of Ferric reducing antioxidant potency of visnagin. The TE value of visnagin was significantly increased in a dose dependant manner, maximum of 75 mM TE value was obtained with 100 µM visnagin concentration. DPPH assay results of visnagin were illustrated in the Fig. 1B. The maximum of 7.2 ± 0.8 mM TE value was obtained at 100 µM visnagin concentration. Fig. 1C represents the results of chemiluminescence assay of visnagin. Control shown 1.8 mM TE value during chemiluminescence whereas it significantly increased in different concentrations of visnagin and 100 µM visnagin shown 7.8 mM TE value. Oxygen Radical Absorbance Capacity of visnagin was analyzed with ORAC assay and the results were depicted in the Fig. 1D. Control exhibited 2 mM TE value and the highest concentration 100 µM shown TE value of 17 mM.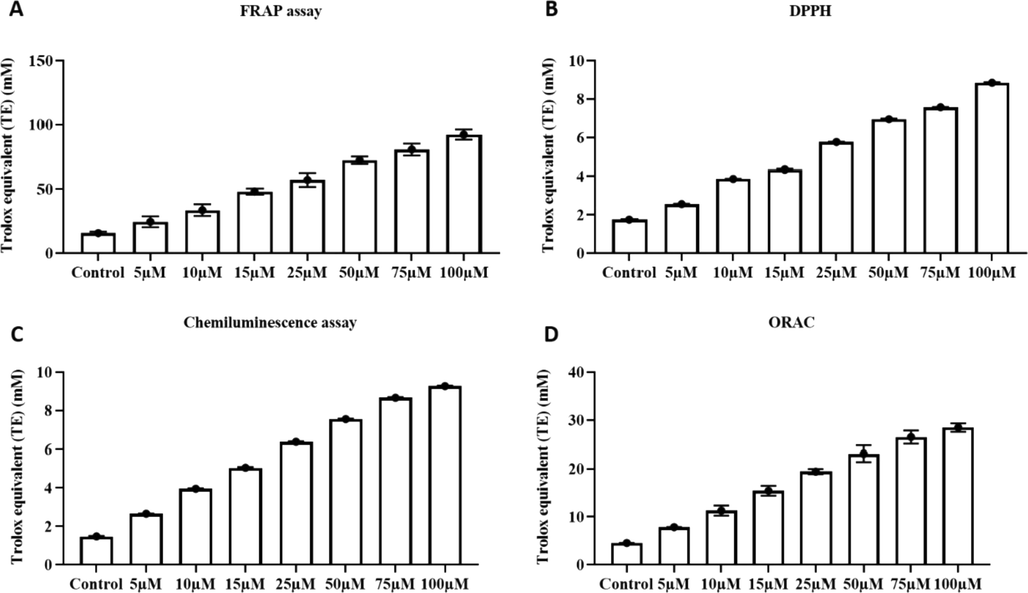
Antioxidant potency of furanochromone Visnagin. A- Ferric reducing antioxidant potency of visnagin. B - DPPH assay results of visnagin. C- Chemiluminescence assay results of visnagin. E - Oxygen Radical Absorbance Capacity of visnagin. The data were statistically analyzed with One Way ANOVA followed by Student’s Newman Keuls test. The results were expressed as mean ± SEM. p < 0.05, statistically significant.
4.2 Cytotoxic potency of furanochrome visnagin
Fig. 2A illustrates the MTT results of visnagin treated HeLa cells. The cytotoxic potency visnagin increased in dose dependant manner and about 80% of dead cells were viewed in 100 µM visnagin treated cells. 50% of cell death was observed with 10 µM visnagin treatment. To compare the cytotoxic effect of visnagin in normal epithelial cells, visnagin was treated Vero kidney epithelial cells (Fig. 2B). Even the highest concentration 100 µM visnagin treatment shown only 10% of cell death and only 6–8% of cell death was observed in 10 µM & 25 µM visnagin treatment which shown 50% cell death in HeLa carcinoma cells.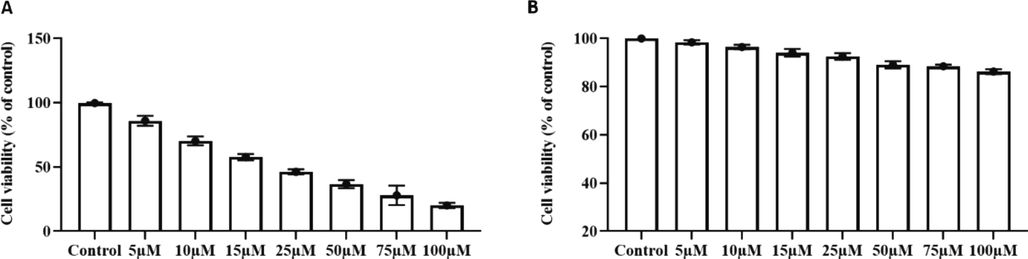
Cytotoxic potency of furanochrome visnagin. The cytotoxic potency of visnagin on carcinoma cells and normal epithelial cells were assessed with MTT assay. A – Human cervical carcinoma HeLa cells. B – Animal normal epithelial cells Vero cell line. Results were subjected to statistical analysis with One Way ANOVA followed by Student’s Newman Keuls test. The values does not share a common superscript and significantly differ at p < 0.05.
4.3 Effect of furanochrome visnagin on antioxidant system in HeLa cells
Visnagin significantly increased the levels of TBARS in HeLa which is the indicator lipid peroxidation in cells. Compared to 10 µM visnagin, the 25 µM visnagin treatment shown drastic increase in TBARS level (Fig. 3A). Increased in antioxidant levels is also key factor for induction of cancer cell induction. Visnagin treatment decreased enzymatic antioxidant superoxide dismutase (Fig. 3B) and non enzymatic antioxidant glutathione (Fig. 3C) levels. The reduction in antioxidant levels was significant and it was in dose dependent manner.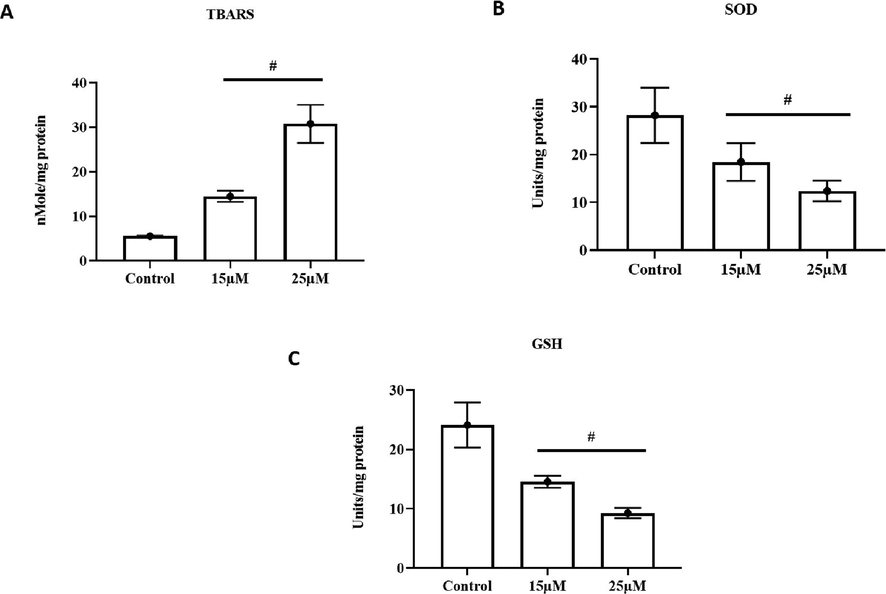
Effect of furanochrome visnagin on antioxidant system in HeLa cells. HeLa cells were treated with 15 µM and 25 µM visnagin for 24 h, assessed for A- Thiobarbituric acid reactive substances (TBARS), B- Enzymatic activity of superoxide dismutase (SOD), C- Level of non-enzymatic antioxidant glutathione (GSH). The assays were performed with commercially available colorimetric kits as per the manufacturer’s protocol. The results demonstrated that visnagin treatment effectively improved the TBARS content, decreased the SOD activity and GSH level in the HeLa cells. Results were subjected to statistical analysis with One Way ANOVA followed by Student’s Newman Keuls test. p < 0.05, statistically significant, respectively.
4.4 Free radical generation of furanochrome visnagin on HeLa cells
Fig. 4 illustrates the results of DCFH-DA stained HeLa cells which shows the ROS generation induced by visnagin. Increased green fluorescence emission was observed in visnagin treated HeLa cells compared to the control cells which indicate the generation of free radicals by visnagin treatment. Compared to 10 µM visnagin treatment, the higher dose 25 µM visnagin treatment shown increased ROS generation.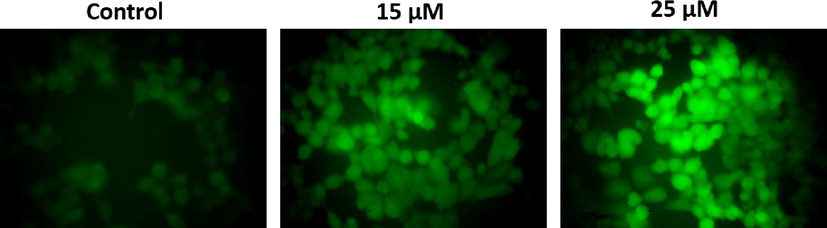
Free radical generation of furanochrome visnagin on HeLa cells. HeLa cells were treated with 15 µM and 25 µM visnagin for 24 h and assessed for ROS generation by DCFH-DA staining technique. The emission of green fluorescence were viewed and photographed under microscope. The visnagin treated HeLa cells showed a increased green fluorescence, which indicates the substantial increment in the ROS production. Representative images were depicted as Control, 15 µM visnagin treated HeLa cells, and 25 µM visnagin treated HeLa cells, respectively.
4.5 Apoptotic induction of furanochrome visnagin on HeLa cells
The induction of apoptosis by visnagin on cancer cell line HeLa cells were assessed with AO/EtBr stain and PI staining. AO/EtBr dual staining results were shown in the Fig. 5A. AO/EtBr stain was performed to differentiate live and dead cells in a culture. Acridine orange stain penetrate both the live and dead cells, stains the live cells in green whereas ethidium bromide can only penetrate into the dead cells and stains red. Necrotic cells with normal nuclear morphology will be stained with orange. Our AO/EtBr staining results of visnagin treated HeLa cells shown increased red fluorescence indicating the presence of increased number of dead cells. The red fluorescence increases in dose dependant manner.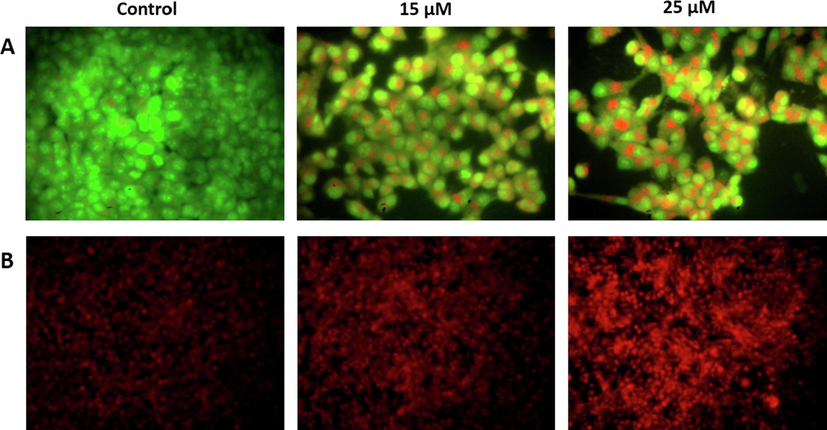
Apoptotic induction of furanochrome visnagin on HeLa cells. HeLa cells were treated with 15 µM and 25 µM visnagin for 24 h, assessed for apoptotic induction with A- AO/EtBr dual staining technique, B- Propidium iodide nuclear staining technique. The dual staining revealed the increased yellow and orange fluorescence in the visnagin treated HeLa cells. The PI staining images revealed the increased red fluorescence in the visnagin treated HeLa cells. These findings indicates the increased apoptotic cell death. The emission of green and red fluorescence were viewed and photographed under microscope. Representative images were depicted as Control, 15 µM visnagin treated HeLa cells, 25 µM visnagin treated HeLa cells, respectively.
Fig. 5B depicts the representative propidium iodide stained images of control and visnagin treated HeLa cells. Propidium iodide is a nuclear stain which is permeable only in dead cells and emits red fluorescence. Visnagin treatment increased the cell death in dose dependant manner which is depicted by the increased red fluorescence emission in both 10 µM visnagin and 25 µM visnagin treated HeLa compared to the control untreated HeLa cells.
4.6 Effect of furanochrome visnagin on cancer cell migration
Migration is a key event of cancer cell progress, hence targeting the cancer cell migration with a drug can effectively inhibit the progression of cancer. Fig. 6 represents the inhibitory potency of visnagin on HeLa cells migration assessed with scratch wound assay. Compared to control cells the visnagin treated HeLa cells decreased cell migration. The number of migrated cells in the scratched area is less in visnagin treated group than the control untreated cells.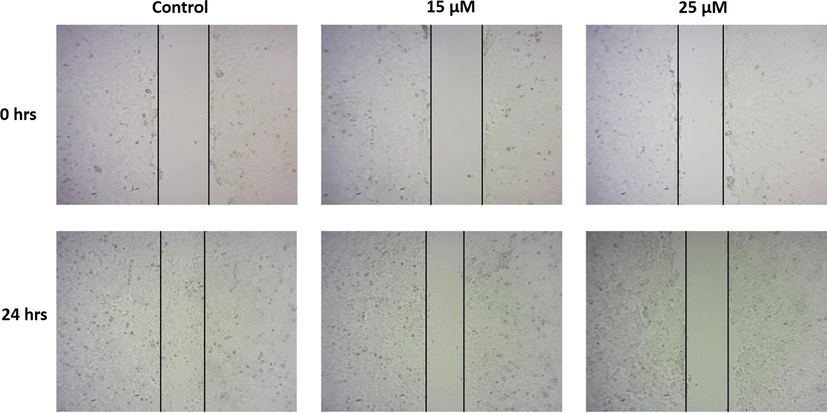
Effect of furanochrome visnagin on cancer cell migration. HeLa cells were treated with 15 µM and 25 µM visnagin for 24 h, analyzed for cell migration using scratch wound assay. The migration of cells in scratched area were viewed and photographed under microscope. The findings revealed that the visnagin treatment effectively decreased the migration of HeLa cells. Representative images were depicted as Control, 15 µM visnagin treated HeLa cells, and 25 µM visnagin treated HeLa cells, respectively.
4.7 Anticarcinogenic potency of furanochrome visnagin on HeLa cells
PI3K/AKT/mTOR pathway is most important intracellular signaling pathway which regulates numerous functions of cells such cell proliferation, cell quiescence and cancer induction in cells. Fig. 7A depicts the results of gene expression of PI3K/AKT/mTOR pathway signaling proteins which were estimated with real time PCR analysis. Visnagin treatment significantly decreased the gene expression of PI3K, AKT, mTOR proteins compared to the untreated control cells. Visnagin treatment also decreased the extracellular cell proliferation signaling pathway MAPK pathway. Visnagin treated decreased ERK1/2, p38 and JNK1/2 protein compared to the control HeLa cells (Fig. 7B).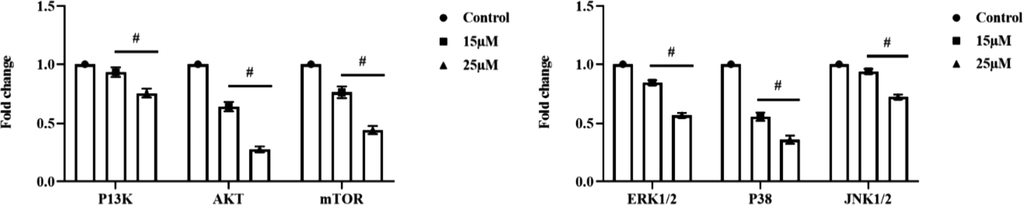
Anticarcinogenic potency of furanochrome visnagin on HeLa cells. HeLa cells were treated with 15 µM and 25 µM visnagin for 24 h, assessed for gene expression of cell cycle signaling protein using real time PCR technique. The visnagin treated HeLa cells demonstrated the decreased expression of PI3K, AKT, and mTOR proteins. The visnagin treatment also decreased the ERK1/2, P38, and JNK1/2 expressions in the HeLa cells. A- PI3K/AKT/mTOR pathway signaling proteins, B- MAPK pathway signaling proteins. Results were subjected to statistical analysis with One Way ANOVA followed by Student’s Newman Keuls test. p < 0.05, statistically significant.
5 Discussion
World health Organization in the year 2019 estimated cancer related mortalities were rapidly increasing globally compared to the mortalities of stroke and cardiovascular diseases (WHO, 2020). Among all cancer types cervical is the 2nd most common cancer among women and the 4th leading cause of mortality among global women population. Lower-middle income countries are prone to cervical cancer related mortality compared to high income countries. Synthetic chemotherapy drugs which are prescribed for cervical cancer are expensive and the recurrence of cancer is more common in cancer patients (Chen & Huang, 2019). At present plant and microbe derived drugs such vincristine, paclitaxel, vinblastine, etoposidem, doxorubicin, bleomycin were used as anticancer drug to treat various cancers (Roussi et al., 2012; Cragg & Pezzuto, 2016).
Antioxidant supplementation has proven to be an effective cure for various diseases. Antioxidants protect the cells from oxidative stress induced damage via scavenging the free radicals. Usage of antioxidant supplements for cancer patients have increased rapidly since it renders a promising curative effect (VandeCreek et al., 1999; Block et al., 2008). Therefore we examined the antioxidant potency of furanochrome visnagin with four different antioxidant potency estimating assays. FRAP Assay was performed to detect the potency of visnagin to reduce ferric-tripyridyltriazine (Fe3+-TPTZ) complex to ferrous tripyridyltriazine (Fe2+-TPTZ). Fe2+-TPTZ forms intense blue color which is measured at 593 nm (Benzie & Strain, 1996). The free radical reducing ability of visnagin was examined with DPPH assay which is organic nitrogen free radical. When an antioxidant reduces DPPH to hydrazine the deep purple colour of DPPH changes to yellow color confirming the presence of hydrazine which is measured at 515 nm. FRAP and DPPH assays were spectophotometric assay whereas ORAC assay is a flurometric assay used to measure the antioxidant scavenging property of peroxyl radical generated by AAPH. Trolox a water soluble analog of vitamin E was used as a standard for all the four assays. Visnagin significantly increased the trolox equivalent capacity in a dose dependent which confirms visnagin is a potent antioxidant.
Once confirming the antioxidant potency of visnagin we have examined the cytotoxic effect of visnagin in cancerous epithelial cells and normal epithelial cells. Since most of the anticancer drug are not target specific and renders adverse effects on normal cells. HeLa, human cervical carcinoma cells and Vero, animal normal epithelial cells were used for the current study. The MTT assay results of both visnagin treated HeLa and Vero cells shows visnagin significantly induces cells death only in cancerous and only very less cytotoxic effect of visnagin was observed in normal epithelial cells. Even at dose of 100 µM concentration visnagin induced 8% cell death which confirms the target specific cytotoxic effect of visnagin against cancerous cells.
Oxidative stress is a vital inducer causes cellular damage and tends to be a key factor of cancer pathogenesis. During aerobic metabolism of cells various reactive oxygen species such as singlet oxygen, hydrogen peroxide, hydroxyl radicals were generated (Klaunig and Kamendulis, 2004). These free radicals were scavenged by the antioxidant system thereby the cells maintain balance the oxidative stress levels. Impaired synthesis antioxidant synthesis or increased levels of antioxidants were reported in cancer patients which indicates increases in levels of antioxidants suppresses the aged cell apoptosis thereby stimulates the cancer cell proliferation (Portakal et al., 2000; Ho et al., 2001; Kolanjiappan et al., 2002; Akbulut et al., 2003). Hence in the present study we assessed the levels of enzymatic antioxidant superoxide dismutase and non enzymatic antioxidant glutathione levels in visnagin treated HeLa cells. Both the low and high dose visnagin treatment significantly reduced the levels of antioxidant SOD and GSH compared to the control untreated HeLa cells. Visnagin treatment also increased the MDA levels which are the reactive aldehyde end product of lipid peroxidation (Akbulut et al., 2003) which confirms visnagin have inhibited the cancer cell progression cervical cancer HeLa cell line.
ROS induced by anticancer drugs plays a vital role in inhibition of cancer cell progression. The disproportional increase in the levels of intracellular ROS induced by chemotherapy has been reported to induce cell cycle arrest, senescence and apoptosis in cancer. Increased ROS levels suppress the antioxidant synthesis thereby inducing oxidative stress in cancer cells. Upon apoptotic signal the oxygen free radicals activates JNK protein which in turn phosphorylates the antiapoptotic Bcl-2 and Bcl-XL (Cadenas, 2004) and also increases the homodimer formation of proapoptotic Bax thereby leading apoptosis in cancer cells (Lee et al., 2008; Zhang et al., 2008). Increased ROS generation activates p38 and JNK proteins which in turn activates Ask-1 leading to the ROS induced apoptosis (You et al., 2006). The results of DCFH-DA staining illustrates visnagin treatment has increased the ROS generation in HeLa cells this may be the reason for increase in ERK1/2, p38 and JNK1/2 protein observed in qPCR analysis. The dual staining technique results and propidium iodide staining results confirms visnagin had induced apoptosis in HeLa cells. The increased number of apoptotic cells in visnagin treatment may be due to the ROS induced activation of MAPK signaling proteins.
PI3K/AKT/mTOR signaling pathway is a multi regulator signaling pathway which involves in the cell growth, survival, migration and invasion (Yoeli-Lerner & Toker, 2006; Osaki & Oshimura, 2004). Hyperactivation of PI3K/AKT signaling was evidenced in various types of cancer cells (Ramesh et al., 2004; Kim et al., 2001; Yoeli-Lerner et al., 2005; Tanno et al., 2001). Tumor invasion and migration is the most targeted are for cancer drug discovery since it increases the mortality rate in cancer patients. Therefore we examined the inhibitory property of visnagin against cervical cancer cell migration using scratch wound assay. Visnagin treatment effectively inhibited the HeLa cells migration this may be due to inhibition of PI3K/AKT/mTOR signaling proteins which is evidenced in our qPCR analysis of PI3K/AKT/mTOR signaling protein. Visnagin persuasively induced apoptosis and inhibited the migration of human cervical cancer HeLa cell line.
6 Conclusion
It is need of today to discern a potent inexpensive drug to treat cervical cancer, a global major health concern of middle aged women. In the present study we attempt to investigate one such drug visnagin, furanochrome extracted from the fruits of Ammi visnaga species. Our results confirm visnagin is potent antioxidant which posses target specific anticancer activity. It decreases the antioxidant levels, increases ROS, inhibits the PI3K/AKT/mTOR and MAPK signaling thereby induces apoptosis and cancer cell migration in HeLa cells which are the characteristic features of an ideal anticancer drug.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Daily variations of plasma malondialdehyde levels in patients with early breast cancer. Cancer Detect. Prev.. 2003;27(2):122-126.
- [Google Scholar]
- Batanouny, K.H., 2001. Wild Medicinal Plants in Egypt: An Inventory to Support Conservation and Sustainable Use. Acad. of Scientific Research & Technology, Cairo, Egypt.
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239(1):70-76.
- [Google Scholar]
- Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int. J. Cancer. 2008;123(6):1227-1239.
- [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2018;68:394-424.
- [Google Scholar]
- Mitochondrial free radical production and cell signaling. Mol. Aspects Med.. 2004;25(1–2):17-26.
- [Google Scholar]
- Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol.. 2019;1(141):14-20.
- [Google Scholar]
- The Encyclopedia of Medicinal Plants. London, UK: Dorling Kindersley; 1996.
- Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract.. 2016;25(Suppl 2):41-59.
- [Google Scholar]
- Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res.. 2001;61:8578-8585.
- [Google Scholar]
- Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J.. 2001;15(11):1953-1962.
- [Google Scholar]
- The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxocol.. 2004;44:29-67.
- [Google Scholar]
- Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin. Chim. Acta.. 2002;326(1–2):143-149.
- [Google Scholar]
- Structure-activity relationship in the ability of flavonols to inhibit chemiluminescence. J. Ethnopharmacol.. 1994;41(1–2):121-126.
- [Google Scholar]
- Novel 2-step synthetic indole compound 1,1,3-tri(3-indolyl)cyclohexane inhibits cancer cell growth in lung cancer cells and xenograft models. Cancer. 2008;113(4):815-825.
- [Google Scholar]
- Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells. Arch. Pharm. Res.. 2010 Nov;33(11):1843-1850.
- [Google Scholar]
- Phytoagents for cancer management: regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxid. Med. Cell Longev.. 2013;2013:925804
- [Google Scholar]
- In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc.. 2007;2(2):329-333.
- [Google Scholar]
- Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem.. 2004;85:231-237.
- [Google Scholar]
- Human papilloma virus-associated cervical cancer and health disparities. Cells. 2019;8(6):622.
- [Google Scholar]
- PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9(6):667-676.
- [Google Scholar]
- Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients. Clin. Biochem.. 2000;33(4):279-284.
- [Google Scholar]
- Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol. Ther.. 2004;9(4):510-518.
- [Google Scholar]
- The involvement of a Ca2+ channel blocking mode of action in the pharmacology of Ammi visnaga fruits. Planta Med.. 1994;60(2):101-105.
- [Google Scholar]
- Roussi, R., Gueritte, F., Fahy, J., in: G.M. Cragg, D.G.I. Kingston, D.J. Newman (Eds.), Anticancer Agents from Natural Products, second ed., CRC/Taylor & Francis, Boca Raton, 2012, pp. 177–198.
- AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res.. 2001;61(2):589-593.
- [Google Scholar]
- Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal.. 2006;19:669-675.
- [Google Scholar]
- An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine. 2010;17(8–9):653-658.
- [Google Scholar]
- Use of alternative therapies among breast cancer outpatients compared with the general population. Altern. Ther. Health Med.. 1999;5(1):71-76.
- [Google Scholar]
- World Health Organization (WHO), 2020. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2019. WHO. Accessed December 11, 2020.
- Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle. 2006;5(6):603-605.
- [Google Scholar]
- Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell. 2005;20(4):539-550.
- [Google Scholar]
- Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl. Acad. Sci. U. S. A.. 2006;103(24):9051-9056.
- [Google Scholar]
- In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13(12):1465-1478.
- [Google Scholar]
- Cervical cancer: epidemiology, risk factors and screening. Chin. J. Cancer Res.. 2020;32(6):720-728.
- [Google Scholar]







