Translate this page into:
Vitamin B1 via Nrf-2/TLR4 signaling pathway ameliorates scopolamine-induced memory dysfunction in adult mice
⁎Corresponding authors. shumailanoreen@hu.edu.pk (Shumaila Noreen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by memory impairment and cognitive decline, with oxidative stress and neuroinflammation playing pivotal roles in its pathogenesis. Among potential candidates, vitamin B1 (Vit. B1) has gained attention for its neuroprotective potential due to anti-inflammatory properties and antioxidant properties. This study investigates the therapeutic potential of Vit. B1 in an AD-like mouse model induced by scopolamine (SCOP). Administering Vit. B1 (300 µg/kg) with SCOP (1 mg/kg) is hypothesized to alleviate memory impairment and neuroinflammation. Behavioral tests, including Morris Water Maze (MWM) and Y-maze tests, revealing Vit. B1 ability to restore both long-term and short-term memory, especially in the prob test compared to SCOP-treated mice. Western blot analysis revealed Vit. B1′s role in reversing SCOP-induced changes by suppressing oxidative stress through NrF-2 and HO-1 proteins and inhibiting TLR4 receptor activation that upregulates TNF-α and NF-kβ, contributing to memory dysfunction. Molecular docking studies explored the binding affinity of TLR4 signalling pathway proteins. Results confirmed VitB1′s inhibitory effect on TLR4/md2 activation and its higher binding preference, underscored by experimental confirmation. Overall, these findings highlight Vit. B1′s neuroprotective potential as a therapeutic approach in AD and suggest a potential avenue for novel therapeutic strategies, warranting further preclinical and clinical research.
Keywords
Scopolamine
Neurodegenerative
Alzheimer disease
Vitamin. B1
Oxidative stress
1 Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by gradual cognitive decline and memory loss, affecting millions of individuals worldwide. A projected 152 million people will be affected by it by 2050, quadrupling every five years, leading to a significant burden on healthcare systems and society (Ahmad et al., 2017; Uwishema et al., 2022). The hallmark pathological features of AD include the presence of amyloid-beta (Aβ) peptide in senile plaques and hyperphosphorylated tau protein in neurofibrillary tangles (NFTs), which contribute to cognitive impairment, memory loss, and dementia-related behavior and psychology (Roda et al., 2022).
Oxidative stress is an essential player in the pathogenesis of AD and various other age-related disorders, including neurodegenerative diseases (Mukli et al., 2022). In AD, there is an overproduction of reactive oxygen species (ROS), leading to weakened antioxidant defenses in the body (Olayinka et al., 2022). The administration of scopolamine (SCOP), a muscarinic receptor blocker, has been extensively studied as an AD model due to its ability to induce cognitive impairment and physiological changes similar to those observed in AD (Chen and Yeong, 2020). SCOP exposure results in increased oxidative stress, further exacerbating the memory dysfunction (Lenz et al., 2012; San Tang, 2019).
One critical pathway involved in cellular defense against oxidative stress is the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Nrf2 acts as a transcription factor, regulating the cellular response to free radicals and oxidative stress. Notably, studies have observed reduced levels of Nrf2 and its downstream target heme oxygenase-1 (HO-1) in the hippocampus of mice exposed to SCOP. The absence of Nrf2 and HO-1 has been shown to accelerate neuronal damage, while activation of the Nrf2 pathway exhibits neuroprotective effects (Ding et al., 2022; Huang et al., 2022). Another key factor in AD pathogenesis is Toll-like receptor 4 (TLR4), whose activation by amyloid β-protein (Aβ) triggers downstream signaling pathways, leading to the generation of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and activation of astrocytes, ultimately contributing to amyloid-dependent neuronal death (Wu et al., 2022).
Vitamin B1 (VitB1) has garnered attention for its potential neuropathic effects. As a cofactor for enzymes involved in crucial metabolic processes, including those in the nervous, visual, and cardiac systems, VitB1 plays a significant role in maintaining cell structure in the brain (Božić et al., 2022). Moreover, VitB1 exhibits anti-inflammatory properties, reduces oxidative stress, and enhances the body's resistance to stress (Ramamoorthy et al., 2022). The urgent need for novel AD treatments, the involvement of oxidative stress in AD pathogenesis, and the well-established use of SCOP as an AD model provide strong rationale for investigating VitB1 therapeutic potential. The role of Nrf2/TLR4 signaling pathways in AD, along with VitB1 anti-inflammatory and neuroprotective properties, highlight its promise as a potential therapeutic agent for memory loss and neuroinflammation in AD. Addressing this research gap can contribute to developing novel treatment strategies for this debilitating disorder, considering the increasing global prevalence of AD.
In this study, we aimed to investigate the potential therapeutic benefits of VitB1 in mitigating memory inflammation and SCOP-induced oxidative stress in a mice model. Additionally, we sought to examine the impact of SCOP on behavioral performance and neuroinflammation through the Nrf-2/TLR4 signaling pathways. The effects of SCOP on memory function were also evaluated, and the potential protective role of VitB1 against these effects was investigated. Additionally, to determine the inhibitory potential of Vit. B1, molecular docking studies were performed on proteins of interest within the LPS-induced Toll-like receptor 4 (TLR4) pathway mediated by Toll-like receptor 4 complex (TLR4/MD2). Finally, the docking results successfully describes the competitive binding of examined Vit. B1 vs. SCOP to TLR4 signalling pathway proteins, has been constructed.
2 Materials & methods
2.1 Chemicals
Vitamin B1 (Catalog Number 731188), Scopolamine (Catalog Number 1610001), Phosphate-Buffered Saline (PBS), Sodium Dodecyl Sulfate (SDS), Acrylamide, Bisacrylamide, Ammonium Persulfate (APS), Tris base, Potassium Chloride, and Sodium Chloride. All the necessary chemicals were purchased for my experiment from two reputable sources: Daejung Chemicals & Metals Co. Ltd. located in Gyeonggi-do, South Korea, and Sigma Chemical Co. based in St. Louis, USA.
2.2 Experimental procedure
Eight-week-old male BALB/C albino mice weighing approximately 30–32 g were purchased from Veterinary Research Institute (VRI), Peshawar (KPK, Pakistan). The mice were kept in standard cages and randomly assigned to one of the following groups (n = 8): Control Group (0.9 % saline), mice treated with SCOP at 1 mg/kg, mice treated with SCOP (1 mg/kg) + Vit. B1 (300 µg/kg), mice treated with Vit. B1 (300 µg/kg). All mice were placed in the breeding room with a 12-hour light/dark cycle at 23 ± 1 °C and fed with a standard laboratory diet and tap water ad libitum. Before the experimentation, the mice were acclimatized for 7 days. All procedures were approved by animal ethics committee of the NMMRC, Peshawar, Pakistan.
2.3 Drug administration
Duration and dose of scopolamine and Vit. B1 were determined according to recent investigations (Husn et al., 2023). SCOP was prepared in saline (0.9 %) and injected intraperitoneally over a 21-day period and Vit. B1 was administered on alternate days during the last 14 days of the experiment. Ten days after starting the drug treatment, the mice received training for behavioral tests that lasted three days. Following a two-day rest, the mice underwent final tests for five days, each test occurring 30 min after receiving the drug.
2.4 Behavioral tests
Two standard behavioral tests (Water Maze and Y-Maze) were used to assess the therapeutic efficacy of Vit. B1 on SCOP-mediated memory dysfunction. The researcher conducting the test for behavior remained unsighted to treatment group of mice. Each group contained eight mice.
2.4.1 Morris water maze (MWM) test
This study used the Morris water maze (MWM) to examine hippocampal-dependent long-term spatial memory and the MWM tests equipment are given in detail reported recently (Ali et al., 2021). In the testing tool, an MWM tank (100 × 40 cm) filled with water (26 cm depth) had a temperature of 23 °C. The mice were first trained twice a day for three days. Then the escaped latency of the mice to find the submerged platform was noted for 60 s. In case of the mice were unable to locate the platform, they were manually guided to it and instructed to remain there for a duration of 10 s. The aforementioned practice was sustained until the fifth day, with each day yielding distinct data (seconds) for the four experimental groups. Following a two-day period of rest, the mice were subjected to a probing test in order to locate a concealed platform. In the probe test, the submerged platform was removed and the mice were allowed to locate the submerged quadrant. The duration of time spent by the mice in the target quadrant was subsequently recorded.
2.4.2 Y-maze test
The Y-maze behavioral test is a commonly used method in preclinical research to gain insights into the neurological causes of cognition and memory-related diseases (Sandhu et al., 2022). The Y-maze apparatus is a simple, yet effective piece of laboratory equipment designed in the shape of a Y, havinf 3 arms of 50 × 10 × 20 (cm3 L × W × H) lying at angle of 120◦. The mice were typically allowed to become acclimated to the Y-maze for a short period of time (for 8 mints) prior to the actual experiment being carried out in order to reduce any stress-related behavior that is connected with the novelty of the environment. The mice were trained continuously with each 10-minute period running for three days. The goal of this step was to get the mice used to the maze and reduce any stress or anxiety that could come from something new. After the training period, the mice were subjected to trials for five consecutive days. Each trial lasted for 8 min. During the trials, the mice were placed in the center of the Y-maze and given unrestricted access to all three arms. The researchers carefully observed and recorded the number of times the mice entered each arm. The formula below calculates the alternation behavior (Khan et al, 2019).
2.5 Protein extraction from mice brain
After conducting the required behavioral studies, the mice were anesthetized with chloroform and their brains were extracted. The scientists were carefully divided the brain into two sections, which were then preserved at a temperature of −10 °C. The tissue samples were later subjected to a protein extraction process, after which the protein extracts were stored at −10 °C for further analysis. These extracts will be used to investigate the molecular mechanisms underlying the cognitive and memory-related effects observed in behavioral studies, with a focus on conditions such as Alzheimer's disease and other neurodegenerative disorders. This research could have significant implications for the treatment of such disorders.
2.6 Western blotting analysis
To start the Western blot analysis was used a Bio-Rad protein assay dye (Cat. No. 5000006) to measure the total protein concentration and took absorbance at 595 nm. To ensure accuracy, normalized all protein samples to 10 µg/group. Gel electrophoresis was conducted, and proteins were transferred to a PVDF membrane using the semi-dry transblot technique. Mice-derived monoclonal primary antibodies (Santa Cruz Biotech Inc.) were utilized, including TLR4 (sc-293072), NF-kB (sc-8008), NrF-2 (sc-365949), HO-1 (sc-136960), TNF-α (sc-52746), at 1:1000 dilution in 1X TBST. Finally, 1:2000 anti-mouse HRP conjugated secondary antibody (0000375517) was applied, and X-ray films were used for development (Ali et al., 2021).
2.7 Oxidative enzyme analysis
2.7.1 Glutathione-S-transferase (GSH) and glutathione (GST) analysis
GST and GSH are essential constituents of the cellular defense against antioxidants mechanism. In order to determine how much GSH and GST were present in our samples, we would use published assay techniques. After a PBS wash, a 2-nitrobenzoic acid solution was added, and the samples' absorbance at 630 nm was recorded. To utilize an established assay procedure with a slight modification to measure GST concentrations. These procedures have been used successfully for many years, assuring precise and reliable assessments of GSH and GST levels in the samples are studying (Iqbal et al., 2020). After thorough mixing, equimolar amounts of 1-chloro-2,4-dinitrobenzene and glutathione-S-transferase were added together, and then the mixture was diluted with 0.1 M PBS. This concludes the experiment. After performing successive dilutions of the sample, absorbance readings were obtained from the homogenate using a wavelength of 630 nm as the setting (Shah et al., 2018).
2.7.2 LPO assay
The LPO assay was an important instrument in the study of oxidative stress because it enables investigators to evaluate the degree of lipid peroxidation and the role that it plays in a wide range of processes, both physiological and pathological. The experiment was performed by using a procedure that had been used before in the past, though with a few modifications (Khan et al., 2019). The amount of thiobarbituric acid reactive substances (TBARS) present in the tissue homogenates of mice was used as a measurement for lipid peroxidation.
2.7.3 Catalase assay
The catalase assay was carried out in compliance with the aforementioned specifications, though with a few minor changes (Iqbal et al., 2020). After adding 10 µL of the sample and 290 µL of 3 % hydrogen peroxide to each well, the mixture was mixed thoroughly. After that, the 96-well plate was left untouched at ambient temperature and out of the light for ten minutes before its absorbance was determined at a wavelength of 630 nm.
2.8 Docking studies
2.8.1 Preparation of tlr4/md2 complex
The crystal structure of TLR4/md2 in complex with Neoseptin-3 was taken from Protein Databank (PDB ID: 5IJC) (Wang et al., 2016). The structure was preprocessed by removing the undesired moieties including co-crystal salts, water molecules and other impurities. The preprocessed structure was energy minimized while using the OPLS4 forcefield implanted in Maestro, Schrodinger Suite. Consequently, the well-converged structure of TLR4/md2 complex was employed as receptor during the docking simulation.
2.8.2 Preparation of SCOP and VitB1 structures
The 3D structures of SCOP and VitB1 were downloaded from PubChem DB (https://pubchem.ncbi.nlm.nih.gov/). The initial coordinates of both the ligands were subjected to the LigPrep module implanted in Maestro, Schrodinger Suite, generated their possible bio-isostere conformers at pH 7.4, and eventually, prepared for docking simulation.
2.8.3 Molecular docking simulation of tlr4/md2 complex and ligands
The docking of both the ligands was conducted by Glide docking package. To get the appropriate docking pose of ligands, docking protocol was optimized by docking back the co-crystal ligand (Neoseptin-3) in the LPS-binding site of TLR4/md2 complex (Wang et al., 2016). During docking, the standard precision (SP) scoring function was executed to orient the compatible docking poses for both the tested ligands (SCOP and VitB1). All the results were analyzed by Schrodinger Suite and Discovery Studio Visualizer.
2.9 Statistical analysis
After conducting the western blot experiments, scanned and compiled the X-ray images for analysis. For statistical comparisons of relative protein densities, were used one-way ANOVA and employed software tools such as ImageJ, GraphPad Prism, and Adobe Photoshop. The blots were quantified with Image J software and their density data was normalized with the control. Protein densities were given in A.U.s as Mean ± S.E.M. “#” and “*” indicated significant differences between control and treated groups and scopolamine-treated mice group. *, #p ≤ 0.05, **, ##p ≤ 0.01 and ***, ###p ≤ 0.001.
3 Results
3.1 Vit. B1 restored both short & long-term memory impaired by SCOP in adult albino mice
SCOP is a chemical agent impacting memory and behavior in an animal model. In the MWM test, mice underwent two training days (three sessions daily) and two rest days. Data was collected for five consecutive days. On day one, control mice had low mean escape latency, while SCOP group mice had significantly higher latency (Fig. 1A). However, the group which received SCOP along with Vit. B1 showed lower escape latencies to reach the target platform. This trend was continued in all experimental groups, with even the SCOP group improving slightly. However, their escape latencies remained higher than the control groups. In the case of the Y-maze test, the control group exhibited the highest % spontaneous alternation, while the SCOP-treated group showed the lowest percentage (Fig. 1B). The Vit. B1 treated group showed significant improvement compared to the SCOP group. However, the group was only injected with Vit. B1 showed a slight decrease, possibly indicating anxiety caused by injections. In addition to the Morris water maze activity, a probe test was conducted on day six. Data showed that mice induced by SCOP spent less time in the target quadrant than control mice (Fig. 1C). In contrast, the SCOP-induced group treated with vit. B1 spent much more time in the target quadrant.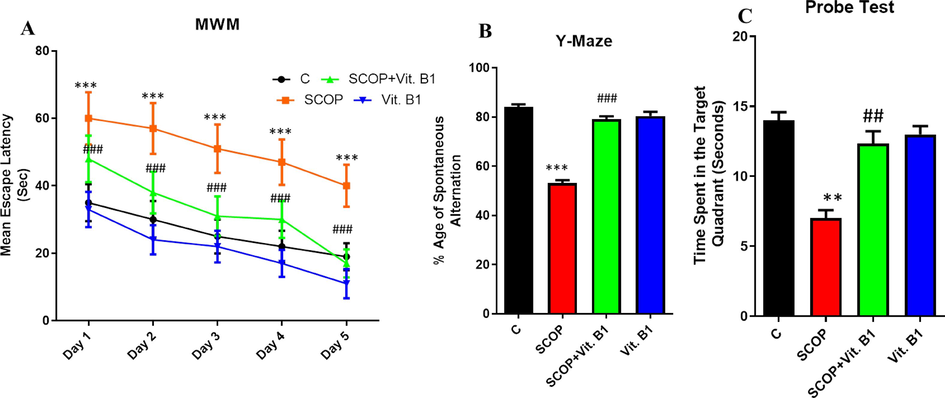
(A) Represent the results from day 1 to day 5 via MWM test while Histogram of Y-maze (B), which specifies the neuroprotective capability of Vit. B1 against SCOP-induced AD in mice (n = 8). (C) A probe test was carried out, during which the hiding surface was removed. Contrary to the SCOP group, the Vit. B1 group spent more time in the targeted region. Mean and standard error of the mean are included in the data set. Differences between the control and SCOP groups are shown with an asterisk (*), while those between the SCOP and SCOP + Drug (Vit.B1) groups are indicated with a hash symbol (#). various degrees of statistical significance are denoted by various symbols; for example, *,# denotes a p-value of ≤ 0.05, **,## denotes a p-value of ≤ 0.01, and ***,### denotes a p-value of ≤ 0.001.
3.2 Vit. B1 reactivated NrF-2/HO-1 as an endogenous antioxidant system against SCOP-induced oxidative stress in adult albino mice
The present study examines the impact of SCOP on oxidative stress in the brains of mice, with a focus on reactive oxygen species (ROS) stability and antioxidant defenses (Chakraborty et al., 2022). Through Western blot analysis (Fig. 2A), it was observed that SCOP induced oxidative stress by downregulating key ROS regulators, namely NrF-2 and HO-1. The downregulation of these markers following SCOP exposure led to mitochondrial dysfunction and memory impairment in the mice.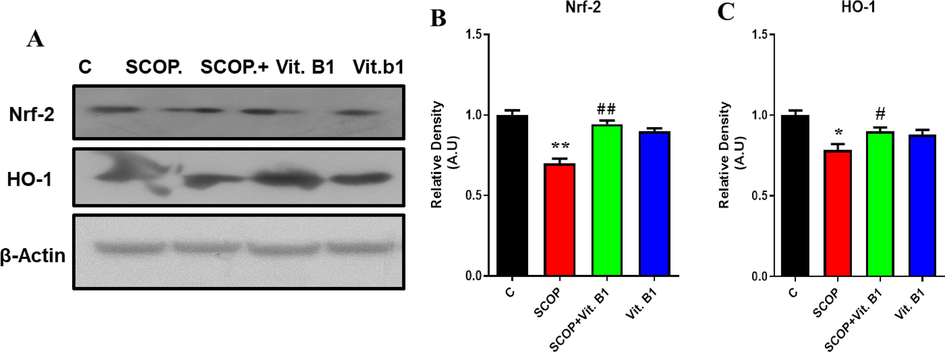
The immunoblot analysis was performed to assess the expression levels of Nrf2 and HO-1 markers, as shown in Figure (A). To quantify the results, relative densities of Nrf2 and HO-1 were measured and represented in histograms as depicted in Figure (B) and (C), respectively. β-actin was used as the loading control for normalization purposes. The data were expressed in arbitrary units (A.U) and were analyzed using Image J software. Mean and standard error of the mean are included in the data set (n = 8). Differences between the control and SCOP groups are shown with an asterisk (*), while those between the SCOP and SCOP + Drug (Vit.B1) groups are indicated with a hash symbol (#). various degrees of statistical significance are denoted by various symbols; for example, *,# denotes a p-value of ≤ 0.05, **,## denotes a p-value of ≤ 0.01, and ***,### denotes a p-value of ≤ 0.001.
The results demonstrated that vitamin B1 supplementation significantly upregulated the expression of NrF-2 (p ≤ 0.01) and HO-1 (p ≤ 0.05) markers, which had been previously suppressed due to SCOP intervention. This study indicates that Vit. B1 exerts a positive influence on the regulation of these antioxidants, potentially counteracting the detrimental effects of SCOP-induced oxidative stress in the brain shown in (Fig. 2B, C). Overall, these findings accentuate the importance of ROS regulators, NrF-2 and HO-1, in maintaining brain health and highlight the auspicious role of Vit. B1 as a neuroprotective agent. The study provides valuable insights into the molecular mechanisms underlying oxidative stress-related brain dysfunction and suggests potential therapeutic avenues for mitigating the harmful effects of neurotoxic substances like SCOP.
3.3 Vit. B1 negatively modulated TLR4 receptor & inhibited both NF-kB/TNF-α
It has been found that SCOP-induced Alzheimer's disease (AD) is a complex model for neurodegeneration that affects multiple cellular and molecular pathways, specifically neuroinflammation that is mediated by NF-kB/TNF-α markers. To investigate the harmful effects of SCOP on the brain, a Western blot analysis was conducted. The results showed that two key inflammatory cytokines, TNF-α and NF-kB, were activated and upregulated in response to SCOP exposure, likely facilitated through the engagement of TLR-4 receptors. These findings unveil the role of particular inflammatory signaling cascades in the development of SCOP-induced AD, which can contribute to a better understanding of the underlying mechanisms driving neurodegenerative processes (Fig. 3A). The findings from the study showed that the control mice had minimal expressions of TLR-4, NF-kB, and TNF-α. However, when the mice were injected with SCOP, there was a substantial upregulation of these markers. The group that was treated with vitamin B1 showed a significant inhibition of TLR-4 activation and the upregulation of NF-kB and TNF-α. This suggests that vitamin B1 has potential neuroprotective effects (Fig. 3B–D). These results highlight the ability of vitamin B1 to counteract the neuroinflammatory response caused by SCOP, making it a promising candidate for neuroprotective interventions against SCOP-induced neurodegeneration.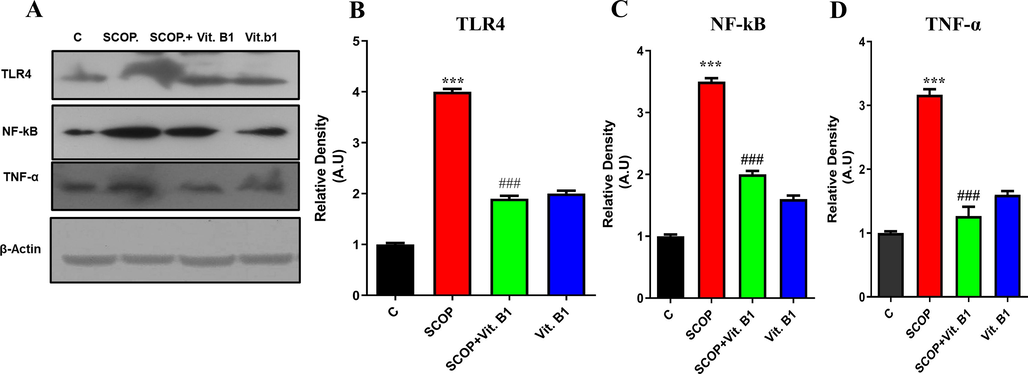
The immunoblot analysis presented the expression levels of TLR4, NF-kB, TNF-α, and β-actin (Figure A), with corresponding histograms for TLR4, NF-kB, and TNF-α (Figure B-D). Mean and standard error of the mean are included in the data set (n = 8). Differences between the control and SCOP groups are shown with an asterisk (*), while those between the SCOP and SCOP + Drug (Vit.B1) groups are indicated with a hash symbol (#). various degrees of statistical significance are denoted by various symbols; for example, *,# denotes a p-value of ≤ 0.05, **,## denotes a p-value of ≤ 0.01, and ***,### denotes a p-value of ≤ 0.001.
3.4 Effect of Vit. B1 on antioxidant assay
Based on the comprehensive analysis of the brain homogenates of the adult albino mice, it was found that the levels of antioxidant enzymes, such as catalase (CAT), glutathione S-transferase (GST), and reduced glutathione (GSH), were significantly increased after three weeks of daily administration of SCOP and Vit. B1 supplementation. Additionally, the lipid peroxidation (LPO) levels assessed through thiobarbituric acid reactive substances (TBARS) were significantly reduced. These findings suggest that the combination of SCOP and Vit. B1 could potentially enhance the antioxidant defence system in the brain of albino mice, leading to a reduction in oxidative stress levels.
Based on the findings presented in Fig. 4, it was evident that the combination of SCOP and Vit. B1 can have a significant impact on reducing oxidative stress in the brain. The results indicate that the activity of antioxidant enzymes such as CAT, GST, and GSH can be increased with the administration of Vit. B1, while SCOP can lead to a decrease in TBARS activity. These findings suggest that a combination of SCOP and Vit. B1 may be an effective way to enhance the antioxidant defence system in the brain and reduce oxidative stress levels. The study shows that vit. B1 has the potential to reduce the oxidative stress caused by SCOP, as seen in the restoration of antioxidant enzyme activities. This was valuable information that sheds light on the neuroprotective benefits of Vit. B1 and its ability to counteract the harmful effects of SCOP-induced oxidative stress in the brain of albino mice.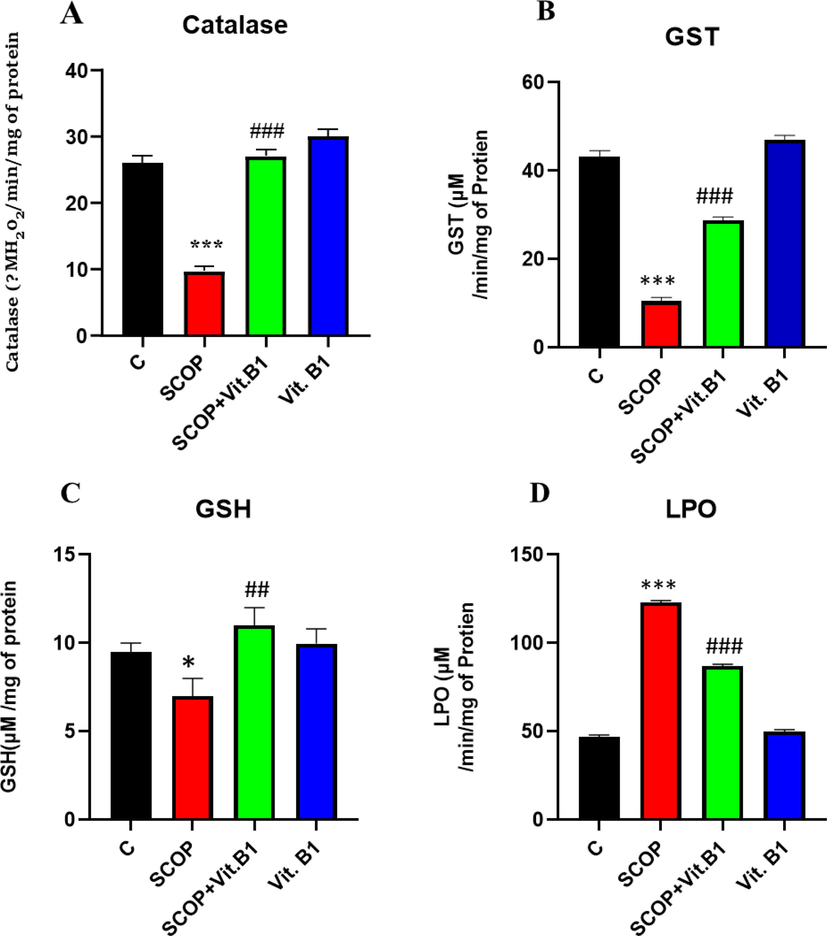
The antioxidant enzyme assays, which was evaluated the levels of catalase, GST, GSH, and LPO in brain homogenates, showed significant improvements in mice treated with Vit. B1 in combination with SCOP. The data was quantified and expressed in arbitrary units (A.U.) using Image J software. This suggests that, Vit. B1 may have potential therapeutic benefits for conditions associated with oxidative stress in the brain. Mean and standard error of the mean are included in the data set (n = 8). Differences between the control and SCOP groups are shown with an asterisk (*), while those between the SCOP and SCOP + Drug (Vit.B1) groups are indicated with a hash symbol (#). various degrees of statistical significance are denoted by various symbols; for example, *,# denotes a p-value of ≤ 0.05, **,## denotes a p-value of ≤ 0.01, and ***,### denotes a p-value of ≤ 0.001.
3.5 Docking analysis reveals VitB1′s inhibitory effect on TLR4/MD2 complex activation
The docking of SCOP and VitB1 suggested that they occupied the LPS binding cavity of TLR4/md2 complex. Since the LPS binding cavity of TLR4/md2 complex is a shallow pocket, which is sub-divided into several sub-pockets. Thus, the docking of both the ligands suggested several orientations of both the ligands. But based on several parameters including docking score, emodel score, clustering analyses, binding interactions and pose orientation, we have selected the final orientation for both the ligands (Fig. 5A, B).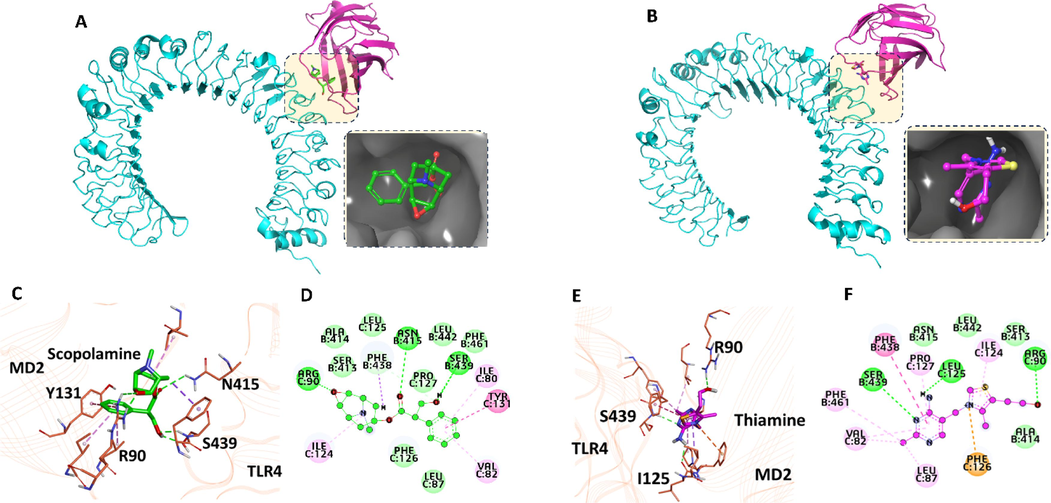
Docking of scopolamine (a) and VitB1 with TLR4/md2 complex. Representation of the interactions map of the best docked pose of scopolamine (C-D) and VitB1 (E-F) with the amino acids of TLR4/md2 complex.
The docking results suggested that SCOP obtained the highest docking score of −6.99 kcal/mol, while the docking score for VitB1 was −6.57 kcal/mol (Table 1). The docking score for both ligands suggests they have a similar tendency/affinity to the tlr4/md2 complex. Despite closely similar docking scores, VitB1 obtained the highest emodel score (-49.1 kcal/mol) than SCOP (-43.82 kcal/mol). Thus, we argue that TLR4/md2 may prefer VitB1 binding to SCOP when both ligands are available.
Comp. Name
Gscore
(kcal/mol)
Emodel Score
MMGBSA dG
Polar interactions
(H-bond)
Non-polar interactions
Lig Atom
Amino acid
Distance (Å)
SCOP
−6.99
−43.82
−44.67
O3
O4
H18
O1
O1N415
S439
S439
R90
R903.0
2.0
2.3
2.9
1.7I124, F438, L125, P127, I80, F461, Y131, V82, L87, F126
VitB1
−6.57
−49.16
−42.58
O1
N2
H12R90
S439
L1252.3
2.3
2.3S413, I124, A414, L442, N415, P127, F438, L87, F461, V82, F126
Further, we computed the binding free energy of TLR44/md2 with docked poses of SCOP and VitB1 by MMGBSA approach in the Schrodinger Suite. These analyses suggested that SCOP and VitB1 have nearly similar binding tendencies towards TLR4/md2 complex. The binding free energy for SCOP and VitB1 with TLR4/md2 complex was −44.47 and −42.58 kcal/mol, suggesting they are not significantly different. Finally, we analyzed the interaction pattern of SCOP and VitB1 with TLR4/md2 complex (Fig. 5A, B). The interactions pattern and visual inspection suggested that both ligands established essential polar interactions (H-bonds) with the critical TLR4/md2 complex residues. For instance, SCOP and VitB1 both have formed h-bonds with S439 of tlr4 and R90 of md2 (Fig. 5C–F). Our findings are in substantial accordance with previous findings that small molecule activators also formed h-bond with these residues (Wang et al., 2016). Thus, we argue that making polar interactions with S439 of tlr4 and R90 of md2 is a prerequisite for activating the TLR4/md complex.
Furthermore, the pose orientation and occupancy of the LPS-binding site of TLR4/md2 complex by SCOP and VitB1 suggested that SCOP has occupied both the large and small hydrophobic regions by extending its benzyl ring and phenoxy groups, respectively (Fig. 5C-F). In parallel, the benzyl ring of SCOP could also establish two pi-pi stacking interactions with the phenyl group of Y131 of md2 in the large sub-pocket hydrophobic region of the LPS-binding site. In contrast, the VitB1 could not occupy the large hydrophobic sub-pocket due to the absence of any such side chain ring (group) and hence unable to establish the mechanism of making hydrophobic interaction with the large hydrophobic region of the LPS-binding site of TLR4/md2 complex.
4 Discussion
The present study aimed to investigate the therapeutic efficacy of Vit. B1 in counteracting SCOP-induced neuropathological alterations, including neuroinflammation, oxidative stress, and memory impairment in adult albino mice. Vit. B1, known as an “anti-stress” is recognized for its immune-boosting properties (Alkahlout et al., 2022), possesses anti-inflammatory and antioxidant properties, activating ROS regulators, Nrf-2, and HO-1 proteins to reduce oxidative stress burden (Farruggia et al., 2018). It also inhibits TLR4 and downstream molecules like NF-kB and TNF-α, mitigating SCOP-induced memory dysfunction (Ahmad et al., 2023). The neuroprotective effects of vit. B1′s through reduced neuronal loss, likely mediated by its antioxidant and anti-inflammatory actions (Bruno and Ziegenfuss, 2005). Furthermore, Vit. B1 is involved in the regulation of acetylcholine levels, a neurotransmitter critical for learning and memory, suggesting a potential mechanism for its memory-enhancing effects (Dief et al., 2015). More research is needed to identify vit. B1′s active compounds involved in alleviating oxidative stress-mediated neuronal cell death and the mechanism behind its memory improvement in animal models. Additionally, exploring the role of other pathways involved in AD pathogenesis, such as the role of microglia and neuroinflammation, would further elucidate Vit. B1′s neuroprotective effects.
The Y-maze and Morris Water Maze (MWM) tests demonstrated that Vit. B1 significantly improved short-term memory deficits induced by SCOP, while general locomotor activity showed no significant improvement. These results align with previous studies highlighting Vit. B1′s potential in memory restoration (Yan et al., 2022;(Ghalibaf et al., 2023). Consumption of fruits and vegetables, rich in neuroprotective compounds like vitamin C, may help improve neurocognition and prevent neurodegenerative disorders (Subash et al., 2014). Notably, a combination of Vit. B1 and vitamin C has been reported to exert synergistic neuroprotective effects in preclinical models of neurodegenerative diseases (Roberson et al., 2023).
SCOP-induced oxidative stress disrupted the balance between ROS and free radicals, suppressing Nrf-2/HO-1 expression, and triggered a pro-inflammatory response, leading to memory dysfunction (Chakraborty et al., 2022). Our study evaluated Vit. B1′s antioxidant capability and found it to be a potent agent. Immunoblot results demonstrated Vit. B1′s involvement in activating the regulatory marker NrF-2/HO-1, effectively balancing free radicals (Fig. 6). Upon activation, it stimulated a pro-inflammatory response at the membrane receptor, suppressing TLR4 activation and downstream NF-kB/TNF-α signaling. This minimized ROS production and its toxic effects, reducing neuroinflammation caused by SCOP. Vit. B1′s antioxidant potential encompasses multiple actions, offering various health advantages, including antioxidant activity and energy production. It has direct interactions with free radicals and hydroperoxides, providing antioxidant benefits (Pandithavidana and Jayawardana, 2019). Additionally, Vit. B1′s involvement in cellular energy production is essential for maintaining proper neuronal function, as impaired energy metabolism has been implicated in AD pathogenesis (Reidling et al., 2010). In addition to scavenging free radicals, Vit. B1 increases endogenous antioxidant proteins CAT, GST, and GSH, while reducing LPO and proinflammatory mediators. Our findings suggest these effects can reduce neuronal damage caused by SCOP, altering cytokine production, the inflammatory cascade, and antioxidant enzymes. Vitamin D3 may also contribute to improved cognitive function in AD rats through other anti-inflammatory mechanisms, potentially reducing Aβ protein buildup (Liu et al., 2015).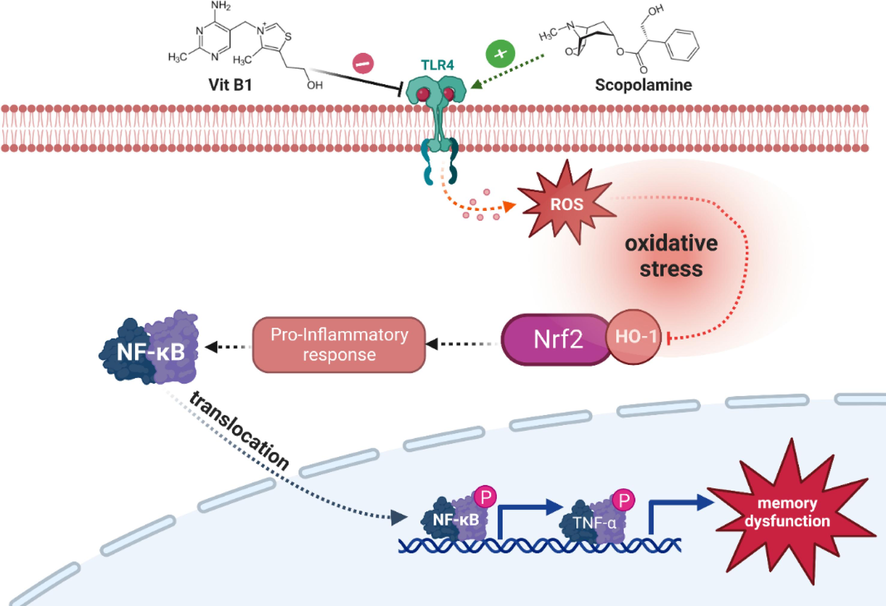
The proposed signaling pathway of neuroprotective Vit. B1 against SCOP animal model. The expression of Vit. B1 suppress the activation of NrF-2/ HO-1 which further deactivated the downstream signaling pathways.
Based on the previously mentioned docking results, we can generate a good idea regarding the binding affinities and corresponding intrinsic activities of SCOP and VitB1 with TLR4/md2 complex binding sites. Collectively, our results are strongly supported by our experimental findings that VitB1 inhibits the activation of TLR4/md2 complex in the synergistic treatment of both SCOP and VitB1. It is worth mentioning that VitB1 exhibited a higher emodel score (−49.1 kcal/mol) than SCOP (−43.82 kcal/mol), suggesting a potential preference for VitB1 binding by TLR4/md2. Experimental findings supported this, showing VitB1′s inhibitory effect on TLR4/md2 activation. SCOP occupied both large and small hydrophobic regions by extending its benzyl ring and phenoxy groups, respectively, while forming pi-pi stacking interactions with Y131 of md2. In contrast, VitB1 could not occupy the large hydrophobic sub-pocket, lacking a suitable side chain ring, inhibiting TLR4/md2 activation. Conclusively, we argue that due to the lack of this hydrophobic interaction and occupation of a large hydrophobic region, VitB1 is not able to activate the tlr4/md2 complex. Instead, VitB1 inhibits the activation of the tlr4/md2 complex. Our preposition is in substantial agreement with the previous finding, where they have conclusively elaborated that the hydrophobic interaction (especially pi-pi stacking interaction) is a pre-requisite for small molecules agonists of TLR4/md2 complex (Wang et al., 2016). Further assessment is needed to elaborate the possible mechanistic understanding of SCOP and VitB1 with TLR4/md2 complex in term of its activation and/or inhibition.
5 Conclusion
In conclusion, our findings reveal the neuroprotective effects of Vit. B1 in restoring memory impairment and mitigating neuroinflammation induced by SCOP in an AD mouse model. The observed activation of the Nrf-2/HO-1 pathway and modulation of TLR4 signaling provide mechanistic insights into Vit. B1′s therapeutic potential. Moreover, the significant increase in antioxidant enzyme levels and the reduction in lipid peroxidation levels further support its antioxidative properties. While these results are promising, further preclinical and clinical research is warranted to validate the efficacy and safety of Vit. B1 as a potential therapeutic agent for AD. Nevertheless, our study contributes to the growing understanding of Vit. B1′s role in neuroprotection and its potential as a novel avenue for AD treatment.
Ethics statement
According to the considerations of the Act 1986 conducted for UK animals, care and treatment of the mice was carried out. Experimental procedures were carried out on mice and were approved (Ref. No. NMMRC/20/2022) by the ethics committee of NMMRC, Peshawar.
Funding
None.
CRediT authorship contribution statement
Abdul Nasir: Conceptualization,Methodology,Investigation,Writing – original draft,Writing – review & editing.Manzar Khan: Conceptualization,Methodology,Investigation,Writing – original draft.Shumaila Noreen: Writing – original draft,Writing – review & editing.Mujeeb Ur Rahman: Conceptualization,Methodology,Investigation.Muhammad Zahid: Conceptualization,Methodology,Investigation.Ghulam Nabi: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol. Neurobiol.. 2017;54:2269-2285.
- [Google Scholar]
- 6-Aminoflavone activates Nrf2 to inhibit the phospho-JNK/TNF-α signaling pathway to reduce amyloid burden in an aging mouse model. ACS Omega 2023
- [Google Scholar]
- Vitamin D exerts neuroprotection via SIRT1/nrf-2/NF-kB signaling pathways against D-galactose-induced memory impairment in adult mice. Neurochem. Int.. 2021;142:104893
- [Google Scholar]
- Classification of a few fruits using deep learning. Int. J. Acad. Eng. Res. (IJAER). 2022;5
- [Google Scholar]
- Therapeutic potential of different natural products for the treatment of Alzheimer’s disease. Oxidative Med. Cellular Longevity 2022
- [Google Scholar]
- Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease. CNS Neurol. Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders). 2020;19:85-93.
- [Google Scholar]
- Impact of exercise and vitamin B1 intake on hippocampal brain-derived neurotrophic factor and spatial memory performance in a rat model of stress. J. Nutr. Sci. Vitaminol.. 2015;61:1-7.
- [Google Scholar]
- Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed. Pharmacother.. 2022;152:113208
- [Google Scholar]
- Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem.. 2018;62:202-209.
- [Google Scholar]
- Minocycline alleviated scopolamine-induced amnesia by regulating antioxidant and cholinergic function. Heliyon.. 2023;9
- [Google Scholar]
- Asafoetida exerts neuroprotective effect on oxidative stress induced apoptosis through PI3K/Akt/GSK3β/Nrf2/HO-1 pathway. Chin. Med.. 2022;17:1-21.
- [Google Scholar]
- Neuroprotective effects of vitamin B1 on memory impairment and suppression of pro-inflammatory cytokines in traumatic brain injury. Metab. Brain Dis. 2023:1-10.
- [Google Scholar]
- Succinamide derivatives ameliorate neuroinflammation and oxidative stress in scopolamine-induced neurodegeneration. Biomolecules. 2020;10:443.
- [Google Scholar]
- Matrine ameliorates anxiety and depression-like behaviour by targeting hyperammonemia-induced neuroinflammation and oxidative stress in CCl4 model of liver injury. Neurotoxicology. 2019;72:38-50.
- [Google Scholar]
- The scopolamine model as a pharmacodynamic marker in early drug development. Psychopharmacology. 2012;220:97-107.
- [Google Scholar]
- In vivo detection of age-and disease-related increases in neuroinflammation by 18F-GE180 TSPO microPET imaging in wild-type and Alzheimer’s transgenic mice. J. Neurosci.. 2015;35:15716-15730.
- [Google Scholar]
- Urinary biomarkers of oxidative stress in aging: implications for prediction of accelerated biological age in prospective cohort studies. Oxidative Med. Cellular Longevity 2022
- [Google Scholar]
- Quercetin mitigates scopolamine-induced memory dysfunction: impact on oxidative stress and cholinergic mechanisms. Metab. Brain Dis.. 2022;37:265-277.
- [Google Scholar]
- Comparative study of antioxidant potential of selected dietary vitamins; computational insights. Molecules. 2019;24:1646.
- [Google Scholar]
- Alzheimer’s disease is associated with disruption in thiamin transport physiology: a potential role for neuroinflammation. Neurobiol. Dis.. 2022;171:105799
- [Google Scholar]
- Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2–deficient mice. Gastroenterology. 2010;138:1802-1809.
- [Google Scholar]
- Association of vitamin C, thiamine, and hydrocortisone infusion with long-term cognitive, psychological, and functional outcomes in sepsis survivors: a secondary analysis of the vitamin C, thiamine, and steroids in sepsis randomized clinical trial. JAMA Netw. Open. 2023;6:e230380.
- [Google Scholar]
- Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res.. 2022;17:1666.
- [Google Scholar]
- The cellular and molecular processes associated with scopolamine-induced memory deficit: a model of Alzheimer’s biomarkers. Life Sci.. 2019;233:116695
- [Google Scholar]
- Friedelin attenuates neuronal dysfunction and memory impairment by inhibition of the activated JNK/NF-κB signalling pathway in scopolamine-induced mice model of neurodegeneration. Molecules. 2022;27:4513.
- [Google Scholar]
- Identification of proteins differentially expressed in the striatum by melatonin in a middle cerebral artery occlusion rat model—a proteomic and in silico approach. Front. Neurosci.. 2018;12:888.
- [Google Scholar]
- Subash, S., Essa, M.M., Al-Adawi, S., Memon, M.A., Manivasagam, T., Akbar, M., 2014. 浆果类植物对神经退行性疾病的神经保护效应. 中国神经再生研究 (英文版) 9, 1557.
- Is Alzheimer’s disease an infectious neurological disease? A review of the literature. Brain Behav. 2022:e2728.
- [Google Scholar]
- TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc. Natl. Acad. Sci.. 2016;113:E884-E893.
- [Google Scholar]
- Toll-Like Receptor 4: a promising therapeutic target for Alzheimer’s disease. Mediator. Inflammation 2022
- [Google Scholar]
- Patchouli alcohol as a selective estrogen receptor β agonist ameliorates AD-like pathology of APP/PS1 model mice. Acta Pharmacol. Sin.. 2022;43:2226-2241.
- [Google Scholar]







