Water mediated oxalic acid catalyzed one pot synthesis of 1,8-dioxodecahydroacridines
⁎Corresponding author. jnsangshetti@rediffmail.com (Jaiprakash N. Sangshetti)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
A simple and convenient one step method for synthesis of acridines and its derivatives from condensation of aromatic aldehydes, cyclic diketones, aryl amines or ammonium acetates using water as a reaction medium is reported. The present protocol provides several advantages such as good yields, cheap catalyst, short reaction time, easy work-up, simplicity in operation. The use of environmentally benign water as a solvent supports the green aspect of method.
Keywords
Water mediated reaction
1,8-Dioxodecahydroacridines
Oxalic acid
Dimedone
Cyclohexanedione
1 Introduction
1,8-Dioxodecahydroacridines and their derivatives are polyfunctionalized 1,4-dihydropyridine (DHP) derivatives (Venkatesan et al., 2009). The DHP derivatives are considered to be very important compounds due to there various pharmacological properties (Klusa, 1995). These compounds are used for treatment of cardiovascular diseases like angina pectoris (Antman et al., 1980) and hypertension (Guazzi et al., 1977). Some of them are employed for the treatment of congestive heart failure as a potential drug candidate (Vo et al., 1995) and also considered as an important class of calcium channel blockers (Janis and Triggle, 1983). DHP is used as an important core for many bioactive compounds (Delfourne et al., 2000; Ferlin et al., 2000; Gordeev et al., 1996; Godfraind et al., 1986). It is also reported that DHP has platelet antiaggregatory activity and is useful in Alzheimer' disease (Bretzel et al., 1992) DHP is also used as dyes (Shanmugasundaram et al., 1996).
One pot multi component reactions (MCRs) are emerged as an effective, efficient and powerful tool in modern synthetic organic chemistry. MCRs lead to efficient formation of product in single step and diversity can be achieved simply by varying reactant components (Weber, 2002).
Various methods for synthesis of 1,8-dioxodecahydroacridines are reported in the literature. These methods involves cyclocondensation of three components aromatic aldehydes, cyclic diketones and nitrogen source like urea (Bakibaev et al., 1991) methyl amine (Hua et al., 2005) different aryl amines and ammonium acetate (Martin et al., 1995) using organic solvents and various catalysts such as triethylbenzylammonium chloride (TEBAC) (Wang et al., 2004), p-dodecylbenzenesulfonic acid (DBSA) (Jin et al., 2004), Proline (Venkatesan et al., 2009), Amberlyst-15 (Das et al., 2006), ammonium chloride (Balalaie et al., 2009), Bronsted acidic imidazolium salts (Shen et al., 2009), under microwave irradiation (Tu et al., 2002), and use of ionic liquids (Li et al., 2005), such as 1-methylimidazolium trifluoroacetate (Dabiri et al., 2008), and by using sulfonic acid functionalized silica (SiO2-Pr-SO3H) (Mohammadi Ziarani et al., 2011).
These reported methodologies have some limitations such as poor yields, prolonged reaction time, multistep synthesis, formation of side products, use of toxic or hazardous organic solvents, harsh reaction conditions, etc. Therefore there is scope for generation of new methodology with milder reaction conditions, short reaction time, better yields, environment friendliness.
2 Result and discussion
Considering the potential of this heterocycle in present work we report the one pot multi component synthesis of 1,8-dioxodecahydroacridines catalyzed by oxalic acid using water as a medium.
The synthesis of derivatives of acridine has been carried out using different mol% of catalyst and the effect of catalyst loading on the yield of product is also investigated in Table 1. From the result it is observed that use (20 mol%) of oxalic acid is more useful giving product with good yield.
| Sr. no. | Catalyst | Quantity (mol%) | Yield (%) |
|---|---|---|---|
| 1 | Oxalic acid | 50 | 97 |
| 2 | Oxalic acid | 40 | 97 |
| 3 | Oxalic acid | 30 | 97 |
| 4 | Oxalic acid | 20 | 97 |
| 5 | Oxalic acid | 10 | 90 |
We have also studied the effect of solvent system on the yield of product (Table 2). It was observed that use of environmentally benign water as a solvent is favorable.
| Sr. no. | Solvent | Temperature | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | Ethanol | Reflux | 60 | 97 |
| 2 | Ethanol + water | Reflux | 60 | 97 |
| 3 | Water | Reflux | 60 | 97 |
1,8-Dioxodecahydroacridines and its derivatives were synthesized from condensation of aldehydes with cyclic diketones and aromatic amines Scheme 1 or ammonium acetate Scheme 2 giving products (4a–4h) and (4i–4j), respectively. The physical data for all synthesized compounds are provided in (Table 3).
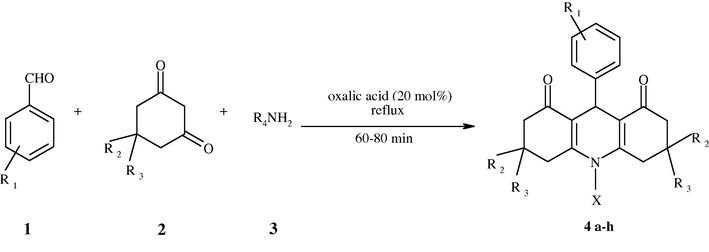
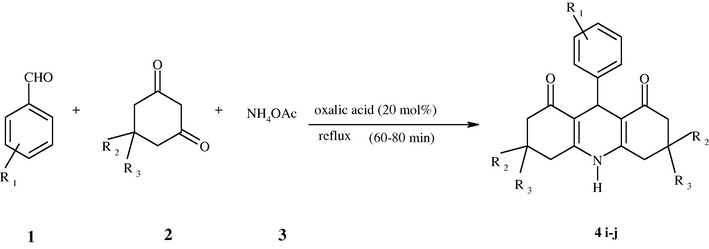
| Product | R1 | R2 and R3 | X (R4 or H) | Time (min) | Yield (%) | M.P. (obtained) | M.P. (reported) | |
|---|---|---|---|---|---|---|---|---|
| 4a | H | CH3 | Ph | 60 | 96 | 250–251 | 254–256 | Shen et al. (2009) |
| 4b | 4-Cl | CH3 | Ph | 60 | 97 | 203–204 | 203–205 | Pyrko (1996) |
| 4c | H | CH3 | Ph 4-CH3 | 75 | 96 | 256–257 | 260–262 | Li et al. (2005) |
| 4d | 4-OCH3 | CH3 | Ph 4-CH3 | 80 | 97 | 280–282 | 281–283 | Li et al. (2005) |
| 4e | 4-Cl | CH3 | Ph 4-CH3 | 65 | 95 | 272–273 | 269–271 | Shen et al. (2009) |
| 4f | 4-OH | CH3 | Ph 4-CH3 | 75 | 96 | 348–349 | 350–352 | Balalaie et al. (2009) |
| 4g | H | H | Ph 4-CH3 | 75 | 97 | 203–204 | 204–206 | Venkatesan et al. (2009) |
| 4h | H | H | Ph | 60 | 94 | 244–246 | 246–248 | Venkatesan et al. (2009) |
| 4i | H | CH3 | H | 70 | 97 | 194–195 | 190–192 | Mora et al. (1995) |
| 4j | 4-Cl | CH3 | H | 80 | 97 | 300–301 | 299–301 | Tu et al. (2002) |
3 Experimental
3.1 General procedure for synthesis of 1,8-dioxo-decahydroacridines
A mixture of aromatic aldehyde 1 (1 mol), diketone 2 (2 mol), aromatic amines (1 mol), or ammonium acetate 3 and oxalic acid (20 mol%) in 10 mL of water was refluxed for appropriate time. After completion of the reaction (as indicated by TLC), the reaction mixture was filtered and dried to get the crude product as a residue. The resulting products were recrystallized using ethanol to get pure products.
3.2 Spectral characterization of representative compounds
3.2.1 10-(4-chlorophenyl)-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4b)
Yellow solid.
ES-MS m/z (%): 461 (M+H).
1H NMR (200 MHz, CDCl3): δ 1.17 (s, 12H), 1.92 (s, 4H), 2.10 (s, 4H), 4.52 (s, 1H), 7.06–7.48 (m, 9H).
4 Conclusion
In conclusion we have developed a new catalytic method for synthesis of 1,8-dioxodecahydroacridines using oxalic acid as a catalyst in aqueous media by one pot three component condensation of aromatic aldehydes, dimedone or cyclohexanedione, aromatic amines or ammonium acetate. The catalyst is inexpensive and easily commercially available. Water is used as a reaction medium which is eco-friendly, inexpensive and abundantly available. There are few advantages of this procedure such as good yields, short reaction time, easy work-up, simplicity in operation, low catalyst loading, use of green solvents, mild reaction conditions.
References
- N. Engl. J. Med.. 1980;302:1269.
- Zh. Org. Khim.. 1991;27:519.
- Chin. J. Chem.. 2009;27:1953.
- Drugs Future. 1992;17:465.
- Catal. Commun.. 2008;9:939.
- J. Mol. Catal. A: Chem.. 2006;247:233.
- J. Org. Chem.. 2000;65:5476.
- Eur. J. Med. Chem. 2000:827.
- Pharmacol. Rev.. 1986;38:321.
- J. Org. Chem.. 1996;61:924.
- Clin. Pharmacol. Ther.. 1977;22:528.
- Chin. J. Chem.. 2005;23:1646.
- J. Med. Chem.. 1983;26:775.
- Synthesis. 2004;12:2001.
- Drugs Future. 1995;20:135.
- J. Chem. Res. (S) 2005:600.
- J. Heterocycl. Chem.. 1995;32:235.
- Arabian J. Chem. 2011:256.
- J. Heterocycl. Chem.. 1995;32:235.
- Chem. Heterocycl. Compd.. 1996;32:742.
- Heteroat. Chem.. 1996;7:17.
- J. Fluorine Chem.. 2009;130:522.
- Chin. J. Org. Chem.. 2002;20:703.
- Synth. Commun.. 2009;39:228.
- J. Med. Chem.. 1995;38:2851.
- Chin. J. Org. Chem.. 2004;24:430.
- Curr. Med. Chem.. 2002;9:2085.







