Translate this page into:
Zinc oxide nanoparticle attenuates chemotherapy resistance by inducing cell stemness progression of colorectal cancer via miR-1321/HIF-2α axis
⁎Corresponding authors at: The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang 524023, Guangdong Province, China (H. Ye, WY. Zou). yehuaishuoxia43@126.com (Hua Ye), weixinzen663318@163.com (Wenying Zou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Colorectal cancer (CRC) is the most malignant cancer type with high morbidity and mortality worldwide. Developed drug resistance severely affected the prognosis of CRC patients. This work aimed to explore the effects of zinc oxide nanoparticles (ZONs) in chemo-resistant CRC.
Methods
We established Oxaliplatin (Oxa)-resistant CRC cells, and in vitro and in vivo model to evaluate the function effect of ZONs. Cell counting kit-8 (CCK-8), colony formation, and 5-ethynyl-2′-deoxyuridine (EDU) assay were performed to detect CRC cell viability and proliferation. CRC cell apoptosis was checked by flow cytometry. Tumor cell proliferation was checked by immunohistochemistry (IHC). Cell stemness was determined by sphere formation assay. Luciferase reporter gene assay was conducted to assess the binding of miR-1321 and HIF-2α 3′UTR region.
Results
ZONs suppressed the viability and proliferation of Oxa-resistant CRC cells both in vitro and in vivo. ZONs suppressed CRC cell stemness and enhanced the sensitivity of CRC cells to chemo-therapy, along with decreased expression of stem cell biomarkers. ZONs elevated level of miR-1321 and reduced level of HIF-2α in CRC cells. MiR-1321 targeted the 3′UTR region of HIF-2α to suppress its expression. ZONs repressed HIF-2α expression by inducing miR-1321 in CRC cells.
Conclusion
ZONs treatment remarkably converted the drug resistance and stemness of CRC cells, via upregulating miR-1321 to repress the expression of HIF-2α. Our findings suggested that ZONs are potential and effective agent for chemo-resistant CRC patients.
Keywords
Colorectal cancer
Zinc oxide nanoparticles
Stemness
miR-1321
HIF-2α
1 Introduction
Colorectal cancer (CRC) belongs to the most malignant cancer type with high morbidity and mortality worldwide (Bray et al., 2018). Even though various therapeutic manners have been developed for CRC treatment, advanced disease progression and the developed therapeutic resistance severely affected the prognosis of CRC patients (Bray et al., 2018; Dekker et al., 2019). Oxaliplatin (Oxa) is a well-recognized reagent for chemotherapy of various cancers, including CRC, and causes cell cycle arrest or cell death through various molecular mechanisms such as disruption of DNA structure and production of free radicals (Wu, 2018; Yamazaki et al., 2021). Resistance to these chemical reagents, also known as chemotherapy, usually leads to treatment failure (Telli et al., 2019). The mechanism of chemoresistance is complicated, and the emergence of cancer stem cells (CSCs) is widely studied (Eun et al., 2017; Vlashi and Pajonk, 2015).
CSCs are a specific cancer cell population with the ability of self-renewal and differentiation into various cell types (Cazet et al., 2018). Studies have indicated that CSCs are responsible for tumor initiation, progression, and heterogeneity (Prasetyanti and Medema, 2017). The CSCs present a survival advantage under chemotherapy and radiotherapy and facilitate cancer recurrence due to the self-renewal ability (Pece et al., 2010). Noteworthy, recent studies indicated that the portion of CSCs was significantly elevated in the tumors resistant to chemotherapy and radiotherapy (Phillips et al., 2006). Thus, targeting cell stemness is a plausible approach to overcome chemoresistance.
Hypoxia-inducible factor 2α (HIF-2α), a member of hypoxia-inducible factor (HIF) family, which functions as a transcriptional factor that response to hypoxia (Hoefflin et al., 2020; Hsu et al., 2020). HIF-2α is not expressed under normoxic conditions, while could be upregulated by hypoxia (Albadari et al., 2019). High level of HIF-2α is observed in multiple cancers, including the CRC (Saint-Martin et al., 2019). Previous studies have indicated the correlation between HIF-2α and the unfavorable prognosis of CRC patients (Han et al., 2018; Ma et al., 2017). And the overexpression of HIF-2α participates in the stemness of CSCs and chemoresistance (Kise et al., 2016).
Zinc oxide nanoparticles (ZONs) are a naturally occurring metal oxide material widely applied in various areas, owing to their high stability, biocompatibility, and low cytotoxicity (Basuthakur and Patra, 2020). Studies have determined the multiple functions of ZONs, including antimicrobial, anti-diabetic, anti-inflammatory, anti-aging, and anti-cancer (Wiesmann et al., 2020). In present study, we evaluated the effects of ZONs on chemoresistance and stemness of CRC, indicated that ZONs enhanced the effects of Oxa on Oxa-resistant CRC cells and inhibited CRC cell stemness, through regulating the micro RNA-1321/HIF-2α axis. Our work suggested that ZON may be an ideal therapeutic agent for the CRC patients resistant to chemotherapy.
2 Materials and methods
2.1 Cell lines
Human CRC cell lines HCT116, HCT8, were brought from the American Type Culture Collection (ATCC; USA), and were maintained in DMEM (Gibco, USA) that contains 10% fetal bovine serum (FBS; Gibco). The Oxa-resistant HCT116 and HCT8 (HCT116/Oxa and HCT8/Oxa) were obtained by constant treatment with elevated Oxa doses (0.1 μM to 10 μM) for eight months.
The HIF-2α overexpressing vector (pcDNA-HIF-2α), small interfering RNA (siHIF-2α), miR-1321 mimics and inhibitors were synthesized by GnenPharma (China). Cell transfection was conducted by using Lipofectamine 2000 reagent (Invitrogen, USA) as per the manufacturer’s protocol.
2.2 Cell counting kit-8 (CCK-8)
CRC cells were seeded in 96-well plates at a density of 5,000 cells per well, and incubated for the indicated time. To determine cell viability, a CCK-8 reagent (20 μL) was added to each well and hatched for 2 h. The absorbance values at 450 nm were measured by a microplate reader (Thermo, USA).
2.3 5-ethynyl-2′-deoxyuridine (EDU) assay
EDU assay was performed to detect the cell proliferation. In brief, CRC cells were stained with EDU (50 µM) for 3 h, after fixation and permeabilization. DAPI was used to counterstain cell nuclei. The images were taken under a fluorescence microscopy (Carl Zeiss, Germany).
2.4 Colony formation
CRC cells were suspended as single cells, and seeded in each well of 12-well plates (500 cells/well). Cells were incubated for 14 days to form visible colonies. After staining with Crystal Violet (Sigma, USA), the colonies were photographed under a dissection microscope (Nikon, Japan).
2.5 Cell apoptosis
Cell apoptosis was checked by using Annexin V/PI detection kit. In short, cells were collected and suspended in binding buffer, incubated with 5 μL Annexin V and PI in dark for 30 min. The samples were then analyzed by using flow cytometry (BD Biosciences, USA).
2.6 Sphere formation
CRC cells (1 × 104 cells/ml) were suspended in DMEM/F12 (Invitrogen, CA) that contains B27 (Sigma, USA), 20 ng/ml EGF (Sigma, USA) and 10 ng/ml bFGF (Invitrogen, USA) in an ultra-low attachment plate (Corning, USA). After incubation for 10 days, the spheroids were captured by using dissection microscope (Nikon, Japan).
2.7 Quantitative real-time PCR
Total RNA was obtained by using Trizol® reagent (Invitrogen, USA) in line with the manufacturer’s protocols. The cDNAs were synthesized by using First-Strand cDNA kit (Transgen, China). PCR amplification was conducted using the SYBR Green method (Qiagen, Germany). The relative RNA levels of genes were evaluated using the 2−ΔΔCT method after normalization to β-actin.
2.8 Luciferase reporter gene assay
The wild type (WT) and mutated (MUT) HIF-2α promoter sequences were cloned into pGL3-Basic vectors. Cells were co-transfected with the WT or MUT reporter gene vectors and miR-1321 mimics for 48 h. Luciferase activity was detected by dual luciferase detection kit (Promega, USA). Results were shown as ratio of firefly luciferase activity to internal control renilla.
2.9 Xenograft model
All experimental operations were conducted following the guidelines of Ethic Committee of Guangdong Medical University. BALB/c nude mice (5-weeks old) were brought from Laboratory Animal Center of Chinese Academy of Sciences (China). HCT116/Oxa cells (5 × 105/mice) were suspended in 0.1 mL PBS and hypodermically injected into fat pad of mice. For treatment, ZONs (dose of 2 mg/kg) were subcutaneously injected to mice. Tumor size was measured using the formula: width2 × length/2. Mice were sacrificed and the tumors were collected.
Histological analysis was performed to determine the proliferation rate of tumors. Tumors were made into 5-µm paraffin-embedded slices, treated with sodium citrate buffer for antigen retrieval. The tissues were then blocked by 5% bovine serum albumin (BSA; Sigma, USA), incubated with anti-KI67 antibody overnight at 4℃, followed by incubation with DAB. The samples were counterstained with hematoxylin.
2.10 Statistics
Statistical analysis was performed using GraphPad Prism (version 7.0) and SPSS (version 19.0). Student’s t test or one-way ANOVA analysis was performed to determine differences between two or more groups. Data were shown as the mean ± the standard deviation (SD) of three independent experiments. P < 0.05 was regarded as statistically significant.
3 Results
3.1 ZONs repress CRC cell growth in vitro
We first assessed the effect of ZONs on the malignant phenotypes of BC cells. The administration of ZONs significantly repressed the viability of HCT116 and HCT8 cells (Fig. 1A). The cell proliferation was validated by colony formation and ECU assay. Obviously, the ZONs reduced the numbers of colonies (Fig. 1B) and EDU-positive CRC cells (Fig. 1C). Conversely, the apoptosis of HCT116 and HCT8 cell was elevated by the ZONs treatment (Fig. 1D).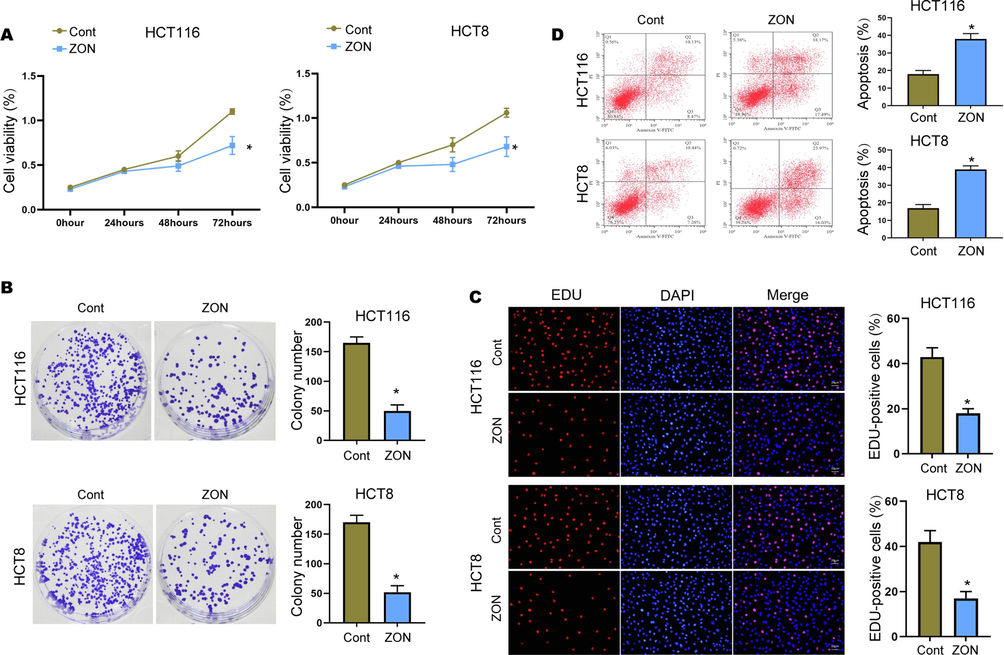
ZONs repress CRC cell growth in vitro. (A) Cell viability was checked by CCK-8 assay. (B) Colony formation to check cell proliferation. (C) EDU assay to determine portion of proliferative CRC cells. (D) Cell apoptosis was checked by flow cytometry. *p < 0.05.
3.2 ZONs attenuate Oxa resistance of CRC cells
Then, we explored the function of ZONs in the modulation of Oxa resistance of CRC cells. We established Oxa-resistant CRC cells HCT116/Oxa and HCT8/Oxa (Fig. 2A). Notably, the IC50 of HCT116/Oxa and HCT8/Oxa was higher than that of parental cells (Fig. 2B), along with elevated level of HIF-2α (Fig. 2C). Moreover, we observed that ZONs treatment decreased the viability of HCT116/Oxa and HCT8/Oxa cells under Oxa treatment (Fig. 2D and E). Meanwhile, ZONs reduced the IC50 of Oxa-resistant CRC cells (Fig. 2D and E).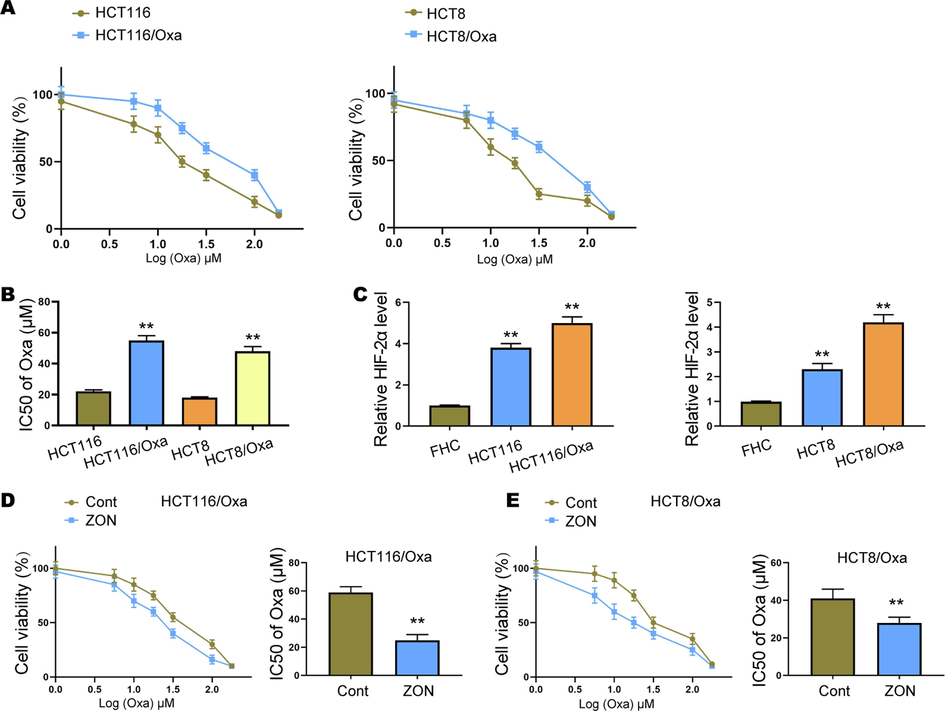
ZONs attenuate Oxa resistance of CRC cells. (A) Cell viability was assessed by CCK-8 assay. (B) IC50 of CRC cells. (C) Level of HIF-2α in CRC cells, Oxa-resistant CRC cells, and normal colon cell line FHC. (D and E) Viability and IC50 of Oxa-resistant CRC cells under ZONs treatment. **p < 0.01.
3.3 ZONs suppress stemness of Oxa-resistant CRC cells
We next evaluated the effect of ZONs on CRC cell stemness. Sphere formation is performed the evaluate the self-renewal ability of OXa-resistant cells. We observed that the expression of Bmi1, c-Myc, Sox2, and Nanog was enhanced in HCT116/Oxa and HCT8/Oxa cells compared with that in HCT116 and HCT8 cells (Fig. 3A). As shown in Fig. 3B and C, ZON treatment notably decreased the size and number of formed spheroids. Drug resistance is usually related with elevated levels of multidrug efflux transporters genes, including the ATP binding cassette subfamily G member 2 (ABCG2). Here, we indicated that treatment with ZONs suppressed the expression of ABCG2 in HCT116/Oxa and HCT8/Oxa cells (Fig. 3D and E). Furthermore, we evaluated the expression of stemness-related genes. Results from Fig. 3C and D suggested that ZONs remarkably decreased the RNA levels of Bmi1, c-Myc, Sox2, and Nanog.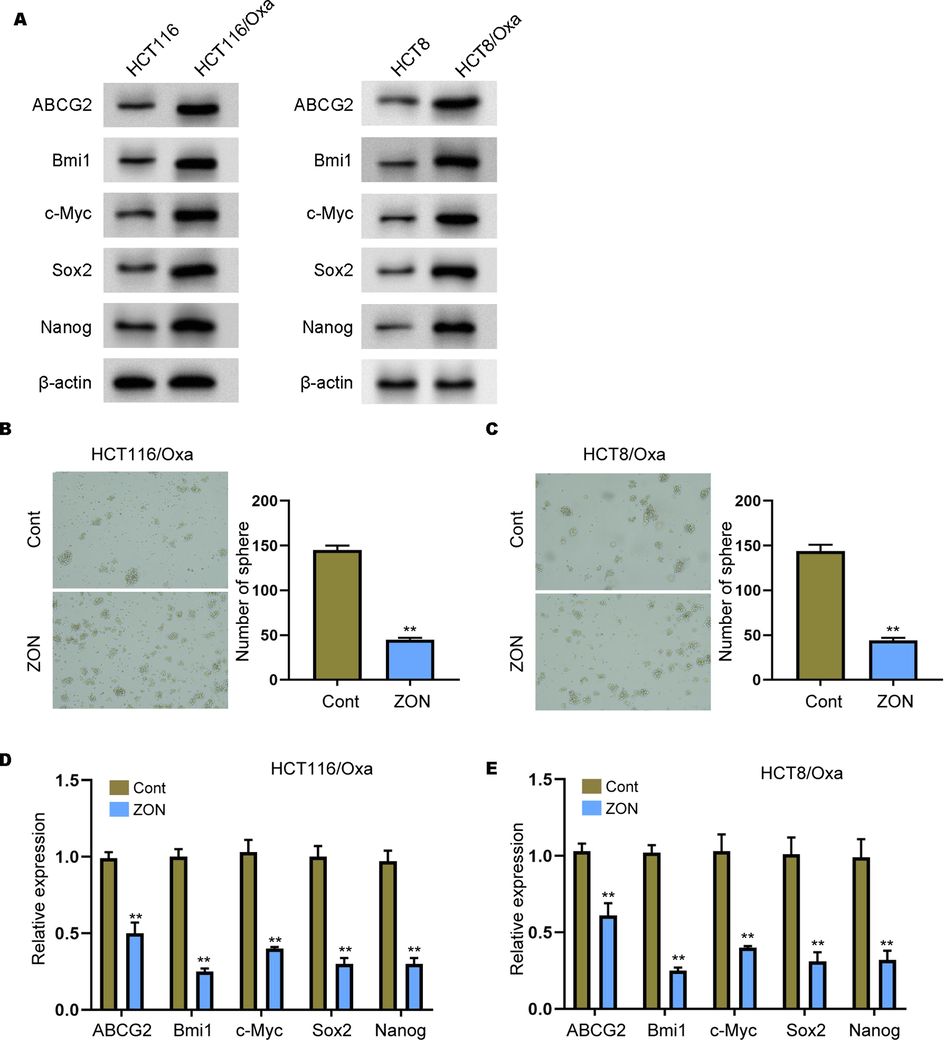
ZONs suppress stemness of Oxa-resistant CRC cells. (A) The expression of ABCG2, Bmi1, c-Myc, Sox2, and Nanog was measured by Western blot analysis in the cells. (B and C) Sphere formation assay to detect self-renewal ability of HCT116/Oxa and HCT8/Oxa cells under ZONs treatment. (D and E) Relative expression of ABCG2, Bmi1, c-Myc, Sox2, and Nanog was detected by qRT-PCR assay. **p < 0.01.
3.4 MiR-1321 acts as sponge of HIF-2α in Oxa-resistant CRC cells
Several studies have identified the effect of ZONs on HIF-1α (Lin et al., 2016; Park et al., 2018), but the correlation of ZONs with HIF-2α is still unclear. To explore the mechanisms underlying the modulation of ZONs in CRC stemness and drug resistance, we determined the expression of HIF-2α and miR-1321. Notably, we observed elevated level of miR-1321 (Fig. 4A) and reduced level of HIF-2α (Fig. 4B) under ZONs treatment. Online prediction indicated the potential binding between miR-1321 on 3′UTR region of HIF-2α (Fig. 4C). Further luciferase reporter gene assay showed that miR-1231 significantly decreased the activity of wild type HIF-2α 3′UTR reporter gene vector, rather than the mutated vectors (Fig. 4D), which manifested the direct interaction between miR-1321 and HIF-2α 3′UTR. Besides, the inhibition of miR-1321 significantly recovered the ZONs-downregulated level of HIF-2α in Oxa-resistant cells (Fig. 4E-G).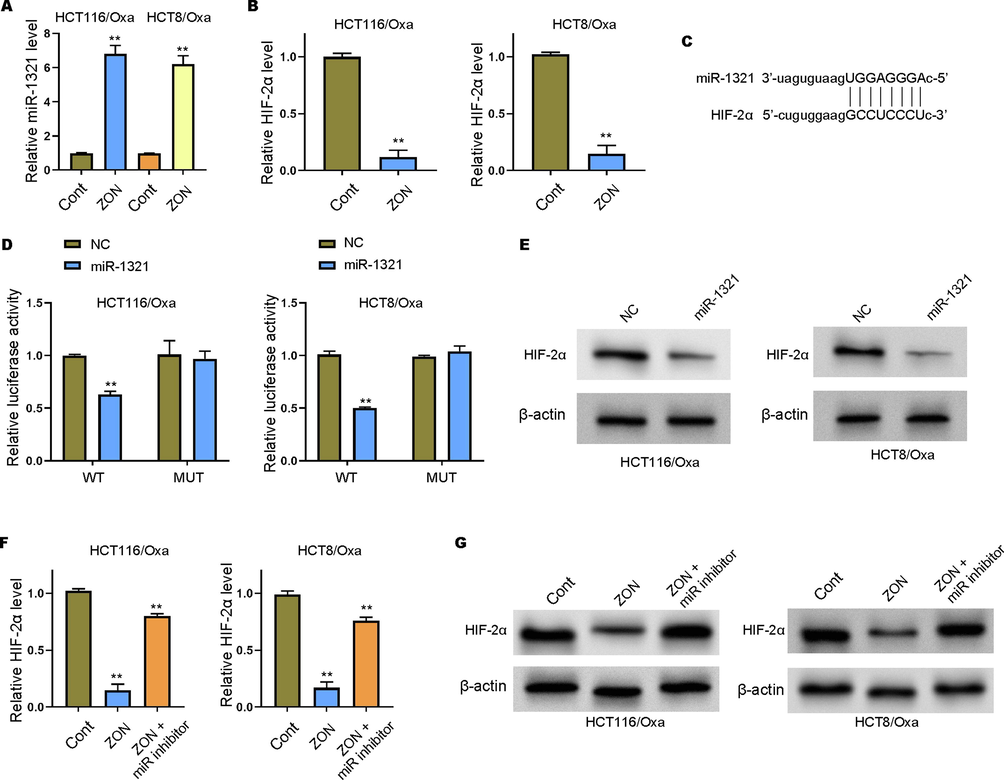
MiR-1321 acts as sponge of HIF-2α in Oxa-resistant CRC cells. (A-B) The qRT-PCR assay to check the RNA levels of miR-1321 and HIF-2α. (C) The predicted interaction site between miR-1231 and HIF-2α. (D) Luciferase reporter gene assay to determine luciferase activity of wild type (WT) and mutated (MUT) HIF-2α 3′UTR promoter vectors. (E-G) Detection of HIF-2α level in Oxa-resistant CRC cells by qRT-PCR and Western blot analysis. **p < 0.01.
3.5 ZONs suppress stemness of Oxa-resistant CRC cells via miR-1231/HIF-2α axis
We next tried to confirm the role of miR-1321/ HIF-2α axis in ZONs-regulated CRC cell proliferation and stemness. We observed significantly enhanced cell proliferation after inhibition of miR-1321 or overexpression of HIF-2α, comparing with the ZONs-treated cells (Fig. 5A and B). Moreover, the ZON treatment notably decreased the sphere formation ability, whereas inhibition of miR-1321 or overexpression of HIF-2α abolished this effect (Fig. 5C and D). The suppressed levels of ABCG2, Bmi1, c-Myc, Sox2, and Nanog in ZONs-treated Oxa-resistant cells were remarkably recovered by miR-1321 inhibitors and HIF-2α overexpression (Fig. 5E and F).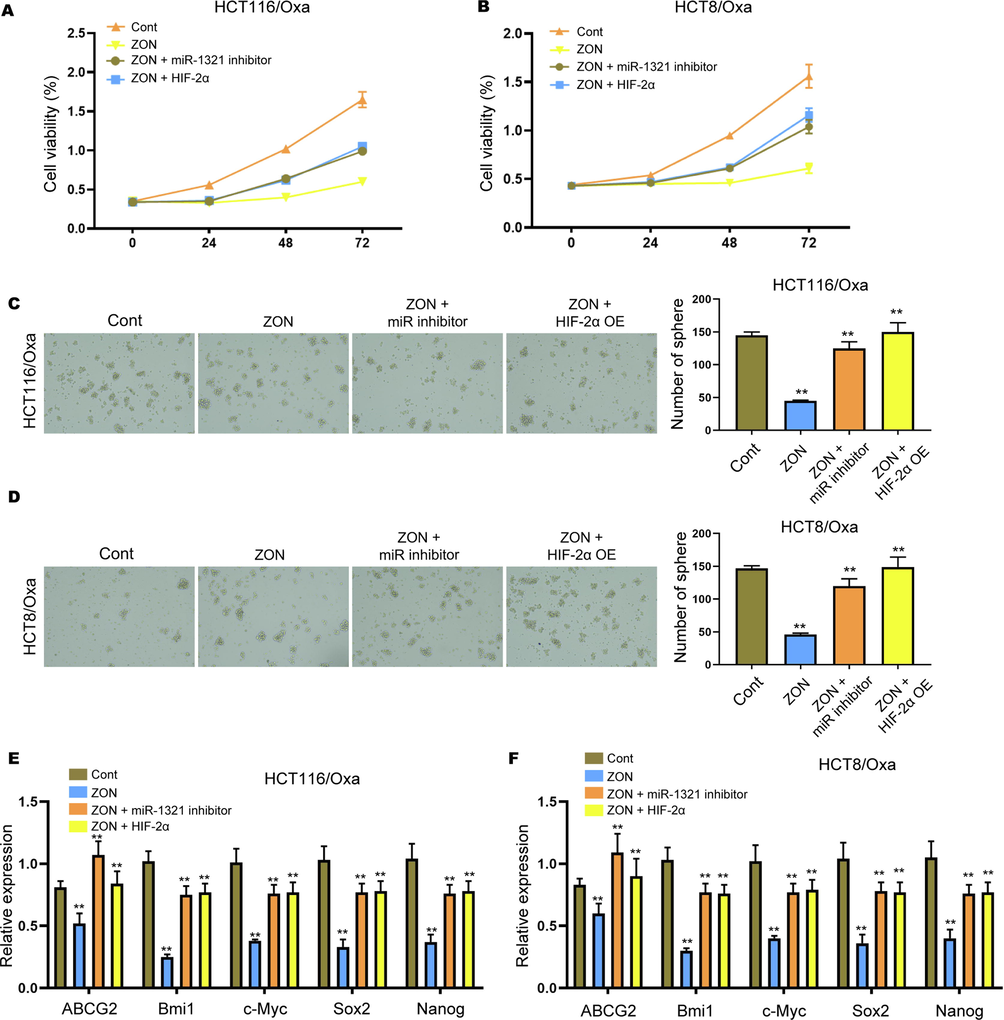
ZONs suppress stemness of Oxa-resistant CRC cells via miR-1231/HIF-2α axis. (A and B) Cell viability was assessed by CCK-8 assay. (C and D) Sphere formation assay to detect self-renewal ability of HCT116/Oxa and HCT8/Oxa cells under treatment of ZONs, miR-1321 inhibitors and HIF-2α overexpressing vectors. (E and F) Relative expression of ABCG2, Bmi1, c-Myc, Sox2, and Nanog was detected by qRT-PCR assay. **p < 0.01.
3.6 ZONs suppress Oxa-resistant CRC cells tumorigenesis in vivo
Subsequently, we evaluated the in vivo function of ZONs in HCT116/Oxa cell tumorigenesis. The administration of ZONs notably repressed the tumor growth, tumor size and tumor weight of HCT116/Oxa cells, whereas miR-1321 inhibition and HIF-2α overexpression reversed these effects (Fig. 6A and B). The decreased RNA level of HIF-2α was also observed in ZONs-treated tumors, and miR-1321 inhibition and HIF-2α overexpression elevated the HIF-2α level (Fig. 6C). Furthermore, the reduced RNA and protein level of KI-67 suggested the suppressed tumor growth under ZON treatment (Fig. 6D and E). And treatment with miR-1321 inhibitors and HIF-2α overexpressing vectors abolished the suppressed expression of KI-67 (Fig. 6D and E).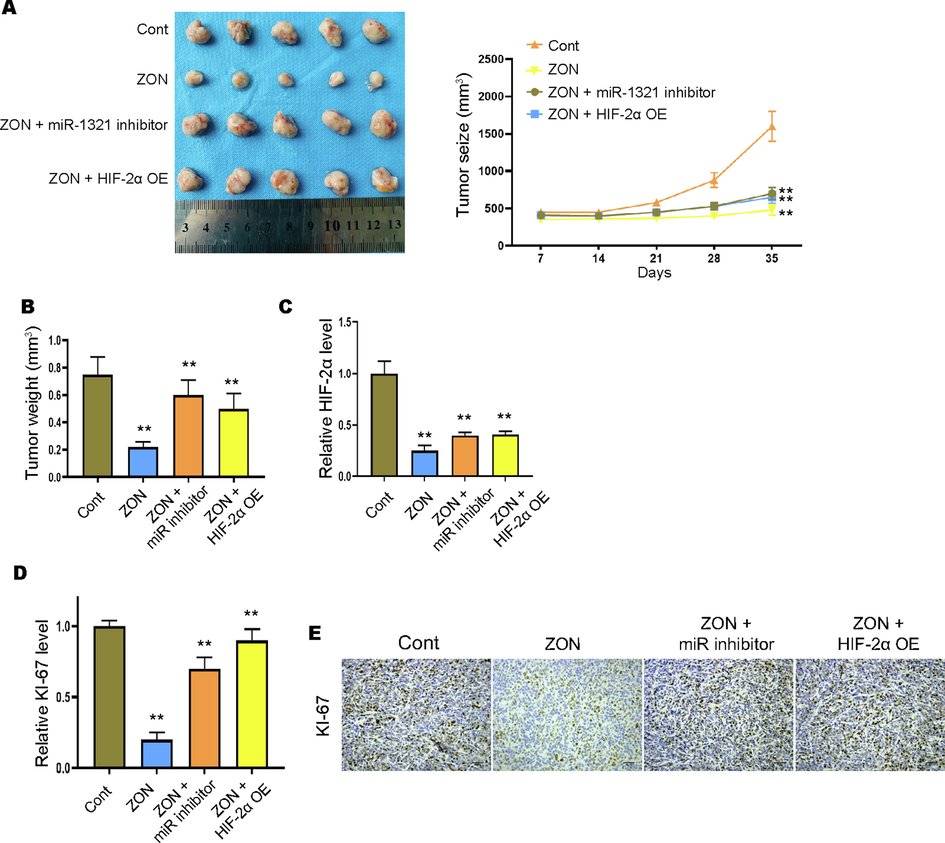
ZONs suppress Oxa-resistant CRC cells tumorigenesis in vivo. Xenograft tumor model was established to determine the in vivo effect of ZONs. Tumor images and growth cure (A) and tumor weight (B) were detected. (C and D) The RNA levels of HIF-2α and KI-67 were detected by qRT-PCR. (E) IHC staining of KI-67 protein. **p < 0.01.
4 Discussion
CRC is the most malignant cancer type that severely threatens human life (Benson et al., 2018). The surgical operation, and chemo- and radio-therapy are the common therapeutic manners for CRC treatment (Cappell, 2008). Nevertheless, cancer cells are capable of escaping the toxicity induced by chemical reagents via multifactorial intrinsic and acquired resistance mechanisms, including the cancer stem cells, abnormal drug metabolism, altered drug transport, and dysregulated receptors, which affect the binding efficacy between drugs to their targets (Mao and Unadkat, 2015). In this study, we explored the effect of ZONs in converting the chemoresistance of CRC cells and the involved mechanisms.
The mechanisms underlying chemoresistance are complicated. Among the mechanisms that affect transport efficacy, drug transporters are widely studied and manipulate the drug efflux (Hsu et al., 2018; Natarajan et al., 2012). The ATP-binding cassette (ABC) transporter family proteins, such as ABCB1, ABCG2, ABCC1 and ABCC10, exhibit great impact on drug efflux (Nakanishi and Ross, 2012). Moreover, cell stemness is also involved in the chemoresistance (Wang et al., 2015). We first determined that ZONs alone could notably suppress the growth of CRC cells and induced cell apoptosis. The chemo-resistant CRC cells were obtained by constant incubation with Oxa, and confirmed by the elevated IC50 values and enhanced expression of HIF-2α. Moreover, the administration of ZONs notably enhanced the sensitivity of Oxa-resistant CRC cells to Oxa, along with decreased level of ABCG2. The overexpression of ABCG2 has been discovered in multiple cancers and is associated with prognosis of patients (Hasanabady and Kalalinia, 2016; Ishikawa and Nakagawa, 2009; Nielsen et al., 2017). Analysis on biopsy specimens demonstrated that high level of ABCG2 suggested short survival of non-small cell lung cancer patients that received platinum-based chemotherapy (Ota et al., 2009). ABCG2 is also found to participate in the development of drug resistance in colon cancer both in vitro and in vivo (Zhang et al., 2019).
Noteworthy, studies have revealed that ABCG2 is correlated with the activity and side population (SP) phenotype of stem cells, suggesting that ABCG2 modulates the self-renewal and differentiation ability of CSCs (An and Ongkeko, 2009; Ding et al., 2010). Our data showed that ZONs treatment significantly alleviated the stemness of Oxa-resistant CRC cells, manifested by impaired sphere formation ability. ZONs treatment also decreased the expression of multiple CRC-related hallmarks, namely the Bmi1, c-Myc, Sox2, and Nanog (Peiris-Pagès et al., 2016; Sun et al., 2016). Further molecular mechanism study demonstrated that ZONs upregulated the level of miR-1321, which sponges HIF-2α to decrease its expression. MiR-1321 is found to be abnormally expressed in ovarian cancer and glioma, and modulates cell migration and invasion (Liu et al., 2013; Luo et al., 2020). HIF-2α has been widely indicated in multiple drug resistance (Lai et al., 2017; Méndez-Blanco et al., 2018; Ahmed et al., 2018). Inhibition of HIF-2α expression could reverse the resistance of lung cancer cells to cisplatin (Gao et al., 2018).
Moreover, there are some limitations in the current study. For example, in this work, we just investigated the oxaliplatin resistance in CRC, but some other common chemical drugs, such as 5-fluorouracil, were not analyzed. The effect of ZONs on 5-fluorouracil resistance of CRC cells should be confirmed in future studies. Several studies have identified the effect of ZONs on HIF-1α (Lin et al., 2016; Park et al., 2018), but the correlation of ZONs with HIF-2α is still unclear. In this work, we found that identified that ZONs inhibited HIF-2α by miR-1321. However, miR-1321/ HIF-2α may be just one of the downstream mechanisms underlying ZONs-mediated CRC and other factors involved in ZONs-mediated CRC should be explored in future investigations. Importantly, miRNAs are known to work in packs, and other miRNAs or non-coding RNAs may regulate HIF-2α and participate in ZONs-mediated CRC development.
5 Conclusion
In conclusion, our data indicated that ZONs effectively inhibit CRC cells proliferation and enhance their sensitivity to chemical drugs, simultaneously suppressing cancer cell stemness. Further analysis on molecular mechanisms exposed that ZONs upregulated the level of miR-1321 in CRC cells and enhanced the expression of HIF-2α. Our finding provides novel evidence to convert the drug resistance during CRC therapy.
Funding
This study was supported by Natural Science Foundation of Guangdong Province(2016B030309002, 2018A0303130252), Science and technology program of Guangdong Province (2019B090905011), Major scientific research projects in Colleges and universities of Guangdong(2017KTSCX081), Medical Research Foundation of Guangdong Province(A2018495), Administration of Traditional Chinese Medicine of Guangdong Province(20182069), Science and Technology Fund of Zhanjiang(2017A06012, 2019A01019), the Fund of Southern Marine Science and Engineering Guangdong Laboratory(Zhanjiang)(ZJW-2019-007).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell Oncol. (Dordr).. 2018;41:319-328.
- [Google Scholar]
- The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert. Opin. Drug Discov.. 2019;14:667-682.
- [Google Scholar]
- ABCG2: the key to chemoresistance in cancer stem cells? Expert. Opin. Drug Metab. Toxicol.. 2009;5:1529-1542.
- [Google Scholar]
- Zinc oxide nanoparticles: future therapy for cerebral ischemia. Nanomed. (London, England).. 2020;15:2729-2732.
- [Google Scholar]
- Benson, A.B., Venook, A.P., Al-Hawary, M.M., Cederquist, L., Chen, Y.J., Ciombor, K.K., et al. (2018). NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Canc. Netw. 16 359–369.
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin.. 2018;68:394-424.
- [Google Scholar]
- Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol. Clin. North Am.. 2008;37(1–24):v.
- [Google Scholar]
- Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun.. 2018;9:2897.
- [Google Scholar]
- ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci.. 2010;86:631-637.
- [Google Scholar]
- Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep.. 2017;50:117-125.
- [Google Scholar]
- Downregulation of HIF-2α reverse the chemotherapy resistance of lung adenocarcinoma A549 cells to cisplatin. Med. Sci. Monit.. 2018;24:1104-1111.
- [Google Scholar]
- Association between hypoxia-inducible factor-2α (HIF-2α) expression and colorectal cancer and its prognostic role: a systematic analysis. Cell Physiol. Biochem.. 2018;48:516-527.
- [Google Scholar]
- ABCG2 inhibition as a therapeutic approach for overcoming multidrug resistance in cancer. J. Biosci.. 2016;41:313-324.
- [Google Scholar]
- HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat. Commun.. 2020;11:4111.
- [Google Scholar]
- Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell Physiol.. 2018;233:5458-5467.
- [Google Scholar]
- HIF-2α is indispensable for regulatory T cell function. Nat. Commun.. 2020;11:5005.
- [Google Scholar]
- Human ABC transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J. Experiment. Therap. Oncol.. 2009;8:5-24.
- [Google Scholar]
- Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev.. 2016;99:197-205.
- [Google Scholar]
- HAF mediates the evasive resistance of anti-angiogenesis TKI through disrupting HIF-1α and HIF-2α balance in renal cell carcinoma. Oncotarget. 2017;8:49713-49724.
- [Google Scholar]
- The role of hypoxia-inducible factor-1alpha in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Part Fibre Toxicol.. 2016;13:52.
- [Google Scholar]
- Identification of aberrant microRNA expression pattern in pediatric gliomas by microarray. Diagn. Pathol.. 2013;8:158.
- [Google Scholar]
- Long non-coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR-1321 and regulating tight junction protein 3 expression. Mol. Med. Rep.. 2020;22:3429-3439.
- [Google Scholar]
- Hypoxia-inducible factor 2α (HIF-2α) promotes colon cancer growth by potentiating Yes-associated protein 1 (YAP1) activity. J. Biol. Chem.. 2017;292:17046-17056.
- [Google Scholar]
- Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport–an update. AAPS J.. 2015;17:65-82.
- [Google Scholar]
- Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp. Mol. Med.. 2018;50:1-9.
- [Google Scholar]
- Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer. 2012;31:73-99.
- [Google Scholar]
- Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem .Pharmacol.. 2012;83:1084-1103.
- [Google Scholar]
- Implications of ABCG2 expression on irinotecan treatment of colorectal cancer patients: a review. Int. J. Mol. Sci.. 2017;18
- [Google Scholar]
- Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung cancer (Amsterdam, Netherlands). 2009;64:98-104.
- [Google Scholar]
- Zinc oxide nanoparticles induce HIF-1alpha protein stabilization through increased reactive oxygen species generation from electron transfer chain complex III of mitochondria. J. Dermatol. Sci.. 2018;91:104-107.
- [Google Scholar]
- Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62-73.
- [Google Scholar]
- The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst.. 2006;98:1777-1785.
- [Google Scholar]
- Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16:41.
- [Google Scholar]
- Functional interaction of hypoxia-inducible factor 2-alpha and autophagy mediates drug resistance in colon cancer cells. Cancers (Basel). 2019;11
- [Google Scholar]
- Liver cancer stem cell markers: Progression and therapeutic implications. World J. Gastroenterol.. 2016;22:3547-3557.
- [Google Scholar]
- NCCN guidelines updates: breast cancer. J. Natl. Compr. Canc. Netw.. 2019;17:552-555.
- [Google Scholar]
- Cancer stem cells, cancer cell plasticity and radiation therapy. Semin. Cancer Biol.. 2015;31:28-35.
- [Google Scholar]
- Cancer stem cell targeted therapy: progress amid controversies. Oncotarget.. 2015;6:44191-44206.
- [Google Scholar]
- Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B. 2020;8:4973-4989.
- [Google Scholar]
- Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6 months) for high-risk stage II colon cancer: the randomized phase III ACHIEVE-2 trial. Ann. Oncol.. 2021;32:77-84.
- [Google Scholar]
- Regorafenib antagonizes BCRP-mediated multidrug resistance in colon cancer. Cancer Lett.. 2019;442:104-112.
- [Google Scholar]







