Translate this page into:
ZrOCl2·8H2O as an efficient and recyclable catalyst for the clean synthesis of xanthenedione derivatives under solvent-free conditions
*Corresponding author. Tel.: +98 632 4233322; fax: +986 632 42333 mosaddegh_e@yahoo.com (Elaheh Mosaddegh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 29 July 2010
Abstract
ZrOCl2·8H2O was found to be an efficient and recyclable catalyst for the reaction of aromatic aldehydes with dimedone to afford 1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthenes under solvent-free conditions. Short reaction time, excellent yields and simple work-up are the advantages of this procedure. The interaction obtained from XRD studies was shown that the catalyst loses H2O during the reaction but it did not affect catalytic activity of the catalyst and the catalyst could be reused several times.
Keywords
ZrOCl2·8H2O
XRD
Dimedone
1,8-Dioxo-1,2,3,4,5,6,7,8-octahydroxanthene
Solvent-free condition
1 Introduction
Xanthene derivatives have attracted considerable attention to organic synthesis in recent years (Robak and Gryglewski, 1996; Wang et al., 1997; Rakavishnikov et al., 1998). They are an important class of compounds which are used as dyes, fluorescent materials for visualization of bio-molecules and in laser technologies due to their useful spectroscopic properties (Menchen et al., 2003a,b; Banerjee and Makherjee, 1981; Reynolds et al., 1971a,b). Also, these compounds have been investigated for agricultural bactericidal activity (Hideu, 1981a,b), photodynamic therapy (Ion et al., 2000), anti-inflammatory effect (Poupelin et al., 1978) and antiviral activity (Lambert et al., 1997a,b). In addition, xanthenediones are important building blocks in a number of natural products (Hatakeyaa et al., 1988; Cingolant and Pigini, 1969; O’Callaghan and McMurry, 1955).
Various methods for the synthesis of xanthenes are described in the literature including palladium catalyzed cyclization of polycyclic aryl triflate esters (Wang and Harvey, 2002), intramolecular trapping of benzynes by phenols (Knight and Little, 2001, 1998) and reaction of aryloxymagnesium halides with triethyl orthoformate (Casiraghi et al., 1973). Recently, the synthesis of 1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthenes has been reported by the condensation of dimedone and aldehydes in the presence of TEBA (Shi et al., 2000), p-dodecylbenzene sulphonic acid (Jin et al., 2004), InCl3/ionic liquid (Fan et al., 2005), Fe3+-montmorillonite (Song et al., 2007), polyaniline-p-toluenesulfonate salt (John et al., 2006), amberlyst-15 (Das et al., 2006), NaHSO4·SiO2 and silica chloride (Das et al., 2007), TiO2/ (Jin et al., 2005), Dowex-50 W (Imani-Shakibaei et al., 2007), and silica sulfuric acid (Seyyedhamzeh et al., 2008).
In spite of the potential utility of the afore mentioned routes for the synthesis of xanthene derivatives many of these methods involve expensive reagents, strongly acidic conditions, long reaction time, low yields, use of excess of reagents/catalyst and use of toxic organic solvents. We hoped to develop a more general protocol for the efficient synthesis of xanthenes via zirconium salts, which have recently attracted much attention as catalysts to organic synthesis due to their easy availability and low toxicity (Ghosh et al., 2006; Firouzabadi et al., 2006; Sun et al., 2006; Eftekhari-Sis et al., 2006; Rodriguez-Dominguez and Kirsch, 2006; Mosaddegh et al., 2007). Herein we present our method toward that goal using ZrOCl2·8H2O as a catalyst (Scheme 1).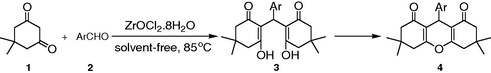
2 Experimental
Melting points were recorded on a Gallenkamp melting point apparatus and are uncorrected. NMR spectra were recorded at 500 (1H) and 125.77 (13C) MHz on a Bruker DRX-500 Avance spectrometer respectively. Chemical shifts are reported (δ) relative to TMS (1H) and CDCl3 (13C) as the internal standards. IR spectra were measured on a Mattson 1000 FT-IR spectrophotometer. Powder X-ray diffraction (XRD) measurements were performed using D8 Advance diffract meter made by Bruker axs company in Germany.
2.1 General procedure for the preparation of 1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthenes
A mixture of dimedone (2 mmol, 0.28 g), aldehyde (1 mmol) and ZrOCl2·8H2O (2 mol%, 12 mg) was stirred at 85 °C for appropriate time. Completion of the reaction was indicated by TLC (n-heptane-ethyl acetate). The mixture was cooled to 25 °C and dichloromethane (10 mL) was added. Then the resulting mixture was stirred for 5 min. The catalyst was separated by filtration. The solvent was removed by distillation and the product was obtained as a solid, which was recrystallized from ethanol and characterized by IR, 1H NMR and 13C NMR in comparison with the literature data. The selected spectral data of five representative 1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthenes are given below.
2.1.1 3,3,6,6-Tetramethyl-9-(4-chlorophenyl)-1,8-dioxo-1,2,3,4,5,6,7,8-octahydro-xanthene (entry 2)
IR (KBr) νmax: 3080, 2982, 1691, 1666, 1640, 1617, 1492, 1368, 1195, 1145, 1120, 1095, 1021, 847 cm−1. 1H NMR (CDCl3, 500 MHz) δ: 0.88 (s, 6H, 2CH3), 1.01 (s, 6H, 2CH3), 2.19 (dd, 4H, J = 16.4, 16.1 MHz, 2CH2), 2.51 (dd, 4H, J = 15.2, 12.0 MHz, 2CH2), 4.72 (s, 1H), 7.52–8.10 (m, 4H, ArH); 13C NMR (CDCl3, 125.77 MHz) δ: 26.4, 28.5, 30.8, 31.7, 49.9, 113.9, 127.7, 129.8, 130.6, 143.1, 162.9, 195.9.
2.1.2 3,3,6,6-Tetramethyl-9-(3-chlorophenyl)-1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (entry 3)
IR (KBr) ν: 3081, 2982, 2882, 1667, 1617, 1592, 1492, 1368, 1219, 1170, 1145, 1120, 897, 698 cm−1; 1H NMR (CDCl3, 500 MHz) δ: 0.89 (s, 6H, 2CH3), 1.02 (s, 6H, 2CH3), 2.22 (dd, 4H, J = 16.1, 16.2 MHz, 2CH2), 2.54 (s, 4H, 2CH2), 4.50 (s, 1H), 7.09–7.26 (m, 4H, ArH); 13C NMR (CDCl3, 125.77 MHz) δ: 26.4, 28.4, 31.2, 31.7, 49.9, 113.7, 126.1, 126.5, 128.0, 129.7, 132.3, 146.5, 163.1, 195.9.
2.1.3 3,3,6,6-Tetramethyl-9-(2,4-dichlorophenyl)-1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (entry 4)
IR (KBr) ν: 3081, 2982, 1691, 1617, 1592, 1567, 1492, 1368, 1195, 1170, 1120, 1095, 1070, 1021, 872, 847 773, 574 cm−1; 1H NMR (CDCl3, 500 MHz) δ: 0.90 (s, 6H, 2CH3), 1.02 (s, 6H, 2CH3), 2.13 (dd, 4H, J = 16.0, 16.1 MHz, 2CH2), 2.51 (t, 4H, 2CH2), 4.78 (s, 1H), 7.23–7.38 (m, 4H, ArH); 13C NMR (CDCl3, 125.77 MHz) δ: 26.4, 28.5, 30.1, 31.6, 49.9, 112.7, 126.6, 128.6, 131.3, 133.0, 133.7, 139.9, 163.2, 195.7.
2.1.4 3,3,6,6-Tetramethyl-9-(4-nitrophenyl)-1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (entry 5)
IR (KBr) ν: 3081, 2957, 1666, 1641, 1542, 1492, 1368, 1219, 1170, 1145, 1121, 822, 748 cm−1; 1H NMR (CDCl3, 500 MHz) δ: 0.96 (s, 6H, 2CH3), 1.09 (s, 6H, 2CH3), 2.15 (dd, 4H, J = 15.8, 15.9 MHz, 2CH2), 2.59 (s, 4H, 2CH2), 4.48 (s, 1H), 7.16–7.26 (m, 4H, ArH); 13C NMR (CDCl3, 125.77 MHz) δ: 26.4, 27.0, 29.0, 32.3, 50.3, 113.8, 123.6, 129.9, 146.4, 152.2, 163.9, 196.5.
2.1.5 3,3,6,6-Tetramethyl-9-(3-nitrophenyl)-1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (entry 6)
IR (KBr) ν: 3080, 2957, 2907, 1667, 1641, 1542, 1368, 1219, 1170, 1145, 1021, 822, 748, 698 cm−1; 1H NMR (CDCl3, 500 MHz) δ: 0.89 (s, 6H, 2CH3), 1.02 (s, 6H, 2CH3), 2.17 (dd, 4H, J = 16.1, 16.2 MHz, 2CH2), 2.56 (s, 4H, 2CH2), 4.63 (s, 1H), 7.52–7.99 (m, 4H, ArH); 13C NMR (CDCl3, 125.77 MHz) δ: 26.4, 28.4, 31.5, 31.8, 49.8, 113.3, 121.3, 122.5, 129.4, 134.7, 146.3, 147.3, 163.4, 196.0.
3 Results and discussion
In continuation of our interest in the application of heterogeneous catalysts to development of a useful synthetic methodology (Mosaddegh et al., 2007), here we report a simple and highly efficient route for the synthesis of 1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthene derivatives using ZrOCl2·8H2O as an efficient catalyst with high catalytic activity under solvent-free conditions at 85 °C (Scheme 1).
It was found that a mixture of the products was obtained in the absence of the catalyst. Compound 4 was obtained in good yields in the presence of a catalytic amount of ZrOCl2·8H2O. Mechanism of the reaction between aldehyde and dimedone has been described in literature (Song et al., 2007). In this reaction, intermediate 3 was formed through Knoevenagel reaction between dimedone and aldehyde, and subsequently, elimination of water occurred from the intermediate 3 to give compound 4. In these processes, ZrOCl2·8H2O plays a crucial role in accelerating the reaction.
Our attempts to use 1,2-dichloroethane as solvent for the synthesis of compound 4 at room temperature produced compound 3. No cyclization product was obtained. We next investigated the effect of temperature on the reaction of 4-chlorobenzaldehyde with dimedone in the presence of ZrOCl2·8H2O as catalyst in 1,2-dichloroethane. The reaction mixture was refluxed and only 4% yield of the corresponding product was obtained after 1 h (Table 1, entry 2).
Entry
Aldehyde
Time (min)
Yielda (%)
m.p.
[m.p. reported]
References
1
C6H5CHO
35
90
199–200
[201–203]
John et al. (2006)
2
4-ClC6H4CHO
35
96
229–230
[231–233]
John et al. (2006)
3
3-ClC6H4CHO
30
93
179–181
[182–184]
Jin et al. (2004)
4
2,4-Cl2C6H3CHO
30
90
248–250
[251–252]
Jin et al. (2004)
5
4-NO2C6H4CHO
25
95
221–223
[224–226]
Jin et al. (2004)
6
3-NO2C6H4CHO
20
96
163–164
[168–170]
Jin et al. (2004)
7
4-OCH3C6H4CHO
15
94
241–242
[241–243]
John et al. (2006)
8
4-CH3C6H4CHO
14
95
193–195
[217–218]
Jin et al. (2004)
9
4-OHC6H4CHO
12
92
247–248
[245–247]
John et al. (2006)
10
3-OHC6H4CHO
25
90
223–225
[225–227]
Song et al. (2007)
11
4-N(CH3)2C6H4CHO
30
97
218–220
[221–222]
Jin et al. (2005)
12
4-OH-3-OCH3C6H3CHO
35
94
226–227
[224–226]
Jin et al. (2004)
13
C6H5C2H2CHO
10
95
176–177
[175–177]
Jin et al. (2004)
We have also examined the effect of temperature in a solvent-free condition. Rising temperature leads to decrease in yields. For example the reaction of 3-nitrobenzaldehyde with dimedone at 110 °C in a solvent-free condition gave the corresponding product (Table 1, entry 6) in 94% yield, while decreasing the temperature to 80–85 °C leads to the product in 96% yield. Therefore, our optimized condition is 2 mol% of ZrOCl2·8H2O and 80–85 °C without solvent.
To investigate the versatility of the catalyst, the reaction of dimedone and various aromatic aldehydes was carried out under solvent-free conditions in 80-85 °C using 2 mol% of ZrOCl2.8H2O. 1,8-Dioxo-octahydro xanthene derivatives containing electron-withdrawing groups such as nitro and halide groups or electron-donating groups such as hydroxyl and alkoxy groups were formed in a short experimental time (10–35 min) with high yields (90–97%). The catalyst was easily regenerated by filtering the reaction mixture after completion of reaction.
The reusability of the catalyst is one of the most important benefits and makes it useful for commercial applications. Thus the recovery and reusability of ZrOCl2·8H2O was investigated. In these experiments, the reaction mixture was isolated with CH2Cl2.The catalyst was easily reused by filtration after washing with CHCl3 and drying at 60 °C. The recycled catalyst has been examined in the next run in the reaction between 4-chlorobenzaldehyde and dimedone. The ZrOCl2·8H2O catalyst could be reused four times without any loss of its activity.
3.1 Catalytic behavior in the reaction process
X-ray diffraction analysis (XRD) has been used to determine the structure of catalyst both before and after the reaction. The interaction obtained from XRD studies is given in Fig. 1.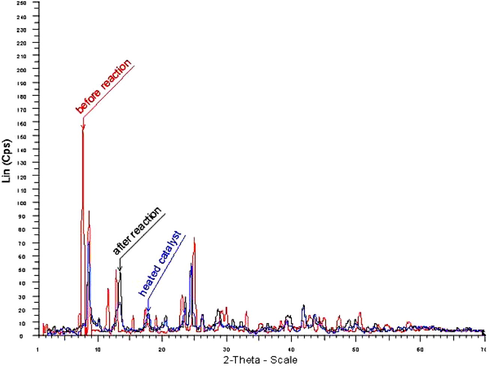
X-ray diffraction analysis (XRD) of ZrOCl2·8H2O; red line: before the reaction, gray line: after the reaction and reused the catalyst, blue line: after heating in the absence of starting materials.
The peak position and intensity distributions in the XRD pattern for the catalyst before the reaction are similar to those after the reaction. A peak at eight disappeared from the XRD pattern after using the catalyst and drying at 60 °C. Also, we obtained the XRD pattern of the catalyst after heating at 85 °C for an appropriate time without using it as a catalyst. Its XRD pattern is similar to that of the used catalyst. Thus, it seems that the catalyst loses some H2O during the reaction but it did not affect catalytic activity of the catalyst and the catalyst could be reused several times.
4 Conclusion
In conclusion, the present method is an operationally simple and clean procedure for the synthesis of compound 4 using a catalytic amount of ZrOCl2.8H2O. In addition, low cast, easy availability, recyclability, low toxicity, moderate Lewis acidity and moisture compatibility of the catalyst, excellent yields of products and short reaction time make this methodology a valid contribution to the existing processes in the field of 1,8-dioxo-1,2,3,4,5,6,7,8-octahydroxanthene derivatives synthesis. Also, the catalytic activity of ZrOCl2·8H2O remains constant during the reaction.
Acknowledgement
We are thankful to the Office of Graduate Studies of the University of Kerman for its financial support.
References
- Stain Technol.. 1981;56:83.
- Tetrahedron Lett.. 1973;14:679.
- J. Med. Chem.. 1969;12:531.
- J. Mol. Catal. A. 2006;247:233.
- Catal. Commun.. 2007;8:535.
- Eur. J. Org. Chem. 2006:5152.
- Can. J. Chem.. 2005;83:16.
- J. Mol. Catal.. 2006;252:150.
- Tetrahedron. 2006;62:4059.
- J. Chem. Soc., Chem. Commun. 1988:1202.
- Hideu, T., 1981. Jpn. Tokkyo Koho JP 56005480.
- Chem. Abstr.. 1981;95:80922b.
- Appl. Catal., A. 2007;325:188.
- Ion, R.M., Albulescu, C., Sirkecioglu, O., Talinli, N., 2000. Intenet Photochem. Photobiol.
- Synlett 2004:866.
- Synth. Commun.. 2005;35:2339.
- J. Mol. Catal. A. 2006;248:121.
- Synlett 1998:1141.
- J. Chem. Soc., Perkin Trans. 1. 2001;14:1771.
- Lambert, R.W., Martin, J.A., Merrett, J.H., Parkes, K.E.B., Thomas, G.J., 1997. PCT Int. Appl. WO 9706178.
- Chem. Abstr.. 1997;126:p212377y.
- Menchen, S.M., Benson, S.C., Lam, J.Y.L., Zhen, W., Sun, D., Rosenblum, B.B., Khan, S.H., Taing, M., 2003. US Patent 6583168.
- Chem. Abstr.. 2003;139:54287f.
- Lett. Org. Chem.. 2007;4:524.
- J. Chem. Res. 1955:214.
- Eur. J. Med. Chem.. 1978;13:67.
- Tetrahedron Lett.. 1998;39:6637.
- Reynolds, G.A., Teccio, S.A., Peterson, O.G., Specht, D.P., 1971. Ger. Offen. DE2109040.
- Chem. Abstr.. 1971;71:p81334c.
- Pol. J. Parmacol.. 1996;48:555.
- Synthesis 2006:1895.
- Dyes Pigm.. 2008;76:836.
- Synth. Commun.. 2000;30:713.
- Catal. Commun.. 2007;8:673.
- Molecules. 2006;11:263.
- Tetrahedron. 2002;58:5927.
- Med. Res. Rev.. 1997;17:367.







