Translate this page into:
ZnS nanoparticle synthesis in 1-butyl-3-methylimidazolium tetrafluoroborate by simple heating
⁎Corresponding author. abdulkareem.t@gmail.com (T. Abdul Kareem),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pure ZnS nanoparticles were prepared in 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4) ionic liquid by simple heating without any sophisticated technology or any other solvents or any other stabilizing or capping agents. Particles of size below 10 nm were obtained by this method and found that the final product is free from the [BMIM]BF4 ionic liquid. It is deduced from the analysis that the growth of the ZnS nanoparticle was controlled by the combined intrinsic high charge and the steric effects of [BMIM]BF4 ionic liquid.
Keywords
ZnS nanoparticles
Ionic liquid
1 Introduction
Ionic liquids (IL) have received much attention recently due to their potential use as green recyclable alternatives to traditional organic solvents. Endres et al. (2008) defined the ionic liquid as “an ionic material that is liquid below 100 °C” with a large number of cations and anions. ILs have high ionic conductivity, low viscosity, high thermal stability, wide electrochemical windows up to 7 volts and have negligible vapor pressure as well as they are environmentally friendly (Endres et al., 2008). Ionic liquids can form ionic liquid–organic solvent system so that it can dissolve a wide spectrum of organic, organometallic, and inorganic compounds (Wang et al., 2008; Zhao and Malhotra, 2002). Physical properties of ionic liquids eliminate environmental and other safety problems due to volatilization, as is the case in the conventional organic solvents. Published results indicate that most of the ionic liquids can also be recycled for reuse (Zhao and Malhotra, 2002) and therefore, ILs are considered as novel and green solvents. Further, the thermodynamic and kinetic behavior of the ionic liquids is different, so that the rates of reaction are often enhanced and selectivity is even better.
Moreover ionic liquids are miscible with the substances having very wide range of polarities and can simultaneously dissolve organic and inorganic substances. These features of ionic liquids offer numerous opportunities for novel material preparations and the modification of existing processes. The great potential of the ionic liquid is the charged nature which can influence the synthesis itself. Ionic liquids are usually composed of large asymmetric organic cations and inorganic or organic anions. Most commonly used ionic liquid is the ionic liquid with cations based on the imidazolium or pyridinium ring with one or more alkyl groups attached to the nitrogen or carbon atoms. Anions include halide ions, tetrafluoroborate (BF4−), tetrachloroaluminate (AlCl4−), hexafluorophosphate (PF6−), and bis(perfluoromethyl-sulfonyl)imide anion (CF3SO2)2N− (also known as bistriflate imide (Tf2N−)) and many new cations have recently been proposed. Imidazolium-based ionic liquids with stable anions (e.g., tetrafluoroborate or trifluoromethyl sulfonate) are the best materials for applications because of its stability, so that [BMIM] BF4 (Fig. 1) has been chosen for the synthesis of ZnS nanoparticles. The basic properties of the of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) are given in Table 1 (http://www.sigmaaldrich.com/catalog/product/fluka/39931).![Chemical structure of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) http://www.sigmaaldrich.com/catalog/product/fluka/39931.](/content/184/2019/12/8/img/10.1016_j.arabjc.2015.06.008-fig1.png)
Chemical structure of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) http://www.sigmaaldrich.com/catalog/product/fluka/39931.
Name
[BMIM] BF4 or 1-Butyl-3-methylimidazolium tetrafluoroborate
Empirical formula
C8H15BF4N2
Molecualr weight (g/mol)
226.02
Color
Light yellow
Density
1.21 g/mL at 20 °C
MP/freezing point
−71.0 °C
Flash point
288 °C
Vapor pressure
<0.000125 hPa
[BMIM][BF4] ionic liquid is used as an electrolyte in electrochemical applications (Abdul Kareem and Anu Kaliani, 2012) and as a capping agent for nanoscale materials (Biswas and Rao, 2007) processing. It is a fact that various size quantization effects such as widening of band gap and the formation of discrete orbitals appear at the nanoscale especially in the case of semiconductors. ZnS is one of the most important materials in the electronic/optoelectronic industry with prominent applications in flat-panel displays (Sakshi et al., 2013), white light LEDs (Nizamoglu and Demir, 2009), electroluminescence devices (Muller et al., 1990), sensors (Wang et al., 2012), lasers (Sorokina et al., 2002), infrared windows (Sumitomo Electric Industries, Ltd, 2010), and as an antireflection coating on solar cells (Gangopadhyay et al., 2004). Generally ZnS nanoparticles are prepared with the help of capping or stabilizing agents by dissolving the ZnS precursors in the solvent using sophisticated technologies. While the group of Biswas and Rao (2007) experimented ionic liquid for processing nanoscale materials and reported the preparation of CdS, CdSe, ZnS, ZnSe and PbS nanoparticles in [BMIM]BF4 ionic liquid and they have synthesized ZnS nanoparticles by keeping the ZnS precursor in autoclave at 180 °C for 5 h. Further, Shahid et al. (2012) report the synthesis of ZnS quantum dots and nanorods by heating the precursor solution to 250 °C under micro wave irradiation. Our experiment shows that ZnS nanoparticles synthesis in [BMIM]BF4 ionic liquid is simple as making a tea and the results are discussed below.
2 Materials and methods
All chemicals were purchased from Sigma Aldrich Chemicals, Bangalore, and used without any further purification. Three ZnS samples were made in three beakers by adding 0.2726 g of ZnCl2 and 0.0156 g of Na2S in 4 ml of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) in each beaker, where the concentration of ZnS precursors was fixed based on our previous experiments. Each of the three beakers was kept for 1 h (sample H1), 2 h (sample H2) and 3 h (sample H3) at 90 °C respectively on a heater without any stirring. It was seen that the precursors were dissolving and the color of the liquid changed from light yellow to a cream color. After washing the cream colored precipitate in ethanol and in water for four times, it was washed again in ethanol to make the precipitate to dry at 80 °C in air to get fine powder. X-ray diffractogram of the samples was obtained by X-ray Powder Diffractometer of Bruker AXS D8 Advance while scanning the samples from 10° to 80° in steps of 0.020° per 31.2 s at 25 °C. SEM analysis was performed by the SEM of JEOL Model JSM – 6390LV and FTIR spectra of the samples were obtained using Fourier Transform Infra Red spectrometer (FTIR) of Avatar 370- Thermo Nicolet with spectral range from 400 to 4000 cm−1 which has a resolution of 4 cm−1. Transmission Electron Microscopic images were obtained from Jeol model JEM-2100 having accelerating voltage about 200 kV.
3 Results and discussion
ZnS nanoparticle growth in [BMIM]BF4 ionic liquid was studied by heating the mixture of the ZnS precursors in [BMIM]BF4 ionic liquid for different time periods. The samples were analyzed by their X-ray diffractograms, SEM and TEM images and by FTIR spectra. X-ray diffractogram of the samples H1, H2 and H3 is shown in Fig. 2 and their crystal information is given in Tables 2–4. Their X-ray diffractograms are well correlated with the ASTM 77-2100 and the peaks are assigned to the (1 1 1), (2 2 0) and (3 1 1) planes. Crystallite size and strain were calculated from the Williamson–Hall plot (Williamson and Hall, 1953) and the crystallite sizes are 2.88 nm in H1 sample, 3.10 nm in H2 and 2.87 nm in H3 sample. It is seen that there was not any remarkable change in crystallite size while increasing the reaction time but the compressive strain was seen to decrease with time, an indication of particle growth with time.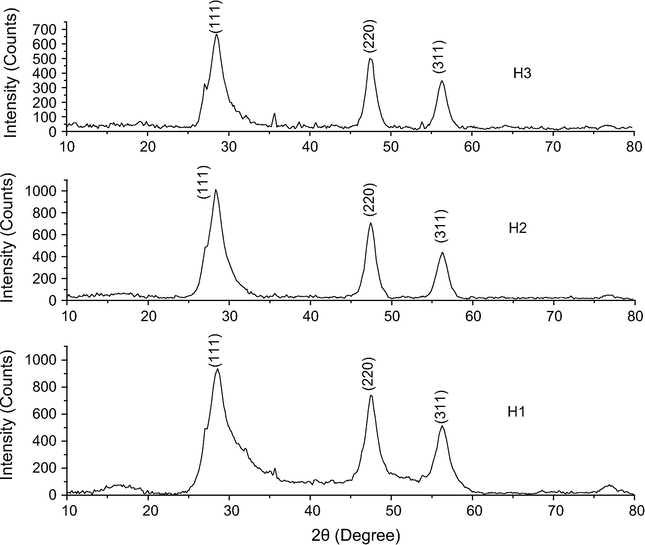
XRD of H1, H2 and H3 samples.
2θ (Degree)
hkl
Lattice constant (d) Å
Strain
Average crystallite size (D) nm
Lattice parameters (a = b = c) Å
Volume of the crystals (a3) × 10−28 m3
Observed
ASTM 77–2100
Observed
ASTM 77–2100
Observed
ASTM 77–2100
Observed
ASTM 77–2100
28.443
28.53
(1 1 1)
3.1355
3.12613
−0.0587
2.8796
5.42934
5.41461
1.60044
1.5875
47.318
47.454
(2 2 0)
1.9195
1.9144
56.157
56.307
(3 1 1)
1.6365
1.6326
2θ (Degree)
hkl
Lattice constant (d) Å
Strain
Average crystallite size (D) nm
Lattice parameters (a = b = c) Å
Volume of the crystals (a3) × 10−28 m3
Observed
ASTM 77–2100
Observed
ASTM 77–2100
Observed
ASTM 77–2100
Observed
ASTM 77–2100
28.198
28.53
(1 1 1)
3.16218
3.12613
−0.0631
3.1020
5.44474
5.41461
1.6141
1.5875
47.318
47.454
(2 2 0)
1.91955
1.91436
56.157
56.307
(3 1 1)
1.63657
1.63256
2θ (Degree)
hkl
Lattice constant (d) Å
Strain
Average crystallite size (D) nm
Lattice parameters (a = b = c) Å
Volume of the crystals (a3) × 10−28 m3
Observed
ASTM 77–2100
Observed
ASTM 77–2100
Observed
ASTM 77–2100
Observed
ASTM 77–2100
28.443
28.53
(1 1 1)
3.13549
3.12613
−0.0749
2.8747
5.42934
5.41461
1.60044
1.5875
47.318
47.454
(2 2 0)
1.91955
1.91436
56.157
56.307
(3 1 1)
1.63657
1.63256
The dried powder samples dispersed in ethanol for TEM analysis. The TEM, HRTEM and SAED images are shown in Figs. 3, 4 and in Fig. 5. It is obvious from the TEM images that the particles sizes are below 10 nm and are well dispersed in ethanol. SAED images show that the samples are polycrystalline and the diffraction rings are assigned to (1 1 1), (2 2 0) and (3 1 1) planes after comparing the interplanar spacing obtained from their X-ray diffractograms. Interplanar spacing calculated from SAED, HRTEM and XRD are compared in Table 5. Variations observed in interplanar spacing and particle sizes calculated from the TEM, SAED and XRD are due to the method of analyzing the samples, that the precipitate was heated to make it powder for XRD and the powder was dispersed in ethanol for TEM and SAED analyses. This work is intended to study the ZnS nanoparticle formation in ionic liquid so that the optical and other properties of the ZnS would not be studied here.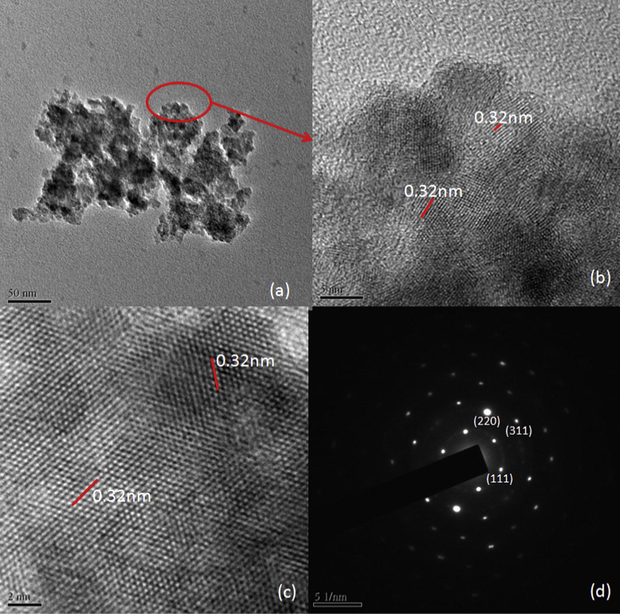
TEM, HRTEM and SAD images of H1 sample.
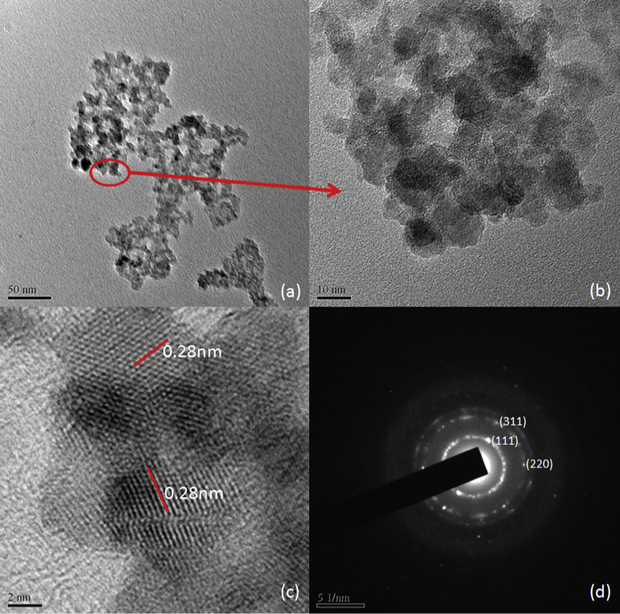
TEM, HRTEM and SAD images of H2 sample.
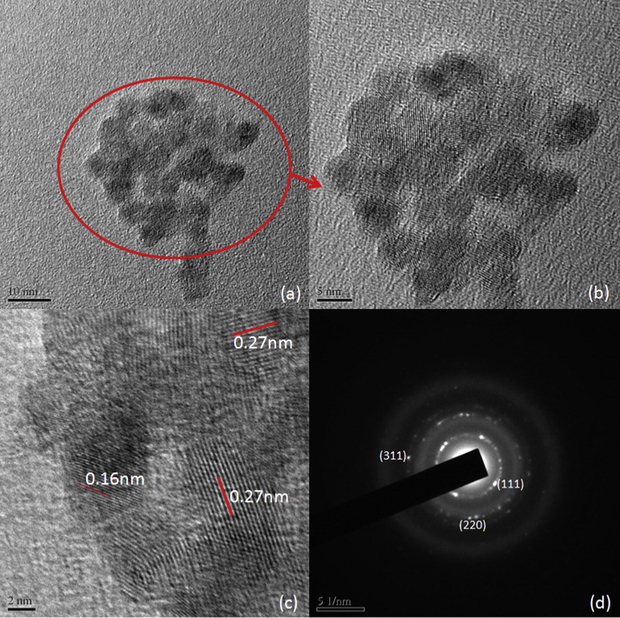
TEM, HRTEM and SAD images of H3 sample.
Sample
Crystallite size from XRD, nm
Particle size from TEM, nm
Interplanar spacing, “d” from HRTEM, nm
Interplanar spacing, “d” from SAED, nm
Interplanar spacing, “d” from XRD, nm
Plane
H1
5.15
6.81
0.32
0.32
0.3135
(1 1 1)
0.19
0.1919
(2 2 0)
–
0.16
0.1636
(3 1 1)
H2
6.33
8.01
0.28
0.31
0.3162
(1 1 1)
–
0.19
0.1919
(2 2 0)
–
0.16
0.1636
(3 1 1)
H3
6.62
6.27
0.27
0.29
0.3135
(1 1 1)
–
0.18
0.1919
(2 2 0)
0.16
0.16
0.1636
(3 1 1)
It is deduced from their X-ray diffractograms and from their TEM images that [BMIM]BF4 ionic liquid acts as a stabilizer (Biswas and Rao, 2007) and a capping agent that controls the agglomeration of the ZnS particles. Here the reaction of the Zn cations with the sulfur anions in [BMIM]BF4 ionic liquid gives rise to ZnS nanocrystal nucleus and grows to particle. Then these nanocrystals will be coated by the [BMIM]BF4 ionic liquid as soon as the ZnS nanocrystals nuclei form and this coating impedes further growth of the particles. The important fact is that the low interfacial tension of ionic liquids leads to high nucleation rates (Biswas and Rao, 2007), that enables the generation of small nanoparticles which undergo weak Ostwald ripening. The combined intrinsic high charge and the steric effects of this ionic liquid are considered to be responsible for creating an electrostatic and steric (colloid-type) stabilization of the ZnS nanoparticles.
It is also important to find the presence of the ionic liquids in the final product, so that the FTIR of the sample H3 was analyzed after dispersing the dried powder in ethanol and their absorption peaks are shown in Fig. 6 and compared with FTIR of the pure [BMIM]BF4 ionic liquid. Each of the absorption peaks is assigned to the respective vibrations as in Table 6. This analysis showed that the ionic liquid was disintegrated during the heating with Na2S and ZnCl2 and covered the ZnS nanoparticles through free S—H bonding that impeded the growth of the particles (Jeon et al., 2008; Yoonnam et al., 2008). It is obvious that the spectrum has the peaks of ethanol and parts of the ionic liquid having C-N stretching vibrations. This heating experiment shows that it is easy to prepare ZnS nanoparticles in [BMIM]BF4 ionic liquid, that does not need any sophisticated technology or any solvents or any stabilizing or capping agents other than [BMIM]BF4 ionic liquid.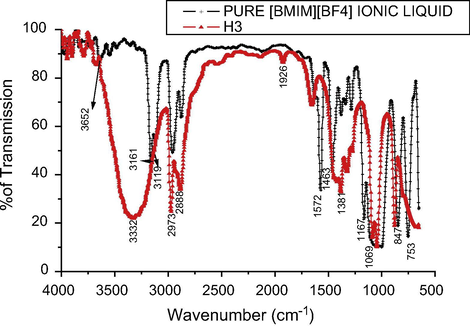
FTIR spectrum of H3 sample.
Peaks
Vibrations
Peaks
Vibrations
3652
Symmetric stretching of the O—H bonds in water
3332
OH stretching vibrations, Intermolecular H Bonds
3161
C—H stretching vibrations in hydrogen bonds formed between C4,5 – H and F− of the cations
2973
C—H asymmetrical stretching
3119
C—H stretching vibrations in hydrogen bonds formed between C2—H and F− of the cations
2888
C—H symmetrical stretching
2960
C—H asymmetric stretching of CH3
2549
SH Stretching vibrations, Free SH
2876
C—H symmetric stretching of CH3
1926
C⚌O Stretching Vibrations, Nonconjugated
1572
C—C stretching of the imidazole ring in BMIM BF4
1652
C⚌C Stretching Vibrations, Nonconjugated
1463
CH3 and CH2 asymmetric bending of alkyl substituent in imidazolium ionic liquids
1417
CH Bending Vibrations, CH2
1381
—C—H bending
1382
CH Bending Vibrations,CH3
1340
C—N stretching
1326
C—N stretch
1285
CH3 deformation
1276
C—O Asymmetrical stretching
1167
C—N stretching
1086
C—O Asymmetrical stretching
1069
C—H deformation in plane
1043
C—O stretching
847
C—H bending
879
CH out-of-plane bending vibrations, C⚌CH2
753
C—H plane bending
678
C—H bending
4 Conclusion
ZnS nanoparticles were prepared in [BMIM]BF4 ionic liquid by simple heating. The analysis shows that the particles are well separated and they are below 10 nm. Absence of the FTIR peaks corresponding to the pristine [BMIM]BF4 ionic liquid in the final product confirms the disintegration of the [BMIM]BF4 during the formation of ZnS. It is deduced that the ZnS nanoparticle growth was stabilized by the combined intrinsic high charge and the steric effects of ionic liquid.
References
- I–V characteristics and the synthesis of ZnS nanoparticles by glow discharge at the metal–ionic liquid interface. J. Plasma Phys.. 2012;78(02):189-197.
- [CrossRef] [Google Scholar]
- Use of ionic liquids in the synthesis of nanocrystals and nanorods of semiconducting metal chalcogenides. Chem. Eur. J.. 2007;13:6123-6129.
- [Google Scholar]
- John Coates, Interpretation of Infrared Spectra, A Practical Approach in Encyclopedia of Analytical Chemistry (2000). R.A. Meyers, (Ed.), John Wiley & Sons Ltd, Chichester, pp. 10815–10837.
- Frank Endres, Douglas MacFarlane and Andrew Abbott (2008) Electrodeposition from ionic liquids, Wiley-VCH Verlag Gmbh&Co.KgaA.
- Application of CBD zinc sulfide (ZnS) film to low cost antireflection coating on large area industrial silicon solar cell. Trans. Electrical Electronic Mater.. 2004;5:1.
- [Google Scholar]
- Structural change of 1-Butyl-3-methylimidazolium tetrafluoroborate + water mixtures studied by infrared vibrational spectroscopy. J. Phys. Chem. B. 2008;112:923-928.
- [Google Scholar]
- Efficient ZnS-like alkaline earth sulfide electroluminescence. J. Crystal Growth. 1990;101:999-1003.
- [Google Scholar]
- Excitation resolved color conversion of CdSe/ZnS core/shell quantum dot solids for hybrid white light emitting diodes. J. Appl. Phys.. 2009;105:083112.
- [Google Scholar]
- Fabrication of ZnS:Cu/PVA nanocomposite electroluminescence devices for flat panel displays. Adv. Mater. Lett.. 2013;4(2):169-173.
- [Google Scholar]
- Microwave assisted synthesis of ZnS quantum dots using ionic liquids. Mater. Lett.. 2012;89:316-319.
- [Google Scholar]
- Further investigation of the intermolecular interactions and component distributions in a [Bmim][BF4]-based polystyrene composite membranes using two-dimensional correlation infrared spectroscopy. Langmuir. 2010;26(13):11427-11434.
- [Google Scholar]
- Broadly tunable compact continuous-wave Cr2+:ZnS laser. Opt. Lett.. 2002;27(12):1040-1042.
- [Google Scholar]
- Sumitomo Electric Industries, Ltd, 2010: Environmentally-resistant ZnS lens for farinfrared cameras (new products and techniques). Sei. Tech. Rev. vol. 71, pp. 113–115.
- Why single-walled carbon nanotubes can be dispersed in imidazolium-based ionic liquids. ACS Nano. 2008;2(12):2540-2546.
- [CrossRef] [Google Scholar]
- Gas sensors, thermistor and photodetector based on ZnS nanowires. J. Mater. Chem.. 2012;22:6845-6850.
- [CrossRef] [Google Scholar]
- X-ray line broadening from filed aluminium and wolfram. Acta Metall.. 1953;1:22.
- [CrossRef] [Google Scholar]
- Structures of ionic liquids with different anions studied by infrared vibration spectroscopy. J. Phys. Chem. B. 2008;112:4735-4740.
- [Google Scholar]







