Translate this page into:
Montmorillonite K10 catalyzed one-pot synthesis of 2-aryl substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)aryl or alkylamide derivatives under ultrasound irradiation
⁎Corresponding authors. Tel.: +91 40 6657 1500. vbrmandava@yahoo.com (Mandava Venkata Basaveswara Rao), manojitpal@rediffmail.com (Manojit Pal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new and greener approach has been developed for the direct and one-pot synthesis of 2-aryl substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)aryl or alkylamide derivatives. The methodology involved reaction of isatoic anhydride, acid hydrazides and aldehydes in the presence of montmorillonite K10 in ethanol under ultrasound irradiation. A number of desired products were synthesized in good to acceptable yields by using this method. The methodology allowed quicker and cost-effective access to this class of compounds under relatively green reaction conditions. The recyclability of the catalyst was tested and a plausible reaction mechanism was proposed.
Keywords
Isatoic anhydride
Acid hydrazides
Aldehydes
Montmorillonite K10
Ultrasound
1 Introduction
Small organic molecules based on 2-aryl substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)aryl or alkylamide framework (A, Fig. 1) have been explored as nonpeptidic antagonists for FPRL1, a member of human formyl peptide receptor (FPR) family of chemoattractant receptors (Zhou et al., 2007; Nanamori et al., 2004). Indeed, the formyl peptide receptor-like 1(FPRL 1) is a G protein-coupled receptor that binds natural and synthetic peptides as well as lipoxin A4 and mediates important biological functions. Antagonists for FPRL1 therefore have obvious therapeutic value especially as potential anti-inflammatory agents. This class of compounds e.g. A-1 was synthesized via a multi-step method involving two key steps starting from anthranilic acid derivative B (Scheme 1) (Zhou et al., 2007; Mayer et al., 1997). Another two-step method has been developed via the reaction of acid hydrazides with isatoic anhydride C followed by treating the resulting compounds with aldehydes (Scheme 2) (Smirnov et al., 2003). A similar two-step strategy has been used to prepare this class of compounds by grinding anthranilhydrazides with aldehydes in the presence of citric acid and substantial amount of acidic Al2O3 at room temperature under solvent-free conditions. Simple workup, short reaction time, cost-effectiveness, and environmentally benign nature of this method have been claimed (Ding et al., 2012). In 1996, chemoselective lithiation of 3-(acylamino)-4(3H)-quinazolinones (by using LDA at −78 °C) at C-2 position was used to prepare a range of 2-substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)alkylamides (Smith et al., 1996). While notable advantages are associated with some of these methods most of them however either involved a multi-step sequence or cumbersome preparation of starting materials. Indeed, a one-pot method could be more convenient in accessing compounds based on the framework A.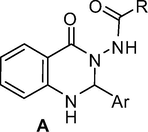
2-Aryl substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)aryl or alkylamide framework.
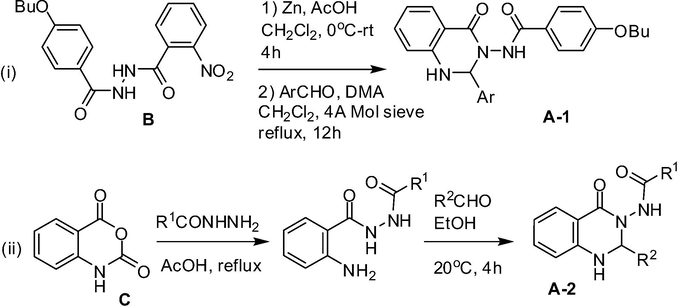
Previous synthesis of (i) compound A-1 and (ii) compound A-2 based on framework A.
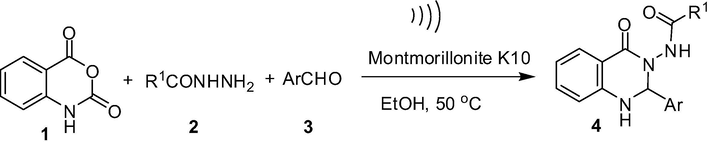
Ultrasound assisted synthesis of 2-aryl substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)aryl or alkylamide derivatives in the presence of montmorillonite K10.
Recently, montmorillonite K10 that belongs to the montmorillonite class of clay attracted particular attention due to its use as an inexpensive, non-corrosive and efficient acid catalyst in organic synthesis (Kaur and Kishore, 2012; Kumar et al., 2014; Cornélis et al., 2004). Indeed, reactions catalyzed by montmorillonite K10 generally involve mild reaction conditions, simple operational procedure and work-up, selectivity and high yields of products. Moreover, montmorillonite catalysts are easily recovered by simple filtration and reused. Thus montmorillonite catalyzed reactions are often termed as environmentally friendly methods. Ultrasound assisted reactions on the other hand are also considered as green techniques in organic and medicinal chemistry (Cella and Stefani, 2012). Indeed, accelerating reactions by using the ultrasound have been recognized as an important strategy for meeting the green chemistry goals of waste minimization and reduction of energy requirements (Pizzuti et al., 2012). The strategy has aided in developing new, energy saving, cost effective and environmentally safe methodologies for the synthesis of various organic molecules (Puri et al., 2013). Considering the impact and importance of montmorillonite catalysis and ultrasound assisted reactions it is not surprising that combination of both in a particular chemical transformation would remain unexplored (Bhor et al., 2008; Li et al., 2004; Safari and Javadian, 2013). However, not much effort has been devoted to this area. Promoted by these observations we now wish to report a one-pot synthesis of compound 4 based on A via the reaction of isatoic anhydride 1, acid hydrazides 2 and aldehydes 3 in the presence of montmorillonite K10 in ethanol under ultrasound irradiation (Scheme 2). While this methodology allowed quicker and cost-effective access to 4 under green reaction conditions the present ultrasound based strategy leading to 4 is not known in the literature.
2 Material and methods
2.1 General methods
Unless stated otherwise, reactions were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254), visualizing with ultraviolet light or iodine spray. Column chromatography was performed on silica gel (60–120 mesh) using distilled petroleum ether and ethyl acetate. 1H and 13C NMR spectra were determined in DMSO-d6 solution using a Varian 400 MHz spectrometer. Proton chemical shifts (δ) are relative to tetramethylsilane (TMS, δ = 0.0) as internal standard and expressed in parts per million. Spin multiplicities are given as s (singlet), d (doublet), t (triplet), and m (multiplet) as well as b (broad). Coupling constants (J) are given in hertz. Infrared spectra were recorded on a JASCO FTIR-4200 spectrometer (Switzerland). Melting points were determined by using a Buchi melting point B-540 apparatus. MS spectra were obtained on a HP-5989A mass spectrometer. Microanalyses were performed using Perkin–Elmer 2400 CHNS/O analyzer.
2.2 General procedure for the preparation of compound 4
A mixture of isatoic anhydride 1 (1.0 mmol), acid hydrazide 2 (1.0 mmol), arylaldehyde 3 (1.0 mmol) and catalytic amount of montmorillonite K10 (5% w/w) in ethanol (5 mL) was heated at 45–50 °C under ultrasound irradiation using a laboratory ultrasonic bath SONOREX SUPER RK 510H model producing irradiation of 35 kHz. The temperature of the bath was maintained by adding cold water from time to time in case an increase in temperature was observed due to the prolonged irradiation. The reaction continued according to the time mentioned in Table 2 (initially effervescence was observed due to the generation of CO2 gas). After completion of the reaction the mixture was cooled and diluted with ethanol (10 mL). The catalyst was filtered off. The filtrate was collected and concentrated. The crude product was recrystallized in hot ethanol, filtered and dried to give the desired product in analytically pure form.
2.3 Recovery of the catalyst
The filtered catalyst was washed with EtOH, cold water followed by ether and then dried under vacuum. This recovered catalyst can be used for the next reaction.
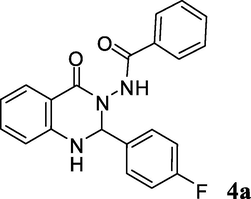
2.3.1 N-(2-(4-fluorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)benzamide (4a)
Pale yellow solid; mp: 240–242 °C; 1H NMR (400 MHz, DMSO-d6): δ 6.20 (s, CH, 1H), 6.83 (m, NH & CH aromatic, 2H), 6.80–7.62 (m, CH aromatic, 12H), 10.44 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 73.8, 113.7, 114.6, 115.0 (d, C-F J = 21 Hz), 117.1, 117.9, 127.3, 128.2 (d, C-F J = 32 Hz), 130.3, 130.4, 131.9, 132.2, 134.0, 147.8, 147.9, 162.5 (d, C-F J = 243 Hz), 163.1, 165.5; IR (KBr, cm−1) 3301 (NH), 3240 (NH), 1686 (C⚌O), 1648 (C⚌O), 1614; MS (ESI, m/z) 361 (M+, 5), 241 (100); found C, 69.43; H, 4.41; N, 11.90; C21H16FN3O2 requires C, 69.80; H, 4.46; N, 11.63%.
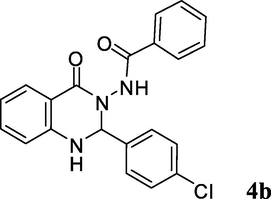
2.3.2 N-(2-(4-chlorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)benzamide (4b)
Ash color solid; mp: 264–266 °C; 1H NMR (400 MHz, DMSO-d6): δ 6.18 (s, CH, 1H), 6.79–6.83 (m, CH aromatic, 2H), 7.34–7.57 (m, CH aromatic + NH, 9H), 7.62 (d, J = 7.2 Hz, CH aromatic, 2H), 7.72 (d, J = 7.6 Hz, CH aromatic, 1H), 10.46 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 73.8, 113.6, 114.6, 117.9, 127.4, 128.0, 128.2, 128.4, 129.9, 131.9, 132.0, 133.7, 134.1, 137.1, 147.7, 162.9, 165.5; IR (KBr, cm−1) 3325 (NH), 3250 (NH), 1685 (C⚌O), 1661 (C⚌O), 1614; MS (ESI, m/z) 378 (M+, 5), 257 (100); found C, 66.45; H, 4.20; N, 11.33; C21H16ClN3O2 requires C, 66.76; H, 4.27; N, 11.12%.
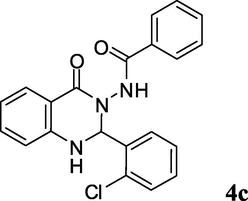
2.3.3 N-(2-(2-chlorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)benzamide (4c)
Off white solid; mp: 219–221 °C; 1H NMR (400 MHz, DMSO-d6): δ 6.66 (s, CH, 1H), 6.79–6.81 (m, CH aromatic, 2H), 7.39–7.42 (m, CH aromatic + NH, 10H), 7.44 (d, J = 7.6 Hz, CH aromatic, 1H), 7.65 (d, J = 7.2 Hz, 1H), 10.50 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 70.6, 113.2, 114.4, 117.8, 127.4, 127.9, 128.3, 129.3, 129.9, 130.6, 131.9, 132.2, 132.9, 134.2, 135.7, 135.8, 147.4, 162.8, 165.6; IR (KBr, cm−1) 3293 (NH), 3195 (NH), 1681 (C⚌O), 1663 (C⚌O), 1615; MS (ESI, m/z) 378 (M+, 10), 257 (100); found C, 66.91; H, 4.26; N, 11.01; C21H16ClN3O2 requires C, 66.76; H, 4.27; N, 11.12%.
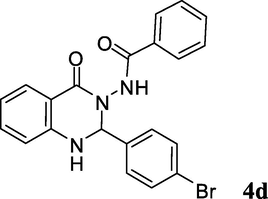
2.3.4 N-(2-(4-bromophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)benzamide (4d)
Brownish white solid; mp: 296–298 °C; 1H NMR (400 MHz, DMSO-d6): δ 6.13 (s, CH, 1H), 6.85 (bs, NH, 1H), 7.35–7.72 (m, CH aromatic, 13H), 10.43 (s, NH); 13C NMR (100 MHz, DMSO-d6): δ 74.3, 114.1, 115.8, 117.7, 119.1, 122.8, 127.8, 128.6, 129.6, 131.3, 132.6, 133.6, 138.0, 148.2, 158.8, 163.4, 166.0; IR (KBr, cm−1) 3312 (NH), 3243 (NH), 1686 (C⚌O), 1649 (C⚌O), 1614; MS (ESI, m/z) 422 (M+, 10), 264 (100); found C, 59.50; H, 3.81; N, 10.10; C21H16BrN3O2 requires C, 59.73; H, 3.82; N, 9.95%.
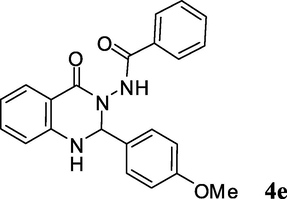
2.3.5 N-(1,2-dihydro-2-(4-methoxyphenyl)-4-oxoquinazolin-3(4H)-yl)benzamide (4e)
Pale yellow solid; mp: 220–222 °C; 1H NMR (400 MHz, DMSO-d6): δ (ppm) 3.74 (s, OCH3, 3H), 6.14 (s, CH, 1H), 6.84 (d, J = 8.4 Hz, CH aromatic, 2H), 6.94 (d, J = 8.8 Hz, CH aromatic, 2H), 7.29 (s, NH, 1H), 7.42–7.48 (m, CH aromatic, 8H), 7.61 (d, J = 6.3 Hz, CH aromatic, 1H), 10.39 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 55.2, 74.0, 113.5, 113.7, 114.6, 117.7, 127.4, 127.7, 128.0, 128.3, 129.5, 129.8, 131.8, 133.9, 148.0, 159.9, 163.3, 165.5; IR (KBr, cm−1) 3391 (NH), 3310 (NH), 1684 (C⚌O), 1651 (C⚌O), 1610; MS (ESI, m/z) 373 (M+, 5), 371, 253 (100); found C, 70.56; H, 5.11; N, 11.30; C22H19N3O3 requires C, 70.76; H, 5.13; N, 11.25%.
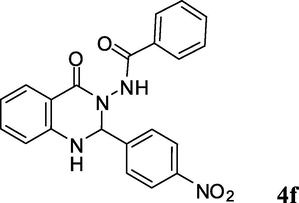
2.3.6 N-(1,2-dihydro-2-(4-nitrophenyl)-4-oxoquinazolin-3(4H)-yl)benzamide (4f)
Off white solid; mp: 258–260 °C; 1H NMR (400 MHz, DMSO-d6): δ 6.32 (s, CH, 1H), 6.81–6.84 (m, CH aromatic, 2H), 7.36–7.66 (m, CH aromatic, 8H), 7.83 (d, J = 8.4 Hz, CH aromatic, 2H), 8.27 (d, J = 8.8 Hz, CH aromatic, 2H), 10.56 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 73.4, 114.6, 114.7, 118.1, 123.3, 127.4, 127.9, 128.4, 129.4, 132.0, 134.2, 134.3, 135.3, 145.5, 147.3, 154.6, 163.7; IR (KBr, cm−1) 3323 (NH), 3272 (NH), 1682 (C⚌O), 1651 (C⚌O), 1614; MS (ESI, m/z) 388 (M+, 10), 268 (100); found C, 64.71; H, 4.14; N, 14.60; C21H16N4O4 requires C, 64.94; H, 4.15; N, 14.43%.
2.3.7 2-Ethoxy-N-(4-oxo-2-phenyl-1,2-dihydroquinazolin-3(4H)-yl)benzamide (4g)
Ash colored solid; 191–193 °C [192–194 °C (Ding et al., 2012)]; 1H NMR (400 MHz, DMSO-d6): δ = 1.14 (t, J = 6.9 Hz, 3H), 4.01 (q, J = 6.9 Hz, 2H), 6.18 (s, 1H), 6.76–6.81 (m, 2H), 6.98–7.07 (m, 2H), 7.32–7.59 (m, 9H), 7.72–7.73 (m, 1H), 9.78 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 14.1, 64.2, 74.2, 113.1, 113.3, 114.4, 117.5, 120.4, 121.1, 127.5, 128.0, 128.3, 129.0, 130.4, 132.8, 134.0, 138.5, 147.4, 156.2, 162.6, 164.1; IR (KBr, cm−1): 3305 (NH), 3242 (NH), 1709 (C⚌O), 1677 (C⚌O); MS (ESI, m/z) 387 (M+, 100).
2.3.8 2-Ethoxy-N-(4-oxo-2-p-tolyl-1,2-dihydroquinazolin-3(4H)-yl)benzamide (4h)
Pale yellow solid; 203–205 °C [209–211 °C (Ding et al., 2012)]; 1H NMR (400 MHz, DMSO-d6): δ = 1.14 (t, J = 6.8 Hz, 3H), 2.27 (s, 3H), 4.0 (q, J = 6.8 Hz, 2H), 6.13 (s, 1H), 6.74–6.79 (m, 2H), 6.98–7.07 (m, 2H), 7.18–7.20 (m, 2H), 7.30–7.45 (m, 5H), 7.59–7.71 (m, 2H), 9.71 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 14.0, 20.6, 64.3, 74.0, 113.1, 113.3, 114.4, 117.5, 120.4, 121.1, 127.4, 127.7, 128.8, 130.4, 132.8, 134.0, 135.5, 138.4, 147.5, 156.2, 162.6, 164.1; IR (KBr, cm−1): 3288 (NH), 3240 (NH), 1681 (C⚌O), 1643 (C⚌O); MS (ESI, m/z) 401 (M+, 100).
2.3.9 N-(2-(2-bromophenyl)-4-oxo-1,2-dihydroquinazolin-3(4H)-yl)-2-ethoxybenzamide (4i)
Off white solid; 193–195 °C [195–197 °C (Ding et al., 2012)]; 1H NMR (400 MHz, DMSO-d6): δ = 1.09 (t, J = 6.8 Hz, 3H), 4.00 (q, J = 6.8 Hz, 2H), 6.61 (s, 1H), 6.76–6.79 (m, 2H), 6.98–7.07 (m, 2H), 7.30–7.47 (m, 5H), 7.62–7.74 (m, 4H), 9.75 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 14.1, 64.3, 73.4, 113.0, 113.1, 114.4, 117.7, 120.5, 120.7, 122.9, 128.0, 128.1, 129.5, 130.6, 131.0, 132.8, 133.0, 134.1, 137.3, 147.0, 156.3, 162.5, 164.2; IR (KBr, cm−1): 3290 (NH), 3243 (NH), 1675 (C⚌O), 1652 (C⚌O); MS (ESI, m/z) 467 ([M+2]+, 95), 465 (M+, 100).
2.3.10 2-Ethoxy-N-(4-(nitrophenyl)-4-oxo-1,2-dihydroquinazolin-3(4H)-yl)benzamide (4j)
Light brown solid; 206–208 °C [209–211 °C (Ding et al., 2012)]; 1H NMR (400 MHz, DMSO-d6): δ 1.08 (t, J = 6.8 Hz, 3H), 3.97 (q, J = 6.8 Hz, 2H), 6.34 (s, 1H), 6.77–6.82 (m, 2H), 6.96–7.05 (m, 2H), 7.33–7.57 (m, 4H), 7.71–7.82 (m, 3H), 8.25–8.28 (m, 2H), 9.90 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 14.1, 64.1, 73.4, 113.0, 113.3, 114.5, 118.1, 120.4, 121.4, 123.4, 128.0, 129.2, 130.3, 132.8, 134.2, 145.7, 147.0, 148.0, 156.1, 162.3, 164.7; IR (KBr, cm−1): 3274 (NH), 3240 (NH), 1677 (C⚌O), 1651 (C⚌O), 1609; MS (ESI, m/z) 432 (M+, 100).
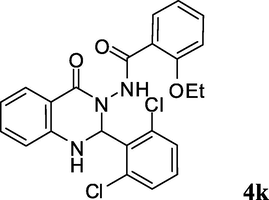
2.3.11 N-(2-(2,6-dichlorophenyl)-4-oxo-1,2-dihydroquinazolin-3(4H)-yl)-2-ethoxybenzamide (4k)
Off white solid; 220–222 °C [223–225 °C (Ding et al., 2012)]; 1H NMR (400 MHz, DMSO-d6): δ = 1.15 (t, J = 6.6 Hz, 3H), 4.01 (q, J = 6.6 Hz, 2H), 6.66–6.72 (m, 2H), 7.01–7.07 (m, 2H), 7.21 (s, 1H), 7.29–7.32 (m, 1H), 7.40–7.51 (m, 5H), 7.67–7.70 (m, 2H), 9.66 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 14.1, 64.3, 71.0, 111.7, 113.1, 113.6, 116.8, 120.3, 120.5, 127.7, 130.7, 131.1, 132.0, 133.2, 134.0, 135.7, 147.2, 156.4, 161.6, 164.8; IR (KBr): 3319 (NH), 3241 (NH), 1665 (C⚌O), 1645 (C⚌O), 1614 cm–1; MS (ESI, m/z) 459 ([M+4]+, 11), 457 ([M+2]+, 63), 455 (M+, 100).
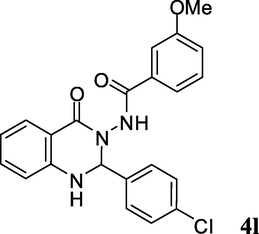
2.3.12 N-(2-(4-chlorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)-3-methoxybenzamide (4l)
Off white solid; mp: 195–197 °C; 1H NMR (400 MHz, DMSO-d6): δ 3.75 (s, OCH3, 3H), 4.65 (bs, NH, 1H), 6.38 (s, CH, 1H), 6.70 (d, J = 8 Hz, CH aromatic, 1H), 6.92–6.98 (m, CH aromatic, 2H), 7.06–7.17 (m, CH aromatic, 3H), 7.28–7.32 (m, CH aromatic, 2H), 7.37–7.39 (m, CH aromatic, 1H), 7.41–7.51 (m, CH aromatic, 2H), 7.94 (d, J = 8 Hz, CH aromatic, 1H), 8.97 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 55.3, 74.4, 111.8, 114.7, 114.7, 118.9, 119.3, 119.8, 129.1, 129.1, 129.2, 129.3, 129.5, 131.5, 132.6, 134.6, 135.7, 135.9, 146.4, 159.5, 164.5, 166.4; IR (KBr, cm−1) 3415 (NH), 3197 (NH), 1660 (C⚌O), 1652 (C⚌O); MS (ESI, m/z) 408 (M+, 10), 257 (100); found C, 64.93; H, 4.41; N, 10.14; C22H18ClN3O3 requires C, 64.79; H, 4.45; N, 10.30%.
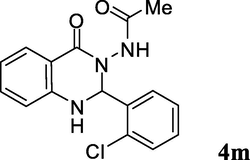
2.3.13 N-(2-(2-chlorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)acetamide (4m)
Pale yellow solid; mp: 265–267 °C; 1H NMR (400 MHz, DMSO-d6): δ 1.75 (s, CH3, 3H), 6.45 (s, CH, 1H), 6.73–6.77 (m, CH aromatic, 2H), 7.30–7.66 (m, CH aromatic + NH, 6H), 7.69 (d, J = 8.0 Hz, CH aromatic, 1H), 9.92 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 20.3, 70.5, 112.9, 114.3, 114.4, 117.6, 127.6, 127.9, 129.2, 129.6, 130.6, 134.1, 136.3, 146.9, 162.3, 168.3; IR (KBr, cm−1) 3213 (NH), 3178 (NH), 1690 (C⚌O), 1635 (C⚌O), 1607; MS (ESI, m/z) 316 (M+, 10), 257 (100); found C, 60.53; H, 4.46; N, 13.20; C16H14ClN3O2 requires C, 60.86; H, 4.47; N, 13.31%.
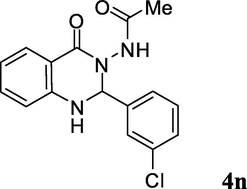
2.3.14 N-(2-(3-chlorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)acetamide (4n)
Ash color solid; mp: 198–200 °C; 1H NMR (400 MHz, DMSO-d6): δ 1.75 (s, CH3, 3H), 6.02 (s, CH, 1H), 6.76–6.78 (m, CH aromatic, 2H), 7.30–7.49 (m, CH aromatic + NH, 5H), 7.52 (s, CH aromatic, 1H), 7.68–7.70 (m, CH aromatic, 1H), 9.97 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 20.2, 73.3, 113.4, 114.5, 117.8, 126.2, 127.2, 127.9, 128.9, 130.3, 133.0, 134.0, 141.3, 147.1, 162.3, 168.4; IR (KBr, cm−1) 3307 (NH), 3241 (NH), 1704 (C⚌O), 1637 (C⚌O), 1615; MS (ESI, m/z) 316 (M+, 5), 257 (100); found C, 60.53; H, 4.50; N, 13.25; C16H14ClN3O2 requires C, 60.86; H, 4.47; N, 13.31%.
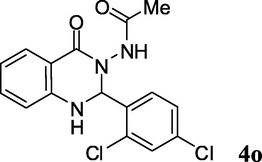
2.3.15 N-(2-(2,4-dichlorophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)acetamide (4o)
Pale yellow solid; mp: 234–236 °C; 1H NMR (400 MHz, DMSO-d6): δ 1.77 (s, CH3, 3H), 6.42 (s, CH, 1H), 6.71–6.78 (m, CH aromatic, 2H), 7.31–7.35 (m, CH aromatic + NH, 2H), 7.51–7.54 (m, CH aromatic, 1H), 7.66–7.69 (m, CH aromatic, 3H), 9.91 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 20.8, 70.9, 113.4, 115.5, 117.6, 118.9, 129.1, 130.2, 131.9, 134.2, 134.7, 135.9, 135.9, 147.3, 162.6, 168.8; IR (KBr, cm−1) 3258 (NH), 3242 (NH), 1701 (C⚌O), 1642 (C⚌O), 1611; MS (ESI, m/z) 350 (M+, 15), 291 (100).
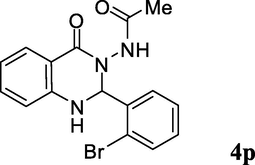
2.3.16 N-(2-(2-bromophenyl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)acetamide (4p)
Pale yellow solid; mp: 270–272 °C; 1H NMR (400 MHz, DMSO-d6): δ 1.76 (s, CH3, 3H), 6.42 (s, CH, 1H), 6.73–6.77 (m, CH aromatic + NH, 2H), 7.29–7.36 (m, CH aromatic, 3H), 7.44–7.47 (m, CH aromatic, 1H), 7.64–7.70 (m, CH aromatic, 3H), 9.90 (s, NH, 1H); 13C NMR (100 MHz, DMSO-d6): δ 20.3, 73.1, 112.9, 114.3, 117.6, 122.9, 127.9, 128.2, 129.4, 130.9, 132.8, 134.1, 137.9, 146.9, 162.2, 168.3; IR (KBr, cm−1) 3335 (NH), 3230 (NH), 1698 (C⚌O), 1637 (C⚌O), 1612; MS (ESI, m/z) 360 (M+, 10%), 301 (100); found C, 53.43; H, 3.91; N, 11.50; C16H14BrN3O2 requires C, 53.35; H, 3.92; N, 11.67%.
3 Results and discussion
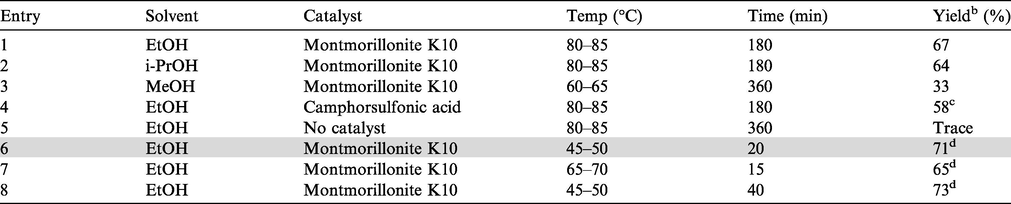
Our initial focus was to establish the optimal reaction conditions for the synthesis of compound 4. Accordingly, the one-pot reaction of isatoic anhydride 1 with benzohydrazide 2a and 4-fluorobenzaldehyde 3a was chosen as a model reaction that was run under various conditions (Table 1). The reaction afforded desired product 4a in moderate yield when performed in EtOH at refluxing temperature (entry 1, Table 1). While similar result was obtained when i-PrOH was used as a solvent (entry 2, Table 1), the yield of 4a was decreased when MeOH was used (entry 3, Table 1). Change of catalyst i.e. the use of camphorsulfonic acid in place of montmorillonite K10 did not afford 4a in better yield (entry 4, Table 1). The reaction did not proceed well in the absence of catalyst indicating key role played by montmorillonite K10 in the present one-pot reaction (entry 5, Table 1). Nevertheless, though we were satisfied by identifying montmorillonite K10 as an effective catalyst for the synthesis of 4a, the duration of the reaction appeared to be lengthy. A quicker access to 4a was more desirable. We therefore focused on the use of ultrasound for accelerating the montmorillonite K10 catalyzed reaction of 1 with 2a and 3a using a laboratory ultrasonic bath SONOREX SUPER RK 510H model producing irradiation of 35 kHz. To our delight the ultrasound assisted reaction was completed within 30 min without affecting the product yield (entry 6, Table 1). To improve the yield of 4a further the reaction was performed at a higher temperature (entry 7, Table 1). However, no improvement was observed except the reaction was completed within 15 min. Increase of reaction time was also proved to be not beneficial though very marginal improvement in yield was observed (entry 8, Table 1). We also performed the reaction using various quantities of catalyst e.g. 1, 3, 5 and 7% w/w of montmorillonite K10 under the conditions of entry 6 of Table 1 when the product 4a was isolated in 33%, 59%, 71%, 70% yield respectively. The product 4a was not formed when the reaction was performed in the absence of catalyst under ultrasound. Thus the condition of entry 6 of Table 1 appeared as the best among all other conditions studied and was used for further studies. In a separate study, the recyclability of the catalyst was examined. Thus montmorillonite K10 recovered from the reaction of entry 6 of Table 1 by simple filtration was reused and the reaction afforded 4a in 69%, 65%, and 64% yield after 2nd, 3rd, and 4th run confirming the recyclability of the catalyst without affecting the yield significantly. bIsolated yield. cCamphorsulfonic acid (4.5 mol%) was used in place of montmorillonite K10. dThe reaction was performed under ultrasound irradiation.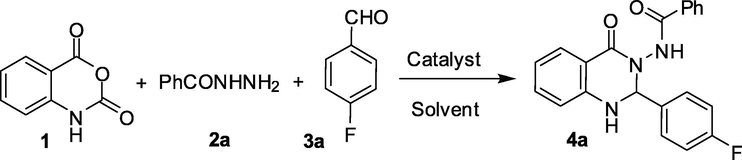
To expand the generality and scope of the present montmorillonite K10 catalyzed reaction assisted by ultrasound a number of acid hydrazides 2 and a variety of arylaldehydes 3 were employed along with the isatoic anhydride 1 (Table 2). Indeed, substituent like F (3a), Cl (3b, 3c, 3k) or Br (3d, 3i) or an electron donating group like OMe (3e) or Me (3h) or electron withdrawing group like NO2 (3f) present on the aromatic ring of aldehydes was used. A di-substitution on the aromatic ring e.g. 3j, 3l was also used. Both benzohydrazides e.g. 2a–c and acetohydrazide 2d were used as acid hydrazides in these reactions. The reaction progressed well in all these cases affording acceptable to good yields of desired products. All the compounds were characterized by spectral data. For example, both the amide carbonyl groups of compounds 4a–l appeared near 166 and 164 ppm in 13C NMR spectra and in the range 1680–1660 and 1660–1650 cm−1 in IR spectra. These groups appeared near 168 and 162 ppm in 13C NMR spectra and near 1700 and 1640 cm−1 in the IR spectra in case of compounds 4m-p. The proton at C-2 of the quinazoline ring appeared as a singlet in the region δ 6.6–6.1 in the 1H NMR spectra of 4. For compounds 4m–p the —COCH3 protons appeared near δ 1.7 in their 1H NMR spectra. All these observations especially appearance of signals for two amide carbonyl groups in both IR and NMR spectra suggest that the compound 4 perhaps exists predominantly in tautomeric form 4-I rather than 4-II (Scheme 3).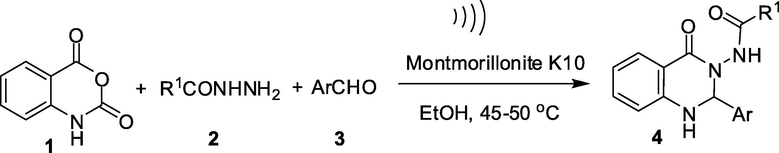
Entry
2; R1⚌
3; Ar⚌
Products (4)
Time (min)
Yieldb (%)
1
2a; Ph
3a; C6H4F-p
20
71
2
2a
3b; C6H4Cl-p
15
83
3
2a
3c; C6H4Cl-o
20
72
4
2a
3d; C6H4Br-p
15
85
5
2a
3e; C6H4OMe-p
15
79
6
2a
3f; C6H4NO2-p
20
77
7
2b; C6H4OEt-o
3g; Ph
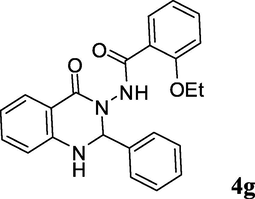
15
92
8
2b
3h; C6H4Me-p
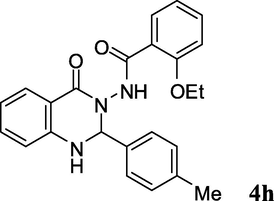
15
93
9
2b
3i; C6H4Br-o
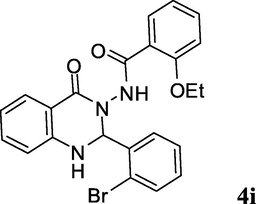
25
88
10
2b
3f
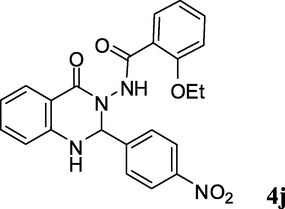
20
90
11
2b
3j; C6H3Cl2-(o,o)
30
81
12
2c; C6H4OMe-m
3b
15
84
13
2d; Me
3c
20
73
14
2d
3k; C6H4Cl-m
15
63
15
2d
3l; C6H3Cl2-(o,p)
20
80
16
2d
3i
20
85
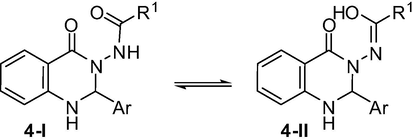
Tautomeric forms of compound 4.
A proposed reaction mechanism for the formation of compound 4 via the one-pot reaction of 1, 2 and 3 on the surface of montmorillonite K10 catalyst is presented in Scheme 4. The reaction seemed to proceed via activation of isatoic anhydride 1 on the lewis acidic surface of montmorillonite clay that facilitated the nucleophilic attack by 2 leading to the intermediate E-3 with the release of CO2. A similar activation of aldehyde 3 on the surface of clay allowed E-3 to react with 3 through its —NH2 moiety. The corresponding imine intermediate thus formed then undergoes intramolecular cyclization (aided by the acidic surface of montmorillonite clay) to give the product 4. While it was evident from Table 1 that the overall reaction rate was accelerated when the reaction was performed under ultrasound irradiation it was however not clear whether all or any particular step was facilitated by the ultrasound. The ultrasound might have played a role in the (i) reaction of 2 with E-1, (ii) initiation of imine formation and (iii) intramolecular cyclization step. It is known that cavitation is the phenomenon responsible for the favorable effects of ultrasound on chemical reactions (Suslick and Flannigan, 2008; Mason, 1997; Luche, 1998). The propagation of ultrasonic waves takes place via alternating compressions and rarefactions induced in the transmitting medium through which they pass. As a result bubbles are generated that subsequently collapse (rapid and violent implosions) in the compression cycle leading to the generation of short-lived regions with local temperatures ∼5000 °C, pressures ∼1000 atm and heating and cooling rates that can exceed 10 billion °C per second. Thus the mechanical energy of sound is transformed into a useful chemical form in these regions. Additionally, the violent collapse can also produce mechanical effects. Overall, the reaction rate acceleration by ultrasound in the present case can be explained by these effects. Nevertheless, to gain further evidence on the intermediacy of E-4, the compound 2-amino-N′-(2-ethoxybenzoyl)benzohydrazide 5 was prepared according to the known procedure (Smirnov et al., 2003; Ding et al., 2012) and was treated with benzaldehyde 3g in the presence of montmorillonite K10 (5% w/w) in EtOH at 45–50 °C under ultrasound irradiation. The reaction was completed within 10 min affording the desired product 4g in 91% yield (Scheme 5) indicating that the reaction proceeded via the intermediate E-4. The formation of compound 5 was also observed when the reactant 1 was treated with 2b under the same reaction conditions [i.e. in the presence of montmorillonite K10 (5% w/w) in EtOH at 45–50 °C under ultrasound irradiation] indicating the intermediacy of E-3 in the present reaction.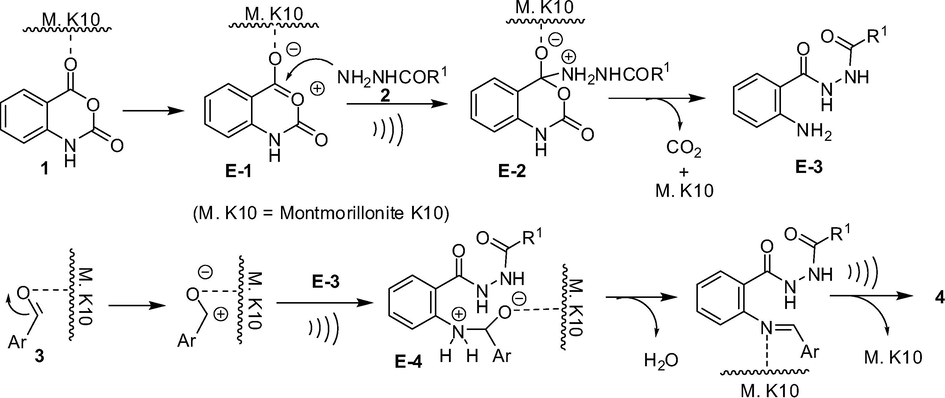
Proposed reaction mechanism for the formation of compound 4.
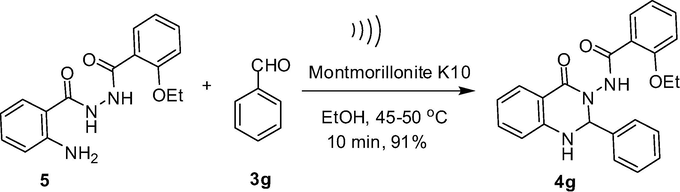
The reaction of compound 5 with aldehyde 3g.
4 Conclusion
In conclusion, we have developed a new and greener approach for the direct and one-pot synthesis of 2-aryl substituted N-(4-oxo-1,2-dihydroquinazolin-3(4H)-yl)aryl or alkylamide derivatives from readily available starting materials. The methodology involved reaction of isatoic anhydride, acid hydrazides and aldehydes in the presence of montmorillonite K10 in ethanol under ultrasound irradiation. A number of acid hydrazides and aldehydes were employed to afford a range of desired products in good to acceptable yields. The methodology allowed quicker and cost-effective access to this class of compounds under relatively green reaction conditions. The recyclability of the catalyst was tested and confirmed. A proposed reaction mechanism showing the role of clay catalyst and ultrasound was presented. Overall, the present methodology may find usage in the preparation of compounds of potential medicinal value.
Acknowledgments
The authors thank the management of Dr. Reddy’s Institute of Life Sciences, Hyderabad, India, for continuous support and encouragement.
References
- Ultrasound promoted selective synthesis of 1,1′-binaphthyls catalyzed by Fe impregnated pillared Montmorillonite K10 in presence of TBHP as an oxidant. Ultrason. Sonochem.. 2008;15:195-202.
- [Google Scholar]
- Montmorillonite K10. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2004
- [CrossRef] [Google Scholar]
- Ultrasonic reactions. In: Zhang W., Cue B.W., eds. Green Techniques for Organic Synthesis and Medicinal Chemistry. Chichester, UK: John Wiley & Sons, Ltd; 2012.
- [CrossRef] [Google Scholar]
- Tandem synthesis of 2,3-dihydroquinazolin-4(1H)-ones on grinding under solvent-free conditions. J. Heteroc. Chem.. 2012;49:375-380.
- [Google Scholar]
- Montmorillonite: an efficient, heterogeneous and green catalyst for organic synthesis. J. Chem. Pharm. Res.. 2012;4:991-1015.
- [Google Scholar]
- K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol.. 2014;4:2378-2396.
- [Google Scholar]
- An efficient and environmentally friendly method for synthesis of arylmethylenemalononitrile catalyzed by Montmorillonite K10–ZnCl2 under ultrasound irradiation. J. Chem. Technol. Biotechnol.. 2004;79:1275-1278.
- [CrossRef] [Google Scholar]
- Synthetic Organic Sonochemistry. New York: Plenum Press; 1998.
- A novel nonpeptide ligand for formyl peptide receptor-like 1. Mol. Pharmacol.. 2004;66:1213-1222.
- [Google Scholar]
- Pizzuti, L., Franco, M.S.F., Flores, A.F.C., Quina, F.H., Pereira, C.M.P., 2012. Recent Advances in the Ultrasound-Assisted Synthesis of Azoles, Green Chemistry – Environmentally Benign Approaches, Dr. Mazaahir Kidwai (Ed.), ISBN: 978-953-51-0334-9.
- Applications of ultrasound in organic synthesis – a green approach. Curr. Org. Chem.. 2013;17:1790-1828.
- [Google Scholar]
- Lithiation of 3-(acylamino)-2-unsubstituted-, 3-(acylamino)-2-ethyl-, and 3-(acylamino)-2-propyl-4(3H)-quinazolinones: convenient syntheses of more complex quinazolinones. J. Org. Chem.. 1996;61:647-655.
- [Google Scholar]
- Montmorillonite K-10 as a catalyst in the synthesis of 5,5-disubstituted hydantoins under ultrasound irradiation. J. Chem. Sci.. 2013;125:981-987.
- [Google Scholar]
- Reactions of N″-acyl-and N″-tosyl-substituted hydrazides of 2-aminobenzoic acid with carbonyl compounds. Russ. Chem. Bull. Intl. Ed.. 2003;52:2444-2453.
- [Google Scholar]
- Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem.. 2008;59:659-683.
- [Google Scholar]
- Pharmacological characterization of a novel nonpeptide antagonist for formyl peptide receptor-like 1. Mol. Pharmacol.. 2007;72:976-983.
- [Google Scholar]







