Translate this page into:
Trace analysis of amines in cheese serum with liquid chromatographic potentiometric detection by using amine-selective electrode
⁎Corresponding author. Fax: +90 212 3834625. isildak@yildiz.edu.tr (Ibrahim Isildak)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A trace quantification liquid chromatographic-potentiometric method for the determination of amines in cheese serum is described. An all solid-state contact polyvinyl chloride membrane amine-selective electrode was prepared and used as a detector in a low-dead volume flow through detection cell. Potentiometric performance characteristics of the proposed electrodes were then examined. A slope of 52.4±3.8 mV per decade toward amines was received at the concentration range of 1.0 × 10−2–8.0 × 10−6 M (r2 = 0.99) under dynamic conditions. Six amine compounds were separated, with a good resolution in the chromatographic system by using acetonitrile-methane sulfonic acid mobile phase gradient at the flow-rate of 1 mL min−1 in 30 min. The potentiometric detector was successfully applied to the analysis of amines in cheese serum samples. The relative standard deviations were ranged from 2.2% to 4.0% and recoveries were ranged from 88% to 101% for the determination. The average concentrations of ethylamine, butylamine and hexylamine in cheese serum samples calculated were 6.46, 4.51 and 8.37 μg L−1 respectively.
Keywords
Amine-selective electrodes
Amine analysis
Potentiometric detection
High performance liquid chromatography

1 Introduction
Amines have been widely used in the oil and food industries, agriculture and pharmaceuticals (McCabe-Sellers et al., 2006; Sciancalepore et al., 2013; Flasarová et al., 2016). Some components of crude oil or natural gas, which can cause technical problems, require the use of amines or amine-containing chemicals during production, transport or purification (Engel et al., 2015). However amines can cause pollution and some serious diseases (Wang et al., 2016), most seriously cancer (Liang et al., 2016). Their presence in high concentrations in food may induce several health disorders in vulnerable people, such as nausea, respiratory discomfort, hot flushes, cold sweats, palpitations, headaches, red rash, and hyper/hypo tension (Giuliano and Rampin, 2004; Grandy, 2007; Perez-Serradilla and Luque de Castro, 2008). Amino compounds are also nitrogenous, low molecular weight, organic compounds which are derived mainly from amino acids through substrate-specific decarboxylase enzymes (Hernandez-Orte et al., 2008; Izquierdo Canas et al., 2008).
Amines (especially biogenic amines) are normal constituents of many foods and beverages and have been found to occur in cheese, wine, beer, fishery products and aged meat (Komprda et al., 2007; Lorenzo et al., 2007; Bernardeau et al., 2008; Marques et al., 2008; Ozogul et al., 2008; Anderson, 2008; Ntzimani et al., 2008). They occur in a wide variety of foods and beverages, especially in protein-rich foods as a result of enzymatic degradation or fermentation processes. They are usually produced by decarboxylation of amino acids or by the amination and transamination of aldehydes and ketones. Several methods have been used for the determination of aliphatic amines, including flow-injection analysis (FIA) (Waite et al., 2013), gas chromatography (GC) (Singh et al., 2011) and high performance liquid chromatography (HPLC) (Gosetti et al., 2013; Li et al., 2014). In liquid chromatography, different techniques have been used to detect trace amount of amines, including conductometry (Kvasnička and Voldřich, 2006), spectroscopy (Milhazes et al., 2007), potentiometry (Basozabal et al., 2014) and amperometry (Lamba et al., 2008).
Amine-selective electrodes were constructed for high performance characteristics with respect to detection limit, linear range, response time, easy fabrication and low cost and were applied as detectors in liquid chromatography for the detection of amines (Poels and Nagels, 2001; Erim, 2013). In the present study, micro-sized electrodes and low-dead volume flow-through cells, sensitive to several amine compounds, were developed using various ionophores based on PVC membrane cocktails. Using this method, three amine compounds were successfully identified in cheese serum samples.
2 Methods and materials
2.1 Reagent and solutions
All chemicals used were of analytical reagent grade or of the highest purity available. The amine compounds (ethylamine, propylamine, butylamine, pentylamine, hexylamine, heptylamine) were provided by Fluka (Bucks, Switzerland). Acetic acid was purchased from Merck (Darmstadt, Germany). Tetrahydrofuran (THF), high molecular weight poly(vinyl chloride) (PVC), graphite and various ionophores used in the construction of the ion selective electrode (ISE) were obtained from Fluka (Bucks, Switzerland). Epoxy (Macroplast Su 2227) and hardener (Desmodur RFE), used in the preparation of the solid contact, were purchased from Henkel (Istanbul, Turkey) and Bayer AG (Darmstadt, Germany). For the analysis of amine compounds, neutral forms of amines were converted to the cationic form. The stock solutions of each amine were prepared in 1 × 10−2 M acetate buffer (pH 4.5) and stored in the dark. Standard solutions of each amine were prepared by diluting the stock solutions, using sufficient buffer solution. To determine the pH working range of the electrodes, 5 × 10−3 M amine solutions in phosphate buffers from pH 2.0 to 8.0 were prepared. Distilled deionized water was used throughout the preparation of the aqueous solution.
2.2 Apparatus
The potentiometric measurements were recorded using a computer-controlled, high-input impedance, multi-channel, potentiometric measurement system (Biyomarge, Istanbul, Turkey). Throughout the measuring process, a micro-sized, solid-state, silver/silver chloride electrode was used as the reference electrode. Solutions at required concentrations were homogenized using an Ultrasonic LC30 stirrer (Germany). The pH of the solutions was measured by a glass pH electrode (Schott) with a Jenway 3040 ion analyzer (Staffordshire, UK). For the chromatographic analysis, a Dionex Ionpac CS12A column connected to Dionex P680 HPLC pump, consisting of a Rheodyne injection valve with a 20 μL sample loop, was used. The dimensions of the column were 4 × 250 mm. 27 mM methane sulfonic acid solution with 10% acetonitrile was used as a mobile phase in FIA and LC conditions. The examined flow rate of the carrier was 0.75, 1.0 and 1.5 mL min−1. The HPLC pump itself was also used for flow injection analysis studies. All measurements were carried out at room temperature (25 ± 5 °C).
2.3 Preparation of all solid-state contact membrane amine-selective electrodes and flow cells
The construction of all solid-state contact, PVC-membrane, amine-selective electrodes, without an inner reference solution, was carried out. In the preparation of the PVC-membrane amine-selective electrodes, a solid-state contact surface was used. The combination of solid-state contact was 50% (w/w) graphite, 35% (w/w) epoxy and 15% (w/w) hardener. A copper wire tip with a length of 10 cm and radius of 0.6 mm, was first sandpapered. The sandpapered tip of the copper wire was then dipped into this mixture, waited for a few seconds and left to dry. This step was repeated three times in order to obtain a solid-state contact. A membrane cocktail, mainly consisting of ionophores, high relative molecular mass polyvinyl chloride (PVC) and dioctyl sebacate (DOS) as plasticizer, was constructed. Five different membrane compositions were applied in the construction of the electrodes (Table 1). The structures of the amino-selective ionophores used are shown in Fig. 1. The amine-selective membrane cocktail was deposited directly onto the all solid-state contact of the copper wire five times using a Pasteur pipette. Each time 50 μL of cocktail was applied. The coated electrode surface was left to dry in laboratory conditions overnight. The dried amine-selective electrode was then conditioned by soaking in the main ion solution for at least 6 h before use. When not in use, the electrodes were stored in laboratory conditions. The electrodes were reconditioned for at least 2 h in the main ion solution before individual measurement processes. PVC: Polyvinyl chloride. DOS: Dioctyl sebacate. Ionophore 1: Potassium tetrakis (p-chlorophenyl)borate. Ionophore 2: Dibenzo-18-crown-6-KI. Ionophore 3: Dicyclo-18-crown-6-KBr. Ionophore 4: Calix-6-arene-hexaethylacetate.
Electrodes
PVC (mg)
DOS (mg)
Ionophore 1 (mg)
Ionophore 2 (mg)
Ionophore 3 (mg)
Ionophore 4 (mg)
Electrode 1
32
66
2
–
–
–
Electrode 2
31
64
1
4
–
–
Electrode 3
31
64
1
–
4
–
Electrode 4
31
64
1
–
–
4
Electrode 5
31
63
1
–
1
4
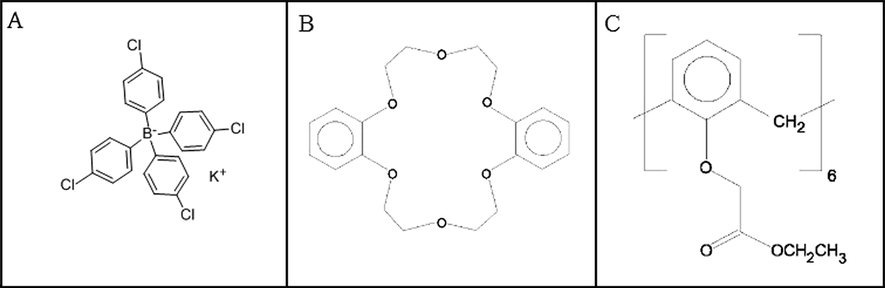
The structure of the amine-selective ionophores used, (A) potassium tetrakis (p-chlorophenyl) borate; (B) dibenzo-18-crown-6 KI and KBr; and (C) calix-6-arene-hexaethylacetate.
An all solid-state PVC-membrane, amine-selective electrode was prepared and combined with a low-dead volume, flow-through cell to be used as a detector in chromatography and this is shown in Fig. 2. The miniature detector cell was made of polycarbonate and has a flow path of 0.8 mm diameter.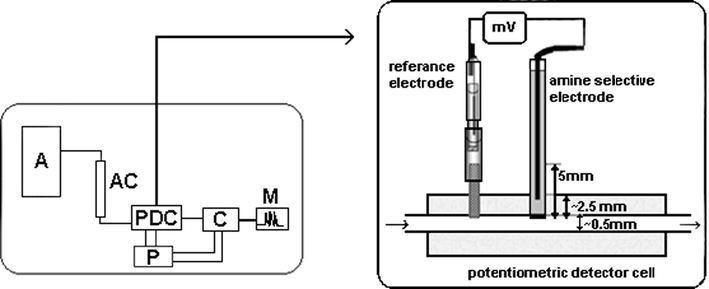
Schematic diagram of the chromatographic system for the determination of aliphatic amines (A: HPLC system; AC: analytical column; PDC: potentiometric detector cell; P: potentiometer; C: computer; M: monitor).
3 Results
3.1 Potentiometric performance characteristics of the amine-selective electrode in FIA conditions
The potentiometric performance characteristics of the flow-through electrode detector were firstly investigated for six alkyl amines under FIA conditions. A mobile phase made up of 27 mM methane sulfonic acid aqueous solution with 10% acetonitrile was used as mobile phase in FIA. Afterward, chromatographic studies were undertaken to evaluate the detection capability of the sensor for standard amines at concentrations between 1.0 × 10−2 and 8.0 × 10−6 M prepared in 1 × 10−2 M acetate buffer solution. All solid-state contact PVC-membrane amine-selective electrode based on potassium tetrakis(p-chlorophenyl)borate exhibited a selectivity sequence toward more lipophilic organic ions in FIA. This is in agreement with the findings when a cation-exchanger-based membrane electrode was used for the detection of amines (Schnierle et al., 1998). All solid-state contact PVC-membrane amine-selective electrodes containing neutral ionophores showed a response pattern toward the shorter-chain amine compounds. With regard to the potentiometric performances observed, the most appropriate combination of the membrane was found with mixed ionophores as 1% potassium tetrakis(p-chlorophenyl)borate and 4% dibenzo-18-crown-6-KI (electrode 2) within others. Amine-selective electrode 2 membrane consisting of the two ionophores performed a linear behavior in the logarithmic concentration range of 1.0 × 10−2–8.0 × 10−6 M standard amine solutions when used as a detector in FIA. Therefore, all flow analyses were carried out with electrode 2. The limit of detection (LOD) for each amine was calculated, using a signal to noise ratio of 3 in the flowgram, according to the IUPAC definition (Buck and Lindner, 1994; Umezawa et al., 2000). The detection limits of the amine-selective electrode 2 were found to be 1.2 × 10−6 M for ethylamine, 7.6 × 10−5 M for propylamine, 5.2 × 10−6 M for butylamine, 5.1 × 10−6 M for pentylamine, 3.4 × 10−5 M for hexylamine and 7.9 × 10−6 M for heptylamine in FIA mode (Table 2). Biogenic amines are formed as a consequence of metabolic processes in microorganisms. Three biogenic amines, putrescine, spermine and spermidine were examined with the FIA method although many kinds of biogenic amines present in fermented food. With the electrode 1 that was consisted of potassium tetrakis(p-chlorophenyl)borate no response was observed for these compounds. On the other hand, the mixed ionophore-based electrodes showed a reasonable response to the biogenic amines. But the separation and detection of these biogenic amine compounds were not further studied in chromatographic experiments due to long separation time of spermine.
Amine
Electrode 1
Electrode 2
Electrode 3
Electrode 4
Electrode 5
Ethylamine
3.2 × 10−5
1.2 × 10−6
7.9 × 10−6
7.3 × 10−5
8.7 × 10−5
Propylamine
4.6 × 10−5
7.6 × 10−5
6.1 × 10−4
3.4 × 10−5
7.9 × 10−5
Butylamine
3.8 × 10−5
5.2 × 10−6
3.1 × 10−5
9.8 × 10−5
8.9 × 10−6
Pentylamine
4.3 × 10−5
5.1 × 10−6
1.29 × 10−4
2.1 × 10−5
7.1 × 10−6
Hexylamine
7.9 × 10−6
3.4 × 10−5
3.4 × 10−5
3.1 × 10−6
6.71 × 10−5
Heptylamine
2.1 × 10−5
7.9 × 10−6
9.8 × 10−4
5.6 × 10−5
3.8 × 10−5
The lifetime of the electrode 2 as a detector was determined while the change in the slope of the peak heights measured in the flowgrams was taken into account. The results of the behavior of the amine-selective electrode in ethylamine solution indicated that the electrode detector did not display any significant change in the slope throughout the usage period of 3 months. However, after this period a significant difference was observed. The same procedure was applied to the other electrodes and these provided similar results. Therefore, the lifetime of the electrode detector was determined as almost 3 months.
To determine the response time of the amine-selective electrode detector, the time to reach maximum peak height potential was examined for 1 × 10−5–1 × 10−4 M, 1 × 10−4–1 × 10−3 of each amine solution. The response time of the electrode was found to be less than 15 s for all the amines. The amine-selective electrode provided a very good performance for amines in FIA and exhibited almost a linear response with near-Nernstian slope of 52.4 ± 3.8 mV per decade, within a concentration range of 1.0 × 10−2–8.0 × 10−6 M (r2 = 0.99). The results obtained in FIA conditions indicated that the flow-through amine-selective electrode can be applied as a detector of amines. However, the sensor was applied for the separation and detection of the amines in LC.
3.2 LC separation and analytical data
To evaluate the potentiometric responses of the amine-selective electrode detector, several chromatograms of a mixture of six amine compounds were obtained and then injected at different concentrations into the LC system. The amine-selective electrode detector exhibited almost similar sensitivity to the studied amines, providing an overall chromatogram. Factor affecting signal shape, retention time and intensity such as composition of the carrier solution, injection volume and flow-rate was optimized. The effect of sample injection volume (10–20 μL) and mobile phase flow-rate (0.5–1.5 mL min−1) was examined. It was observed from the chromatograms that longer retention time elapsed for long chain amines (e.g. 60 min for heptylamine at 0.5 mL min−1) at the flow-rate of 0.75 mL min−1. When the flow rate of 1.5 mL min−1 was applied, the peak heights were decreased and also ethylamine and propylamine peaks overlapped at this flow rate. The reason for the decrease in the peak heights was thought to be the insufficient time allowed for the interaction between these amine compounds at the electrode surface. Therefore, it can be deduced that the amine compounds left the amine-selective electrode detector cell without obtaining enough equilibrium in the run. Overall, the amine-selective electrode detector responded linearly on the logarithmic activities of amines, especially at low concentrations almost of between 9.0 × 10−6 and 1.0 × 10−3 M together with a detection limit of 3 × 10−6 M toward amines in liquid chromatography. This linear behavior was found to be nearly in agreement with the behavior of the amine-selective electrode used in FIA conditions. These chromatographic results from the amine-selective electrode were also found to be in parallel with the results of Poels and Nagels (2001). Although, in the present study, the attained detection limits were found to be lower than the results achieved by Poels and Nagels, that could be due to the lower dead volume of the flow-through detection cell used in the present study. The chromatographic separation of the amines was studied by changing the acetonitrile and methane sulfonic acid composition of the mobile phase. A long retention time of the amines was required when using isocratic elution so it was applied in gradient elution. A study of several combinations for obtaining the optimum gradient profile resulted in a mixture of pump A: 5% acetonitrile containing 20 mM methane sulfonic acid composition for 15 min and pump B: 5% acetonitrile containing 50 mM methane sulfonic acid composition for 15 min. Under such conditions, the six amines studied can be separated with a reasonable resolution in 30 min at a mobile phase flow-rate of 1 mL min−1. A chromatogram for the analysis of a standard mixture of amines is shown in Fig. 3.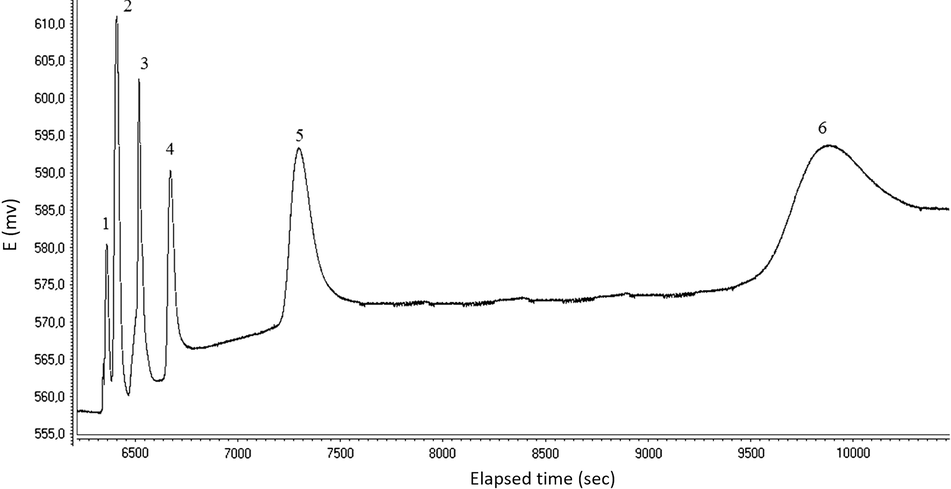
Chromatogram of standard mixture of six aliphatic amines: (1) ethylamine; (2) propylamine; (3) butylamine; (4) pentylamine; (5) hexylamine; (6) heptylamine; each of them was 1.25 × 10−4 M. Experimental conditions: Mobile phase (Gradient elution; A pump: 5% acetonitrile containing 20 mM methane sulfonic acid solution (for 15 min.); B pump: 5% acetonitrile containing 50 mM methane sulfonic acid solution (for 15 min.); Flow rate: 1.0 mL min−1; Injection volume: 20 μL; Dionex CS12A column (4 × 250 mm)).
3.3 Quantitative analysis
Calibration curves were obtained from the peak heights for each amine in LC. Calibration curves obtained for six amines using standard sample solutions are shown in Fig. 4. Contents of each amine in the analyzed sample were determined from these curves. The linearity relationship between the amine concentrations and the peak height at the above mentioned separation conditions was checked. All graphs exhibited a limited degree of linearity in the investigated concentration range of 1.0 × 10−2–8.0 × 10−6 M. At high and very low amine concentrations injected there were deviations from linearity. However they were still quite useful because of their good reproducibility. The regression equation is y = a logx + b, where x is the concentration of amine (mol L−1) and y is the peak height (cm) and correlation coefficients (r) of the amines. All other analytical parameters under chromatographic conditions were as given in Table 3. The results suggested that the method proposed is sufficiently sensitive to detect the amines in a cheese serum sample. For the determination of amines in real samples, known amounts of amines were added to a 100 mL of cheese serum sample determined by the proposed procedure. The recoveries of the amines from cheese serum sample were around 88–101% with a relative standard deviation of 2.2–4.0%, as shown in Table 4.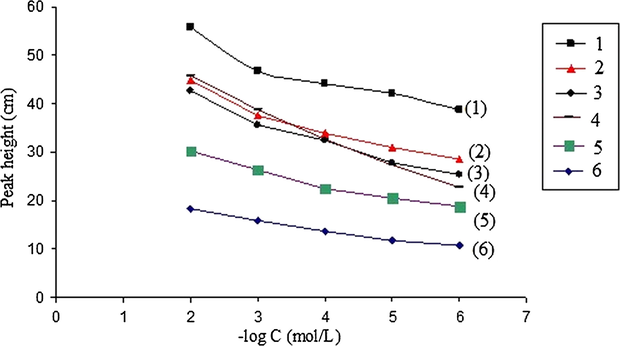
Calibration curves for aliphatic amines: (1) propylamine; (2) butylamine; (3) pentylamine; (4) heptylamine; (5) hexylamine; (6) ethylamine under the optimized chromatographic conditions.
Amine
tR(min)
LODa (μg L−1)
y = a log x + b
r
Ethylamine
6.6
3.92
y = 2.09 log x + 22.32
0.9952
Propylamine
7.5
4.37
y = 3.86 log x + 61.08
0.9002
Butylamine
10.2
4.11
y = 3.95 log x + 50.94
0.9446
Pentylamine
12.5
6.19
y = 4.28 log x + 49.96
0.9705
Hexylamine
16.6
6.23
y = 2.84 log x + 35.04
0.9633
Heptylamine
28.3
4.38
y = 5.81 log x + 56.76
0.9945
Amine
Added
Founda
Recovery%
RDSb
Ethylamine
60
58
97
2.2
Propylamine
74
75
101
2.3
Butylamine
88
81
92
2.2
Pentylamine
102
90
88
2.7
Hexylamine
116
108
93
3.5
Heptylamine
130
115
88
4.0
3.4 Analytical application
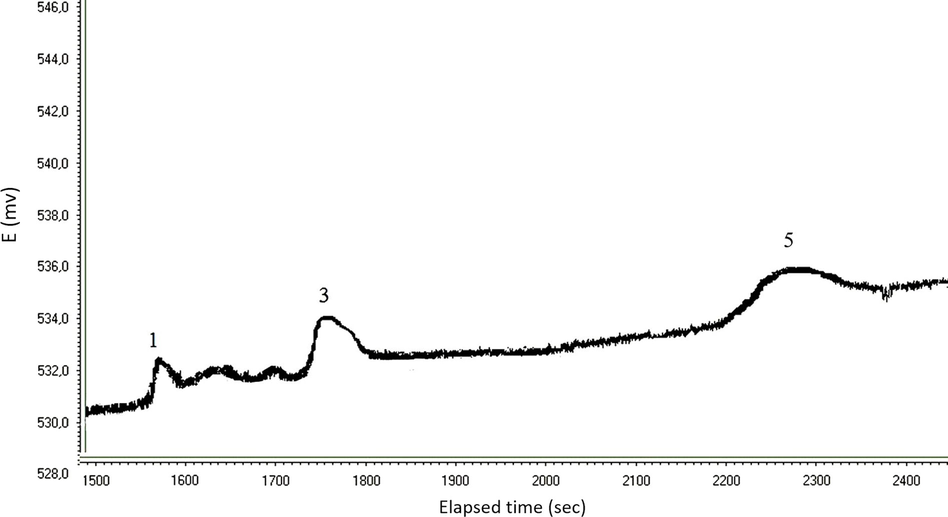
The prepared electrode was used to determine the amines in a cheese serum samples which were obtained from the Ondokuz Mayis University Faculty of Agriculture, Turkey. Firstly, the samples were filtered with a membrane of 0.45 μm pore and 47 mm diameter and then directly injected into the LC system. The procedure applied to the cheese samples was identical to the procedure which was previously applied to the standard amines. The total elution time was 30 min. Optimization of the chromatographic method was achieved by the cooperation of amine standard solutions, and then the optimized method was used for real samples in a similar way. A chromatogram obtained from 20 μL injection of a cheese serum sample is shown in Fig. 5.
From the chromatogram it can be suggested that low levels of amines in the cheese serum sample could be successfully determined with the developed method. In the chromatogram, the peaks for ethylamine, butylamine and hexylamine correspond to 6.46, 4.51 and 8.37 μg L−1 concentrations respectively. The analytical results obtained with the chromatographic system for the cheese serum sample are summarized in Table 5.
Amine
Average value (
)
Standard deviation (SD)
(1) Ethylamine
6.46
2.94
(3) Butylamine
4.51
1.63
(5) Hexylamine
8.37
2.31
4 Discussion
In this study, amine-selective electrodes based on potassium tetrakis(p-chlorophenyl)borate, dibenzo-18-crown-6-KI, dicyclo-18-crown-6-KBr and calix-6-arene-hexaethylacetate as ionophores were developed for the detection of amine compounds in LC. The developed LC method with the amine-selective electrode detector based on potassium tetrakis(p-chlorophenyl)borate and dibenzo-18-crown-6-KI was found to be quite good in elution time that was 30 min for 6 amines in gradient elution, and satisfactory for the detection of amines between 9.0 × 10−6 and 1.0 × 10−3 M. This method exhibited high selectivity toward ethylamine, propylamine, butylamine, pentylamine, hexylamine and heptylamine with LOD of 3.92, 4.37, 4.11, 6.19, 6.23 and 4.38 μg L−1 respectively. Consequently, a low-dead volume flow-through detection cell embodied with the all solid-state contact amine-selective electrode based on potassium tetrakis(p-chlorophenyl)borate and dibenzo-18-crown-6-KI as ionophores was successfully applied for the sensitive detection of ethylamine, butylamine and hexylamine in cheese serum samples. Accuracy was evaluated by the standard addition method and calculated mathematically based on the sample recovery. The recovery results obtained were in the range of 88–101%, which implies a high level of accuracy for the method. The RSD values of the analytical results did not exceed 4%.
5 Conclusion
A trace quantification liquid chromatographic-potentiometric method for the determination of amines in cheese serum was developed. An all solid-state contact polyvinyl chloride membrane amine-selective electrode based on mixed ionophores was prepared and used as a detector in a low-dead volume flow through detection cell in LC. The developed method is robust, since small changes in the method did not cause significant variations in the results. However, the described LC-amine-selective electrode-potentiometric detection method is comparable with that in the literature (Barreira et al., 1987; Vale and Gloria, 1997) that includes tedious and time-consuming derivatization steps or post-column reactions, and presents a simple, specific, precise and accurate amine detection in cheese serum samples.
Acknowledgments
The authors would like to acknowledge the financial support of the Science and Technology Research Council of Turkey (TUBITAK) (Project Number: 107T336).
References
- Biogenic and volatile amine-related qualities of three popular fish species sold at Kuwait fish markets. Food Chem.. 2008;107:761-767.
- [Google Scholar]
- Separation and determination of aliphatic amines by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. A. 1987;398:381-386.
- [Google Scholar]
- Direct potentiometric quantification of histamine using solid-phase imprinted nanoparticles as recognition elements. Biosens. Bioelectron.. 2014;58:138-144.
- [Google Scholar]
- Safety assessment of dairy microorganisms: the Lactobacillus genus. Int. J. Food Microbiol.. 2008;126:278-285.
- [Google Scholar]
- Recomendations for nomenclature of ion-selective electrodes. Pure Appl. Chem.. 1994;66:2527-2536.
- [Google Scholar]
- Recent analytical approaches to the analysis of biogenic amines in food samples. TrAC, Trends Anal. Chem.. 2013;52:239-247.
- [Google Scholar]
- Biogenic amine production by Lactococcus lactis subsp. cremoris strains in the model system of Dutch-type cheese. Food Chem.. 2016;194:68-75.
- [Google Scholar]
- Simultaneous determination of sixteen underivatized biogenic amines in human urine by HPLC-MS/MS. Anal. Bioanal. Chem.. 2013;405:907-916.
- [Google Scholar]
- Trace amine-associated receptor 1–family archetype or iconoclast. Pharmacol. Ther.. 2007;116:355-390.
- [Google Scholar]
- Biogenic amine determination in wine fermented in oak barrels: factors affecting formation. Food Res. Int.. 2008;41:697-706.
- [Google Scholar]
- Amino acids and biogenic amines during spontaneous malolactic fermentation in Tempranillo red wines. J. Food Compos. Anal.. 2008;21:731-735.
- [Google Scholar]
- Content and distribution of biogenic amines in Dutch-type hard cheese. Food Chem.. 2007;102:129-137.
- [Google Scholar]
- Determination of biogenic amines by capillary zone electrophoresis with conductometric detection. J. Chromatogr. A. 2006;1103:145-149.
- [Google Scholar]
- Determination of aliphatic amines by high-performance liquid chromatography-amperometric detection after derivatization with naphthalene-2,3-dicarboxaldehyde. Anal. Chim. Acta. 2008;614:190-195.
- [Google Scholar]
- Simultaneous determination of biogenic amines and estrogens in foodstuff by an improved HPLC method combining with fluorescence labeling. LWT – Food Sci. Technol.. 2014;55:355-361.
- [Google Scholar]
- Degradation of aromatic amines in textile-dyeing sludge by combining the ultrasound technique with potassium permanganate treatment. J. Hazard. Mater.. 2016;314:1-10.
- [Google Scholar]
- Biogenic amine content during the manufacture of dry-cured lacón, a Spanish traditional meat product: effect of some additives. Meat Sci.. 2007;77:287-293.
- [Google Scholar]
- Biogenic amines in wines: influence of oenological factors. Food Chem.. 2008;107:853-860.
- [Google Scholar]
- Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. J. Food Compos. Anal.. 2006;19:S58-S65.
- [Google Scholar]
- Electrochemical and spectroscopic characterisation of amphetamine-like drugs: application to the screening of 3, 4-methylenedioxymethamphetamine (MDMA) and its synthetic precursors. Anal. Chim. Acta. 2007;596:231-241.
- [Google Scholar]
- Formation of biogenic amines and relation to microbial flora and sensory changes in smoked turkey breast fillets stored under various packaging conditions at 4 °C. Food Microbiol.. 2008;25:509-517.
- [Google Scholar]
- Seasonal effects in the nutritional quality of the body structural tissue of cephalopods. Food Chem.. 2008;108:847-852.
- [Google Scholar]
- Potentiometric detection of amines in ion chromatography using macrocycle-based liquid membrane electrodes. Anal. Chim. Acta. 2001;440:89-98.
- [Google Scholar]
- Capillary electrophoretic determination of different classes of organic ıons by potentiometric detection with coated-wire ion-selective electrodes. Anal. Chem.. 1998;70:3585-3589.
- [Google Scholar]
- Microdroplet-based multiplex PCR on chip to detect foodborne bacteria producing biogenic amines. Food Microbiol.. 2013;35:10-14.
- [Google Scholar]
- Determination of aliphatic amines by gas chromatography–mass spectrometry after in-syringe derivatization with pentafluorobenzoyl chloride. J. Chromatogr. A. 2011;1218:5683-5687.
- [Google Scholar]
- Potentiometric selectivity coefficients of ion-selective electredes. Pure Appl. Chem.. 2000;72:1851-2082.
- [Google Scholar]
- Chemiluminescence detection of piperazine designer drugs and related compounds using tris (2, 2′-bipyridine) ruthenium (III) Talanta. 2013;116:1067-1072.
- [Google Scholar]
- An analytical method for the field investigation of environmental amines released by industrial processes. Process. Saf. Environ.. 2016;102:328-335.
- [Google Scholar]