Translate this page into:
Molecular modeling, synthesis and characterization of branched geminal zwitterionic liquids for enhanced oil recovery
⁎Corresponding authors at: Instituto Mexicano del Petróleo, Eje Central Lázaro Cárdenas 152, Col. San Bartolo Atepehuacan, México D.F. 07730, Mexico. Tel.: +52 55 91758191 (R. Cisneros-Dévora), +52 55 91758134 (L.S. Zamudio-Rivera). rcisnerosde@conacyt.mx (R. Cisneros-Dévora), lzamudio@imp.mx (L.S. Zamudio-Rivera)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A Sulfobetaine-based Branched Geminal Zwitterionic Liquid (SBGZL) and another one based on hydroxysultaine (HBGZL), were synthesized and their performance was evaluated in the enhancement of the oil recovery (EOR) when they are injected through flooding water into carbonate reservoirs. SBGZL obtained 39.4% and 215.5% more recovery factor for light- and heavy-oil, respectively, than obtained by a synthetic brine used as a reference; conversely, HBGZL obtained 37.5% and 44.7%, respectively. Density-Functional-Theory calculations of interaction energies reveal that both the SBGZL and HBGZL molecules adsorb on the carbonate surfaces stronger than an asphaltene molecule, implying that the good EOR performance of the zwitterionic liquids is due to the induced wettability change of the firstly-oil-wet carbonate surfaces.
Keywords
Enhanced oil recovery
Density Functional Theory
Sulfobetaine-based zwitterionic geminal liquid
Supramolecular chemistry
1 Introduction
Despite there exist in the world very-important and significant advances within the development of wettability-modifier chemicals to be applied onto hydrocarbon extraction, currently in Mexico there exist remaining oil deposits difficult to treat due to they are naturally fractured, exhibit low permeability, present heterogeneous lithologies, have high temperatures (greater than 90 °C), have high salinity (generally higher than 60,000 ppm as sodium chloride) and, especially, exhibit high content of divalent ions such as calcium and magnesium (more than 5000 ppm) (Hernandez-Altamirano et al., 2014 and references contained there).
In the field of Enhanced Oil Recovery (EOR) processes, chemicals have been designed to achieve wettability alteration to a more water-wet state in oil carbonate reservoirs by means of spontaneous imbibition (Pons-Jiménez et al., 2015). Nowadays, the design of novel and new Branched Geminal Zwitterionic Liquids (BGZL) structures has been addressed to be applied in EOR since they are tolerant to extreme conditions of high-temperatures, high-pressures, high-water salinities and high-hardnesses (Lopez-Chavez et al., 2012a,b; Lopez-Chavez et al., 2014). In particular, the Branched Geminal Zwitterionic Liquids based either in sulfobetaine (SBGZL) or in hydroxysultaine (HBGZL) are characterized to be electrically neutral, possess hydrophobic chains and zwitterionic polar groups and exhibit chirality.
As far as we know, there are not in the literature any references about SBGZL and HBGZL suggesting their applications to production processes nor their use as modifiers of wettability and corrosion inhibitors, although these chemicals can be exposed to brines with high content of divalent ions such as calcium, magnesium, barium and strontium (150,000 ppm), temperatures up to 220 °C and pressures up to 300 kg/cm2. At present paper, through experimental and theoretical approaches, we investigate the promising application of them to alter the rock wettability and then to favorably enhance the recovery of crude oil embedded in rocks such as calcite (Cal) and dolomite (Dol). Also, we briefly describe a possible chemical mechanism explaining the increase in oil recovery factor (ORF) found in this study.
2 Experimental
2.1 Materials and methods
All salts for brine, reagents, and solvents were acquired from Aldrich Chemical Co. (reagent grade) and used without additional purification. All Nuclear Magnetic Resonance (NMR) spectra 1H and 13C were recorded on a VARIAN Mercury 200-BB spectrometer in CDCl3. The chemical shifts (δ) are measured in ppm and referenced to the internal SiMe4 (TMS) peak. Coupling constants (J) are measured in Hz. IR Spectra were recorded on a Bruker-Tensor 27 FT-IR using ATR mode. The heavy crude oil (HCO) utilized in this study was provided by the Mexican state-owned petroleum company PEMEX from a marine well drilled in the south of the Gulf of Mexico. The samples were characterized by the following standard procedure: Saturated, Aromatics, Resins and Asphaltenes ASTM D-2007-03 content (ASTM, 2011), whereas the Stiff and Davis Stability Index was calculated for the brines.
2.2 Synthesis of zwitterionic liquids
The general supramolecular synthesis of SBGZL and HBGZL is shown in Scheme 1.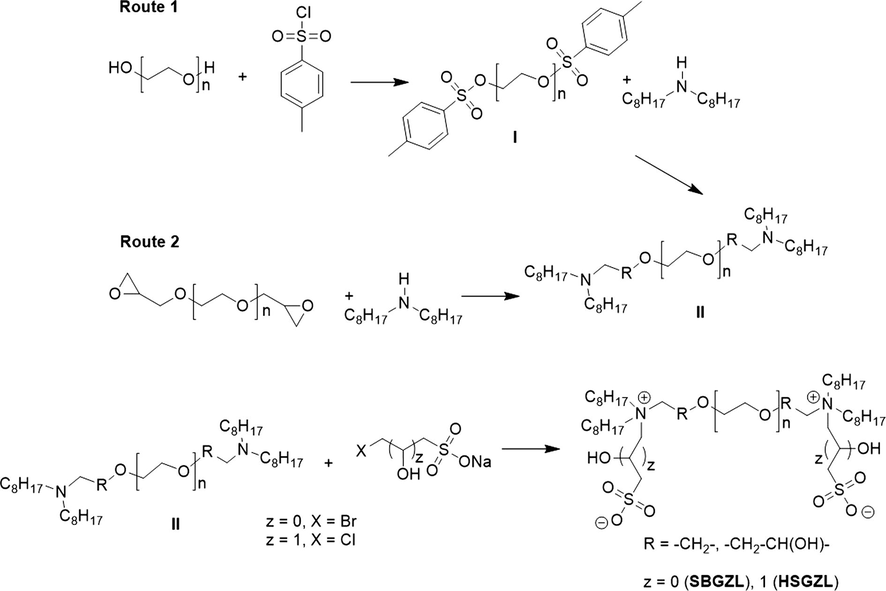
Synthetic route for prepare zwitterionic liquids base sulfobetaine (SBGZL) and hydroxysultaine (HBGZL).
2.2.1 Synthesis of SBGZL
2.2.1.1 First stage
In a 50 mL round bottom flask containing 5.0 g of an aqueous 17% w/w solution of sodium hydroxide (0.85 g, 21.25 mmol), it is added 4.24 g (7.75 mmol) of polyethylene glycol, whose number average molecular weight is 600 g/mol. The mixture was stirred for 20 min. After this time it is slowly added, at room temperature (20 °C) and atmospheric pressure, 7.37 g of a 40% w/w solution of tosyl chloride (2.95 g, 15.47 mmol) in tetrahydrofuran. Then, the reaction mixture was stirred for one hour at room temperature and subsequently the reaction mixture was placed in a separation funnel to recover the organic phase. The solvent was removed under reduced pressure, obtaining 6.27 g (7.47 mmol, yield 96%) of the product ditosylate polyether (TS-PE, I) as a clear yellow viscous liquid.
2.2.1.2 Second stage
In a 50 mL two neck round-bottom flask provided with a condenser, magnetic stirrer and thermometer, it is added dioctylamine (1.97 g, 8.16 mmol), TS-PE (6.27 g, mmol), potassium carbonate (4.77 g, 34.51 mmol) and 23 mL of acetonitrile. The reaction mixture was heated to reflux vigorously stirred for 8 h.
Once the reaction time was completed, the mixture was washed with water and carried out an extraction of the organic phase, which was evaporated under reduced pressure to obtain 6.18 g (6.12 mmol, yield 82%) of the product bis-N,N-dioctyl-N-polyether as a viscous amber liquid (II).
2.2.1.3 Third stage
In a 100 mL round-bottom flask provided with condenser, magnetic stirrer and thermometer, it is placed bis-N,N-dioctyl-N-polyether (6.18 g) and a solution of sodium 2-bromo ethane sulfonate (2.77 g, 13.13 mmol) in 50 g of water was added. The reaction mixture was placed under reflux and stirred vigorously for 48 h. Once the reaction time was completed, the aqueous phase was separated and evaporated under reduced pressure. The residue was washed with chloroform to remove the salts by filtration. The organic fraction was evaporated under reduced pressure to obtain 6.84 g (5.58 mmol, yield 91%) of a viscous and amber-in-color zwitterionic liquid, labeled as bis-N,N-dioctyl-N-sulfobetaine polyether (SBGZL).
2.2.2 Synthesis of HBGZL
2.2.2.1 First stage
In a 50 mL round-bottom flask, 10 g (19 mmol) of polyethylene glycol diglycidyl ether (526 g/mol of average molecular weight) and 3.5 g (14.5 mmol) of dioctylamine were stirred for 1 h at 95 °C to obtain 13.5 g (13.37 mmol, yield 92%) of the bis-N,N-dioctyl-N-hydroxypolyether as a transparent yellow viscous liquid.
2.2.2.2 Second stage
In a 100 mL round-bottom flask, provided with a condenser, magnetic stirrer and a thermometer, it was placed 2 g (1.98 mmol) of bis-N,N-dioctyl-N-hydroxypolyether and a solution of 0.86 g (4.38 mmol) of sodium 3-chloro-2-hydroxypropane sulfonate in 15 g of water. The reaction mixture was placed under reflux conditions and stirred vigorously for 72 h.
Once the reaction time was completed, the aqueous phase was separated and evaporated under reduced pressure. The dry product was washed with chloroform to remove the salts by filtration. The solvent was removed to obtain 1.4 g (1.01 mmol, yield 51%) of the zwitterionic geminal liquid as a viscous and amber labeled bis-N,N-dioctyl-N-polyether hydroxysultaine (HBGZL).
2.3 Computational construction of molecular structures
Theoretical studies on supramolecular interactions between BGZLs, asphaltene (Asph) and reservoir rocks in a water environment, were performed via Density Functional Theory (DFT). The water environment is taken into account since SBGZL and HBGZL are injected into oil reservoir through an aqueous solution, and it was simulated through its dielectric continuum (permeability constant: 78.54), as treated in the Conductor-like Screening Model (COSMO) approach (Klamt and Schüürmann, 1993).
It was used the Materials Studio (MS) interface (Version 7.0, 2012) to build molecular models of Asph, SBGZL, HBGZL, as well as Cal and Dol surfaces. SBGZL has been modeled consisting of a central curved alkyl chain, which contains 12 ether groups, at whose each of the two ends was attached one zwitterionic moiety (formed by a sulfobetaine group) bridging the curved alkyl chain ends with a pair of straight alkyl tail branches having 8 carbons in length (Fig. 1A). HBGZL has been modeled in a similar manner as SBGZL but includes a hydroxyl group at each zwitterionic head. Asph molecule has been modeled following the literature (Fig. 1B) (Buenrostro-Gonzalez et al., 2004), i.e., through fused aromatic and heteroaromatic rings such as phenolic (OH), thiophene and pyridine groups. This asphaltene molecule represents the average characteristics of a HCO from the Marina District in Mexico (Hernandez-Altamirano et al., 2014).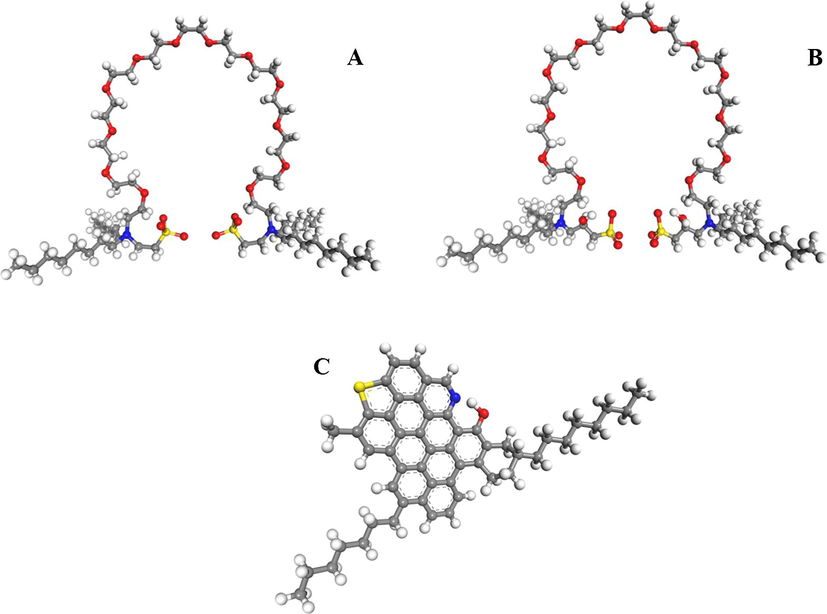
Perspective view of molecular models of branched geminal zwitterionic liquid based on sulfobetaine (A), hydroxysultaine (B) and asphaltene (C). Atom colors stand for the following: gray, carbon; white, hydrogen; red, oxygen; blue, nitrogen; and yellow, sulfur.
In order to investigate adsorption of BGZLs and Asph on carbonate rocks of oil reservoirs, (104) surfaces of Cal and Dol were modeled (Pons-Jiménez et al., 2014).
To obtain the optimized molecular geometries for all complexes, DFT computations through MS DMol3 application were executed in water environment. The molecular model of SBGZL, HBGZL or Asph was put over Cal and Dol surfaces in an orthorhombic simulation cell in such a manner that the adjacent molecule images, which appear due to the periodic boundaries, were separated by around 10 Å; so, the chosen cell dimensions were a = 32.38, b = 24.95 and c = 41.00 Å for Cal, and a = 30.79, b = 24.03 and c = 41.00 Å for Dol. The cells having these dimensions are denoted herein as Cal cell and Dol cell, respectively.
DMol3 runs were performed within the Local-Density Approximation (LDA), using the functional by Vosko S.H., Wilk L. and Nusair M. (VWN) (Vosko et al., 1980), the Double Numerical (DN) basis set, the Ortmann, Bechstedt and Schmidt method for the DFT-Dispersion correction (Ortmann et al., 2006), the Effective Core Potentials for core treatment (Dolg et al., 1987; Bergner et al., 1993), spin-polarized electrons, and water as the solvent. All run parameters were set at the coarse accuracy option.
Interaction energy ΔE calculations for SBGZL, HBGZL and Asph molecules on carbonate surfaces in water environment can give insights into the performance of SBGZL and HBGZL in an oil field, and they were quantified for the pairs SBGZL/Cal, SBGZL/Dol, HBGZL/Cal, HBGZL/Dol, Asph/Cal and Asph/Dol through ΔE = EM/S − (EM + ES), where EM, ES and EM/S are the total energies of individual molecules, pristine surfaces, and interacting molecule/surface system, respectively.
2.4 Spontaneous imbibition tests to determine ORF
The effects on the ORF by addition of SBGZL and HBGZL to brines were determined through tests of spontaneous imbibition over oil-containing Bedford Cal limestone cores in contact with the brine (Hernandez-Altamirano et al., 2014). Spontaneous imbibition tests using brine with added 0.03 wt% (300 ppm) of SBGZL and HBGZL were performed, and the results were compared with those of a reference consisting in brine without BGZL.
All tests were performed in a standard Amott cell (Hernandez-Altamirano et al., 2014). The Cal cores were impregnated separately with light and heavy oils at 120 °C under atmospheric pressure. After cooling to room temperature, the cores were placed into Amott cells at 90 °C, and then poured into either the pure brine or the surfactant-added brine. All systems were maintained at 90 °C temperature during 15 days and the oil recovery was monitored all of the time.
At the end of the experiment, the oil produced was gravimetrically determined using the equation:
3 Results and discussion
3.1 Spectroscopic characterization of SBGZL and HBGZL
All compounds were obtained in high yields through two synthetic routes and were characterized by NMR and infrared spectroscopies. The corresponding signals were observed for each intermediate involved in the reactions step. For SBGZL, there were observed 1H NMR signals around 3.94 ppm corresponding to the ether groups, as well as from 1.28 to 0.86 ppm due to the alkyl chains. Also, the 13C NMR signal at 70.4 ppm reveals the presence of carbon linked to sulfonate group, and peaks at 65.4 and 53.3 ppm mean there are C–N bonds. Infrared spectra show a band in 1221 cm−1 corresponding to sulfonate group. Besides, 1641 cm−1 confirms again C–N bonds.
On the other hand, infrared spectrum for HBGZL shows a peak at 3423 cm−1 evidencing the OH group, whereas peaks at 1647 and 1222 cm−1 reveal ammonium and sulfonate groups, respectively. The HBGZL NMR spectrum is analogous to the SBGZL one. The experimental confirmation on the presence of the above functional groups gives confidence on the molecular structures modeled at present paper. Below are the details of the NMR spectra:
3.1.1 SBGZL
1H NMR: 3.94 (m, –O(CH2CH2O)n–), 3.61 (m, –NCH2CH2O–), 3.27 (m, –NCH2CH2O–), 3.05 (m, –NCH2CH2–), 1.75 (m, –NCH2CH2–), 1.28 (m, alkyl chain), 0.86 (m, Me). 13C NMR: 70.4 (CSO3−), 65.4, 53.3 (C–N), 52.1 (C–N), 31.1, 22.3 (RCH2Me), 13.9 (Me). IR, ATR (cm−1): 2922 (νasymm CH), 2853 (νsymm CH), 1641 (νC–N), 1464 (δCH2), 1221 (νSO3), 1098 (νsymm ether linkage).
3.1.2 HBGZL
1H NMR: 3.85 (m, –O(CH2CH2O)n–), 3.58 (m, –NCH2CH2O–), 3.25 (m, –NCH2CH2O–), 3.20 (m), 3.03 (m, –NCH2CH2–), 2.99 (m), 1.26 (m, –NCH2CH2–), 1.24 (m, alkyl chain), 0.83 (m, Me). 13C NMR: 70.3 (CSO3−), 65.5, 53.2 (C–N), 52.1 (C–N), 31.0, 22.3 (RCH2Me), 13.8 (Me). IR, ATR (cm−1): 3423 (νO–H) 2922 (νasymm CH), 2853 (νsymm CH), 1647 (νC–N), 1465 (δCH2), 1222 (νSO3), 1098 (νsymm ether linkage).
3.2 Oil and carbonate cores properties
Spontaneous imbibition tests used real light and heavy oils extracted from Mexican southeastern fields, whose saturated, aromatic, resin and asphaltene (SARA) analyses are shown in Table 1.
Properties
Oil
Light
Heavy
Saturated
30.68
13.40
Aromatics
28.62
24.76
Resins
39.35
51.01
Asphaltenes
1.32
10.44
Acid number
0.21
1.83
Basic number
1.70
2.12
Two natural brines contained also at Mexican reservoirs, possessing high salinity and high content of calcium (Table 2) were used in all experiments to resemble conveniently the conditions of the oil fields. They are labeled as Brine 1 and Brine 2, and are used to recover light and heavy oils, respectively.
Brine 1
Brine 2
Density (g/cm3)
1.0043
1.0216
pH
7.65
6.68
Turbidity (FTU)
4
15
Cations (mg/L)
Na+
1703.66
11,630.06
Ca2+
416.00
1976.00
Mg2+
106.95
427.86
Fe2+
0.06
0.25
Ba2+
35
–
Anions (mg/L)
Cl−
3200.00
22,000.00
SO42−
350.00
825.00
HCO3−
405.04
122.00
Hardness as CaCO3
1480.00
6700.00
Salinity as NaCl
5275.00
36,265.59
A number of cylindrical carbonate cores were employed to perform imbibition tests simulating the matrix rock of real reservoirs. Their permeabilities to helium are around 66 mD, the average porosity to water is 20%, and the diameter and height dimensions are around 3.8 and 7.0 cm, respectively.
Experimental results show that BGZL performs better than the reference brine since former recovers more oil (Fig. 2A and B, and Table 3), i.e., SBGZL and HBGZL recover about 1.3 times more light oil than the reference brine (Table 3). In the case of heavy oil, SBGZL recovers more than 3 times and HBGZL about 1.5 times the oil amount recovered by the reference brine. 15-day duration of the imbibition tests.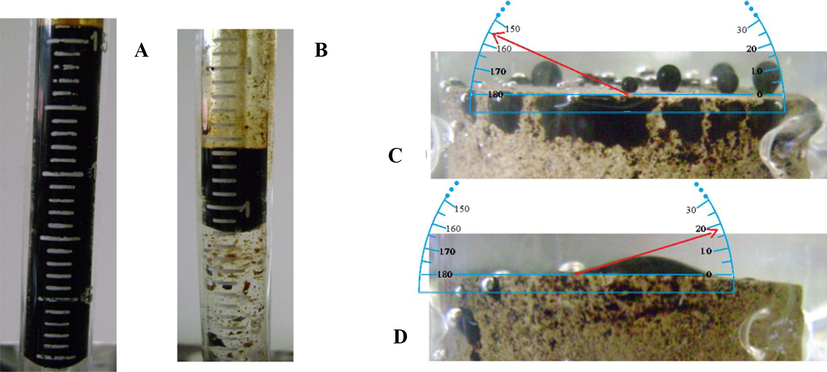
Recovered heavy oil using (A) a GZL-containing brine and (B) the reference brine. Contact angles produced by using (C) a GZL-containing brine and (D) the reference brine.
Chemical dissolved in brines
Light oil
Heavy oil
Impregnated (g)
Recovered (g)
Fr (%)
Impregnated (g)
Recovered (g)
Fr (%)
None
12.2874
4.5796
37.3
6.8124
0.8364
12.3
SBGZL
12.1547
6.3253
52.0
6.2834
2.4394
38.8
HBGZL
11.8760
6.0987
51.3
6.3646
1.1320
17.8
Moreover, BGZL alters rock wettability but reference brine does not, from being oil-wet to water-wet (Fig. 2C and D). Latter fact reveals that BGZL molecules replace oil ones over the carbonate surfaces, it being their ether groups responsible for the induced affinity of carbonate surfaces to water.
3.3 Theoretical results
DFT results show that an Asph molecule approaches more closely onto Cal surface than onto Dol surface because the Asph OH-group interacts more intensively with Ca2+ than with Mg2+ atoms, as it is revealed by the OH–Ca2+ distance (Fig. 3E) which is shorter than the OH–Mg2+ distance (Fig. 3F). This suggests a more intense adsorption of Asph by Cal surface and it is confirmed through the interaction energies among Asph molecule and carbonate surfaces (Table 4) since Cal surface yields an interaction energy 9.15% more negative than Dol surface. Besides, bearing in mind the relative sizes among Asph (110 atoms) and water (3 atoms) molecules, it is expected that the interaction energy among Asph and carbonate surface is much more negative than that among water and the same carbonate surface, explaining the known tendency of the carbonate rock to be oil-wet (Pons-Jiménez et al., 2015; Lopez-Chavez et al., 2014).
DFT optimized molecular models for SBGZL and HBGZL over A, C Cal and B, D Dol surfaces respectively, and for Asph over E Cal and F Dol surfaces. Atom colors are as in Fig. 1; additionally, dark and light green colors stand for Ca and Mg atoms, respectively.
Individual or interacting systems
Carbonate-surface simulation cell
Cal cell
Dol cell
Total energy (kcal/mol)
SBGZL
−2,926,840.81
−2,926,839.73
HBGZL
−3,069,493.08
−3,069,494.20
Asph
−1,605,263.02
−1,605,263.16
Carbonate surface
−70,629,863.94
−52,685,361.02
SBGZL on the carbonate surface
−73,556,996.58
−55,612,462.60
HBGZL on the carbonate surface
−73,699,632.38
−55,755,154.16
Asph on the carbonate surface
−72,235,338.79
−54,290,818.26
Interacting systems
Interaction energy (kcal/mol)
SBGZL on the carbonate surface
−291.83
−261.85
HBGZL on the carbonate surface
−275.36
−298.93
Asph on the carbonate surface
−211.83
−194.08
However, interaction energies among SBGZL, HBGZL and the carbonate surfaces are more negative than those yielded by Asph (Fig. 3 and Table 4). In fact, SBGZL adsorbs more strongly to Cal surface than HBGZL, leading to the better EOR performance of SBGZL over HBGZL when they act on Cal cores. It is worthy to note that theoretical results predict that on Dol cores HBGZL performs better than SBGZL instead. The theoretical result for Cal cores explains the above experimental result about the better EOR performance of SBGZL.
Following the molecular mechanism proposed by Mirna (Pons-Jiménez et al., 2014), there would exist supramolecular interactions among either SBGZL or HBGZL and the oil molecules to form zwitterion–dipole pairs. These pairs travel within the oil phase and arrive to the pore walls of cores, where BGZLs attractively interact with the carbonate surface more strongly than the adsorbed oil molecules and then latter are removed. Ether groups of BGZLs allow rock surface to become water-wet, emerging capillary forces that lead to water imbibition and thus the oil displacement. Details of this molecular mechanism explaining the enhancement of the oil recover will be given in a paper we are preparing.
4 Conclusions
Smart chemicals SBGZL and HBGZL were designed to have a greater adherence than oil to the oil reservoir rocks and then to change the rock wettability. The adsorption energies (i.e., the negative of the interaction energies) of SBGZL molecules over carbonate rock surfaces, in comparison with the ones of the Asph molecule, are around 38% and 35% greater for Cal and Dol surfaces; in the case of the HBGZL, this tendency is reversed with around of 54% and 30% for Dol and Cal surface, respectively. Experimentally, SBGZL-containing flooding water injected within Cal cores obtained 39.4% and 215.5% more recovery factor for light- and heavy-oil, respectively. Conversely, HBGZL obtained an oil recovery factor greater than that of the reference by 37.5% and 44.7% for light- and heavy-oil, respectively. The above theoretical and experimental results suggest that both SBGZL and HBGZL increase the oil recovery factor, regardless of the light or heavy nature of the oil, by alteration of the wettability of the firstly-oil-wet carbonate rock, and that the ab initio adsorption-energies computations of BGZLs and Asph molecules over carbonate rock could be of vital importance to infer the technical feasibility and moneymaking of BGZLs as chemicals for application in the EOR.
Acknowledgments
The authors express gratitude to the Instituto Mexicano del Petróleo for both providing facilities and granting permission to publish results. This work was supported by the project IMP-D.00509. R. Cisneros-Dévora, R. Cerón-Camacho and M. Pérez-Álvarez thank Dirección de Cátedras CONACyT for the financial support granted during the research reported at this manuscript.
References
- ASTM, D2007-11, 2011. Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petroleum-Derived Oils by the Clay-Gel Absorption Chromatographic Method 1, 1–8.
- Ab-initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys.. 1993;80:1431.
- [Google Scholar]
- Buenrostro-Gonzalez, E., Garcia-Martinez, J., Ruiz-Morales, Y., 2004. Una aproximación a la estructura molecular de asfaltenos separados de aceites crudos mexicanos, Instituto Nacional del Derecho de Autor, número de registro 03-2004-040212361000-01, México, Instituto Mexicano del Petróleo, 16 de abril de 2004.
- Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys.. 1987;86:866-872.
- [Google Scholar]
- Hernandez-Altamirano, R., Mena-Cervantes, V.Y., Zamudio Rivera, L.S., Flores-Sandoval, C.A., Ramírez-Estrada, A., Cisneros-Dévora, R., Martínez-Magadan, J.M., Oviedo-Roa, R., Ramírez-Pérez, J.F., 2014. Líquidos Zwitteriónicos Geminales Base Sulfobetaína e Hidroxisultaína, Proceso de Obtención y uso como Modificadores de la Mojabilidad con Propiedades Inhibitorias de la Corrosión, MX/a/2014/015226.
- COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans.. 1993;2:799-805.
- [Google Scholar]
- Quantum chemical characterization of zwitterionic structures: Supramolecular complexes for modifying the wettability of oil–water–limestone system. J. Mol. Graph. Model.. 2014;51:128-136.
- [Google Scholar]
- Density functional theoretical study of the interaction of geminal zwitterionic liquids with limestone, regarding the behavior of the wettability parameter. J. Chem. Eng. Data. 2012;57(12):3538-3542.
- [Google Scholar]
- Computational modeling of the influence of geminal zwitterionic liquids on changes in the parameters of wetting of oil-rock system. Mater. Res. Soc. Symp. Proc.. 2012;1473:32-37.
- [Google Scholar]
- Semiempirical van der Waals correction to the density functional description of solids and molecular structures. Phys. Rev. B. 2006;73:205101.
- [Google Scholar]
- Theoretical and experimental insights on the true impact of C12TAC cationic surfactant in enhanced oil recovery for heavy oil carbonate reservoirs. Colloids Surf., A. 2014;455:76-91.
- [Google Scholar]
- Supramolecular pairing among heteroaromatic compounds and the cationic surfactant C12TAC. Fuel. 2015;149:174-183.
- [Google Scholar]
- Version 7.0, Accelrys Software Inc., accelrys.com, 2012.
- Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys.. 1980;58:1200-1211.
- [Google Scholar]







