Translate this page into:
A highly active copper-based metal-organic framework catalyst for a friedel–crafts alkylation in the synthesis of bis(indolyl)methanes under ultrasound irradiation
⁎Corresponding authors. ntkphuong@inomar.edu.vn (Phuong T.K. Nguyen), thphuong@hcmus.edu.vn (Phuong Hoang Tran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An efficient and robust copper-based metal-organic framework catalyst for a first preparation of bis(indolyl)methanes under ultrasound irradiation.
Abstract
A robust copper-based metal-organic framework (MOF) catalyst, termed MOF-891, was successful applied to the synthesis of the family of bioactive compounds, bis(indolyl)methanes, through a Friedel–Crafts alkylation under ultrasound irradiation. The remarkable catalytic activity of MOF-891 was proven by the successful synthesis of bis(indolyl)methanes in high yield (up to 96%) and purity. In MOF-891, there exist two types of copper clusters, one discrete and one infinite rod, both are formed through water molecules triggering hydrogen bonds demonstrated the participation as catalytic active site for the Friedel–Crafts alkylation reaction. The well-defined construction of hydrogen bonds in MOF-891 along with Lewis copper sites attributed a stable catalysis activity and exhibited the heterogeneous nature with no significant loss of catalytic performance after six cycles.
Keywords
Heterogeneous catalysis
Metal-organic framework
Indoles
Bis(indolyl)methanes
Sonochemistry
1 Introduction
Bis-heterocyclic compounds, such as bis(indolyl)methanes, are well-known fine chemicals for their vast biomedical properties as well as their use in colorimetric sensing applications (He et al., 2006; Kamal et al., 2009; Safe et al., 2008; Tietze et al., 2002). The synthesis of bis(indolyl)methanes occurs through a Friedel–Crafts alkylation reaction between indoles and carbonyl compounds in the presence of an acidic catalyst (Bartoli et al., 2010; Dalpozzo, 2015; Das et al., 2013; Shiri et al., 2010). This method combines high atom economy with the capability to affect a wide-range of functionalized substrates, and the acid promoted electrophilic substitution of indoles with carbonyl compounds is the favourable methodology. Although numerous homogeneous (Abe et al., 2013; Ganesan et al., 2015; Safe et al., 2008; Zhao et al., 2013) and heterogeneous (Karmakar et al., 2016; Ramesh et al., 2003; Sandtorv, 2015; Satam et al., 2008; Shirini et al., 2016) catalysts have been reported, there remains a noticeable absence of synthesizing heterogeneously robust catalysts that can combine multiple active acidic sites prompting to enhance the catalytic performance. In fact, the high catalytic activity (high yield, short reaction time, and under mild reaction conditions), the applicability of green energy sources, for instance, ultrasound irradiation, as well as the facile recyclability over many consecutive reactions are the prominent demands in the development of new heterogeneous catalyst (Shiri et al., 2010). Accordingly, the approach of preparing the feasible catalytic platforms offer the multiple catalytic sites is a much-desired methodology.
Metal-organic frameworks (MOFs) represent a viable material for achieving this objective. MOFs are designable, crystalline compounds that are constructed from linking inorganic metal clusters with organic units via strong bonds (Furukawa et al., 2013). As a result of their tunable pore diameters, judicious internal environment, chemical stability, and high density of active sites (either from the inorganic units, organic units, or a combination of both) offer MOFs tremendous opportunities for use as heterogeneous catalysts for fine chemical synthesis (Chughtai et al., 2015) such as N-containing heterocycles compounds with the remarkably biological and pharmacological activities (Dhakshinamoorthy and Garcia, 2014), including pyrimidines (Heys et al., 2000), propargylamines (Konishi et al., 1990), quinolines (Marco-Contelles et al., 2009), indoles (Cacchi and Fabrizi, 2005; Huang et al., 2011; Li et al., 2011), triazoles (Luz et al., 2010), and heterocyclic amides (Allen and Williams, 2011; Bromberg et al., 2012).
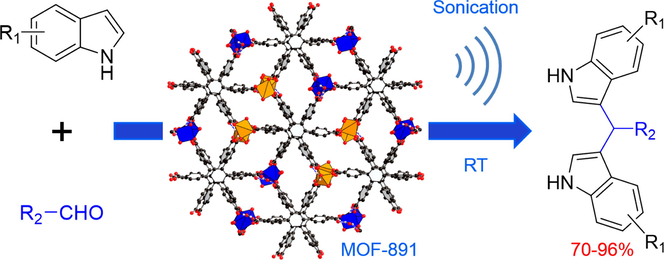
MOF formed of hydrogen bonds inducing metal cluster (Custelcean et al., 2010; Horike et al., 2009; Schneemann et al., 2014), a highly functionalized class of porous material, has been exploited in various applications, such as separation (Ye et al., 2016), adsorption (Uemura et al., 2006), catalysis (Hall et al., 2016), chemical sensing (Azhdari Tehrani et al., 2017), conductivity (Gao et al., 2015), and drug release (Liu et al., 2016). In fact, the hydrogen bonding-function materials have been demonstrated the importance in the activation of electrophilic components toward nucleophilic addition in the Friedel–Crafts alkylation reactions (McGuirk et al., 2015; Zhang et al., 2016). In this contribution, we describe the first use of a copper-based MOF, termed MOF-891 (Nguyen et al., 2015), in which copper clusters are formed of water molecules triggering hydrogen bonds, as an heterogeneous catalyst to promote the electrophilic substitution of indoles with aromatic aldehydes under ultrasonic irradiation (Fig. 1). It is a crucial distinction that MOF-891 was proven to be an effective catalyst under ultrasonic irradiation, which led to short reaction times (1.5–2.5 h), mild conditions (room temperature), and high yield (up to 96%) for the synthesis of bis(indolyl)methane derivatives. Through this work, we further demonstrate MOF-891s recyclability and significant outperformance of other MOFs and commonly used homogeneous and heterogeneous catalysts.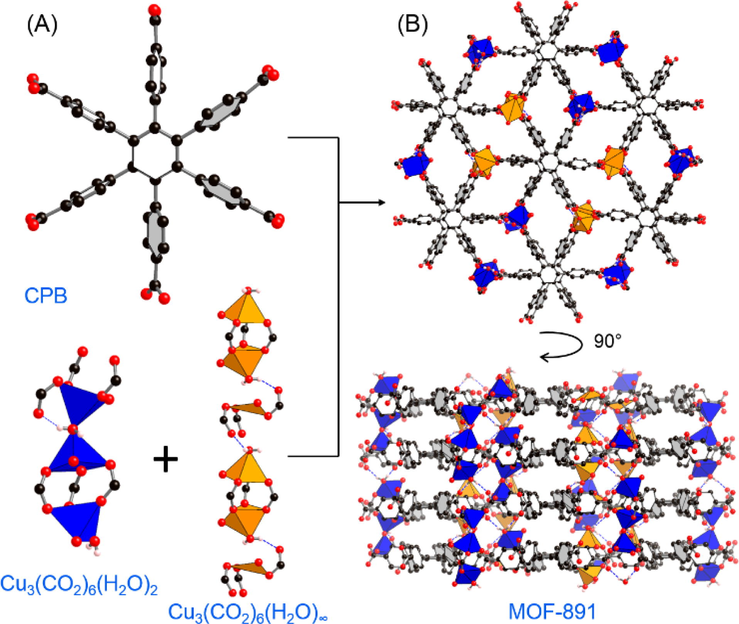
Crystal structure of MOF-891. (A) Trigonal prism and infinite rod-shaped secondary building units are connected by a hexagonal CPB linker to produce (B) MOF-891. Color code: Cu, blue and orange polyhedra; C, black, O, red. All H atoms of the benzene units are omitted for clarity. Redrawn from CCDC depositions 1428731.
2 Experimental
2.1 Instrumentation and general procedures
Fourier transform infrared (FT-IR) spectra were measured using KBr pellets on a Bruker Vertex 70 system. Powder X-ray diffraction (PXRD) data were collected using a Bruker D8 Advance employing Ni-filtered Cu Kα (λ = 1.54178 Å). Nitrogen adsorption isotherms were recorded on a Micromeritics 3Flex. Helium (99.999% purity) was used to estimate the dead space and ultrahigh-purity-grade N2 (99.999% purity) was used throughout the adsorption experiments. Thermal gravimetric analysis (TGA) was performed on a TA Q500 thermal analysis system with the sample held in a platinum pan in a continuous airflow. Ultrasonic irradiation was performed on an Elma S30H Ultrasonic cleaning unit (ultrasonic frequency of 37 kHz, nominal power of 280 W and output of 80 W). Gas chromatography-mass spectrometry (GC–MS) measurements were carried out on an Agilent GC System 7890 equipped with a mass selective detector (Agilent 5973 N) and a capillary DB-5MS column (30 m × 250 µm × 0.25 µm). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Advance II 500 MHz NMR spectrometer. Chemical shifts were quoted in parts per million (ppm) and referenced to the appropriate solvent peak or 0 ppm for tetramethylsilane (TMS). High-resolution electrospray-ionization mass spectrometry (HR-ESI-MS) was conducted in negative ionization mode on an Agilent 1200 series high-performance liquid chromatography coupled to a Bruker micrOTOF-QII mass spectrometer detector. Inductively coupled plasma mass spectroscopy (ICP-MS) data was collected on an Agilent ICP-MS 7700x instrument. Field-emission Scanning Electron Microscope (FE-SEM) was performed on an ultralow voltage imaging with Hitachi's S-4800 FE-SEM operating at an accelerating voltage of 1 kV.
2.2 Synthesis of MOF-891 and other catalysts
MOF-891 was synthesized according to reported procedure (Nguyen et al., 2015). A solid mixture of H6CPB (20 mg, 0.025 mmol) and Cu(NO3)2·3H2O (2.42 mg, 0.01 mmol) was dissolved in a mixture of DEF/H2O (0.5/3.0 mL) in an 8-mL glass vial. The mixture reaction solution was then heated at 85 °C for 16 h to yield blue, block-shaped crystals. The crystals were thoroughly washed with DMF (3 × 10 mL) per day for three days total. To obtain guest-free material, the DMF was decanted, MOF-891 was then immersed in anhydrous MeOH (3 × 10 mL) per day over a total of three days. The solvent-exchanged sample was activated under vacuum at ambient temperature for 16 h, followed by heating at 150 °C under vacuum for an additional 24 h, obtained a green crystal phase (80% yield based on H6CPB linker).
MOF-890, Cu-MOF-2. Full synthetic and characterization details can be found in the Supporting Information (SI, Section S2). HKUST-1 (Basolite C300), MOF-177 (Basolite Z377), and ZIF-8 (Basolite Z1200) were purchased from Sigma-Aldrich. These were activated under vacuum (10−3 Torr) and heated at 120, 150, 100 °C for 24 h, respectively, to obtain guest-free materials. Structure and porosity of all MOF catalysts were confirmed by powder X-ray diffraction, TGA, and N2 adsorption measurements at 77 K.
2.3 Catalytic studies
In a model reaction, an indole (0.234 g, 2 mmol) and benzaldehyde (0.106 g, 1 mmol) in m-xylene (2 mL) were added into a flask pre-charged with MOF-891 catalyst (0.013 g, 0.01 mmol, the molecular weight of MOF unit: 1322.76 g mol−1). The reaction was then sonicated at RT for 1.5 h and monitored by TLC and GC–MS. After completion of reaction, the MOF-891 catalyst was filtered from the reaction mixture and the organic layers were evaporated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (90:10 acetone/petroleum ether) in order to afford the pure product 3,3′-(phenylmethylene)bis(1H-indole) (1), which was confirmed via FTIR, 1H NMR, 13C NMR, HR-ESI-MS and GC–MS. For the recycling experiment, the recovered catalyst was washed with acetone (3 × 2 mL) and ethanol (3 × 2 mL) before being isolated by centrifugation. The catalyst was then dried in air and re-applied to the next cycle.
3 Results and discussion
The MOF-891 framework consists of two types of Cu(II) clusters: one that forms infinite rods, [Cu3(CO2)6(H2O)2]∞, and the other, a trigonal prism, [Cu3(CO2)6(H2O)2]. Each discrete unit is built and connected, at least in part, through hydrogen bonding (Fig. 1A). As a result, MOF-891 is formulated as [Cu3(CPB)(DEF)0.4(H2O)3.8 (calculated from elemental analysis). Two important structural characteristics are Lewis acidic Cu(II) units (Alonso et al., 2008; Poulsen and Jørgensen, 2008; Singh and Singh, 2008) as well as water inducing hydrogen bonds, hypothetically served as strong Brønsted acidic sites (Jiang et al., 2014), which offer permanent and highly active catalytic sites along the available windows of MOF-891. Taken together, the interesting structure of MOF-891 potentially provide a catalytic platform for acid-catalyzed reactions.
Following structural analysis, initial catalytic activity was assessed through an exemplary reaction between indole and benzaldehyde. Specifically, the solvent conditions were first screened with 1 mol% of the MOF-891 catalyst at room temperature under ultrasound irradiation.
Reasonable yields were obtained in all polar protic solvents and almost all polar aprotic solvents as evidenced by 68–82% isolated yields of 1 (Table 1, entries 1–5). For nonpolar solvents, 1,4-dioxane and toluene afforded similar results (75 and 85% yields for 1, respectively) as the polar solvents with the exception of m-xylene, which achieved the highest yield of 92% for 1 after 1.5 h. Besides, there were no traces of byproduct detected (Table 1, entry 9). Moreover, to demonstrate the effect of ultrasound irradiation on the reaction of MOF-891-based catalyst, control experiments were then carried out in the absence of ultrasound irradiation. These reactions, performed with magnetic stirring at 80 °C for both 1.5 and 3 h, resulted in product 1 being obtained with lower yields of 73% and 80%, respectively.
Entrya
Type of solvent
Solvent
Yieldb (%)
1
Polar protic
Ethanol
82
2
t-butanol
80
3
n-butanol
78
4
Polar aprotic
Ethyl acetate
80
5
THF
80
6
DMF
68
7
Non polar
1,4-dioxane
75
8
Toluene
85
9
m-xylene
92
10
p-xylene
70
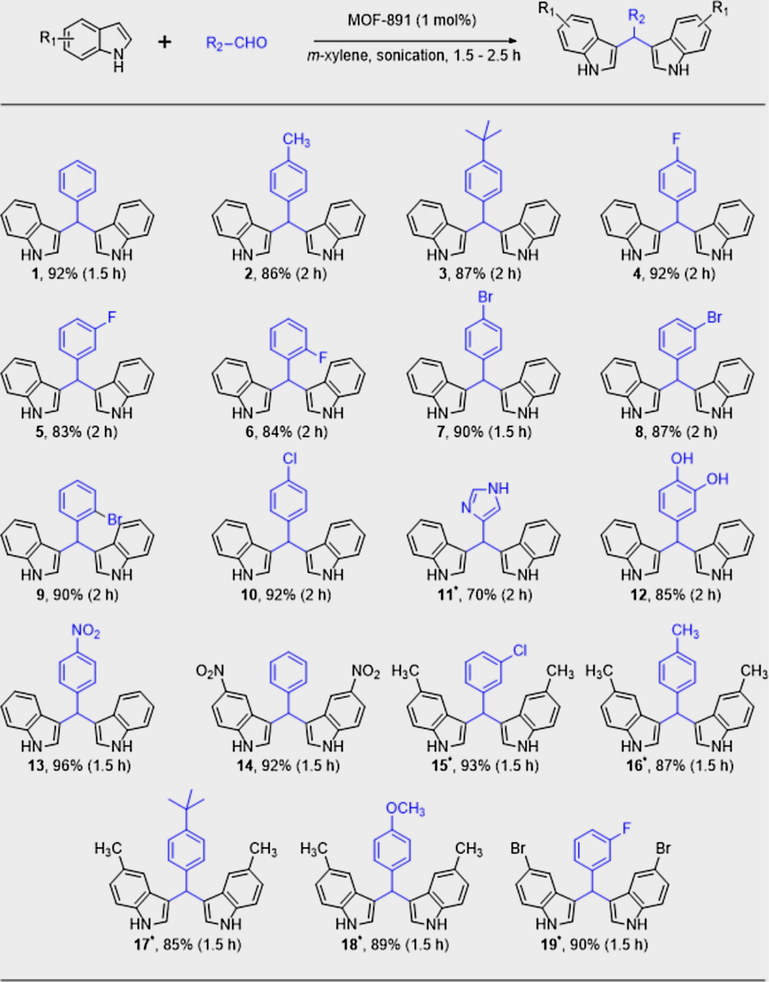
After optimizing the solvent system and demonstrating the importance of ultrasound irradiation, the versatility of the catalyst toward a wide substrate scope was investigated (Table 2, Section S3). As a result, there were no significant differences in reactivity when electron-withdrawing or electron-donating substituents were present in the aromatic ring of benzaldehyde substrates (Table 2, 1–10, 12–19). In fact, pharmacologically relevant substitution patterns on the aromatic ring can be targeted with high efficiency by using this procedure. Heterocyclic aldehydes, such as 1H-imidazole-4-carbaldehyde, were transformed to the desired product 11 in a moderate yield, presumably due to a minor interaction of the amine with the MOF-891 catalyst. The alkylation was also proceeded smoothly with 3,4-dihydroxybenzaldehyde provided 12 with 85% isolated yield. Finally, higher reactivity for 5-methyl-1H-indole and 5-bromo-1H-indole was observed, when using MOF-891, to the respective bis(indolyl)methane products in high yield. Therefore, suffice to say, the exceptional activity of MOF-891 was confirmed for a wide range of substrates.
Entry
Type
Catalyst
Yieldb (%)
1
MOFs
MOF-891
92 (91)c
2
MOF-890
83
3
HKUST-1
85
4
Cu-MOF-2
55
5
ZIF-8
60
6
MOF-177
80
7
Heterogeneous catalysts
CuO
60
8
ZnO
55
9
Al2O3
56
10
MgO
57
11
Fe2O3
52
12
TiO2
38
13
Homogeneous catalysts
CuSO4
75
14
Cu(NO3)2·3H2O
80
15
CuCl2·2H2O
81
16
Cu(CH3COO)2
63
17
AlCl3·6H2O
73
18
FeCl3
76
19
HfCl4
72
20
No catalyst
0
Entry
Catalyst (mol%)
Temp. (°C)
Time (h)
Yield (%)a
Ref.
1
MOF-891 (1)
r.t
1.5
92
This work
2
PdCl2(MeCN)2 (5)
r.t
10
80
Mohapatra et al. (2015)
3
Dy(OTf)3 (10)
r.t
24
84
Mi et al. (2004)
4
[DABCO-H][HSO4] (10)
90 °C
2
91
Xu et al. (2016)
5
[Cu(3,4-tmtppa)](MeSO4)4 (1)
90 °C
2
92
Sobhani et al. (2009)
6
Cu(BF4)2·SiO2 (5)
80 °C
0.5
96
Meshram and Patil (2009)
7
Cu/MWCNT-GAA@Fe3O4 (0.2)
70 °C
1.3
88
Shaabani et al. (2016)
8
Cu-isatin Schiff base-γ-Fe2O3 (0.5)
80 °C
2
81
Sobhani et al. (2016)
9
HY-Zeolite, 500 mg
r.t
2
80
Karthik et al. (2005)
10
AS-13, 50 mg
Reflux
4
84
Kubczyk et al. (2011)
11
Indion Ina 225H resin, 100 mg
50 °C
2.5
90
Surasani et al. (2013)
12
GO, 150 mg
40 °C
3
76
Wang et al. (2017)
To insure the catalytic activity did not originate from any leaching of Cu(II) ions from MOF-891 into the reaction mixture, a model reaction was stopped after 0.5 h, and MOF-891 was separated from the reaction by centrifugation. The reaction solution was further sonicated for 1 h and afforded only 52% isolated yield of the product 3,3′-(phenylmethylene)bis(1H-indole). The same experiment was taken and stopped at 1 h. The reaction was continuously sonicated for 0.5 h and afforded 78% isolated yield of 1. Consequently, the formation of 1 could not proceed after the MOF-891 catalyst was separated from the reaction solution, while 92% yield of 1 was obtained in the heterogeneous catalysis under ultrasonic irradiation for 1.5 h (Section S4, Table S2).
To confirm that the catalytic activity was a result of MOF-891 (as opposed to leach Cu(II) ions), the catalyst was filtered off at the end of the reaction, and an aliquot was taken from the reaction mixture filtrate for analysis by inductively coupled plasma mass spectrometry (ICP-MS). Accordingly, ICP-MS analysis revealed the Cu(II) concentration to be <2 ppm (Section S4), again confirming that the source of catalytic activity was in fact MOF-891.
Following this, the heterogeneous nature of MOF-891 was assessed through recycling studies carried out with the facile preparation for the next catalytic reactions under identical conditions. As expected, the MOF-891 catalyst could be successfully reused for at least 6 consecutive runs without significant loss in catalytic activity (Fig. 2). PXRD (Fig. 3a) and FT-IR analysis (Fig. 3b) of MOF-891 revealed that the crystallinity and integrity of this material was maintained after the sixth cycle.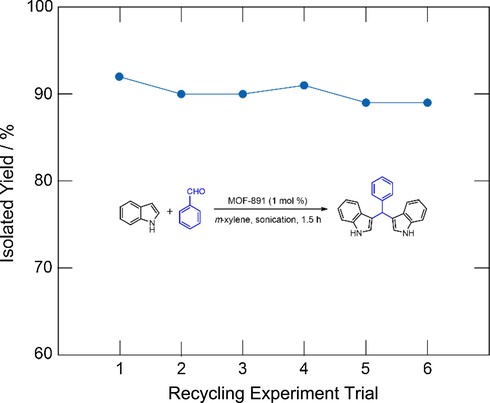
Catalytic activity of MOF-891 in the synthesis of 3,3′-(phenylmethylene)bis(1H-indole) over six recycling steps.
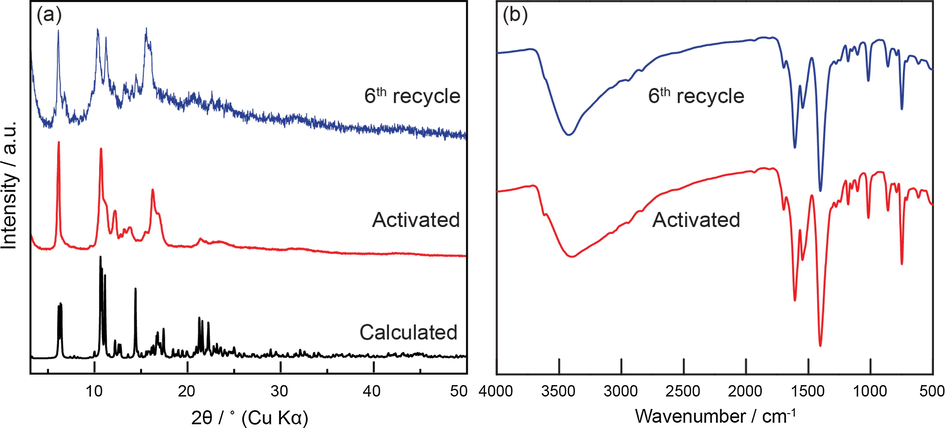
PXRD (a) and FT-IR (b) of an activated MOF-891 (red) and after 6th time recycling and reusing of MOF-891 (blue).
Furthermore, SEM images of the guest-free parent (Fig. 4a) and recovered MOF-891 after catalytic reactions (Fig. 4b) confirmed the layered-like morphology of the catalyst was retained after the several recycle reactions.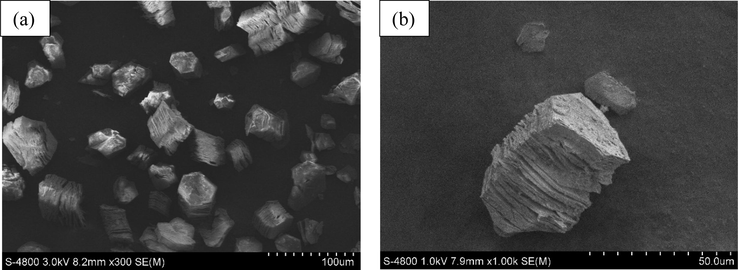
SEM images of the MOF-891 catalyst after activation (a) and 6th recycle experiment (b).
The importance of MOF-891 as a heterogeneous catalyst was elaborated by carrying out comparative studies, in which the catalytic activity of other MOFs as well as relevant homogeneous and heterogeneous catalysts were assessed (Table 3). Overall, the homogeneous metal salts and heterogeneous metal oxides exhibited lower yields than MOF-891 in the case study of 3,3′-(phenylmethylene)bis(1H-indole) synthesis. The most active homogeneous catalyst was CuCl2·2H2O, which yielded 81% product in comparison to the 92% yield achieved by MOF-891. It is noted that MOF-891 has the added advantage of being recyclable where CuCl2·2H2O does not. Furthermore, CuO was found to be the most active heterogeneous catalyst (60%), which was significantly lower than MOF-891. To compare catalytic activity with other microporous, Lewis acidic MOFs, MOF-890 (Nguyen et al., 2015), HKUST-1 (Furukawa et al., 2011), Cu-MOF-2 (Carson et al., 2009; Kim et al., 2001), ZIF-8 (Wang et al., 2008), and MOF-177 (Furukawa et al., 2007) were chosen as representative examples. Indeed, MOF-891 again outperformed all as evidenced by the highest yield of 1, while MOF-890, HKUST-1, and MOF-177 afforded moderate yields for the desired product (Table 3, entries 2, 3, and 6). The catalytic activity of Cu-MOF-2 and ZIF-8 were observed to have the lowest performance of all MOFs tested for this transformation under the same conditions.
In order to clarify the relationship between the pore size metrics of the MOF catalysts and the catalytic performance, the pore size distribution of MOF catalysts were calculated from their N2 isotherm measurements used a Non-Local Density Functional Theory (NLDFT) (Section S2). The experimental results were observed in line with that of the values estimated from the crystal structure models (Section S2, Figs. S4–S5, Table S1). Accordingly, MOF-891, MOF-890, and Cu-MOF-2, existing the narrow pore-type materials (Table S1), the layered-liked structures, and serving the Lewis acid sites of copper clusters. However, MOF-890 and Cu-MOF-2 exhibited noticeably less catalytic activity when compared to that of MOF-891. The other Lewis acid MOFs, HKUST-1, ZIF-8, and MOF-177, with higher surface area as well as exceeding pore size metric, which did not readily appear to impact the catalytic activities. In short, the pore size metric of MOF catalyst was not a significant function controlling the catalysis reaction in the synthesis of bis(indolyl)methanes.
Futhermore, it is important to note that MOF-890, which is structurally similar to MOF-891, comprises an identical rhomboidal channels, and offers discrete Cu(II) units linked by the CPB linker (Fig. S1) (Nguyen et al., 2015). However, there is one important difference: the water molecules inducing hydrogen bonds within the Cu(II) units is noticeably absent in MOF-890. These structure-function relationships provide insight into the remarkable catalytic bis(indolyl)methanes formation. Accordingly, we attribute the origin of the outperformed catalytic activity of MOF-891 to the clusters of water molecules triggering hydrogen bond linking the infinite [Cu3(CO2)6(H2O)2]∞ rod units. These acidic sites originated from hydrogen bonds are oriented and active, which affords them with the opportunity to promote interactions between the substrates and active sites. Therefore, we emphasize the importance of MOF-891s well-oriented Cu(II)-based rods with hydrogen bonds in the formation of bis(indolyl)methanes.
In addition, the efficiently catalytic performance of MOF-891 catalyst for the synthesis of bis(indolyl)methanes was compared with those of other reported catalysts summarized in Table 4. Overall, MOF-891 again showed, short reaction time, mild condition (room temperature) and excellent yield of product with a rational amount of employed catalyst in the comparison with other reported compounds. All in all, MOF-891 was demonstrated as an efficient heterogeneous catalyst in the synthesis of bis(indolyl)methanes.
From the rationalization of the structure-function relationship, we identify a plausible mechanism for the preparation of bis(indolyl)methanes in the presence of the heterogeneous catalyst (Scheme 1) (Shiri et al., 2010). In MOF-891, the active sites derived from the Cu(II) clusters, [Cu3(CO2)6(H2O)2] and [Cu3(CO2)6(H2O)2]∞, are lied on water molecules yielding the hydrogen bonds. These copper-based clusters provide the highly active acidic sites affording an activation of the carbonyl group of benzaldehyde. This activated carbonyl then proceeds to a condensation reaction. The appropriate indole attacks the activated carbonyl group, followed by elimination of H2O, to form the intermediate. The intermediate is subsequently attacked by a second indole, which inevitably leads to the formation of the final bis(indolyl)methane product (Scheme 1).![A proposed mechanism for the synthesis of indoles with different carbonyl compounds in the presence of MOF-891s active site: [Cu3(CO2)6(H2O)2] taken from Cu(II) infinite rods, [Cu3(CO2)6(H2O)2]∞. The principal catalysis is influenced by water molecules inducing hydrogen bonds, which are present in the Cu(II) active sites contained in MOF-891.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.11.009-fig6.png)
A proposed mechanism for the synthesis of indoles with different carbonyl compounds in the presence of MOF-891s active site: [Cu3(CO2)6(H2O)2] taken from Cu(II) infinite rods, [Cu3(CO2)6(H2O)2]∞. The principal catalysis is influenced by water molecules inducing hydrogen bonds, which are present in the Cu(II) active sites contained in MOF-891.
According to the proposed mechanism, in order to gain more insight about the position where catalytic reaction could undergo, we evaluated the dimensional calculations of products 1, product 17, which are respected to be the smallest and largest products, respectively, and their corresponding substrates including indoles and benzaldehydes. All compounds were optimized by using the Gaussian09 computational suite (Frisch et al., 2009) with the B3LYP method at the 6-311G(d,p) level (Boese and Martin, 2004) (Table S3) and pore sizes of MOF aperture were estimated from pore size distribution measurement (Table S1). As shown in Table S3, the sizes of the calculated substrates as well as the short dimension of product 1 (11.5 Å) were obviously smaller than those of accessible MOF’s aperture (8.2 × 12.3 Å2), while the metrics of a product 17 (12.9 × 16.4 Å2) are totally larger than those of the MOF’s window. Thus, we demonstrated that the molecules of substrate and product 1 could take the diffusions into MOF pores during the catalytic reactions, but the molecule of product 17 would not be able to access and synthesized inside the MOF pores. Moreover, the production of 17 was obtained efficiently with a high yield of product (85%), regardless of the restricted pore size of MOF for the conformation of the bulky product. These points supported us to conclude that the synthesis of bis(indolyl)methanes were mainly catalyzed by the catalytic active sites exposed to the outside surface rather than in the interior pores of MOF (Jiang and Yaghi, 2015; Sun et al., 2009).
4 Conclusion
In summary, MOF-891 was demonstrated to exhibit remarkable catalytic activity in the bis(indolyl)methanes synthesis due to the synergy of the Lewis acidic copper sites and the infinite hydrogen bond-linking the copper-based clusters. The method was carried out under mild conditions (room temperature, 1.5–2 h) with the effective assistance of ultrasound irradiation leading to excellent product yields (up to 92% yield). MOF-891 as a heterogeneous catalyst was proven to be superior over other structurally similar MOFs as well as MOFs with varying dimensionality and pore sizes. Moreover, MOF-891 outperformed commonly used homogeneous metal salts and heterogeneous Lewis acidic catalysts. Finally, the heterogeneous nature of the MOF-891 catalyst was proven as evidenced by no significant loss in performance when employed as a catalyst for six successive runs.
Acknowledgements
We thank Dr. H.T.C. Ho and Dr. Q.T. Ton at the University of Science (VNU-HCM) for their valuable input. This work was financially supported by VNU-HCM (No. A2015-50-01-HĐ-KHCN) and the United States Office of Naval Research Global: Naval International Cooperative Opportunities in Science and Technology Program (No. N62909-15-1N056).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Org. Lett.. 2013;15:3622-3625.
- Chem. Soc. Rev.. 2011;40:3405-3415.
- J. Org. Chem.. 2008;73:6401-6404.
- Inorg. Chem.. 2017;56:1446-1454.
- Chem. Soc. Rev.. 2010;39:4449-4465.
- J. Chem. Phys.. 2004;121:3405-3416.
- Chem. Mater.. 2012;24:1664-1675.
- Chem. Rev.. 2005;105:2873-2920.
- Eur. J. Inorg. Chem.. 2009;2009:2338-2343.
- Chem. Soc. Rev.. 2015;44:6804-6849.
- J. Am. Chem. Soc.. 2010;132:7177-7185.
- Chem. Soc. Rev.. 2015;44:742-778.
- RSC Adv.. 2013;3:14308-14311.
- Chem. Soc. Rev.. 2014;43:5750-5765.
- Furukawa, H., Cordova, K.E., O’Keeffe, M., Yaghi, O.M., 2013. Science 341.
- Inorg. Chem.. 2011;50:9147-9152.
- J. Mater. Chem.. 2007;17:3197-3204.
- Frisch, M.J., et al., 2009. Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT.
- RSC Adv.. 2015;5:28597-28600.
- J. Mater. Chem. A. 2015;3:22347-22352.
- ACS Catal.. 2016;6:3248-3252.
- Org. Lett.. 2006;8:333-336.
- Chem. Soc. Rev.. 2000;29:57-67.
- Nat. Chem.. 2009;1:695-704.
- Chem. – Eur. J.. 2011;17:12706-12712.
- J. Am. Chem. Soc.. 2014;136:12844-12847.
- Chem. Rev.. 2015;115:6966-6997.
- J. Enzyme Inhib. Med. Chem.. 2009;24:559-565.
- Org. Lett.. 2016;18:5200-5203.
- Appl. Catal. A. 2005;286:137-141.
- J. Am. Chem. Soc.. 2001;123:8239-8247.
- J. Am. Chem. Soc.. 1990;112:3715-3716.
- Green Chem.. 2011;13:2320.
- ACS Catal.. 2011;1:1604-1612.
- Int. J. Nanomed.. 2016;11:1187-1200.
- J. Catal.. 2010;276:134-140.
- Chem. Rev.. 2009;109:2652-2671.
- J. Am. Chem. Soc.. 2015;137:919-925.
- Synth. Commun.. 2009;40:29-38.
- Tetrahedron Lett.. 2004;45:4567-4570.
- Tetrahedron Lett.. 2015;56:5709-5713.
- Inorg. Chem.. 2015;54:10065-10072.
- Chem. Rev.. 2008;108:2903-2915.
- Adv. Synth. Catal.. 2003;345:557-559.
- Cancer Lett.. 2008;269:326-338.
- Adv. Synth. Catal.. 2015;357:2403-2435.
- Catal. Commun.. 2008;9:1071-1078.
- Chem. Soc. Rev.. 2014;43:6062-6096.
- RSC Adv.. 2016;6:18113-18125.
- Chem. Rev.. 2010;110:2250-2293.
- RSC Adv.. 2016;6:48469-48478.
- Org. Lett.. 2008;10:4121-4124.
- J. Organomet. Chem.. 2016;822:154-164.
- J. Organomet. Chem.. 2009;694:3027-3031.
- J. Am. Chem. Soc.. 2009;131:1883-1888.
- Green Chem. Lett. Rev.. 2013;6:113-122.
- Eur. J. Org. Chem.. 2002;2002:1634-1645.
- J. Am. Chem. Soc.. 2006;128:16122-16130.
- Wang, B., Cote, A.P., Furukawa, H., O/'Keeffe, M., Yaghi, O.M., 2008. Nature 453, 207–211.
- Catal. Commun.. 2017;89:138-142.
- Synthesis. 2016;48:3559-3566.
- Inorg. Chem.. 2016;55:292-299.
- Chem. Commun.. 2016;52:8585-8588.
- RSC Adv.. 2013;3:10272-10276.
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2017.11.009.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







