Translate this page into:
Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems
⁎Corresponding author. andre.fajardo@pq.cnpq.br (André R. Fajardo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
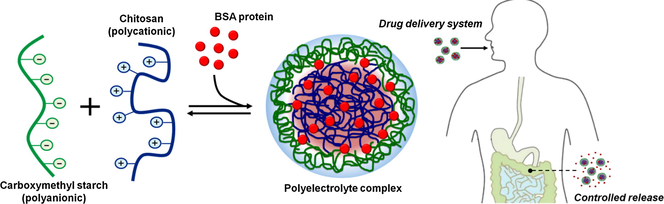
Microparticles with oval-shape morphology and rough and porous surfaces were prepared by polyelectrolyte complexation of carboxymethyl starch (CMS) and chitosan (Cs). CMS with DS of 0.5, the polyanionic moiety, was synthesized from rice starch with low content of amylose (6%). A preliminary investigation revealed that this kind of starch is more susceptive to esterification than rice starches with higher contents of amylose. The CMS/Cs microparticles showed higher chemical and thermal stability than microparticles prepared by conventional ionotropic crosslinking of Cs with TPP ions. The carboxymethyl groups of CMS are more efficient to neutralize the positive groups of Cs and, also, enhance the entrapment of bovine serum albumin (BSA) in the CMS/Cs matrix as compared to Cs/TPP. In vitro experiments conducted in simulated gastric fluid (pH 1.2) and simulated intestinal fluid (pH 6.8) with the testing microparticles revealed that the CMS/Cs-BSA microparticles exhibit a highlighted pH-dependent release profile. This desirable property allows controlling the release of BSA more efficiently, which minimizes undesirable issues (e.g. burst effect and non-sustained release). Furthermore, the BSA release from CMS/Cs-BSA microparticles in SIF follows an ideal Zero-order kinetics, which is very attractive for a drug delivery system. Therefore, microparticles based on CMS/Cs polyelectrolyte complex may be promising to control the drug release in specific regions of the gastrointestinal tract.
Keywords
Carboxymethyl starch
Chitosan
Polyelectrolyte complex
Drug release
Controlled release
Bovine serum albumin
1 Introduction
Drug delivery systems (DDS) can improve the efficacy and safety of drug administration, since they control the rate, time, and place of drug release (Fu and Kao, 2010; Gombotz and Wee, 2012). Several studies in the literature describe the use of polymeric-based materials as efficient DDS (Al Dalaty et al., 2016; Nayak et al., 2016). Biopolymers and/or their derivatives are especially attractive to DDS formation due to their renewability, low-cost, non-toxicity, good biological performance, and controlled biodegradability (Facchi et al., 2017; Garcia-Gonzalez et al., 2015). Starch is the most abundant storage biopolymer constituted by two macromolecular complexes, amylose and amylopectin. The proportion of between these macromolecules varies according to the botanic origin of starch (Masina et al., 2017). Amylose is a linear polysaccharide of glucose units linked by α-1,4 glycosidic bonds, while the amylopectin, compared to amylose, has additional branched parts in α-1,6 glycosidic links (Ismail et al., 2013). Native starch shows some undesirable characteristics such as insolubility in cold water, excessive viscosity after heating and tendency to retrogradation, which impairs its use (Colussi et al., 2014; Sangseethong et al., 2015). In this way, to overcome such limitations, different strategies to modify chemically the starch backbone have been described by different studies (Ashogbon and Akintayo, 2014; Chen et al., 2015; Yousefi and Razavi, 2015). Carboxymethyl starch (CMS), one of the most important starch-derivatives, is synthesized by the etherification of free hydroxyl groups of starch with carboxymethyl groups (—CH2COOH) (Assaad and Mateescu, 2010; Zdanowicz et al., 2014). This attractive derivative has been widely used in DDS formulation as reported elsewhere (Assaad et al., 2011; Ispas-Szabo et al., 2017). In contrast to the native starch, CMS is soluble in cold water and it does not exhibit the undesirable tendency to retrogradation. Besides that, depending on the degree of substitution (DS), the presence of carboxymethyl groups in CMS backbone ensure mucoadhesivity and a highly pH responsiveness, two important characteristics to target delivery of drugs (Saboktakin et al., 2009; Saikia et al., 2015).
There is recent growing interest in polyelectrolyte complexes (PEC) as DDS due to their easy preparation protocol generally performed under mild and non-toxic conditions (Meka et al., 2017). Moreover, the pH-dependent property presented by these complexes allows controlling the release of several drugs, proteins, and genes (Assaad et al., 2011; Lopes et al., 2013; Vehlow et al., 2016). PEC can be formed when polymers with opposite charges are mixed in an aqueous medium. Electrostatic interactions between these charged polymers gain in entropy due to the dissociation of the counter ions to the medium leading to PEC formation (Sarika and James, 2016). Other forces like hydrogen bonding (H-bond), van der Waals forces, hydrophobic, and dipole interactions are also relevant to the PEC formation. These interactions between the polymeric chains led to the formation of non-permanent tridimensional networks without the need for chemical crosslinkers. The formation and stability of PEC depend on many factors including the pH, temperature, and ionic strength of complexation medium. Furthermore, the ratio of polymers/charges, the degree of ionization of the polymers, the polymer charge density, the molecular weight, and polymer chain flexibility affects the PEC formation (Fajardo et al., 2011). Usually, when one of the components is in excess, PEC formation can lead to a stable dispersion (Sarika and James, 2016). Another important aspect of PEC formation is related to the interactions between the polymers that could be a result of the polymer friction charge. According to the Flory-Huggins interaction theory, the repulsion between the backbone chains of polymers is predominant when the friction charge is low (Lodge and Muthukumar, 1996). In this condition, occurs a biphasic separation in the solution, each containing a predominant polymer. For the high charge fraction, the electrostatic interactions between the polymers dominate and they precipitate to form a stable complex (PEC) (Meka et al., 2017).
Taking into account these aspects, this study demonstrates the formation of PEC microparticles from anionic CMS and chitosan. Chitosan (Cs) is a well-known polymer derived from the biopolymer chitin that possesses protonable amino groups in its backbone. Under acidic conditions, these cationic groups (—NH3+) favor the PEC formation between Cs and anionic polymers (Fajardo et al., 2011). PECs based on Cs has been extensively used as DDS likely due to the advantageous properties of this polymer. The biodegradation of Cs-based materials show to be nontoxic, non-immunogenic, and non-carcinogenic. Generally, Cs is biodegraded in harmless products such as amino sugars, which are absorbed completely by the human body (Ngo et al., 2015). Besides that, Cs-based materials present a pH-dependent swelling and release behavior, which makes them appropriate for the delivery of drugs, proteins, or vaccines in the gastric cavity (Assaad et al., 2011; Kofuji et al., 2005; Walke et al., 2015). Herein, microparticles based on CMS/Cs PEC were loaded with bovine serum albumin (BSA), a model therapeutic protein. Recently, therapeutic proteins have gained considerable attention because of their use in the treatment of gastric injuries and/or major gastric defensive factors, in tissue regeneration and tumor therapy (Niu et al., 2016; Wu and Jin, 2008; Zhang et al., 2016a,b). To the best of your knowledge, this is the first study to report the formation and characterization of microparticles based on CMS/Cs PEC and investigates their potential as a DDS.
2 Materials and methods
2.1 Materials
Rice starches with different amylose content (6, 18, and 30%) were kindly donated by LabGrãos/UFPel (Pelotas-Brazil). Chitosan (Cs, 85% deacetylated, Mv 87,000 g/mol) was purchased from Golden-Shell Biochemical (China). Monochloroacetic acid (99%) and bovine serum albumin (BSA, lyophilized powder > 98%) were purchased from Sigma (USA). Isopropyl alcohol, methanol, acetic acid glacial and sodium tripolyphosphate (TPP) were purchased from Synth (Brazil). All chemicals of analytical grade were used as received without further purification.
2.2 Synthesis of carboxymethyl starch (CMS)
CMS was synthesized in an alcoholic medium as previously described by Wilpiszewska et al. (2015) with minor modifications (Scheme 1). Briefly, monochloroacetic acid (880 mg) was dissolved in isopropyl alcohol (150 mL) in a reactor and, then, NaOH (500 mg) was added. The system was stirred up to the mixture became white and homogeneous. Next, the starch with an amylose content of 6% (1.45 g) and the remaining NaOH (250 mg) was added. The resulting mixture was magnetically stirred for 4 h at 50 °C. Finally, the synthesized CMS was recovered by vacuum filtration, neutralized with acetic acid, and washed several times with methanol (80 v/v-%) to remove the unreacted chemicals. Purified CMS was allowed to dry in an oven at 40 °C (overnight). Similarly, CMS was synthesized using the rice starches with amylose contents of 18% and 30%. The degree of substitution (DS) of each CMS sample was estimated according to Nattapulwat et al. (2009). CMS (300 mg) was completely solubilized in distilled water (30 mL) and, then, NaOH solution (0.2 M, 20 mL) was added. The resulting mixture was transferred to a volumetric flask (100 mL) and the volume was adjusted to the mark with distilled water. An aliquot (25 mL) was withdrawn and titrated with HCl standard solution (0.04 M) using phenolphthalein as the indicator. A blank sample was also titrated. The DS values were calculated per Eqs. (1) and (2):
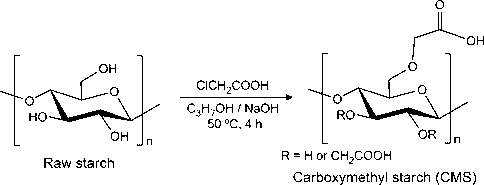
Synthesis of carboxymethyl starch (CMS) from rice starch.
2.3 Preparation of the microparticles based on CMS/Cs polyelectrolyte complex
Microparticles were prepared by polyelectrolyte complexation between CMS and Cs. Briefly, Cs was completely solubilized in acetic acid solution (1 w/v-%) and the resulting solution (3 w/v-%, pH 5) was transferred to a plastic syringe equipped with a needle (inner diameter 1.2 mm). Subsequently, the CS solution was gently drop-wise (rate 1 mL/min and drop height of 1 cm) into a CMS solution (4 w/v-%, pH 8.5 adjusted with 0.1 M NaOH solution) containing TPP (0.1 w/v-%), an anionic salt used as an auxiliary crosslinker of Cs. In the further studies, the CMS with the highest DS value was utilized for the preparation of microparticles. The instantaneously formed CMS/Cs microparticles were kept under mild stirring (50 rpm) for 1 h at room temperature for maturation. Next, the as-prepared CMS/Cs microparticles were recovered, purified in distilled water and dried in a vacuum desiccator at room temperature. Microparticles based on Cs ionically crosslinked with TPP (Cs/TPP) were prepared for comparative purposes. In this sense, similar procedures were carried out; however, the Cs solution was drop-wise into a TPP solution (0.75 w/v-%, pH 8.5) without CMS.
2.4 Analytical methods
Fourier transform infrared (FTIR) spectra were recorded in the range of 400–4000 cm−1 on KBr pellets using a Shimadzu Affinity spectrometer (Japan). Thermogravimetric analyses (TGA) were performed using a Shimadzu DTG 60 Analyzer (Japan) from room temperature up to 600 °C under a nitrogen atmosphere at a heating rate of 10 °C/min. Scanning electron microscopy (SEM) was used to investigate the surface morphology of the prepared microparticles. The microparticles were gold-coated by sputtering before the SEM visualization in a JEOL JSM-6610LC microscope (USA). The pH of zero charges (pHPZC) was estimated as described by Gomes et al. (2015). Each sample (50 mg) was placed in vials filled with NaCl solution (0.1 M, 50 mL) where the pH was adjusted to different values (2–12) with HCl or NaOH (0.1 M) using a pH meter. The vials containing the samples were stirred in an orbital shaker (100 rpm) for 24 h at room temperature. After equilibrium, the samples were withdrawn and the final pH of the solution (pHf) was measured. Next, changes in pH (ΔpH = pHi - pHf) were calculated and plotted against pHi (the initial pH). The pHPZC of each sample was set to the pH where ΔpH is zero (i.e. without variation).
2.5 Preparation of BSA-loaded microparticles
CMS/Cs and Cs/TPP microparticles were prepared using the same procedures described in the previous section; however, BSA (30 mg) was premixed with Cs solution before dropping in CMS or TPP solutions. The BSA-loaded microparticles were labeled as CMS/Cs-BSA and Cs/TPP-BSA, respectively. The encapsulation efficiency and BSA loading were determined by a digestion method (Anal et al., 2003; Yuan et al., 2017). A certain amount of the BSA-loaded microparticles was dissolved in HCl (0.1 M, 200 mL) for 24 h at room temperature. Next, the suspension was centrifuged (4000 rpm for 30 min) and filtrated. The supernatant was analyzed at 280 nm in a UV–Vis Micronal BS82 spectrometer (Brazil). BSA content in the microparticles sample was estimated using a previously built calibration curve (R2 > 0.99). Supernatant from the pristine microparticles (without BSA) was taken as blank. The encapsulation efficiency and BSA loading were calculated per Eqs. (3) and (4) (Yuan et al., 2017). All samples were analyzed in triplicate.
2.6 BSA release experiments
In vitro BSA release experiments were performed in two separated media; simulated gastric fluid (SGF, pH 1.2) and simulated intestinal fluid (SIF, pH 6.8) both without the presence of enzymes (Pereira et al., 2013). For this, BSA-loaded microparticles (200 mg) were placed into a dialysis membrane bag (MWCO 12,000) and, then, the bag was tied and soaked in the release medium (SGF or SGF, 100 mL). The entire system was incubated at 37 ± 1 °C with mild stirring (50 rpm) over the whole experiment. At pre-determined time intervals, an aliquot (4 mL) of the release medium was withdrawn and its absorbance at 280 nm was measured using a UV–Vis spectrometer. An equivalent volume (4 mL) of fresh release medium was refilled in the system. The amount of BSA in the release medium was determined using a calibration curve. All measurements were performed in triplicate.
3 Results and discussion
3.1 Characterization of CMS synthesized from rice starches with different amylose contents
Carboxymethyl groups (—CH2COOH) were introduced in the starch backbone by an etherification reaction between starch and monochloroacetic acid in the presence of NaOH. This reaction is built on the conversion of free hydroxyl groups (—OH) in glucose units to alkoxide groups, a more reactive form, by the strong alkaline medium. Then, the glucose units of starch were etherified by carboxymethyl groups as illustrated in Scheme 1. As aforementioned, this reaction was performed using rice starches with different amylose content (6, 18, and 30%) aiming to evaluate the effect of this parameter on the DS value. The insertion of the —CH2COOH groups in the starch samples was investigated by FTIR analysis (Fig. S1(a-c), Supporting information). According to the FTIR spectra obtained from the raw starches, no obvious discrepancies were observed. Overall, the spectra of raw starches show bands at about 3420 cm−1, 2936 cm−1, 1155 cm−1, and 1018 cm−1 associated with the stretching vibration of the O—H bond (—OH groups), asymmetric stretching vibration of the C—H bond (CH2 groups), asymmetric vibration of the C—O—C bond and C—O vibration of glycosidic units and the pyranoid ring (Pereira et al., 2013; Zhang et al., 2012). Moreover, the band at 1644 cm−1 is assigned to the scissoring vibration mode absorbed water molecules (Zhang et al., 2012). The presence of —CH2COOH groups in the modified starches was confirmed by the appearing of bands related to the asymmetric and symmetric vibration modes of the C⚌O bond. These bands were observed at 1608 cm−1 and 1426 cm−1 to the low-amylose starch (Fig. S1a), at 1607 cm−1 and 1423 cm−1 to the medium-amylose starch (Fig. S1b) and at 1610 cm−1 and 1426 cm−1 to the high-amylose starch (Fig. S1c) (Spychaj et al., 2013). Furthermore, the band related to the absorbed water molecules was not found in the FTIR spectra of the CMS samples. Finally, as compared to the raw starches, all spectra of CMS show a broadening in the band associated with the asymmetric stretching vibration of the CH2 groups and the band related to the O—H stretching vibration shifts to lower wavenumber. Taken together, these finds suggest the increasing of esterification of the starches with different amylose content and, therefore, the presence of carboxymethyl groups in the starch backbone (Wang et al., 2009).
The degree of substitution (DS) of CMS samples was estimated using a back titration method (Nattapulwat et al., 2009). Briefly, the carboxymethyl groups present in the starch backbone were protonated and, then, neutralized with NaOH. The excess of NaOH was titrated with HCl and the DS value was calculated per Eq. (2). According to this method, the DS values calculated for the low, medium and high-amylose starches were 0.5, 0.4, and 0.3, respectively. These results are in agreement with some previous studies reported in the literature (Bhattacharyya et al., 1995; Singh et al., 2004). As evidenced, the low-amylose starch exhibited a greater DS value compared to medium and high-amylose starches. An import aspect to a highly efficient carboxymethylation reaction is the availability of reactive sites (i.e. —OH groups) in starch grain. According to Stojanovic et al. (2005) starches with a smaller grain size presents a high surface area increasing the presence of reactive —OH groups on the surface of starch, which facilitates the carboxymethylation reaction. Another significant concern is related to the different reactivity of the three free —OH groups present in the starch (Wu, 2011). The primary —OH (at the C6 position) is more reactive than the secondary ones (at C2 and C3 positions) due to the steric hindrance. Since the carboxymethylation reaction is a second-order nucleophilic substitution, the primary —OH groups have been the main sites for carboxymethylation of starch (Colussi et al., 2014; Wu, 2011). These aspects can explain the highest DS value achieved for the low-amylose starch. Furthermore, the starch sample with the lowest content of amylose shows higher reaction efficiency (RE) (48.1%) as compared to the medium and high-amylose starches (38.5% and 28.0%, respectively). Herein, the RE parameter is defined as the fraction of monochloroacetic acid that had reacted with starch to form CMS (Bhandari and Hanna, 2011). Taking into account the previously discussed aspects, it is suggested that the monochloroacetic acid molecules react more efficiently with the low-amylose starch than with medium and high-amylose starches. Consequently, the CMS synthesized from the low-amylose starch exhibits the higher DS value. The low-amylose starch granules surfaces were investigated by SEM (Fig. S2a) and they showed spherical shape with a smooth surface and particle size of 8–14 μm. The SEM image recorded from the CMS synthesized from this starch sample (Fig. S2b) showed eruption and coalescence of the starch granules suggesting that the carboxymethylation reaction affects the structural arrangement of the rice starch. Some authors propose that the strong alkaline condition used for the CMS synthesis promotes such granular disintegration (Colussi et al., 2014; Zhang et al., 2012). Then, to prepare the polyelectrolyte microparticles, the CMS synthesized from the starch with lowest amylose content was selected, since it possesses the highest negative charge density.
3.2 Characterization of the CMS/Cs microparticles
CMS, an anionic polymer, can form PEC with Cs, a cationic polymer, by the simple dropping of one solution to other. As result, CMS/Cs microparticles can be obtained and their use as drug delivery systems (DDS) is attractive from several viewpoints. The DDS as microparticles can provide sustained release properties and a more uniform distribution of solutes (i.e. drugs, proteins, genes, etc.) in the polymeric matrix and in the gastrointestinal tract (Shelke et al., 2014). Here, in addition to CMS and Cs, a small amount of TPP, non-toxic inorganic compost, was utilized as an additional ionic crosslinker to Cs due to the low DS value obtained in the starch modification (DS ≈ 0.5). The anionic phosphate groups of TPP bind the free protonated amino groups of Cs, thereby ensuring the morphological stability of particles. The stability of microparticles is enhanced as a result of the synergistic effect that outcomes from the polyelectrolyte complexation between CMS and Cs and the ionotropic crosslinking of Cs, caused by the presence of TPP ions. These TPP ions can diffuse inward the CMS/Cs microparticle, crosslinking the excess of protonated amino groups of internal Cs chains, stabilizing the microparticle structure (Dima et al., 2015). Further, varieties of auxiliary crosslinking agents are generally used to enhance the efficiency of controlled release systems (Saikia et al., 2015).
Fig. 1 shows the FTIR spectra of CMS, Cs, and microparticles CMS/Cs and Cs/TPP. Examining the Cs spectrum is observed a broad band centered at 3438 cm−1 associated with the O—H and N—H stretching vibration (—NH2 and —OH groups), bands in the region of 2930–2870 cm−1 related do the C—H stretching vibration (CH2 and CH3 groups) and bands at 1654 cm−1 and 1596 cm−1 assigned to the C⚌O stretching vibration (amide I band) and N—H deformation (amide II band) (Brugnerotto et al., 2001). Cs/TPP spectrum exhibits characteristics bands proceeding from Cs and, also, a new band at 1216 cm−1 associated with the stretching vibration of the P⚌O bond of PO43− groups in TPP. Moreover, the band at 1596 cm−1 in the Cs spectrum shifts to 1543 cm−1 due to the protonation of the amino groups (—NH3+). On other notes, the band associated with the O—H and N—H stretching vibration is broadened in the Cs/TPP spectrum suggesting an increment of the intramolecular hydrogen bonds. All this finds confirm the ionotropic crosslinking of Cs with TPP as result of electrostatic interactions among the PO43− groups of TPP and the —NH3+ of Cs (Martins et al., 2012; Wu et al., 2005). The CMS/Cs spectrum shows slight discrepancies as compared to the Cs/TPP as observed in Fig. 1. The main changes are related to the appearing of shoulder-type bands at 1635 cm−1 likely due to the stretching vibration of the C⚌O bond of carboxylate groups (—COO−) from CMS and at 1560 cm−1 related to the —NH3+ groups of Cs that interact with CMS. Additionally, the band related to the C—H stretching (∼2920 cm−1) became more intense likely due to the CH2 groups of CMS. Since the CMS cannot penetrate into Cs matrix due its long-chain structure, the interaction between the —NH3+ groups and the —COO− groups of CMS is restricted to the surface of Cs drops, while TPP ions penetrate the drops, as previously discussed (Zhang et al., 2013). This aspect can explain the small changes observed in CMS/Cs spectrum as compared to the Cs/TPP.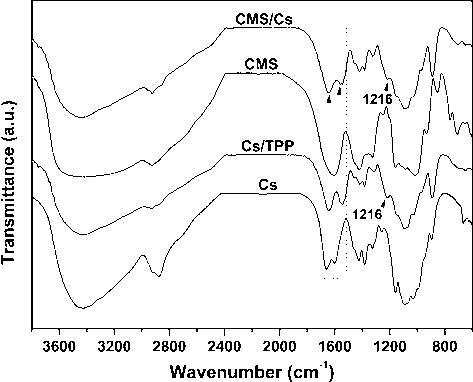
FTIR spectra of pure polysaccharides (CMS and Cs) and microparticles (CMS/Cs and Cs/TPP).
The thermal stability of the CMS, Cs, and microparticles (CMS/Cs and Cs/TPP) was investigated by TGA/DTG analysis (Fig. 2a and b). The TGA curve of CMS revealed three weight loss stages. The first stage (∼14% of weight loss) occurred from 25 °C to 170 °C due to the loss of water and light volatile compounds. The second and third stages (∼36% of weight loss) started at 250 °C and they are attributed to the degradation of the CMS backbone (carbonization and ash formation). According to the literature, the thermal stability of CMS has a straight relationship with its DS. In light of this, CMS samples with high DS show low thermal stability because of the hydrophilic nature of the carboxymethyl groups that favor the thermal decomposition process (Zhang et al., 2015). At 600 °C, the residue of CMS was 50% of its initial weight. The TGA curve of pure Cs showed two weight loss stages. The first stage occurred from 25 °C to 110 °C (∼11% of weight loss) due to loss of water. The second stage is associated with the depolymerization and decomposition of the Cs chains through deacetylation and cleavage of glycosidic linkages and, as noticed, it started at 240 °C (∼60% of weight loss) (Moussout et al., 2016). TGA/DTG curves of CMS/Cs and Cs/TPP microparticles possess a certain similarity (see Fig. 2a and b). Both curves showed two weight loss stages, which occurred in similar temperature ranges. Compared to pure CMS and Cs, the microparticles presented lower thermal stability, which is expected for polymeric networks formed by polyelectrolyte complexation or ionotropic crosslinking (Maciel et al., 2015; Fajardo et al., 2012; Martins et al., 2011). The complexation between CMS/Cs and ionotropic interaction between Cs and TPP ions disrupt some interactions present in the pure polysaccharides (intra and interchains H-bonds, for example) leading to a weakening of the microparticles structure as compared to CMS and Cs (Fajardo et al., 2012; Bigucci et al., 2009). Maciel et al. (2015) calculated the activation energy (Ea) for different stages of degradation of a polyelectrolyte complex (PEC) based on chitosan (Cs) and carboxymethyl cashew gum (CMCG). These authors find lowest Ea for the PEC than those for pure Cs and CMCG, confirming the lower thermal stability of complexes.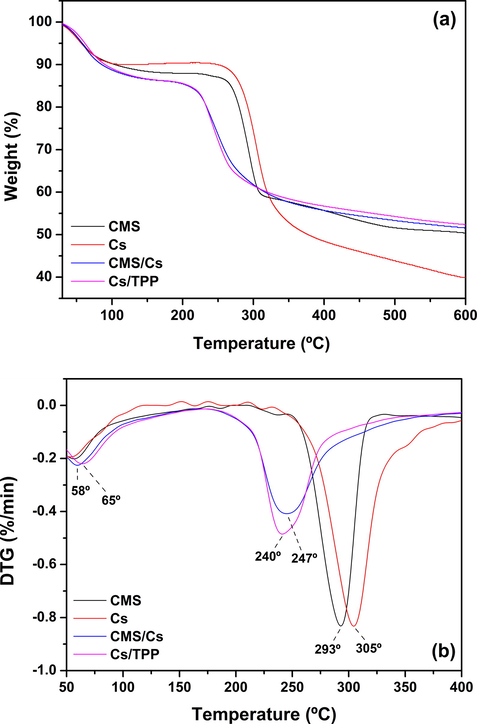
(a) TGA and (b) DTG curves of pure polysaccharides (CMS and Cs) and microparticles (CMS/Cs and Cs/TPP).
Taking into account the two microparticle systems, it is observed from the DTG curves (Fig. 2b) that the CMS/Cs microparticles are thermally degraded at 247 °C, while the Cs/TPP microparticles are degraded at 240 °C. This slight increment in the thermal stability of CMS/Cs as compared to Cs/TPP can be associated with the better stabilization of the hydrophilic groups of Cs by CMS than by TPP ions. The non-stabilized groups of Cs favor the dehydration process with the consequent increment degradation of the polymeric matrix. In other words, the polyelectrolyte complexation between CMS and Cs is more efficient to stabilize the polymeric matrix than the ionotropic interaction between Cs and TPP ions. This hypothesis may explain the increment of the maximum temperature observed for the loss of water from the microparticle matrixes. According to DTG curves (Fig. 2b), the temperature peaks of maximum weight loss due to water elimination are observed at 58 °C for CMS/Cs and at 65 °C for Cs/TPP. As aforementioned, the polyelectrolyte complexation between CMS and Cs reduces the availability of free hydrophilic groups in this matrix impairing the interaction between the microparticles and the water molecules. In contrast, the ionotropic crosslinking between Cs and TPP ions preserves some hydrophilic groups of Cs available to interact with water molecules. Consequently, the temperature to eliminate water molecules sorbed on CMS/Cs microparticles is lower than that required to Cs/TPP microparticles. Overall, the thermal analysis suggests that CMS/Cs complex shows higher stability than the Cs/TPP.
SEM images were taken of CMS/Cs and Cs/TPP microparticles and presented in Fig. 3. As noticed, the CMS/Cs microparticle (Fig. 3a) showed an oval-shaped morphology with some roughness and a non-regular surface. At high magnification (Fig. 3b), it is observed that small pores are distributed heterogeneously through the CMS/Cs surface. On the other hand, the Cs/TPP microparticle showed a spherical-shaped morphology and a smoother surface as compared to CMS/Cs (see Fig. 3c and d). The diffusion of TPP ions inward the Cs matrix contributes to compact the Cs/TPP surface and to reduce the particles size. As estimated, the average sizes of the CMS/Cs and Cs/TPP microparticles are 1.23 ± 0.02 mm and 0.90 ± 0.03 mm, respectively. Taken together, these observations allow suggesting that the polyelectrolyte complexation between CMS and Cs has a straight effect on the particle surface morphology and size.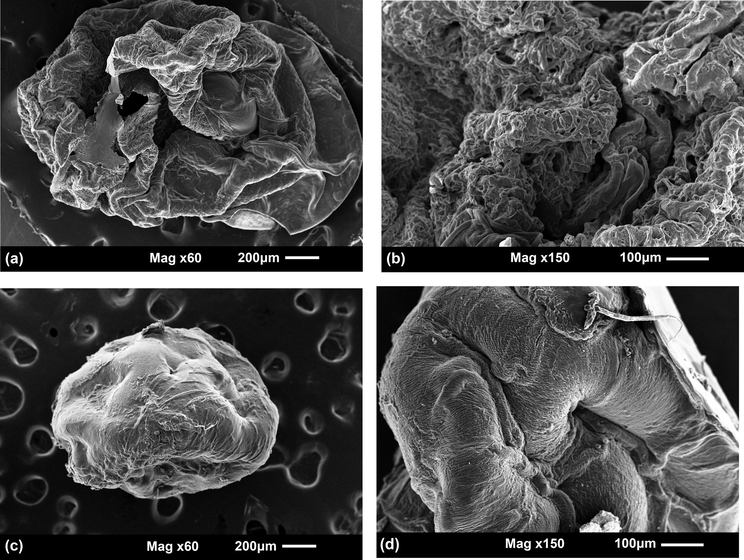
SEM images of CMS/Cs microparticles (a and b) and Cs/TPP microparticles (c and d).
3.3 BSA encapsulation and releasing experiments
BSA, a globular protein frequently used in numerous studies due to its stability and lack of interference within biological reactions, was employed here as a model protein (Li et al., 2017). The encapsulation efficiency, expressed as a percentage of the amount of BSA entrapped in the microparticles in comparison to its initial amount, was around 67% for CMS/Cs and 60% for Cs/TPP. The BSA-loading, expressed as a percentage of the amount of BSA entrapped in comparison to the amount of microparticles, was around 10% for CMS/Cs and 9% for Cs/TPP. These results suggest that the microparticles prepared by polyelectrolyte complexation of CMS and Cs have been more efficient in BSA encapsulation than the Cs/TPP microparticles. The starch-derivative, CMS, has a high-functionalized backbone with hydroxyl and carboxymethyl groups that act as interaction sites to BSA. This interaction restricts the BSA diffusion to the medium during the microparticle formation. Moreover, another important aspect that affects the encapsulation efficiency of BSA is the pH of the medium. Here, BSA was added in an acidic Cs-solution (pH 5) before the microparticles formation. This pH condition is close to the isoelectric point of BSA (pH 4.7) and, as result; this protein exhibits both negative and positive patches. Negative patches of BSA interact with the —NH3+ groups of Cs preventing its release during the microparticle formation. After that, the Cs-solution containing BSA was drop-wised in the CMS or TPP solutions. In this stage, the negative charges of CMS and TPP interact with the positive patches of BSA. Due to its high molecular weight and negative charge density, CMS is more effective to stabilize the BSA than TPP enhancing the encapsulation efficiency (Walke et al., 2015; Zhang et al., 2016a,b). In order to support these inferences, the pH of zero charge (pHPZC) for the microparticles (loaded or not) was estimated (Fig. S3). In the light of drug encapsulation and release applications, this parameter is paramount to describe changes in the charge surface and stability of particles in the aqueous medium. At pHPZC, the Zeta potential (ζ) is zero and the stability is minimum while the solubility of particles is maximum (Bojnanska et al., 2014). Conversely, below the pHPZC, the particle surface is positively charged, while above pHPZC the surface is negatively charged. As assessed, the CMS/Cs microparticles show pHPZC higher than Cs/TPP, which can be explained by the excess of anionic groups on the surface of CMS/Cs. The BSA-loaded in the microparticles caused a slight variation in the pHPZC of both microparticles. For CMS/Cs the pHPZC was shifted from 1.24 to 1.35, while for Cs/TPP the pHPZC was shifted from 1.17 to 1.20. Higher variation in the pHPZC values estimated for the CMS/Cs and CMS/Cs-BSA microparticles can be associated with the decreasing in the number of anionic groups on the microparticles surface due to their interaction with the positive patches of BSA. These experimental results corroborate the previous discussion.
The interaction between the BSA and the microparticles was further investigated by FTIR analysis (Fig. S4). The FTIR spectrum of pure BSA showed the characteristics bands of acetylamino I group at 1650 cm−1, acetylamino II at 1542 cm−1 and acetylamino III at 1245 cm−1 confirming the α-helix of the secondary structure of BSA (Sahu and Prusty, 2010). The broadband centered at 3315 cm−1 is related to the stretching vibration of the O-H and N-H bonds. Comparatively to the spectra of the non-loaded microparticles, the spectra of CMS/Cs-BSA and Cs/TPP-BSA showed changes in the bands related to O—H and N—H stretching vibrations, C⚌O stretching vibration (amide I) and —NH3+ groups. These bands were shifted to lower wavenumber region and increased in intensity. Such slight shifts in the FTIR spectra of the BSA-loaded microparticles suggest changing in the ambient the functional groups (hydroxyl, amino, and carboxymethyl), probably due to the intra and intermolecular H-bonds formed between BSA and the polymeric matrix. Furthermore, the FTIR of BSA-loaded microparticles showed bands at 1650 cm−1 and 1449 cm−1, which proceed from the BSA. The bands of acetylamino I and II groups of BSA can overlap with the bands assigned to the C⚌O bonds and —NH3+ groups, causing an increase in the intensity of these bands. In addition, this overlapping indicates that the interaction between BSA and the polymeric matrix is preferentially due to electrostatic attraction than van der Waals interaction (Swain and Sarkar, 2013).
The morphology of the BSA-loaded microparticles was investigated by SEM (Fig. 4). The images allow noticing that the protein entrapped in the microparticles affects their morphology as compared to the bare microparticles. Overall, the CMS/Cs-BSA microparticles exhibited some spherical shape, rough and dense surface with an average size of 1.53 ± 0.02 mm (Fig. 4a and b). The absence of pores distributed through the CMS/Cs-BSA surface was also noticed. Although the Cs/TPP-BSA microparticles had shown some spherical shape, when compared to the CMS/Cs-BSA, these microparticles showed higher roughness and irregularities on the surface (Fig. 4c and d). Moreover, the estimated average size of Cs/TPP-BSA microparticles was 1.40 ± 0.04 mm. Such discrepancies suggest a higher compatibility between the BSA and the CMS/Cs matrix, likely due to the electrostatic interactions and H-bonds engaged between the protein and polymers (CMS and Cs). It should be mentioned that both microparticles systems had no obvious phase separation indicating good compatibility between BSA and the CMS/Cs and Cs/TPP matrixes.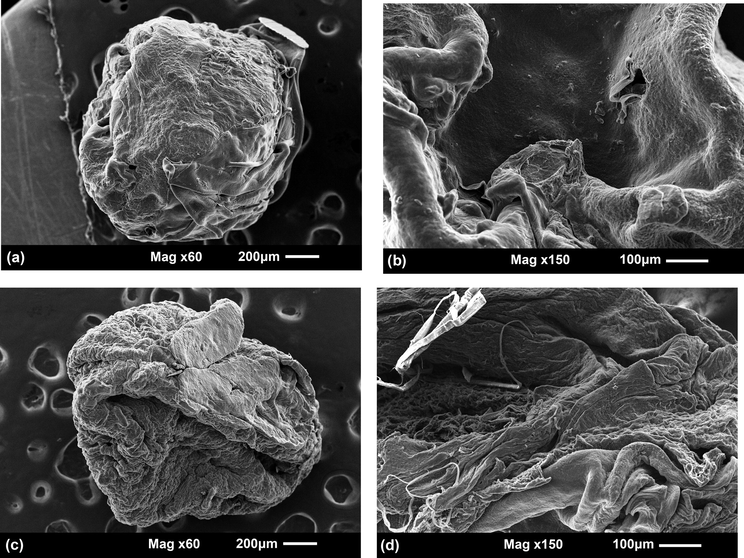
SEM images of CMS/Cs-BSA microparticles (a and b) and Cs/TPP-BSA microparticles (c and d).
The in vitro release profile of BSA from the loaded microparticles was examined in a model enzyme-free simulated gastric and intestinal fluids (SGF and SIF). These media were chosen because they mimic the pH and ionic strength of some human environments, especially stomach and small intestine (Yuan et al., 2017). The effect of enzymes activity was not studied since the Cs cannot be degraded by the digestive enzymes of the human stomach and small intestine (Zhang et al., 2002). Besides, there is some difficult to perform an assay evaluating the influence of digestive enzymes on the release of BSA encapsulated in a DDS due to the lack of a reliable method to quantify intact BSA in the presence of its hydrolyzed derivative from enzymatic digestion (Yuan et al., 2017). However, if BSA is efficiently encapsulated in the polymeric matrix, was expected that the digestive enzymes would not easily diffuse into particles and digest the BSA. The percentages of cumulative release of BSA-loaded microparticles (CMS/Cs-BSA and Cs/TPP-BSA) in SGF and SIF are shown in Fig. 5.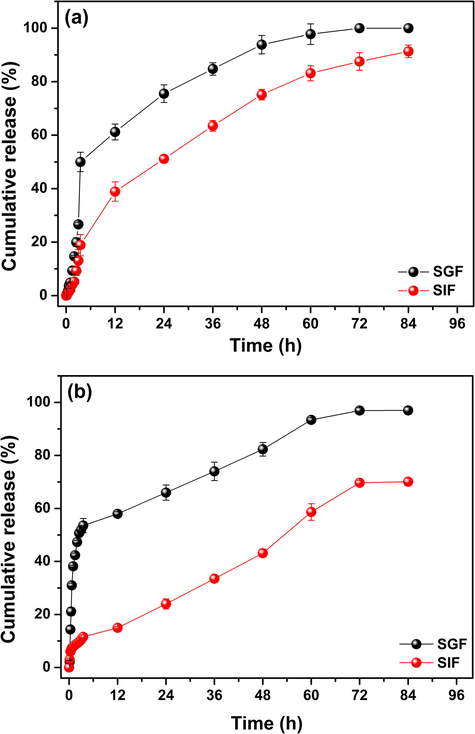
Cumulative release profile of BSA from (a) CMS/Cs-BSA microparticles and (b) Cs/TPP-BSA microparticles in simulated gastric (pH 1.2) and intestinal (pH 6.8) fluids at 37 °C.
The experimental data revealed that the BSA releasing is pH-dependent and it is more pronounced in the acidic environment (SGF, pH 1.2) for both microparticles. After 12 h in SGF, almost 58% and 61% of BSA was released from CMS/Cs-BSA and Cs/TPP-BSA. As the time goes on, the BSA release rate in SGF decreases to both microparticles system and after 84 h the cumulative BSA release achieved 97% for CMS/Cs-BSA and 100% for Cs/TPP-BSA. Conversely, in SIF (pH 6.8) at the first 12 h, about 15% of BSA was released from CMS/Cs-BSA, while 38% were released from Cs/TPP-BSA. At the end of the experiment (84 h), the percentage of BSA released from CMS/Cs-BSA and Cs/TPP-BSA was 70% and 91%, respectively. These release data are in agreement with the study done by Zou et al. (2015) that investigated the BSA release profiled from chitosan microspheres. According to the data exposed in Fig. 5, it is evidenced that SGF is the medium with higher BSA cumulative release independent of the microparticles system. This behavior can be explained by the swelling and partial dissolution of the microparticles caused by the repulsive forces that arise from the repulsion between the free —NH3+ groups on Cs backbone. The repulsive forces weaken the intra and intermolecular bonding interactions engaged between the polymeric network and between the polymeric network and BSA (Keawchaoon and Yoksan, 2011). As assessed, both microparticles system exhibited low values of pHPZC, which can be speculated as result of a great number of negatively ionizable groups on the microparticles surface. Under strong acidic medium, the elevated concentration of protons neutralizes the negative charges on the microparticles surface facilitating the protonation of the free amino groups of Cs and, consequently, the release of BSA (Zou et al., 2015). On the other hand, in SIF the protonation of the amino groups of Cs does not occur preventing the expansion of the polymer network and swelling. As result, the diffusion of BSA to the releasing medium is impaired (Shu and Zhu, 2002). Furthermore, the interaction between the microparticles and the releasing medium is minimal due to the electrostatic repulsion among the OH− ions, available in SIF, and the negatively charged groups on the microparticles surface. Therefore, in SIF the swelling of the microparticles is low, which decreases their surface area and reduces the release of BSA (Keawchaoon and Yoksan, 2011).
Fig. 5 also provides important information about the influences of the microparticle composition in the BSA release profile. As noticed, the CMS/Cs-BSA microparticles have released BSA more slowly than Cs/TPP-BSA microparticles in both tested media (SGF and SIF). Such discrepancy regarding the releasing is more evident in the first 3.5 h of the experiment. The Cs/TPP-BSA microparticles showed a burst effect in SGF and SIF, where 58% and 20% of BSA were released. The burst effect is related to desorption process of BSA bound to the microparticles surface. As the microparticles have a high surface area, incessant contact with the releasing medium promoted the rapid desorption release of BSA (Keawchaoon and Yoksan, 2011; Pereira et al., 2013). In contrast, the CMS/Cs-BSA microparticles just showed a burst in SGF, where 52% of BSA were released at the beginning of the experiment. In SIF, only 11% of BSA was released from CMS/Cs-BSA after 3.5 h. This absence of burst release in SIF indicates a better entrapment of protein in the CMS/Cs microparticles likely due to the presence of CMS that bounds efficiently the BSA chains resulting in a more stable system. In addition, after 12 h the release of BSA in SGF and SIF increased for both microparticles system due to the swelling of the microparticles. It is evident that this increase in the BSA releasing was less pronounced for the CMS/Cs microparticles, especially in SIF, which allows suggesting that this system is able to control the release of BSA more efficiently than the Cs/TPP microparticles. About the 72 h, for all tested conditions and systems, the BSA releasing has reached a plateau stage, probably due to the equilibrium (reduced difference) of concentrations of BSA between the dense phase (microparticles) and the release medium (Agnihotri et al., 2004).
In order to gain insight into the release mechanism of BSA from the microparticles systems, the data presented in Fig. 5 were examined using different kinetic models. This mathematical modeling of BSA release kinetics is a trustable tool to obtain information about the mass transport mechanisms that are involved in these promising DDS. The cumulative release data were fitted to Zero-order (5), First-order (6), Higuchi (7), Hixson-Crowell (8), Korsmeyer-Peppas (9) and Baker-Lonsdale (10) kinetic models (Nayak et al., 2016; Zou et al., 2015):
Model
Parameter
CMS/Cs-BSA
Cs/TPP-BSA
SGF
SIF
SGF
SIF
Zero-order
k0 (mg/h)
0.173
0.161
0.238
0.216
R2
0.754
0.991
0.826
0.909
First-order
k1 (1/h)
0.018
0.032
0.043
0.053
R2
0.286
0.867
0.467
0.482
Higuchi
kH (mg/h1/2)
1.683
1.440
2.307
2.093
R2
0.851
0.939
0.979
0.992
Hixson-Crowell
kHC (mg1/3/h)
0.019
0.011
0.035
0.004
R2
0.946
0.968
0.955
0.982
Korsmeyer-Peppas
kKP
0.244
1.114
0.051
0.024
n
0.370
0.460
0.804
0.981
R2
0.652
0.908
0.903
0.905
Baker-Lonsdale
kBL
0.004
0.001
0.007
0.004
R2
0.955
0.901
0.967
0.977
Following consideration of the R2 values, the BSA release from CMS/Cs microparticles depends on the release medium (SGF or SIF). In SGF, it was found that the Baker-Lonsdale model fits better the experimental data (R2 = 0.955). The Baker-Lonsdale model describes the drug release from spherical matrices using an equation derived from the Higuchi model. In this model, the first controlling mechanism is a combination of swelling and degradation of microparticles matrices, then, after the diffusion phenomenon take parts in the BSA release process (Jafari and Kaffashi, 2016). On the other hand, in SIF the BSA release data are better fitted by the Zero-order model (R2 = 0.991) suggesting that the protein release from CMS/Cs is only time-dependent. According to this kinetic model, the BSA release is attributed to time-dependent changes in microparticles surface area, which is gradually depleted of BSA, and diffusional path length that increases with time (Tang et al., 2015). Moreover, the Zero-order kinetics not depends on the concentration of drug or environment to induce the drug release and, because of this, this kinetics has several advantages to drug therapy (Tang et al., 2015).
Considering the Cs/TPP-BSA microparticles, the experimental release data obtained to both release media (SGF and SIF) are better fitted by the Higuchi kinetic model (R2 = 0.979 and 0.992). Higuchi model assumes that the BSA release process depends on the Fickian diffusion mechanism (Paul, 2011). Further, this model is based on the assumptions that: (i) amount of BSA entrapped in the microparticles is greater than the solubility of the BSA in the release medium; (ii) the size of the microparticles is much greater than the size of the BSA chains; (iii) BSA diffuses outward the microparticles at a constant rate; and (iv) the release medium maintain the sink conditions (Bajpai et al., 2017). Besides that, according to Higuchi model, if the BSA diffusion is the rate-limiting step, the solid/liquid interface will move towards the interior of the microparticles over time (Paul, 2011).
As assessed here, the microparticles formed by polyelectrolyte complexation of CMS and Cs exhibit obvious differences regarding the BSA release profile and release kinetics as compared to the conventional Cs/TPP microparticles. Overall, the PEC microparticles based on CMS and Cs were compatent to control the release of BSA more efficiently. Additionally, the release mechanism of the CMS/Cs microparticles can be tailored according to the release medium, which is a very attractive feature since it allows targeting the drug delivery. Taken together, these findings suggest that PECs based on CMS/Cs are promising vehicles for drug delivery due to its advantages over other conventional systems described in the literature.
4 Conclusions
Rice starches with different amylose content were esterified in order to obtain carboxymethyl starch (CMS), a polyanionic polysaccharide. As assessed, the starch sample with the lowest content of amylose (6%) was the most susceptive of esterification and, as result, a CMS with a DS of 0.5 was synthesized. Microparticles were prepared by polyelectrolyte complexation between CMS and chitosan (Cs), a polycationic polysaccharide, aiming their use as vehicles for drug delivery systems (DDS). For comparative purpose, conventional Cs-based microparticles were prepared by ionotropic crosslinking of Cs with TPP ions. The two microparticles systems, CMS/Cs and Cs/TPP, showed obvious discrepancies regarding network stability (chemical and thermal), morphology, size, and surface charge. Overall, the CMS/Cs microparticles exhibited more attractive properties than the Cs/TPP microparticles. Moreover, the microparticles prepared by polyelectrolyte complexation showed to be more efficient to encapsulate BSA likely due to the presence of CMS in the microparticle formulation. In vitro release experiments conducted in simulated gastric and intestinal human fluids (SGF and SIF) with the BSA-loaded microparticles revealed that the CMS/Cs-BSA microparticles exhibit a more evident pH-dependent release profile and a high control on the BSA release minimizing the burst effect, particularly in SIF. Furthermore, in SIF, the BSA release process from CMS/Cs-BSA follows a Zero-order kinetics, which is highly attractive for a DDS. These results allow suggesting that the CMS/Cs microparticles can provide controlled release of BSA in constant dose to a specific region of the gastrointestinal tract.
Acknowledgements
The authors are thankful to CNPq – Brazil for the financial support (PQ fellowship - Process 305974/2016-5).
References
- Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Rel.. 2004;100:5-28.
- [Google Scholar]
- Effect of non-cross-linked calcium on characteristics, swelling behaviour, drug release and mucoadhesiveness of calcium alginate beads. Carbohyd. Polym.. 2016;140:163-170.
- [Google Scholar]
- Chitosan-alginate multilayer beads for gastric passage and controlled intestinal release of protein. Drug Dev. Ind. Pharm.. 2003;29:713-724.
- [Google Scholar]
- Recent trend in the physical and chemical modification of starches from different botanical sources: a review. Starch. 2014;66(1–2):41-57.
- [Google Scholar]
- The influence of protonation ratio on properties of carboxymethyl starch excipient at various substitution degrees: structural insights and drug release kinetics. Int. J. Pham.. 2010;394:75-84.
- [Google Scholar]
- Polyelectrolyte complex of carboxymethyl starch and chitosan as drug carrier for oral administration. Carbohyd. Polym.. 2011;84:1399-1407.
- [Google Scholar]
- Controlled release of Doxycycline from gum acacia/poly(sodium acrylate) microparticles for oral drug delivery. Int. J. Biol. Macromol.. 2017;104:1064-1071.
- [Google Scholar]
- Preparation of highly substituted carboxymethyl starch using a twin-screw extruder. Starch. 2011;63:771-779.
- [Google Scholar]
- A comparative account of conditions for synthesis of sodium carboxymethyl starch from corn and amaranth starch. Carbohyd. Polym.. 1995;27:247-253.
- [Google Scholar]
- Pectin-based microspheres for colon-specific delivery of vancomycin. J. Pharm. Pharmacol.. 2009;61:41-46.
- [Google Scholar]
- Determination of critical parameters of drug substance influencing dissolution: a case study. Biomed Res. Int.. 2014;2014:1-9.
- [Google Scholar]
- An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569-3580.
- [Google Scholar]
- Recent progress in chemical modification of starch and its applications. RSC Adv.. 2015;5:67459-67474.
- [Google Scholar]
- Structural, morphological, and physicochemical properties of acetylated high-, medium-, and low-amylose rice starches. Carbohyd. Polym.. 2014;103:405-413.
- [Google Scholar]
- Hexavalent chromium removal in contaminated water using reticulated chitosan micro/nanoparticles from seafood processing wastes. Chemosphere. 2015;141:100-111.
- [Google Scholar]
- Polysaccharide-based materials associated with or coordinated to gold nanoparticles: synthesis and medical application. Curr. Med. Chem.. 2017;24:2701-2735.
- [Google Scholar]
- Effect of stoichiometry and pH on the structure and properties of Chitosan/Chondroitin sulfate complexes. Colloid Polym. Sci.. 2011;289:1739-1748.
- [Google Scholar]
- Polyelectrolyte complexes based on pectin-NH2 and chondroitin sulfate. Carbohyd. Polym.. 2012;87:1950-1955.
- [Google Scholar]
- Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv.. 2010;7:429-444.
- [Google Scholar]
- Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohyd. Polym.. 2015;117:797-806.
- [Google Scholar]
- Fast dye removal from water by starch-based nanocomposites. J. Colloid Interface Sci.. 2015;454:200-209.
- [Google Scholar]
- Starch-based hydrogels: present status and applications. Int. J. Polym. Mater. Polym. Biomater.. 2013;62:411-420.
- [Google Scholar]
- Carboxymethyl starch excipients for drug chronodelivery. AAPS Pharmscitech. 2017;18:1673-1682.
- [Google Scholar]
- Mathematical kinetic modeling on Isoniazid release from Dex-HEMA-PNIPAAm nanogels. Nanomed. Res. J.. 2016;1:6-13.
- [Google Scholar]
- Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloid Surf. B. 2011;84:163-171.
- [Google Scholar]
- Sustained insulin release with biodegradation of chitosan gel beads prepared by copper ions. Int. J. Pham.. 2005;303(1–2):95-103.
- [Google Scholar]
- Engineering BSA-dextran particles encapsulated bead-on-string nanofiber scaffold for tissue engineering applications. J. Mater. Sci.. 2017;52:10661-10672.
- [Google Scholar]
- Physical chemistry of polymers: entropy, interactions, and dynamics. J. Phys. Chem.. 1996;100:13275-13292.
- [Google Scholar]
- Incorporation of theophylline in a chitosan/chondroitin sulfate hydrogel matrix: in vitro release studies and mechanical properties according to pH changes. J. Appl. Polym. Sci.. 2013;128(5):3417-3424.
- [Google Scholar]
- Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohyd. Polym.. 2015;132:537-545.
- [Google Scholar]
- Chitosan/TPP microparticles obtained by microemulsion method applied in controlled release of heparin. Int. J. Biol. Macromol.. 2012;51:1127-1133.
- [Google Scholar]
- A review of the chemical modification techniques of starch. Carbohyd. Polym.. 2017;157:1226-1236.
- [Google Scholar]
- A comprehensive review on polyelectrolyte complexes. Drug Discovery Today. 2017;22:1697-1706.
- [Google Scholar]
- Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab.. 2016;130:1-9.
- [Google Scholar]
- preparation and application of carboxymethyl yam (dioscorea esculenta) starch. AAPS Pharmscitech. 2009;10:193-198.
- [Google Scholar]
- Swelling and drug release behavior of metformin HCl-loaded tamarind seed polysaccharide-alginate beads. Int. J. Biol. Macromol.. 2016;82:1023-1027.
- [Google Scholar]
- Biological effects of chitosan and its derivatives. Food Hydrocolloid.. 2015;51:200-216.
- [Google Scholar]
- Protective effect of cod (gadus macrocephalus) skin collagen peptides on acetic acid-induced gastric ulcer in rats. J. Food Sci.. 2016;81:H1807-H1815.
- [Google Scholar]
- Elaborations on the Higuchi model for drug delivery. Int. J. Pham.. 2011;418:13-17.
- [Google Scholar]
- Starch-based microspheres for sustained-release of curcumin: preparation and cytotoxic effect on tumor cells. Carbohyd. Polym.. 2013;98:711-720.
- [Google Scholar]
- Synthesis and characterization of superparamagnetic nanoparticles coated with carboxymethyl starch (CMS) for magnetic resonance imaging technique. Carbohyd. Polym.. 2009;78:292-295.
- [Google Scholar]
- Design and evaluation of a nanoparticulate system prepared by biodegradable polymers for oral administration of protein drugs. Pharmazie. 2010;65:824-829.
- [Google Scholar]
- Carboxymethyl starch-coated iron oxide magnetic nanoparticles: a potential drug delivery system for isoniazid. Iranian Polym. J.. 2015;24:815-828.
- [Google Scholar]
- Influence of reaction parameters on carboxymethylation of rice starches with varying amylose contents. Carbohyd. Polym.. 2015;115:186-192.
- [Google Scholar]
- Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohyd. Polym.. 2016;148:354-361.
- [Google Scholar]
- Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol.. 2014;25:448-460.
- [Google Scholar]
- Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int. J. Pham.. 2002;233:217-225.
- [Google Scholar]
- Effect of acetylation on some properties of corn and potato starches. Starch. 2004;56:586-601.
- [Google Scholar]
- Medium and high substituted carboxymethyl starch: synthesis, characterization and application. Starch. 2013;65:22-33.
- [Google Scholar]
- A comparison of some methods for the determination of the degree of substitution of carboxymethyl starch. Starch. 2005;57:79-83.
- [Google Scholar]
- Study of BSA protein adsorption/release on hydroxyapatite nanoparticles. Appl. Surf. Sci.. 2013;286:99-103.
- [Google Scholar]
- Macromolecular crowding of molecular imprinting: a facile pathway to produce drug delivery devices for zero-order sustained release. Int. J. Pham.. 2015;496:822-833.
- [Google Scholar]
- Polyelectrolyte complex based interfacial drug delivery system with controlled loading and improved release performance for bone therapeutics. Nanomaterials. 2016;6:1-9.
- [Google Scholar]
- Fabrication of chitosan microspheres using vanillin/TPP dual crosslinkers for protein antigens encapsulation. Carbohyd. Polym.. 2015;128:188-198.
- [Google Scholar]
- Carboxymethyl chinese yam starch: synthesis, characterization, and influence of reaction parameters. Carbohyd. Res.. 2009;344:1764-1769.
- [Google Scholar]
- Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohyd. Polym.. 2015;128:82-89.
- [Google Scholar]
- Polymer-based sustained-release dosage forms for protein drugs, challenges, and recent advances. AAPS Pharmscitech. 2008;9:1218-1229.
- [Google Scholar]
- Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pham.. 2005;295:235-245.
- [Google Scholar]
- Reaction kinetics of carboxymethylation of cornstarch. J. Appl. Polym. Sci.. 2011;121:1901-1907.
- [Google Scholar]
- Dynamic rheological properties of wheat starch gels as affected by chemical modification and concentration. Starch. 2015;67:567-576.
- [Google Scholar]
- Entrapment of protein in chitosan-tripolyphosphate beads and its release in an in vitro digestive model. Food Chem.. 2017;229:495-501.
- [Google Scholar]
- Crosslinked carboxymethyl starch: One step synthesis and sorption characteristics. Int. J. Biol. Macromol.. 2014;71:87-93.
- [Google Scholar]
- Synthesis and characterization of carboxymethyl potato starch and its application in reactive dye printing. Int. J. Biol. Macromol.. 2012;51:668-674.
- [Google Scholar]
- Preparation and characterization of carboxymethyl starch microgel with different crosslinking densities. Carbohyd. Polym.. 2015;124:245-253.
- [Google Scholar]
- An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int. J. Pham.. 2002;239:197-205.
- [Google Scholar]
- Effect and mechanism of sodium chloride on the formation of chitosan-cellulose sulfate-tripolyphosphate crosslinked beads. Soft Matter. 2013;9:10354-10363.
- [Google Scholar]
- Encapsulation of pancreatic lipase in hydrogel beads with self-regulating internal ph microenvironments: retention of lipase activity after exposure to gastric conditions. J. Agric. Food Chem.. 2016;64:9616-9623.
- [Google Scholar]
- Protein encapsulation in alginate hydrogel beads: effect of pH on microgel stability, protein retention and protein release. Food Hydrocolloid.. 2016;58:308-315.
- [Google Scholar]
- Preparation and drug release behavior of pH-responsive bovine serum albumin-loaded chitosan microspheres. J. Ind. Eng. Chem.. 2015;21:1389-1397.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2018.04.004.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







