Translate this page into:
Magnetically recoverable γ-Fe2O3 nanoparticles as a highly active catalyst for Friedel–Crafts benzoylation reaction under ultrasound irradiation
⁎Corresponding author. thphuong@hcmus.edu.vn (Phuong Hoang Tran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A simple, facile and efficient method has been developed for the Friedel–Crafts benzoylation of arenes using magnetic γ-Fe2O3 nanoparticles under solvent-free sonication. The γ-Fe2O3 nanoparticles were used as an efficient and magnetically recoverable catalyst for the synthesis of aromatic ketones in good to excellent yields at room temperature under solvent-free. The reaction occurred with high regioselectivity under mild condition. The magnetic γ-Fe2O3 nanoparticles are economically synthesized in large-scale, easily separated from the reaction mixture by an external magnet and able to be reused several times without significant loss of the catalytic performance, which make them easy application to industrial processes.
Keywords
Magnetic γ-Fe2O3 nanoparticles
Friedel–Crafts acylation
Solvent-free sonication
Green catalyst
1 Introduction
Magnetically separable nanoparticles are attracting considerable academic and industrial interest because of cost-effective and non-toxic catalysts (Taylor et al., 2010; Chen et al., 2012; Rossi et al., 2014); especially, in liquid-phase catalytic reactions, magnetic nanoparticles can combine the advantages of homogeneous and heterogeneous catalysts due to their high dispersion, excellent reactivity, and easy separation (Jiang et al., 2015; Xu et al., 2015; Cheng et al., 2014). In this aspect, the magnetic nanoparticles, known as iron oxide nanoparticles (NPs), have emerged as sustainable and inexpensive alternative in organic reactions (Polshettiwar et al., 2011). In the last decade, the direct use of magnetic Fe3O4 nanoparticle has been studied extensively (Polshettiwar et al., 2011; Mrówczyński et al., 2014; Baig and Varma, 2013). Nonetheless, they are highly sensitive to oxidizing reagents and easy to suffer aggregation owing to its high chemical reactivity and large surface area to volume ratio (Jiang et al., 2016; Polshettiwar et al., 2011). Super-paramagnetic γ-Fe2O3 NPs are green and efficient catalyst due to their extreme stability, high surface area and easy synthesis promised as one of the most widely used catalysts in this field (Polshettiwar et al., 2011; Chen et al., 2012). This excellent nanoparticle catalyst demonstrated high activity due to high surface area increasing contacts between reactants and catalyst, good dispersion in the reaction mixture, stability at high temperature reaction (Lu et al., 2007; Bhosale et al., 2015; Santos et al., 2014). In addition to stability and reactivity, magnetic separation by an external magnet can be considered as a green separation tool and has been applied in biomedicine, catalyst support and catalysts (Bhosale et al., 2015; Jiang et al., 2015; Nehlig et al., 2015; Taylor et al., 2010; Xu et al., 2015).
The Friedel–Crafts acylation is a valuable method for the synthesis of aromatic compounds, which are useful precursors in the pharmaceutical, flavor, dye and agrochemical industries (Sartori and Maggi, 2006). However, the traditional Lewis acid-catalyzed Friedel–Crafts acylation has associated with a large number of environmental and economic issues (Olah, 1973). Furthermore, the traditional Lewis acids must be employed more than stoichiometric amounts which could not be recovered and reused after aqueous work-up (Sartori and Maggi, 2010). Consequently, the development of eco-catalysts has attracted much attention in the past two decades (Sartori and Maggi, 2010). Many catalysts for Friedel–Crafts acylation have been developed in literatures such as metal triflates (Tran et al., 2015c; Tran et al., 2015b; Tran et al., 2015a), metal-organic frameworks (Doan et al., 2016; Jiang et al., 2014; Lee et al., 2009; Calleja et al., 2014; Chung et al., 2014), zeolites (Bai et al., 2012), palladium catalysts (Jiang and Wang, 2013; Pan et al., 2013; Tang et al., 2013), ionic liquids (Tran et al., 2016; Martins et al., 2014; Luo et al., 2012; Hallett and Welton, 2011), and sulfate zirconia (Signoretto et al., 2008). In spite of their potential utility, the catalysts are normally used more than stoichiometric quantities and required inert atmosphere, harsh condition, long reaction time and tedious work-up. As a result, the investigations of more efficient and environmentally benign catalysts for production of high yield with low energy and minimum waste are still in progress.
Ultrasonic technology has become an efficient energy source for green organic and pharmaceutical synthesis owing to safety, low energy and simply instrumental setup (Choudhury et al., 2014a; Blanco et al., 2015). Specially, ultrasound has no unexpected action on chemical bonds, but chemical and physical effects of ultrasound can enhance selectivity, yields and generation of active catalyst surfaces under mild reaction conditions in both homogeneous and heterogeneous systems (Shekouhy and Hasaninejad, 2012; Cravotto and Cintas, 2006). Moreover, ultrasound enables the rapid dispersion of solids and forms porous materials as well as prohibits particle aggregation thanks to the numerous explosion of cavitation (Thokchom et al., 2015; Mason, 1997). Additionally, catalytic activity can be also improved under ultrasound activation due to the deformation of fresh and highly active surface (Thokchom et al., 2015; Choudhury et al., 2014b). For these reasons, reactions taken place under ultrasound irradiation have been usually reported with higher yields, lower temperature and shorter reaction time in comparison with traditional stirring methods (Kanchithalaivan et al., 2014; Teo et al., 2010; Zou et al., 2012).
As a consequence of our interests in Friedel–Crafts acylation reaction and in ultrasound-assisted organic synthesis, we report herein the use of magnetic γ-Fe2O3 NPs as the catalyst of the Friedel–Crafts benzoylation under sonication. Under ultrasound irradiation, the collapse of bubbles generates localized microscopic “hot spots” giving the high temperature (5000 K) and the pressure (500 kPa) to provide favorable condition for the Friedel–Crafts reaction (Mason, 1997). These conditions lead to the formation of the desired products even when the reaction is carried out under room temperature and in the presence of only 20 mol% magnetic γ-Fe2O3 NPs. Moreover, γ-Fe2O3 in nano-scale particles are rapidly dispersed in the reaction mixture and γ-Fe2O3 nanoparticles are highly air-stable catalyst under harsh conditions. The fact is that the reaction proceeding via ionic intermediate is influenced by the mechanical effects of cavitation such as surface cleaning, particle size reduction and improved mass transfer (Mason, 1997). For these reasons, the present method is atom-economical and environmentally friendly than previously reported methods and is successful in achieving as a satisfactory solution for Friedel–Crafts reaction problems. To the best of our knowledge, although the Friedel–Crafts acylation was reported using magnetic Fe3O4 (Hoseini et al., 2013) and CuFe2O4 nanoparticles (Parella et al., 2013), there is no report of application of magnetic γ-Fe2O3 NPs to Friedel–Crafts benzoylation reaction under ultrasound irradiation. In this work, γ-Fe2O3 NPs and other traditional Lewis acid catalysts were examined for the benzoylation of anisole with benzoyl chloride, and the best catalytic performance was obtained using γ-Fe2O3 nanoparticles with 97% selective in para-position. The scope of substrates was also investigated under current method and much interesting results in regioselectivity with the benzoylation of 1-methylnaphthalene, anthracene, and indole were observed. In addition, the simple and efficient recovery of magnetic γ-Fe2O3 nanoparticles promises the potential of this material for synthetic and catalytic applications.
2 Material and methods
2.1 Chemical and instrumentation
All chemicals were purchased from Merck and Sigma-Aldrich and used without further purification.
For ultrasound reaction, the ultrasound bath Elma S30H was used. Powder X-ray diffraction (PXRD) patterns were recorded using a Bruker D8 Advance operated at 40 kV and 40 mA with a Ni filtered Cu Kα radiation (λ = 1.54178 Å) source with scanning rate 2° per min and 2θ angle ranging from 10° to 70°. The morphology of the sample was examined by transmission electron microscopy (TEM) using JEOL JEM 1400 with operating voltage at 100 kV. The magnetization of the sample was measured in a varied magnetic field between −16 and 16 kOe at room temperature using a vibrating sample magnetometer (VSM/Microsense, EV11, USA). GC-MS analyses were performed on an Agilent GC System 7890 equipped with a mass selective detector Agilent 5973 N and a capillary DB-5MS column (30 m × 250 μm × 0.25 μm). The 1H and 13C NMR spectra were recorded on a Bruker Advance 500 using CDCl3 as the solvent and TMS as the internal standards.
2.2 Synthesis of magnetic γ-Fe2O3 nanoparticles
The magnetic γ-Fe2O3 nanoparticles were synthesized according to a modification of previous literatures (Lu et al., 2007). In a typical procedure, magnetic Fe3O4 nanoparticles were prepared via co-precipitation. In brief, 150 ml of an aqueous solution of 0.080 M FeCl3.6H2O and 0.040 M FeCl2·4H2O served as a source of iron. The magnetite particles were co-precipitated under vigorous mechanical stirring (2000 rpm) by adding ammonia solution to the iron-containing solution. After that, the reaction was carried out at 80 °C for 2 h under nitrogen atmosphere to prevent oxidation. The black particles were collected by sedimentation with the help of a large magnetic bar. Then, these particles were washed with distilled water, and dried in vacuum oven at 80 °C in order to obtain black powder. Next, Fe3O4 NPs underwent a phase transition to γ-Fe2O3. The obtained black powder was annealed in oven at 200 °C during 3 h. The color of the powder changed from black to brown red.
An X-ray diffraction analysis was carried out on the resulting brown red obtained powder. Its XRD pattern (Fig. 1) showed six characteristic peaks of 2θ, 30.3, 35.6, 43.5, 53.9, 57.2 and 62.7 which indicated the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) planes, respectively. The pattern was in good agreement with the standard XRD pattern COD 9006316, so this confirmed the formation of γ-Fe2O3 nanoparticles with an inverse spinal structure. The XRD patterns of magnetic γ-Fe2O3 nanoparticles were also consistent with the previous literatures (Lu et al., 2007), and no impurity signals were observed. The average crystal size of synthesized NPs calculated by Debye–Scherrer equation was 8.6 nm. The TEM images of γ-Fe2O3 powder are shown in Fig. 2. It is clear from TEM images that the nanoparticles are spherical in shape. TEM result also indicates that the diameter of γ-Fe2O3 NPs is in the range of 5–15 nm, which leads to the availability of high surface area for catalytic activity. Vibrating sample magnetometer (VSM) analysis gives information about the magnetic properties of the materials. The M−H hysteresis loop for γ-Fe2O3 NPs measured at room temperature is shown in Fig. 3. The value of saturation magnetization (Ms) of these NPs is observed to be 69.64 emu/g, which suggested the super paramagnetic nature of the synthesized γ-Fe2O3 NPs.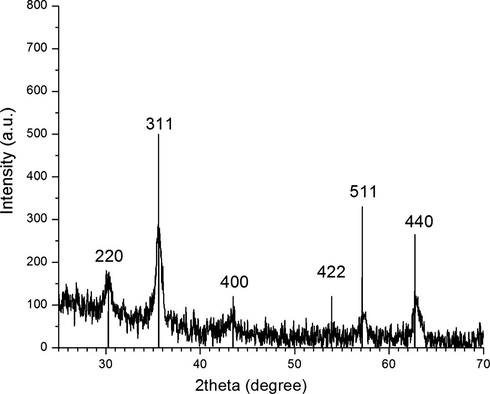
XRD pattern of γ-Fe2O3 nanoparticles.
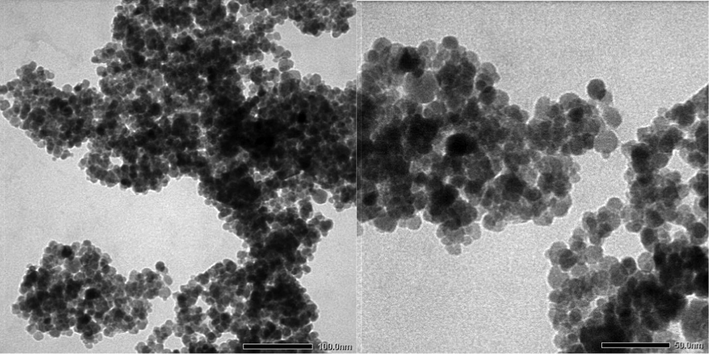
TEM image of γ-Fe2O3 nanoparticles.
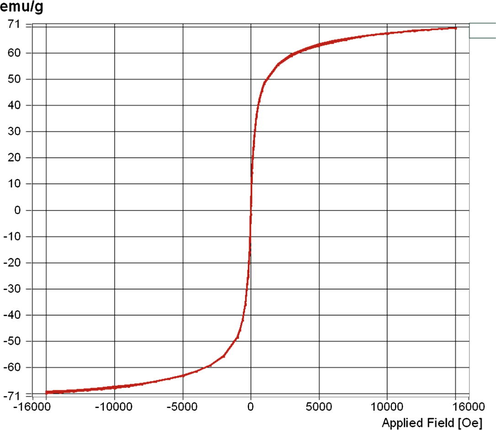
Magnetization curve of γ-Fe2O3 nanoparticles.
2.3 General methods for Friedel–Crafts benzoylation reaction
In a typical procedure for Friedel–Crafts benzoylation, benzoyl chloride (1.0 mmol) was added to a vessel containing the mixture of aromatic compounds (2 mmol) and 0.20 mmol γ-Fe2O3 nanoparticles as a catalyst. Then, the calcium chloride tube was placed onto the reaction vessel to prevent the reaction from moisture in the atmosphere. The reaction mixture was irradiated under solvent-free sonication at room temperature (30–35 °C) for 60 min. The process was monitored by thin-layer chromatography and GC. Upon the completion of reaction, the mixture was diluted with ethyl acetate (10 mL). The catalyst was separated by an external magnet and washed with ethanol (2 × 10 mL) and deionized water for several times. After that, the catalyst was dried at 100 °C and recycled up to six times. The reaction mixture was extracted with ethyl acetate (3 × 15 mL), washed with saturated Na2CO3 solution (3 × 10 mL), followed by water (2 × 20 mL) and then dried over MgSO4. The solvent was removed under vacuum. All the products are well-known compounds, and their identities were confirmed by GC-MS, 1H and 13C NMR spectra by comparison with literatures.
3 Results and discussion
The Friedel–Crafts benzoylation of anisole was chosen as a model reaction to search for the optimal condition. All reactions were carried out at room temperature under solvent-free ultrasound irradiation (Elma S30H, frequency 37 kHz and electric power 200 W). To establish the optimal condition for γ-Fe2O3 NPs, the Friedel–Crafts acylation reaction of anisole and benzoyl chloride was taken place in various conditions under sonication. In general, the solvent plays a significant role on reaction in the presence of heterogeneous solid catalyst. In the present method, owning to the special catalytic reactivity, γ-Fe2O3 NPs afforded the desired products in excellent yield under solvent-free condition. Anisole was benzoylated in good to high yields at room temperature in appropriate reaction times (see below). Thus, the reaction temperature is not optimized for this method. In addition, the increase in reaction temperature enhanced the formation of FeCl3. Consequently, this procedure is designed for energy efficiency; we saved more energy for heating, cooling or solvent removal.
The molar ratios of anisole and benzoyl chloride were screened in the presence of 20 mol% γ-Fe2O3 NPs for 30 min (Fig. 4). It is found that the molar ratio showed a significant effect on the reaction conversion. With 1:1 ratio, the reaction proceeded with only 59% yield. The excellent result was obtained with 2.0 M equivalents of anisole. However, when more than 2.0 M equivalents of anisole was used, the formation of desired product was decreased, presumably due to the excess mole of anisole decreasing the rate of formation of acylium ion (Parida et al., 2009; Chen et al., 2015; Dzudza and Marks, 2008). Consequently, the best reactant molar ratio was chosen with 2.0 M equivalents of anisole. Then, we decided to investigate the effect of catalyst loading in the Friedel–Crafts benzoylation of anisole (2.0 equiv.) at room temperature under sonication (Fig. 5). The Friedel–Crafts benzoylation of anisole with benzoyl bromide was carried out under sonication for 30 min, using 2.0 M equivalents of anisole in the presence of 5 mol%, 10 mol%, 15 mol%, 20 mol%, and 30 mol% γ-Fe2O3 NPs. The catalyst amount was calculated with respect to the γ-Fe2O3 NPs/benzoyl chloride molar ratio. As expected, an increase in the catalyst concentration led to significant enhancement in the reaction conversion. The Friedel–Crafts benzoylation of anisole under ultrasound irradiation did not proceed in the absence of γ-Fe2O3 NPs. The reaction using 5 mol% catalyst afforded the desired product in 45% yield for 30 min under solvent-free sonication. The reaction conversion continued to increase when the catalyst concentration was used in 10 mol% and 15 mol%. In the presence of 20 mol% γ-Fe2O3 NPs, 73% conversion was achieved for the reaction after 30 min. The use of more than 20 mol% γ-Fe2O3 NPs did not improve the reaction rate. The reaction conversion was obtained in 75% with 30 mol% catalyst. The previous report for Friedel–Crafts benzoylation of anisole with benzoyl chloride in the presence of activated α-Fe2O3 gave 95% yield with only 5 mol% of catalyst. However, the difficult separations of α-Fe2O3 was observed using hydrogen peroxide to oxidize chloride ion and precipitate of the catalyst from the organic solution (Sharghi et al., 2010).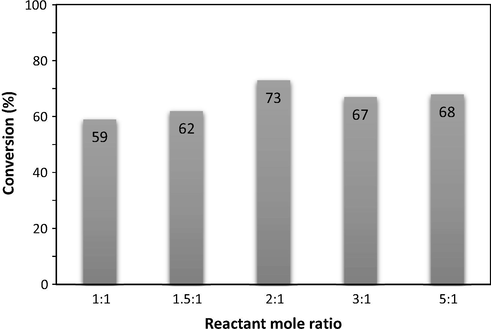
Effect of anisole:benzoyl chloride molar ratio on the reaction conversion.
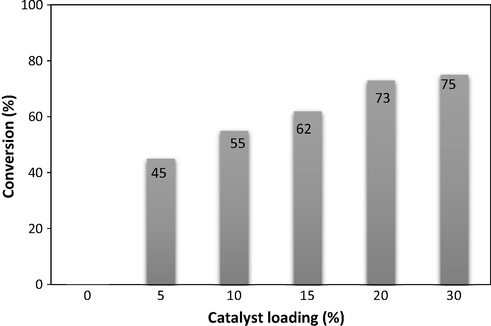
Effect of catalyst loading on the benzoylation of anisole under sonication for 30 min.
Next, we carried out the Friedel–Crafts benzoylation of anisole under solvent-free sonication bath to find out the efficient catalyst. At the beginning, the Friedel–Crafts benzoylation did not proceed in the absence of the catalyst (Table 1, entry 1). Then we investigated the effect of various catalysts using model benzoylation of anisole and 20 mol% catalyst was used. Among these, magnetic γ-Fe2O3 nanoparticles were found to be the best catalyst. Along with magnetic γ-Fe2O3 nanoparticles, we also tested various moisture sensitive Lewis acid catalysts. It is observed that the Friedel–Crafts benzoylation using these catalysts proceeds difficultly (Table 1, entries 2–5). The Friedel–Crafts benzoylation is unsuccessful for metal oxide catalysts in our method (Table 1, entries 7–12). Attempts to increase the conversions by increasing reaction times to greater than 120 min did not lead to significant improvements in conversions. The γ-Fe2O3 nanoparticles with great surface area and easy separation by a magnet could catalyze efficiently the Friedel–Crafts reaction more efficiently than other Lewis acid catalysts in the present method.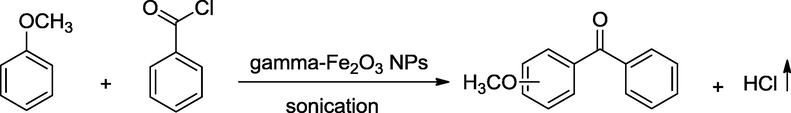
Entry
Catalysta
Conversionb (%)
Regioselectivity (o/p)
1
None
0
–
2
Fe2(SO4)3
10 (62)c
0/100
3
FeSO4
0
–
4
FeCl3
68 (70)d
2/98
5
AlCl3
27 (39)e
3/97
6
γ-Fe2O3 NPsc
88
3/97
7
Fe2O3
4 (5)f
0/100
8
ZnO
15 (17)g
3/97
9
Al2O3
0
–
10
TiO2
0
–
11
CuO
0
–
12
MgO
0
–
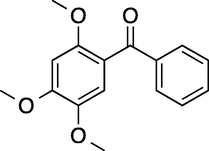
To evaluate the efficiency of the γ-Fe2O3 NPs catalyst under sonication, various arenes were tested in the Friedel–Crafts benzoylation with benzoyl chloride reagent. The scope of benzoylation reaction is presented in Table 2. The reaction time must be optimized for each substrate. Arenes containing electron-donating and without substituent can also be efficiently benzoylated. Owing to low boiling point and less activity, the benzoylation of benzene could not be achieved in previous reports (Ross and Xiao, 2002; Gmouh et al., 2003; Tran et al., 2012). However, benzene was benzoylated with 100% conversion (82% isolated yield), without by-product for 120 min under the present method (Table 2, entry 1). Dialkylbenzenes could be benzoylated in excellent yields in shorter reaction time (Table 1, entries 2–3). Anisole was benzoylated in 92% yield, with only 3% of ortho-benzoylated product (Table 2, entry 4). Dimethoxybenzenes were also benzoylated in excellent yields, with high selectivity of desired products (Table 2, entries 5–7). However, 1,2,4-trimethoxybenzene, in which the aromatic ring bears trimethoxy substituents was benzoylated in moderate yield, presumably due to steric hindrance (Table 2, entry 8). In contrast, three methyl substituents are present on the ring; mesitylene was still reactive, afforded in 87% isolated yield (Table 2, entry 10). Phenetole was also reactive under the present method with only 6% of selectivity in ortho-position (Table 2, entry 9). Interestingly, naphthalene was benzoylated in good yield, with 80% of α-benzoylated product whereas selectivity for β-acetylnaphthalene was reported in the aforementioned literatures (Selvakumar et al., 2010). Remarkably, the benzoylation of 1-methylnaphthalene was afforded in 90% yield, with 100% selectivity in 2-position. In contrast, the benzoylation of 1-methylnaphthalene using benzoyl chloride gave isomeric mixtures of corresponding ketones in moderate yield (Deutsch et al., 2005). The pure isomer product has opened an important synthetic method. Anthracene was also benzoylated in good yield, with 100% selectivity in 9-position and without dibenzoylation (Sharghi et al., 2010). Friedel–Crafts benzoylation reaction was found to be successful for indole without NH protection under present method; the benzoylated product was obtained in high yield with 90% regioselectivity for the C-3 position (Table 2, entry 14). Generally, the 3-acylated products were obtained in high yields within N-protected indoles (Yu et al., 2013; Taylor et al., 2010).
Entry
Aromatic
Product
Time (min)
Converstionb (%)
Yield (%)
1
120
100
82
2
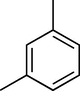
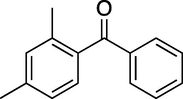
60
99 (2-/4- = 5/95)
88
3
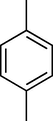
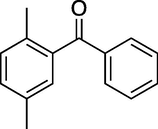
60
100
85
4
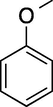

60
99 (o-/p- = 3/97)
92c
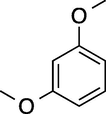
5
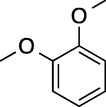
180
100
87
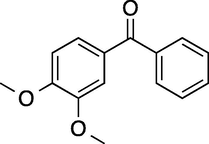
6
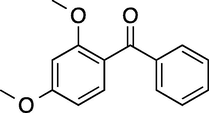
60
100 (2-/4- = 10/90)
88
7
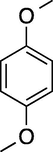
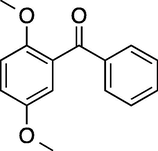
60
100
92
8
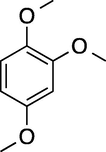
180
100
73
9
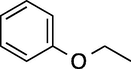

60
100 (o-/p- = 6/94)
86
10
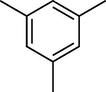
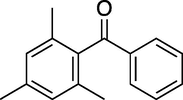
60
100
87
11
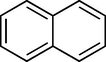
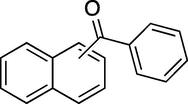
60
100 (α/β = 80/20)
88
12
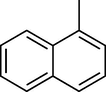
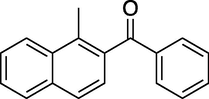
60
100
90
13
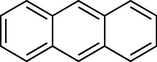
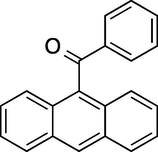
60
90
77
14
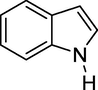
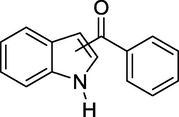
45
100 (1-/2-/3- = 10/0/90)
82
The Friedel–Crafts benzoylation of arenes with benzoyl chloride formed hydrogen chloride as a by-product that can lead to metal leaching. It is noticeable that HCl gas is liberated during the process, so it would escape from the reaction mixture. Moreover, the formation of FeCl3 from HCl and γ-Fe2O3 NPs is an endothermic reaction, and as a result, FeCl3 is not formed in the present method. For the leaching test, a catalytic reaction was stopped after 10 min, the γ-Fe2O3 NPs were separated from the reaction mixture by magnetic decantation, and the reaction mixture was analyzed by GC (Fig. 6). The reaction solution was then stirred for further 90 min. No further conversion was detected for the Friedel–Crafts benzoylation of anisole after the γ-Fe2O3 NPs were removed. The observation proved that there was no contribution from leached γ-Fe2O3 NPs to form FeCl3 in the mixture and the conversion was only possible in the presence of the γ-Fe2O3 nanoparticles.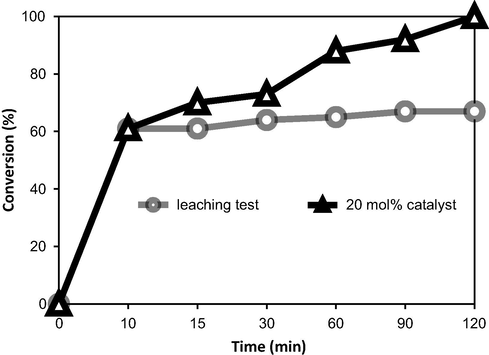
Leaching test showed no contribution from homogeneous catalysis of leached species.
The recycling of γ-Fe2O3 NPs catalyst was observed in the Friedel–Crafts benzoylation of anisole under sonication for 120 min (Fig. 7). The recovery of catalyst is easily assisted by external magnet. Due to its high stability in our method, γ-Fe2O3 NPs catalyst was recovered easily in a quantitative yield (Fig. 8). Then, γ-Fe2O3 NPs were washed with ethyl acetate, dried under vacuum, and used for next cycle without further purification. Owing to the ease of recovery and stability under this method, γ-Fe2O3 NPs catalyst was reused over six times without any significant loss in reactivity and regioselectivity. The present method is promising for its application in an industrial process.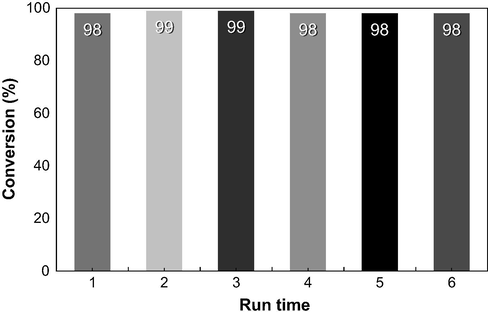
Reusability of γ-Fe2O3 NPs in the acylation reaction.
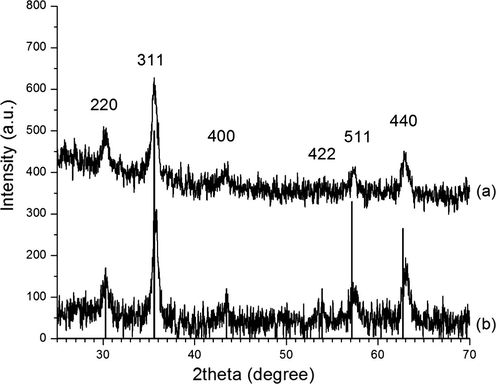
XRD patterns of γ-Fe2O3 NPs (a) and γ-Fe2O3 NPs after using six times (b).
4 Conclusion
In the present work, the performance of magnetic γ-Fe2O3 NPs as a heterogeneous catalyst for the Friedel–Crafts benzoylation under solvent-free sonication was investigated. The desired products were obtained in good to excellent yields under the method. Although the magnetic Fe3O4 and CuFe2O4 nanoparticles catalyzed the Friedel-Crafts acylation were reported in literatures, the present method demonstrates several merits, including highly stable catalyst, excellent catalytic reactivity with high regioselectivity, facile large-scale synthesis of catalyst, operational simplicity, elimination of solvent, no need for inert atmosphere condition. The catalyst is recovered and reused over six times without significant loss of catalytic activity and regioselectivity. This method has an easy work-up procedure, good to excellent yields, mild condition and potentially industrial application.
Acknowledgments
We thank MSc. Duy-Khiem Nguyen Chau (University of Minnesota Duluth, USA) and MSc. Mai Hoang Ngoc Do (IPH-HCM) for their help.
References
- Catal. Commun.. 2012;29:114-117.
- Chem. Commun.. 2013;49:752-770.
- J. Mol. Catal. A: Chem.. 2015;404–405:8-17.
- Chem. Soc. Rev.. 2015;44:5341-5370.
- Catal. Today. 2014;227:130-137.
- ACS Appl. Mater. Interfaces. 2012;4:2303-2309.
- J. Mol. Catal. A: Chem.. 2015;396:231-238.
- Green Chem.. 2014;16:3401-3427.
- Ultrason. Sonochem.. 2014;21:169-181.
- Ultrason. Sonochem.. 2014;21:1050-1064.
- Catal. Lett.. 2014;144:817-824.
- Chem. Soc. Rev.. 2006;35:180-196.
- J. Catal.. 2005;231:269-278.
- Dalton Trans.. 2016;45:7875-7880.
- J. Org. Chem.. 2008;73:4004-4016.
- Org. Lett.. 2003;5:2219-2222.
- Chem. Rev.. 2011;111:3508-3576.
- Synth. Commun.. 2013;43:1683-1691.
- J. Am. Chem. Soc.. 2014;136:12844-12847.
- Catal. Today. 2016;264:83-90.
- Org. Lett.. 2013;15:788-791.
- Appl. Catal. A: Gen.. 2015;502:105-113.
- ACS Comb. Sci.. 2014;16:566-572.
- Chem. Soc. Rev.. 2009;38:1450-1459.
- Angew. Chem.. 2007;46:1222-1244.
- Adv. Mater. Res.. 2012;443–444:917-922.
- Martins, M.A., Frizzo, C.P., Tier, A.Z., Moreira, D.N., Zanatta, N., Bonacorso, H.G., 2014. Chem. Rev. 114, PR1-PR70.
- Chem. Soc. Rev.. 1997;26:443-451.
- RSC Adv.. 2014;4:5927-5952.
- RSC Adv.. 2015;5:104688-104694.
- Friedel-Crafts Chemistry. New York: John Wiley and Sons; 1973.
- Chem. Commun.. 2013;49:2933-2935.
- Tetrahedron Lett.. 2013;54:1738-1742.
- J. Mol. Catal. A: Chem.. 2009;297:93-100.
- Chem. Rev.. 2011;111:3036-3075.
- Green Chem.. 2002;4:129-133.
- Green Chem.. 2014;16:2906-2933.
- RSC Adv.. 2014;4:40008-40018.
- Chem. Rev.. 2006;106:1077-1104.
- Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes. Boca Raton: Taylor & Francis; 2010.
- Appl. Catal. A: Gen.. 2010;372:130-137.
- Adv. Synth. Catal.. 2010;352:3031-3044.
- Ultrason. Sonochem.. 2012;19:307-313.
- Appl. Catal. B. 2008;84:363-371.
- J. Org. Chem.. 2013;78:11163-11171.
- Org. Lett.. 2010;12:5740-5743.
- J. Phys. Chem. B. 2010;114:3178-3184.
- Ultrason. Sonochem.. 2015;27:210-234.
- Tetrahedron Lett.. 2012;53:222-224.
- Tetrahedron Lett.. 2015;56:2187-2192.
- Tetrahedron Lett.. 2015;56:612-618.
- Asian J. Org. Chem.. 2015;4:482-486.
- RSC Adv.. 2016;6:37031-37038.
- Tetrahedron. 2015;71:5254-5259.
- Chem. Commun. (Camb). 2013;49:2368-2370.
- ACS Comb. Sci.. 2012;14:38-43.
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2017.04.008.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







