Translate this page into:
Five undescribed plant-derived bisphenols from Artemisia capillaris aerial parts: Structure elucidation, anti-hepatoma activities and plausible biogenetic pathway
⁎Corresponding authors at: College of Pharmacy, School of Medicine, Hangzhou Normal University, Hangzhou 311121, Zhejiang Province, PR China (Xiao Du) and Center Lab of Longhua Branch and Department of Infectious Disease, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, So uthern University of Science and Technology), Shenzhen 518020, Guangdong Province, PR China (Xiaobin Zeng). duxiao9@999.com.cn (Xiao Du), zeng.xiaobin@szhospital.com (Xiaobin Zeng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
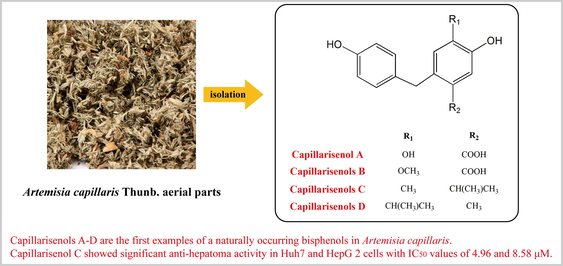
Abstract
Five undescribed bisphenols were isolated from aerial parts of A. capillaris Thunb. Capillarisenols A–E are the type of bisphenols firstly isolated from this plant. Capillarisenol C showed significant anti-hepatoma activity, better than positive control (Lenvatinib)
Abstract
Yin-Chen, which belongs to the Asteraceae family and the genus Artemisia, is among the most abundantly used traditional medicines in China for the treatment of hepatitis and bilious disorder. Herein, five undescribed plant-derived bisphenols, capillarisenols A–E (13, 15, 25, 29, 31), and one undescribed phenolic compound (32), together with 32 known phenolic compounds (1–12, 14, 16–24, 26–28, 30, 33–38), were isolated and identified based on spectroscopic evidence from aerial parts of Artemisia capillaris Thunb. Capillarisenols A–E are the type of bisphenols firstly isolated from this plant. The plausible biogenetic pathway of new compounds was also proposed. In addition, the potential anti-hepatoma effects on Huh7 and HepG2 cell lines of all isolated compounds were evaluated in vitro. Capillarisenol C (25) showed significant anti-hepatoma activity in Huh7 and HepG 2 cells, with IC50 values of 4.96 and 8.58 μM, better than the positive control drug (Lenvatinib). This study provided phytochemical evidence for further development and utilisation of A. capillaris in health products.
Keywords
Artemisia capillaris
Bisphenols
Structure elucidation
Plausible biogenetic pathway
Anti-hepatoma activity
1 Introduction
Yin-Chen, which belongs to the Asteraceae family and the genus Artemisia, is among the most abundantly used traditional medicines in China for the treatment of hepatitis and bilious disorder (Jang, et al., 2015; Zhao, et al., 2014). Two species, Artemisia scoparia Waldst. et Kit and Artemisia capillaris Thunb., are documented in the Chinese Pharmacopoeia as authentic Yin-Chen resources (Chinese Pharmacopoeia Commission, 2015). In Guangdong, China, Yin Chen and fried crucian carp are boiled in soup, which can effectively soothe the liver and clear liver heat. This is a commonly used local soup food therapy. Modern pharmacological studies have shown that A. scoparia and A. capillaris have a broad spectrum of biological activities, including antiviral, antitumor, anti-bacterial, anti-inflammatory, antioxidant, anti-cirrhosis and hepatoprotective effects (Ding, et al., 2021; Hsueh, et al., 2021). Most of the therapeutic effects can be attributed to the major or minor compounds found in this medicinal herbs, for example, flavonoids and coumarins (Cai, et al., 2020; Geng, et al., 2015; Lee, et al., 2007; Wang, et al., 2008). In addition, phytochemical assessments have found that Yin-Chen also contains other compounds, such as flavonoid glycosides, volatile oil, steroids, chromones, coumarin, phenolic acids and terpenoids (Aati, et al., 2020; Ding, et al., 2021; Hsueh, et al., 2021; Tian, et al., 2020).
As part of the ongoing search for promising new anti-liver compounds from traditional medicinal plants, bioactivity-guided isolation and fraction of A. capillaris aerial parts were carried out. Five undescribed bisphenols, capillarisenols A–E (13, 15, 25, 29, 31), and one undescribed phenolic compound (32), together with 32 known phenolic compounds (1–12, 14, 16–24, 26–28, 30, 33–38) were isolated and identified. The chemical structures of all identified compounds are shown in Fig. 1. Capillarisenols A–E (13, 15, 25, 29, 31) are the naturally occurring bisphenols firstly isolatedfrom this plant. Here, we describe the structural elucidation and plausible biogenetic pathway of new compounds. In addition, the potential anti-hepatoma effects of all isolated compounds on Huh7 and HepG 2 cells were evaluated.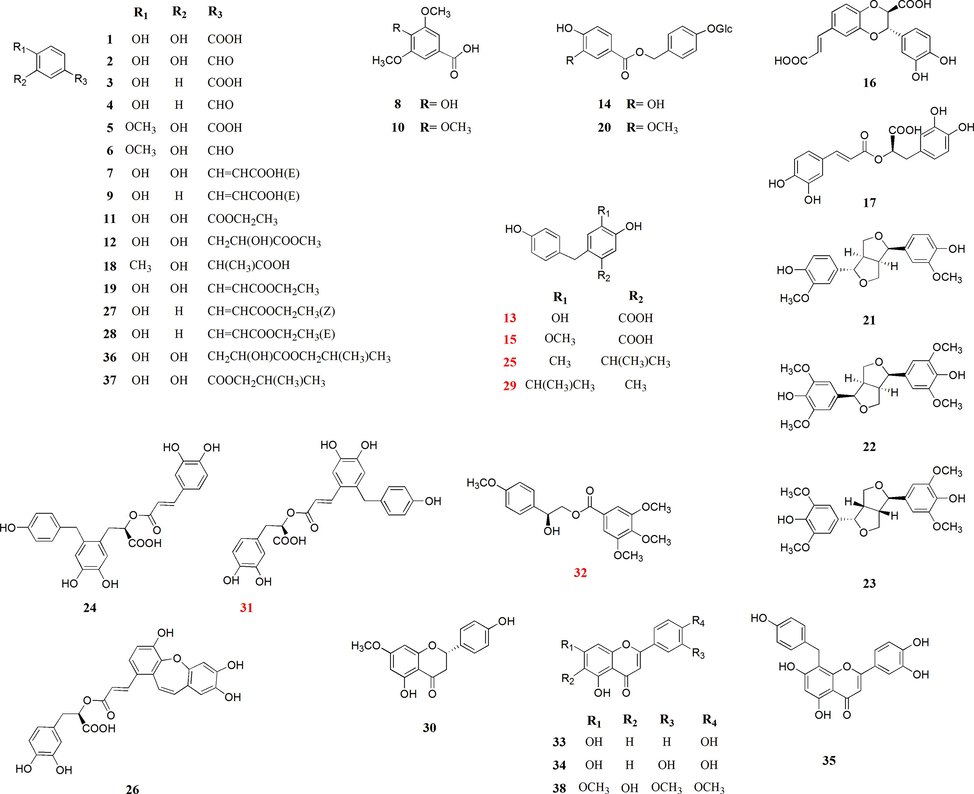
Chemical structure of all compounds (1–38) from A. capillaris aerial parts.
2 Materials and methods
2.1 General experimental procedures
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DPX-400 (1H NMR, 400 MHz; 13C NMR, 100 MHz) spectrometer, with tetramethylsilane (TMS) as the internal standard. Unless otherwise specified, chemical shifts (δ) were quoted in ppm with reference to the residual solvent signal (DMSO‑d6: δH 2.50 ppm, δC 39.52 ppm). Positive- or negative-ion HR-ESI-TOF-MS data were measured using a Bruker microTOF-QII mass spectrometer (Bruker, Karlsruhe, Germany). Analytical HPLC was carried out on a Shimadzu LC-16 instrument, and UV detection was carried out using a YMC-Pack ODS-A C18 (4.6 × 250 mm, 5 μm) column. All samples were purified by preparative HPLC using a Shimadzu HPLC system equipped with a UV detector and a Cosmosil 5C18-MS-II column (20 × 250 mm, 5 μm, Nacalai Tesque, Inc., Nijo Karasuma, Japan). Silica gel (100–200, 200–300, and 300–400 mesh, Qingdao Marine Chemical Factory, Qingdao, China), ODS (50 μm, ODS-A-HG, YMC Co. ltd., Japan), and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Sweden) were used for open column chromatography (CC) to separate samples, and thin-layer chromatography (TLC) analysis were carried out using precoated silica gel GF254 plates. Solvents were of analytical grade (Guanghua Chemical Co., ltd. Guangzhou, P.R. China) for open CC and HPLC grade (Merck, Germany) for HPLC analysis.
2.2 Plant material
The dried aerial parts of A. capillaris Thunb. were gathered in Ganzhou, Jiangxi Province, China, in August 2019 and authenticated by Dr. Xiaobin Zeng of Shenzhen People’s Hospital. A voucher specimen (No. 20190805) has been deposited in the Center Lab of Longhua Branch, Shenzhen People’s Hospital, Second Clinical Medical College of Jinan University, Shenzhen, China.
2.3 Extraction and isolation
The air-dried aerial parts of A. capillaris (7.0 kg) were powered and extracted four times (5 days each) using 80 % EtOH at room temperature. A dark-brown crude extract (1.4 kg, 20 %) was afforded following concentration under reduced pressure. The extract was suspended in water (2 L) and partitioned successively with petroleum ether, ethyl acetate, and n-BuOH. The ethyl acetate fraction (114 g) was separated by ODS column chromatography, eluted with a MeOH − H2O (5–100 %) gradient, and yielded ten fractions. Various column chromatographic separations of the 10 fractions afforded compounds 1–35. The n-BuOH fraction (200 g) was first separated by silina gel column chromatography, eluted with a CH2Cl2-MeOH (5–100 %) gradient, and yielded six fractions. Various column chromatographic separations of FB. 3 afforded compounds 36–38. Fig. 2 shows the extraction and isolation procedures for compounds from the aerial parts of A. capillaris. The purity of these compounds was determined as to be more than 95 % by HPLC.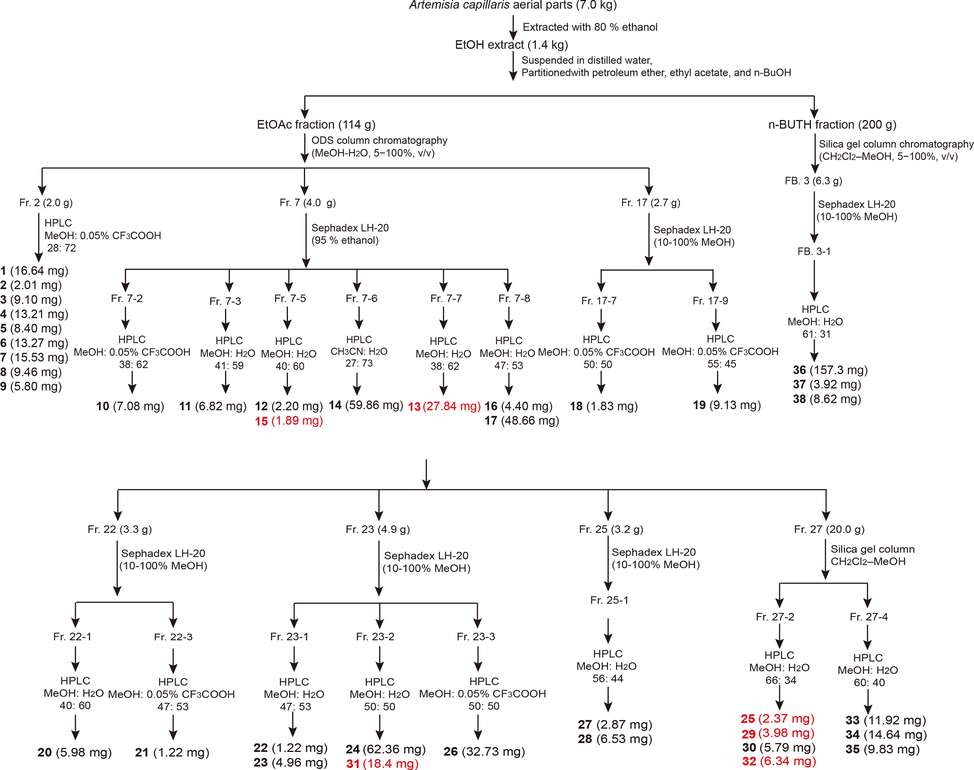
Extraction and isolation procedure of compounds from A. capillaris aerial parts.
2.3.1 Protocatechuic acid (1)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.33 (1H, s, H-2), 7.28 (1H, d, J = 8.0 Hz, H-6), 6.78 (1H, d, J = 8.0 Hz, H-5); 13C NMR (100 MHz, DMSO‑d6) δC: 167.3 (C-7), 150.0 (C-3), 144.9 (C-4), 121.9 (C-1), 121.7 (C-6), 116.6 (C-2), 115.2 (C-5).
2.3.2 Protocatechuyl aldehyde (2)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 9.70 (1H, s, H-7), 7.26 (1H, d, J = 8.0 Hz, H-6), 7.23 (1H, s, H-2), 6.91 (1H, d, J = 8.0 Hz, H-5); 13C NMR (100 MHz, DMSO‑d6) δC: 191.1 (C-7), 152.1 (C-3), 145.9 (C-4), 128.8 (C-1), 124.5 (C-6), 115.5 (C-2), 114.4 (C-5).
2.3.3 4-Hydroxybenzoic acid (3)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.78 (2H, d, J = 7.1 Hz, H-2/6), 6.82 (2H, d, J = 7.1 Hz, H-3/5); 13C NMR (100 MHz, DMSO‑d6) δC: 167.2 (C-7), 161.6 (C-4), 131.5 (C-2/6), 121.4 (C-1), 115.1 (C-3/5).
2.3.4 4-Hydroxybenzaldehyde (4)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.75 (2H, d, J = 8.0 Hz, H-2/6), 6.92 (2H, d, J = 8.0 Hz, H-3/5); 13C NMR (100 MHz, DMSO‑d6) δC: 190.9 (C-7), 163.3 (C-4), 132.1 (C-2/6), 128.4 (C-1), 115.8 (C-3/5).
2.3.5 3-Hydroxy-4-Methoxybenzoic acid (5)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.43 (1H, d, J = 8.0 Hz, H-6),7.42 (1H, s, H-2), 6.84 (1H, d, J = 8.0 Hz, H-5), 3.80 (3H, s, H-OCH3); 13C NMR (100 MHz, DMSO‑d6) δC: 167.2 (C-7), 151.1 (C-3), 147.2 (C-4), 123.5 (C-1), 121.6 (C-6), 115.0 (C-2), 112.7 (C-5), 55.5 (C-OCH3).
2.3.6 3-Hydroxy-4-Methoxy-Benzaldehyde (6)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.41 (1H, d, J = 8.0 Hz, H-6),7.35 (1H, s, H-2), 6.98 (1H, d, J = 8.0 Hz, H-5), 3.82 (3H, s, H-OCH3).
2.3.7 Caffeic acid (7)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.39 (1H, d, J = 15.9 Hz, H-7),7.01 (1H, s, H-2), 6.95 (1H, d, J = 8.0 Hz, H-6), 6.75 (1H, d, J = 8.0 Hz, H-5), 6.16 (1H, d, J = 15.9 Hz, H-8); 13C NMR (100 MHz, DMSO‑d6) δC: 167.9 (C-9), 148.1 (C-3), 145.6 (C-4), 144.6 (C-7), 125.7 (C-1), 121.1 (C-6), 115.7 (C-2), 115.1 (C-5), 114.6 (C-8).
2.3.8 3,5-Dimethoxy-4-Hydroxybenzoic acid (8)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.20 (2H, s, H-2, 6), 3.80 (6H, s, 3,5-OCH3); 13C NMR (100 MHz, DMSO‑d6) δC: 167.3 (C-7), 150.0 (C-3), 144.9 (C-4), 121.9 (C-1), 121.7 (C-6), 116.6 (C-2), 115.2 (C-5).
2.3.9 Cumaric acid (9)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.51 (2H, d, J = 8.0 Hz, H-2, 6), 7.49 (1H, d, J = 15.9 Hz, H-7), 6.79 (2H, d, J = 8.0 Hz, H-3, 5), 6.28 (1H, d, J = 15.9 Hz, H-8).
2.3.10 3,4,5-Trimethoxybenzoic acid (10)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.23 (2H, s, H-2/6), 3.82 (6H, s, H3-8/10), 3.72 (3H, s, H3-9); 13C NMR (100 MHz, DMSO‑d6) δC: 166.9 (C-7), 152.7 (C-3/5), 141.4 (C-4), 125.9 (C-1), 106.6 (C-2/6), 60.1 (C-9), 56.0 (C-8/10).
2.3.11 3,4-Dihydroxybenzoic acid ethyl ester (11)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.37 (1H, s, H-2), 7.29 (1H, d, J = 8.0 Hz, H-6), 684 (1H, d, J = 8.0 Hz, H-5), 4.22 (2H, q, J = 7.0 Hz, H-8), 1.27 (3H, t, J = 7.0 Hz, H-9); 13C NMR (100 MHz, DMSO‑d6) δC: 165.7 (C-7), 150.4 (C-3), 145.1 (C-4), 121.6 (C-1), 120.7 (C-6), 116.3 (C-2), 115.4 (C-5), 60.0 (C-8), 14.3 (C-9).
2.3.12 3,4,α-Trihydroxymethyl phenylpropionate (12)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 6.59 (1H, d, J = 7.6 Hz, H-5), 6.58 (1H, d, J = 1.7 Hz, H-2), 6.42 (1H, dd, J = 7.6, 1.7 Hz, H-6), 4.11 (1H, dd, J = 7.7, 5.3 Hz, H-8), 3.59 (3H, s, H3-10), 2.74 (1H, dd, J = 13.7, 5.3 Hz, H-7a), 2.67 (1H, dd, J = 13.7, 7.7 Hz, H-7b); 13C NMR (100 MHz, DMSO‑d6) δC: 174.0 (C-9), 144.8 (C-3), 143.7 (C-4), 128.2 (C-1), 120.0 (C-6), 116.7 (C-2), 115.2 (C-5), 71.7 (C-8), 51.3 (C-10), 39.7 (C-7).
2.3.13 Capillarisenol a (13)
Pale yellow, amorphous powder; UV (MeOH) λmax (logε) 220, 261, and 297 nm; HR-ESI-TOF-MS m/z 283.0653 [M + Na]+; IR νmax 3386, 1656, 1514, 1027, 955 cm−1; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 1.
No.
13
15
δH
δC
δH
δC
1
–
121.6
–
120.0
2
–
135.3
–
135.8
3
6.77, s
114.6
6.52, s
118.2
4
–
150.4
–
148.9
5
–
144.0
–
142.8
6
7.30, s
117.6
7.31, s
118.1
7
–
168.1
–
168.3
8
3.75, s
55.5
–
–
1′
–
132.0
–
132.0
2′
6.93, d (8.2 Hz)
129.4
6.91, d (8.2 Hz)
129.6
3′
6.61, d (8.2 Hz)
114.9
6.63, d (8.2 Hz)
115.0
4′
–
155.2
–
155.3
5′
6.61, d (8.2 Hz)
114.9
6.63, d (8.2 Hz)
115.0
6′
6.93, d (8.2 Hz)
129.4
6.91, d (8.2 Hz)
129.6
7′
4.16, s
37.3
4.10, s
37.1
2.3.14 Amburoside a (14)
Pale yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.37 (2H, d, J = 8.3 Hz, H-2/6), 7.36 (1H, d, J = 2.0 Hz, H-2′), 7.33 (1H, dd, J = 8.2, 2.0 Hz, H-6′), 7.05 (2H, d, J = 8.3 Hz, H-3/5), 6.80 (1H, d, J = 8.2 Hz, H-5′), 5.19 (2H, s, H2-7), 4.88 (1H, d, J = 7.3 Hz, H-1′’), 3.69 (1H, br d, J = 11.7 Hz, H-6′’a), 3.46 (1H, dd, J = 11.7, 5.7 Hz, H-6′’b), 3.33 (1H, m, H-5′’), 3.26 (2H, t, J = 9.0 Hz, H-2′’/3′’), 3.17 (1H, t, J = 9.0 Hz, H-4′’); 13C NMR (100 MHz, DMSO‑d6) δC: 165.6 (C-7′), 157.3 (C-4), 150.5 (C-4′), 145.1 (C-3′), 129.7 (C-1), 129.6 (C-2/C-6), 121.9 (C-6′), 120.6 (C-1′), 116.3 (C-2′), 116.2 (C-3/C-5), 115.4 (C-5′), 100.3 (C-1′’), 77.1 (C-3′’), 76.7 (C-5′’), 73.3 (C-2′’), 69.8 (C-4′’), 65.4 (C-7), 60.7 (C-6′’).
2.3.15 Capillarisenol b (15)
Pale yellow, amorphous powder; UV (MeOH) λmax (logε) 222, 259, and 296 nm; HR-ESI-TOF-MS m/z 274.2749 [M + H]+; IR νmax 3436, 1655, 1438, 1027, 955 cm−1; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 1.
2.3.16 Trans-6-[(1E)-2-Carboxyethenyl]-3-(3,4-Hihydroxyphenyl)-2,3- Dihydro-1,4-Benzodioxin-2-Carboxlic acid (16)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.48 (1H, d, J = 15.9 Hz, H-7′), 7.29 (1H, d, J = 2.0 Hz, H-2′), 7.20 (1H, dd, J = 2.0, 8.0 Hz, H-5′), 6.95 (1H, d, J = 8.0 Hz, H-6′), 6.80 (1H, s, H-2), 6.70 (2H, s, H-5, 6), 6.37 (1H, d, J = 15.9 Hz, H-8′), 5.26 (1H, d, J = 4.3 Hz, H-7), 5.09 (1H, d, J = 4.4 Hz, H-8); 13C NMR (100 MHz, DMSO‑d6) δC: 168.9 (C-9), 167.7 (C-9′), 145.7 (C-4), 145.2 (C-3), 144.0 (C-3′), 143.5 (C-7′), 142.3 (C-4′), 128.1 (C-1), 126.7 (C-1′), 122.3 (C-6′), 118.4 (C-6), 117.4 (C-5′), 117.1 (C-8′), 116.7 (C-2′), 115.4 (C-2), 114.6 (C-5′), 75.1 (C-8), 74.6 (C-7).
2.3.17 Rosmarinic acid (17)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 2.
No.
17 (Rosmarinic acid)
24
31
δH
δC
δH
δC
δH
δC
1
–
125.4
–
125.4
–
123.0
2
7.09, d (2.1 Hz)
114.9
7.07, br s
115.0
7.10, s
113.1
3
–
145.5
–
145.7
–
144.2
4
–
148.6
–
148.7
–
148.6
5
6.79, d (8.1 Hz)
115.8
6.79, d (8.1 Hz)
115.9
6.60, s
117.6
6
7.02, dd (2.1, 8.1 Hz)
121.4
7.01, d (8.1 Hz)
121.7
–
133.9
7
7.49, d (15.9 Hz)
145.6
7.48, d (15.9 Hz)
146.0
7.80, d (15.7 Hz)
142.5
8
6.27, d (15.9 Hz)
113.6
6.24, d (15.9 Hz)
113.3
6.11, d (15.7 Hz)
114.0
9
–
166.0
–
166.0
–
166.0
1′
–
127.9
–
125.1
–
127.6
2′
6.71, d (2.1 Hz)
116.7
6.66, s
117.7
6.67, d (1.1 Hz)
116.6
3′
–
144.9
–
143.3
–
145.0
4′
–
143.9
–
143.9
–
144.0
5′
6.67, d (8.1 Hz)
115.4
6.46, s
117.5
6.62, d (8.1 Hz)
115.4
6′
6.55, dd (2.1, 8.1 Hz)
119.9
–
131.3
6.49, d (1.1, 8.1 Hz)
120.1
7′
2.93, dd (8.2, 14.4 Hz);
3.01, dd (4.4, 14.4 Hz)36.4
2.90, dd (9.2, 14.5 Hz);
3.03, dd (4.1, 14.5 Hz)33.1
2.87, dd (8.7, 14.4 Hz);
2.98, dd (3.4, 14.4 Hz)36.3
8′
5.06, dd (4.4, 8.2 Hz)
73.6
4.96, dd (4.1, 9.2 Hz)
72.9
4.98, dd (3.4, 8.7 Hz)
73.2
9′
–
171.3
–
171.2
–
171.0
1′'
–
–
–
130.6
–
131.1
2′'
–
–
6.91, d (8.3 Hz)
129.4
6.87, d (8.2 Hz)
129.2
3′'
–
–
6.67, d (8.3 Hz)
115.2
6.65, d (8.2 Hz)
115.2
4′'
–
–
–
155.4
–
155.4
5′'
–
–
6.67, d (8.3 Hz)
115.2
6.65, d (8.2 Hz)
115.2
6′'
–
–
6.91, d (8.3 Hz)
129.4
6.87, d (8.2 Hz)
129.2
7′'
–
–
3.78, s
36.5
3.81, s
36.4
2.3.18 2-(3-Hydroxy-4-Methylphenyl)Propanoic acid (18)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 6.96 (1H, d, J = 7.6 Hz, H-6), 6.70 (1H, d, J = 1.7 Hz, H-3), 6.59 (1H, dd, J = 7.6, 1.7 Hz, H-5), 3.49 (1H, q, J = 7.1 Hz, H-8), 2.06 (3H, s, H3-7), 1.28 (3H, d, J = 7.1 Hz, H3-9); 13C NMR (100 MHz, DMSO‑d6) δC: 175.6 (C-10), 155.3 (C-2), 139.8 (C-4), 130.4 (C-6), 122.2 (C-1), 117.9 (C-5), 113.4 (C-3), 44.4 (C-8), 18.6 (C-9), 15.6 (C-7).
2.3.19 Caffeic acid ethyl ester (19)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.46 (1H, d, J = 15.9 Hz, H-7), 7.04 (1H, d, J = 2.0 Hz, H-2), 6.99 (1H, dd, J = 8.1, 2.0 Hz, H-6), 6.76 (1H, d, J = 8.1 Hz, H-5), 6.25 (1H, d, J = 15.9 Hz, H-8), 4.15 (2H, q, J = 7.1 Hz, H2-1′), 1.24 (3H, t, J = 7.1 Hz, H3-2′); 13C NMR (100 MHz, DMSO‑d6) δC: 166.5 (C-9), 148.4 (C-4), 145.6 (C-7), 145.0 (C-3), 125.5 (C-1), 121.3 (C-6), 115.7 (C-5), 114.8 (C-2), 114.0 (C-8), 59.7 (C-1′), 14.3 (C-2′).
2.3.20 Amburoside b (20)
Yellow, amorphous powder; 1H NMR (400 MHz, DMSO‑d6) δH: 7.44 (1H, dd, J = 8.5, 2.1 Hz, H-6′), 7.38 (2H, d, J = 8.6 Hz, H-2/6), 7.37 (1H, d, J = 2.1 Hz, H-2′), 7.04 (2H, d, J = 8.6 Hz, H-3/5), 7.01 (1H, d, J = 8.5 Hz, H-5′), 5.21 (2H, s, H2-7), 4.87 (1H, d, J = 7.2 Hz, H-1′’), 3.82 (3H, s, H3-8′), 3.69 (1H, br d, J = 11.6 Hz, H-6′’a), 3.45 (1H, dd, J = 11.7, 5.7 Hz, H-6′’b), 3.33 (1H, m, H-5′’), 3.25 (2H, t, J = 8.2 Hz, H-2′’/3′’), 3.17 (1H, t, J = 8.2 Hz, H-4′’); 13C NMR (100 MHz, DMSO‑d6) δC: 165.4 (C-7′), 157.3 (C-4), 152.0 (C-4′), 146.3 (C-3′), 129.7 (C-2/C-6), 129.6 (C-1), 121.9 (C-6′), 121.6 (C-1′), 116.2 (C-3/C-5), 115.7 (C-5′), 111.5 (C-2′), 100.3 (C-1′’), 77.1 (C-3′’), 76.6 (C-5′’), 73.2 (C-2′’), 69.7 (C-4′’), 65.6 (C-7), 60.7 (C-6′’), 55.7 (C-8′).
2.3.21 (+)-Epi-Pinoresinol (21)
Yellow amorphous solid; 1H NMR (400 MHz, DMSO‑d6) δH: 8.85 (1H, s, OH), 8.81 (1H, s, OH), 6.90 (2H, s, H-2′’/2′), 6.75 (4H, overlapped, H-5′/5′’/6′’/6′), 4.77 (1H, d, J = 5.9 Hz, H-2), 4.32 (1H, d, J = 6.8 Hz, H-6), 4.05 (1H, d, J = 9.2 Hz, H-4ex), 3.77 (6H, s, OCH3 × 2), 3.73 (2H, overlapped, H-8ex, 4ax), 3.38 (1H, overlapped, H-8ax), 3.10 (1H, t, J = 8.5 Hz, H-1), 2.83 (1H, q, J = 7.1 Hz, H-5); 13C NMR (100 MHz, DMSO‑d6) δC: 147.5 (C-3′’), 147.3 (C-3′), 146.0 (C-4′’), 145.4 (C-4′), 132.3 (C-1′’), 129.6 (C-1′), 118.6 (C-6′’), 117.9 (C-6′), 115.1 (C-5′’/5′), 110.3 (C-2′’), 109.8 (C-2′), 87.0 (C-2), 81.4 (C-6), 70.3 (C-8), 68.8 (C-4), 55.6 (OCH3 × 2), 53.9 (C-1), 49.4 (C-5).
2.3.22 (+)-Diasyringaresinol (22)
Yellow amorphous solid; 1H NMR (400 MHz, DMSO‑d6) δH: 8.25 (2H, s, OH × 2), 6.59 (4H, s, H-2′/6′/2′’/6′’), 4.82 (1H, d, J = 4.6 Hz, H-2/6), 3.75 (12H, s, OCH3 × 4), 3.46 (4H, d, J = 3.6 Hz, H2-4/8), 3.23 (2H, m, H-1/5); 13C NMR (100 MHz, DMSO‑d6) δC: 147.8 (C-3′/5′/3′’/5′’), 134.3 (C-4′/4′’), 129.3 (C-1′/1′’), 103.6 (C-2′/6′/2′’/6′’), 83.3 (C-2/6), 68.1 (C-4/8), 55.9 (OCH3 × 4), 48.6 (C-1/5).
2.3.23 (+)-Lirioresinol a (23)
Yellow amorphous solid; 1H NMR (400 MHz, DMSO‑d6) δH: 8.26 (1H, s, OH), 8.23 (1H, s, OH), 6.61 (2H, s, H-2′’/6′’), 6.59 (2H, s, H-2′/6′), 4.76 (1H, d, J = 5.9 Hz, H-2), 4.32 (1H, d, J = 6.9 Hz, H-6), 4.09 (1H, d, J = 9.3 Hz, H-4ex), 3.77 (2H, overlapped, H-8ex, 4ax), 3.75 (12H, s, OCH3 × 4), 3.38 (1H, overlapped, H-8ax), 3.10 (1H, t, J = 8.6 Hz, H-1), 2.83 (1H, q, J = 7.1 Hz, H-5); 13C NMR (100 MHz, DMSO‑d6) δC: 147.9 (C-3′’/5′’), 147.8 (C-3′/5′), 134.9 (C-1′’), 134.2 (C-4′’), 131.5 (C-4′), 128.8 (C-1′), 103.6 (C-2′’/6′’), 103.0 (C-2′/6′), 87.1 (C-6), 81.5 (C-2), 70.2 (C-4), 68.9 (C-8), 56.0 (OCH3 × 4), 53.9 (C-5), 49.3 (C-1).
2.3.24 2-Caffeoyloxy-3-[2-(4-Hydroxybenzyl)-4,5-Dihydroxy]Phenyl propionic acid (24)
Pale yellow, oil; HR-ESI-TOF-MS m/z 465.1054 [M - H]-; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 2.
2.3.25 Capillarisenol C (25)
Pale yellow, amorphous powder; UV (MeOH) λmax (logε) 204, 225, and 279 nm; HR-ESI-TOF-MS m/z 255.1310 [M - H]–; IR νmax 3436, 1660, 1513, 1052, 955 cm−1; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 3.
No.
25
29
δH
δC
δH
δC
1
–
153.9
–
152.4
2
–
120.7
–
131.2
3
6.77, s
132.4
6.84, s
127.3
4
–
127.9
–
129.7
5
–
144.7
–
133.5
6
6.67, s
111.6
6.53, s
116.7
7
2.98, m
28.2
3.11, m
26.1
8
1.00, d (6.8 Hz)
23.8
1.11, d (6.9 Hz)
22.6
9
1.00, d (6.8 Hz)
23.8
1.11, d (6.9 Hz)
22.6
10
2.04, s
15.6
2.02, s
18.9
1′
–
132.0
–
131.3
2′
6.86, d (8.4 Hz)
129.1
6.87, d (8.4 Hz)
129.1
3′
6.64, d (8.4 Hz)
115.0
6.63, d (8.4 Hz)
115.0
4′
–
155.2
–
155.1
5′
6.64, d (8.4 Hz)
115.0
6.63, d (8.4 Hz)
115.0
6′
6.86, d (8.4 Hz)
129.1
6.87, d (8.4 Hz)
129.1
7′
3.73, s
36.5
3.68, s
37.4
2.3.26 Isosalvianolic acid C (26)
Yellow, oil; HR-ESI-TOF-MS m/z 491.0654 [M - H]-; 1H NMR (400 MHz, DMSO‑d6) δH: 7.82 (1H, d, J = 15.7 Hz, H-15), 7.46 (1H, d, J = 8.4 Hz, H-11), 6.90 (1H, d, J = 8.4 Hz, H-12), 6.86 (2H, d, J = 4.8 Hz, H-7/8), 6.85 (1H, s, H-2), 6.69 (1H, d, J = 2.0 Hz, H-2′), 6.67 (1H, d, J = 8.0 Hz, H-5′), 6.65 (1H, s, H-5), 6.56 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 6.33 (1H, d, J = 15.7 Hz, H-16), 5.05 (1H, dd, J = 8.2, 4.4 Hz, H-8′), 3.01 (1H, dd, J = 14.2, 4.4 Hz, H-7′b), 2.93 (1H, dd, J = 14.2, 8.2 Hz, H-7′a); 13C NMR (100 MHz, DMSO‑d6) δC: 170.7 (C-9′), 165.6 (C-17), 151.1 (C-13), 150.0 (C-4), 147.2 (C-1), 145.1 (C-14), 145.0 (C-3′), 144.1 (C-4′), 142.6 (C-3), 141.6 (C-15), 131.9 (C-7), 131.1 (C-9), 127.2 (C-1′), 124.3 (C-11), 123.2 (C-8), 122.5 (C-10), 121.0 (C-6), 120.1 (C-6′), 116.8 (C-12), 116.7 (C-2′), 116.2 (C-16), 115.4 (C-5), 114.5 (C-5′), 108.9 (C-2), 72.9 (C-8′), 36.1 (C-7′).
2.3.27 2-cis-p-Hydroxyl ethyl cinnamate (27)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.62 (2H, d, J = 8.7 Hz, H-2/6), 6.84 (1H, d, J = 12.8 Hz, H-7), 6.75 (2H, d, J = 8.7 Hz, H-3/5), 5.75 (1H, d, J = 12.8 Hz, H-8), 4.11 (2H, q, J = 7.1 Hz, H2-1′), 1.20 (3H, t, J = 7.1 Hz, H3-2′); 13C NMR (100 MHz, DMSO‑d6) δC: 166.0 (C-9), 158.8 (C-4), 143.0 (C-7), 132.5 (C-2/6), 125.5 (C-1), 115.6 (C-8), 114.9 (C-3/5), 59.6 (C-1′), 14.1 (C-2′).
2.3.28 trans-p-Hydroxyl ethyl cinnamate (28)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.56 (1H, d, J = 15.9 Hz, H-7), 7.55 (2H, d, J = 8.3 Hz, H-2/6), 6.79 (2H, d, J = 8.3 Hz, H-3/5), 6.37 (1H, d, J = 15.9 Hz, H-8), 4.16 (2H, q, J = 7.0 Hz, H2-1′), 1.24 (3H, t, J = 7.0 Hz, H3-2′); 13C NMR (100 MHz, DMSO‑d6) δC: 166.6 (C-9), 159.9 (C-4), 144.6 (C-7), 130.3 (C-2/6), 125.1 (C-1), 115.8 (C-8), 114.3 (C-3/5), 59.7 (C-1′), 14.3 (C-2′).
2.3.29 Capillarisenol d (29)
Yellow, amorphous powder; UV (MeOH) λmax (logε) 204, 226, and 279 nm; HR-ESI-TOF-MS m/z 255.1309 [M - H]–; IR νmax 3418, 1658, 1438, 1027, 954 cm−1; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 3.
2.3.30 Sakuranetin (30)
Yellow, amorphous powder; 1H NMR (400 MHz, DMSO‑d6) δH: 12.12 (1H, s, 5-OH), 9.61 (1H, s, 4′-OH), 7.33 (2H, d, J = 8.5 Hz, H-2′/6′), 6.80 (2H, d, J = 8.5 Hz, H-3′/5′), 6.11 (1H, d, J = 2.3 Hz, H-8), 6.08 (1H, d, J = 2.3 Hz, H-6), 5.49 (1H, dd, J = 12.8, 2.9 Hz, H-2), 3.79 (3H, s, H3-11), 3.32 (1H, dd, J = 17.1, 12.8 Hz, H-3a), 2.73 (1H, dd, J = 17.1, 2.9 Hz, H-3b); 13C NMR (100 MHz, DMSO‑d6) δC: 197.0 (C-4), 167.4 (C-7), 163.2 (C-5), 162.9 (C-9), 157.8 (C-4′), 128.7 (C-1′), 128.4 (C-2′/6′), 115.1 (C-3′/5′), 102.6 (C-10), 94.6 (C-6), 93.8 (C-8), 78.6 (C-2), 55.9 (C-11), 42.0 (C-3).
2.3.31 Capillarisenol E (31)
Pale yellow, amorphous powder; [α]25D + 76.7° (c = 1.5, MeOH); UV (MeOH) λmax (logε) 215 and 267 nm; HR-ESI-TOF-MS m/z 465.1051 [M - H]–; IR νmax 3436, 1660, 1437, 1050, 955 cm−1; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 2.
2.3.32 (7′S)-2-Hydroxy-2-(4-Methoxyphenyl)Ethyl3,4,5-Trimethoxy benzoate (32)
Pale yellow, amorphous powder; [α]25D −4.6° (c = 1.0, MeOH); UV (MeOH) λmax (logε) 215 and 267 nm; HR-ESI-TOF-MS m/z 385.1203 [M + Na]+; IR νmax 3445, 1660, 1438, 1028, 955 cm−1; 1H NMR (400 MHz, DMSO‑d6) and 13C NMR (100 MHz,DMSO‑d6) spectrum information, see Table 4.
No.
32
δH
δC
1
–
124.8
2
7.20, s
106.5
3
–
152.7
4
–
141.7
5
–
152.7
6
7.20, s
106.5
7
–
165.1
8
3.81, s
56.0
9
3.73, s
60.2
10
3.81, s
56.0
1′
–
134.0
2′
7.37, d (8.5 Hz)
127.5
3′
6.92, d (8.5 Hz)
113.5
4′
–
158.6
5′
6.92, d (8.5 Hz)
113.5
6′
7.37, d (8.5 Hz)
127.5
7′
4.88, m
69.8
8′
4.25, m
69.4
9′
3.74, s
55.4
2.3.33 Luteolin (33)
Yellow, amorphous powder; 1H NMR (400 MHz, DMSO‑d6) δH: 12.97 (1H, s, 5-OH), 7.41 (1H, dd, J = 8.1, 1.8 Hz, H-6′), 7.40 (1H, d, J = 1.8 Hz, H-2′), 6.89 (1H, d, J = 8.1 Hz, H-5′), 6.66 (1H, s, H-3), 6.45 (1H, d, J = 2.0 Hz, H-8), 6.19 (1H, d, J = 2.0 Hz, H-6); 13C NMR (100 MHz, DMSO‑d6) δC: 181.6 (C-4), 164.1 (C-2), 163.9 (C-7), 161.4 (C-5), 157.3 (C-9), 149.7 (C-3′), 145.7 (C-4′), 121.5 (C-1′), 119.0 (C-6′), 116.0 (C-5′), 113.4 (C-2′), 103.7 (C-10), 102.8 (C-3), 98.8 (C-6), 93.8 (C-8).
2.3.34 Apigenin (34)
Yellow, amorphous powder; 1H NMR (400 MHz, DMSO‑d6) δH: 12.96 (1H, s, 5-OH), 7.92 (2H, d, J = 8.7 Hz, H-2′/6′), 6.92 (2H, d, J = 8.7 Hz, H-3′/5′), 6.78 (1H, s, H-3), 6.48 (1H, d, J = 2.1 Hz, H-8), 6.19 (1H, d, J = 2.1 Hz, H-6); 13C NMR (100 MHz, DMSO‑d6) δC: 181.7 (C-4), 164.1 (C-2), 163.7 (C-7), 161.4 (C-5), 161.2 (C-4′), 157.3 (C-9), 128.5 (C-2′/6′), 121.2 (C-1′), 116.0 (C-3′/5′), 103.7 (C-10), 102.8 (C-3), 98.8 (C-6), 94.0 (C-8).
2.3.35 C-p-Hydroxybenzylluteolin (35)
Yellow, oil; HR-ESI-TOF-MS m/z 391.0544 [M - H]-; 1H NMR (400 MHz, DMSO‑d6) δH: 12.95 (1H, s, 5-OH), 7.43 (1H, d, J = 2.2 Hz, H-2′), 7.35 (1H, dd, J = 8.2, 2.2 Hz, H-6′), 7.06 (2H, d, J = 8.1 Hz, H-2′’/6′’), 6.88 (1H, d, J = 8.2 Hz, H-5′), 6.67 (1H, s, H-3), 6.60 (2H, d, J = 8.1 Hz, H-3′’/5′’), 6.32 (1H, s, H-6), 3.98 (1H, s, H3-7′’); 13C NMR (100 MHz, DMSO‑d6) δC: 182.4 (C-4), 164.2 (C-2), 162.3 (C-7), 159.7 (C-5), 155.8 (C-4′’), 154.9 (C-9), 150.1 (C-4′), 146.2 (C-3′), 131.2 (C-1′’), 129.4 (C-2′’/6′’), 122.2 (C-1′), 119.3 (C-6′), 116.5 (C-5′), 115.4 (C-3′’/5′’), 113.8 (C-2′), 107.1 (C-8), 104.1 (C-10), 103.0 (C-3), 98.9 (C-6), 27.4 (C-7′’).
2.3.36 3,4,α-Trihydroxy-Isobutylphenylpropionate (36)
Yellow, oil; HR-ESI-TOF-MS m/z 253.1135 [M - H]-; 1H NMR (400 MHz, DMSO‑d6) δH: 6.63 (1H, d, J = 8.1 Hz, H-5), 6.62 (1H, d, J = 2.1 Hz, H-2), 6.45 (1H, dd, J = 8.1, 2.1 Hz, H-6), 4.17 (1H, dd, J = 7.4, 5.6 Hz, H-8), 3.79 (2H, d, J = 6.6 Hz, H2-10), 2.78 (1H, dd, J = 13.7, 5.6 Hz, H-7a), 2.69 (1H, dd, J = 13.7, 7.4 Hz, H-7b), 1.82 (1H, m, H-11), 0.83 (6H, d, J = 6.6 Hz, H3-12/H3-13); 13C NMR (100 MHz, DMSO‑d6) δC: 174.0 (C-9), 145.1 (C-3), 144.0 (C-4), 128.6 (C-1), 120.4 (C-6), 117.1 (C-2), 115.6 (C-5), 72.1 (C-8), 70.2 (C-10), 40.1 (C-7), 27.6 (C-11), 19.1 (C-12/13).
2.3.37 Isobutyl protocatechuate (37)
Yellow, oil; 1H NMR (400 MHz, DMSO‑d6) δH: 7.37 (1H, d, J = 2.1 Hz, H-2), 7.32 (1H, dd, J = 8.2, 2.1 Hz, H-6), 6.81 (1H, d, J = 8.2 Hz, H-5), 3.97 (2H, d, J = 6.5 Hz, H2-8), 1.98 (1H, m, H-9), 0.95 (6H, d, J = 6.5 Hz, H3-10/H3-11); 13C NMR (100 MHz, DMSO‑d6) δC: 165.7 (C-7), 150.4 (C-3), 145.1 (C-4), 121.7 (C-1), 120.7 (C-6), 116.2 (C-2), 115.4 (C-5), 69.8 (C-8), 27.5 (C-9), 19.0 (C-10/11).
2.3.38 5,6-Dihydroxy-7,3′,4′-Trimethoxyflavone (38)
Yellow, amorphous powder; 1H NMR (400 MHz, DMSO‑d6) δH: 7.54 (1H, s, H-3), 7.02 (1H, s, H-4), 7.01 (1H, s, H-7), 4.05 (2H, d, J = 6.6 Hz, H2-9), 2.00 (1H, m, H-10), 0.95 (6H, d, J = 6.6 Hz, H3-11/H3-12); 13C NMR (100 MHz, DMSO‑d6) δC: 158.8 (C-8), 150.3 (C-7a), 148.2 (C-6), 144.1 (C-5), 143.1 (C-2), 118.1 (C-3a), 114.7 (C-3), 106.0 (C-4), 97.8 (C-7), 70.2 (C-9), 27.4 (C-10), 18.9 (C-11/12).
2.4 Anti-Hepatoma activity assay in vitro
Huh 7 cells and HepG 2 cells were maintained in DMEM containing 10 % FBS (foetal bovine serum) and cultured at 37 °C (5 % CO2, 95 % relative humidity). A cytotoxicity assay was performed, according to the CCK8 method, using 96-well microplates. Briefly, 200 μL of adherent cells were seeded into each well of the 96-well cell culture plates and allowed to adhere for 24 h before drug addiction, with an initial density of 5 × 103 cells each well. Each tumor cell line was exposed to the test compounds at different concentrations for six times within 48 h.
3 Results and discussion
3.1 Structural elucidation of five new bisphenols (13, 15, 25, 29, 31)
Compound 13 was isolated as white amorphous powder. Its molecular formula was determined as C14H12O5 by the HR-ESI-TOF-MS at m/z 283.0653 [M + Na]+. The UV maximum absorptions at 220, 261, and 297 nm indicated the existence of aromatic moieties. In the 1H NMR spectrum of 13, characteristic signals corresponding to a 1,2,4,5-tetrasubstituted benzene ring [δH 6.52 (s, H-3) and 7.31 (s, H-6)], a 1,4-disubstituted benzene ring [δH 6.91 (2H, d, J = 8.2 Hz, H-2′/H-6′) and 6.63 (2H, d, J = 8.2 Hz, H-3′/H-5′)], and methylene [δH 4.10 (2H, s, H2-7′)] were observed. The 13C NMR spectrum of 13 revealed the presence of 14 carbon signals, which were assigned based on the DEPT-135 spectrum to a carbonyl group [δC 168.3 (C-7)], eight aromatic carbons [δC 120.0 (C-1), 135.8 (C-2), 118.2 (C-3), 148.9 (C-4), 142.8 (C-5), 118.1 (C-6), 132.0 (C-1′), 129.6 (C-2′/C-6′), 115.0 (C-3′/C-5′), and 155.3 (C-4′)], and a methylene carbon [δC 37.1 (C-7′)]. Based on the comprehensive analysis of 1H–1H COSY, HSQC, and HMBC spectra, the 1H and 13C NMR spectral data of 13 were assigned (Table 1).
Interpretation of the 1H–1H COSY correlations (Fig. 3) led to the assignment of two spin-coupling systems (H-2′ to H-3′ and H-5′ to H-6′) in 13. In the HMBC spectrum, correlations between H-3 and C-1/C-5/C-7′, as well as between H-6 and C-2/C-4/C-7, allowed the establishment of a 1,2,4,5-tetrasubstituted benzene ring moiety (a), in which C-4 and C-5 were oxygenated due to its obvious downfield shift (δC 148.9 and 142.8). Meanwhile, HMBC correlations between H-2′/H-6′ and C-4′ and between H-3′/H-5′ and C-1′, in which C-4′ was oxygenated due to its obvious downfield shift (δC 155.3), indicated the existence of a p-hydroxybenzene unit (b). Furthermore, the HMBC correlations between H-3/H-2′/H-6′ and C-7′ as well as between H2-7′ and C-1/C-3/C-2′/C-6′ implied that two fragments (a and b) were connected by methylene (C-2 − C-7′−C-1′ bond). Moreover, the remaining carbonyl and hydroxyl groups could be assigned to a carboxyl moiety based on molecular formula information, and this moiety was attached to the C-1 position on the basis of the HMBC correlation between H-6 and C-7. Hence, the planar structure of 13 was confirmed (Fig. 3) by the analysis, and compound 13 was identified as 2-C-p-hydroxybenzylprotocatechuic acid, which was renamed capillarisenol A.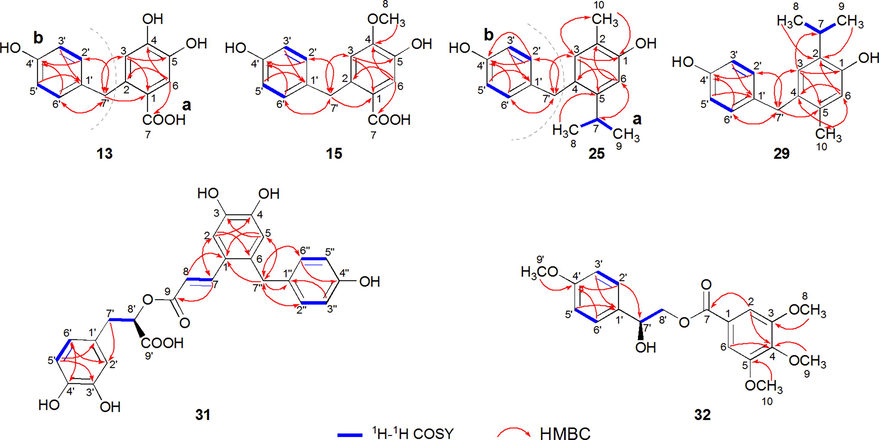
Key 1H–1H COSY, HMBC and NOESY correlations of five new bisphenols (13, 15, 25, 29, 31) and one new aromatic compound 32.
Compound 15 was obtained as an off-white amorphous powder. Its molecular formula was determined to be C15H14O5 according to the HR-ESI-TOF-MS data at m/z 274.2749 [M + H]+. The UV spectrum of 15 showed the absorption maxima at 222, 259, and 296 nm. In the 1H and 13C NMR spectra of 15, a 1,2,4,5-tetrasubstituted aromatic fragment, a 1,4-disubstituted aromatic fragment, a carboxyl group, a methylene, and a methoxyl group were observed. Extensive analysis of the 1D- and 2D NMR data (Table 1) of 15 indicated that its skeletal structure was identical to that of 13, and the difference between them is that the hydroxyl group (δC 148.9, C-4) in 13 was replaced by a methoxyl group (δC 150.4, C-4; δC 55.5, C-8) in 15, which was supported by the HMBC correlations from H3-8 (s, δH 3.75) to C-4 (Fig. 2). Accordingly, compound 15 was determined to be 2-C-p-hydroxybenzylisovanillic acid, which was renamed capillarisenol B.
Compound 25 was isolated as yellow oil. Its molecular formula was established as C17H20O2 based on its negative HR-ESI-TOF-MS data (m/z 255.1310 [M - H]–). The UV maximum absorptions at 204, 225, and 279 nm supported the presence of aromatic moieties. The 1H NMR spectrum of DMSO‑d6 indicated a 1,2,4,5-tetrasubstituted benzene ring due to the presence of two aromatic proton singlets at δ 6.67 (1H, s) and 6.77 (1H, s) and a 1,4-disubstituted benzene ring due to aromatic protons at δ 6.86 (2H, d, J = 8.4 Hz) and 6.64 (2H, d, J = 8.4 Hz), one two-proton singlet at δ 3.73 (2H, s), and one three-proton singlet at δ 2.04 (3H, s) assignable to the methylene and methyl groups likely attached to the aromatic ring, respectively, as well as two overlapping three-proton doublets at δ 1.00 (6H, d), assignable to two methyl groups likely attached to the methine group. Furthermore, the 17 carbons were resolved as 14 carbon signals in the 13C NMR, confirming the presence of structurally symmetric subunits in the compound. Based on 1D- and 2D NMR experiments, the 1H- and 13C NMR data of 25 were assigned and listed (Table 3).
The 1H–1H COSY correlations led to the establishment of three spin-coupling systems in 25, as shown in blue bold lines in Fig. 3. In the HMBC spectrum, key correlations between H-3 and C-1/C-10, between H-6 and C-2/C-4/C-7, between H-7 and C-6, between H3-8/H3-9 and C-5, and between H3-10 and C-1/C-3 established a carvacrol motif (a). In addition, the HMBC correlations between H-2′/H-6′ and C-4′ and between H-3′/H-5′ and C-1′ suggested the existence of an p-hydroxybenzene unit (b), in which C-4′ was oxygenated due to its obvious downfield shift (δ 155.2). Furthermore, the HMBC correlations between H-3/H-6′ and C-7′ as well as between H2-7′ and C-2′/C-6′ implied that two substructures (a and b) were also connected through the C-4 − C-7′−C-1′ bond. Consequently, compound 25 was identified as 4-C-p-hydroxybenzylcarvacrol, which was renamed capillarisenol C.
The molecular formula of compound 29 was determined to be C17H20O2 based on HR-ESI-TOF-MS analysis (m/z 255.1309 [M - H]–), which was indicative of eight degrees of unsaturation. The UV spectrum of 29 showed the absorption maxima at 204, 226, and 279 nm. The 1D- and 2D NMR data (Table 3) of 29 resembled those of 25, and one of the major differences was the replacement of a methyl group at C-2 in 25 by an isopropyl motif (δC 26.1, C-7; δC 22.6, C-8/C-9) at the same position in 29, as supported by the HMBC correlations from H3-8/H3-9 (δH 1.11) to C-2 and from H-3 (δH 6.84) to C-7 as well as a COSY correlation of H-7 (δH 3.11) to H3-8/H3-9. The other major difference was the replacement of an isopropyl motif at C-5 in 29 by a methyl group (δC 18.9, C-10) at the same position in 29, which was confirmed by the HMBC correlations from H3-10 (δH 2.02) to C-4 and C-6. The planar structure of 29 was further confirmed with the aid of 2D NMR spectroscopic analysis (Fig. 3). Thus, compound 29 was assigned to be 4-C-p-hydroxybenzylthymol, which was renamed capillarisenol D.
The molecular formula of compound 31 was determined to be C25H22O9 by its HR-ESI-TOF-MS at m/z 465.1051 [M - H]–. The UV absorption at 215 and 267 nm implied the existence of an aromatic ring in 31. The 13C NMR spectrum of 31 revealed that this compound contained 25 carbons, which consisted of two methylene, one methine, two olefinic, eighteen aromatic, and two carbonyl carbons. In the 1H NMR spectrum, two double doublets at δH 2.87 (dd, J = 8.7, 14.4 Hz) and 2.98 (dd, J = 3.4, 14.4 Hz) were attributed to the geminal protons of the benzylic methylene, which were coupled with a double doublet at δH 4.98 (dd, J = 3.4, 8.7 Hz), assignable to the oxymethine proton. The characteristic ABX-type aromatic proton signals at δH 6.49 (dd, J = 1.1, 8.1 Hz), 6.62 (d, J = 8.1 Hz), and 6.67 (d, J = 1.1 Hz) suggested the presence of a 1,3,4-trisubstituted aromatic ring. These data indicated that compound 31 had a similar structure to rosmarinic acid (compound 17) (Petersen and Simmonds, 2003). In comparison with the 1H NMR spectrum of rosmarinic acid, two doublets at δH 6.11 and 7.81 (J = 15.7 Hz) and two singlets at δH 6.60 and 7.10 were observed in the spectrum of 31 instead of the characteristic ABX-type aromatic proton signals in that of rosmarinic acid. Based on this finding, a 1,2,4,5-tetrasubstituted aromatic ring of trans-caffeoyl moiety was present in the molecule of 31 in place of the 1,3,4-trisubstituted one of rosmarinic acid, which was also supported by λmax at 288 and 340 nm in the UV spectrum as well as by 13C NMR data (Table 2). In addition, a pair ortho-coupling protons were observed at δH 6.65 and 6.87, accompanied by diphenyl methylene protons at δH 3.81. Therefore, it was suggested that a 4-hydroxybenzylic moiety was attached to C-6 of rosmarinic acid, which was further supported by the presence of signals at δC 36.4, 115.2 (2C), 129.2 (2C), 131.1, and 155.4 in 13C NMR spectrum and by the HMBC correlations of H-7′’ to C-1/C-5 and H-5 to C-7′’ (Fig. 2). Compound 31 showed a specific rotation of + 76.7° (in methanol), which was the same behaviour as that of rosmarinic acid ([α]D25: + 70.8°). Therefore, it was proposed that the stereochemistry at C-8′ was R configuration. Thus, compound 31 was deduced to be 6-C-p-hydroxybenzylrosmarinic acid, which was renamed capillarisenol E.
3.2 Structural elucidation of another new aromatic compound 32
Compound 32 was obtained as yellow oil, with the molecular formula C19H22O7 based on its HR-ESI-TOF-MS at m/z 385.1203 [M + Na]+. The UV absorption at 215 and 267 nm of 32 indicated the presence of an aromatic ring. The 1H NMR spectrum of 32 exhibited six aromatic [δH 7.20 (2H, s, H-2 and H-6), 7.37 (2H, d, J = 8.5 Hz, H-2′ and H-6′), 6.92 (2H, d, J = 8.5 Hz, H-3′ and H-5′)], one methine [δH 4.88 (1H, m, H-7′)], one methylene [δH 4.25 (2H, m, H-8′)], and four methoxyl [δH 3.81 (6H, s, H3-8 and H3-10), 3.74 (3H, s, H3-9′), 3.73 (3H, s, H3-9)] proton signals. The 13C NMR and DEPT-135 spectra showed 19 carbon resonances shared by twelve aromatic, four methoxyl, one carbonyl, one methine, and one methylene carbons. The 1H- and 13C NMR spectral data of 32 are listed in Table 4, and the signal assignments were determined by the HSQC technique. The 1H–1H COSY correlations led to the establishment of three spin-coupling systems in 32 (blue bold lines in Fig. 3). Combined with 1H NMR, 13C NMR, and HRESIMS, one hydroxyl group and one carbonyl group were found in compound 32. The hydroxyl group was attached to C-7′, which was confirmed by the chemical shifts of the methine carbon of C-7′ (69.8) as well as the HMBC correlation of H-2′/C-7′. One carbonyl group was located at C-1, which was confirmed by the HMBC correlation of H-2/C-7. Four methoxyl groups were attached to C-3, C-4, C-5, and C-4′ based on the HMBC correlations of H-8/C-3, H-9/C-4, H-10/C-5, and H-9′/C-4′, respectively. To further determine the absolute configuration at C-7′, the molecular optical rotation value of compound 32 were compared with that of R-suspensaside and S-suspensaside (Guo, et al., 2007). Compound 32 showed a specific rotation of −4.6° (methanol), which was nearly equal to that of S-suspensaside ([α]25D = -4.7°). This method was successfully applied previously for the configuration confirmation (Ge, et al., 2018; Ma, et al., 2016; Xiao, et al., 2022). Therefore, it was proposed that the stereochemistry at C-7′ was S configuration. Based on the analysis, the structure of 32 was thus determined to be (7′S)-2-hydroxy-2-(4-methoxyphenyl)ethyl3,4,5-trimethoxy benzoate.
The other known compounds were identified as protocatechuic acid (1) (Li, et al., 2022), protocatechuyl aldehyde (2) (Li, et al., 2005), 4-hydroxybenzoic acid (3) (Zhang, et al., 2021), 4-hydroxybenzaldehyde (4) (Zhang, et al., 2021), 3-hydroxy-4-methoxybenzoic acid (5) (Yang, et al., 2017), 3-hydroxy-4-methoxy-benzaldehyde (6) (Yang, et al., 2017), caffeic acid (7) (Ge, et al., 2018), 3,5-dimethoxy-4-hydroxybenzoic acid (8) (Feng, et al., 2011), cumaric acid (9) (Ge, et al., 2018), 3,4,5-trimethoxybenzoic acid (10) (Feng, et al., 2011), 3,4-dihydroxybenzoic acid ethyl ester (11) (Yin, et al., 2013), 3,4,α-trihydroxy-methyl phenylpropionate (12) (Gu, et al., 2007), amburoside A (14) (Sauvain, et al., 1999), trans-6-[(1E)-2-carboxyethenyl]-3-(3,4- dihydroxyphenyl)-2,3-dihydro-1,4-benzodioxin-2-carboxlic acid (16) (Wan, et al., 2008), rosmarinic acid (17) (Refaey, et al., 2021), 2-(3-hydroxy-4-methylphenyl) propanoic acid (18) (Austgulen, et al., 1987), caffeic acid ethyl ester (19) (Ge, et al., 2018), amburoside B (20) (Sauvain, et al., 1999), (+)-epi-pinoresinol (21) (Ge, et al., 2019), (+)-diasyringaresinol (22) (Chang, et al., 1998), (+)-lirioresinol A (23) (Liu, et al., 2013), 2-caffeoyloxy-3-[2-(4-hydroxybenzyl)-4,5-dihydroxy]phenylpropionic acid (24) (Kikuzaki and Nakatani, 1989), isosalvianolic acid C (26) (Liu, et al., 2014), cis-p-hydroxyl ethyl cinnamate (27) (Lu, et al., 2015), trans-p-hydroxyl ethyl cinnamate (28) (Lu, et al., 2015), sakuranetin (30) (Ferreira, et al., 2020), luteolin (33) (Ferreira, et al., 2020), apigenin (34) (Ferreira, et al., 2020), 8-C-p-hydroxybenzylluteolin (35) (Merghem, et al., 1995), 3,4,α-Trihydroxy- isobutylphenylpropionate (36) (Gu, et al., 2007), isobutyl protocatechuate (37) (Li, et al., 2022), and 5,6-dihydroxy-7,3′,4′-trimethoxyflavone (38) (Sato and Tamura, 2015).
3.3 Hypothetical biogenetic pathway for new bisphenols
The hypothetical biogenetic pathway for four new bisphenols (13, 15, 25, 29) is shown in Scheme 1. The reaction may be derived from the precursor of 4-hydroxybenzaldehyde (4), with a subsequent attack on protocatechuic acid (1), 3-hydroxy-4-methoxy benzoic acid (5), carvacrol, and thymol, respectively, through a Knoevenagel condensation reaction and eduction of the exocyclic double bond, then yielded capillarisenols A-D. Furthermore, 4-hydroxybenzaldehyde (4) and rosmarinic acid (17) could also follow the same hypothetical biogenetic pathway to generate compounds 24 and 31 (capillarisenol E). This biogenetic pathway plausibly elucidates the structures of new bisphenols.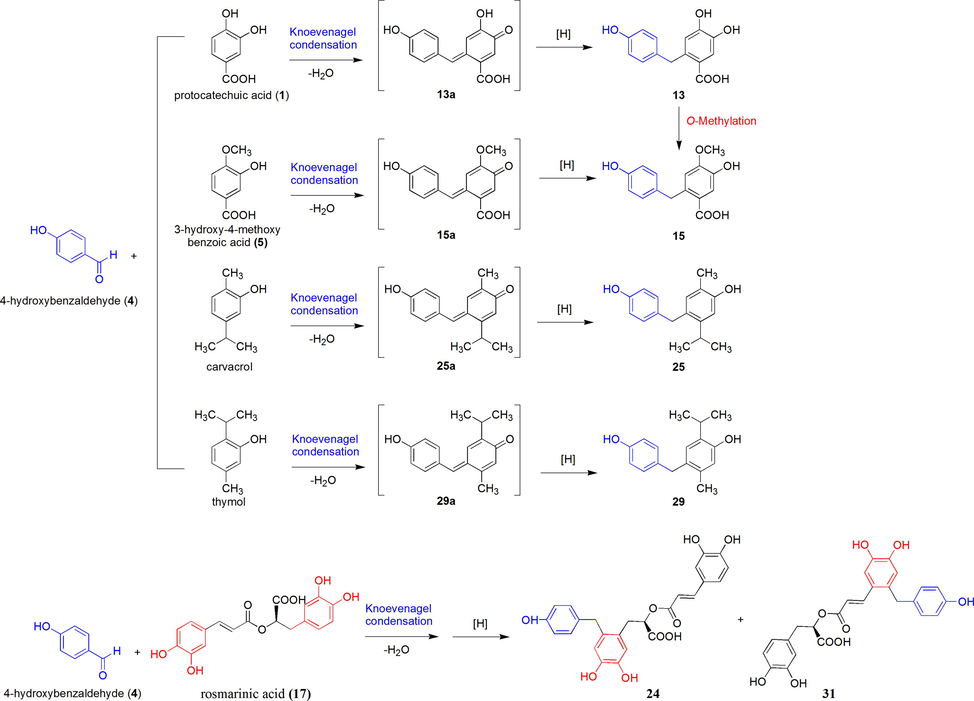
Hypothetical biogenetic pathway for new bisphenols (13, 15, 25, 29, 31).
3.4 Anti-hepatoma activities of all compounds (1–38)
The anti-hepatoma activities of all compounds are shown in Table 5. Of the five new bisphenols (capillarisenols A-E), only capillarisenol C (25) exhibited significant inhibition of Huh7 and HepG2 cells proliferation, with IC50 values of 4.96 and 8.58 μM, respectively. While the first-line anti-HCC drug lenvatinib exhibited anti-hepatoma activities against Huh7 and HepG2 cells with IC50 values of 29.85 and 30.17 μM, respectively. In addition, new compound 32 also showed significant inhibition of Huh7 and HepG2 cells proliferation, with IC50 values of 40.27 and 16.56 μM, respectively. Luteolin (33) is a common flavonoid, and its IC50 values for Huh7 and HepG2 cells inhibition were 38.14 and 21.07 μM. These results indicated that capillarisenol C is a potential anti-cancer compound, and its mechanism is under study. The IC50 of other compounds were greater than 50 μM.
Compounds
Huh7
IC50 (μM)
HepG2
IC50 (μM)
25
4.96
8.58
32
40.27
16.56
33
38.14
21.07
34
> 50
41.82
Lenvatinib
29.85
30.17
4 Conclusion
Yin-Chen, which belongs to the Asteraceae family and the genus Artemisia, is among the most abundantly used traditional medicines in China for treating hepatitis and bilious disorder. In this study, chemical investigation of aerial parts of Artemisia capillaris Thunb. in Ganzhou resulted in the isolation of five novel bisphenols, capillarisenols A–E (13, 15, 25, 29, 31), and one new phenolic compound (32), along with 32 eight known phenolic compounds (1–12, 14, 16–24, 26–28, 30, 33–38).
Bisphenol A (BPA; 2,2-bis(4-hydroxyphenol) propane) is a bisphenol that was first developed as a synthetic oestrogen by Dianin in 1891, and its oestrogenic activity was discovered in 1936 (Dodds and Lawson, 1936). Although BPA is recognised as a synthetic oestrogen, it is rapidly becoming one of the most produced and used chemicals worldwide. Because BPA can be polymerised into polycarbonate plastic due to its light weight, transparency, colouring, impact resistance, heat resistance, chemical resistance, lack of change over time, and easy moulding and thermoforming. BPA can leach out of food or beverage containers and be ingested (Zalko, et al., 2011), causing many human diseases, such as diabetes, obesity, cardiovascular, chronic respiratory, and kidney disease, and breast cancer (Rezg, et al., 2014; Rochester, 2013; Vandenberg, et al., 2012). Therefore, BPA has been banned from use in the food industry, especially in baby food. Currently, there are a series of bisphenol derivatives (e.g., BPB, BPF, BPS) that are potential substitutes for BPA, a widely studied typical endocrine-disrupting chemical, but they have shown little success (Eladak, et al., 2015; Wang, et al., 2021).
Capillarisenols A-E are the first examples of naturally occurring bisphenols in Artemisia capillaris Thunb. They were not cytotoxic. Only Capillarisenol C showed significant anti-hepatoma activity in Huh7 and HepG2 cells, with IC50 values of 4.96 and 8.58 μM, better than the positive control drug (Lenvatinib). These results provide new ideas for the study of BPA substitutes as well as phytochemical evidence for the further development and utilisation of A. capillaris in health products.
5 Notes
The authors declare no competing financial interest.
Funding
This work was supported by National Natural Science Foundation of China (81503221, and 81903760), Natural Science Foundation of Guangdong Province (2017A030313659, and 2021A1515220185), and Shenzhen Fundamental Research and Discipline Layout Project (JCYJ20170413093108233, JCYJ20190806151816859, JCYJ20190806153401647 and JCYJ20220818102609021).
References
- Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab. J. Chem.. 2020;13:8209-8217.
- [Google Scholar]
- Metabolism in rats of p-cymene derivatives: carvacrol and thymol. Pharmacology & toxicology. 1987;61:98-102.
- [Google Scholar]
- Recent progress in the study of Artemisiae Scopariae Herba (Yin Chen), a promising medicinal herb for liver diseases. Biomed. Pharmacother.. 2020;130:110513
- [Google Scholar]
- Chemical constituents from Cassytha f iliformis II. J. Nat. Prod.. 1998;61:863-866.
- [Google Scholar]
- Pharmacopoeia of the People's Republic China. Beijing, China: China Medical Science Press; 2015.
- Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol.. 2021;273:113960
- [Google Scholar]
- Synthetic oestrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996.
- [Google Scholar]
- A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril.. 2015;103:11-21.
- [Google Scholar]
- Chemical constituents of Selaginella stautoniana. Chin. J. Nat. Med.. 2011;9:108-111.
- [Google Scholar]
- Nitrogen-containing naringenin derivatives for reversing multidrug resistance in cancer. Bioorg. Med. Chem.. 2020;28:115798
- [Google Scholar]
- Novel caffeoylquinic acid derivatives from Lonicera japonica Thunb. flower buds exert pronounced anti-HBV activities. RSC Adv.. 2018;8:35374-35385.
- [Google Scholar]
- Chemical constituents from Lonicera japonica flower buds and their anti-hepatoma and anti-HBV activities. Bioorg. Chem.. 2019;92:103198
- [Google Scholar]
- Three new anti-HBV active constituents from the traditional Chinese herb of Yin-Chen (Artemisia scoparia) J. Ethnopharmacol.. 2015;176:109-117.
- [Google Scholar]
- Studies on chemical constituents of Prunella vulgaris. China Journal of Chinese Materia Medica. 2007;32:923-926.
- [Google Scholar]
- Simultaneous determination of 12 major constituents in Forsythia suspensa by high performance liquid chromatography-DAD method. J. Pharmaceut. Biomedi.. 2007;43:1000-1006.
- [Google Scholar]
- The pharmacological effects and pharmacokinetics of active compounds of Artemisia capillaris. Biomedicines. 2021;9:1412.
- [Google Scholar]
- A survey of therapeutic effects of Artemisia capillaris in liver diseases. Evid-Based Compl. Alt.. 2015;2015:728137
- [Google Scholar]
- Structure of a new antioxidative phenolic acid from oregano (Origanum vulgare L.) Agricultural and Biological Chemistry. 1989;53:519-524.
- [Google Scholar]
- Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J. Ethnopharmacol.. 2007;109:318-324.
- [Google Scholar]
- Bioactive chemical constituents of Cercis chinensis. Chem. Nat. Comp.. 2022;58:138-140.
- [Google Scholar]
- Evaluation of antiviral activity of compounds isolated from Ranunculus sieboldii and Ranunculus sceleratus. Planta Med.. 2005;71:1128-1133.
- [Google Scholar]
- Chemical constituents from Qianliang tea. Journal of Chinese Pharmaceutical Sciences. 2013;22:427-430.
- [Google Scholar]
- Phenolic acids and antioxidant activities of Danshen injection. Chem. Nat. Comp.. 2014;50:83-87.
- [Google Scholar]
- Chemical constituents from shoots of Phyllostachys edulis (I) Chinese Traditional and Herbal Drugs. 2015;46:334-338.
- [Google Scholar]
- Hepatoprotective phenylethanoid glycosides from Cirsium setosum. Nat. Prod. Res.. 2016;30:1824-1829.
- [Google Scholar]
- Five 8-C-benzylated flavonoids from Thymus hirtus (Labiateae) Phytochem.. 1995;38:637-640.
- [Google Scholar]
- Bioactive constituents from Thunbergia erecta as potential anticholinesterase and anti-ageing agents: Experimental and in silico studies. Bioorg. Chem.. 2021;108:104643
- [Google Scholar]
- Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ. Int.. 2014;64:83-90.
- [Google Scholar]
- Bisphenol A and human health: a review of the literature. Reprod. Toxicol.. 2013;42:132-155.
- [Google Scholar]
- High antiallergic activity of 5, 6, 4’-trihydroxy-7, 8, 3’-trimethoxyflavone and 5, 6-dihydroxy-7, 8, 3’, 4’-tetramethoxyflavone from eau de cologne mint (Mentha× piperita citrata) Fitoterapia. 2015;102:74-83.
- [Google Scholar]
- Quantitative analysis of six phenolic acids in Artemisia capillaris (Yinchen) by HPLC-DAD and their transformation pathways in decoction preparation process. J. Anal. Methods Chem.. 2020;2020:8950324.
- [Google Scholar]
- Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev.. 2012;33:378-455.
- [Google Scholar]
- Biotransformation of caffeic acid by Momordica charantia peroxidase. Can. J. Chem.. 2008;86:821-830.
- [Google Scholar]
- Bisphenol A (BPA), BPS and BPB-induced oxidative stress and apoptosis mediated by mitochondria in human neuroblastoma cell lines. Ecotox. Environ. Safe.. 2021;207:111299
- [Google Scholar]
- Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. J. Pharmaceut. Biomed.. 2008;46:477-490.
- [Google Scholar]
- Tubuloside B, isolated from Cistanche tubulosa, a promising agent against M1 macrophage activation via synergistically targeting Mob1 and ERK1/2. Biomed. pharmacother.. 2022;153:113414
- [Google Scholar]
- Constituents of Dianthus superbus and their cytotoxic activity. Chem. Nat. Comp.. 2017;53:740-741.
- [Google Scholar]
- A new benzophenone derivatives from Hypericum sampsonii. Natural Product Research and Development. 2013;25:875-877.
- [Google Scholar]
- Viable skin effificiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82:424-430.
- [Google Scholar]
- Polyacetylenes and anti-hepatitis B virus active constituents from Artemisia capillaris. Fitoterapia. 2014;95:187-193.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104580.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Spectroscopic data of all compounds.







