Translate this page into:
A small library of C–H⋯O hydrogen bonds based on supramolecular architectures of 1,5-diketone malonates in the solid state
⁎Corresponding authors. dsalazar@mixteco.utm.mx (Domingo Salazar-Mendoza), jimenezf@imp.mx (Federico Jiménez-Cruz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Structural supramolecular architectures in the solid state of 1,5-diketone malonates 1a-g are proposed to construct a small library of hydrogen bonds by C–H⋯O interactions by crystal structure analysis. A possible route of crystallization mechanism at different levels of hierarchy considers the next strategic steps: a) Intramolecular interactions provide a kite-like molecular conformation. b) Intermolecular interactions provide molecular recognition between the structural unit, synthon, and macrocycles. c) The supramolecular architectures obtained from the conjunction of the multiple functions performed by the C–H⋯O interactions. X-ray studies and Hirshfeld Surface Analysis are described.
Keywords
1, 5-diketone malonates
Supramolecular architectures
Synthons
Intermolecular hydrogen bonding
Intramolecular hydrogen bonding
Hirshfeld surface analysis

1 Introduction
1,5-diketones are typical precursors in intramolecular aldol condensation (Nielsen et al., 1968) and pinacol coupling reactions. (Harrowven et al., 1998) Their synthesis can be achieved by the addition of activated methylene compound to α,β-unsaturated ketones, (March 1992) condensation of ketone enolates and the Mannich base derived from methyl ketones, (Rao et al., 2002) or with the reported methods: The ruthenium-catalyzed coupling between α,β -unsaturated ketones, alkynes and water, (Trost et al., 1997) the aryllithium addition to 3,4-dihydropyranones and TMSCl in THF, (Harrowven et al., 1998, 1999), or more specifically, the addition of difluoroenoxysilane to α,β -unsaturated ketones to produce 2,2-difluoro-1,5-diketones. (Lefebvre et al., 1998) In addition, other reported syntheses are the ferrocenyl substituted 1,5-diketone synthesis under ultrasound irradiation, (Ji et al., 2004) a tandem cross-coupling reaction of ketones with aldehydes promoted by barium hydride or barium alkoxides, (Takahashi et al., 2006) the asymmetric direct Michael addition of α,β-unsaturated aldehydes with acetophenone catalyzed by a Jørgensen-Hayashi catalyst in methanol, (Li et al., 2011) a catalyzed reaction by aqueous KOH between aryl methyl ketones and aldehydes and the subsequent dimerizations. (Liu et al., 2015).
Likewise, regarding structural studies, the 1,5-diketone moiety is present in benzamarone (1,2,3,4,5-pentaphenylpentan-1,5-dione), which presents two and three stereocenters in their structure evidenced by X-ray and NMR spectroscopy. (Mufato et al., 2011) In this context, we reported that 1,5-diketone malonate 1 performed a rearrangement to 1,3-diketone 3 via an aldol intermediate 2. (Jiménez-Cruz et al., 1998, 2000). In these cases, the aldol-dehydration product 4 is undetected, Fig. 1. The presence of the 2,2-bis-methoxycarbonyl adjacent to the aroyl moiety is the key to the C–C bond cleavage in compound 2.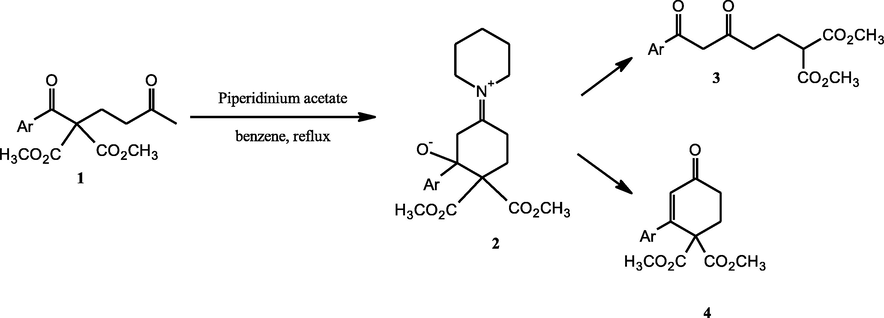
The reaction of 1,5 diketone malonate 1 to 1,3-diketone rearrangement 3 versus aldol-dehydration products 4.
It should be noted that 1,5-diketones with one methoxycarbonyl and even with two electron-withdrawing fluorine atoms produced the expected cyclohexenone. (Walker, 1955, Hernández-Ortega, 2001).
On the other hand, the study of spatial arrangements of intermolecular and intramolecular interactions marks a milestone in crystal engineering to recognize 1D, 2D and 3D spatial configurations in crystalline molecular structures. Supramolecular synthons in crystals are structural units formed with intermolecular interactions to analyze the complex interplay between close packing, hydrogen bonding and other interactions in crystal structures. (Desiraju, 2013) A significant role of C–H⋯O hydrogen bonds are associated with intramolecular and intermolecular interactions constituting supramolecular synthons and supramolecular architectures. (Dunitz et al., 2005) In particular, C–H⋯O interactions are of interest in molecular recognition (Sutor, 1962, 1963, Taylor et al., 1982, Desiraju, 1996) and crystal engineering. (Desiraju, 1996).
This work describes supramolecular architectures in various crystals of 1,5-diketone malonates 1a-g, Fig. 2.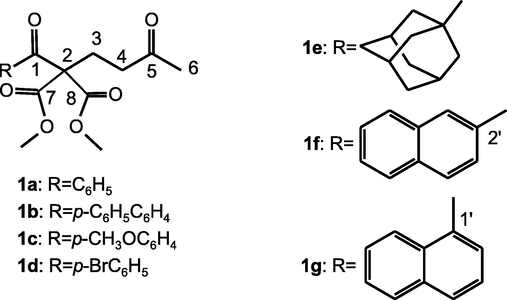
1,5 diketone malonates 1 a-g of this study.
Considering the adjacent quaternary carbon to the carbonyl in the of the titled 1,5-diketone malonates 1a-g (Fig. 2) and the particular crystals architecture for the aryl, naphthyl and adamantyl moieties in the molecular structure, we are motivated to show a small library of hydrogen bonds exclusively by C–H⋯O interactions. From the results obtained on the crystalline structure of these compounds, a structural hierarchy is proposed that allows exploring and tracing a possible route of the elements that constituted their crystallization mechanism: a) Intramolecular interactions provide an unexpected kite-like molecular conformation through the complementary association between hydrogen bond interactions and electronic delocalization. b) Intermolecular interactions provide molecular recognition between the structural units, synthons, and macrocycles, through C–H⋯O hydrogen bond interactions, and c) The supramolecular architectures obtained from the conjunction of the C–H⋯O cooperative hydrogen bonds at different levels of hierarchy, supported by experimental and Hirshfeld surface analysis.
2 Experimental
2.1 Preparation, isolation, and crystallization
1,5-diketone malonates 1a-g were synthesized according to the procedure previously described. (Jiménez-Cruz et al., 1998, 2000) It consists of the C-aroylation of the dimethyl malonate anion (NaH in THF) with the corresponding aroyl chlorides to produce an aroylmalonate. Chemical reagents and solvents were provided by Sigma Aldrich and were used as received. Then, the Michael type addition of the aroylmalonates to methyl vinyl ketone using Triton B or Et3N in THF affording the 1,5-diketones malonates. 1H and 13C NMR for the unreported 1,5 diketones 1e-g are described here, which were recorded in a Varian Unity Spectrometer at 300 MHz for proton and 75.4 MHz for carbon. The 1H NMR chemical shifts are expressed in ppm relatives to tetramethylsilane (TMS), and the 13C NMR chemical shifts were referenced with the triplet of CDCl3 (δ = 77 ppm). NMR results and the corresponding spectra are shown in the supplementary section (Figures S1-S6). The characterization for 1a–d was reported elsewhere. (Jiménez-Cruz et al., 1998, 2000) Crystals were grown from 2:1 ethanol–water solutions by slow evaporation for all 1,5-diketone malonates 1a-g.
2.2 X-ray structural determination
The X-ray data for the single crystal of 1,5-diketone dimethyl malonates 1a and 1c-g were collected at 293 K using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) on a Siemens P4/PC diffractometer. The data collection, (Siemens, 1994) cell refinement and data reduction were carried out using XSCANS. The structure was solved with direct methods and refined by full-matrix least-square methods based on F2 using SHELXS97 and SHELXL97 packages. (Sheldrick, 1997, 2008) In addition, 1,5-diketone dimethyl malonate 1b was performed with Cu Kα radiation (λ = 1.54178 Å) at 293 K on a Nicolet P3/F diffractometer; and cell refinement and data reduction were performed with Nicolet P3 Software 1980.
3 Results and discussion
3.1 X-ray crystallographic data for 1,5-diketone malonates 1 a-g
Data for 1,5-diketone malonates 1a-g are shown in Table 1, and geometry parameters such as bond lengths and angles are depicted in Table S1 (Supplementary information). a α = 97.05 (1), β = 92.83 (2), γ = 93.06 (2). b α = 72.973 (3), β = 75.842 (3), γ = 79.745 (3). c α = 81.420 (11), β = 85.153 (9), γ = 80.846 (11).
Compound (temp, K)
Crystal data
1a (293)
1b (293)
1c (293)
1d (291)
1e (293)
1f (293)
1 g (293)
Cryst. Syst.
Monoclinic
Triclinic
Triclinic
Monoclinic
Orthorhombic
Triclinic
Monoclinic
Space group
P21/n
P1
P1
P21/c
Pbca
P1
P21/c
Formula
C16H18O6
C22H22O6
C17H20O7
C16 H17 BrO
C20H28O6
C20H20O6
C20H20O6
M, gmol−1
306.30
382.40
336.33
385.21
364.42
356.36
356.36
a, Å
8.134 (1)
8.8501 (3)
8.259 (2)
8.1988(11)
9.391 (3)
8.7366 (11)
8.4703 (5)
b, Å
16.618 (1)
8.9253 (3)
9.905(2)
19.9380(16)
19.261 (3)
10.0008 (12)
13.7770 (12)
c, Å
11.436 (1)
13.5517 (5)
21.203 (2)
10.6674(14)
21.290 (3)
10.5306 (11)
15.7530 (15)
β, deg
98.67 (1)
b
a
96.531(14)
90
c
102.003 (6)
d, g/cm3
1.331
1.288
1.302
1.477
1.257
1.320
1.316
Crystal form
Colorless block
Colorless prism
Colorless prism
Colorless prism
Colorless prism
Colorless block
Colorless
block
Crystal size, mm
0.68x0.60x
0.400.40x0.20x
0.200.60x0.40x
0.240.38 × 0.20 × 0.12
0.44x0.24x
0.120.40x0.16x
0.100.40x0.16x
0.04
Z
4
2
4
4
8
2
4
Diffractometer
Siemens P4/PC
Radiation
Mo Kα
Cu Kα
Mo Kα
Mo Kα
Mo Kα
Mo Kα
Mo Kα
μ, mm−1
0.102
0.774
0.101
0.2399
0.092
0.098
0.097
Scan mode
θ-2θ
θ-2θ
ω
ω
ω
ω
ω
Measured Reflections
3051
2839
6490
3274
3382
5527
5048
Independent Reflections
2845
(Rint = 0.0528)2632
(Rint = 0.0171)6032
(Rint = 0.0635)3051
(Rint = 0.0441)3382
(Rint = 0.000)5210
(Rint = 0.0422)4779
(Rint = 0.0375)
Refinement on F2
R
0.0525
0.0505
0.0476
0.0461
0.0561
0.0510
0.0485
Rw
0.1128
0.1304
0.1025
0.0612
0.0885
0.0760
0.0827
GOF
1.011
1.043
0.899
0.835
0.875
0.890
0.869
Crystals suitable for X-ray diffraction were obtained from 1,5-diketone malonates 1a-g; their structural parameters are shown in Table 1. The structure of the compounds 1a-g; is formed by 1,5-diketone and dimethyl malonate, also with substituent groups linked at C1: phenyl, 1,1′-biphenyl, 4-methoxyphenyl, 4-bromophenyl, 1-adamantyl, l-naphthyl and 2-naphthyl, for 1a, 1b, 1c, 1d, 1e, 1f and 1 g, respectively. Fig. 3 shows a drawing displacement ellipsoids of 1,5-diketone malonates 1a-g.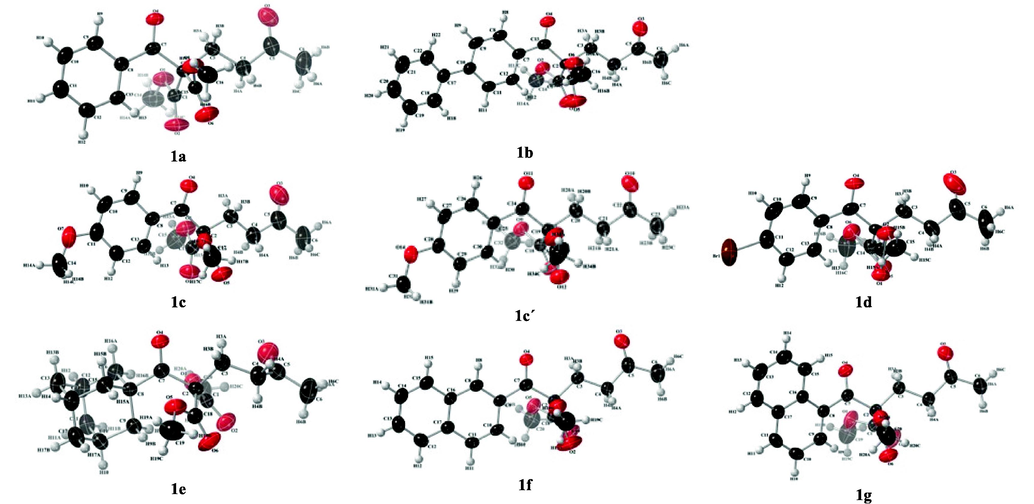
View of compounds 1a-g, the displacement ellipsoids are drawn at the 50 % probability level.
3.2 Structural hierarchy of the small library of hydrogen bonds based on supramolecular architectures of 1,5-diketone malonates 1a-g
From the results obtained on the crystalline structure of these compounds, a structural hierarchy is proposed that allows exploring and tracing a possible route of the elements that constituted their crystallization mechanism leading to the construction of a small library of hydrogen bonds exclusively by C–H⋯O interactions. The crystal structure of the 1,5-diketone malonates 1a-g was analyzed according to the strategy steps: a) Intramolecular hydrogen bonds, b) Intermolecular hydrogen bonds in synthons and macrocycles, and c) Supramolecular architectures.
The strategic sequence of steps for the hierarchy that helps assess the supramolecular architecture in the titled 1,5-diketones is described below.
3.2.1 Intramolecular hydrogen bonds in monomer molecular structure of 1,5-diketones 1a-g
The first approach is to analyze the presence of intramolecular hydrogen bonds observed in the monomer molecular structure of 1,5-diketones 1a-g. Regarding Fig. 4, these bonds generate the kite conformation of each compound and the quasi-perpendicular conformation between the two molecular fragments that constitute the molecule. Thus, the 1,5-diketones and dimethyl malonate moieties resemble two perpendicular molecular axes with a total angle between planes near φ = 90° for the seven molecular structures: the angle between planes(φ) is 90.223, 89.016,90.330, 87.652, 89.845, 93.930 and 89.175° for 1a, 1b, 1c, 1d, 1e, 1f and 1 g, respectively.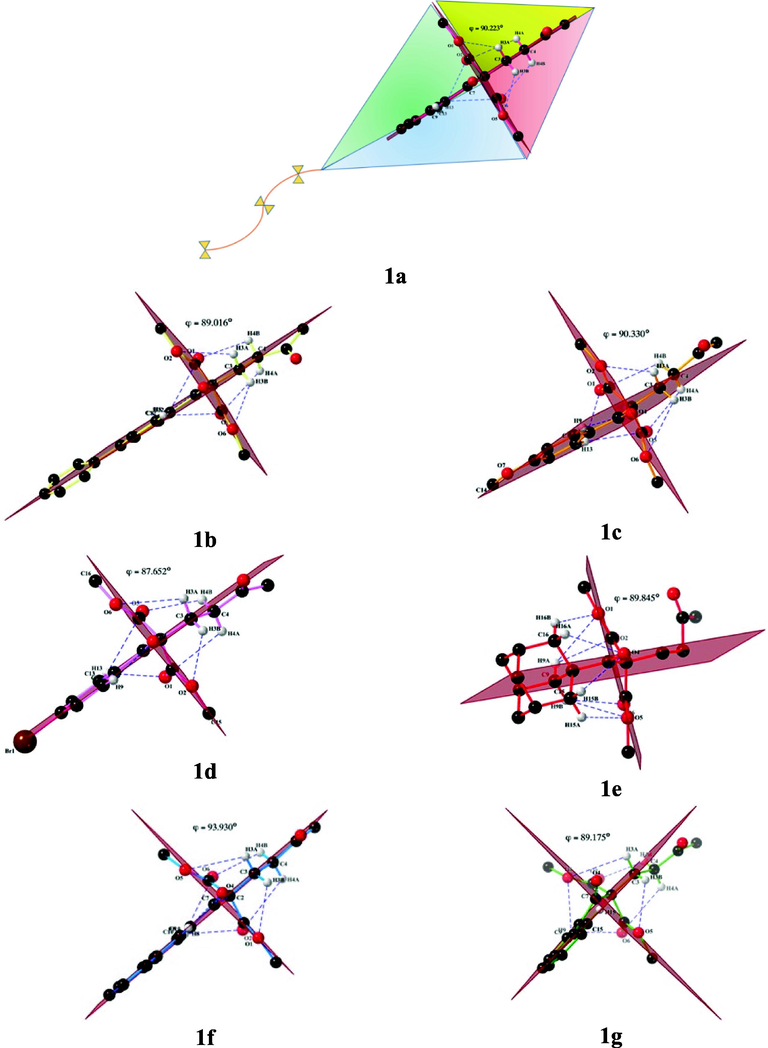
Intramolecular hydrogen bonds in molecular structures of compounds 1a-g (blue dashed lines) and inspired kite conformation morphology.
The compounds 1a-g have an average of seven intramolecular hydrogen bonds formed from the carbonyl group of the 1,5-diketones and the functional group –COO in the malonate with the -C–H of the molecule. As a result of this interaction, compounds 1a-g have a kite-like topology given the two perpendicular molecular axes, which are constituted on average by the seven intramolecular hydrogen bonds indicated by blue dashed lines between molecular planes, Fig. 4. Remarkably, C–H⋯O hydrogen bond interactions are key to supporting the kite conformation. Tables 2 show the intramolecular hydrogen bonds in the molecular structure of 1a-g compounds.
Compound 1a
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3A┄O1
2.52(3)
2.851(3)
99.4(17)
C3─H3B┄O5
2.64(3)
2.967(4)
100(2)
C4─H4A┄O2
3.09(3)
3.556(4)
113(2)
C4─H4B┄O6
2.72(3)
3.277(4)
118(2)
C9─H9┄O4
2.42(3)
2.771(4)
102(2)
C13─H13┄O2
2.57(3)
3.325(4)
138(3)
C13─H13┄O6
2.98(3)
3.708(4)
135(2)
Compound 1b
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3A┄O2
2.92
3.137(3)
94
C3─H3B┄O6
2.58
2.904(3)
100
C4─H4A┄O5
2.84
3.500(4)
126
C4─H4B┄O1
2.71
3.126(5)
107
C8─H8┄O4
2.44
2.758(4)
100
C12─H12┄O1
3.25
3.966(3)
135
C12─H12┄O5
2.74
3.495(4)
138
Compound 1c
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3A┄O2
2.56
2.838(4)
97
C3─H3B┄O6
2.99
3.213(4)
94
C4─H4A┄O5
2.52
3.089(3)
118
C4─H4B┄O1
2.94
3.525(3)
120
C9─H9┄O4
2.51
2.790(4)
98
C13─H13┄O1
2.72
3.362(4)
127
C13─H13┄O5
3.18
3.968(4)
144
Compound 1d
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3A┄O6
2.92
3.181(6)
96
C3─H3B┄O2
2.56
2.795(6)
94
C4─H4A┄O1
3.09
3.554(6)
111
C4─H4B┄O5
2.56
3.187(6)
123
C9─H9┄O4
2.45
2.744(7)
99
C13─H13┄O1
2.66
3.346(7)
131
C13─H13┄O5
3.19
3.957(7)
141
Compound 1e
D─H┄A
H┄A
D┄A
∠D─H┄A
C9─H9A┄O2
3.13
3.825(5)
130
C9─H9B┄O6
2.87
3.584(5)
131
C9─H9A┄O1
2.71
3.347(5)
123
C9─H9B┄O5
2.71
3.364(5)
125
C15─H15A┄O5
2.61
3.287(5)
127
C15─H15B┄O4
2.57
2.878(5)
98
C16─H16A┄O4
2.63
2.926(5)
98
Compound 1f
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3A┄O5
3.01
3.218(3)
93
C3─H3B┄O1
2.64
2.927(3)
97
C4─H4A┄O2
2.97
3.492(3)
115
C4─H4B┄O6
2.52
3.111(3)
119
C8─H8┄O4
2.42
2.740(3)
100
C10─H10┄O2
2.90
3.609(3)
134
C10─H10┄O6
3.25
3.999(3)
139
Compound 1g
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3A┄O1
3.25
3.383(3)
90
C3─H3B┄O5
2.63
2.887(2)
96
C4─H4A┄O6
2.95
3.513(3)
118
C4─H4B┄O2
2.41
3.016(3)
120
C9─H9┄O1
2.67
3.138(3)
112
C9─H9┄O6
2.74
3.378(3)
126
C15─H15┄O4
2.31
2.868(3)
118
C3─H3A┄O1
3.25
3.383(3)
90
3.2.2 Dimers based on synthons and macrocycles by intermolecular hydrogen bonds of 1,5-diketones 1a-g
Hierarchical analysis of the supramolecular structure of 1,5-diketone dimers 1a-g was performed from a synthon and macrocycle approach. A synthon is defined as a representative structural unit that links molecules and crystals and is implicated in all the stages through which molecules progress as they form crystals. (Desiraju, 2013, 1995, 2003) On another side, the motifs generated from intermolecular hydrogen bonds are defined as a type of graph set, such as C (chain), R (ring), D (dimer), and S denotes an intramolecular hydrogen bond. Additionally, the number of donors (d) and acceptors (a) used in motifs are assigned as subscripts and superscripts. In contrast, the size of the motif corresponding to the number of atoms in the repeat unit is indicated in parentheses. (Etter, 1990).
By applying the former concepts, the dimeric structure of compound 1a is formed by the C12-H12┄O4 interaction (Fig. 5), which is a simple hydrogen bond due to the donor interacting with an acceptor. However, because of the long-range hydrogen bonding, a donor may interact with more than one acceptor simultaneously. Also, the 1a dimeric structure presents a bifurcated arrangement of hydrogen bonds (Steiner, 2002) constituted by the C10-H10 donor and the O2 and O6 acceptors. The dimer formed a 16-atom ring with three acceptors and two donors:
. Table 3 shows the distance (Å) and the angle (°) of the intermolecular hydrogen bonds in the structure of dimeric structure 1a.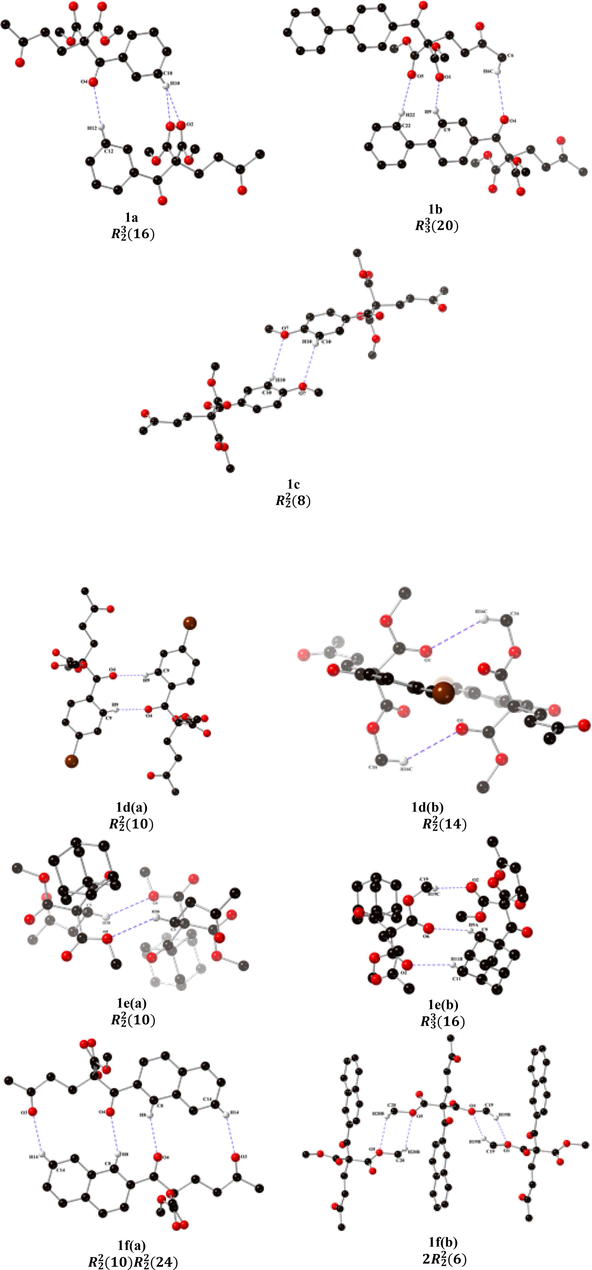
Crystal structure of dimers formed by two supramolecular synthons through the two hydrogen bonds in diketone malonates 1a-g.
Crystal structure of dimers formed by two supramolecular synthons through the two hydrogen bonds in diketone malonates 1a-g.
Compound 1a
D─H┄A
H┄A
D┄A
∠D─H┄A
C10─H10┄O2
2.69(3)
3.347(4)
131.6(18)
C10─H10┄O6
2.77(3)
3.586(4)
152.2(19)
C12─H12┄O4
2.63(3)
3.634(4)
174.9(16)
Compound 1b
D─H┄A
H┄A
D┄A
∠D─H┄A
C22─H22┄O5
2.77
3.690(5)
170
C9─H9┄O1
2.41
3.221(4)
145
C6─H6C┄O4
3.01
3.802(8)
140
Compound 1c
D─H┄A
H┄A
D┄A
∠D─H┄A
C10─H10┄O7
2.94
3.480(4)
118
Compound 1d
D─H┄A
H┄A
D┄A
∠D─H┄A
C9─H9┄O4
2.38
3.280(6)
164
C16─H16C┄O1
2.96
3.754(6)
140
Compound 1e
D─H┄A
H┄A
D┄A
∠D─H┄A
C3─H3B┄O5
2.71
3.513(5)
140
C11─H11B┄O2
2.99
3.779(5)
139
C9─H9A┄O6
2.69
3.464(5)
137
C19─H19C┄O2
2.47
3.260(5)
140
Compound 1f
D─H┄A
H┄A
D┄A
∠D─H┄A
C8─H8┄O4
2.59
3.449(3)
154
C14─H14┄O3
2.62
3.431(3)
146
C20─H20B┄O5
2.81
3.485(3)
128
C19─H19B┄O1
2.75
3.598(4)
148
Compound 1g
D─H┄A
H┄A
D┄A
∠D─H┄A
C19─H19B┄O1
2.77
3.601(3)
145
C9─H9┄O6
2.94
3.561(3)
125
C13─H13┄O3
2.61
3.285(3)
130
C11─H11┄O4
2.86
3.678(3)
148
For structure 1b, the supramolecular dimer is formed by three simple intermolecular hydrogen bonds: C6─H6C┄O4, C9─H9┄O1 and C22─H22┄O5. The dimer formed a 20-atom ring with three acceptors and three donors: , Fig. 5. Table 3 presents the distance (Å) and angle (°) of the intermolecular hydrogen bonds in structure 1b; the values of distances and bond angles are like those described in the literature. (Sutor,1962, 1963, Steiner, 2002).
The dimeric compound 1c is formed by the interaction C10─H10┄O7. This compound is an example of the role of hydrogen bonds in molecular recognition patterns by structural units called supramolecular synthons, Fig. 5. The synthon constitutes a dimer, an 8-atom ring, with two acceptors and two donors: . Table 3 presents the distance (Å) and angle (°) of intermolecular hydrogen bonds in the structure of compound 1c. The compound 1d has two dimers formed by two supramolecular synthons through C9─H9┄O4 and C16─H16C┄O1 interactions, Fig. 5. Each of the dimers 1d(a) and 1d(b) formed a 10-atom ring with two acceptors and two donors, and respectively. Table 3 presents the distance (Å) and angle (°) of the intermolecular hydrogen bonds in compound 1d.
The molecular structure of dimer 1e(a) is formed by the structural unit C3─H3B┄O5, a 10-atom ring with two acceptors and two donors: . A supramolecular dimer is present in the crystal structure of 1e(b), constituted by three acceptors and three donors: a ring with sixteen members. Table 3 presents the distance (Å) and angle (°) of intermolecular hydrogen bonds in the structure of compound 1e.
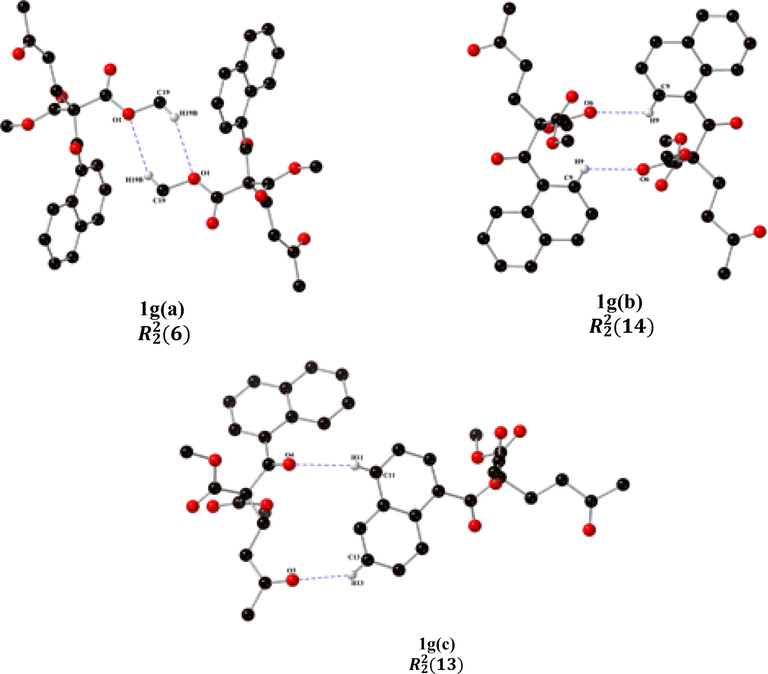
The molecular structure of 1f(a) has a particular dimer formed by two supramolecular synthons through C8─H8┄O4 and C14─H14┄O3 interactions, with a synthon inside the other. This dimer is formed of two rings, one with 10 atoms and the other with 24 atoms, with two acceptors and two donors in each ring: respectively; furthermore, two small 1f(b) synthons, each which with a 6-atoms ring by C19─H19B┄O1 and C20─H20B┄O5 interactions are observed. The dimeric compound 1 g(a) with a supramolecular synthon shows the C19─H19B┄O1 interaction, forming a 6-membered ring with two acceptors and two donors: , Fig. 5.
The second supramolecular structural unit 1 g(b) is formed by the C9─H9┄O6 interaction that assembles two molecules, the synthon formed by a 14-atom ring with two acceptors and two donors: he third supramolecular structure, 1 g(c), corresponds to a macrocycle with C13─H13┄O3 and C11─H11┄O4 interactions. These interactions comprise a 13-membered ring with two acceptors and two donors: . Table 3 presents the distance (Å) and angle (°) of intermolecular hydrogen bonds in the structure 1 g.
3.3 Hirshfeld surface analysis of 1,5-diketones
Hirshfeld surface analysis (Wolff et al., 2012) was performed to quantify and visualize the close intermolecular atomic contacts in the crystal structure and the associated fingerprint plots of the molecule showing the significant contributions of the different intermolecular interactions on the Hirshfeld surface. (Spackman & Jayatilaka, 2009) These results show the importance of the intramolecular and the intermolecular C–H⋯O hydrogen-bonds non-covalent interaction in the primary kite-like conformation and the dimer motifs for the 1,5-diketone malonates can be highlighted.
Remarkably H⋯O inside and outside interactions are depicted in the fingerprint plots of the 1,5-diketones 1a–g, Fig. 6. It is noticeable that inside interactions are slightly greater than outside contributions, the first mainly attributed to the carbonyl group of the 1,5-diketones and the functional group –COO in the malonate to the -C–H of the molecule by supporting the kite-like topology. In addition, outside contributions represent C–H⋯O hydrogen bond interactions with intermolecular hydrogen bonds (dimers, synthons and macrocycles). Fig. 7 describs the Hirshfeld surfaces for the diketones, enhancing the inside interactions, Fig. 8 shows the surfaces for dimers synthons.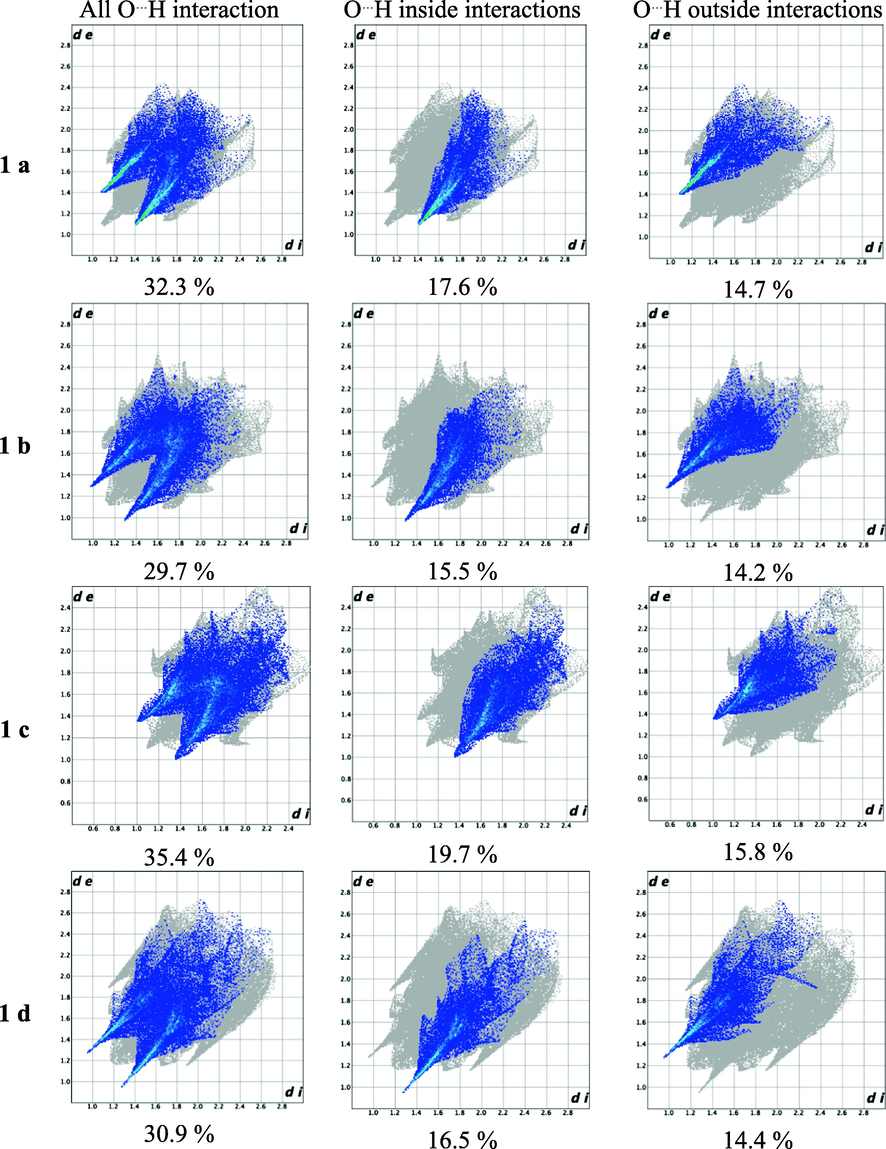
O–H Atom-atom interactions and their contribution to the Hirshfeld fingerprint plot for 1,5-diketones 1a – g.
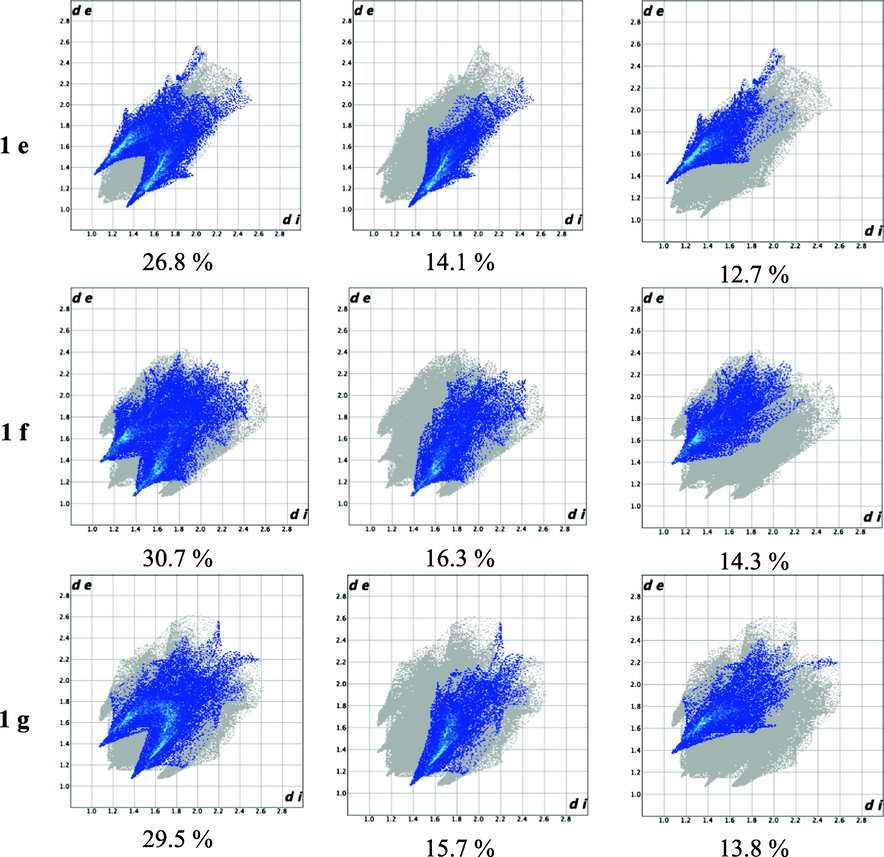
O–H Atom-atom interactions and their contribution to the Hirshfeld fingerprint plot for 1,5-diketones 1a – g.
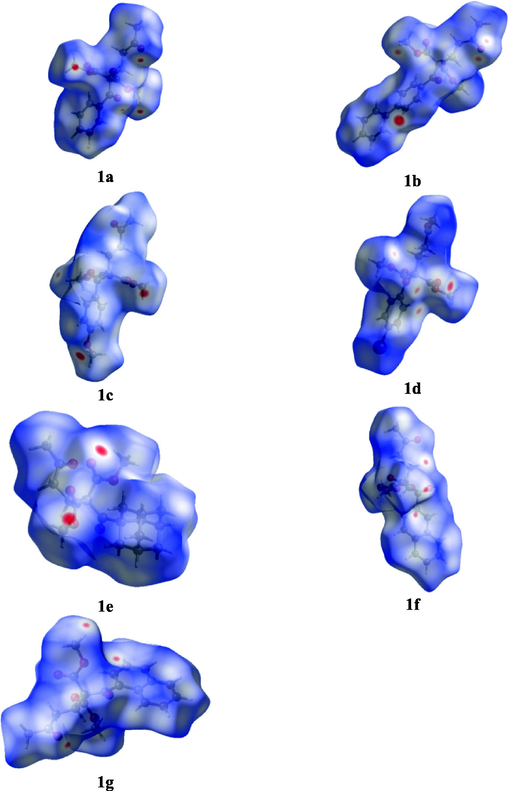
The Hirshfeld surface of 1,5-diketones 1a – g mapped with dnorm.
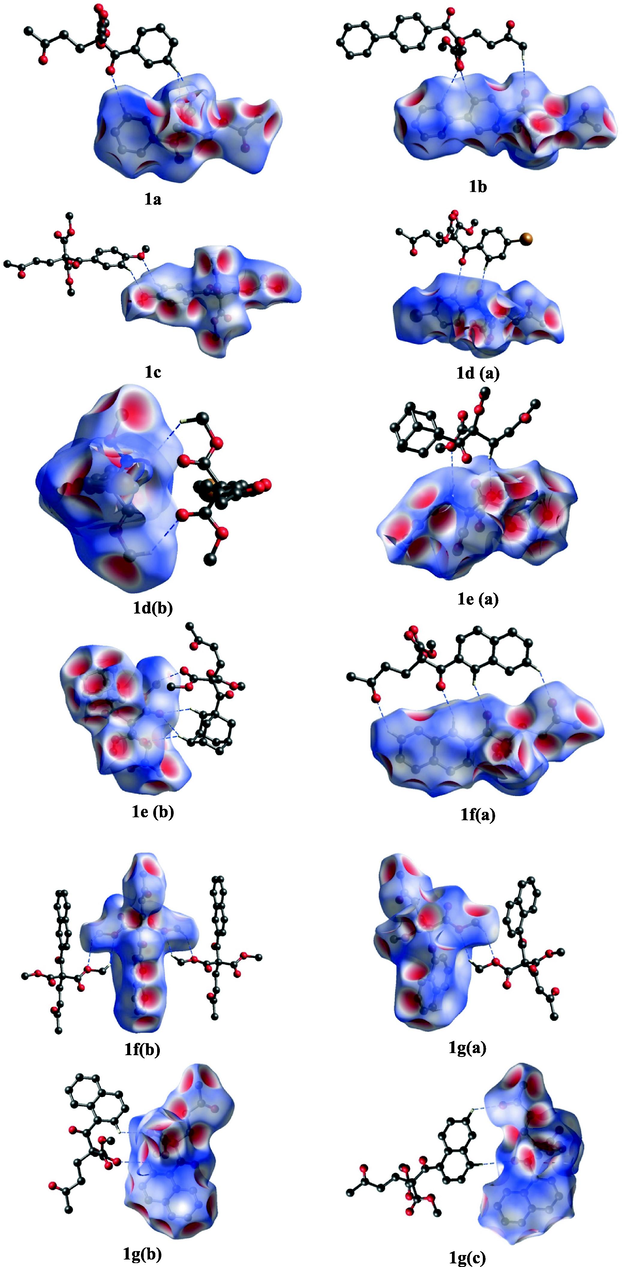
The Hirshfeld surface of 1,5-diketones 1a–g of dimers synthons and macrocycles.
3.4 Supramolecular architectures
Highlighting the importance of intramolecular c–h⋯o hydrogen bonds in the primary kite-like conformation, the intermolecular C–H⋯O hydrogen bond interactions in dimer motifs for the 1,5-diketone malonates are also important non-covalent interaction at different levels of hierarchy in crystal engineering. (Sutor, 1963, Desiraju, 2003) a small library of supramolecular architectures in diketones 1a-g can be described in the crystal structures in conjunction with the cooperative C–H⋯O hydrogen bonds, Fig. 9.ing the importance of intramolecular C–H⋯O hydrogen bonds in the primary kite-like conformation, the intermolecular C–H⋯O hydrogen bond interactions in dimer motifs for the 1,5-diketone malonates are also important non-covalent interaction at different levels of hierarchy in crystal engineering. (Sutor, 1963, Desiraju, 2003) A small library of supramolecular architectures in diketones 1a-g can be described in the crystal structures in conjunction with the cooperative C–H⋯O hydrogen bonds, Fig. 9.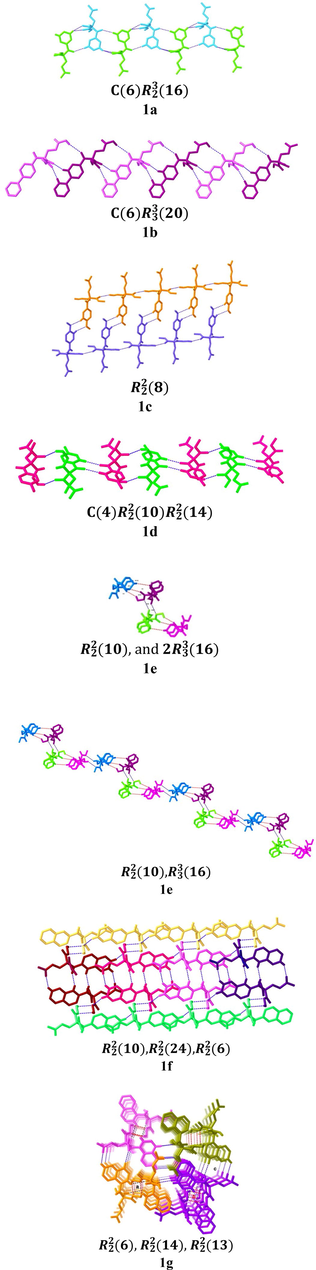
Supramolecular structures through hydrogen bonds of 1,5-diketones 1a-g.
According to Fig. 9, the motif dimers 1a and 1b with intermolecular hydrogen bonds show a supramolecular chain through 6 hydrogen bond interactions C(6) represented by stick model topology, for 1a and for 1b. The 1c supramolecular synthon shows a two-dimensional supramolecular structure with three interactions. The 1c synthon is represented by two series of molecules in parallel using the sticks model, . The compound 1d, with two dimers, generated a supramolecular structure with four C(4) interactions represented by the sticks model, .
The supramolecular structure (with adamantyl moiety) of 1e has a macrocycle in conjunction with the supramolecular synthon. This structural unit has 4 molecules that form a 1D zigzag supramolecular structure with five interactions each, .
For compound 1f, the three synthons generated a 3D supramolecular structure with four interactions represented by the sticks model, . In the case of 1 g, , the interaction of four stick motifs is notable.
4 Conclusions
In conclusion, the 1,5-diketone malonates 1a-g are illustrative examples of crystal packing determined exclusively by weak C–H⋯O hydrogen bonds. The plausible route of construction of crystalline solid has been explored to understand the mechanism of crystallization: a) Intramolecular C–H⋯O interactions provide 1,5-diketone malonates 1a-g a kite-like topology because the seven intramolecular hydrogen bonds constituted, on average, the two perpendicular molecular axes. b) The intermolecular interactions of C–H⋯O generate molecular recognition by forming ten synthons and four macrocycles as structural units in the crystallization process. c) The supramolecular architectures of compounds 1a-g are three-dimensional and obtained from the conjunction of cooperative hydrogen bonds C–H⋯O at different hierarchy levels.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CCDC number: 2204857, 2204858, 2204859, 2204860, 2204861, 2204862, 2204863, for the reported structures (1a - g) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from Cambridge Crystallographic Data Center via https://www.ccdc.cam.ac.uk/data_request/cif.
CRediT authorship contribution statement
Domingo Salazar-Mendoza: Conceptualization. José Luis García-Gutiérrez: Investigation. Federico Jiménez-Cruz: Conceptualization, Writing – original draft.
References
- Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl.. 1995;34:2311-2327.
- [CrossRef] [Google Scholar]
- The CH···O Hydrogen Bond: Structural Implications and Supramolecular Design. Acc. Chem. Res.. 1996;29:441-449.
- [CrossRef] [Google Scholar]
- Crystal engineering. From molecules to materials. J. Mol. Struct.. 2003;656:5-15.
- [CrossRef] [Google Scholar]
- Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc.. 2013;135:9952-9967.
- [CrossRef] [Google Scholar]
- Molecular Recognition in Organic Crystals: Directed Intermolecular Bonds or Nonlocalized Bonding? Angew. Chem. Int. Ed.. 2005;44:1766-1787.
- [CrossRef] [Google Scholar]
- Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res.. 1990;234:120-126.
- [CrossRef] [Google Scholar]
- A short synthesis of α-herbertenol featuring the use of a dihydropyranone as a 1,5-diketone synthon. Tetrahedron Lett.. 1998;39:9573-9574.
- [CrossRef] [Google Scholar]
- 1,5-Diketones from 3,4-dihydropyranones: An application in the synthesis of (±)-α-herbertenol. Tetrahedron. 1999;55:9333-9340.
- [CrossRef] [Google Scholar]
- 4-Ethoxycarbonyl-3-hydroxy-3-phenylcyclohexanone, Acta Cryst., C. 2001;57:425-427.
- [CrossRef]
- An efficient synthesis of ferrocenyl substituted 1,5-diketone and cyclic α, β-unsaturated ketones under ultrasound irradiation. J. Organomet. Chem.. 2004;689(10):1843-1848.
- [CrossRef] [Google Scholar]
- A simple 1,5 → 1,3-diketone rearrangement. Tetrahedron Lett.. 1998;39:2685-2688.
- [CrossRef] [Google Scholar]
- Synthesis of 1-Aryl-1,3-Diketones Containing the Dimethyl Malonate Moiety. Synth. Commun.. 2000;30:3439-3450.
- [CrossRef] [Google Scholar]
- Mixed organofluorine-organosilicon chemistry. 8. One-pot synthesis of 2,2-difluoro-1,5 diketones from acylsilanes, trifluoromethyltrimethylsilane and enones, and their annulation reaction. Tetrahedron. 1998;54:5939-5948.
- [CrossRef] [Google Scholar]
- Asymmetric Direct Michael Addition of Acetophenone to α, β-Unsaturated Aldehydes. Synthesis. 2011;1085–1091
- [CrossRef] [Google Scholar]
- A green synthesis of highly substituted 1,5-diketones. RSC Adv.. 2015;5:56949-56953.
- [CrossRef] [Google Scholar]
- Advanced Organic Chemistry. New York: Wiley; 1992. p. :1283.
- Mufato, J. D., Vega, D. R., J. M. Aguirre, J. M., Faba, D. J., Lantaño, B., 2011, Synthesis and structural study of benzamarones by X-ray and NMR spectroscopy, J. Mol. Struct., 987, 1–3, 119-125. 10.1016/j.molstruc.2010.12.005.
- The Aldol Condensation. In: Adams R., Blatt A.H., Boekelheide V., Cairns T.L., Cram D.J., House H.O., eds. Organic Reactions. New York: Wiley; 1968.
- [Google Scholar]
- Novel domino products from the reaction of phenyl vinyl ketone and its derivatives with cyclic ketones. Tetrahedron. 2002;58:2189-2199.
- [CrossRef] [Google Scholar]
- SHELXS97 and SHELXL97. Germany: University of Göttingen; 1997.
- Siemens, 1994, XSCANS, Version 2.1b, Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- checkCIF validation ALERTS: what they mean and how to respond. Acta Cryst.. 2020;E76:1-11.
- [CrossRef] [Google Scholar]
- Steiner, T., The Hydrogen Bond in the Solid State, 2002, Angew. Chem. Int. Ed., 41, 48–76. 10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U.
- Evidence for the existence of C-H⋯O hydrogen bonds in crystals. J. Chem. Soc.. 1963;1105–1110
- [CrossRef] [Google Scholar]
- 1,5-Diketone Synthesis Promoted by Barium Hydride or Barium Alkoxides. Synlett.. 2006;17:2833-2835.
- [CrossRef] [Google Scholar]
- Taylor, R., Kennard, O., J. Am. Chem. Soc., 1982, Crystallographic evidence for the existence of CH⋯O, CH⋯N and CH⋯Cl hydrogen bonds, 104, 19, 5063–5070. doi:10.1021/ja00383a012.
- A Ru-Catalyzed Three-Component Addition To Form 1,5-Diketones. J. Am. Chem. Soc.. 1997;119:836-837.
- [CrossRef] [Google Scholar]
- Triton B in Synthesis of 3-Phenylcyclohexenones. J. Am. Chem. Soc.. 1955;77:3664-3667.
- [CrossRef] [Google Scholar]
- Crystal explorer. Australia: University of Western Australia Crawley; 2012.
Appendix A
Supplementary data
Supplementary information: data associated with X-ray and 1H and 13C NMR data and spectra for 1,5-diketone malonates 1e-g.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104843.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1






