Translate this page into:
Design, synthesis and biological activity of chalcone derivatives containing pyridazine
⁎Corresponding authors. wyjbio@163.com (Yongjun Wu), wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Inspired by the application of fungicide from natural products, a series of chalcone derivatives containing pyridazine were designed, synthesized, and evaluated for their antifungal activities against nine plant fungi and antibacterial activity against three plant bacteria. The antibacterial results revealed that B8 showed the best activity against Xanthomonas campestris pv. citri (Xac) with an EC50 value of 78.89 μg/mL, superior to Bismerthiazol (EC50 = 86.72 μg/mL). The antifungal bioactivity results showed that some of the compounds had good bioactivity against fungi, such as B4 showed the best bioactivity against Botrytis cinerea (BC) with an EC50 value of 8.91 μg/mL, which was better than the azoxystrobin (EC50 = 20.28 μg/mL). Compounds B4, D2 and D3 showed good biological activity against Rhizoctonia solani (RS), with an EC50 value of 18.10, 20.18 and 20.60 μg/mL, comparable to the azoxystrobin. Antifungal mechanism studies of BC by B4 suggest that B4 disrupts the cell membrane of the mycelium and thus inhibits the growth of the fungus. The above indicates that chalcone derivatives containing pyridazine have the potential to become fungicides.
Keywords
Chalcone derivatives
Pyridazine
Antifungal activity
Antibacterial activity
Mechanism of action
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- HRMS
-
High-resolution mass spectrometry
- EC50
-
Median effective concentration
- Xoo
-
Xanthomonas oryzae pv.oryzae
- Xac
-
Xanthomonas axonopodis pv. Citri
- Psa
-
Pseudomonas syringae pv. actinidiae
- RS
-
Rhizoctonia solani
- FG
-
Fusarium graminearum
- SS
-
Sclerotinia sclerotiorum
- BD
-
Botryosphaeria dothidea
- CG
-
Colletotrichum gloeosporioides
- PS
-
Phomopsis sp
- BC
-
Botrytis cinerea
- PC
-
Phytophthora Capsici
- CC
-
Colletotrichum capsici
- CA
-
Colletotrichum acutatum
Abbreviations
1 Introduction
Plant-pathogenic fungi cause huge economic losses in global agricultural production every year and seriously threaten to human health and food safety (Silva et al., 2019). Botrytis cinerea, the causal agent of gray mold in plants, has a wide host range and is one of the most damaging diseases in the world currently (Dean et al., 2012). It can infect plants' roots, leaves, flowers, and fruits, causing yield reduction in many crops and fruit rot (Williamson et al., 2007, Li et al., 2018). In addition, Xoo, Xac, and Psa can cause yield reduction of major crops every year (Shasmita et al., 2019, Wu et al., 2021). Currently, plant pathogenic fungi and bacteria are still mainly controlled by means of chemical fungicides, which are not only easy to cause environmental pollution (Muller et al., 2019, Zubrod et al., 2019), but also prone to resistance (Lu et al., 2014, Ma et al., 2018), so there is a need to continuously develop new high-efficiency, low-toxicity fungicides.
Chalcone is precursor for the biosynthesis of natural product flavonoids. Chalcone is part of the plants most prominent class of secondary metabolites. (Tomar et al., 2009, Funakoshi-Tago et al., 2015). Chalcone and its derivatives are widely used in fungicides because of their good anti-fungal (Zhou et al., 2022), anti-bacterial (Dkhar et al., 2020), anti-viral (Gan et al., 2017), insecticidal (Yan et al., 2018), herbicidal (Duarte et al., 2022), anti-cancer (Coskun et al., 2017) and other biological activities (Li et al., 2018), in addition, chalcone has little toxic side effects. Compared with other natural products such as carvacrol and therapeutic, chalcone has the characteristics of simple synthesis (Aljelehawy et al., 2023, Alavi et al., 2023). Thus, chalcone is an important class of lead compounds on which compounds with excellent biological activity can be exploited.
In addition, pyridazine, also known as o-diazabenzene, is a class of nitrogen-containing heterocyclic compounds with a special structure and a wide range of biological activities. Pyridazine compounds exist with good biological activities in fungicides and pharmaceuticals (Fig. 1), such as anti-fungal (Cui et al., 2020, Dang et al., 2020), anti-bacterial (Mustafa and Mostafa 2020), herbicidal (Zou et al., 2017), insecticidal (Buysse et al., 2017), anti-viral (Galtier et al., 2003) and anti-cancer (Ahmed et al., 2021, He et al., 2021). Pyridazine is one of the more important active fragments in pesticides, and some commercial pyridazine pesticides have been successfully developed. Therefore, we introduced the pyridazine fragment.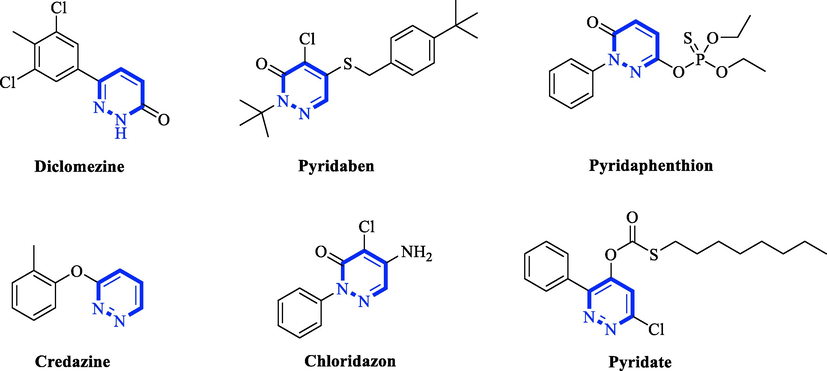
The structures of some commercialized pyrimidine fungicides and herbicide.
Based on the considerations mentioned above and our continuing interest in developing high-efficiency, low-toxicity fungicides using the chalcone backbone, we envisaged a combination of the chalcone backbone and the pyridazine heterocycle to discover new fungicides. A series of chalcone derivatives containing pyridazine were designed, synthesized, and evaluated for their in vitro and in vivo antifungal activities against nine plant fungi and antibacterial activity against three plant bacteria. The compound B4, which possesses superior antifungal activity, was subjected to scanning electron microscopy, spore germination assay and in vivo antifungal assay to conduct a preliminary explore of its antifungal mechanism.
2 Materials and methods
2.1 Instruments and chemicals
1H, 13C, and 19F nuclear magnetic resonance (NMR) spectra were obtained using Bruker Ascend 400 (Bruker Optics, Switzerland) and 500 MHz (Bruker Optics, Switzerland). High-resolution mass spectrometry (HRMS) data were conducted using a Thermo Scientific Q Exactive (Thermo Scientific, St. Louis, MO, U.S.A.). Scanning electronmicroscopy (SEM) data were obtained on FEI Nova Nano 450(Hillsboro, OR, U.S.A.). Microscope (Olympus Ltd, Japan). Melting point apparatus (Shanghai Yi Dian Physical Optics Instrument Co, Ltd, China).
2.2 Synthesis of target compounds
2.2.1 Synthesis of intermediates 1
Intermediates 1 were synthesized by the reported methods (Zhou et al., 2022). Aqueous sodium hydroxide solution (5% NaOH, 5 mmol) was added to a round-bottomed flask containing 1-(4-hydroxyphenyl)ethan-1-one (4 mmol) and differently substituted aldehydes (6 mmol). The mixture was stirred at ambient temperature for 12 h. The resulting dark-yellow mixture was acidified by HCl (5% HCl, 5 mmol) after the reaction was completed. Finally, the mixture was filtered under vacuum, and the residue was dried to yield intermediate 1.
2.2.2 Synthesis of intermediates 2
Intermediates 2 were synthesized by the reported methods (Liu et al., 2017). Maleic hydrazide or phthalhydrazide (17.84 mmol) was added to POCl3 or POBr3 (30 mL), and the abovementioned mixture was heated to reflux for 2 h. After cooling to room temperature, add to a 250 mL ice bath beaker, decompress and filter to obtain the crude product, and rinse with clean water three times. Finally, recrystallize with DMF/water system to obtain intermediate 2.
2.2.3 Synthesis of A1-A5, B1-B9 and C1
The intermediate 1 (1.93 mmol), intermediate 2 (2.32 mmol), and K2CO3 (5.80 mmol) were reacted in DMF for 5–8 h at 110 ℃, and the reaction was detected using TLC (Petroleum ether/ EtOAc = 1:1, V/V). After the reaction was completed, the system was poured into the water by cooling to room temperature, and the solid precipitated. The crude product was purified by column chromatography and eluted with (Petroleum ether/ EtOAc = 3:1, V/V) to give compounds A1-A5, B1-B9, and C1.
2.2.4 Synthesis of D1-D5
The target compound B1-B5 (1.43 mmol) and sodium acetate (5.70 mmol) were poured into acetic acid (30 mL) and reacted at 110 ℃ for 5–6 h. Reaction detection by TLC (Petroleum ether/ EtOAc = 2:1, V/V). The crude product was purified by column chromatography and eluted with (Petroleum ether/ EtOAc = 3:1, V/V) to give compounds D1-D5.
The physical date, 1H NMR, 13C NMR, and 19F NMR and HRMS data of the target compounds were provided in the Supporting Information.
2.3 Biological assays
2.3.1 Antibacterial activity in vitro
The in vitro anti-bacterial activities of target compounds A1-A5, B1-B9, C1, and D1-D5 against the plant pathogenic bacteria Xanthomonas oryzae pv. Oryzae (Xoo), Xanthomonas axonopodis pv. citri (Xac) and Pseudomonas syringae pv. actinidiae (Psa) were evaluated by a slightly modified 96-well plate method(Moghaddam et al., 2014). Bismerthiazol (BT)and thiodiazole-copper (TC) were used as positive control agents.
2.3.2 Antifungal activity in vitro
The in vitro antifungal effects of the target compounds A1-A5, B1-B9, C1, and D1-D5 against the plant pathogenic fungi Rhizoctonia solani (RS), Botrytis cinerea (BC), Phomopsis sp. (PS), Colletotrichum acutatum (CA), Botryosphaeria dothicdea (Bd), Fusarium graminearum (FG), Colletotrichum gloeosporioides (CG), Sclerotinia sclerotiorum (SS) and Phytophthora capsica (PC) were evaluated by a mycelial growth rate method (Wu et al., 2019, Long et al., 2021, Tian et al., 2021).
2.3.3 Antifungal activity in vivo
The in vivo biological activity of B4 against rice sheath blight disease was determined by the reported in vitro leaf test and greenhouse experiments with slight modifications (Zhang et al., 2018) with the rice cultivar Fengyouxiangzhan. Azoxystrobin (AZ) was used as a positive control agent.
2.3.4 Spore germination assay
The inhibitory activity of B4 on the spore germination of B. cinerea was assessed by microscopic observation. Spore suspensions (2 × 105 spores/mL) were prepared by seeding conidia in a 0.1% Tween 20 solution. The DMSO solution of the test compound B4 was diluted with the conidial suspension to obtain five different final concentrations of 0, 25, 50, 100, and 200 μg/mL. Then, 30 μL of these cultures were removed to a concave slide and incubated in a humidity chamber at 28 °C and 90% relative humidity. Three replicates were performed, and a conidial suspension containing a corresponding concentration of acetone in water was regarded as the control. After incubation at 28 °C for 12 h, the number of germinated spores was examined by counting and measuring approximately 100 conidia in each field of three randomly selected fields under a biological microscope photographic system at 25 × magnification. The experiments were repeated three times. (Zhang et al., 2014).
2.3.5 Scanning electron microscopy (SEM)
To investigate in more detail the anti-bacterial and anti-fungal effects of the compounds, we used the Scanning electron microscopy (SEM) method reported in the literature (Peng et al., 2021, Tang et al., 2022).
2.3.6 Determination of MDA contents
Mycelia of BC were cultured on PDB medium on a rotary shaker (200 rpm) at 25 ℃. Then, prepared solutions of compound B4 at different concentrations (0, 25, 50, 100 μg/mL) were added and incubated with the mycelia for 24 h at 25 °C. The medium was filtered, and the hyphae were collected in a vacuum freeze dryer. Mycelia (0.1 g) were homogenized in an ice bath with 1 mL of MDA extract (produced by Solarbio Technology Co., Ltd., Beijing, China) and centrifuged at 8000 g and four °C for 15 min. The supernatant was removed, added to each reagent according to the kit instructions, held in a 100 °C water bath for 60 min, cooled in an ice bath, and centrifuged at 10,000 g for 10 min at room temperature. The supernatant was aspirated, and then the absorbance of each sample was measured at 532 nm and 600 nm. Each group was repeated three times. MDA content in mycelia was determined using an MDA assay kit (Beijing Solarbio Science & Technology Co.) (Mo et al., 2021).
3 Results and discussion
3.1 Chemistry
As shown in Scheme 1, compounds A1-A5, B1-B9, C1 and D1-D5 were synthesized, and characterized their structures by 1H NMR, 13C NMR, 19F NMR and HRMS. All synthetic compounds were original.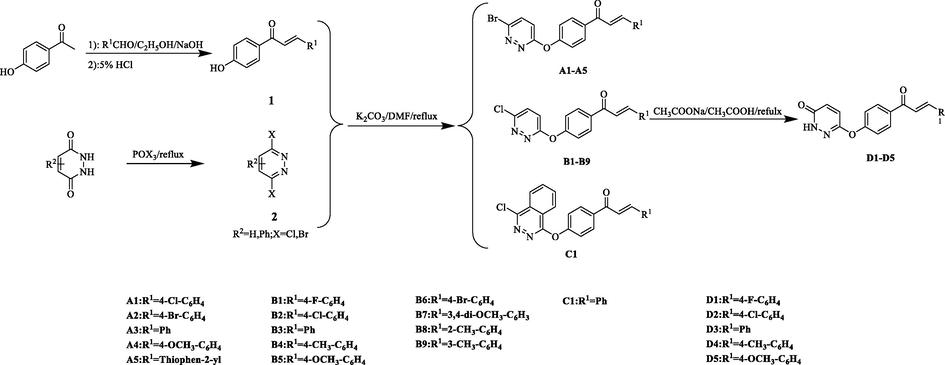
Synthetic route of the target compounds.
3.2 Antibacterial activity
3.2.1 Antibacterial activity in vitor
The antibacterial effects of target compounds against Xac, Xoo, and Psa were detected by the turbidimetric method, as shown in Table 1. The against Xac activity of B8 (55.7%) at 100 μg/mL was superior to BT and slightly lower than TC.
Compd.
Xac (%)
Psa (%)
Xoo (%)
100 μg/mL
50 μg/mL
100 μg/mL
50 μg/mL
100 μg/mL
50 μg/mL
A1
16.99 ± 2.57
15.07 ± 1.59
8.52 ± 0.85
7.83 ± 0.06
12.37 ± 4.19
10.48 ± 3.41
A2
34.15 ± 1.11
15.76 ± 1.13
10.06 ± 0.45
7.92 ± 0.10
18.56 ± 1.04
17.59 ± 1.24
A3
6.76 ± 0.72
5.96 ± 2.21
7.10 ± 0.87
6.10 ± 0.81
6.39 ± 6.10
4.09 ± 1.16
A4
35.66 ± 4.76
15.39 ± 0.76
14.34 ± 0.53
12.07 ± 0.15
35.43 ± 1.20
14.80 ± 2.74
A5
32.32 ± 3.94
15.34 ± 3.96
11.16 ± 0.98
9.70 ± 0.87
16.62 ± 0.96
15.49 ± 7.39
B1
10.26 ± 3.54
3.84 ± 4.16
14.62 ± 0.17
9.34 ± 0.32
16.16 ± 1.57
10.38 ± 0.12
B2
24.05 ± 1.86
23.57 ± 1.19
10.84 ± 0.42
8.20 ± 0.56
23.72 ± 0.49
19.02 ± 0.60
B3
15.07 ± 2.76
13.52 ± 4.72
12.80 ± 3.15
10.20 ± 0.49
9.41 ± 2.28
4.70 ± 0.12
B4
24.00 ± 1.80
13.95 ± 1.50
7.79 ± 0.79
5.56 ± 0.61
40.34 ± 0.26
36.91 ± 0.84
B5
24.80 ± 4.74
17.48 ± 3.88
8.70 ± 0.57
8.42 ± 1.21
9.36 ± 6.26
4.34 ± 0.41
B6
42.40 ± 1.15
6.78 ± 10.1
8.14 ± 0.31
8.10 ± 0.66
22.91 ± 0.59
21.03 ± 0.15
B7
16.19 ± 1.25
8.76 ± 1.53
7.29 ± 4.52
5.37 ± 0.99
11.61 ± 1.85
9.97 ± 0.10
B8
55.66 ± 3.35
23.89 ± 2.42
10.56 ± 1.36
9.56 ± 0.59
41.24 ± 0.56
20.49 ± 0.79
B9
9.72 ± 1.87
4.04 ± 1.13
6.29 ± 0.36
4.16 ± 1.04
28.51 ± 1.08
12.88 ± 3.26
C1
18.81 ± 0.46
17.21 ± 1.08
10.20 ± 0.31
8.29 ± 0.40
15.64 ± 2.46
12.32 ± 4.13
D1
33.04 ± 0.76
25.23 ± 5.54
21.45 ± 1.35
16.12 ± 1.10
26.07 ± 1.93
24.62 ± 2.60
D2
29.72 ± 6.62
22.02 ± 7.86
14.77 ± 0.89
10.70 ± 0.78
35.66 ± 1.31
29.50 ± 2.11
D3
35.07 ± 3.22
32.08 ± 8.55
8.48 ± 0.06
7.91 ± 0.86
36.35 ± 0.70
34.59 ± 1.10
D4
47.59 ± 0.95
15.71 ± 4.86
16.62 ± 2.74
11.46 ± 0.91
27.68 ± 0.53
24.11 ± 0.70
D5
50.64 ± 2.60
33.25 ± 4.48
13.07 ± 0.75
9.47 ± 1.06
22.28 ± 2.36
21.16 ± 1.14
TCb
63.36 ± 5.57
44.35 ± 8.46
69.34 ± 0.86
35.98 ± 0.46
63.25 ± 3.23
39.76 ± 2.70
BTb
51.68 ± 6.63
45.21 ± 2.92
51.91 ± 1.27
35.30 ± 0.66
49.44 ± 2.36
43.53 ± 1.06
The EC50 values of B8 against Xac was further measured according to the method mentioned above (Table 2). From the data, the EC50 value of B8 (EC50 = 78.89 μg/mL) was excellent than BT (EC50 = 86.72 μg/mL) and approaching TC (EC50 = 70.43 μg/mL).
The structure–activity relationship (SAR) was analyzed on the basis of the antibacterial activities against Xac, Xoo, and Psa, shown in Table 1. When R1 was the Ph group, the antibacterial activity was A3 < B3 < C1 < D4. When R1 was an electron-donating group, such as the 4-OCH3-Ph group, the antibacterial activity was B5 < A4 < D5. When R1 was an electron-absorbing group such as the 4-F-Ph group, the antibacterial activity was B1 < D1. When R1 was CH3-Ph group, the antibacterial activity was B9 < B4 < B8.
3.2.2 Scanning electron microscopy
We further investigated the mechanism of action of B8 on Xac and B4 on Xoo by scanning electron microscopy. As shown in Fig. 2, when the concentration of B8 was 0 μg/mL (A), the Xac cells were fully packed, and the surface was smooth; when the concentration of B8 was 50 μg/mL (B), a few Xac cells showed a certain degree of fold and flattening on the surface of the cell membrane. When the concentration increased to 100 μg/mL(C), many Xac cells showed flattening on the surface of the cell membrane, and the folded condition increased, eventually leading to cell death.
Scanning electron micrographs of Xac in the presence of different concentrations of B8 (A) 0 µg/mL; (B) 50 µg/mL and (C) 100 µg/mL. Scale bar for (A, B and C) are 2 µm.
The effect of different concentrations of drugs interacting with Xac can be seen in Fig. 3. At a concentration of 0 μg/mL (A), all Xac cells are full and have a smooth surface. At a concentration of 50 μg/mL (B), a few Xac cells showed a certain degree of ruffling and flattening. When the concentration was increased to 100 μg/mL (C), a large number of Xac cells showed flattened surface, and the folded condition increased, but no membrane damage occurred. Compared to compound B8, B4 does not cause cell membrane damage, resulting in cell death.
Scanning electron micrographs of Xoo in the presence of different concentrations of B4 (A) 0 µg/mL; (B) 50 µg/mL and (C) 100 µg/mL. Scale bar for (A, B and C) are 2 µm.
3.3 Antifungal activity
3.3.1 Antifungal activity in vitro
According to the results of antifungal activity evaluation (Table 3), chalcone derivatives A1-A5, B1-B9, C1, and D1-D5 were screened against nine plant fungi (RS, BC, PS, CA, FG, CG, SS, PC, BD) at 100 μg/mL. The mycelial growth inhibition assay was used to determine the inhibition of the target compounds against the nine fungi at a concentration of 100 μg/mL. The data in Table 3 showed that some of the target compounds had excellent antifungal activity. Among them, B4 showed excellent inhibitory activity against most of the tested fungi and was superior to the AZ (87.45% and 76.30%) at 100 μg/mL concentration against BC (100%) and RS (85.67%) and close to the AZ against PS (82.66%), SS (75.74%), and FG (65.66%). To further determine the activity of the target compounds, we performed EC50 determination of the target compounds, which is shown in Table 4. From Table 4, we know that the EC50 values of B4 against BC and RS were 8.91 µg/mL and 18.10 µg/mL better than the AZ, and the EC50 values of D2 and D3 were 20.18 µg/mL and 20.60 µg/mL and comparable to AZ. Therefore, we selected BC and RS for the mechanism study.
Compd.
BC
PS
CG
CA
RS
PC
SS
BD
FG
A1
20.53 ± 3.36
35.06 ± 2.68
21.25 ± 4.14
0.00 ± 0.00
53.70 ± 3.27
15.28 ± 2.32
42.13 ± 1.32
38.60 ± 3.80
0.00 ± 0.00
A2
22.81 ± 4.19
39.85 ± 1.67
37.92 ± 2.94
0.00 ± 0.00
59.26 ± 1.81
18.98 ± 1.13
39.57 ± 3.09
23.53 ± 1.14
17.74 ± 3.60
A3
34.22 ± 3.03
55.72 ± 0.00
27.08 ± 5.84
16.97 ± 1.67
49.63 ± 3.04
36.11 ± 1.76
50.64 ± 2.08
9.40 ± 2.21
9.06 ± 1.66
A4
22.05 ± 3.93
38.75 ± 3.33
22.50 ± 6.26
5.50 ± 2.29
59.63 ± 0.91
30.09 ± 1.13
41.28 ± 3.23
26.47 ± 2.28
11.32 ± 3.25
A5
20.15 ± 6.92
40.22 ± 1.98
32.08 ± 7.46
0.00 ± 0.00
52.96 ± 0.91
27.31 ± 1.13
49.36 ± 1.92
0.00 ± 0.00
21.89 ± 3.62
B1
36.12 ± 1.44
52.40 ± 7.51
39.58 ± 2.18
12.84 ± 1.14
56.30 ± 1.15
26.39 ± 1.52
47.23 ± 2.08
36.40 ± 8.65
35.09 ± 1.14
B2
22.81 ± 0.93
43.54 ± 2.32
42.92 ± 5.13
0.00 ± 0.00
55.19 ± 0.91
14.35 ± 1.13
49.79 ± 1.32
6.71 ± 3.34
32.83 ± 1.14
B3
41.44 ± 2.76
52.03 ± 2.68
37.08 ± 6.68
25.23 ± 1.67
31.48 ± 0.91
37.50 ± 1.52
45.96 ± 1.92
25.37 ± 3.25
32.83 ± 4.97
B4
100 ± 0.00
82.66 ± 1.67
48.75 ± 3.64
51.83 ± 1.21
85.67 ± 1.67
35.65 ± 1.13
75.74 ± 2.14
11.41 ± 2.55
65.66 ± 0.90
B5
25.10 ± 4.94
47.23 ± 1.67
22.50 ± 2.80
22.48 ± 3.26
57.41 ± 1.67
29.17 ± 1.52
42.13 ± 2.64
25.00 ± 3.95
22.26 ± 1.80
B6
31.94 ± 3.93
42.07 ± 2.59
22.50 ± 5.42
0.92 ± 1.40
57.04 ± 1.15
13.89 ± 1.76
40.85 ± 2.51
12.75 ± 9.06
1.51 ± 1.21
B7
32.32 ± 8.62
46.13 ± 4.57
28.75 ± 3.05
11.93 ± 4.43
47.04 ± 1.67
36.57 ± 2.09
45.53 ± 1.32
22.43 ± 1.66
35.85 ± 1.14
B8
12.17 ± 2.79
45.76 ± 1.21
25.42 ± 3.55
0.00 ± 0.00
25.10 ± 2.08
8.80 ± 2.73
16.23 ± 2.98
0.00 ± 0.00
16.98 ± 2.28
B9
24.71 ± 4.20
45.39 ± 3.33
24.58 ± 2.94
0.00 ± 0.00
24.69 ± 2.68
12.50 ± 2.32
15.79 ± 1.61
0.00 ± 0.00
18.49 ± 6.54
C1
37.26 ± 1.25
70.11 ± 1.85
26.67 ± 4.35
38.53 ± 1.14
58.52 ± 1.81
29.17 ± 1.52
39.57 ± 3.09
12.75 ± 4.16
13.96 ± 3.69
D1
52.85 ± 1.86
53.14 ± 3.26
39.17 ± 5.72
29.36 ± 3.88
55.19 ± 1.67
43.98 ± 1.13
48.94 ± 1.61
18.79 ± 3.03
20.75 ± 0.00
D2
45.68 ± 2.89
59.78 ± 0.90
40.00 ± 1.40
11.47 ± 1.67
62.59 ± 0.91
44.44 ± 1.76
56.60 ± 1.61
20.13 ± 4.71
6.04 ± 1.85
D3
44.19 ± 3.36
57.93 ± 2.43
36.67 ± 4.45
47.71 ± 3.13
62.22 ± 1.41
42.59 ± 1.43
60.85 ± 2.64
0.00 ± 0.00
21.89 ± 1.85
D4
40.30 ± 3.03
46.86 ± 3.43
31.25 ± 6.06
5.96 ± 3.55
59.26 ± 1.15
32.41 ± 1.43
48.09 ± 2.08
18.12 ± 2.08
8.68 ± 5.86
D5
37.26 ± 3.46
40.96 ± 3.02
30.83 ± 2.68
2.29 ± 1.21
57.78 ± 1.41
29.17 ± 2.32
44.26 ± 3.39
5.37 ± 5.55
3.77 ± 2.31
AZb
87.45 ± 1.25
91.51 ± 1.67
63.33 ± 4.12
73.85 ± 1.21
76.30 ± 1.15
60.65 ± 4.78
88.51 ± 3.13
79.04 ± 2.31
66.79 ± 1.14
Fungi
Compd
EC50 (µg/mL)
r2
Regression equation
BC
B4
8.91
0.9889
y = 2.3932x + 2.7194
AZ
20.28
0.9453
y = 1.0088x + 3.9899
RS
B4
18.10
0.9443
y = 1.2111x + 3.4755
D2
20.18
0.9767
y = 0.7602x + 4.0080
D3
20.60
0.9289
y = 0.8345x + 3.9036
AZ
10.23
0.9417
y = 0.9366x + 4.0541
SS
B4
20.62
0.9404
y = 0.7288x + 4.0421
AZ
17.46
0.9394
y = 0.8942x + 3.8893
PS.
B4
20.82
0.9774
y = 1.2576x + 3.3440
AZ
10.67
0.9637
y = 0.5070x + 4.4788
3.3.2 Antifungal activity in vivo
In order to further examine the application prospect of the potent B4, its’ protective activity and curative activity against RS were first measured by the detached leaf assay in vivo. As shown in Fig. 4, Fig. 5 and Table 5, the obtained experimental results indicated B4 could significantly inhibit RS growth. Fig. 4 shows the control (C) and negative control (F) rice leaves with large spot lengths and yellowing leaves. The rice leaves treated with B4 (A) at 200 µg/mL (63.15%) had shorter spot lengths and greener leave colours, which were close to the AZ (66.53%). As shown in Fig. 5, the rice leaves treated with B4 (A) at 200 µg/mL (with an impressive curative efficacy of 86.85%) had a shooter spot length, which was better than AZ (78.38%). The curative activity of B4 (B) at 100 µg/mL was 67.35%, which was slightly lower than that of the AZ (68.41%), but there was still a significant quality effect compared to the control (C) and negative control (F). Therefore, these experiments indicated that B4 could effectively control rice sheath blight disease caused by RS.
The protective efficacy of B4 against rice sheath blight on detached rice leaves, (A) B4 at 200 μg/ mL; (B) B4 at 100 μg/ mL; (C) Control, 0.1% DMSO; (D) AZ at 200 μg/ mL; (E) AZ at 100 μg/ mL; (F) Negative control.

The Curative efficacy of B4 against rice sheath blight on detached rice leaves (A) B4 at 200 μg/mL; (B) B4 at 100 μg/mL; (C) control, 0.1% DMSO; (D) AZ at 200 μg/mL; (E) AZ at 100 μg/mL; (F) Negative control.
Treatment
Concentration(μg/mL)
Curative activity
Protective activity
Lesion
length (cm)Control
efficacy (%)Lesion
length (cm)Control
efficacy (%)
B4
100
2.20 ± 0.45
67.35
3.86 ± 0.28
44.05
200
0.89 ± 0.42
86.85
2.54 ± 0.24
63.15
AZ
100
2.12 ± 0.55
68.41
3.79 ± 0.25
45.06
200
1.45 ± 0.34
78.38
2.31 ± 0.30
66.53
Negative control
6.74 ± 0.44
–
7.23 ± 0.56
–
3.3.3 Spore germination assay
The target compound B4 was assayed against in-spore germination of BC at concentrations of 0, 25, 50, 100, and 200 µg/mL. BC infects hosts through RNA disruption of plant defence mechanisms with the help of conidia so that B4 can reduce damage to host plants by inhibiting spore germination. As shown in Fig. 6 and Fig. 7, B4 effectively inhibited spore germination in a concentration-dependent manner. As shown in Fig. 6, the spores in the control group (A) all germinated. But the number of spores germinated began to decrease when the concentration of compound B4 increased. Only half the spores germinate when B4 (B) at 25 µg/mL, while when the concentration rises to 100 µg/mL (D), only one tenth of the spores germinate, with a significant increase in the inhibition rate. When B4 (F) at 200 µg/mL, the relative inhibition of spore germination was 100%. As shown in Fig. 7, the relative inhibition of spore germination was 100, 90.77, 72.58 and 51.51% at concentrations of 200, 100, 50 and 25 μg/mL, respectively. The EC50 value of B4 was 26.55 μg/mL, indicating a good inhibition rate against the spores. In contrast to the mycelium inhibition experiment, the inhibition rate of B4 on spore germination was lower than on mycelium, indicating that the compound inhibited mycelium better.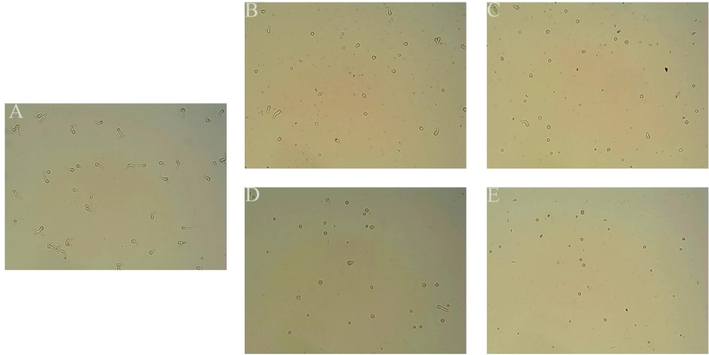
Effects of B4 on spore germination of BC (A) 0 µg/mL; (B)25 µg/mL; (C) 50 µg/mL; (D) 100 µg/mL and (E) 200 µg/mL.
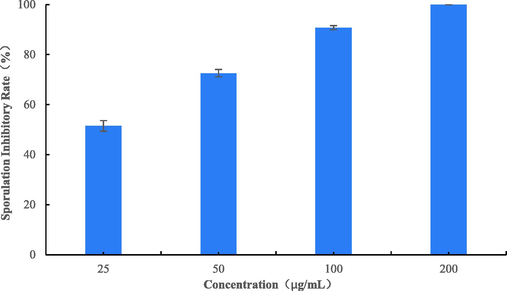
In vitro effect of B4 against spore germination of BC.
3.3.4 Phospholipid peroxidation of BC induced by B4
Malondialdehyde (MDA) is a critical metabolic executive in the peroxidation process of biological cell membranes and can also reflect the extent of damage to cell membranes by oxidative stress. After the effect of B4 Fig. 8, the MDA content increased with increasing concentration and was 1.83, 2.05 and 2.41 times higher than that of the control when the concentration of B4 was 25, 50, and 100 μg/mL, respectively. Therefore, it can be shown that B4 can significantly increase the degree of lipid peroxidation of BC cell membrane, and the degree of lipid peroxidation was positively correlated with the concentration of the compound.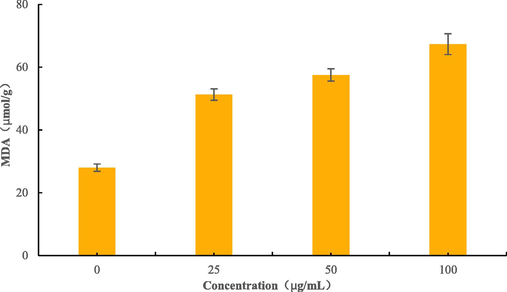
MDA contents of BC treated with B4.
3.3.5 Effect of compound B4 on the hyphae morphology of BC
As shown in Fig. 9, scanning electron microscopy revealed that the mycelium of the blank control group had normal morphology, uniform thickness, smooth surface and good growth. In contrast, after 50 μg/mL and 100 μg/mL B4 treatment, the mycelium was wrinkled, collapsed, severely deformed and varied in thickness. Therefore, B4 can destroy the morphology of the mycelium of BC, thus affecting the average growth of mycelium.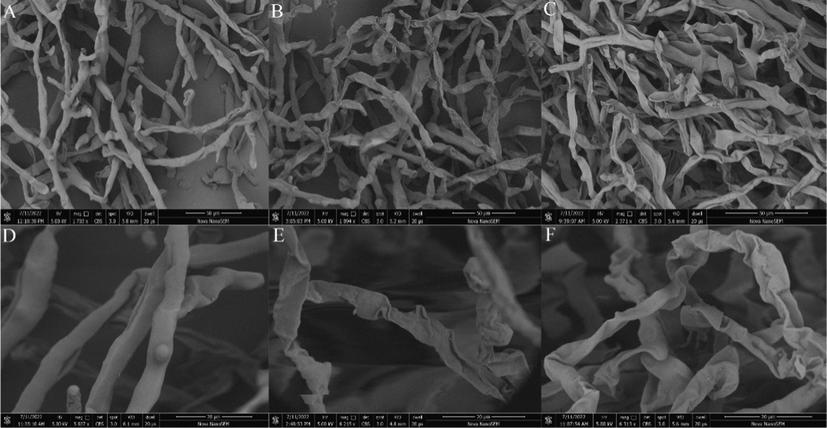
Scanning electron micrographs of BC mycelia. (A,D) negative control, 0.1% DMSO; (B,E) treated with 0.1% DMSO plus B4 at 50 μg/mL;(C,F) treated with 0.1% DMSO plus B4 at 100 μg/mL;Scale bar for (A, B and C) are 50 μm. Scale bar for (D, E and F) are 20 μm.
4 Conclusion
In summary, a series of chalcone derivatives containing pyridazine moiety had been designed and synthesized and their structures had been identified by NMR and HRMS. All the target compounds were assayed for their biological activities against three bacteria and nine fungi. The results showed excellent antibacterial and antifungal activities for some of the target compounds. Antibacterial activities result show B8 possessed the best bacterial activity with an EC50 value of 78.89 μg/mL against Xac, which was superior to BT (EC50 = 86.72 μg/mL). Antifungal activities result show B4 at 100 μg/mL concentration showed excellent inhibitory activity against most of the tested fungi and was superior to the AZ (87.45% and 76.30%) against BC (100%) and RS (85.67%). In vivo protective activity and curative activity against RS of B4 results indicated that compound B4 was able to significantly inhibit rice sheath blight disease, having a curative efficacy of 86.85% at 200 μg/mL. Scanning electron microscopy, spore germination assay and phospholipid peroxidation assay were taken for the preliminary study of the antifungal mechanism. Scanning electron microscopy and phospholipid peroxidation assay results showed that B4 could destroy the hyphal morphology of BC and affect cell membrane permeability. Spore germination assay results showed that B4 could effectively inhibit spore germination of BC. In short, the present work implied that B4 could be regarded as promising candidate for developing more effective fungicides to fight against the pathogen BC.
Acknowledgements
This research was completed with the support of the National Natural Science Foundation of China (No. 32072446), the Science Foundation of Guizhou Province (No. 20192452).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development of pyridazine derivatives as potential EGFR inhibitors and apoptosis inducers: Design, synthesis, anticancer evaluation, and molecular modeling studies. Bioorg. Chem.. 2021;106:104473
- [Google Scholar]
- Micro and nanoformulations of catechins for therapeutic applications: recent advances and challenges. Micro Nano Bio Aspects.. 2023;2(1):8-19.
- [Google Scholar]
- Anticancer, antineurodegenerative, antimicrobial, and antidiabetic activities of carvacrol: recent advances and limitations for effective formulations. Nano Micro Biosystems.. 2023;2(1):1-10.
- [Google Scholar]
- Synthesis and biological activity of pyridazine amides, hydrazones and hydrazides. Pest. Manage. Sci.. 2017;73(4):782-795.
- [Google Scholar]
- Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem.. 2017;136:212-222.
- [Google Scholar]
- Simple Analogues of Quaternary Benzo[c]phenanthridine Alkaloids: Discovery of a Novel Antifungal 2-Phenylphthalazin-2-ium Scaffold with Excellent Potency against Phytopathogenic Fungi. J Agric Food Chem.. 2020;68(52):15418-15427.
- [Google Scholar]
- Design, synthesis, and bioactivities of novel pyridazinone derivatives containing 2-phenylthiazole or oxazole skeletons. J. Heterocycl. Chem.. 2020;57(11):4088-4098.
- [Google Scholar]
- The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol.. 2012;13(4):414-430.
- [Google Scholar]
- Ru, Rh and Ir metal complexes of pyridyl chalcone derivatives: Their potent antibacterial activity, comparable cytotoxicity potency and selectivity to cisplatin. Polyhedron. 2020;185:114606
- [Google Scholar]
- A new quinolinone-chalcone hybrid with potential antibacterial and herbicidal properties using in silico approaches. J. Mol. Model.. 2022;28(6):176.
- [Google Scholar]
- Anti-inflammatory activity of flavonoids in Nepalese propolis is attributed to inhibition of the IL-33 signaling pathway. Int. Immunopharmacol.. 2015;25(1):189-198.
- [Google Scholar]
- Synthesis and Antiviral Activities of 3-Aralkyl-Thiomethylimidazo[1,2-b]Pyridazine Derivatives. Antiviral Chem. Chemother.. 2003;14:177-182.
- [Google Scholar]
- Novel trans-Ferulic Acid Derivatives Containing a Chalcone Moiety as Potential Activator for Plant Resistance Induction. J. Agric. Food. Chem.. 2017;65(22):4367-4377.
- [Google Scholar]
- Pyridazine as a privileged structure: An updated review on anticancer activity of pyridazine containing bioactive molecules. Eur. J. Med. Chem.. 2021;209:112946
- [Google Scholar]
- Li, H., Chen, Y., Z, Z, Q., Li, B, Q., G, Z., Tian, S, P., 2018. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2(3), 111-119.
- Design, synthesis, biological evaluation, and molecular docking of chalcone derivatives as anti-inflammatory agents. Bioor. Med. Chem Lett.. 2018;27(3):602-606.
- [Google Scholar]
- Synthesis and the interaction of 2-(1H-pyrazol-4-yl)-1H-imidazo[4,5-f][1,10]phenanthrolines with telomeric DNA as lung cancer inhibitors. Eur. J. Med. Chem.. 2017;133:36-49.
- [Google Scholar]
- Fabrication of Versatile Pyrazole Hydrazide Derivatives Bearing a 1,3,4-Oxadiazole Core as Multipurpose Agricultural Chemicals against Plant Fungal, Oomycete, and Bacterial Diseases. J. Agric. Food. Chem.. 2021;69(30):8380-8393.
- [Google Scholar]
- Small Changes Result in Large Differences: Discovery of (-)-Incrustoporin Derivatives as Novel Antiviral and Antifungal Agents. J. Agric. Food. Chem.. 2014;62(35):8799-8807.
- [Google Scholar]
- Detection and Characterization of Qol-Resistant Phytophthora capsici Causing Pepper Phytophthora Blight in China. Plant Dis.. 2018;102(9):1725-1732.
- [Google Scholar]
- Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Physiol.. 2021;178:104942
- [Google Scholar]
- Chemical composition and antibacterial activity of essential oil of Ocimum ciliatum, as a new source of methyl chavicol, against ten phytopathogens. Ind. Crops Prod.. 2014;59:144-148.
- [Google Scholar]
- Long-term effects of the fungicide pyrimethanil on aquatic primary producers in macrophyte-dominated outdoor mesocosms in two European ecoregions. Sci. Total Environ.. 2019;665:982-994.
- [Google Scholar]
- Antimicrobial Pyridazines: Synthesis, Characterization, Cytotoxicity, Promiscuity, and Molecular Docking. Chem. Biodivers.. 2020;17:6.
- [Google Scholar]
- Antibacterial and Antiviral Activities of 1,3,4-Oxadiazole Thioether 4H-Chromen-4-one Derivatives. J. Agric. Food. Chem.. 2021;69(67):11085-11094.
- [Google Scholar]
- Priming with salicylic acid induces defense against bacterial blight disease by modulating rice plant photosystem II and antioxidant enzymes activity. Physiol. Mol. Plant Pathol.. 2019;108:101427.
- [Google Scholar]
- Trichoderma/pathogen/plant interaction in pre-harvest food security. Fungal Biol.. 2019;123:565-583.
- [Google Scholar]
- Synthesis, bioactivity and preliminary mechanism of action of novel trifluoromethyl pyrimidine derivatives. Arabian J. Chem.. 2022;15(9):104110
- [Google Scholar]
- Toad Alkaloid for Pesticide Discovery: Dehydrobufotenine Derivatives as Novel Agents against Plant Virus and Fungi. J. Agric. Food. Chem.. 2021;69(34):9754-9763.
- [Google Scholar]
- Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem.. 2009;45(2):745-751.
- [Google Scholar]
- Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol.. 2007;8(5):561-580.
- [Google Scholar]
- Synthesis, Antibacterial Activity, and Mechanisms of Novel 6-Sulfonyl-1,2,4-triazolo 3,4-b 1,3,4 thiadiazole Derivatives. J. Agric. Food. Chem.. 2021;69(16):4645-4654.
- [Google Scholar]
- Novel 1,3,4-Oxadiazole-2-carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. J. Agric. Food. Chem.. 2019;67(50):13892-13903.
- [Google Scholar]
- Synthesis and Study on Insecticidal Activity of New Heterocyclic Chalcone Derivatives. Chin. J. Org. Chem.. 2018;38(7):1763-1771.
- [Google Scholar]
- Antifungal Activity of Compounds Extracted from Cortex Pseudolaricis against Colletotrichum gloeosporioides. J. Agric. Food. Chem.. 2014;62(21):4905-4910.
- [Google Scholar]
- Design, synthesis, fungicidal property and QSAR studies of novel-carbolines containing urea, benzoylthiourea and benzoylurea for the control of rice sheath blight. Pest. Manage. Sci.. 2018;74(7):1736-1746.
- [Google Scholar]
- Design, Synthesis, and Antifungal Activity of Novel Chalcone Derivatives Containing a Piperazine Fragment. J. Agric. Food. Chem.. 2022;70(4):1023-1036.
- [Google Scholar]
- Design, Synthesis, and Bioactivity Evaluation of Novel 3-(Substituted Phenoxy)-6-Methyl-4-(3-Trifluoromethylphenyl) Pyridazine Derivatives. J. Heterocycl. Chem.. 2017;54(1):670-676.
- [Google Scholar]
- Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol.. 2019;53(7):3347-3365.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104852.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







