Translate this page into:
Green solvent selection and extractin protocol for selective recovery of anti-diabetic components from T. crispa
⁎Corresponding author. Artiwan.Sh@chula.ac.th (Artiwan Shotipruk)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
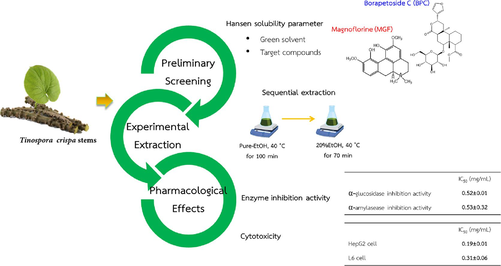
This study systematically explores a green extraction process of T. crispa stems to selectively isolate therapeutic compounds, borapetoside C (BPC) and magnoflorine (MGF), recognized for their anti-diabetic properties. In agreement with Hansen solubility parameters prediction, H2O and EtOH were found to be suitable solvents for extraction of BPC and MGF, respectively, resulting in 33 % and 45 % extractabilities after 60 min. Further investigations demonstrated considerable increase in BPC extractability using 20 % EtOH:H2O mixture at 40 °C, with complete extraction achieved after 60 min. While for MGF, pure-EtOH at 40 °C, was the most suitable solvent, showing the highest selectivity and complete extraction after 100 min. The BPC-rich extract from 20 % EtOH:H2O exhibited higher anti-diabatic activity (IC50 = 0.63 ± 0.03 and 0.72 ± 0.04 mg/mL, respectively, for α-glucosidase and α-amylase enzymes inhibition activity), and considerably lower cytotoxicity to L6 and HepG2 cells, with IC50 = 0.26 ± 0.16 and 0.24 ± 0.02 mg/mL, respectively, compared with the MGF-rich extract obtained with pure-EtOH. Based on these results, a sequential extraction scheme was proposed involving initial pure-EtOH extraction to selectively and completely remove MGF, followed by extraction with a 20 % EtOH:H2O mixture to recover the remaining BPC, which was approximately 80 % of the BPC originally present. The obtained MGF-free BPC-rich extract showed significantly lower cytotoxicity (IC50 = 0.31 ± 0.06 mg/mL against L6 cell) and higher enzyme inhibition activities (IC50 = 0.53 ± 0.32 and 0.52 ± 0.02 mg/mL for α-glucosidase and α-glucosidase enzymes inhibition activity), comparable to acarbose (IC50 = 0.43 ± 0.02 and 0.83 ± 0.03 mg/mL for α-glucosidase and α-glucosidase enzymes inhibition activity), the result that potentially leads to the development of a promising industrial process to harness T. crispa for diabetes prevention and treatment.
Keywords
Borapetoside C
Magnoflorine
Anti-diabetic
Hansen solubility parameters
Extraction
1 Introduction
Diabetes is one of the most serious chronic diseases, which currently interferes the lives and wellbeing of more than 642 million people worldwide, and causes 1.5 million deaths each year, making it one of the top ten causes of mortality (WHO, 2020). With exponentially increasing numbers of affected individuals, diabetes has become a global crisis and its impact on the health of the future generations is far reaching (Silva et al., 2018). As a result, global market for diabetic medications is expected to also be continuously growing, with a predicted rate of 7.8 % by 2027. Of these, anti-diabetic medications derived from natural resources are gaining increasing importance due to growing customer awareness of detrimental side effects of many of the chemical-based ingredients (Ekor, 2014; Cvetanović et al., 2018; Wang et al., 2022).
Borapetoside C (BPC, Fig. 1(a)), a diterpenoid glycosides found as the main active constituent in Tinospora crispa (T. crispa) stems, has been demonstrated to possess excellent anti-diabetic activity through its inhibition of the α-glucosidase and α-amylase enzymes, that regulate postprandial blood glucose levels (Lam et al., 2012; Ruan et al., 2012). In addition, BPC has also been shown to be the most efficient α-glucosidase inhibitor (exhibiting the lowest IC50 value) when compared to the other potential anti-diabetic natural products (Kanetkar et al., 2007; Wang et al., 2014; Hamid et al., 2015). Another important compound found also in the stems of T. crispa, a polycyclic aporphine alkaloid, namely MGF (Fig. 1(b)), is as well known for its ability to exhibit inhibition of α-glucosidase (Xu et al., 2020), and has been used to treat diabetes (Patel and Mishra, 2012; Cherku, 2019; Ahmad et al., 2022). The mechanism of action on the anti-diabetic property of BPC and MGF was illustrated in a recent study by Khanal et al. (2019). In addition to anti-diabetic property, MGF inhibits proliferation of various cancer cells such as lung, breast and liver by suppressing the expression and secretion of vascular endothelial growth factor (VEGE) (Patel and Mishra, 2012; Okon et al., 2020). This VEGF inhibition activity is advantageous for the development of the anti-cancer drugs, it nevertheless causes adverse toxic effects to normal cells, including bone marrow, bone and myoblast cells (Germani et al., 2003; Arsic et al., 2004). As a result, for effective anti-diabetic treatment, it is preferable that BPC and MGF be selectively and separately extracted from T. crsipa stems to minimize the toxicity of the resulting extract, while maintaining its ability to inhibit α-glucosidase and α-amylase enzymes.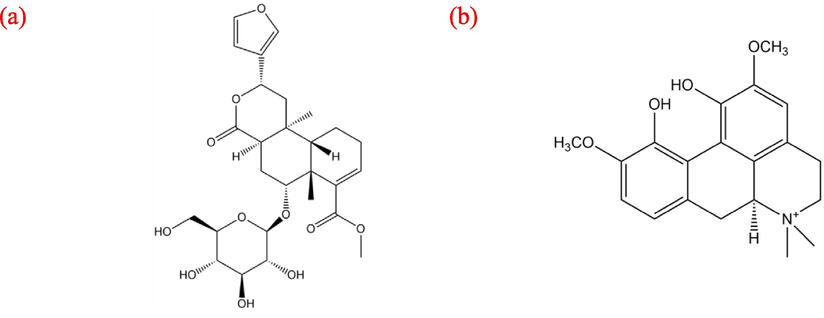
Molecular structures of (a) BPC and (b) MGF.
In previous studies, various solvents such as H2O, methanol (MeOH), ethanol (EtOH), ethyl acetate (EtOAc), acetone and dichloromethane (DCM), as well as mixtures of H2O and EtOH or MeOH have been employed to obtain T. crispa stem extracts, containing active compounds including BPC and MGF (Martin et al., 1996; Lam et al., 2012; Adnan et al., 2018; Ahmad et al., 2022; Zhu et al., 2023; Zuhri et al., 2024). In most of these studies, emphasis was placed on analytically identifying active components and pharmacological effects (Ahmad et al., 2018). To the authors’ knowledge, there have been no research studies that concern the systematic determination of extraction solvents and conditions that yield the highest efficiencies for selective extraction of BPC and MGF from T. crispa, particularly with an interest on the anti-diabetic activity and cytotoxicity of the extract obtained.
In terms of extraction efficiency of a solvent for a target compound, the solute–solvent solubilization properties play a crucial role (Soh and Eckelman, 2016; Milescu et al., 2020). Hansen solubility parameters (HSPs), developed as an extension from the Hilderbran solubility parameter to predict the miscibility of two species, by considering their intermolecular interactions, has been extensively used as a powerful tool for the estimation of solvent compatibility for dissolution of a target solute, such as a polymer (Sambrailo and Kunst, 1987; Bacon et al., 2014; Poleo and Daugulis, 2014) or a biological material such as DNA or protein (Ursin et al., 1995). The HSP theory has moreover gained the popularity as seen in an increasing number of research publications (Park et al., 2010; Hong et al., 2015; Laboukhi-Khorsi et al., 2017; Daisuk and Shotipruk, 2020; Milescu et al., 2020; Chen et al., 2023; Kanda et al., 2024), as a simple yet powerful tool for preliminary evaluation of solvents for extraction of natural products. By using this theory, it is possible to minimize time and resource consuming screening experiments that lead to the generation of large amount of waste solvents.
The aim of this study was to develop an efficient and selective process for extraction of BPC and MGF from T.crispa stems. Firstly, by applying the HSPs theory, the solubility of BPC and MGF in different solvents were compared, and suitable extraction solvents for these compounds were selected. Secondly, the possibility to further enhance the extractability of BPC and MGF from T. crispa stems by mixing two selected solvents was experimentally evaluated. In so doing, the effects of solvent mixture composition, extraction temperature and time on the extractability and selectivity for the two compounds were determined. Thirdly, the α-glucosidase and α-amylase inhibition activities and cytotoxicity of the extracts obtained with selected solvents at the most suitable extraction conditions were determined and were compared with those of the BPC standard (BPC-STD) and MGF standard (MGF-STD), and with a commercial diabetic drug (acarbose), as positive controls. Lastly, sequential extraction with two optimum extraction conditions for BPC and MGF was demonstrated as a process scheme to obtain an extract with relatively high anti-diabetic activity and considerably reduced cytotoxicity without the need for complicated purification steps.
2 Materials and methods
2.1 Plant materials, chemical and reagents
The stems of T. crispa were purchased from herbal drug store (Vejpongosot pharmacy Co., Ltd, Bangkok, Thailand), and identified by one of the authors (C.C.). A voucher specimen (CC-TC-0720) was deposited at the herbarium of the Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Thailand. The stems were oven-dried (50 °C, 72 h) before being cut into small pieces and processed with a hammer mill to yield a uniform powder (granule diameter 2 mm). The powder was kept in a sealed container at 25 °C and protected from light and moisture until further use. The sample moisture content was measured to be 5.2–6.0 % over the course of this study.
Extraction solvents: MeOH (purity 99 %), EtOH (purity 99 %), EtOAc (purity 99 %), DCM (purity 99 %), and acetone (purity 99 %) were purchased from Carlo Erba reagents. Co., Ltd. Barcelona, Spain. All organic extraction solvents were of analytical reagent (AR) grade, while those used for chromatographic analysis were of HPLC grade. α-glucosidase (from Saccharomyces cerevisiae), α-amylase (from porcine pancreas), p-nitrophenyl α-D glucopyranoside (pNPG), red starch, MGF-STD and acarbose were purchased from Sigma Aldrich Co., Ltd. St. Louis, MO, USA. Dulbecco’s Modified Eagle’s Medium (DMEM), MTT [3-(4, 5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide], L-glutamine, penicillin G sodium salt and streptomycin sulfate were obtained from GIBCO Invitrogen (NY, USA). Fetal bovine serum (FBS) was purchased from Biochrom AG (Berlin, Germany). BPC used as a BPC-STD was extracted and purified (96 % purity, Fig. S1) at our laboratory. The chemical structure of the obtained BPC-STD was confirmed by 1H NMR (Fig. S2 and Table S1 and), 13C NMR (Fig. S3 and Table. S2), and LC-MS (Fig. S4).
2.2 Hansen solubility parameter (HSP) theory for prediction of solute–solvent solubility
2.2.1 HSP theory
The HSPs were developed based on the concept that the total cohesive energy density (Et⁄V) comprising three individual energy densities required to overcome the dispersion force, δd2; the polar force, δp2; and the hydrogen bonds between molecules, δh2 (Hansen, 2007), as given by the following equations:
2.2.2 Determination of HSPs for BPC and MGF
As the HSP values for BPC and MGF are not available in literature, the estimation of their HSPs values was achieved by employing the Stefanis & Panayiotou group contribution method, which has been shown to be suitable for natural products containing complicated multi-ring, heterocyclic, and aromatic structures (Stefanis and Panayiotou, 2008), described as follows:
2.2.3 Evaluation of solute–solvent solubility
The solubility of a solute in a solvent can be evaluated based on the deviation between the HSPs of the solvent (A) and the solute (B), the so-called Ra value, defined as follows:
2.3 Experimental T. crispa stems extraction
2.3.1 Extraction procedure
To evaluate the predictability of HSP theory in suggesting suitable solvents for extraction of BPC and MGF, T. crispa stems powder samples (1.0 g) were extracted at the solid to solvent ratio of 1:10 (w/v), 25 °C for 60 min with various pure solvents (H2O, EtOH, MeOH, EtOAc, DCM and acetone) or selected solvent mixtures. The most suitable solvents or solvent mixtures were then selected for subsequent studies based on the experimental extractability and selectivity of the solvents for BPC and MGF. Furthermore, with the selected solvents, the most suitable extraction conditions were determined by varying extraction temperature (25, 40 and 60 °C) and time (between 0 and 100 min). For each experiment, the solid to solvent ratio was fixed at 1:10 (w/v), which was preliminary found to be the most suitable among the various ratios between 1:5 to 1:15 (w/v) studied. The extraction temperature was controlled by a hot plate (IKA, Staufen, Germany). Following a specified extraction time, the extract was then separated from the T. crispa stems residue by filtering (cheesecloth followed by a Whatman No.1 filter paper). The clarified extract was then evaporated using a rotary vacuum evaporator (Rotavapor® R-100, Büchi, Switzerland). The dried extract was then kept at 4 °C until the HPLC analysis for the BPC and MGF contents. The experiments were performed in triplicate and the average extractability and selectivity of the solvents for BPC and MGF were determined based on Eqs. (7–9):
As a control experiment, reflux extraction was conducted in a round-bottom flask connected to a reflux condenser. Extraction was performed using boiling H2O or boiling EtOH as solvent to extract BPC and MGF, respectively. Extraction was also carried out repeatedly in 5 cycles (8 h/ each cycle) to completely extract BPC and MGF. The total amount extracted was used as a basis for the calculation of the extractability.
2.4 Quantification of BPC and MGF in crude T. crispa extracts
Quantification of BPC and MGF in the extracts were conducted using reverse phase UHPLC (Waters, Milford, MA, USA), consisting of a quaternary pump, equipped with a 2998 photodiode array detector. The analysis was performed according to a previous protocol (Cachet et al., 2018), with some modifications. Briefly, samples of the extracts dissolved in MeOH (20 µL at 1 mg/mL) were injected into a Poroshell 120 column (2.1 mm × 100 mm, 2.7 µm, Agilent Technologies (CA, USA)). The mobile phases comprised 0.05 % trifluoroacetic acid (TFA) in deionized water (eluent A) and MeOH (eluent B). The elution gradient was from 90(A):10(B) to 20(A):80(B) at a flow rate of 0.6 mL/min for 60 min. The UV detection of BPC and MGF were carried out at 214 and 320 nm, and the corresponding absorption spectra were compared to those of the BPC-STD (Fig. S5) and MGF-STD (Fig. S6) respectively. The chromatographic separation pattern was analyzed using Empower Pro 2 Software (Waters, Milford, MA, USA).
The content of BPC and MGF in the extracts were calculated by a BPC-STD and MGF-STD standard curve performed with different concentrations. Calibration curves were prepared using BPC-STD and MGF-STD. BPC-STD (3.0 mg) was dissolved in 1.0 mL of MeOH to yield a stock solution of 3.0 mg/mL, which was diluted to give 3.0, 2.5, 2.0, 1.5, 1.0 0.5, and 0.2 mg/mL solutions. For MGF-STD, 0.8 mg of MGF-STD was dissolved in 1.0 mL of MeOH, yielding a stock solution of 0.8 mg/mL, which was then diluted to give 0.4, 0.2, 0.1, 0.05, 0.025 and 0.0125 mg/mL solutions. Standard solutions were injected into the UHPLC to give the calibration curves for BPC (slope 13104, intercept −489.89, R2 0.9988) and MGF (slope 4743.5, intercept −316.38, R2 0.9995).
2.5 In vitro enzyme inhibitory activity assays
2.5.1 α-glucosidase inhibition
The inhibitory activity of α-glucosidase was evaluated using a method previously described (Hamid et al., 2015), with some modifications. Initially, 10 mg of the extracts were dissolved and diluted in 20 mM phosphate buffer at pH 6.9 containing 6.7 mM NaCl, resulting in concentration ranging from 0.1 to 10 mg/mL. Subsequently, 20 µL of each concentration was transferred to a well plate. In each well, 10 µL of 0.1 U/mL α-glucosidase solutions and 20 µL of 0.5 mM pNPG dissolved in 0.1 M phosphate buffer (pH 6.9) were added (total reaction mixture volume = 50 µL), which was then incubated at 37 °C for 20 min. The enzymatic reaction was stopped by the addition of sodium carbonate solution (7.5 %, 150 µL), and the released substrate (p-nitrophenol) was then measured at 400 nm using a microplate reader. Controls were carried out in the same manner, but with only 0.1 M phosphate buffer (pH 6.9) instead of plant extracts. The inhibitory activity of α-glucosidase was assessed by the following equation:
2.5.2 α-amylase inhibition
Inhibition of α-amylase activity was determined using a previously described method (Forester et al., 2012). Briefly, 20 µL of sample crude extracts at various concentrations, ranging from 0.1 to 10 mg/mL, dissolved in 20 mM phosphate buffer pH 6.9 containing 6.7 mM NaCl were added into a well plate. To each well, 20 µL of 0.3 U/mL α-amylase solution and 20 µL of red starch solution (7 mg/mL) were added (total reaction mixture volume = 60 µL), and the reaction mixture was incubated 37 °C for 10 min. The reaction was stopped by the addition of 140 µL of 95 % EtOH solution, and the absorbance was then measured at 540 nm using a microplate reader. Controls were carried out in the same manner, but with 20 mM phosphate buffer pH 6.9 instead of the plant extracts. The inhibitory activity of α-amylase was also assessed according to Eq. (10).
2.6 Cytotoxicity assay
The viabilities of myoblast cells isolated from the skeletal muscle of a rat (L6; ATCC; CRL1458) and hepatocellular carcinoma (HepG2; ATCC HB-8065) cells were evaluated using a method previously described (Suktham et al., 2018). Briefly, the cells were seeded at a density of 1 × 104 cells/well with a complete DMEM medium containing 10 % FBS, 100 µg/mL L-glutamine, 100 µg/mL streptomycin and 100 µg/mL penicillin. The cells were incubated in a 37 °C incubator containing 5 % CO2 until confluence was reached (24 h). The extracts at various concentrations, ranging from 0.015 to 2.0 mg/mL, diluted in 100 µL of DMEM media without FBS were added into the wells, which were further incubated at 37 °C for 24 h. The treated cells were then washed with 100 µL PBS pH 7.4 before being incubated with 100 µL DMEM media containing 20 µL of 5 mg/mL MTT for 4 h, after which, the media and MTT were removed. The remaining contents in the well were solubilized with DMSO, and the absorbance of the solutions was measured at 550 nm using a microplate reader. The cell viability (%) was calculated based on Eq. (11).
For the comparison of both enzymes inhibitory and cytotoxic activities of the various T. crispa extracts, BPC-STD, MGF-STD and acarbose, a diabetes commercial drug, were used as benchmarks and/or positive controls. The results were expressed as half maximum inhibitory concentrations (IC50), the concentration of the crude extract required for 50 % inhibition of enzyme activity or cell viability. All experiments were carried out in triplicate; data presented are the mean ± one standard deviation (SD).
2.7 Statistical analysis
The significance of differences between the means of various data groups was evaluated using one-way analysis of variance (ANOVA) and posthoc Turkey’s test using the IBM SPSS statistical software. Significance was accepted at the p < 0.05 level.
3 Results and discussion
3.1 HSPs prediction of BPC and MGF solubility in various solvents
The literature HSPs values at 25 °C of commonly used pure solvents (Hansen, 2007) and those of BPC and MGF, calculated using group contribution theory (Stefanis and Panayiotou, 2008), are summarized in Table 1. Detailed calculations are presented in Table S3-4. It can be noted that δh is the dominant parameter for BPC due to the presence of four sugar –OH groups in each molecule (Fig. 1(a)). On the other hand, the contributions to the three interactions were more evenly distributed for MGF. Specifically, these are two –OH and two –OCH3 groups contributing to hydrogen bonding, dimethylated nitrogen and consequent quaternery ammonium cation that facilitate polar interactions, and two –CH3 groups that contribute to dispersion (Fig. 1(b)).
Solvents
HSPs parameters (MPa1/2)
BPC
MGF
δd
δp
δn
Ra (MPa1/2)
Ra (MPa1/2)
H2O
15.5
16.0
42.3
5.6
25.6
MeOH
14.7
12.3
22.3
17.7
5.4
EtOH
15.8
8.8
19.4
20.7
3.4
EtOAc
15.8
5.3
7.2
33.2
11.5
Acetone
15.5
10.4
7.0
32.9
10.5
DCM
17.8
3.1
5.7
33.3
14.9
BPC
15.1
10.9
39.9
MGF
14.7
10.2
17.3
The Ra values in Table 1 calculated from Eq. 6 suggest that BPC is the most soluble in H2O (having the lowest Ra = 5.6 MPa1/2), followed by MeOH and then EtOH, acetone, EtOAc and DCM. It is noted that since δh is the dominant solubility parameter for BPC, H2O, which has the closest δh to that of BPC (42.3 vs. 39.9 MPa1/2), is therefore the most compatible “green” solvent for BPC for that reason. Acetone, EtOAc and DCM, having the highest Ra values for BPC (Ra approximately 33 MPa1/2), have considerably lower δh (δh 5.7–7.2 MPa1/2), implying that they would be poor solvent matches for the compound. For MGF, EtOH (having the lowest Ra = 3.4 MPa1/2) appears most soluble, due to the closet of δh (19.4 vs 17.3 MPa1/2) to that of MGF followed by MeOH. Acetone, EtOAc, DCM and H2O, which have considerably different δp and δh values from MGF, have relatively high Ra values, which render them poor solvents for the compound.
3.2 Experimental evaluation of HSPs prediction
T. crispa stems extraction experiments were carried out with various solvents at 25 °C for 60 min, whose results are shown in Fig. 2. As seen from Fig. 2(a), the highest extractability of BPC (33 %) was achieved using H2O (0.91 mg extracted out of a total of 2.71 mg BPC in a 1.0 g sample). This extraction rate exceeded that of MeOH, in agreement with the HSP prediction. When extracting MGF at 25 °C, MeOH displayed the highest extractability (52 %) after 60 min (0.21 mg out of the total 0.37 mg of MGF in 1.0 g sample), followed by EtOH (45 %), despite MeOH having a slightly larger Ra value when compared to EtOH (5.4 vs 3.4 MPa1/2). The deviation of experimental results from HSP predictions are possible, as theoretical Ra value were determined based on the solute–solvent pair, while in actual extraction, other compounds that are present could influence the solubility and extractability of the interested compound. Nevertheless, the statistical analysis of our results showed no significant difference between MGF extractability obtained with MeOH and EtOH (Fig. 2(a)). The next smallest Ra values suggested by the HSPs theory were those of acetone (10.5 MPa1/2), EtOAc (11.5 MPa1/2) and DCM (14.9 MPa1/2), and the results agreeably showed lower MGF extractability for all these solvents.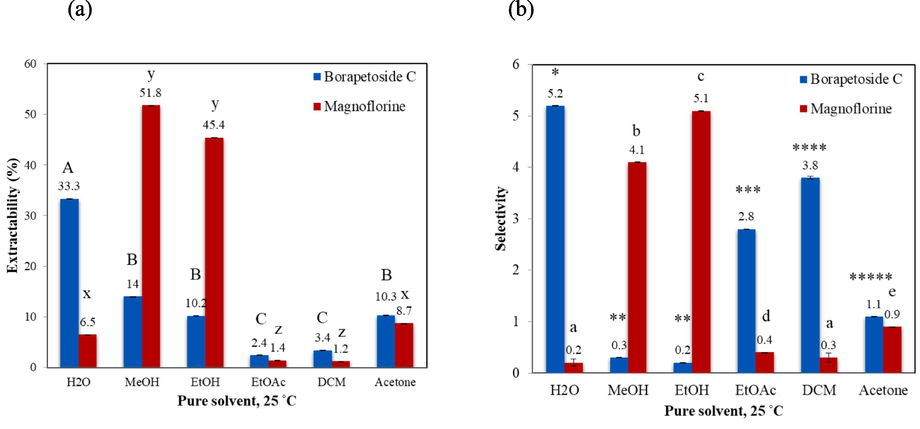
(a) Extractability (%) and (b) selectivity of BPC and MGF by pure solvents at 25 °C for 60 min. Data are presented as means ± SD (n = 3). Different letters and symbols indicate significant differences between samples within the same category at the level of p < 0.05.
In relation to selectivity, Fig. 2(b) demonstrated that H2O showed the highest BPC selectivity, as H2O highly selectively extracted BPC, compared to MGF. For MGF, EtOH provided the highest MGF selectivity followed by MeOH. It was observed that the highest MGF extractability and selectivity were obtained with MeOH and EtOH, respectively, as these solvents were predicted to have the highest solvency for MGF with relatively low Ra values when compared to other solvents (5.4 and 3.4, respectively vs. 11.5–25.6 MPa1/2). Nevertheless, since MeOH raises safety and health concerns owing to its acute oral toxicity, EtOH was deemed preferable for extracting MGF. According to these results, H2O and EtOH were selected as suitable solvents for BPC and MGF, respectively.
3.3 Determination of suitable conditions for selective extraction of BPC and MGF
In this study, the effect of different factors including EtOH:H2O solvent mixture composition, extraction temperature and time were evaluated to further enhance the extraction efficiency for BPC and MGF, respectively.
3.3.1 Effect of composition of EtOH:H2O solvent mixture
The extraction results with EtOH:H2O solvent mixtures at various compositions shown in Fig. 3(a) revealed that mixing EtOH into H2O in the range of 10 up to 60 % (v/v) could enhance the BPC extractability when compared to pure-H2O. Beyond this point, the BPC content gradually decreased and showed the lowest BPC extractability using pure-EtOH. The enhanced extractability could be a result of a complex interaction between the EtOH and H2O molecules, particularly the hydrogen bonding formations between the molecules (Güçlü-Üstündağ and Temelli, 2005), that helps enhance the solubility of BPC in their mixtures. Our results correspond to a previous work that reported improved extraction of triterpene glycosides: madecassoside and asiaticoside, using solvent mixtures of EtOH in H2O in the range of 15–50 % (v/v) (Yingngam et al., 2020). The highest extractability for BPC (87 %), a 2.6 folds increase from the pure-H2O extraction, was obtained with 20 % EtOH:H2O mixture. For MGF, the use of EtOH and H2O mixtures in the range of 70–90 % (v/v) significantly enhanced the MGF extractability by 59 to 70 % when compared to pure-EtOH (45 %). The highest MGF extractability (70 %) was achieved with 90 % EtOH:H2O solvent mixture (Fig. 3(b)).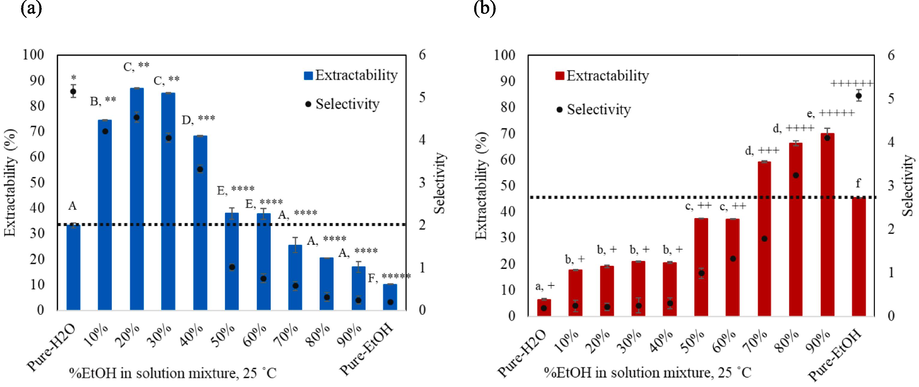
Experimental extractability (%) and selectivity of (a) BPC and (b) MGF by EtOH: H2O mixtures of various compositions at 25 °C and 60 min. The data is presented as the mean ± SD (n = 3). Different letters and symbols indicate significant differences in extractability and selectivity, respectively at the level of p < 0.05.
In terms of selectivity for BPC (Fig. 3(a)), the BPC selectivity tends to decrease with increasing EtOH content to the lowest selectivity of 0.19 with pure-EtOH. Nevertheless, for the solvent mixtures with EtOH up to 30 %, the BPC selectivity remained relatively high (4–4.5). On the other hand, as seen in Fig. 3(b), the MGF selectivity favored the solvent mixtures with high EtOH content, the highest selectivity (5.1) observed for pure-EtOH extraction.
From the results herein, it could be summarized that 20 % EtOH:H2O mixture provided the highest BPC extractability with slight drop in the selectivity, while for MGF, the highest extractability was observed with 90 % EtOH:H2O mixture, and the highest selectivity with pure-EtOH. These solvents were therefore selected for further investigation to enhance extraction efficiency by determining the effect of extraction temperature and time.
3.3.2 Effect of extraction temperature
The effects of extraction temperature on BPC extractability and selectivity were evaluated with 20 % EtOH:H2O mixture as shown in Fig. 4(a). For MGF, experiments were carried out with 2 different solvent ratios which are 90 % EtOH:H2O solvent mixture (Fig. 4(b)) and pure-EtOH (Fig. 4(c)). As seen in Fig. 4(a) for extraction with 20 % EtOH:H2O mixture, BPC extractability increased with increasing temperature, reaching maximum at 40 °C where BPC could be completely extracted after 60 min. The BPC selectivity, however, decreased as the temperature increased for extraction with 20 % EtOH:H2O mixture (Fig. 4(a)) since the solvent extracted increased amount of MGF (Fig. S7(a)).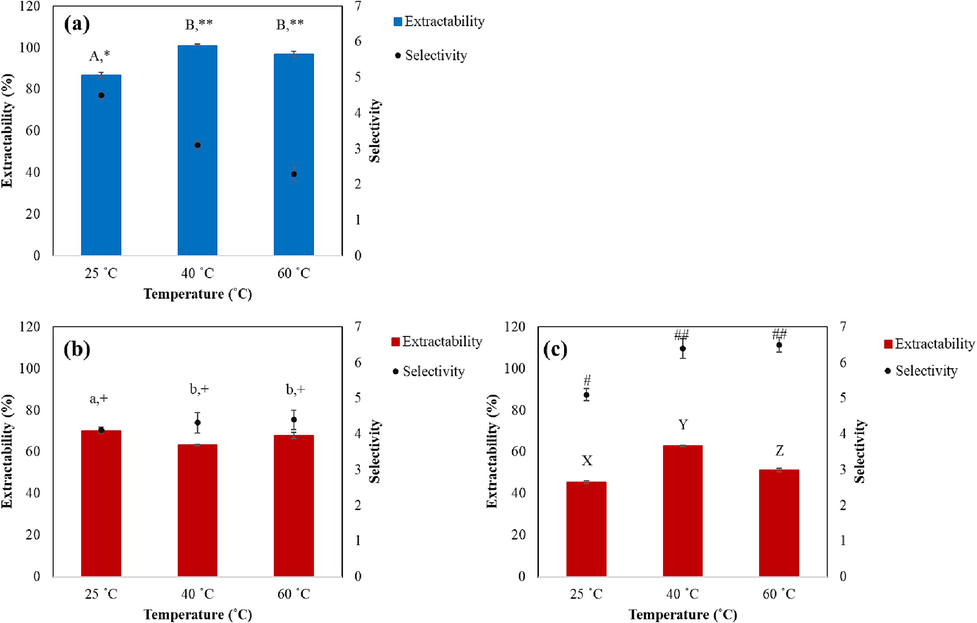
Effects of extraction temperature on the extractability (%) and selectivity of BPC using (a) 20 % EtOH:H2O and MGF using (b) 90 % EtOH:H2O and (c) pure-EtOH for 60 min. Data are presented as the mean ± SD (n = 3). Different letters and symbols indicate significant differences between samples within the same category at the level of p < 0.05.
For MGF, the extractability and selectivity do not change considerably when using 90 % EtOH:H2O solvent mixture within the temperature range of 25 to 60 °C (Fig. 4(b)). However, with pure-EtOH, the change in temperature affected MGF extractability and selectivity to significant levels, particularly for temperature increase from 25 to 40 °C (Fig. 4(c)). Although at 40 °C, MGF extractability was lower with pure-EtOH than with 90 % EtOH:H2O mixture, at 25 °C, the MGF extractability was comparable to that achieved with 90 % EtOH: H2O. On the other hand, the MGF selectivity increased significantly from 5.1 at 25 °C to 6.4 at 40 °C. The reason for this is that pure-EtOH is highly favorable for MGF, but less favorable for BPC (Fig. S7(c)). As temperature increases, EtOH becomes even less polar, improving the solubility for MGF, while decreasing the solubility for BPC, thus MGF selectivity increased.
In the subsequent study, further investigation was carried out with 20 % EtOH:H2O solvent mixture at 40 °C (giving the highest extractability, and relatively high selectivity for BPC) and pure-EtOH at 40 °C (giving the highest MGF selectivity, and relatively high extractability) to determine the suitable extraction time.
3.3.3 Effects of extraction time
As shown in Fig. 5(a), the time profiles of T. crispa stems extraction suggest that, with 20 % EtOH:H2O solvent mixture at 40 °C, BPC was completely extracted within 60 min, together with a small of MGF (22 % of the MGF in the original sample). With pure-EtOH at 40 °C on the other hand (Fig. 5(b)), MGF extraction was favored and could be completely extracted after 100 min. Along with it, approximately 20 % of BPC originally present in the sample was co-extracted.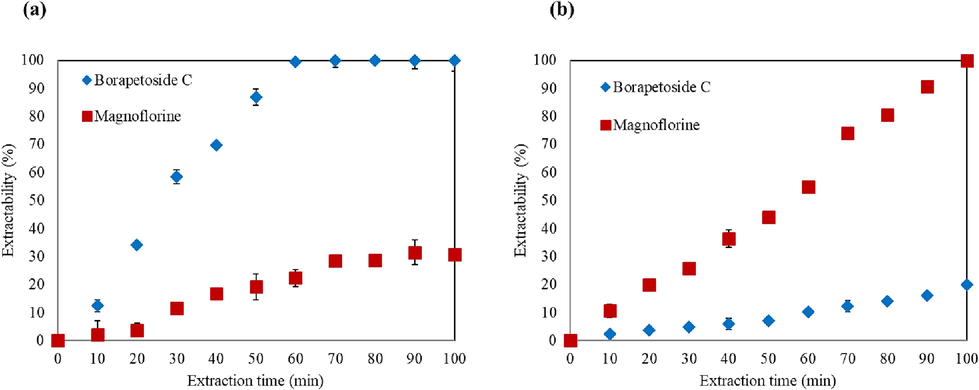
Effect of extraction time at 40 °C using: (a) 20 % EtOH:H2O solvent mixture, (b) pure-EtOH at 40 °C. Data are presented as the mean ± SD (n = 3).
In view of extracting T. crispa stems to enhance anti-diabatic effects, while minimizing the toxicity of the extract, a sequential extraction scheme involving initially removing MGF, and subsequently extracting the remaining BPC under an optimized conditions can be implemented. To illustrate, an experiment was carried out in which MGF-rich extract is first obtained by extraction with pure-EtOH at 40 °C for 100 min, followed by extraction with 20 % EtOH:H2O solvent mixture at 40 °C for additional 70 min. As shown in Fig. 6, MGF was completely extracted after 100 min with pure-EtOH at 40 °C, together with approximately 20 % of BPC originally present in the T. crispa stems sample (0.54 mg/g DW). Without MGF contamination, the remaining 80 % of BPC was subsequently extracted with 20 % EtOH:H2O solvent mixture at 40 °C within the next 40 min.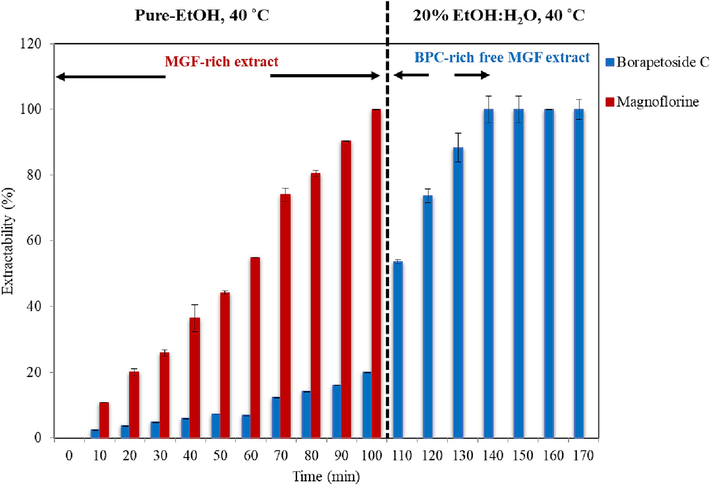
Sequential extraction of BPC and MGF. The stems powder was initially extracted with pure-EtOH at 40 °C for 100 min before being extracted with 20 % EtOH:H2O solvent mixture at 40 °C for another 70 min. The data is presented as the mean ± SD (n = 3).
3.4 In vitro α-glucosidase and α-amylase inhibitory activities
The α-glucosidase and α-amylase inhibitory activities of the extracts obtained with various solvents were compared to those of BPC-STD, MGF-STD, and anti-diabatic drug, acarbose. As shown in Table 2, comparing between the BPC and MGF-STDs, BPC-STD exhibited considerably higher activities than that observed for MGF-STD (low IC50 values for both α-amylase and α-glucosidase inhibition assays). The enzyme inhibition activities of BPC were found to be comparable to those of acarbose, the commercial anti-diabatic drug. On the other hand, MGF-STD exhibited lower inhibition activities for both enzymes than those of BPC-STD and acarbose. As expected, the extract obtained with 20 % EtOH:H2O solvent mixture had higher BPC content (lower MGF content), its enzymatic inhibition activity for both enzymes was higher than that obtained with pure-EtOH (which had high MGF level). The results for sequential extraction showed that the BPC-rich fraction obtained during the latter 40 min period following MGF removal yielded higher inhibition activities to both enzymes than those extracts obtained with either pure-EtOH or 20 % EtOH:H2O solvent mixture. The anti-diabatic activities of this extract were comparable to those of BPC-STD. Data are presented as the mean ± SD (n = 3). Different letters and symbols indicate significant differences between samples within the same category (column) at the level of p < 0.05.
Sample
IC50 (mg/mL)
Enzyme inhibitory activity
Cytotoxicity
α-amylase
α-glucosidase
L6
HepG2
Crude extracts 20 % EtOH:H2O
0.72 ± 0.04*
0.63 ± 0.03a
0.26 ± 0.16X
0.24 ± 0.02A
Crude extracts Pure-EtOH
0.83 ± 0.22**
1.10 ± 0.27b
0.08 ± 0.04Y
0.06 ± 0.01B
Crude extracts from sequential stage 2
0.53 ± 0.32***
0.52 ± 0.01c
0.31 ± 0.06X
0.19 ± 0.01A
Acarbose
0.43 ± 0.02****
0.83 ± 0.03d
0.32 ± 0.02X
0.46 ± 0.07C
BPC-STD
0.53 ± 0.04***
0.47 ± 0.03c
0.32 ± 0.07X
0.18 ± 0.02A
MGF-STD
0.68 ± 0.05*
0.95 ± 0.02b
0.15 ± 0.03Y
0.13 ± 0.07A
3.5 In vitro cytotoxic activity
The cytotoxicity of the extracts obtained with 20 % EtOH:H2O solvent mixture, pure-EtOH, and that from the sequential extraction were evaluated against L6 and HepG2 cell lines, using MTT assay (photographs shown in Fig. S8 and S9, respectively). The results in Table 2, comparing the BPC and MGF-STDs, indicated that MGF-STD not only showed lower enzyme inhibition activity but also exhibited higher toxicity than BPC (IC50 = 0.15 mg/mL vs 0.32 mg/mL against L6 cell and 0.13 mg/mL vs 0.18 mg/mL against HepG2 cell, respectively). This implied that the extract obtained with 20 % EtOH:H2O solvent mixture (containing high BPC content) would be less toxic than the extract obtained with pure-EtOH (containing high MGF content). As expected, the IC50 values for the cytotoxicity tests against L6 (0.26 mg/mL vs 0.08 mg/mL) and HepG2 (0.24 mg/mL vs 0.06 mg/mL) suggested that the extract with 20 % EtOH:H2O exhibited approximately 3.3–3.9-folds lower toxicity. Furthermore, by completely removing MGF in the first step of the sequential extraction, the final extract was found to have considerably reduced cytotoxicity, particularly reflected by the IC50 values for L6 cells that are comparable to those of BPC-STD and acarbose.
4 Conclusion
HSPs theory was shown to give reasonable prediction of solubility of BPC and MGF in various solvents and selected solvent mixtures. Comparing the pure solvents, the most suitable solvents for extraction of BPC and MGF were H2O and EtOH respectively. In this study, the effectiveness of further improving the extraction efficiency utilizing EtOH:H2O solvent mixtures was evaluated. Extraction with 20 % EtOH:H2O solvent mixture at 40 °C was found to be most suitable for BPC, while extraction with pure-EtOH at 40 °C was the most suitable for MGF. This result suggested therefore that MGF and BPC can be extracted sequentially first with pure-EtOH at 40 °C, then followed 20 % EtOH:H2O solvent mixture at 40 °C. Compared with the extract obtained with either pure-EtOH or 20 % EtOH:H2O solvent mixture, the MGF-free BPC-rich fraction obtained at later times from the sequential extraction exhibited considerably lower cytotoxicity comparable to those of BPC-STD, while the in vitro α-glucosidase and α-amylase inhibition activities were significantly increased. These findings provided useful information regarding the suitable extraction solvents and conditions, important for the future development of effective herbal anti-diabatic drug.
CRediT authorship contribution statement
Kunat Suktham: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. Chaisak Chansriniyom: Formal analysis, Methodology. Duangporn Polpanich: Formal analysis. Artiwan Shotipruk: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing.
Acknowledgement
The work was supported by the National Research Council of Thailand (NRCT) (grant numbers N42A650258). The first author also thanks the Royal Thai Government, Thailand for the Ph. D. scholarship (grant numbers 009/2562) and to Dr. Thomas Gale, University of Greenwich, UK, for proofreading.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory activity of tinocrisposide by inhibiting nitric oxide production in lipopolysaccharide-stimulated raw 264.7 cells. Asian J. Pharm. Clin. Res.. 2018;11:149.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of Tinospora crispa extract and its major compounds on the immune functions of RAW 264.7 macrophages. Int. Immunopharmacol.. 2018;60:141-151.
- [CrossRef] [Google Scholar]
- Magnoflorine from Tinospora crispa upregulates innate and adaptive immune responses in Balb/c mice. Int. Immunopharmacol.. 2022;11:109081
- [CrossRef] [Google Scholar]
- Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther.. 2004;10:844-854.
- [CrossRef] [Google Scholar]
- A framework to predict and experimentally evaluate polymer–solute thermodynamic affinity for two-phase partitioning bioreactor (TPPB) applications. J. Chem. Technol. Biotechnol.. 2014;89:948-956.
- [CrossRef] [Google Scholar]
- Clerodane furanoditerpenoids as the probable cause of toxic hepatitis induced by Tinospora crispa. Sci. Rep.. 2018;8:13520
- [CrossRef] [Google Scholar]
- Experimental analysis and thermodynamic modelling of Nitroxynil solubility in pure solvent and binary solvent. Arab. J. Chem.. 2023;16:104531
- [CrossRef] [Google Scholar]
- Inhibitory activity of leaf extract of Tinospora cordifolia and magnoflorine on aldose reductase for control of diabetes. Int. J. Green Pharm.. 2019;13
- [CrossRef] [Google Scholar]
- A new source for developing multi-functional products: biological and chemical perspectives on subcritical water extracts of Sambucus ebulus L. J. Chem. Technol. Biotechnol.. 2018;93:1097-1104.
- [CrossRef] [Google Scholar]
- Recovery of γ-oryzanol from rice bran oil soapstock derived calcium soap: consideration of Hansen solubility parameters and preferential extractability in various solvents. LWT.. 2020;134:110238
- [CrossRef] [Google Scholar]
- The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol.. 2014;4:177.
- [CrossRef] [Google Scholar]
- Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol. Nutr. Food Res.. 2012;56:1647-1654.
- [CrossRef] [Google Scholar]
- Vascular endothelial growth factor modulates skeletal myoblast function. Am. J. Pathol.. 2003;163:1417-1428.
- [CrossRef] [Google Scholar]
- Solubility behavior of ternary systems of lipids, cosolvents and supercritical carbon dioxide and processing aspects. J. Supercrit. Fluids.. 2005;36:1-15.
- [CrossRef] [Google Scholar]
- α-Glucosidase and α-amylase inhibitory constituents of Tinospora crispa: isolation and chemical profile confirmation by ultra-high performance liquid chromatography-quadrupole time-of-flight/mass spectrometry. J. Funct. Foods. 2015;16:74-80.
- [CrossRef] [Google Scholar]
- Hansen solubility parameters A user's handbook (2nd ed.). CRC Press Taylor & Francis Group; 2007.
- Extraction of natural dye from Gardenia and chromaticity analysis according to chi parameter. J Ind Eng Chem.. 2015;24:326-332.
- [CrossRef] [Google Scholar]
- Extraction of fucoxanthin, antioxidants and lipid from wet diatom Chaetoceros simplex var. calcitrans by liquefied dimethyl ether. Arab. J. Chem.. 2024;17:105538
- [CrossRef] [Google Scholar]
- In silico antidiabetic screening of borapetoside C, cordifolioside A and magnoflorine. Indian J. Pharm. Sci.. 2019;81(3):550-555.
- [CrossRef] [Google Scholar]
- Efficient solvent selection approach for high solubility of active phytochemicals: application for the extraction of an antimalarial compound from medicinal plants. ACS Sustain. Chem. Eng.. 2017;5:4332-4339.
- [CrossRef] [Google Scholar]
- Hypoglycemic diterpenoids from Tinospora crispa. J. Nat. Prod.. 2012;75:153-159.
- [CrossRef] [Google Scholar]
- Furanoid diterpene glucosides from Tinospora rumphii. Phytochem.. 1996;42:153-158.
- [CrossRef] [Google Scholar]
- Sustainable single-stage solid–liquid extraction of hesperidin and rutin from agro-products using cyrene. ACS Sustain. Chem. Eng.. 2020;8:18245-18257.
- [CrossRef] [Google Scholar]
- Magnoflorine-isolation and the anticancer potential against NCI-H1299 lung, MDA-MB-468 breast, T98G glioma, and TE671 rhabdomyosarcoma cancer cells. Biomolecules. 2020;10
- [CrossRef] [Google Scholar]
- Solubility prediction of bioantioxidants for functional solvent by group contribution method. J. Ind. Eng. Chem.. 2010;16:490-495.
- [CrossRef] [Google Scholar]
- Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats. J. Funct. Foods. 2012;4:79-86.
- [CrossRef] [Google Scholar]
- A comparison of three first principles methods for predicting solute–polymer affinity, and the simultaneous biodegradation of phenol and butyl acetate in a two-phase partitioning bioreactor. J. Chem. Technol. Biotechnol.. 2014;89:88-96.
- [CrossRef] [Google Scholar]
- Borapetoside C from Tinospora crispa improves insulin sensitivity in diabetic mice. Phytomedicine. 2012;19:719-724.
- [CrossRef] [Google Scholar]
- Asymmetric membrane formation. is the evaporation step necessary? Desalination. 1987;64:321-328.
- [CrossRef] [Google Scholar]
- Diagnosis of diabetes mellitus and living with a chronic condition: participatory study. BMC Public Health. 2018;18:699.
- [CrossRef] [Google Scholar]
- Green solvents in biomass processing. ACS Sustain. Chem. Eng.. 2016;4:5821-5837.
- [CrossRef] [Google Scholar]
- Prediction of Hansen solubility parameters with a new group-contribution method. Int. J. Thermophys.. 2008;29:568-585.
- [CrossRef] [Google Scholar]
- Efficiency of resveratrol-loaded sericin nanoparticles: promising bionanocarriers for drug delivery. Int. J. Pharm.. 2018;537:48-56.
- [CrossRef] [Google Scholar]
- Permeability of commercial solvents through living human skin. Am. Ind. Hyg. Assoc. J.. 1995;56:651-660.
- [CrossRef] [Google Scholar]
- Differential anti-diabetic effects and mechanism of action of charantin-rich extract of Taiwanese Momordica charantia between type 1 and type 2 diabetic mice. Food Chem. Toxicol.. 2014;69:347-356.
- [CrossRef] [Google Scholar]
- Recovery of natural active molecules using aqueous two-phase systems comprising of ionic liquids/deep eutectic solvents. Green Chem. Eng.. 2022;3:5-14.
- [CrossRef] [Google Scholar]
- WHO, 2020. Diabetes. https://www.who.int/health-topics/diabetes?gad_source=1&gclid=Cj0KCQjwwYSwBhDcARIsAOyL0fiGAG4z-hTYmS42TdiLsHodpoNJFcNDB3HPABsjDSMYkKfGehr5HSUaAj8iEALw_wcB#tab=tab_1. (accessed 25 March 2024).
- Magnoflorine: a review of its pharmacology, pharmacokinetics and toxicity. Pharmacol. Res.. 2020;152:104632
- [CrossRef] [Google Scholar]
- Modeling and optimization of microwave-assisted extraction of pentacyclic triterpenes from Centella asiatica leaves using response surface methodology. Ind. Crops Prod.. 2020;147:112231
- [CrossRef] [Google Scholar]
- Zhu, Y. L., Deng, L., Dai, X. Y., Song, J. Q., Zhu, Y., Liu, T., Kong, X. Q., Zhang, L. J., Liao, H. B., 2023. Tinopanoids K-T, clerodane diterpenoids with anti-inflammatory activity from Tinospora crispa. Bioorg Chem. 140, 106812. doi: 10.1016/j.bioorg.2023.106812.
- Exploration of the main active metabolites from Tinospora crispa (L.) Hook. f. & Thomson stem as insulin sensitizer in L6.C11 skeletal muscle cell by integrating in vitro, metabolomics, and molecular docking. J. Ethnopharmacol.. 2024;319:117296
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105778.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







