Translate this page into:
Inhibitory effects of propylene glycol alginate sodium sulfate derivatives on atrial fibrosis in atrial fibrillation
⁎Corresponding authors at: School of Biology and Food Engineering, Changshu Institute of Technology, Changshu 215500, China (Y.T. Xue); Department of Cardiology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University of Medicine, Shanghai 200093, China (Y.G. Li). xueyiting@cslg.edu.cn (Yiting Xue), liyigang@xinhuamed.com.cn (Yigang Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
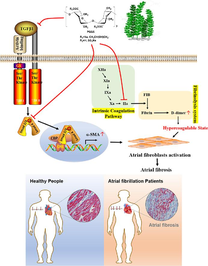
Abstract
Atrial fibrosis is the hallmark of structural remodeling in the pathogenesis of atrial fibrillation (AF). Meanwhile, AF causes a hypercoagulable state, and then provokes pro-fibrotic response. To discover a potential effective AF treatment targeting both coagulation and atrial fibrosis, this study investigated the structure–activity relationship of propylene glycol alginate sodium sulfate (PSS) derivatives with heparin-like activity on TGF-β1-induced atrial fibrosis. We found that PSS derivatives had significantly inhibitory effects on proliferation, migration, phenotypic transformation, and secretion/deposition of extracellular matrix of atrial fibroblasts. Among them, PGGS showed the optimal anti-atrial fibrotic activity by suppressing TGF-β1-induced activation of Smad2/3 signaling pathway. Furthermore, the study in vivo indicated that PGGS treatment displayed a reduced atrial fibrosis and AF inducibility, and attenuated the hypercoagulable state by decreasing D-dimer level and thrombin (FIIa) activity in MHC-TGF-β1 cys33ser transgenic mice, which had increased fibrosis in atrium but not in the ventricles. Our results demonstrated that PSS derivatives, especially PGGS, were potential anti-atrial fibrosis and anti-coagulant agents for AF prevention. Our study is beneficial in extending the current understandings of the function of PSS on atrial fibrosis and vulnerability to AF.
Keywords
Propylene glycol alginate sodium sulfate (PSS)
Structure-activity relationship
Atrial fibrosis
Atrial fibrillation
Anticoagulation
MHC-TGF-β1 cys33ser transgenic mice
1 Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, leading to a high morbidity and mortality (Dzeshka et al., 2015; Fan et al., 2015). Studies have shown that atrial fibrosis contributes to the maintenance of AF (Lai et al., 2022), and is the vital factor that makes AF refractory to rhythm control (Nattel, 2017). Atrial fibrosis results from the abnormal accumulation and disproportion of fibrillar collagen induced by proliferation, phenotypic differentiation, migration and secretion of atrial fibroblasts, which is a hallmark of arrhythmogenic structural remodeling (Ma, Chen, and Ma, 2021; Sygitowicz, Maciejak-Jastrzebska, and Sitkiewicz, 2021). Besides, AF causes activation of blood coagulation to promote pro-inflammatory and pro-fibrotic response in atrial fibroblasts. Spronk et al. (Spronk et al., 2017) found inhibition of coagulation via nadroparin could prevent stroke, attenuate atrial fibrosis, and further alleviate the complexity of the AF substrate. Apparently, it is of vital importance to explore drugs with both anticoagulant and anti-fibrotic effects for the treatment of AF. However, drugs clinically targeting both blood coagulation and atrial fibrosis have been rarely proved.
Since the first antifibrotic polysaccharide from Ganoderma lucidum was found (Park et al., 1997), more than thirty polysaccharides derived from natural resources were reported to be involved in the treatment of fibrosis-related diseases (Wang et al., 2022). Alginate extracted from brown seaweeds, a linear polysaccharide composed of mannuronic acid (M) and guluronic acid (G), was discovered to suppress liver fibrosis through inhibiting the nuclear factor-κB signaling pathway (Heyraud et al., 1996; Xia et al., 2020). As we know, sulfated derivatives of alginate showed excellent anticoagulant and antithrombotic activities (Xin et al., 2016; Guan, 1999). Interestingly, as the sulfated derivative of low-molecular-weight alginate, propylene glycol alginate sodium sulfate (PSS) presented hepatic fibrosis by suppressing inflammation, promoting extracellular matrix (ECM) decomposition and inactivating hepatic stellate cells (Xu et al., 2020). Moreover, PSS was safer than heparin in clinical application because its biological potency was about 1/10 of heparin (Xin et al., 2014; Ma et al., 2019). We hypothesized PSS could not only inhibit atrial fibrosis but reduce the threshold of AF inducibility. It was thoroughly studied that the bioactivities of PSS derivatives were closely relevant with its molecular weight (Mw), monosaccharide composition (M/G ratio) and degree of sulfation (DS). The anticoagulant and anti-selectin activities of PSS derivatives increased with Mw and DS (Xin et al., 2016; Ma et al., 2019; Wu et al., 2016), and PSS fraction with low M/G ratio showed optimal anticoagulant and anti-selectin activity (Xue et al., 2018; Ma et al., 2019). However, the relationship between the structures of PSS derivatives and their effects on atrial fibrosis was unknown.
Substantial clinical and experimental evidences support a central role for TGF-β1 to be an important mediator of atrial fibrosis in the pathogenesis of AF. As compared to angiotensin II, TGFβ1 is a more direct stimulator of fibrosis. Among numerous models for atrial fibrillation, TGFβ1 overexpression model (MHC-TGF-β1 cys33ser transgenic mice, Tx) is analogous to structural heart disease in human, which is more consistent with the etiology of AF patients in clinical practice. Considering the above reasons, this study intends to explore the inhibitory potential of PSS derivatives on atrial fibrosis in AF using TGF-β1-treated atrial fibroblasts and cardiac-specific TGF-β1-transgenic mice, namely Tx mice manifested by increased fibrosis in atrium but not in the ventricles. Furthermore, two series of PSS derivatives with varied Mw and M/G ratio were prepared by pH fractionation and chemical modification. Through exploring the structure–activity relationship of PSS derivatives on TGF-β1-induced proliferation, phenotypic differentiation, migration and secretion of atrial fibroblasts in vitro, we identified the structural fragment in PSS derivatives with optimal anti-atrial fibrotic activity. Meanwhile, we evaluated the inhibition effect of this structural fragment on atrial fibrosis and AF inducibility using Tx mice.
2 Materials and methods
2.1 Reagents
Low-molecular-weight alginic acid (PA, M/G ratio = 1.78, and Mw = 7.5 kD) was provided by Chia Tai Haier Pharmaceutical Co., Ltd. (Qingdao, China). 1-phenyl-3-methyl-5-pyrazolone (PMP), transforming growth factor β1 (TGF-β1) and chloral hydrate were obtained from Sigma-Aldrich (St. Louis. MO). D-dimer kit and hydroxyproline assay kit were purchased from Nan Jing Jian Cheng Technology Co. (Nanjing, China). Dulbecco's Modified Eagle's medium (DMEM)/F12, fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Grand Island, NY, USA). The primary antibodies against p-Smad2, p-Smad3, Smad2/3, Smad4, α-SMA and GAPDH proteins were purchased from Cell Signaling Technology (Danvers, MA, USA), Service-bio Technology and ABclonal Biotechnology (Wuhan, China). The primary antibody against TGF-β1 protein and FITC-labeled secondary antibody was obtained from Abcam (USA). All other chemicals and solvents were analytical grade.
2.2 Preparation of PSS derivatives
PSS derivatives with different M/G ratio (PGGS, PMGS, PSS) were consistent with the samples used in our previous studies (Xue et al., 2018; Xue et al., 2016) according to the synthetic procedure in Scheme 1.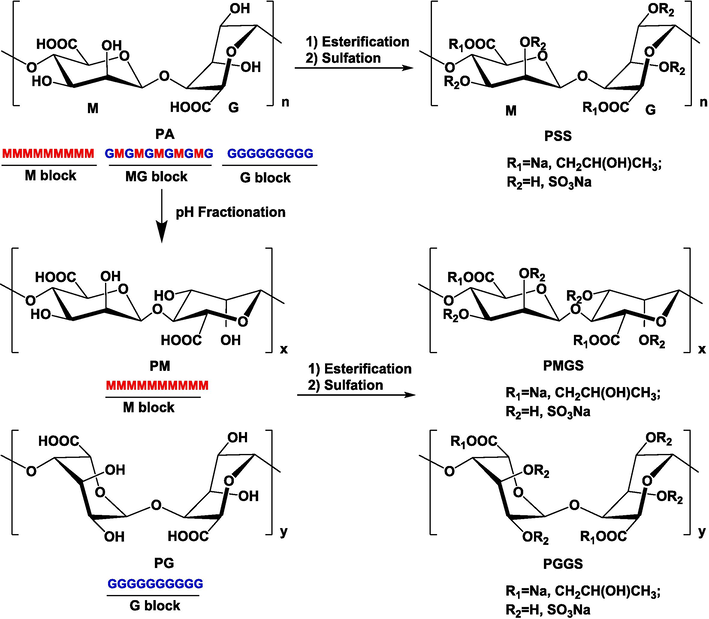
The synthesis of PSS derivatives.
PMGS samples with different Mw were prepared by gel permeation chromatography method (Xue et al., 2016). Briefly, 5 g of PMGS powder was dissolved in 10 ml NH4HCO3 solution (0.2 M) and separated on a Sephadex G75 gel chromatography column (5.0 × 100 cm). The column was performed on a GE AKTA purifier system (UPC-100, USA) and eluted with 0.2 mol/L NH4HCO3 solution at a flow rate of 2 ml/min. Each fraction (12 mL/tube) was collected with a fraction collector (CU-950) and detected by a refractometer (R1-102, Shodex). Four PMGS fractions with different Mw (PMGS-1, PMGS-2, PMGS-3, PMGS-4) were obtained after evaporating and freeze-drying.
2.3 Chemical and structural characterization of PSS derivatives
The relative Mws of PSS derivatives were analyzed by high performance gel permeation chromatography (HPGPC, Agilent 1260, USA) (Xue et al., 2016). The M/G ratios of PSS derivatives were detected by pre-derivatization HPLC (Dionex UltiMateTM 3000, USA) after the degradation with trifluoroacetic acid (TFA) (Wu et al., 2014). The organic sulfur contents of PSS derivatives were determined by ion chromatography (IC, Qingdao Shineha, China) (Xue, Li, and Guan, 2014). The structures of PSS derivatives were tested by fourier transform infrared spectroscopy (FT-IR) on a Nicolet Nexus 470 infrared spectrometer in the range of 400 to 4000 cm−1 with a KBr pellet (Xin et al., 2018).
2.4 Isolation and culture of atrial fibroblasts
Primary atrial fibroblasts were isolated from the 3-day-old SD rats as previously described (Chen et al., 2017). Atrial fibroblasts were maintained at 37 °C in 5 % CO2 incubator and cultured in DMEM/F12 with 10 % FBS and 1 % penicillin/streptomycin. To establish a model of atrial fibrosis, atrial fibroblasts were placed in serum-free medium for 12 h and then treated with 5 ng/mL TGF-β1 for 24 h.
2.5 Transwell migration assay and scratch wound assay
Mediums (600 μL) with or without TGF-β1 (5 ng/mL) were added to the bottom chamber of 24-well plates. Atrial fibroblasts in serum-free medium were pretreated with 100 μg/mL PSS derivatives for 2 h and then harvested to add into transwell inserts (100 μL, 2 × 104 cells) with polycarbonate membranes (8.0 μm pore size, Costar). Atrial fibroblasts were allowed to migrate for 24 h. Then atrial fibroblasts were fixed by 10 % formalin, stained with 0.25 % crystal violet, rinsed and dried. High power fields (×200) were counted to determine the average number of migrated atrial fibroblasts per field.
Atrial fibroblasts were cultured in 6-well plates at a density of 3 × 105/well. After forming the confluent monolayer, atrial fibroblasts were pretreated with 100 μg/mL PSS derivatives for 2 h. Then a sterile 200 μL micropipette was used to generate a horizontal scratch in each well. The cellular debris was washed with PBS and replaced with 2 mL fresh medium with or without TGF-β1 (5 ng/mL). Photographs of a 4 × magnification were taken on 0 h and 24 h after the TGF-β1 treatment. The covered area was considered as the uncovered area from images obtained on 0 h minus the uncovered area from images obtained on 24 h after the TGF-β1 treatment using Image J software. Quantification of the covered area (%) was counted as the covered area divide the uncovered area from images obtained on 0 h.
2.6 Cell counting Kit-8 (CCK-8) assay
The cell viability of atrial fibroblasts pretreated by PSS derivatives was measured by CCK-8 assay (CK04, Dojindo) and cell counting assay as previously described (Chen et al., 2017; Cui et al., 2016). For CCK-8 assay, atrial fibroblasts were plated in 96-well plates at a density of 5 × 103 cells/well. For cell counting assay, atrial fibroblasts were plated in 6-well plates, with 1 × 104 cells per well. The cells were serum starved and then exposed to 5 ng/mL TGF-β1 for 24 h. In the PSS derivatives treatment group, atrial fibroblasts were pre-incubated with PSS derivatives (100 μg/mL) for 2 h and then treated with 5 ng/mL TGF-β1 for 24 h. After the cells in 6-well plates were stained by trypan blue staining, cell counts were evaluated using a hematocytometer. And each well in 96-well plates was added with 10 μL CCK-8 solution. After incubated at 37 °C for 2 h, the optical density was detected at 450 nm using the Biotek Synergy 2 plate reader. Untreated cells were used as control.
2.7 Immunofluorescence staining
Atrial fibroblasts were seeded in 24-well plates with glass coverslips. Once reaching 60 % confluency, the cells were pretreated with PSS derivatives (100 μg/mL) and then stimulated by 5 ng/mL TGFβ-1 for up to 24 h. After rinsed with cold PBS thrice, the cells were fixed with 4 % paraformaldehyde, permeabilized with 0.5 % Triton-X 100, and then blocked with 5 % bovine serum albumin (BSA). Subsequently, the cells were incubated with the primary anti-α-SMA antibody (1:400) at 4 °C overnight. The following day, the cells were incubated with FITC-labeled secondary antibody for 1 h. Finally, after nuclei were stained with DAPI (Beyotime, China) for 30 min, the immunofluorescence images were photographed using a fluorescence microscope (Olympus). The images were analyzed using Image Pro Plus 6.0 software.
2.8 Western blot and quantitative real-time PCR (qRT-PCR)
Proteins were extracted from the cultured atrial fibroblasts and underwent the Western blot analysis as previously described (Wang et al., 2017). The primary antibodies against TGF-β1 (1:1000), Smad2/3 (1:1000), p-Smad2 (1:1000), p-Smad3 (1:1000) and Smad4 (1:1000) were used in this study. GAPDH (1:1000) was used as an internal control. Gel Imaging System (Tanon) and AlphaView software were used to image and analyze Western blots.
Total RNA was extracted from cultured atrial fibroblasts or mice atrium using TRIzol (Takara). qRT-PCR was performed as we previously described (Wang et al., 2017). The sequences of primer in this study were listed in Table 1.
Gene
Forward Primer
Reverse Primer
α-SMA
5′-ACTGGGACGACATGGAAAAG-3′
5′-CATCTCCAGAGTCCAGCACA-3′
CTGF
5′-GAGTCGTCTCTGCATGGTCA-3′
5′-CCACAGAACTTAGCCCGGTA-3′
Collagen I
5′-TCGATTCACCTACAGCACGC-3′
5′-GACTGTCTTGCCCCAAGTTCC-3′
GAPDH
5′-ACCACAGTCCATGCCATCAC-3′
5′-TCCACCACCCTGTTGCTGTA-3′
IL-6
5′-CTGCAAGAGACTTCCATCCAG-3′
5′-AGTGGTATAGACAGGTCTGTTGG-3′
IL-1β
5′-GAAATGCCACCTTTTGACAGTG-3′
5′-TGGATGCTCTCATCAGGACAG-3′
TNF-α
5′-CAGGCGGTGCCTATGTCTC-3′
5′-CGATCACCCCGAAGTTCAGTAG-3′
2.9 Animal studies
The Tx mice, presenting selective atrial fibrosis, was a gift from Dr. Loren J. Field, Wells Center for Pediatric Research, Indiana University School of Medicine (Nakajima et al., 2006). We used 3.5 months-old male Tx mice and wild type littermates in our study. The Tx mice were intraperitoneally injected with 25 mg/kg PGGS and 50 mg/kg PGGS which showed the optimal activity daily for 28 d and then were sacrificed at the 28th day.
2.10 Cardiac electrophysiology study
All the mice were anaesthetized with 2 % isoflurane inhalation. The mice were placed on a heated pad in order to maintain 37 °C body temperature. Surface electrocardiogram (ECG) and intracardiac electrogram were recorded simultaneously. A 1.1 octopolar catheter (Millar Inc., USA) was inserted into the right atrium through the right jugular vein. Atrial vulnerability was assessed by burst stimulation lasting for 5 s, starting with a cycle length of 50 ms, gradually decreasing to 48, 46, 44, 42 ms, and then shortened up to 10 ms in serial continuous bursts. AF was defined as a period of rapid irregular heart rhythm with irregular R-R intervals exceeding 1 s. AF was considered to be inducible if one or more bursts in the series evoked an AF episode. AF inducibility was deemed as the number of mice successfully induced AF divided by the total number of mice in each group. The time from the end-point pacing to the occurrence of the first detected P wave was defined as the duration of AF. The number of AF episodes and the duration of AF were analyzed (Wang et al., 2017).
2.11 Collection of serum samples and ELISA
The mice in each group were anesthetized with 2 % isoflurane at the 28th day. Blood samples were collected from the heart by opening the thoracic cavity. Serum was separated by centrifugation at 1000 rpm at 4 °C for 15 min. The D-dimer which was closely associated with the hypercoagulable state and thrombin (FIIa) activity were measured using ELISA kits (R&D Systems) according to the manufacturer’s instructions.
2.12 Hydroxyproline content assay
Atrial fibroblasts cultured in 6-well plates were pre-incubated with PSS derivatives (100 μg/mL) for 2 h and then treated with 5 ng/mL TGF-β1 for 24 h. The supernatants of cultured atrial fibroblasts were collected for hydroxyproline content assay. At the 28th day post PGGS intraperitoneal injection in Tx mice, serums of all the mice were collected to determine hydroxyproline content with hydroxyproline assay kit. The OD values of each sample were measured at 550 nm using a microplate reader (BioRad550, USA) (Zhao et al., 2018).
2.13 Histological analysis
Fixed atrium tissues of mice were cut into 5 μm sections. Picrosirius red staining was used to evaluate the fibrotic areas in atrium. The ratio of atrial fibrotic area to the total atrium area was calculated.
2.14 Statistical analysis
All the data are presented as the mean ± SEM, and the significance was calculated using GraphPad Prism 8.0 (San Diego, CA, USA). Comparison between groups was performed using One-Way ANOVA analysis followed by Tukey's test, with p < 0.05 considered significant.
3 Results
3.1 Chemical and structural characterization of PSS derivatives
PSS derivatives with different M/G ratio, including PGGS (fraction with low M/G ratio), PSS (fraction with middle M/G ratio) and PMGS (fraction with high M/G ratio), were prepared. Three samples shown in Table 2 (PGGS, PSS and PMGS) possessed similar Mw. In addition, we also obtained four PMGS fractions with different Mw (PMGS1 ∼ 4). PMGS1 ∼ 4 had higher M/G ratio as compared to PSS, and their Mw were 11858 Da, 8484 Da, 5195 Da and 3399 Da, respectively. Moreover, there was no obvious difference in degree of sulfate substitution (DS) of PSS derivatives, and their DS were 10.42 %, 11.48 %, 11.16 %, 11.20 %, 11.16 %, 11.50 % and 10.20, respectively. Mw, molecular weight; M/G ratio, monosaccharide composition; MwD, molecular weight distribution; DS, degree of sulfation (DS).
PSS fractions
Mw (Da)
MwD
M/G
DS
PSS derivatives with different M/G
PGGS
8403
1.35
0.42
10.42
PSS
9446
1.30
1.77
11.48
PMGS (PMGS-2)
8484
1.12
6.70
11.16
PMGS fractions with different Mw
PMGS-1
11,858
1.21
5.89
11.20
PMGS-2
8484
1.12
6.70
11.16
PMGS-3
5195
1.14
8.35
11.50
PMGS-4
3399
1.12
8.22
10.20
The structures of PSS derivatives were further characterized based on FT-IR. As shown in Fig. 1, the strong absorption peaks at 1608 cm−1 and 1442 cm−1 corresponded to vCOO- (asymmetric) and vCOO- (symmetric), respectively (Leal et al., 2008; Xin et al., 2018). The bands at 1221 cm-1and 827 cm−1 were ascribed to the S = O and C-O-S bonds, which indicated that the hydroxyl groups were successfully sulfated (Li et al., 2013). The characteristic bands were exhibited to correspond to the characteristic C1-H of M and G residues at 801 cm−1 and 742 cm−1 (Wang et al., 2020). In Fig. 1A, the vibration peak at 801 cm−1 became stronger with the increase of G content, which confirmed the result of M/G ratio in Table 2. These samples were used to test the relationship between structure of PSS derivatives and the activity of TGFβ1-stimulated atrial fibrosis in atrial fibrillation.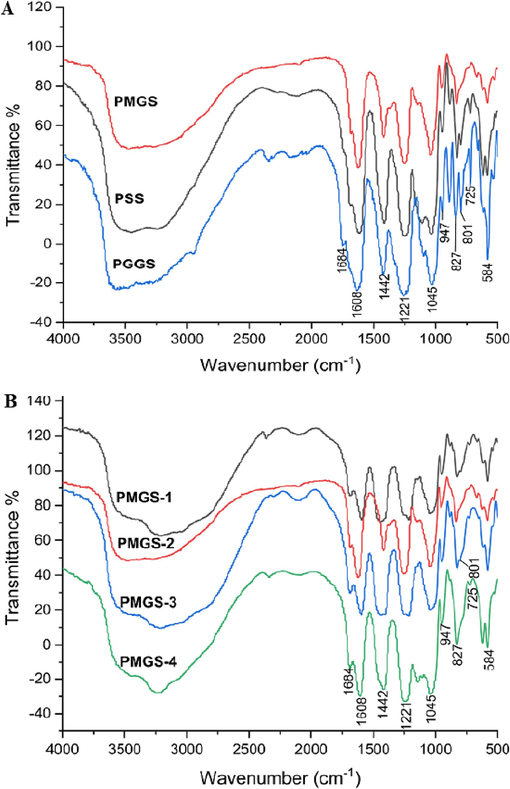
FT-IR spectra of PSS derivatives.
3.2 The structure–activity relationship of PSS derivatives on inhibiting atrial fibrosis
Atrial fibroblasts, highly plastic population of resident cells, are the predominant contributor in the atrial fibrotic response during the development of AF (Nattel, 2017). During the pathogenesis of AF, atrial fibroblasts are activated by increasing systemic or paracrine expression of TGF-β1. TGF-β1 enhanced the proliferation and migration of atrial fibroblasts, promoted phenotypic differentiation of atrial fibroblasts to myofibroblasts, as well as excessive production and deposition of extracellular matrix (ECM) and collagen (Pappritz et al., 2018). In order to evaluate the anti-fibrotic effects of PSS derivatives, we firstly tested their cytotoxicity on atrial fibroblasts by CCK-8 assay. The results showed that PSS derivatives had no obvious cytotoxicity at the concentration of 1000 μg/mL (Fig. 2A).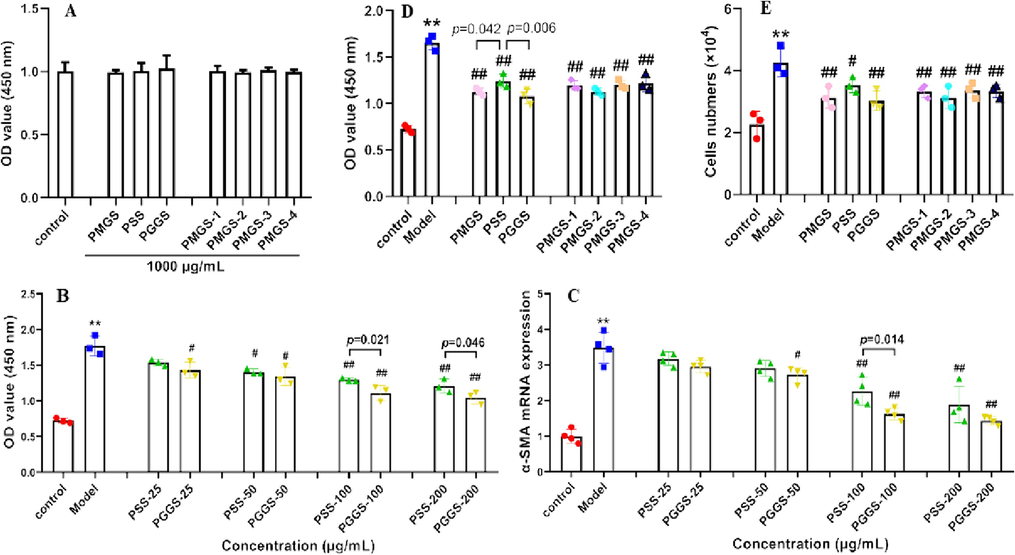
Cytotoxicity and inhibition of cellular proliferation and ɑ-SMA mRNA expression of PSS derivatives on atrial fibroblasts. (A) Cell cytotoxicity was tested by CCK-8 assay after treatment with or without PSS derivatives (1000 μg/mL) for 24 h. (B-E) Atrial fibroblasts were pretreated with PSS derivatives for 2 h, and then co-incubated with 5 ng/mL TGF-β1 for 24 h. (B) Cell proliferation was tested by CCK-8 assay. (C) α-SMA mRNA expression was assessed by qRT-PCR. (D) Quantification of cell proliferation by CCK-8 assay. (E) Quantification of cell proliferation by cell counting. Data are presented with mean ± SEM (n = 3). *p < 0.05 and **p < 0.01 versus control; #p < 0.05 and ##p < 0.01 versus model group (TGF-β1-treated group).
As a key profibrotic mediator in fibrotic diseases including atrial fibrosis, TGF-β1 is commonly used as a stimulator to make fibrosis models in vivo and in vitro (Meng, Nikolic-Paterson, and Lan, 2016; Wang et al., 2017). Since PGGS showed the best activity in previous structure–activity relationship studies (Ma et al., 2019; Xue et al., 2018), PGGS and PSS were chosen to screen the optimum intervention concentration by investigating their effects on TGF-β1-stimulated atrial fibroblasts. As shown in Fig. 2B-C, PSS and PGGS exerted inhibitory effects on TGF-β1-induced cellular proliferation and ɑ-SMA mRNA expression with a dose-dependent manner in 25 ∼ 200 μg/mL. At 100 μg/mL concentration, we observed PSS and PGGS possessed significantly reduced proliferative and fibrosis-promoting properties compared with the model group (TGF-β1-treated group). Moreover, there was a significant difference in inhibitory effects between PSS and PGGS. In view of above findings, 100 μg/mL was selected for subsequent structure–activity relationship study.
Next, the structure–activity relationship of PSS derivatives on TGF-β1-induced proliferation of atrial fibroblasts was determined by cell counting and CCK-8 assay. As shown in Fig. 2D-E, all PSS derivatives significantly reduced TGF-β1-stimulated cell proliferation (p<0.05). However, there was no distinct rule in different Mw or different M/G ratio. Overall, compared with PSS, PGGS and PMGS revealed obvious inhibition on TGF-β1-induced proliferation of atrial fibroblasts.
During the pathogenesis of atrial fibrosis, the expression of α-SMA, a widely characterized cytoskeletal protein, is a hallmark of transdifferentiation of atrial fibroblasts into myofibroblasts (Wang et al., 2017). The highly specialized synthetic and contractile myofibroblasts further increase the secretion of ECM and collagen, and targeting myofibroblasts has the potential to reduce atrial fibrosis (Lai et al., 2022). As shown in Fig. 3C, treatment with PSS derivatives could reverse TGF-β1-induced α-SMA mRNA expression. Meanwhile, immunofluorescence assay also confirmed the same results (Fig. 3A-B). Among the PSS derivatives, PGGS possessed strongest inhibition on phenotypic differentiation of atrial fibroblasts versus PSS and PMGS (Fig. 3A-B). To provide more evidence to support this result, we verified the effect of PGGS on TGF-β1-induced α-SMA protein expression by Western Blot. Results showed that PGGS remarkably inhibited TGF-β1-induced α-SMA protein expression (Fig. 3D-E). These results indicated that PGGS exerted the most remarkable improvement effect on TGF-β1-induced myofibroblast differentiation. In addition, we found the structure–activity relationship of PSS derivatives on TGF-β1-induced differentiation of atrial fibroblasts was same as their effects on proliferation of atrial fibroblasts. Consistent with anticoagulant and antitumor activities in previous studies (Ma et al., 2019; Xue et al., 2018), PGGS had an advantage on anti-atrial fibroblasts proliferation and differentiation compared with PSS. On the contrary, Mw was not proportional to the antagonistic activity against atrial fibrosis. PMGS with a stronger effect may be related with its anti-inflammatory effect versus PSS (Xue et al., 2018) because atrial fibroblasts were recognized as inflammatory supporter cells (Pappritz et al., 2018). In Fig. 2D and Fig. 3C, it demonstrated a positive relationship between the inhibition on proliferation and differentiation of atrial fibroblasts and Mw of PMGS2 ∼ 4. The activity of PMGS-1 was less than PGMS-2 due to its lower M/G ratio.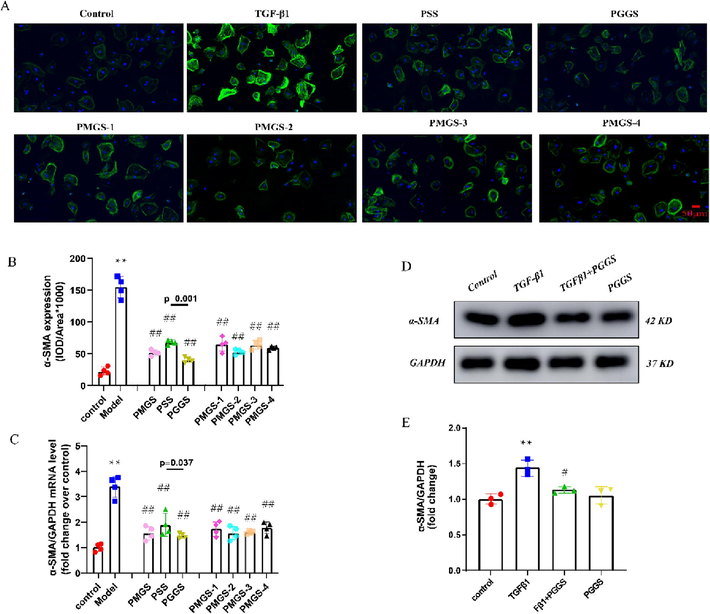
Inhibitory effects of PSS derivatives against TGF-β1-induced fibroblast-to-myofibroblast differentiation. (A) The expression of α-SMA in atrial fibroblasts were determined by immunofluorescence assay. Green represents α-SMA-positive atrial fibroblasts, and blue represents DAPI-stained nuclei. Scale bar = 50 µm. (B) Quantitative data on α-SMA expression in atrial fibroblasts of the indicated group. (C) mRNA level of α-SMA in atrial fibroblasts assessed by qRT-PCR. (D) Representative Western blot of α-SMA protein expression in atrial fibroblasts. (E) Quantification of α-SMA protein expression in atrial fibroblasts (n = 3). Results were presented as the mean ± SEM. *p<0.05 and **p<0.01 versus control; #p<0.05 and ##p<0.01 versus model.
Acquiring migratory capacity is also of vital importance for activated atrial fibroblasts, so we investigated the effects of PSS derivatives on this property. As shown in Fig. 4A-D, transwell assay and scratch wound assay revealed that two series of PSS derivatives pretreatment could effectively alleviate TGF-β1-induced cell migration. The structure–activity relationship of PSS derivatives on cellular migration of atrial fibroblasts was consistent with their effects on proliferation and differentiation. PGGS and PMGS-2 possessed more significant improvement effects. However, scratch wound assay showed that there was no obvious difference between PSS and PMGS, and the wound healing property of PMGS had a positive relationship with Mw. In any event, PGGS had the strongest ability to antagonize TGF-β1-enhanced migration of atrial fibroblasts.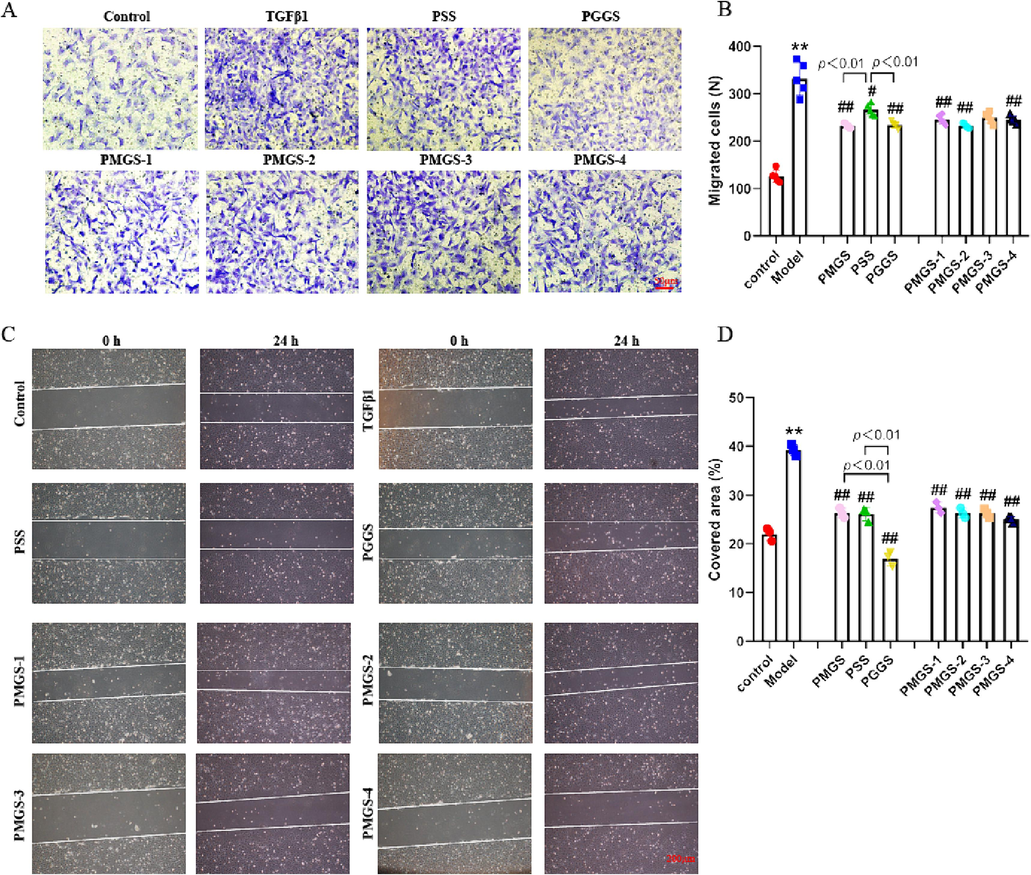
Inhibitory effects of PSS derivatives against TGF-β1-induced migration capacity. (A) Representative staining of atrial fibroblasts in Transwell assay (scale bar 50 μm). (B) Quantification of migrated atrial fibroblasts. (C) Wound was set into a confluent cell layer and then submitted to different treatments. Photos at time 0 and after 24 h were shown. The area delimited with the white line represents the uncovered area. (D) The percentage of covered area after respective treatments was measured by a wound-healing assay (scale bar 100 μm). Results were presented as the mean ± SEM. *p<0.05 and **p<0.01 versus control; #p<0.05 and ##p<0.01 versus model group (TGF-β1-treated group).
As the predominant component of cardiac extracellular matrix, collagen contained a large amount of hydroxyproline (Yamashita et al., 2004; Bruckner-Tuderman and Bruckner, 1998). In this study, the hydroxyproline content on the cell supernatant were measured to represent the amount of collagen secreted by atrial fibroblasts. Results showed that pretreatment with two series of PSS derivatives attenuated TGF-β1-enhanced hydroxyproline content (Fig. 5A) compared with the model group. However, this result possessed no exactly same trend with Figs. 2-4. Though PGGS was still the best performer, hydroxyproline content declined with the increase of Mw for PMGS. Interestingly, PMGS1 exerted a stronger inhibitory effect than PSS, which was second only to PGGS. We further evaluated the mRNA expressions of Collagen I and connective tissue growth factor (CTGF) (Liu et al., 2017; Song et al., 2017). Both genes could promote fibroblast division and collagen deposition and were closely related to the occurrence and development of various organ fibrosis. Fig. 5B-C revealed that PSS derivatives suppressed TGF-β1-induced mRNA expressions of Collagen I and CTGF. Compared with PSS, PGGS and PMGS-1 showed obvious inhibitory effects on TGF-β1-induced mRNA expressions of Collagen I and CTGF, and PMGS-2 only significantly decreased CTGF mRNA expressions. These results indicated all PSS derivatives can weaken TGF-β1-induced collagen overproduction by reducing the expression of fibrosis-related genes, including Collagen I and CTGF. Among them, PGGS and PMGS-1 exerted the most obvious effects on extracellular matrix synthesis by inhibition of both Collagen I and CTGF genes, especially PGGS.
Inhibitory effects of PSS derivatives against TGF-β1-induced hydroxyproline secretion and mRNA expressions of fibrosis-related genes in atrial fibroblasts. (A) Analysis of hydroxyproline content in supernatants of dedicated atrial fibroblasts. (B) mRNA expressions of Collagen I. (C) mRNA expressions of CTGF. Results were presented as the mean ± SEM. *p<0.05 and **p<0.01 versus control; #p<0.05 and ##p<0.01 versus model group (TGF-β1-treated group); ×p<0.05 and ××p<0.01 versus PSS.
Taking a broader look at the results in Figs. 2-5, PSS derivatives attenuated TGF-β1-induced proliferation, migration, phenotypic transformation of atrial fibroblasts, and secretion/deposition of ECM, which proved that PSS derivatives possessed anti-atrial fibrotic activities. But there was no obvious or uniform structure–activity relationship for PSS derivatives. It was certain that PGGS exhibit the strongest anti-atrial fibrotic activity in vitro.
3.3 Effect of PGGS on the activation of TGF-β1/Smad2/3 signaling pathway
As we know, TGF-β1 played a key role in the pathogenesis of atrial fibrosis by activating the canonical Smad2/3/4 signaling pathway (Dzeshka et al., 2015). To identify the downstream targets of PGGS-mediated inhibition of atrial fibrosis, we examined TGF-β1/Smad2/3/4 activities in atrial fibroblasts. As displayed in Fig. 6, TGF-β1 stimulation significantly increased TGF-β1, p-Smad2 and p-Smad3 protein levels. However, PGGS treatment remarkably attenuated TGF-β1-enhanced p-Smad2 and p-Smad3 protein expressions. These results revealed that PGGS can significantly attenuate TGF-β1-mediated proliferation, differentiation, migration and secretion capacities of atrial fibroblasts by inhibition of TGF-β1/Smad2/3 signaling pathway.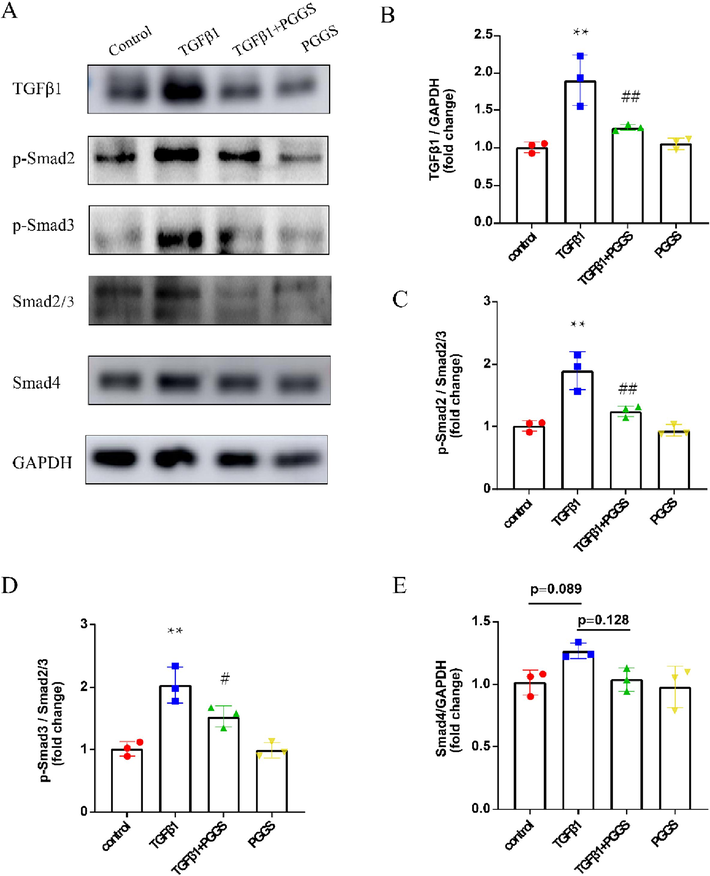
The Inhibitory effect of PGGS on TGF-β1/Smad2/3 signaling pathway in atrial fibroblasts. (A) Representative Western blot of TGF-β1, p-Smad2, p-Smad3, Smad2/3, Smad4 and GAPDH in atrial fibroblasts. (B-E) Quantification of TGF-β1, p-Smad2, p-Smad3 and Smad4 protein expressions. Results were presented as the mean ± SEM. *p<0.05 and **p<0.01 versus control; #p<0.05 and ##p<0.01 versus TGF-β1.
3.4 Ameliorative effect of PGGS on hypercoagulable state, atrial fibrosis and susceptibility to AF in vivo
As reported in the literature, Tx mice showed increased interstitial fibrosis in the atrium but not in the ventricles due to elevated levels of TGF-β1. Selective atrial fibrosis in Tx mice is sufficient to produce a substrate for AF and increase AF inducibility (Choi et al., 2012; Nakajima et al., 2006). Aiming to evaluate the effect of PGGS, the PSS derivative with the optimal anti-fibrosis activity, on atrial fibrosis in vivo, Tx mice were used as the model of atrial fibrosis and AF vulnerability. Referred to the published articles (Wang et al., 2022), two commonly used effective intervention dose of PGGS (25 mg/kg and 50 mg/kg) were selected for study. In addition, valsartan was used as a positive control drug for improving atrial fibrosis. As shown in Fig. 7A, picrosirius red staining showed that TGF-β1-enhanced fibrotic area in left atrium was remarkably attenuated by PGGS treatment (Fig. 7B-C). In addition, compared with wild type mice, higher hydroxyproline content in serum of Tx mice portended the excessive production and deposition of collagen in the pathogenesis of atrial fibrosis. Conversely, two doses of PGGS treatment significantly suppressed the upward trend in this transgenic mouse model, and the improvement was similar to that of the positive control drug (Fig. 7D-G). The higher dose of PGGS (50 mg/kg) had a more obvious tendency to inhibit atrial fibrosis, however, but was no statistical difference in the ameliorating of fibrosis between two doses of PGGS (p = 0.895). To further confirm whether the significant differences in atrial fibrosis was associated with those in threshold for AF, we carried out electrophysiological studies in vivo (Wang et al., 2017). Results revealed that all the mice showed no spontaneous arrhythmia. However, Tx mice significantly increased susceptibility to AF versus wild type mice, and its main principal manifestation was increased episodes of AF and prolonged AF durations. In contrast, PGGS treatment inhibited TGF-β1-induced AF episodes and durations (Fig. 9A-D). These results demonstrated that PGGS treatment exhibited a protective effect on atrial fibrosis and attenuated susceptibility to AF. To further verify the underlying mechanism by which PSS derivates exerted its anti-fibrosis response in left atrium post TGF-β1, we assessed the regulatory role of PSS derivates on Smad cascade activation in vivo using left atrium of mice in dedicated group. Western Blot results showed that (Fig. 8) both Smad2 phosphorylation and Smad3 phosphorylation as well as the Smad4 expressions were remarkedly increased in left atrium of Tx mice. Two doses of PGGS treatment significantly attenuated the increase of Smad2/3 phosphorylation and Smad4 expressions in Tx transgenic mouse model, and the improvement was similar to that of the positive control drug (Fig. 8A-E). The higher dose of PGGS (50 mg/kg) had a more obvious tendency to inhibit the activation of Smad2/3/4 signaling pathway, however, there was no statistical difference between two doses of PGGS (Fig. 8).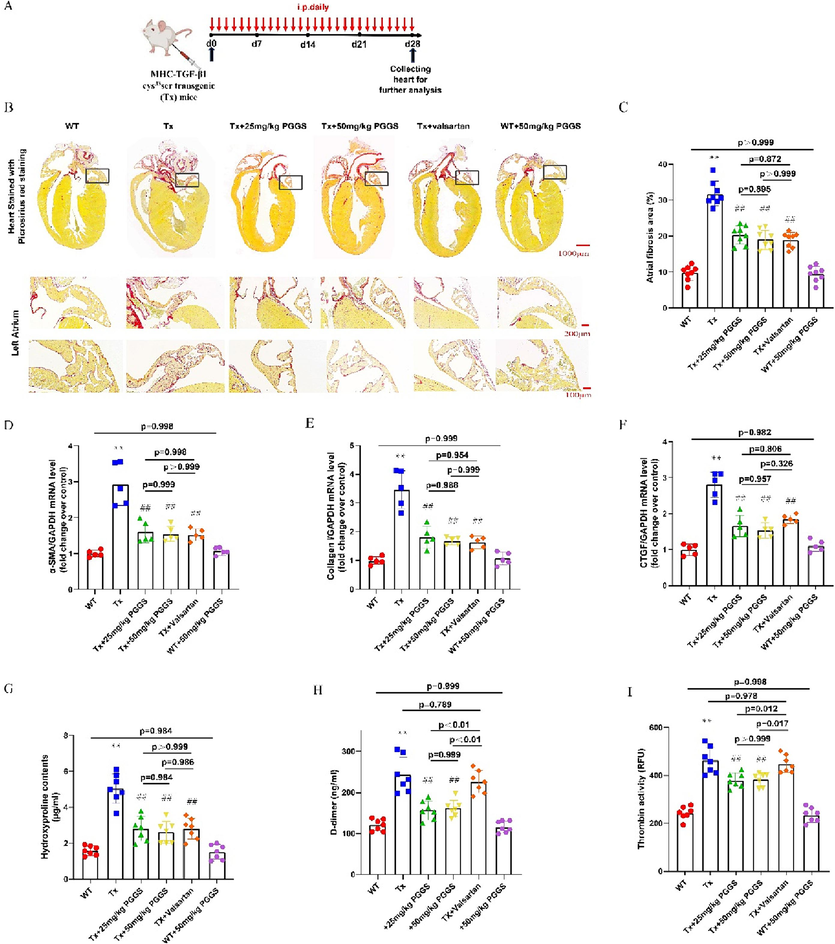
The Inhibitory effect of PGGS on atrial fibrosis and hypercoagulable state in vivo. (A) Schematic description of the experimental design. (B) Representative Picrosirius red staining of atrium tissues (Scale bar = 1000 μm for the first line panel; scale bar = 200 μm for the second line panel; scale bar = 100 μm for the second line panel). (C) Quantification of atrial fibrosis. (D-F) The mRNA expression of α-SMA, Collagen I and CTGF determined by qRT-PCR. (G) Analysis of hydroxyproline content in serum of mice in each group. (H) Analysis of D-dimer content in serum of mice in each group. (I) Analysis of thrombin activity in serum of mice in each group. Results were presented as the mean ± SEM. *p<0.05 and **p<0.01 versus WT; #p<0.05 and ##p<0.01 versus Tx. WT, wild type; Tx, MHC-TGF-β1 cys33ser transgenic mice; Tx + PGGS, PGGS-treated MHC-TGF-β1 cys33ser transgenic mice.
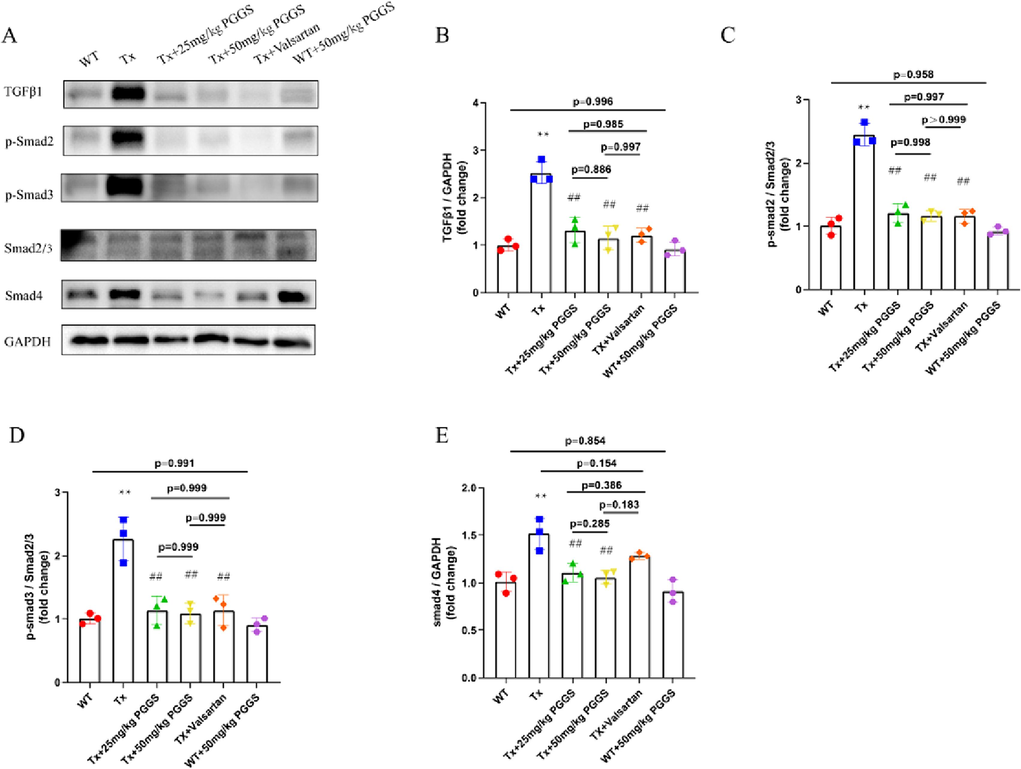
The Inhibitory effect of PGGS on TGF-β1/Smad2/3/4 signaling pathway in the atrium tissues of mice. (A) Representative Western blot of TGF-β1, p-Smad2, p-Smad3, Smad2/3, Smad4 and GAPDH in atrium tissues of mice in each group. (B-E) Quantification of TGF-β1, p-Smad2, p-Smad3 and Smad4 protein expressions. Results were presented as the mean ± SEM. *p<0.05 and **p<0.01 versus WT; #p<0.05 and ##p<0.01 versus Tx. WT, wild type; Tx, MHC-TGF-β1 cys33ser transgenic mice; Tx + PGGS, PGGS-treated MHC-TGF-β1 cys33ser transgenic mice.
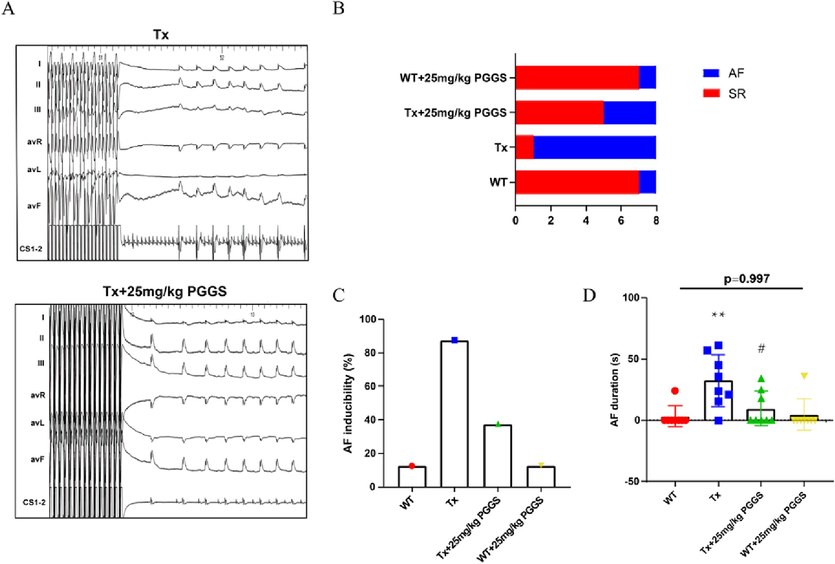
The Inhibitory effect of PGGS on vulnerability to atrial fibrillation in vivo. 25 mg/kg PGGS was used as the representative intervention dose for cardiac electrophysiology study. (A) Representative surface ECG (lead I、II、III、aVR、aVL and aVF) and intracardiac ECG (CS1-2) tracings of mice receiving electrophysiological studies. (B-D) AF inducibility (B-C) and AF durations (D) in WT, Tx, Tx + 25 mg/kg PGGS and WT + 25 mg/kg PGGS group during the electrophysiological studies. Data were presented as the mean ± SEM. *p<0.05 and **p<0.01 vs WT group; #p<0.05 and ##p<0.01 vs Tx group. WT, wild type; Tx, MHC-TGF-β1 cys33ser transgenic mice; Tx + PGGS, PGGS-treated MHC-TGF-β1 cys33ser transgenic mice.
Hypercoagulability in AF patients causes atrial fibrosis and promotes AF (Spronk et al., 2017). In vivo, Tx mice indeed possessed high D-dimer level, an important marker of hypercoagulability and fibrinolysis hyperactivity (Sener et al., 2021), compared with wild type mice (Fig. 7H). As shown in Fig. 7I, the thrombin (FIIa) activity significantly elevated in Tx mice compared with the wild type mice. Moreover, in line with our expectation, two doses of PGGS treatment decreased the thrombin activity compared with that in the Tx mice. We also examined the anticoagulant property of PSS derivatives especially PGGS in vitro. Among two series of PSS derivatives, PGGS had highest anticoagulant activity by activated partial thromboplastin time (APTT) and thrombin time (TT) assay in vitro (Supplementary Fig. S1). Above mentioned results indicated that PGGS could effectively decrease the blood hypercoagulability during the pathogenesis of atrial fibrosis. The inflammatory response also plays a key role in the process of TGF-β1-induced atrial fibrosis. We also analyzed the mRNA expressions of IL-6, IL-1β and TNF-α using left atrium of mice in dedicated group. mRNA expressions of IL-6, IL-1β and TNF-α were significantly higher in left atrium of Tx mice compared with that of wild type mice. PGGS treatment partially blocked TGF-β1-induced upregulation of above mentioned pro-inflammatory cytokines. (Supplementary Fig. S2). Given the above, PGGS had a potential to alleviate both atrial fibrosis and its accompanied hypercoagulable and inflammatory state.
4 Discussion
AF is the most common serious cardiac arrhythmia, and its prevalence increases dramatically with advancing age. AF acts as a major international health challenge in developed and developing countries, and places health services under great stress in an aging population. Besides, continued increase in AF-related morbidity and mortality raised the need for therapies to prevent or delay AF onset and progression. Intervening the evolution of arrhythmogenic atrial substrate, characterized by structural remodeling processes especially atrial fibrosis provides the basis for so-called upstream therapies which could halt AF onset or delay its progression from paroxysmal AF to persistent forms. Furthermore, AF causes a hypercoagulable state. During the pathogenesis of AF, thrombus formation is promoted by both the hypercoagulable state and blood stasis in poorly contractile atrium. AF treatment with an anticoagulant could dramatically reduce the burden of stroke. Importantly, atrial fibrosis and hypercoagulation interact and promote each other. The changes of coagulation factors not only acted as the secondary event of fibrosis, but also played an important role in the occurrence and development of fibrosis (Spronk et al., 2017). So it is highly expected to discover an effective atrial fibrillation treatment targeting both coagulation and atrial fibrosis. This study taken this as a breakthrough to discover a potential AF treatment targeting both coagulation and atrial fibrosis.
As highly negatively charged polymers, PSS derivatives are known to have anticoagulant, antithrombotic activities due to the heparin-like structure (Wu et al., 2016; Xue et al., 2016). Recently, PSS has been reported to have an inhibitory effect on liver fibrosis by suppressing inflammation and inactivating hepatic stellate cells (Xu et al., 2020). This portended that PSS is likely to participate in the treatment of organ fibrosis through the synergistic effect of cells inactivation and anticoagulation. The structural diversity and heterogeneity of polysaccharides make their characterization highly challenging, which limit their pharmaceutical development (Wang et al., 2020). Based on our previous studies, we prepared two series of PSS derivatives with different M/G ratio and different Mw to find the best structural fragment to antagonize atrial fibrosis in this study. We found PSS derivatives showed inhibitory effects on proliferation, phenotypic differentiation, migration and secretion of atrial fibroblasts with very low toxicity (Figs. 2-5). Consistent with previous studies (Xue et al., 2016; Ma et al., 2019), the higher content of G, the stronger anti-fibrotic activity the PSS derivatives showed. Among them, PGGS possessed the optimal anti-atrial fibrosis effect, which may be related to its conformations and advanced structures, due to C-5 epimers of G (Atkins et al., 1973a,1973b). However, there was no obvious structure–activity relationship between anti-atrial fibrosis activity of PSS derivatives and their Mw. PMGS-2 had stronger anti-atrial fibrosis effect than PSS, which was second only to PGGS. The inflammatory response can both increase the pathogenesis of AF and the propensity for coagulation in AF (Esmon, 2004). We also found that PGGS treatment partially blocked TGF-β1-induced upregulation of pro-inflammatory cytokines, hinting that anti-inflammatory properties of PSS derivatives possessed a certain additive effect on their anti-atrial fibrosis activity.
Substantial numbers of clinical and experimental evidences support a critical role for TGF-β1, a profibrotic biomarker, in AF-related atrial fibrosis (Lai et al., 2022). TGF-β1/activin receptor-like kinase 5 (ALK5)/Smad2/3/4 pathway is classic downstream effector of TGF-β1 (Wang et al., 2017). Taking PGGS, a PSS derivative with the optimal anti-fibrotic activity, as an example, our studies implied the inhibitory effects of PGGS on TGF-β1-induced activation of Smad2/3/4 signaling pathway in vivo and in vitro. In view of the fact that some heparinoid polysaccharides could enter into the cells after long time incubation (Gao et al., 2018; Wu et al., 2016), we supposed that the heparinoid polysaccharide PGGS with the rigid structure (Atkins et al., 1973b) may be easier to bind to cellular surface ALK5 to play a competitive inhibitory role or bind to other receptors such as EGFR to induce the internalization of PGGS into cytoplasm to depress the phosphorylation of Smad2/3 in TGF-β1/Smad2/3/4 signaling pathway (Fig. 10). Furthermore, cardiac electrophysiology study and histological analysis showed that PGGS can reverse TGF-β1-enhanced atrial fibrosis, AF duration, AF inducibility and serum hydroxyproline contents in the Tx transgenic mouse model (Figs. 7-9). Our results collectively demonstrated that PGGS with the optimal anti-atrial fibrotic activity among PSS and its derivatives prevented atrial fibrosis and AF inducibility by suppressing activation of atrial fibroblasts through the TGF-β1/Smad2/3/4 signaling pathway.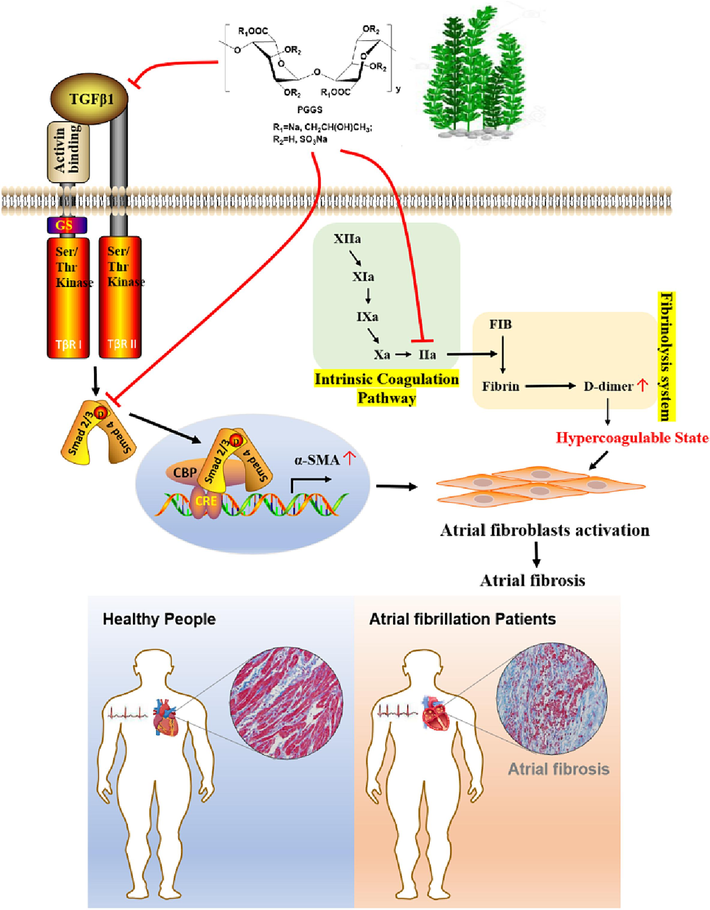
Schematic illustration of the molecular mechanisms of PGGS against atrial fibrosis in AF and accompanied hypercoagulable state.
In addition, the accepted scenario linked AF and thromboembolism. Clinical guideline recommend anticoagulation treatment is warranted in men and women who have AF with a CHA2DS2-VASc score of 2 or 3, respectively (January et al., 2019). In 2010, dabigatran, a direct thrombin (FIIa) inhibitor and the first novel oral anticoagulant, became available for the anticoagulation treatment of AF. Similarly, PSS and derivative have heparin-like anticoagulant activity due to its heparin-like structure, and those with low Mw also had an inhibitory effect on FIIa in vitro (Xue et al., 2018; Xin et al., 2016). We supposed that PGGS treatment could also improve hypercoagulable state in Tx mice in vivo. Further studies validated that PGGS treatment effectively reduced thrombin (FIIa) activity. Among variety of prothrombotic markers, D-dimer is the gold standard which could mirror the hypercoagulable state of AF. As we expected, PGGS treatment also significantly abrogated the elevated serum D-dimer content in Tx mice in vivo, indicating the relieved hypercoagulability.
In conclusion, PSS and its derivatives, especially PGGS, had potentials to alleviate both atrial fibrosis and the accompanied hypercoagulable state. Together with their easily accessible property, PSS derivatives, especially PGGS, are potential upstream therapy and anti-coagulation agents for AF prevention. More importantly, this study extends the present understanding of PSS function on atrial fibrosis and vulnerability to AF, which might effectively act as a potential clinical substitute for the treatment of AF.
PSS derivatives had significantly inhibitory effects on proliferation, migration, phenotypic transformation, and secretion/deposition of extracellular matrix of atrial fibroblasts. Among them, PGGS showed the optimal anti-atrial fibrotic activity by suppressing TGF-β1-induced activation of Smad2/3 signaling pathway. Furthermore, the study in vivo indicated that PGGS treatment displayed a reduced atrial fibrosis and AF inducibility, and attenuated the hypercoagulable state by decreasing D-dimer level and thrombin (FIIa) activity in MHC-TGF-β1 cys33ser transgenic mice. In conclusion, PSS derivatives, especially PGGS, are potential upstream therapy and anti-coagulation agents for AF prevention.
Author contributions
Conceptualization, project administration, supervision, writing original draft, Q.W.; methodology, investigation, J.Q.; methodology, data curation, validation, X.H.; investigation, Methodology, K.D.; methodology, data curation, J.Z.; data curation, methodology, B.L.; resources, supervision, Y.Y. and J.Y.; methodology, Z.W.; validation, C.L.; software, supervision, Q.S. and L.W.; preparation of PSS and its derivatives, writing original draft, Y.X.; writing, review and editing, C.L. and Y.L.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82370317 and 81900293 to Qian Wang), the Fundamental Research Funds for the Central Universities (YG2023QNB12 to Qian Wang), University Basic Science Research Project of Jiangsu Province (21KJB350022 to Yiting Xue), National Natural Science Foundation of China (82130009 and 82070515 to Yigang Li), Shandong Medical and Health Technology Development Program (2019WS595 to Qijuan Sun).
7 Institutional Review Board Statement
All experimental procedures were performed with the approval of the Ethics Committee as well as the Animal Care and Use Committee of Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine.
8 Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due as public availability violates the consent given by the study participants.
CRediT authorship contribution statement
Qian Wang: . Junhao Qiu: Investigation, Methodology. Xiaoliang Hu: Data curation, Methodology, Validation. Kangfei Ding: Investigation, Methodology. Jun Zhang: Data curation, Methodology. Bo Liu: Data curation, Methodology. Yuli Yang: Resources, Supervision. Zhixing Wei: Methodology. Cheng Li: Validation. Qijuan Sun: Software, Supervision. Jianfeng Yu: Resources, Supervision. Lingtian Wu: Software, Supervision. Chunxia Li: Writing – review & editing. Yiting Xue: Writing – review & editing. Yigang Li: Writing – review & editing.
Acknowledgement
We thank Dr. Loren J. Field, Wells Center for Pediatric Research, Indiana University School of Medicine, and Dr. Shengzhong Duan, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, for providing us with the MHC-TGF-β1 cys33ser transgenic mice.
Limitations
This study selected atrial fibrosis, the hallmark of structural remodeling in AF as the entry point. The effects of PSS derivatives on electrical remodeling of AF in vivo and in vitro were not concerned. In addition, atrial fibrosis could also cause heterogeneity of cardiac electrical conduction, and this study did not measure the atrial effective refractory period and the atrial action potential duration in vivo.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Structural components of alginic acid. I. The crystalline structure of poly-beta-D-mannuronic acid. Results of x-ray diffraction and polarized infrared studies. Biopolymers. 1973;12(8):1865-1878.
- [Google Scholar]
- Structural components of alginic acid. II. The crystalline structure of poly-alpha-L-guluronic acid. Results of x-ray diffraction and polarized infrared studies. Biopolymers. 1973;12(8):1879-1887.
- [Google Scholar]
- Genetic diseases of the extracellular matrix: more than just connective tissue disorders. J. Mol. Med. (Berlin, Germany. 1998;76(3–4):226-237.
- [Google Scholar]
- Haplodeficiency of activin receptor-like kinase 4 alleviates myocardial infarction-induced cardiac fibrosis and preserves cardiac function. J. Mol. Cell. Cardiol.. 2017;105:1-11.
- [Google Scholar]
- Triggered firing and atrial fibrillation in transgenic mice with selective atrial fibrosis induced by overexpression of TGF-beta1. Circ J. 2012;76(6):1354-1362.
- [Google Scholar]
- Orphan nuclear receptor Nur77 inhibits angiotensin II-induced vascular remodeling via downregulation of beta-catenin. Hyperyension. 2016;67:153-162.
- [Google Scholar]
- Cardiac fibrosis in patients with atrial fibrillation mechanisms and clinical implications. J. Am. Coll. Cardiol.. 2015;66(8):943-959.
- [Google Scholar]
- The impact of the inflammatory response on coagulation. Thromb. Res.. 2004;114:321-327. 0049–3848 (Print)
- [Google Scholar]
- Atrial overexpression of angiotensin-converting enzyme 2 improves the canine rapid atrial pacing-induced structural and electrical remodeling. Basic Res. Cardiol.. 2015;110(4):25.
- [Google Scholar]
- The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym.. 2018;198:329-338.
- [Google Scholar]
- NMR spectroscopy analysis of oligoguluronates and oligomannuronates prepared by acid or enzymatic hydrolysis of homopolymeric blocks of alginic acid. Application to the determination of the substrate specificity of Haliotis tuberculata alginate lyase. Carbohyd. Res.. 1996;289:11-23.
- [Google Scholar]
- AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical Practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e151.
- [Google Scholar]
- miR-181b targets semaphorin 3A to mediate TGF-β-induced endothelial-mesenchymal transition related to atrial fibrillation. J. Clin. Invest.. 2022;132(13):e142548.
- [Google Scholar]
- FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohyd. Res.. 2008;343(2):308-316.
- [Google Scholar]
- Preparation, characterization and antioxidant activities of polymannuronic acid phosphate, H-phosphonate and sulfate. Int. J. Biol. Macromol.. 2013;62:281-286.
- [Google Scholar]
- Osthole inhibits the expressions of collagen I and III through Smad signaling pathway after treatment with TGF-beta 1 in mouse cardiac fibroblasts. Int. J. Cardiol.. 2017;228:388-393.
- [Google Scholar]
- Left atrial fibrosis in atrial fibrillation: mechanisms, clinical evaluation and management. J. Cell Mol. Med.. 2021;25(6):2764-2775.
- [Google Scholar]
- Structure-activity relationship of propylene glycol alginate sodium sulfate derivatives for blockade of selectins binding to tumor cells. Carbohydr. Polym.. 2019;210:225-233.
- [Google Scholar]
- Cardiomyocyte cell cycle activation ameliorates fibrosis in the atrium. Circ. Res.. 2006;98(1):141-148.
- [Google Scholar]
- Molecular and cellular mechanisms of atrialfibrosis in atrialfibrillation. JACC. Clin. Electrophysiolo.. 2017;3(5):425-435.
- [Google Scholar]
- Cardiac (myo)fibroblasts modulate the migration of monocyte subsets. Sci. Rep.. 2018;8:5575.
- [Google Scholar]
- Antifibrotic effects of a polysaccharide extracted from ganoderma lucidum, glycyrrhizin, and pentoxifylline in rats with cirrhosis induced by biliary obstruction. Biol. Pharm. Bul.. 1997;20(4):417-420.
- [Google Scholar]
- The impact of using different age-adjusted cutoffs of D-dimer in the diagnosis of pulmonary thromboembolism. Am. J. Emerg. Med.. 2021;43:118-122.
- [Google Scholar]
- TGF-beta-independent CTGF induction regulates cell adhesion mediated drug resistance by increasing collagen I in HCC. Oncotarget. 2017;8(13):21650-21662.
- [Google Scholar]
- Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur. Heart J.. 2017;38(1):38-50.
- [Google Scholar]
- A review of the molecular mechanisms underlying Cardiac fibrosis and atrial fibrillation. J. Clin. Med.. 2021;10(19):4430.
- [Google Scholar]
- The inhibitory effects and mechanisms of polymannuroguluronate sulfate against human papillomavirus infection in vitro and in vivo. Carbohydr. Polym.. 2020;241:116365
- [Google Scholar]
- Natural polysaccharides as potential anti-fibrotic agents: a review of their progress. Life Sci.. 2022;308:120953
- [Google Scholar]
- The crucial role of activin A/ALK4 pathway in the pathogenesis of Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation. Basic Res. Cardiol.. 2017;112(4):47.
- [Google Scholar]
- Determination of M/G ratio of propylene glycol alginate sodium sulfate by HPLC with pre-column derivatization. Carbohydr. Polym.. 2014;104:23-28.
- [Google Scholar]
- Anticoagulant and FGF/FGFR signal activating activities of the heparinoid propylene glycol alginate sodium sulfate and its oligosaccharides. Carbohydr. Polym.. 2016;136:641-648.
- [Google Scholar]
- Alginate suppresses liver fibrosis through the inhibition of nuclear factor-kappa B signaling. Drug Des. Dev. Ther.. 2020;14:1295-1305.
- [Google Scholar]
- Study on the bioassay methods of the biological potency of propylene glycol alginate sodium sulfate (PSS) Chin. J. Mar. Drugs. 2014;33:65-69.
- [Google Scholar]
- Anticoagulant and antithrombotic activities of low-molecular-weight propylene glycol alginate sodium sulfate (PSS) Chin. J. Mar. Drugs. 2016;114:33-40.
- [Google Scholar]
- Propylene glycol guluronate sulfate (PGGS) reduces lipid accumulation via AMP-activated kinase activation in palmitate-induced HepG2 cells. Int. J. Biol. Macromol.. 2018;114:26-34.
- [Google Scholar]
- TGF-beta/Smad and JAK/STAT pathways are involved in the anti-fibrotic effects of propylene glycol alginate sodium sulphate on hepatic fibrosis. J. Cell Mol. Med.. 2020;24(9):5224-5237.
- [Google Scholar]
- Determination of the free and linked sulfate content of propylene glycol alginate sodium sulfate. Chin. J. Mar. Drugs. 2014;33:1-5.
- [Google Scholar]
- Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS) Carbohydr. Polym.. 2016;144:330-337.
- [Google Scholar]
- The mechanisms of sulfated polysaccharide drug of propylene glycol alginate sodium sulfate (PSS) on bleeding side effect. Carbohydr. Polym.. 2018;194:365-374.
- [Google Scholar]
- Activin A is a potent activator of renal interstitial fibroblasts. J. Am. Soc. Nephrol. JASN. 2004;15(1):91-101.
- [Google Scholar]
- Oxymatrine inhibits transforming growth factor beta 1 (TGF-beta 1)-induced cardiac fibroblast-to-myofibroblast transformation (FMT) by mediating the notch signaling pathway in vitro. Med. Sci. Monit.. 2018;24:6280-6288.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105792.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







