Translate this page into:
Network pharmacology of Dracaena sp. in Guangxi and its related species leaf secondary metabolites possess antioxidant properties
⁎Corresponding authors at: College of Life Science and Technology, Guangxi University, No.100 Daxue East Road, Nanning, Guangxi 530004, China. shenpeihong@gxu.edu.cn (Peihong Shen), ynhuangld@gxu.edu.cn (Luodong Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Performed comparative metabolome in the leaves of six Dracaena species. Secondary metabolism of Dracaena is both species-specific and individual-specific. Network pharmacology revealed the antioxidant mechanism in Dracaena leaves. Dracaena sp. in Guangxi is cryptic specie and contain potentially medicinal value.
Abstract
In this study, metabolite maps were conducted on leaf samples accessions of Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3) and six related species including D. angustifolia (CH), D. elliptica (XZ), D. cochinchinensis (CB), D. cambodiana (HN), D. marginata (HB), and Yucca schidigera (SL). We identified 2,971 compounds including carboxylic acids and their derivatives, organooxygen compounds, prenol lipids, benzenes and their substituted derivatives, and flavonoids. Of those, 73 characteristic secondary metabolites were screened out by weighted correlation network analysis (WGCNA). Pterostilbene, 9-cis-retinal, and dracorubin were unique to the Dracaena sp. in Guangxi, and 3-hydroxytridecanoic acid and chrysin were highly abundant in them. An in vitro antioxidant activity assay disclosed that all 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) scavenging rates and all fluorescence recovery after photobleaching (FRAP) rates were in the ranges of 75–87 % and 3.10–3.25 μmol/mL, respectively, for the Dracaena sp. in Guangxi. Hence, the latter have relatively high and stable foliar antioxidant activity. A network pharmacological analysis of the top 20 metabolites in Dracaena leaf and their 446 antioxidant-related disease targets revealed ten core action targets, including GAPDH, AKT1, MAPK3, VEGFA, CASP3, TNF, MAPK1, SRC, EGFR, and MAPK8. An antioxidant-related compound-target-pathway network was constructed and verified by molecular docking. The results of this study provide a theoretical basis for mining and exploiting the foliar secondary metabolites of Dracaena.
Keywords
Antioxidant
Dracaena
Metabolomics
Molecular docking
Network pharmacology
Guangxi
1 Introduction
Dracaena spp. (Class Liliopsida, Order Asparagales, Family Asparagaceae) are rare plant species in tropical and sub-tropical regions. At least seven wild species including D. cochinchinensis, D. cambodiana, D. angustifolia (Synonym: D. menglaensis), D. elliptica, D. terniflora, D. impressivenia, and D. houkouensis, occur in Yunnan, Hainan, Guangdong, Guangxi, and Taiwan Provinces of China (Zheng et al., 2009; Zhang et al., 2019).
Since ancient times, the red resin (dragon’s blood) secreted by the stems of certain Dracaena spp. has been prized as a traditional Chinese medicine (TCM) (Parson and Prendergast, 2001). However, only D. cambodiana and D. cochinchinensis have been reported as sources of dragon’s blood sources in China. The Compendium of Materia Medica and the Shennong Materia Medica state that the red resin of Dracaena spp. activates the removes stasis, converges and stops bleeding, causes detumescence, alleviates pain, recuperates the blood, removes putrefaction, and alters muscle tone. The stems of Dracaena spp. contain flavonoids, phenols, terpenoids, steroids, esters, glycosides, and other secondary metabolites and its leaves contain flavonoids, feruloamides, resinols, steroids, and other medicinal compounds (Thu et al., 2020; Zhang et al., 2019). These aforementioned substances have antioxidant, antitumor, anti-inflammatory, antithrombotic, analgesic, hemostatic, and antidiabetic efficacy (Gupta et al., 2007; Mensor et al., 2001; Sun et al., 2019).
Most earlier studies focused on the secondary metabolites in the stems but paid little attention to the natural plant products in the leaves of Dracaena spp. The latter constitute the bulk of the shoot and form clusters on the stem apices (Fan et al., 2014). The leaves of Dracaena spp. have been administered for medicinal purposes (Knight and Joseph, 2000) and have been processed into beverages, powders, and other preparations. The Dai “Palm-Leaf Sutra” writes that Dracaena leaf and dragon’s blood have similar curative effects and are highly efficacious against diabetes. Local people often use the leaves of D. cochinchinensis to prepare a tea that alleviates stomachache and abdominal pain. The Food and Drug Administration of Yunnan Province has published standards for Dracaena cambodiana leaves (Dai Medicine Name: BaiMaiGaShai) (Yun YNZYC‑0114‑2007) (Yunnan Food and Drug Administration, 2005). The leaves are processed into a powder that is prescribed to clears away fire, detoxify, eliminate wind and soreness, promote blood circulation and remove stasis, reduce swelling, alleviate pain, and renew muscles and joints.
Dracaena has important medicinal value and effectively treats several chronic diseases. However, few studies have been conducted on the secondary metabolites in the leaves of different Dracaena spp.. Hence, there is a lack of information on new varieties with useful medicinal properties. Liquid chromatography-mass spectrometry (LC-MS) is now the principal technique by which metabolic changes occurring in living organisms are detected. LC-MS can identify most metabolites, analyze a wide variety of complex biological samples, and generate comprehensive metabolome data (Rinschen et al., 2019; Plumb et al., 2023). Thus, it can effectively perform qualitative and quantitative analyses of the metabolic components of Dracaena spp.
Antioxidants inhibit the biosynthesis of reactive oxygen species (ROS). The leaves of Dracaena spp. have strong antioxidant activity and could effectively treat various diseases (Morrogh-Bernard et al., 2017). The antioxidant efficacy of Dracaena leaves is closely linked to their flavonoid, phenolic compound, and other secondary metabolites content (Ong et al., 2016). Network pharmacology systematically integrates drug, target, and disease interactions (Zhao et al., 2023). Molecular docking validates the interactions among active compounds and their disease targets (Asiamah et al., 2023). Researchers widely apply metabonomics, network pharmacology, and molecular docking to elucidate the molecular mechanisms by which antioxidant plant extracts treat various human diseases.
The ambient environment has a significant impact on plant secondary metabolite biosynthesis and accumulation. Altitude, temperature, and other physical factors affect metabolite yield, quality, composition, and efficacy (Li et al., 2020). Dracaena sp. (previously named D. cochinchinensis) is widely distributed in Guangxi, China, and is recognized there as an important source of dragon’s blood. Nevertheless, we discovered that Dracaena sp. in Guangxi is cryptic species, which had unique shapes and characteristics intermediate between those of D. cambodiana and D. cochinchinensis (Wang, 2023). Here, we used UPLC-MS/MS to identify and compare the secondary metabolites of six wild Dracaena sp. in Guangxi and six related species, clarify the molecular mechanisms of these substances, and evaluate the characteristics of all foliar antioxidants. We integrated metabolomics, network pharmacology, and molecular docking, and provided a theoretical basis for elucidating the phytochemical characteristics of the leaves of Dracaena sp. in Guangxi and selecting and breeding these plants.
2 Materials and methods
2.1 Plant materials and phylogenetic trees
Six accessions of Dracaena sp. were collected at various sites in Nanning City (voucher No. RM1), Daxin County (C6, C13), Chongzuo City (CZ4), Jingxi City (JX2), and Bama County (BM3) of Guangxi Province, China. Six related species including D. angustifolia (CH), D. elliptica (XZ), D. cochinchinensis (CB), D. marginata (HB), D. cambodiana (HN), and Yucca schidigera (SL) (Fig. 1) were acquired from the Dracaena Germplasm Resource Bank of Guangxi University (Nanning City, Guangxi Province, China; Dr. Luodong Huang, ynhuangld@gxu.edu.cn). More detailed information about the sample collection area is shown in Table S1. All leaf samples were stored at −80 °C until the subsequent DNA extraction and metabolome analyses.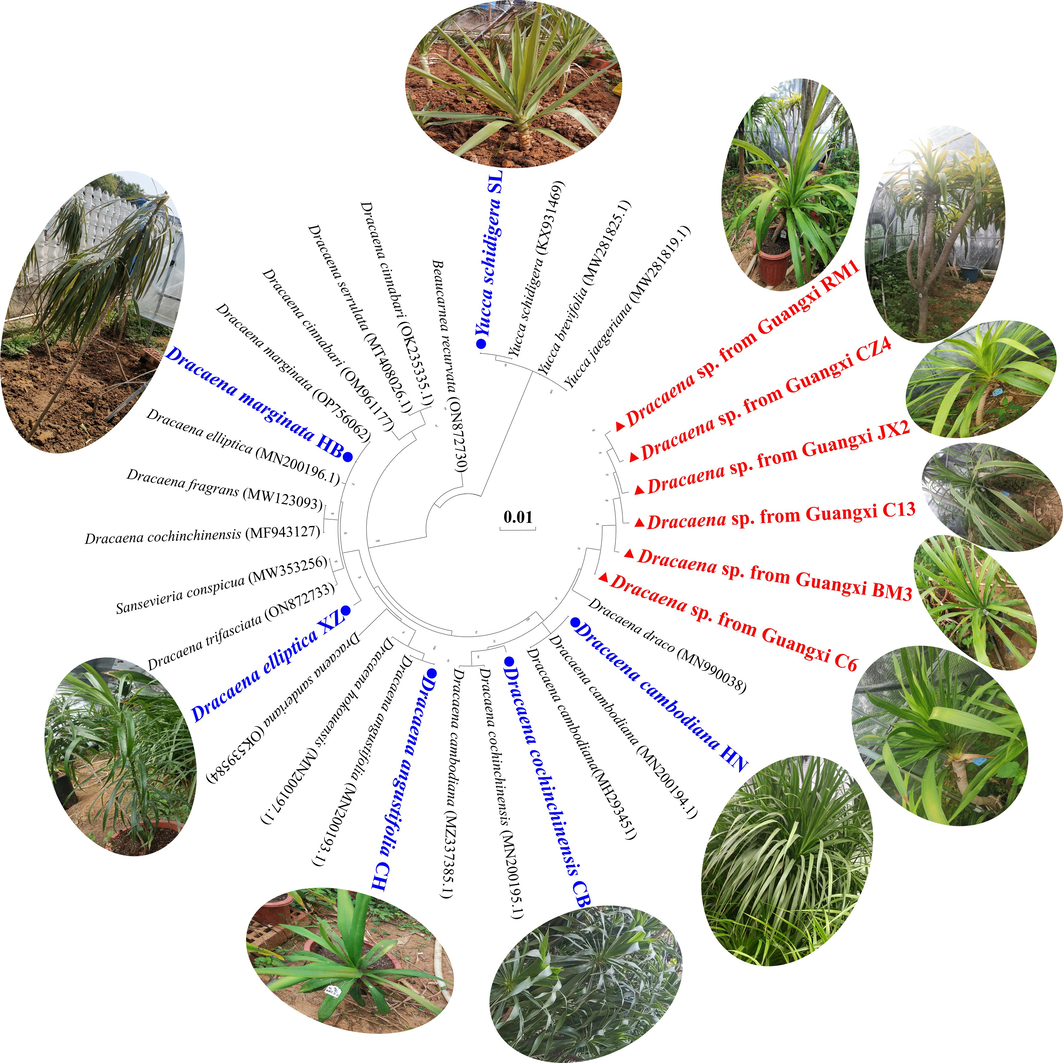
Plant characteristics, experimental materials, and phylogenetic relationships among Dracaena sp. in Guangxi and its related species.
The chloroplast clpP gene primers and PCR amplification conditions used here were those previously described by Zhang et al. (2019). The DNA was isolated with a MiniBEST Plant Genomic DNA Extraction Kit (TaKaRa, China) and the amplified products were sequenced at Sangon Biotech Co. Ltd., Shanghai, China. The sequencing data were artificially corrected with the SeqMan package in LaserGene (DNASTAR Inc., Madison, WI, USA) according to the method of Li et al. (2022). Mega v. 11 (https://www.megasoftware.net/) was then used for multiple comparison rounds and to construct a phylogenetic tree for various Dracaena spp. and their relatives.
2.2 Component extraction and metabolome analysis
For the metabolome analysis, 5 g of each sample was placed in a lyophilizer (LICHEN, China), vacuum freeze-dried for 72 h, and pulverized. Then 400 mg powder was accurately weighed out, dissolved in 10 mL of 70 % (v/v) ethanol solution, extracted overnight with a magnetic stirrer (LICHEN, China), subjected to ultrasonic extraction for 2 h, and centrifuged at 10,000 × g for 10 min. The total metabolite extraction rate reached 25–40 %. The supernatants were then passed through a 0.22 μm filter membrane and stored in sample injection bottles until they were subjected to UPLC-MS/MS.
The data acquisition instruments included a Q-ExactiveTM quadrupole electrostatic field orbital trap high-resolution mass spectrometry system (Thermo Fisher Scientific, Waltham, MA, USA) and an UltiMateTM 3000 ultrahigh performance liquid chromatography system (Thermo Fisher Scientific). The chromatographic column was an ACQUITY UPLCBEH C18 (50 mm × 2.1 mm, 1.7 μm) (Thermo Fisher Scientific). The column and automatic sampler temperatures were 30 °C and 10 °C, respectively. Mobile phases A and B were 0.1 % (v/v) formic acid and methanol, respectively, and negative ion (ESI-) mode was used. The sample elution gradient was as follows: 0–2 min, 95 % A-95 % A; 2–13 min, 95 % A-0 % A; 13–16 min, 0 % A-0 % A; 16–16.1 min, 0 % A-95 % A; and 16.1–19 min, 95 % A-95 % A. The injection volume and velocity were 2 μL and 0.3 mL/min, respectively. The MS conditions were as follows: ion source, heating electrospray ionization (HESI); temperature, 350 °C; spray voltage in negative ion mode, 3.0 kV; capillary temperature, 320 °C; sheath gas pressure, 35 psi; auxiliary airflow rate, 10 psi; scan mode, full MS/dd-MS2; mass range, 70–1,000 m/z; primary and secondary scan resolutions, 70,000 and 17,500, respectively; and collision gas, high-purity nitrogen. The foregoing optimized operating parameters were derived from Llorach et al. (2019).
2.3 Computational analysis of metabonomics data
Raw data files generated by UPLC-MS/MS using Compound Discoverer v.3.2 (Thermo Fisher Scientific) and Xcalibur v. 2.2.0 (Thermo Fisher Scientific) and processed for the peak comparison and selection and the quantification of each metabolite. The peak intensities were normalized to the total spectral intensity, the molecular formulae were predicted based on the addition ions, the molecular ion peaks, and the fragment ions, and the results were then compared with the mzCloud (https://www.Mzcloud.org/), mzVault (https://thermo-mzvault.updatestar.com/en), and ChemSpider (https://www.chemspider.com) databases. Background ions were removed with blank samples, the original quantitative results were normalized, and the metabolites were identified and comparatively quantified.
The leaf secondary metabolites were quantified using the multiple reaction monitoring (MRM) model in a triple quadrupole mass spectrometer. The precursor (parent) ions of the target substances were screened by the quadrupole, and ions corresponding to substances with different molecular weights (MWs) were eliminated. The precursor ions were generated and ion fragments were formed in the collision chamber, and ions with the required characteristics were screened by the triple quadrupole. Mass spectral (MS) data were obtained for the various metabolites, the MS peaks were integrated, and those corresponding to the same metabolite were corrected. The relative metabolite content of each sample was determined by integrating the peak areas (Fraga et al., 2010).
Using public databases such as HMDB (https://hmdb.ca/), METALIN (https://metlin.scripps.edu/index.php), LIPIDMAPS (https://lipidmaps.org/), PubChem (https://pub.ncbi.nlm.nih.gov.cn/), and MasschemBank (https://massbank.jp/) were consulted for metabolite comments. A weighted correlation network analysis (WGCNA) package (https://horvath.genetics.ucla.edu/html/CoexpressionNetwork/Rpackages/WGCNA) was applied to build common expression modules according to the method of Ning et al. (2022). TBtools-II v.2.083 (Chen et al., 2023) was used to plot Venn diagrams,visualize the relative metabolite content, and compare metabolites among groups. The metabolites were annotated and subjected to a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis.
2.4 Antioxidant capacity determination and network pharmacology
The 2,2‘-azinobis-(3- ethylbenzthiazoline- 6-sulphonate) (ABTS), FRAP, and DPPH scavenging rates of the leaf extracts were determined per the method of Rumpf et al. (2023). The targets of the top 20 metabolites were predicted based on the Swiss Target Prediction database (https://www.swisstargetprediction.ch). The target protein data were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://www.tcmsp-e.com/tcmsp.php). DisGeNET (https://disgenet.org/), GeneCards (https://genecards.org/), Online Mendelian Inheritance in Man (OMIM, https://omim.org/), PharmGKB (https://pharmgkb.org/), and the Therapeutic Target Database (TTD, https://db.idrblab.net/ttd/) were used to screen disease targets. Antioxidant target proteins were searched using the keyword “antioxidant”. Targets with scores ≥ 1 were selected and merged from the preceding databases, and duplicate disease targets were deleted.
A protein–protein interaction (PPI) network diagram of the target proteins was plotted by importing the composition-disease intersection target data into the STRING database (https://string-db.org). The PPI results were visualized with Cytoscape (https://cytoscape.org). Gene ontology (GO) category and KEGG pathway enrichment analyses of the core targets were conducted on the Metascape platform (https://metascape.org/gp/index.html#/main/step1) to determine their potential functions. False discovery rate (FDR) < 0.05 was the threshold significance level for screening relevant GO categories and KEGG pathways. Cytoscape was then used to construct a compound-target-pathway network.
2.5 Molecular docking analysis of metabolites
The 3D structures of the selected compounds were acquired from PubChem (https://pubchem.ncbi.nlm.nih.gov) and optimized with SYBYL Molecular Modeling Software (https://labs.chem.ucsb.edu/gerig/thomas/chem142a/sybyl_intro.html), and 3D minimum free energy conformations were obtained for each compound. The crystal structures of the core target proteins glyceraldehyde 3-phosphate dehydrogenase (GAPDH), protein kinase B (AKT1), mitogen-activated protein kinase-3 (MAPK3), vascular endothelial growth factor-A (VEGFA), caspase-3 (CASP3), tumor necrosis factor (TNF), MAPK1, SRC, epidermal growth factor receptor (EGFR), and mitogen-activated protein kinase-8 (MAPK8) were downloaded from the Protein Data Bank (PDB, https://www.rcsb.org) using the PDB IDs 6ynf, 7apj, 6ges, 6zbr, 7rn7, 7khd, 7e73, 7nxe, 6lud, and 4ux9. The crystal structures were then optimized for dehydration, hydrogenation, and docking grid boxes using AutoDock Tools (https://autodocksuite.scripps.edu/adt/). Autodock Vina (https://vina.scripps.edu/) was used to study molecular docking. Each molecular docking yielded 20 conformations, and the one with the highest affinity was used in the final docking.
3 Results and discussion
3.1 Phylogenetic analysis
The clpP genes were amplified and sequenced to understand the phylogenetic relationships among six accessions of wild Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3) as well as those among other accessions from six related species (HN, CB, CH, XZ, HB, and SL). The clpP sequence length was in the range of 1,513–1,584 bp. The Sansevieria conspicua and Beaucarnea recurvata were used as outgroups. The optimal maximum likelihood (ML) model was generated by the MaGe v. 10 algorithm for the clpP sequences of 33 accessions. The default values were applied for all parameters except the site coverage limit which was set to 85 %. The Bayesian information criterion (BIC) score indicated that the ideal ML model was “T92 + G (Tamura3-Parameter)”. The ML phylogenetic tree was constructed with Mega v. 11 according to the model prediction results, revealed two distinct clades, and showed that D. angustifolia (CH), D. elliptica (XZ), and D. marginata (HB) are genetically distant from D. cochinchinensis (CB), D. cambodiana (HN) and Dracaena sp. in Guangxi. Then, the Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3) were isolated and most of the nodes had relatively high support values. Hence, they might differ from other existing Dracaena. They formed a large branch with D. cochinchinensis (CB) and D. cambodiana (HN) (Fig. 1). These three Dracaena species are sources of dragon’s blood. Moreover, the six wild Dracaena accessions collected from various regions in Guangxi all belonged to the same species and significantly differ from other Dracaena spp.
3.2 Metabolomics analysis of 12 accessions
Metabonomics is the study of small endogenous molecules with relative MW < 1,000. Here, we detected 2,971 metabolites including 410 carboxylic acids and their derivatives, 271 fatty acyl compounds, 206 prenol lipids, 155 steroids and their derivatives, 154 flavonoids, 58 glycerophospholipids, 46 cinnamic acids and their derivatives, 45 phenols, 44 coumarins and their derivatives, and other compounds in C6, C13, CZ4, JX2, RM1, BM3, HN, CB, CH, XZ, and HB. Sample compositions are illustrated in Fig. 2.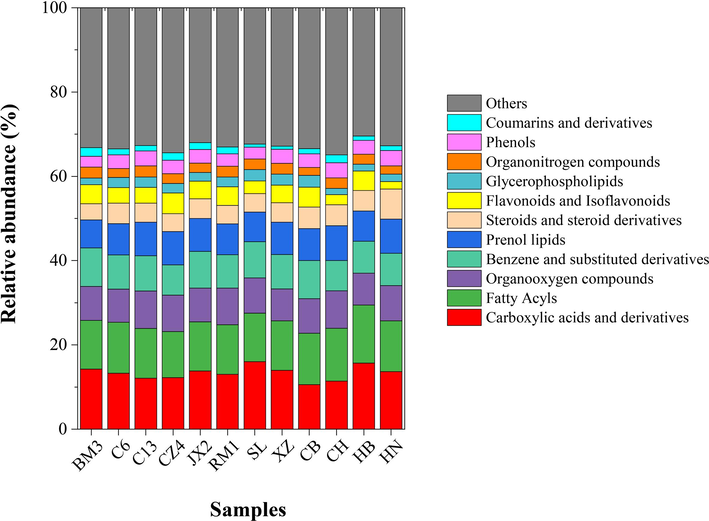
Untargeted metabolomics analysis identifying secondary metabolites in leaves of 12 accessions. Differentially accumulating metabolites were assigned to various secondary metabolite categories.
There were 1,520 metabolites in all 12 accessions, 565 endemic metabolites in the Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3), and 886 endemic metabolites (Fig. 3A) in the six related species (HN, CB, CH, XZ, HB, and SL). Of the 565 metabolites unique to Dracaena sp. in Guangxi, the first five were carboxylic acids and their derivatives followed by organooxygen compounds, prenol lipids, benzenes and their substituted derivatives, and flavonoids. There were 25 stable and abundant metabolites in the six wild accessions of Dracaena sp. in Guangxi, and their structures and spectral data are listed in Supplementary Table S2. Pterostilbene, an active component of dragon’s blood, has anti-inflammatory and antitumor efficacy, and inhibits platelet aggregation (McCormack and McFadden, 2013; Qu et al., 2023). Vitamin A and its derivative 9-cis-retinal were abundant in the Dracaena sp. in Guangxi. They regulate the cell cycle and have anticancer, anti-inflammatory, and neuroprotective efficacy. Moreover, 9-cis-retinal is used to treat visual impairment (Manzano et al., 2000; Yang et al., 2019).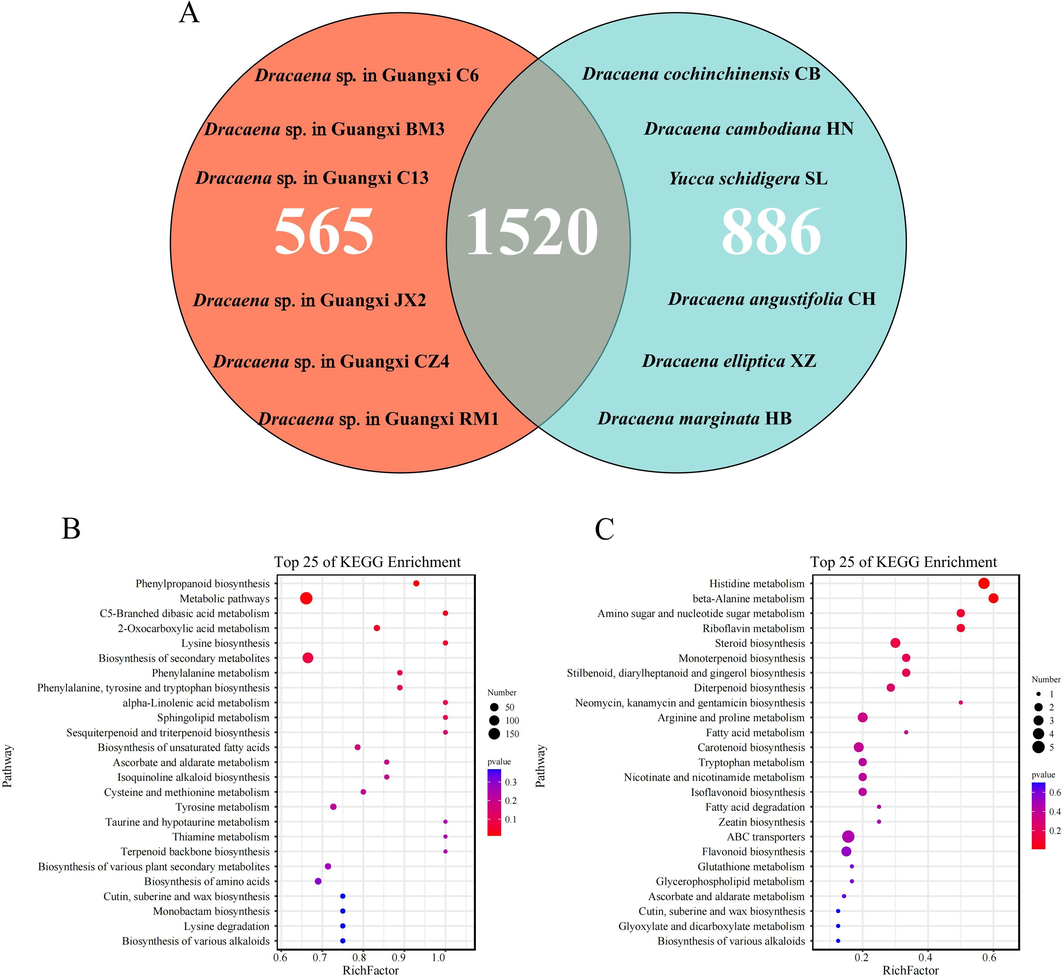
KEGG pathway analysis based on secondary metabolite biosynthesis in Dracaena sp. in Guangxi and their related species. (A) Venn diagram showing 1,520 secondary metabolites common to all accessions, 565 unique to six wild accessions of Dracaena sp. in Guangxi, and 886 unique to the remaining accessions. (B) KEGG pathway enrichment analysis of secondary metabolites common to all 12 accessions. (C) KEGG pathway enrichment of secondary metabolites unique to six wild accessions of Dracaena sp. in Guangxi.
The present study focused on flavonoids as they are the main active components in dragon’s blood. We detected 97 flavonoids which accounted for 4.65 % of the total secondary metabolite profile in Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3). We also found 34 unique flavonoids in them (Supplementary Table S3). The flavonoid profile was the most abundant and complex of all, and numerous dihydrochalcones, chalcones, flavonoids, isoflavones, and other compounds were isolated from the aforementioned Dracaena sp. in Guangxi. Proanthocyanidin-dracorubin (Gong et al., 2008) was extracted exclusively from the resin of CZ4. These results suggest secondary metabolism is both species- and individual-specific in Dracaena. Diosmetin is anti-inflammatory, antioxidant, antibacterial, and analgesic (Choi et al., 2019; Li et al., 2022). Chrysin (Li et al., 2022; Naz et al., 2019) and daidzein (Das et al., 2018; Laddha and Kulkarni, 2023) are administered for the treatment of cancers, neurodegenerative, and heart diseases, diabetes and its complications, and osteoporosis.
The abovementioned findings suggest that each Dracaena sp. in Guangxi and other regions produces specific secondary metabolites. We can speculate that preponderance of flavonoids in Dracaena is more effectively protect and defend the host plants against predation, pathogenesis, ROS, etc. than other natural plant products. Hence, there are regional differences among Dracaena spp. in terms of the profiles, combinations, interactions, and biological characteristics of their metabolites.
3.3 Metabolic pathway analysis
The composition and content of the accumulated metabolites in the Dracaena sp. in Guangxi differ from those of other related species. A KEGG pathway enrichment analysis was conducted on the 1,520 metabolites in the 12 accessions and the 565 metabolites in the Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3). It has discovered that the 25 most important pathways included Phenylpropanoid biosynthesis, C5-branched diacid basic metabolism, 2-Oxocarboxylic acid metabolism, Lysine biosynthesis, Phenalylanine, tyrosine and tryptophan synthesis, and Sesquiterpinoid and monoterpenoid synthesis (Fig. 3B). Some of the 25 most important pathways in the six accessions of Dracaena sp. in Guangxi included Histidine metabolism, β-alanine metabolism, Amino sugar and nucleotide sugar metabolism, Riboflavin metabolism, Sesquiterpinoid synthesis, Monoterpenoid synthesis, Isoflavonoid synthesis, and Flavonoid biosynthesis (Fig. 3C). The Dracaena sp. in Guangxi were significantly enriched in the downstream metabolic pathways of certain antioxidant and anti-inflammatory pharmacologically active substances. These discoveries provides insights into the biosynthesis and accumulation of the active metabolites in the Dracaena sp. in Guangxi.
3.4 Identification of closely related metabolite modules
Different plant species produce their specific secondary metabolites and thousands of intermediate metabolites with unique characteristics. WGCNA uses data for numerous metabolites to identify those that are synergistic (Langfelder and Horvath, 2008), and those that form clusters of interest. WGCNA also reveals metabolites that are significantly correlated with particular plant phenotypes (Yang et al., 2023). Here, WGCNA analyzed 2,971 metabolites and explored their associations with the Dracaena sp. in Guangxi and its related species. Fig. 4 shows the compositions of the 20 modules in which the metabolites were aggregated. Unaggregated metabolites are shown in gray. Details of the metabolites in each module are listed in Supplementary Table S4.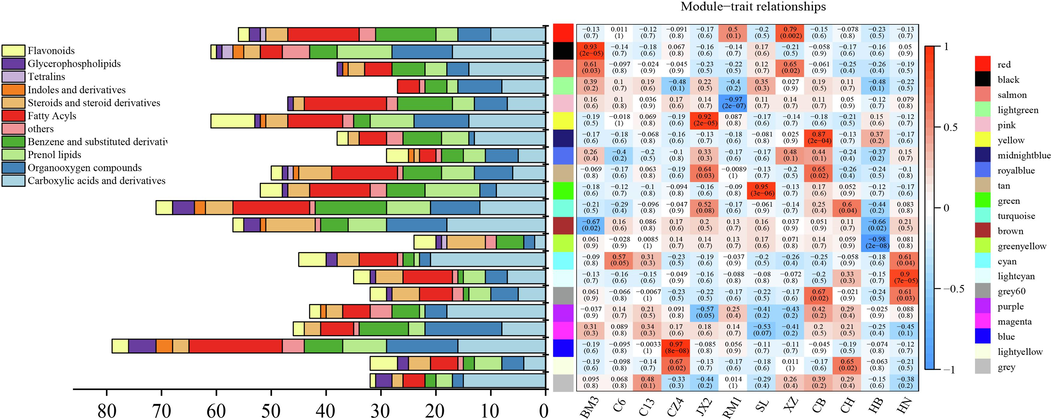
WGCNA of secondary metabolites among 12 accessions and various categories of secondary metabolites in each module.
The Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3) were strongly positively correlated with the magenta module which contained 73 metabolites. SL had the weakest correlation with the magenta module (cor = -0.53, p = 0.07), the strongest correlation with green module (cor = 0.95, p = 3e-06), and the highest abundance of prenol lipids. Thus, SL was a Yucca genus rather than a Dracaena.
The magenta module had the highest abundance of organooxygen compounds including chlorogenic and neochlorogenic acids. The latter also occur in other medicinal plants such as honeysuckle, eucommia leaves and chrysanthemum. These substances are antioxidant, hepatoprotective, renoprotective, antibacterial, antitumor, glucoregulatory, liporegulatory, anti-inflammatory, and neuroprotective (Wang et al., 2022; Mei et al., 2019). They also have significant antioxidant, antibacterial, antiviral, and antipyretic efficacy (Metwally, 2020; Yan et al., 2020; Yu et al., 2021). Other constituents included 2,4-diacetylphloroglucinol (2,4-DAPG) which is a broad-spectrum antibiotic (Biessy and Filion, 2021; Zhang et al., 2022) and vanillin acetone (Hart et al., 2014; Graton et al., 2019) which is anti-inflammatory and antihypertensive. The JX2 sample was strongly positively correlated with the yellow module which included flavonoids such as rutin, nictoflorin, xanthorhamnin, and afzelin. The CZ4 sample was strongly positively correlated with the blue module which had the highest abundance of nearly all metabolites including fatty acyls and glycerophospholipids. Hence, the sample of Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3) have a unique metabolite profiles. It were significantly correlated in the metabolite clusters and differed from other species of Dracaena in terms of their compounds with unique pharmacological effects. These discoveries lay a theoretical foundation for breeding Dracaena germplasm resources and studying their pharmacological properties.
The XZ sample was strongly positively correlated with the red (cor = 0.79, p = 0.002) and salmon (cor = 0.65, p = 0.02) modules. The CB sample was strongly positively correlated with the midnight blue (cor = 0.87, p = 2e-04), tan (cor = 0.65, p = 0.02) and gray 60 (cor = 0.67, p = 0.02) modules. The CH sample was strongly positively correlated with the turquoise (cor = 0.6, p = 0.04) and light yellow (cor = 0.65, p = 0.02) modules. The HB sample was, in general, more weakly correlated with the modules than all other samples. The HN sample was strongly positively correlated with the cyan (cor = 0.61, p = 0.04), light cyan-gray 60 (cor = 0.9, p = 7e-05), and gray 60 (cor = 0.61, p = 0.03) modules. The preceding results indicate that each Dracaena spp. had a unique metabolite composition and these differences explain the various medicinal characteristics of Dracaena.
3.5 Identification metabolites of Dracaena sp. in Guangxi
We analyzed the differences in the chemical constituents of the various accessions of Dracaena sp. in Guangxi had a unique metabolite composition and pharmacological characteristics. We identified the common and the unique metabolites of C6, C13, CZ4, JX2, RM1, and BM3 via a Venn diagram (Fig. 5A). We discovered that 514 metabolites were common to all six accessions while 111, 75, 131, 160, 108, and 118 metabolites were unique to C6, C13, CZ4, JX2, RM1, and BM3, respectively. The top 12 classes of common metabolites were fatty acyls, benzenes and their substituted derivatives, carboxylic acids and their derivatives, prenol lipids, organooxygen compounds, steroids and their derivatives, organonitrogen compounds, flavonoids, piperidines, phenols, coumarins and their derivatives, phenols, and others (Fig. 5B) such as the flavone hispidulin which has significant anticancer and antimutagenic efficacy (Liu et al., 2020; Patel and Patel, 2017; Wang et al., 2020). Hyperoside prevents certain cancers and protects the brain, neurons, heart, kidneys, lungs, blood vessels, bones, joints, and liver (Fan et al., 2021; Sun et al., 2021). Kaempferol is antibacterial, anti-inflammatory, antioxidant, antitumor, cardioprotective, neuroprotective, and antidiabetic (Devi et al., 2015; Nejabati and Roar, 2022). Quercetin has strong antioxidant activity (Alizadeh and Ebrahimzadeh, 2022; Di Petrillo et al., 2022). Certain unstudied compounds have potential medicinal activity and will be further explored in future research (Mollica et al., 2014).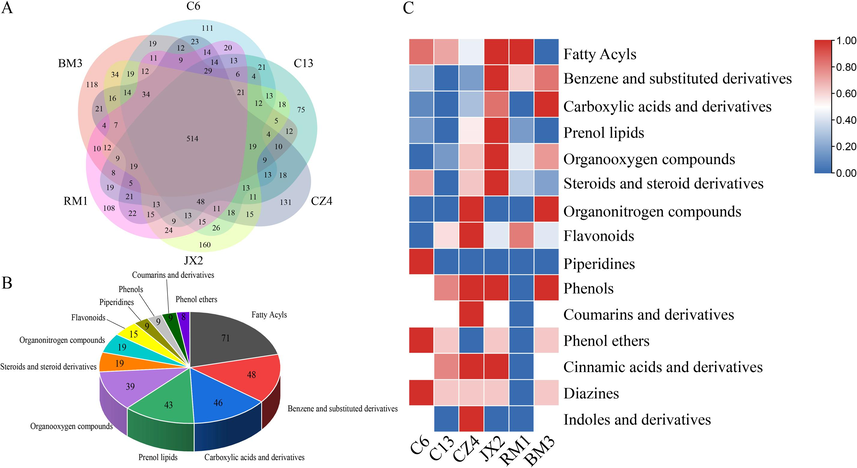
Analysis of secondary metabolite characteristics of six wild accessions of Dracaena sp. in Guangxi. (A) Venn diagram. (B) Classification of secondary metabolites common. (C) Heat map of secondary metabolite composition.
Several substances with a wide range of biological activity were detected in the leaves of the Dracaena sp. in Guangxi. We plotted the unique constituents of C6, C13, CZ4, JX2, RM1, and BM3 in a heat map (Fig. 5C). The JX2 sample had the greatest number of unique metabolites (1 6 0) including fatty acyls, benzenes and their substituted derivatives, prenol lipids, organooxygen compounds, and steroids. The C6 sample had the most piperidines and phenol ethers while the CZ4 sample had the most flavonoids and coumarins and their derivatives.
3.6 Analysis of the antioxidant activity in the leaves of 12 accessions (7 species)
We then envaluated the antioxidant activity of the Dracaena sp. in Guangxi and its related species. The DPPH and ABTS scavenging rates were in the ranges of 48–90 % and 75–90 %, respectively, and the FRAP was in the range of 2.15–3.25 μmol/mL (Fig. 6). For the leaf extracts from the 12 accessions, the order of the DPPH scavenging rates was SL > JX2 > CZ4 > C13 > CH > C6 > RM1 > BM3 > HN > HB > CB > XZ, the order of the ABTS scavenging rates was SL > CB > C13 > JX2 > RM1 > CH > C6 > C4 > HB > HN > BM3 > XZ, and the order of the FRAP was CZ4 > RM1 > SL > JX2 > C6 > BM3 > C13 > HN > CB > XZ > HB. The DPPH scavenging rates of the Dracaena sp. in Guangxi (C6, C13, CZ4, JX2, RM1, and BM3) were in the range of 75–87 % and were substantially higher than those for D. cambodiana (HN) and D. cochinchinensis (CB) (74 % and 62 %, respectively). The latter two are sources of dragon’s blood. The ABTS scavenging rates of the six Dracaena sp. in Guangxi were in the range of 77–89 % whereas those for HN and CB were 81 % and 89 %, respectively. The FRAP values were as follows: CZ4, 3.25 μmol/mL; JX2, 3.19 μmol/mL; RM1, 3.24 μmol/mL; C13, 3.10 μmol/mL; BM3, 3.18 μmol/mL; C6, 3.19 μmol/mL; HN, 2.74 μmol/mL; and CB, 2.67 μmol/mL. The FRAP value tends to increase with the DPPH and ABTS scavenging rates (Ghalloo et al., 2022).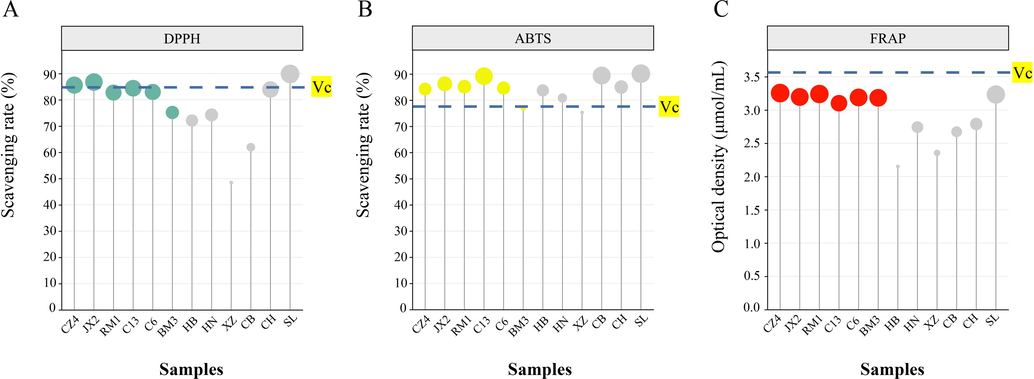
DPPH (A), ABTS (B), and (FRAP) (C) assays of leaf extracts from 12 accessions.
The preceding assays revealed the antioxidant activity of various Dracaena leaf extracts. It markedly differed among Dracaena accessions but was strong and stable in them all. The leaf extracts of SL had higher antioxidant activity than those of all other accessions possibly because SL was enriched in prenol lipids. Pterostilbene and vitamin A and its derivatives might have also contributed to the antioxidant activity of the Dracaena sp. in Guangxi.
3.7 Network pharmacological analysis
For all 12 accessions (7 species) selected the top 20 most abundant and stable compounds (Supplementary Table S5) and identified 446 of their targets. We also screened 1,056 disease targets from various antioxidant gene databases and obtained 138 potential antioxidant targets (Fig. 7) by intersecting the aforementioned substances and their disease targets.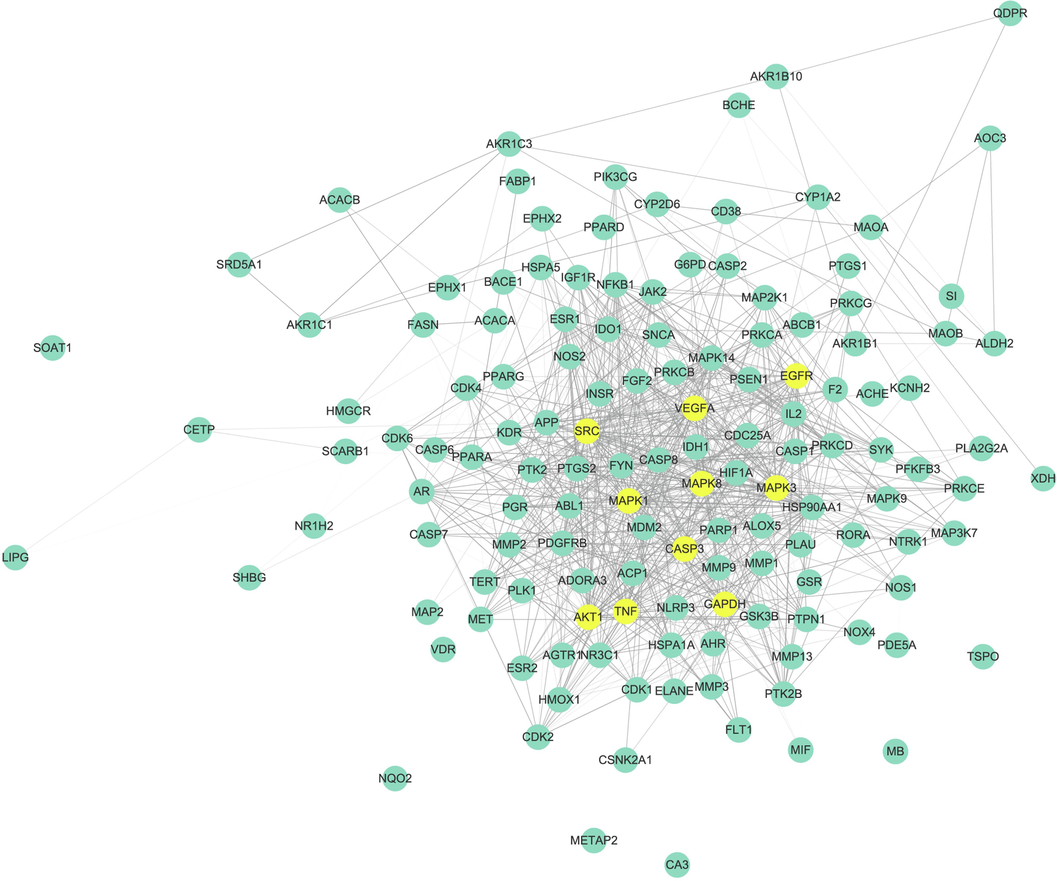
Top 20 most stable and abundant compounds in 12 accessions selected as research objects, network pharmacological analysis of their antioxidant activity, and cross-target PPI networks of antioxidant constituents of Dracaena sp. in Guangxi and their related species elsewhere.
We plotted a PPI network for the 138 target proteins and designated the first 10 with degree ≥ 10 (GAPDH, AKT1, MAPK3, VEGFA, CASP3, TNF, MAPK1, SRC, EGFR, and MAPK8) as core targets (Fig. 7). CASP3 is widely used as an apoptosis biomarker (Lin et al., 2016; Zhang et al., 2021). AKT1 is implicated in metabolism, proliferation, and angiogenesis (Thirumal Kumar et al., 2019; Wang et al., 2023). VEGFA induces endothelial cell formation in arteries, veins, and lymphatic vessels, promotes angiogenesis, and is anti-inflammatory (Claesson-Welsh and Welsh, 2013; Liu et al., 2023). EGFR activates the binding and receptor activity of epidermal growth factor, participates in the cellular response to amino acid stimulation, and positively regulates fibroblast proliferation (Jutten and Rouschop, 2014; Ma et al., 2015; Liu et al., 2017).
We conducted GO category and KEGG pathway enrichment analyses on the 10 core targets. The first 20 enriched GO terms are shown in Fig. 8. Response to growth factor, Response to reactive oxygen species, Response to molecules of bacterial origin, and Response to oxidative stress were significantly enriched (Fig. 8B). The KEGG analysis showed that AGE-RAGE signaling pathway in diabetic complications, Fluid shear stress and atherosclerosis, Proteoglycans in cancer, and Human CMV infection pathways were significantly enriched pathways (Fig. 8C). We then plotted a composition-target-pathway interaction network based on the aforementioned 20 components, 10 core targets, and first 20 KEGG pathways (Supplementary Figure S1). For all 12 accessions, numerous stable compounds were closely associated with antioxidant and antibacterial efficacy as well as growth factors. These results lay a theoretical foundation for elucidating the antioxidant properties of leaf extracts of Dracaena sp. in Guangxi and its related species.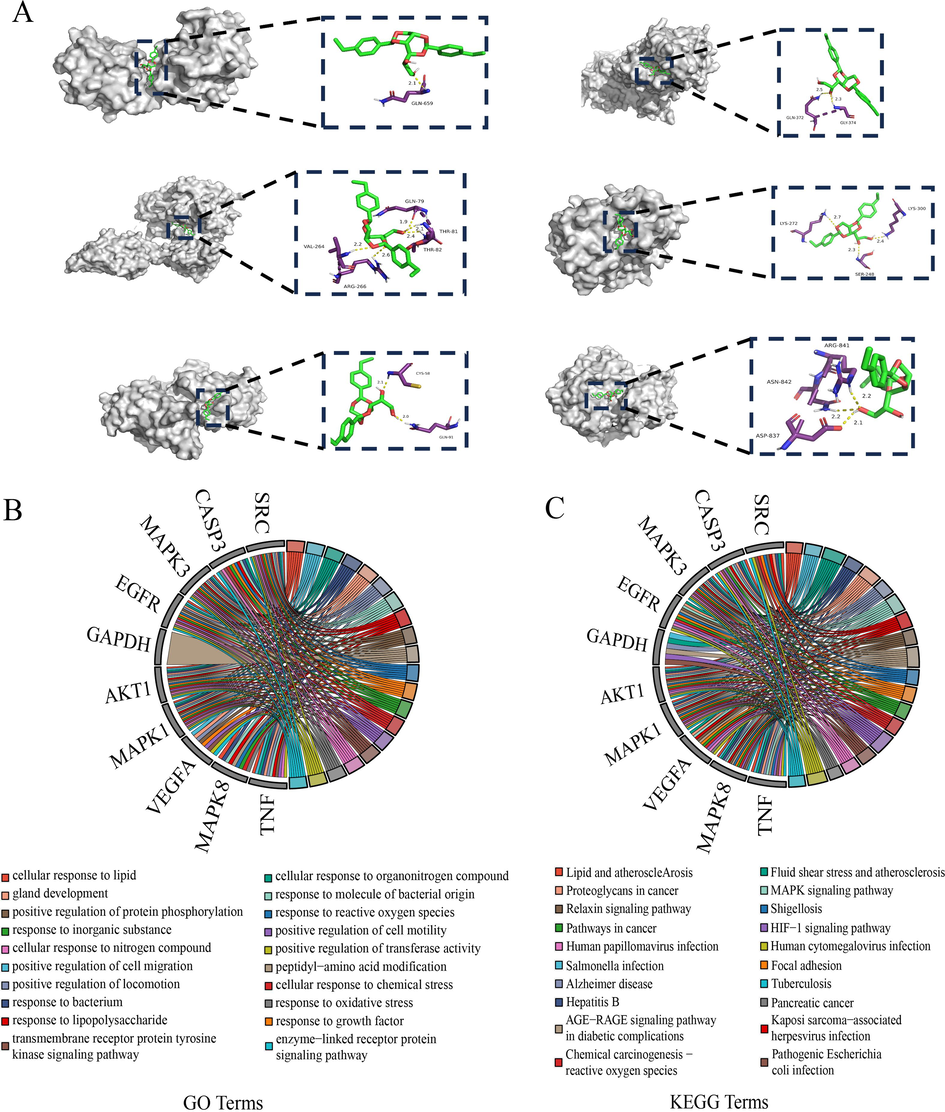
Molecular docking results. (A) Schematic diagram of partial molecular docking between active components META977 (bis(4-ethylbenzylidensil) and antioxidant-related core target proteins. (B)–(C) Top 20 GO terms and KEGG pathways of hub genes.
3.8 Molecular docking study
Molecular docking is commonly implemented in computer-aided drug design. It analyzes interactions between small and large molecules, identifies active precursor compounds, and provides evidence for structural optimization (Huang and Zou, 2010). Here, we performed molecular docking on the 20 selected compounds and 10 antioxidant-related core target proteins. A binding energy heat map disclosed that most of the selected compounds could bind the 10 target proteins (Supplementary Figure S2), and META1391 (rutin) most tightly bound them followed by META977 (bis(4-ethylbenzylidensil)sorbitol), META1847 (N,N‘-dicyclohexylylurea), and META2847 (3-β,24R,24‘R-fucosterol epoxide) (Supplementary Fig. S2).
We visualized 44 conformations with binding energy < -6 kcal/mol in PyMOL to detect interactions between the compounds and their target proteins. There were one, five, two, two, three, and three hydrogen bonds between META977 (bis(4-ethylbenzylidensil)) and the target proteins (SRC, AKT1, TNF, MAPK3, MAPK1, EGFR) (Fig. 8A). META977 could potentially treat various stomach diseases (Yu et al., 2022), and its high abundance in Dracaena leaf might explain the strong antioxidant activity potential pharmacological activity of this plant material.
Supplementary Figure S3 shows molecular docking between selected compounds and their antioxidant-related core target proteins. Thirty-seven form at least one hydrogen bond and have good binding affinities for their core targets. These results indicate that the compounds extracted from the leaves of the 12 accessions can bind several antioxidant targets. Future experiments should aim to optimize the medical implementation of Dracaena leaf extracts via in silico techniques (Stefanucci A et al., 2019) and derive novel antioxidant products from them.
4 Conclusion
Here, metabolomics identified 2,971 secondary metabolites in the leaves of 11 accessions of Dracaena (6 species) and one accessions of Yucca schidigera for the first time. We detected interspecies and intervarietal differences in the composition and content of these compounds. WGCNA was applied to identify the natural plant products characteristic of 12 accessions. The six accessions of Dracaena sp. in Guangxi were highly similar in terms of their secondary metabolite compositions. We identified certain metabolites that are unique to each of them and significantly differ from those of the other species. These findings form the basis for mapping the metabolites of various Dracaena spp. We used network pharmacology and molecular docking to investigate the antioxidant properties of Dracaena, and target correlation analyses screened META1391, META977, META1847, and META2847 which have potential pharmacological activity. The present work indicated that the Dracaena sp. in Guangxi are potential germplasm resources with strong pharmacological efficacy, identified certain metabolites with potential pharmacological activity, and provided a theoretical basis for selecting novel medicinally active Dracaena varieties and developing natural medicinal active ingredients.
Funding
This research was funded by the Project of Bama County for Talents in Science and Technology, China (No.20210036, 202101269) and Innovation Projects of Guangxi Graduate Research, China (YCSW2022028).
CRediT authorship contribution statement
Jiale Guo: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. Zihao Wang: Formal analysis, Investigation, Validation, Writing – review & editing. Yihan Xiang: Formal analysis, Writing – review & editing. Zhixin Wei: Software, Validation, Visualization. Wei Zheng: Methodology, Software, Visualization. Peihong Shen: Conceptualization, Project administration, Supervision. Luodong Huang: Conceptualization, Funding acquisition, Methodology, Resources, Writing – review & editing.
Acknowledgement
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem.. 2022;229:114068
- [CrossRef] [Google Scholar]
- Applications of molecular docking in natural products-based drug discovery. Sci. Afr.. 2023;20:e01593
- [CrossRef] [Google Scholar]
- Phloroglucinol derivatives in plant-beneficial Pseudomonas spp.: Biosynthesis, regulation, and functions. Metabolites. 2021;11(3):182.
- [CrossRef] [Google Scholar]
- TBtools-II: A “One for All, All for One” bioinformatics platform for biological big-data mining. Mol. Plant. 2023;16(11):1733-1742.
- [CrossRef] [Google Scholar]
- Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed. Pharmacother.. 2019;117:109091
- [CrossRef] [Google Scholar]
- Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. Biofactors. 2018;44(5):405-417.
- [CrossRef] [Google Scholar]
- Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res.. 2015;99:1-10.
- [CrossRef] [Google Scholar]
- Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res.. 2022;36(1):266-278.
- [CrossRef] [Google Scholar]
- Hyperoside reduces rotenone-induced neuronal injury by suppressing autophagy. Neurochem. Res.. 2021;46(12):3149-3158.
- [CrossRef] [Google Scholar]
- A systematic review of the botanical, phytochemical and pharmacological profile of dracaena cochinchinensis, a plant source of the ethnomedicine dragon’s blood. Molecules. 2014;19(7):10650-10669.
- [CrossRef] [Google Scholar]
- Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem.. 2010;82:4165-4173.
- [CrossRef] [Google Scholar]
- Phytochemical profiling, in vitro biological activities, and in silico molecular docking studies of Dracaena reflexa. Molecules. 2022;27(3):913.
- [CrossRef] [Google Scholar]
- Analysis of pharmaceutical samples of Resina Draconis by HPLC-PAD. Phytochem. Anal.. 2008;19(6):499-505.
- [CrossRef] [Google Scholar]
- Apocynin alters redox signaling in conductance and resistance vessels of spontaneously hypertensive rats. Free Radic. Biol. Med.. 2019;134:53-63.
- [CrossRef] [Google Scholar]
- Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol.. 2007;115(3):361-380.
- [CrossRef] [Google Scholar]
- ’T Hart, B. A., Copray, S., Philippens, I., 2014. Apocynin, a low molecular oral treatment for neurodegenerative disease. BioMed Res. Int. 2014, 298020. Doi: 10.1155/2014/298020.
- Advances and challenges in protein-ligand docking. Int. J. Mol. Sci.. 2010;11(8):3016-3034.
- [CrossRef] [Google Scholar]
- EGFR signaling and autophagy dependence for growth, survival, and therapy resistance. Cell Cycle. 2014;13(1):42-51.
- [CrossRef] [Google Scholar]
- Review: Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci.. 2000;30(2):145-158. PMID:10807157
- [Google Scholar]
- Pharmacokinetics, pharmacodynamics, toxicity, and formulations of daidzein: An important isoflavone. Phytother. Res.. 2023;37(6):2578-2604.
- [CrossRef] [Google Scholar]
- WGCNA: An R package for weighted correlation network analysis. BMC Bioinform.. 2008;9:559.
- [CrossRef] [Google Scholar]
- Li HL, Wei YY, Li XH, Zhang SS, Zhang RT, Li JH, Ma BW, Shao SB, Lv ZW, Ruan H, Zhou HG, Yang C. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis., 2022. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol.Sin. 43(4), 919–932. Doi: 10.1038/s41401-021-00726-0.
- The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem.. 2020;148:80-89.
- [CrossRef] [Google Scholar]
- Chrysin reduces inflammation and oxidative stress and improves ovarian function in D-gal-induced premature ovarian failure. Bioengineered. 2022;13(4):8291-8301.
- [CrossRef] [Google Scholar]
- Plastome comparison and phylogenomics of Fagopyrum (Polygonaceae): insights into sequence differences between Fagopyrum and its related taxa. BMC Plant Biol.. 2022;22(1):339.
- [CrossRef] [Google Scholar]
- Genetic polymorphisms in the apoptosis-associated gene CASP3 and the risk of lung cancer in Chinese population. PLoS One. 2016;11(10):e0164358.
- [CrossRef] [Google Scholar]
- Network pharmacology and molecular docking combined with widely targeted metabolomics to elucidate the potential compounds and targets of Euphorbia helioscopia seeds for the treatment of pulmonary fibrosis. Comput. Biol. Med.. 2023;160:107007
- [CrossRef] [Google Scholar]
- Epidermal growth factor receptor (EGFR): A rising star in the era of precision medicine of lung cancer. Oncotarget. 2017;8(30):50209-50220.
- [CrossRef] [Google Scholar]
- Hispidulin: A promising flavonoid with diverse anti-cancer properties. Life Sc.. 2020;259:118395
- [CrossRef] [Google Scholar]
- Comparative metabolite fingerprinting of legumes using LC-MS-based untargeted metabolomics. Food Res. Int.. 2019;126:108666
- [CrossRef] [Google Scholar]
- Decoding the EGFR mutation-induced drug resistance in lung cancer treatment by local surface geometric properties. Comput. Biol. Med.. 2015;63:293-300.
- [CrossRef] [Google Scholar]
- Human renal mesangial cells are a target for the anti-inflammatory action of 9-cis retinoic acid. Br. J. Pharmacol.. 2000;131(8):1673-1683.
- [CrossRef] [Google Scholar]
- A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell Longev.. 2013;2013:575482
- [CrossRef] [Google Scholar]
- Target discovery of chlorogenic acid derivatives from the flower buds of Lonicera macranthoides and their Mao B inhibitory mechanism. Fitoterapia. 2019;134:297-304.
- [CrossRef] [Google Scholar]
- Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother. Res.. 2001;15(2):114-118.
- [CrossRef] [Google Scholar]
- Metwally, D.M., Alajmi, R.A., El-Khadragy, M.F., Yehia, H.M., AL-Megrin, W.A., Akabawy, A.M.A., Amin, H.K., Abdel Moneim, A.E., 2020. Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J.Funct.Foods. 75(5), 104202. Doi: 10.1016/j.jff.2020.104202.
- Synthesis and anti-cancer activity of naturally occurring 2,5-diketopiperazines. Fitoterapia. 2014;98:91-97.
- [CrossRef] [Google Scholar]
- Self-medication by orang-utans (Pongo pygmaeus) using bioactive properties of Dracaena cantleyi. Sci. Rep.. 2017;7(1):16653.
- [CrossRef] [Google Scholar]
- Chrysin: Pharmacological and therapeutic properties. Life Sc.. 2019;235:116797
- [CrossRef] [Google Scholar]
- Kaempferol: A potential agent in the prevention of colorectal cancer. Physiol. Rep.. 2022;10(20):e15488.
- [Google Scholar]
- Metabolomics analysis revealed the characteristic metabolites of hemp seeds varieties and metabolites responsible for antioxidant properties. Front. Plant Sci.. 2022;13:904163
- [CrossRef] [Google Scholar]
- Pharmacognostic and antioxidant properties of Dracaena sanderiana leaves. Antioxidants. 2016;5(3):28.
- [CrossRef] [Google Scholar]
- Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Tradit. Complement. Med.. 2017;7(3):360-366.
- [CrossRef] [Google Scholar]
- Advances in high throughput LC/MS based metabolomics: A review. Trends Analyt. Chem.. 2023;160:116954
- [CrossRef] [Google Scholar]
- Pterostilbene as a therapeutic alternative for central nervous system disorders: A review of the current status and perspectives. J. Agric. Food Chem.. 2023;71(40):14432-14457.
- [CrossRef] [Google Scholar]
- Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol.. 2019;20(6):353-367.
- [CrossRef] [Google Scholar]
- Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol.. 2023;233:123470
- [CrossRef] [Google Scholar]
- Discovery of novel amide tripeptides as pancreatic lipase inhibitors by virtual screening. New J. Chem.. 2019;43:3208-3217.
- [CrossRef] [Google Scholar]
- Phenolic constituents, pharmacological activities, quality control, and metabolism of Dracaena species: A review. J. Ethnopharmacol.. 2019;244:112138
- [CrossRef] [Google Scholar]
- Hyperoside attenuates non-alcoholic fatty liver disease through targeting Nr4A1 in macrophages. Int. Immunopharmacol.. 2021;94:107438
- [CrossRef] [Google Scholar]
- Thirumal Kumar, D., Jain, N., Evangeline, J., Kamaraj, B., Siva, R., Zayed, H., George Priya Doss, C., 2019. A computational approach for investigating the mutational landscape of RAC-alpha serine/threonine-protein kinase (AKT1) and screening inhibitors against the oncogenic E17K mutation causing breast cancer. Comput.Biol.Med. 115, 103513. Doi: 10.1016/j.compbiomed.2019.103513.
- Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and bioactivities. Molecules. 2020;25(11):2608.
- [CrossRef] [Google Scholar]
- PKMYT1 inhibits lung adenocarcinoma progression by abrogating AKT1 activity. Cell. Oncol.. 2023;46(1):195-209.
- [CrossRef] [Google Scholar]
- The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr.. 2022;29(9):943911
- [CrossRef] [Google Scholar]
- Hispidulin attenuates cardiac hypertrophy by improving mitochondrial dysfunction. Front. Cardiovasc. Med.. 2020;7:582890
- [CrossRef] [Google Scholar]
- Wang, Z.H. 2023. Investigation on the germplasm reources and evolutionary genome of Dracaena sp. in Guangxi. Master's Dissertation. College of Life Science and Technology, Guangxi University, Nanning, China.
- Use of chlorogenic acid against diabetes mellitus and its complications. J. Immunol. Res.. 2020;2020:968508
- [CrossRef] [Google Scholar]
- Effects of 9-cis-retinoic acid on the proliferation and apoptosis of cutaneous T-cell lymphoma cells. Anticancer Drugs. 2019;30(1):56-64.
- [CrossRef] [Google Scholar]
- Global transcriptome and co-expression network analyses revealed hub genes controlling seed size/weight and/or oil content in peanut. Plants. 2023;12(17):3144.
- [CrossRef] [Google Scholar]
- Neochlorogenic acid attenuates hepatic lipid accumulation and inflammation via regulating mir-34a in vitro. Int. J. Mol. Sci.. 2021;22(23):13163.
- [CrossRef] [Google Scholar]
- Investigating the active substance and mechanism of San-Jiu-Wei-Tai granules via UPLC-QE-Orbitrap-MS and network pharmacology. Evid. Based Complement. Alternat. Med.. 2022;2022:1487903.
- [CrossRef] [Google Scholar]
- Regulatory roles of RpoS in the biosynthesis of antibiotics 2,4-diacetyphloroglucinol and pyoluteorin of Pseudomonas protegens FD6. Front. Microbiol.. 2022;13:993732
- [CrossRef] [Google Scholar]
- Species identification of Dracaena using the complete chloroplast genome as a super-barcode. Front. Pharmacol.. 2019;10:1441.
- [CrossRef] [Google Scholar]
- Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J. Cell. Mol. Med.. 2021;25(17):8159-8168.
- [CrossRef] [Google Scholar]
- Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol.. 2023;309:116306
- [CrossRef] [Google Scholar]
- Research advances in Dragon's blood plants in China. Chin. Wild Plant Resour.. 2009;28(6):15-20.
- [CrossRef] [Google Scholar]
- Yunnan Food and Drug Administration, 2005. Yunnan provincial standard of Chinese medicinal materials, third edition. Yunnan Scientific Publishing Press, Kunming, China, pp.29,186-189.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105812.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







