Translate this page into:
A pair of new enantiomeric hybrid phthalide–adenines with a rare 5-oxa-1-azaspiro[3,4]octane moiety and two pairs of new enantiomeric hybrid paraethyl phenol–adenines from Ligusticum chuanxiong
⁎Corresponding authors at: State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. guoli@cdutcm.edu.cn (Li Guo), pengcheng@cdutcm.edu.cn (Cheng Peng), xiling@cdutcm.edu.cn (Liang Xiong)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
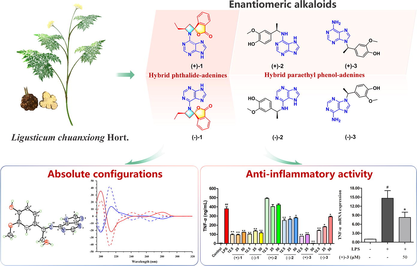
Three pairs of new enantiomeric adenine alkaloids, (+)/(−)-liguadenines A–C [(+)/(−)-1–3], were isolated from the rhizome of Ligusticum chuanxiong Hort. The structures and absolute configurations of these compounds were determined using spectroscopic data, single-crystal X-ray diffraction, and electronic circular dichroism analyses. To the best of our knowledge, compounds (+)-1 and (−)-1 are the first hybrid phthalide–adenines to be ever reported. The linkage between the phthalide and adenine units in these compounds forms a rare 5-oxa-1-azaspiro[3,4]octane moiety. Analyses of the anti-inflammatory activities of the isolated compounds show that (+)/(−)-1 and (+)-3 exhibit significant inhibitory activity against LPS-induced TNF-α and IL-6 production in RAW264.7 cells. RT-qPCR analysis confirms that the most active compound, (+)-3, exerts anti-inflammatory activity by downregulating the mRNA expressions of TNF-α and IL-6 in the cells.
Keywords
Ligusticum chuanxiong
Enantiomers
Adenine alkaloids
Hybrid phthalide–adenines
Hybrid paraethyl phenol–adenines
Anti-inflammatory activity
1 Introduction
The rhizome of Ligusticum chuanxiong Hort. (synonyms L. striatum DC.) (Apiaceae), used as medicine for nearly 2,000 years in China, plays an important role in clinical practice due to its remarkable effects in promoting blood circulation and removing blood stasis (Chen et al., 2018). Thus far, various types of secondary metabolites, such as phthalides (Huang et al., 2022, Zhang et al., 2020, Zhang et al., 2021), alkaloids (Pu et al., 2022, Zhang et al., 2018a), polysaccharides (Wang et al., 2022, Wang et al., 2019), and organic acids (Chen et al., 2018, Zhang et al., 2018b), have been detected in L. chuanxiong. In light of their significant and effective biological activities, the phthalides and alkaloids extracted from L. chuanxiong have attracted great attention. The phthalides, a major class of secondary metabolites in L. chuanxiong, exhibit excellent anti-platelet aggregative, anticoagulant, vasorelaxant, and uterine smooth muscle relaxant activities (Chen et al., 2018, Huang et al. 2022, Liu et al., 2022), whereas the alkaloids, including pyrazines, carbolines, adenines, nucleosides, and phenylpropanoid-amino acid adducts, exhibit significant antiplatelet aggregative and anti-cerebral ischemia reperfusion injury activities (Pu et al., 2022, Zhang et al., 2018a).
Adenine, an important natural alkaloid, has been used as a scaffold for the synthesis of many medicinal compounds, such as fludarabine, cladribine, adefovir, and tenofovir (Buyens et al., 2017, Goswami et al., 2017, Luo et al. 2018). Adenine derivatives, most of which are substituted at N-9 or N-10 have also been detected in many plants. Although the N-9-substituted derivatives such as adenosine and nucleoside are much more common than the N-10-substituted counterparts (Araya et al., 2011, Bakr et al., 2021, Ma et al., 2008, Ma et al., 2007, Wang et al., 2021), we have succeeded in isolating two N-10-substituted nucleoside alkaloids from L. chuanxiong (Pu et al., 2019). We have also isolated butylphthalides, the most significant phthalides in L. chuanxiong, and phthalide dimers from this plant (Huang et al. 2022, Liu et al., 2022). However, phthalide–adenine hybridization has not yet been reported.
As part of our ongoing investigation regarding the rhizome of L. chuanxiong, three pairs of new enantiomeric adenine alkaloids (1–3) have been isolated in this study (Fig. 1), and their absolute configurations have been elucidated using X-ray diffraction analysis and electronic circular dichroism (ECD) calculations. Notably, (+)-1 and (−)-1 are the first hybrid phthalide–adenines to ever be reported. The linkage between the phthalide and adenine units in these compounds results in the formation of a rare 5-oxa-1-azaspiro[3,4]octane moiety that has been reported in four synthesis studies only (Cordero et al. 2011, Capraro et al., 1983, Shipov and Baukov, 1988, Roth, 1979). As for compounds (+)-2, (−)-2, (+)-3, and (−)-3, they are hybrid paraethyl phenol–adenines. Herein, the isolation method, structure, absolute configuration, and anti-inflammatory activity of rare hybrid adenines in L. chuanxiong are discussed.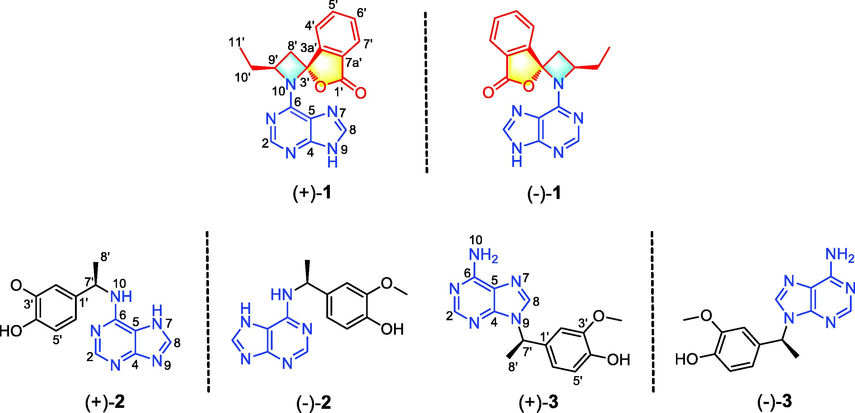
Structures of compounds 1–3.
2 Experimental
2.1 General experimental procedures
Optical rotations and IR data were measured using an Anton Paar MCP 200 automatic polarimeter (Anton Paar GmbH, Graz, Austria) and an Agilent Cary 600 FT-IR microscope (Agilent Technologies Inc., CA, USA). ECD spectra were recorded using an Applied Photophysics Chirascan and Chirascan-plus circular dichroism spectrometer (Applied Photophysics ltd., Leatherhead, England). NMR spectra were obtained by a Bruker-AVIIIHD-600 spectrometer or a Bruker Avance NEO 600 spectrometer (Bruker Corporation, Billerica, MA, USA) with solvent peaks as reference standards. X-ray structure analyses were performed on a Bruker D8 QUEST diffractometer (Bruker Corporation, Billerica, MA, USA). HRESIMS measurements were carried out using a Waters Synapt G2 HDMS instrument (Waters Corporation, Milford, MA, USA) or a Bruker tims-TOF-MS instrument (Bruker Corporation, Billerica, MA, USA). TLC was performed using glass plates precoated with silica gel GF254 (Qingdao Marine Chemical Inc., Qingdao, China). Column chromatography separation was performed using D-101-type macroporous adsorbent resin (Chengdu Kelong Chemical Reagent Factory, Chengdu, China), 732-type strong acid cation exchange resin (Chengdu Chron Chemicals Co., ltd., Chengdu, China), silica gel (200–300 mesh, Yantai Institute of Chemical Technology, Yantai, China), and Sephadex LH-20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden). Meanwhile, HPLC separations were carried out using an Alltech 426 instrument. Reversed-phase semipreparative HPLC analyses were conducted on a Zorbax SB-C18 (250 × 9.4 mm2, 5 μm) column, while normal-phase and reversed-phase enantioseparations were conducted on Daicel Chiralpak AD-H (250 × 4.6 mm2, 5 μm) and Daicel Chiralpak IG (250 × 4.6 mm2, 5 μm) columns, respectively. The uterine smooth muscle relaxant activity and vasorelaxant activity of the isolated compounds were assessed using a PL3508B6/C-V Panlab 8 Chamber Organ Bath System (AD Instruments, Australia). RAW264.7 cells were obtained from Obio Technology Corp (Changsha, China), while the lipopolysaccharide was obtained from Sigma-Aldrich (St. Louis, MO, United States). Curcumin was purchased from Sichuan Weikeqi Biological Technology Co., ltd. (Sichuan, China). Mouse IL-6 Elisa and Mouse TNF-α Elisa kits were purchased from Elabscience Biotechnology Co., ltd. (Wuhan, Hubei, China). Total RNA Isolation Kit, 2 × RT OR-EasyTM Mix, and 2 × Real Time PCR EasyTM MIX-SYBR Green I were obtained from Chengdu Foregene Biological Technology Co., ltd. (Chengdu, China).
2.2 Plant material
Ligusticum chuanxiong Hort. was obtained from NeoGreen Pharmaceutical Technology Development Co., ltd. (Pengzhou, China) in October 2016. The sample was identified by Dr. Jihai Gao and deposited in the School of Pharmacy at Chengdu University of Traditional Chinese Medicine (specimen: LC-20161030).
2.3 Extraction and isolation
The L. chuanxiong rhizome (100 kg) was decocted with H2O three times (2.5 h each time). The residue (28.8 kg) obtained by evaporating the water extract was suspended in warm water and partitioned with n-BuOH. The n-BuOH portion (9.3 kg) was separated by column chromatography on D101 macroporous adsorption resin, and six fractions were eluted with H2O, 10%, 30%, 50%, 70%, and 95% ethanol. The 30% EtOH fraction (701.0 g) was further separated by elution on strong acid cation exchange resin with H2O, 75% EtOH, and 75% EtOH containing 2 M ammonia. Of the three major fractions obtained, the one eluted with 75% EtOH containing 2 M ammonia water (120.0 g) was further separated into nine fractions (F1–F9) by silica gel column chromatography using gradient elution with CH2Cl2/MeOH (100:0–0:100). Separation of the F6 fraction (3.1 g) by RP-MPLC (gradient elution with 10:90–100:0 MeOH/H2O) afforded nine subfractions (F6-1–F6-9).
The F6-3 subfraction was further separated on a Sephadex LH-20 column (85% MeOH in H2O), and seven subfractions F6-3-1–F6-3-7 were obtained. F6-3-3 was successively purified by Sephadex LH-20 column chromatography (CH2Cl2/MeOH, 1:1), preparative TLC (CH2Cl2/MeOH, 9:1), and semipreparative HPLC (25% MeOH in H2O) to yield compound 1 (1.5 mg). In addition, compounds 2 (6.3 mg) and 3 (3.2 mg) were obtained by purifying F6-3-4 on a silica gel column (CH2Cl2/MeOH, 80:1–1:1), followed by semipreparative HPLC (40% MeOH in H2O).
Since compounds 1–3 show no cotton effects in their ECD spectra, and since their specific optical rotations are close to zero, these compounds were subjected to HPLC enantioseparation on a Daicel Chiralpak IG column or a Daicel Chiralpak AD-H column. Accordingly, three pairs of enantiomers, (+)-1 (0.59 mg)/(−)-1 (0.61 mg), (+)-2 (2.60 mg)/(−)-2 (2.50 mg), and (+)-3 (1.54 mg)/(−)-3 (1.60 mg), were obtained (Table 1).
Compound
Chiral phase
Mobile phase
Flow rate (mL/min)
Retention time (min)
Amount (mg)
Peak area ratio [(+)/(−)]
1
(+)-1
IG
MeOH/H2O, 19:1
1.0
12.9
0.59
0.96/1
(–)-1
10.2
0.61
2
(+)-2
AD-H
n-hexane/ethanol, 10:1
1.0
31.4
2.60
1.04/1
(–)-2
36.4
2.50
3
(+)-3
AD-H
n-hexane/ethanol, 2:1
1.0
21.1
1.54
0.97/1
(–)-3
24.2
1.60
2.4 Physicochemical properties and spectroscopic data of compounds 1–3
2.4.1 (+)-Liguadenine A and (−)-liguadenine A [(+)-1 and (−)-1]:
white amorphous powder; {[α]25D +40.0 (c 0.04, MeOH); ECD (MeCN) λmax (Δε) 201 (−0.37), 219 (+9.45), 237 (+0.95), 279 (+1.29) nm; (+)-1}; {[α]25D −47.5 (c 0.04, MeOH); ECD (MeCN) λmax (Δε) 201 (+1.29), 219 (−9.70), 237 (−0.51), 280 (−1.02) nm; (−)-1}; UV (MeCN) λmax (log ε) 198 (4.44), 278 (3.68) nm; IR(ATR) νmax 3030, 2920, 1769, 1716, 1618, 1571, 1466, 1400, 1285, 1264, 1085, 916, 797, 767, 735, 691 cm−1; 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data see Table 2; HR-ESI-MS m/z: 344.1126 [M + Na]+, calcd. for C17H15N5O2Na, 344.1123.
Position
1
2
3
1H
13C
1H
13C
1H
13C
2
8.40 s
153.7
8.22 s
153.8
8.21 s
153.6
4
—
160.7
—
151.3
—
150.4
5
—
113.9
—
118.8
—
120.2
6
—
151.8
—
154.7
—
157.3
8
8.60 s
145.7
8.08 s
140.6
8.17 s
140.9
1′
—
169.9
—
136.8
—
133.1
2′
—
—
7.03 s
111.2
7.00 d (2.4)
111.7
3′
—
95.3
—
149.0
—
149.3
3a′
—
151.8
—
—
—
—
4′
7.85 d (7.2)
123.8
—
146.7
—
147.8
5′
7.90 t (7.2)
136.6
6.75 d (7.8)
116.2
6.77 d (8.4)
116.4
6′
7.75 t (7.2)
132.2
6.88 d (7.8)
119.7
6.84 dd (8.4, 2.4)
120.3
7′
7.94 d (7.2)
126.6
5.43 m
51.0
5.80 q (7.2)
55.4
7a′
—
126.4
—
—
8′
3.20 dd (15.6, 11.4)
2.57 dd (15.6, 1.8)43.3
1.60 d (7.2)
23.1
1.95 d (7.2)
21.2
9′
4.97 m
56.5
—
—
—
—
10′
2.37 m
2.22 m26.6
—
—
—
—
11′
0.97 t (7.2)
7.8
—
—
—
—
OMe-3′
—
—
3.84 s
56.4
3.82 s
56.4
2.4.2 (+)-Liguadenine B and (−)-liguadenine B [(+)-2 and (−)-2]:
colorless crystals; m.p. 148–149 °C; {[α]25D +60.2 (c 0.07, MeOH); ECD (MeCN) λmax (Δε) 200 (+35.71), 214 (−21.93), 281 (+5.67) nm; (+)-2}; {[α]25D −58.6 (c 0.06, MeOH); ECD (MeCN) λmax (Δε) 200 (−22.49), 214 (+13.87), 281 (−3.66) nm; (−)-2}; UV (MeCN) λmax (log ε) 200 (4.22), 268 (3.72) nm; IR(ATR) νmax 3362, 3334, 2975, 2944, 1613, 1521, 1454, 1431, 1260, 1224, 1138, 1055, 1009, 900, 851, 796, 707 cm−1, 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data see Table 2; HR-ESI-MS m/z: 308.1127 [M + Na]+; calcd. for C14H15N5O2Na, 308.1123.
2.4.3 (+)-Liguadenine C and (−)-liguadenine C [(+)-3 and (−)-3]:
white amorphous powder; {[α]25D +20.1 (c 0.06, MeOH); ECD (MeCN) λmax (Δε) 202 (+6.83), 253 (−0.85) nm; (+)-3}; {[α]25D −14.7 (c 0.05, MeOH); ECD (MeCN) λmax (Δε) 201 (−5.51), 252 (+0.71) nm; (−)-3}; UV (MeCN) λmax (log ε) 202 (3.70), 261 (2.98) nm; IR(ATR) νmax 3327, 3178, 3125, 1643, 1515, 1466, 1276, 1212, 1027, 975, 853, 798, 708 cm−1; 1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD) data see Table 2; HR-ESI-MS m/z: 308.1106 [M + Na]+, calcd. for C14H15N5O2Na, 308.1123.
2.5 X-ray crystallography data of compound 2
Compound 2 was dissolved with dichloromethane/methanol (4:6, V/V) and then slowly evaporated to a single crystal at 4℃. Crystal data for 2: C14H15N5O2·H2O, M = 303.32, triclinic, a = 7.8867(2) Å, b = 8.9792(2) Å, c = 11.4775(3) Å, α = 81.829(10)°, β = 73.211(10)°, γ = 68.117(10)°, V = 721.54(3) Å3, space group P-1, T = 293(2) K, Z = 2, μ(Cu Kα) = 0.845 mm−1, 22,147 reflections measured, 2652 independent reflections (Rint = 0.0765), average redundancy 8.351, completeness = 99.5 %. Final R indices (I > 2σ(I)): R1 = 0.0428, wR2 = 0.1140. Final R indices (all data): R1 = 0.0637, wR2 = 0.1256. The goodness of fit on F2 was 1.073. CCDC number: 2157588. The results of X-ray diffraction analysis showed that 2 is an enantiomeric racemate.
2.6 Bioactivity assays
2.6.1 ELISA for TNF-α and IL-6
RAW264.7 cells were cultured according to a previously described method (Li et al., 2021), then they were dispensed into a 24-well plate at the density of 2 × 106 cells per well. After incubation at 37 °C for 24 h, the cells were treated with 1 µg/mL LPS for 24 h, with or without the tested compounds (12.5, 25, and 50 μM). The collected cell supernatants were spun at 1000 × g for 20 min to remove the particulate matter, then the levels of TNF-α and IL-6 in each sample were measured according to the manufacturer’s instructions. In addition, the cytotoxic effects of the compounds on RAW264.7 cells were evaluated by CCK-8 assay. Curcumin was used as a positive control (1.56, 3.13, 6.25, 12.5, and 25 μM).
2.6.2 RNA extraction and RT-qPCR analysis
Cultured RAW264.7 cells were dispensed into 6-well plates. After 24 h treatment, the supernatant was removed, and the cells were washed twice with PBS. Subsequently, the total RNA was extracted and dissolved in 75 μL of RNase-free ddH2O. The purity of total RNA was assessed by measuring the value of OD260/280 using a Nucleic Acid/Protein Analyzer. A 2 × RT OR-EasyTM Master Premix reverse transcription kit was used to reverse-transcribe the RNA, and the obtained target cDNA was quantified using the EvaGreen 2 × qPCR MasterMix- No Dye kit, according to the manufacturer’s instructions. Primers used for RT-qPCR see Table 3.
Primers
Forward (5′→3′)
Reverse (5′→3′)
GAPDH
GGCCTTCCGTGTTCCTACC
TGCCTGCTTCACCACCTTC
TNF-α
ATGAGAAGTTCCCAAATGGC
CTCCACTTGGTGGTTTGCTA
IL-6
GGGACTGATGCTGGTGACAA
TCCACGATTTCCCAGAGAACA
2.6.3 Other activity assay
The inhibitory effects of the isolates against OT-induced contractions of rat uterine smooth muscle (Liu et al., 2022, Ni et al., 2021), KCl-induced contractions of rat aorta rings (Chen et al., 2022, Liu et al., 2019), and ADP-induced rabbit platelet aggregation (Pu et al., 2019) were measured as described previously.
3 Results and discussion
3.1 Structure elucidation
Compound 1 was isolated as a white amorphous powder. Its HR-ESI-MS presents a positive quasi-molecular ion at m/z 344.1126 (calcd. for C17H15N5O2Na, 344.1123), which suggests that the molecular formula of this compound is C17H15N5O2 possessing 13 degrees of unsaturation. The 1H NMR and 1H–1H COSY data (Table 2 and Fig. 2) suggest that compound 1 possesses an adenine moiety [δH 8.40 (1H, s, H-2) and 8.60 (1H, s, H-8)] (Li et al., 2009), an ortho-substituted benzene ring [δH 7.94 (1H, d, J = 7.2 Hz, H-7′), 7.90 (1H, t, J = 7.2 Hz, H-5′), 7.85 (1H, d, J = 7.2 Hz, H-4′), and 7.75 (1H, t, J = 7.2 Hz, H-6′)], and a substituted n-butyl unit [δH 3.20 (1H, dd, J = 15.6, 11.4 Hz, H-8′a), 2.57 (1H, dd, J = 15.6, 1.8 Hz, H-8′b), 4.97 (1H, m, H-9′), 2.37 (1H, m, H-10′a), 2.22 (1H, m, H-10′b), and 0.97 (3H, t, J = 7.2 Hz, H3-11′)]. The 13C NMR (Table 2) and DEPT spectra show 17 carbon signals corresponding to the above units, with seven quaternary carbons, three of which (δC 160.7, 151.8, 113.9) are attributed to the adenine unit. The remaining four quaternary carbons include a carbonyl carbon (δC 169.9), two aromatic carbons (δC 151.8 and 126.4), and a carbon (δC 95.3) bonded to two hetero atoms. The resonance signals detailed above reveal that compound 1 is an unusual hybrid phthalide–adenine. The HMBC correlations (Fig. 2) of H-2 with C-4 and C-6; and H-8 with C-4 and C-5 confirm the presence of an adenine unit. Meanwhile, the n-butylphthalide fragment is evidenced by the HMBC correlations of H-4′ with C-3′, C-6′, and C-7a′; H-5′ with C-3a′ and C-7′; H-6′ with C-4′ and C-7a′; H-7′ with C-1′, C-3a′, and C-5′; and H-8′ with C-3′, C-3a′, C-9′, and C-10′, as well as by the 1H–1H COSY signals of H-4′/H-5′/H-6′/H-7′ and H-8′/H-9′/H-10′/H-11′. The adenine and butylphthalide units of 1 account for 12 degrees of unsaturation. The 13th degree of unsaturation suggests a ring linkage between the two units. In addition, compared to the common n-butylphthalide compound, C-3′ in compound 1 is changed to a quaternary carbon (δC 95.3) bonded to two hetero atoms, while C-9′ in compound 1 is changed to a CH group (δH 4.97, δC 56.5) linked to a N atom. Overall, the spectroscopic data indicate that a C-3′–N-10–C-9′ linkage between the adenine and butylphthalide units in 1 forms a four-membered aza-heterocycle, resulting in the formation of a rare 5-oxa-1-azaspiro[3,4]octane moiety. Based on these results, the planar structure of compound 1 is established.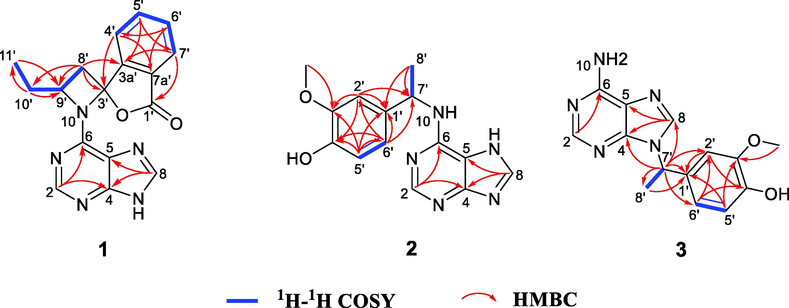
Key 1H–1H COSY and HMBC correlations of compounds 1–3.
The relative configuration of compound 1 was elucidated based on the analysis of NOESY cross-peaks. The NOESY correlations of H-4′ (δH 7.85)/H-8′a (δH 3.20) and H-8′a (δH 3.20)/H-10′a (δH 2.37) demonstrate that H-4′, H-8′a, and H-10′ have the same orientation (Fig. 3). Interestingly, no cotton effect is observed in ECD spectrum of compound 1, which suggests that this compound may be a racemic mixture. Indeed, chiral separation of 1 on a Daicel Chiralpak IG column yields (+)-1 and (−)-1 (Table 1), and based on the comparison of experimental and calculated ECD spectra, the absolute configurations of these enantiomers are 3′R,9′S and 3′S,9′R, respectively (Fig. 4). Hence, (+)-1 and (−)-1 were established as (+)-(3′R,9′S)-liguadenine A and (−)-(3′S,9′R)-liguadenine A, respectively.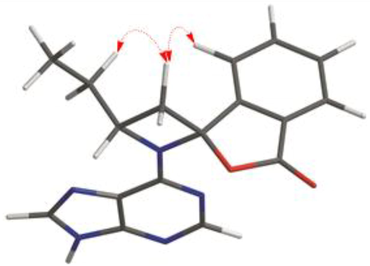
Key NOESY correlations of compound 1.
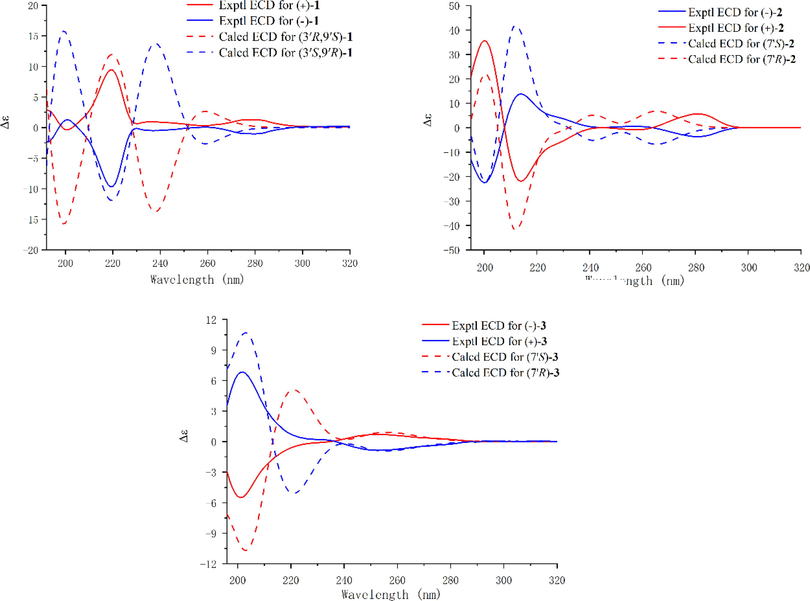
The experimental and calculated ECD spectra of compounds 1–3.
Based on HR-ESI-MS analysis (m/z 308.1127 [M + Na]+, calcd. for C14H15N5O2Na, 308.1123), the molecular formula of compound 2 is C14H15N5O2. 1H and 13C NMR analyses reveal that 2 is an adenine hybrid that comprises a 4-ethyl-2-methoxyphenol moiety with C-7′ substituted [δH 7.03 (1H, s, H-2′), 6.88 (1H, d, J = 7.8 Hz, H-6′), 6.75 (1H, d, J = 7.8 Hz, H-5′), 5.43 (1H, m, H-7′), and 1.60 (3H, d, J = 7.2 Hz, H-8′); δC 149.0, 146.7, 136.8, 119.7, 116.2, 111.2, 51.0, and 23.1], as well as a methoxy group [δH 3.84 (3H, s, OMe-3′); δC 56.4]. This is confirmed by the HMBC correlations of H-2′ with C-1′, C-3′, C-4′, and C-7′; OMe-3′ with C-3′; H-6′ with C-1′, C-2′, C-4′, and C-7′; and H3-8′ with C-1′ and C-7′, together with the 1H–1H COSY correlations of H-5′/H-6′ and H-7′/H-8′ (Fig. 2). Although no HMBC correlation of H-7′/C-6 is observed, the adenine and 4-ethyl-2-methoxyphenol substructures must be connected by a C-6–N-10–C-7′ linkage in order to match the molecular formula and chemical shifts of C-6 and C-7′. This linkage is verified by X-single crystal diffraction analysis (Fig. 5). Considering that the specific optical rotation of 2 is zero, this compound was subjected to enantioseparation on a Daicel Chiralpak AD-H column (Table 1). As described for compound 1, ECD calculations were used to determine the absolute configuration of 2, and as shown in Fig. 4, the experimental ECD spectra of (+)-2 and (−)-2 are consistent with the calculated ECD spectra of (7′R)-2 and (7′S)-2, respectively. Therefore, compounds (+)-2 and (−)-2 are labelled (+)-(7′R)-liguadenine B and (−)-(7′S)-liguadenine B.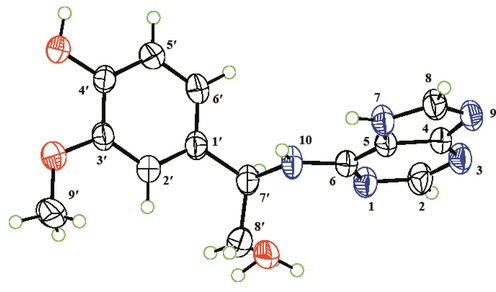
X-ray crystallographic structure of compound 2.
The spectroscopic data of compound 3 are similar to those of 2. Indeed, HR-ESI-MS analysis reveals that the two compounds possess the same molecular formula, which indicates that they are isomers. The 1H and 13C NMR data of 3 (Table 2) suggest that this compound is also a hybrid, and that it is composed of adenine and 4-ethyl-2-methoxyphenol moieties, like compound 2. However, the C-4, C-5, C-6, C-1′, C-7′, and C-8′ resonances in the 13C NMR spectrum of 3 are shifted by ΔδC −0.9, +1.4, +2.6, −3.7, +4.4, and −1.9 ppm, respectively, compared to the same signals in the spectrum of 2. Therefore, the difference between compounds 3 and 2 lies in the linkage between the two moieties. Based on the HMBC correlations of H-7′ with C-4, C-8, C-1′, C-2′, C-6′, and C-8′, the adenine and 4-ethyl-2-methoxyphenol substructures in 3 are connected by a N-9–C-7′ linkage. The specific optical rotation and ECD spectrum of 3 suggest that it is a racemic mixture, and the absolute configurations of the separated (+)-3 and (−)-3 enantiomers are 7′R and 7′S, respectively, as determined by ECD calculations (Fig. 4). Therefore, compounds (+)-3 and (−)-3 are established as (+)-(7′R)-liguadenine C and (−)-(7′S)-liguadenine C, respectively.
3.2 Effects of compounds 1–3 on TNF-α and IL-6 levels in RAW 264.7 cells
First, all compounds showed no cytotoxic activity against RAW264.7 cells in the range of experimental concentrations. Then, the anti-inflammatory effects of the three pairs of enantiomeric alkaloids isolated from L. chuanxiong were assayed using LPS-stimulated RAW 264.7 macrophages, and the concentrations of inflammatory factors (TNF-α and IL-6) in the supernatant were detected by ELISA. Except for (+)-2, all compounds exhibit significant inhibitory activity against LPS-induced TNF-α production, with (+)-3 being the most active (Fig. 6). Compounds (+)-1/(−)-1 and (+)-3 also show significant inhibitory effects against the production of IL-6. Interestingly, the anti-inflammatory activity of (+)-3 is better than that of its enantiomer. In addition, a comparison of the anti-inflammatory effects of (±)-2 and (±)-3 indicates that the linkage between the adenine and paraethyl phenol moieties in these compounds plays an important role in anti-inflammatory activity (N-9 linkage > N-10 linkage). Curcumin was used as a positive control and showed a significant inhibitory effect on TNF and IL-6 release (Fig. 7).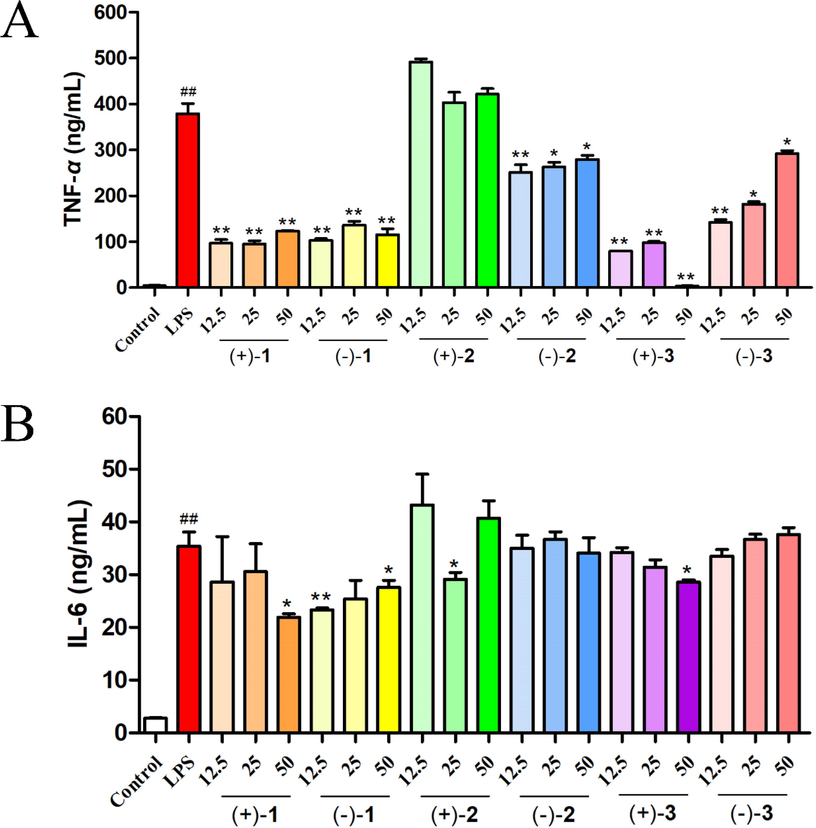
Effects of compounds 1–3 on the levels of TNF-α (A) and IL-6 (B) in LPS-stimulated RAW 264.7 cells. The data represent the mean ± SD of three experiments. ##P < 0.01 vs the control group; *p < 0.05, **p < 0.01 vs the LPS group.
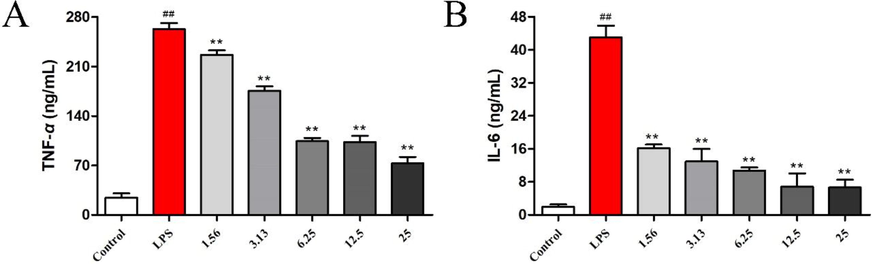
Effects of curcumin on the levels of TNF-α (A) and IL-6 (B) in LPS-stimulated RAW 264.7 cells. The data represent the mean ± SD of three experiments. ##P < 0.01 vs the control group; **p < 0.01 vs the LPS group.
3.3 Effect of compound (+)-3 on the mRNA expression of LPS-induced inflammatory cytokines in RAW264.7 cells
The effect of (+)-3, the most active compound, on the mRNA expression of LPS-induced inflammatory cytokines in RAW264.7 cells was further explored. As shown in Fig. 8, LPS prominently induces the mRNA expressions of TNF-α and IL-6 compared to the normal control. However, treatment with 50 μM (+)-3 significantly downregulates the IL-6 and TNF-α mRNA expressions induced by LPS. These results suggest that (+)-3 suppresses inflammatory response by downregulating the mRNA expressions of TNF-α and IL-6.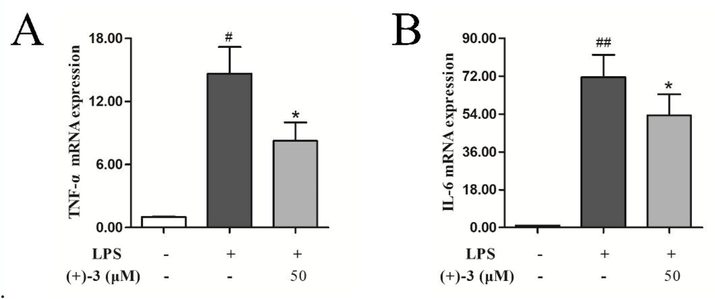
Effects of compound (+)-3 on the mRNA expression of TNF-α (A) and IL-6 (B) in LPS-stimulated RAW264.7 cells. The data represent the mean ± SD of three experiments. #P < 0.05, ##P < 0.01 vs the control group; *p < 0.05, vs the LPS group.
3.4 Other activity assay
None of the tested compounds showed inhibitory effects against OT-induced contractions of rat uterine smooth muscle, KCl-induced contractions of rat aorta rings, and ADP-induced rabbit platelet aggregation.
4 Conclusion
In summary, three pairs of new enantiomeric adenine hybrids (1–3) were isolated from the rhizome of L. chuanxiong. (+)/(−)-Liguadenines B and C [(+)/(−)-2 and 3] are unusual hybrid paraethyl phenol–adenines, while (+)/(−)-liguadenine A [(+)/(−)-1] are novel hybrid phthalide–adenines possessing a rare 5-oxa-1-azaspiro[3,4]octane moiety. Compounds (+)/(−)-1 and (+)-3 exert anti-inflammatory effects by inhibiting the production of TNF-α and IL-6 in LPS-stimulated RAW264.7 cells. In addition to enriching the literature with data concerning rare types of alkaloids in L. chuanxiong, this study provides a reference for the research of anti-inflammatory compounds in L. chuanxiong.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82022072 and U19A2010), the Fok Ying Tung Education Foundation (Grant No. 171037), the Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC1557), the Science and Technology Innovation Talent Program of Sichuan (Grant No. 2023JDRC0041), the “Ten Thousand Talents” Plan of Sichuan Province (Grant No. 168), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-D-202209), and the Scientific and Technological Industry Innovation Team of Traditional Chinese Medicine of Sichuan Province (Grant No. 2022C001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification of unprecedented purine-containing compounds, the zingerines, from ginger rhizomes (Zingiber officinale Roscoe) using a phase-trafficking approach. Phytochemistry. 2011;72:935-941.
- [CrossRef] [Google Scholar]
- New adenosine derivatives from Aizoon canariense L.: in vitro anticholinesterase, antimicrobial, and cytotoxic evaluation of its extracts. Molecules. 2021;26:1198.
- [CrossRef] [Google Scholar]
- Solvent-directed regioselective benzylation of adenine: characterization of N9-benzyladenine and N3-benzyladenine. J. Heterocyclic Chem.. 2017;54:2946-2950.
- [CrossRef] [Google Scholar]
- [2 + 2]-Cycloaddition of ‘maleic isoimides’ with ketenes and conversion of spiro-β-lactams to muconic and tetramic acid derivatives. Helv. Chim. Acta. 1983;66:362-378.
- [Google Scholar]
- Vasorelaxant effect of curcubisabolanin A isolated from Curcuma longa through the PI3K/Akt/eNOS signaling pathway. J. Ethnopharmacol.. 2022;294:115332
- [CrossRef] [Google Scholar]
- A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong) Food Chem. Toxicol.. 2018;119:309-325.
- [CrossRef] [Google Scholar]
- Mechanism of the acid-mediated thermal fragmentation of 5-spirocyclobutane-isoxazolidines. Eur. J. Org. Chem.. 2011;28:5608-5616.
- [CrossRef] [Google Scholar]
- Rational design of selective adenine-based scaffolds for inactivation of bacterial histidine kinases. J. Med. Chem.. 2017;60:8170-8182.
- [CrossRef] [Google Scholar]
- Six pairs of enantiomeric phthalide dimers from the rhizomes of Ligusticum chuanxiong and their absolute configurations and anti-inflammatory activities. Bioorg. Chem.. 2022;127:105970
- [CrossRef] [Google Scholar]
- Chemical constituents in n-butanol extract from the seeds of Alpinia katsumadai. Chin. J. Nat. Med.. 2009;7:417-420.
- [CrossRef] [Google Scholar]
- Polyacetylene glucosides from the florets of Carthamus tinctorius and their anti-inflammatory activity. Phytochemistry. 2021;187:112770
- [CrossRef] [Google Scholar]
- Isoindolines and phthalides from the rhizomes of Ligusticum chuanxiong and their relaxant effects on the uterine smooth muscle. Phytochemistry. 2022;198:113159
- [CrossRef] [Google Scholar]
- Curcumanes A and B, two bicyclic sesquiterpenoids with significant vasorelaxant activity from Curcuma longa. Org. Lett.. 2019;21:1197-1201.
- [CrossRef] [Google Scholar]
- C−H amination of purine derivatives via radical oxidative coupling. J. Org. Chem.. 2018;83:3710-3718.
- [CrossRef] [Google Scholar]
- Two new constituents from Artemisia capillaris Thunb. Molecules. 2008;13:267-271.
- [CrossRef] [Google Scholar]
- Bromophenols coupled with nucleoside bases and brominated tetrahydroisoquinolines from the red alga Rhodomela confervoides. J. Nat. Prod.. 2007;70:337-341.
- [CrossRef] [Google Scholar]
- Chemical composition and uterine smooth muscle relaxant activity of essential oils from 10 kinds of blood-activating and stasis-resolving Chinese medicinal herbs. J. Ethnopharmacol.. 2021;269:113713
- [CrossRef] [Google Scholar]
- Nucleoside alkaloids with anti-platelet aggregation activity from the rhizomes of Ligusticum striatum. Nat. Prod. Res.. 2019;33:1399-1405.
- [CrossRef] [Google Scholar]
- Total alkaloids from the rhizomes of Ligusticum striatum: a review of chemical analysis and pharmacological activities. Nat. Prod. Res.. 2022;36:3489-3506.
- [CrossRef] [Google Scholar]
- Spiro-β-lactame durch [2+2]-cycloaddition von ketenen an iminolactone. Helv. Chim. Acta. 1979;62:1966-1977.
- [CrossRef] [Google Scholar]
- Cheminform abstract: reaction of spiro-β-lactams, e.g. (I), with O-silyl substituted enols (II) ChemInform. 1988;19:46.
- [Google Scholar]
- Protective effect of polysaccharide from Ligusticum chuanxiong hort against H2O2-induced toxicity in zebrafish embryo. Carbohydr. Polym.. 2019;221:73-83.
- [CrossRef] [Google Scholar]
- Monoterpene indole alkaloids from the roots of Bousigonia mekongensis and their anti-diabetic nephropathy activity. Fitoterapia. 2021;153:104964
- [CrossRef] [Google Scholar]
- The isolation, structural features and biological activities of polysaccharide from Ligusticum chuanxiong: A review. Carbohydr. Polym.. 2022;285:118971
- [CrossRef] [Google Scholar]
- Chemical constituents from the rhizome of Ligusticum chuanxiong Hort. and their Nrf2 inducing activity. Chem. Biodivers.. 2021;18:e2100302.
- [Google Scholar]
- Novel phenylpropanoid-amino acid adducts from Ligusticum chuanxiong. Org. Chem. Front.. 2018;5:1423-1430.
- [CrossRef] [Google Scholar]
- Ferulic acid derivatives from Ligusticum chuanxiong. Fitoterapia. 2018;125:147-154.
- [CrossRef] [Google Scholar]
- Neophathalides A and B, two pairs of unusual phthalide analog enantiomers from Ligusticum chuanxiong. Org. Biomol. Chem.. 2020;18:5453-5457.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
https://doi.org/10.1016/j.arabjc.2022.xxxxxx
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104696.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







