Translate this page into:
Design, synthesis of N-thianyl indole acetamide derivatives as potential plant growth regulator
⁎Corresponding author. jwu6@gzu.edu.cn (Jian Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The design, synthesis, and testing of acetamide derivatives containing indole structures (C) were carried out to determine their potential for regulating plant growth. The root growth of Arabidopsis thaliana was strongly inhibited by certain compounds, with compounds C2, C4, C6, and C7 achieving a 100 % inhibitory rate at a concentration of 100 µM. The growth of lateral roots in A. thaliana was significantly promoted by compounds C2 and C6 at a concentration of 50 µM. Additionally, these compounds have been found to increase the expression of genes associated with lateral root development, including IAA14, LAX3, AXR3, GH3.1, GH3.3, and BRU6. These genes are involved in the auxin signaling pathway and IAA-amino synthase-related genes. In brief, these target compounds may be promising lead compounds for developing agents that can regulate plant growth.
Keywords
Indoleacetamide derivative
Synthesis
Plant growth regulation
Mechanism of action
- IAA
-
indole-3-acetic acid
- Ac6c
-
1-Aminocyclohexane carboxylic acid
- DEGs
-
Differentially expressed genes
- LBD
-
Lateral organ binding domain
- SHY2
-
SHORT HYPOCOTYL 2
- ARFs
-
Auxin-responsive factors
- GATA
-
GATA transcription factor
- PLA2
-
PECTIN LYASE2
- EXP17
-
EXPASIN17
- PG
-
Polygalacturonase
- PAA
-
Phenylacetic acid
Abbreviations
1 Introduction
Phytohormones are essential for plant life processes, and the manipulation of phytohormones to achieve specific crop phenotypes has become a popular tool in agricultural production (Zhao et al., 2024). Exogenous phytohormones and their related compounds offer specific advantages over alternative agrochemical agents through their ability to enhance crop resistance to biotic and abiotic stresses, improve crop morphology, and increase crop yield (Rademacher, 2015). As a result, agricultural chemists have become interested in the chemical modification of phytohormones to discover valuable compounds (Frackenpohl et al., 2023).
Auxin has been identified through classical coleoptile bending tests and plays an integral role in seedling and root growth. Auxin affects plants throughout the entire process of plant growth and development, including gametogenesis, embryogenesis, seedling growth, vascular patterning, and flower development (Zhao, 2010). Exogenous auxin has many uses in agriculture, which can be used in tissue culture to stimulate rooting of cuttings, promote fruit setting, and thin fruit (Rademacher, 2015). Indole-3-acetic acid (IAA) is a heterocyclic monocarboxylic acid compound and is the predominant natural auxin. Its structure was identified in 1934. After that, agricultural chemists developed a large number of derivatives around the indole skeleton and evaluated their auxin activity (Nigović et al., 1996; Porter and Thimann, 1965; Thimann, 1958). However, the auxin compounds that have been widely used so far are still mainly IAA, naphthalene acetic acid (NAA) and 2,4-D.
Scientists have proposed various theories to guide the structural modification of highly active auxins. These theories include the two-point attachment theory (Two PA), the three-point attachment theory (Three PA), the separation charge theory (SCT), the conformational change theory (CCT), the physico-chemical influence of a boundary (PCIB), and others. However, each theory has its limitations. For example, the Two PA theory does not account for the reversibility of enzymatic processes, the Three PA theory does not consider benzoic acid derivatives, and SCT fails to explain multidimensional physiological phenomena related to auxins. Additionally, both CCT and PCIB also have their own constraints (Ferro et al., 2010).
Therefore, we attempted to modify IAA using the traditional drug development concept of backbone conversion and active fragment splicing to obtain analogues of auxin. The potential of indole compounds as plant growth regulators has been well-documented, and potential schemes for aminoacyl derivatization have been proposed (Sun et al., 2024, 2023). Tetrahydrothiopyran is a key six-membered ring system with a sulfur atom that has demonstrated potential in drug development. Compounds with this structure display diverse biological activities, including antimicrobial properties (Hada and Sharma, 2018; Łączkowski et al., 2016), anti-herpesvirus activity (Kontani et al., 2005), anticonvulsant activity (Łączkowski et al., 2016) and anti-bacterial infection treatment (Fu et al., 2014). Additionally, they have been used as phosphodiesterase inhibitors for cancer, diabetes, inflammation, psychiatric, and neurological disorders (Clauss et al., 2010), as well as potential penicillin-binding protein inhibitors (Rajalakshmi et al., 2020) and plant growth regulators (Kast et al., 1988). The sulfur atom found in this structure is noteworthy for its ability to improve compound selectivity and target adaptability (Beno et al., 2015). It functions as a potential hydrogen bond acceptor (Mustafa and Winum, 2022; Platts et al., 1996), making it a crucial and beneficial component in drug molecules.
Moreover, 1-aminocyclohexane carboxylic acid (Ac6c) is an important non-protein quaternary α-amino acid that plays a crucial role in the development of new drugs (Abercrombie et al., 2015). Certain compounds have displayed remarkable effects on plant growth regulation (Dejonghe et al., 2018; Vaidya et al., 2019). It was observed that some compounds exhibited promising anti-pest activity on plants in our previous work (Zhang et al., 2021).
After being inspired by the descriptions mentioned above, we aimed to create new non-protein quaternary α-amino acid derivatives containing sulfur by combining the structure of indole-3-acetic acid and tetrahydrothiopyran. This was achieved through a reaction involving the hydroxyl and amine groups to form an amide linker. The resulting compounds, N-thianyl indole acetamide derivatives (Fig. 1), were synthesized for potential use in regulating plant growth. Biological activity tests were carried out on these compounds to identify any that showed promising effects on plant growth regulation.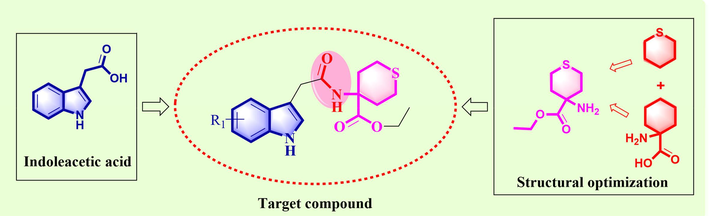
Design of N-Thianyl indole acetamide derivatives.
2 Materials and methods
2.1 Chemicals, reagents, and instrumentation
All reagents and solvents were purchased from commercial companies. The melting point of the compound was determined using an XT-4 micro melting point instrument from Beijing Tech Instrument Co., Beijing, China. The NMR data of the synthesized compounds were recorded with a 400-MHz instrument (Bruker Advance III, Fallanden, Switzerland) or a JEOL-ECX 500 MHz (JEOL, Tokyo, Japan). High-resolution mass spectrometry (HRMS) data were conducted using an Orbitrap LC-MS instrument (Thermo Fisher Scientific, Waltham, MA, USA). The qRT-PCR kits were purchased from Sangon Biotech (Shanghai) Co., and the experiments were performed using a CFX96TM Real-Time System and an Eppendorf Centrifuge 5417R (Eppendorf AG, Hamburg, Germany). Additionally, we used a BIOBASE BBS-SDC Medical clean bench (Shandong Boke Regenerative Medicine Co., Ltd, Zaozhuang, China), an RXM type artificial climate box (Ningbo Jiangnan Instrument Factory, Ningbo, China), and a BIOBASE fully automatic autoclave (Shandong Boke Regenerative Medicine Co., Ltd, Zaozhuang, China) for our experiments.
2.2 Chemistry
As depicted in Scheme 1, compound C was easily obtained by reacting indole analogue A with ethyl 4-aminotetrahydro-2H-thiopyran-4-carboxylate in the presence of the condensing agent HATU.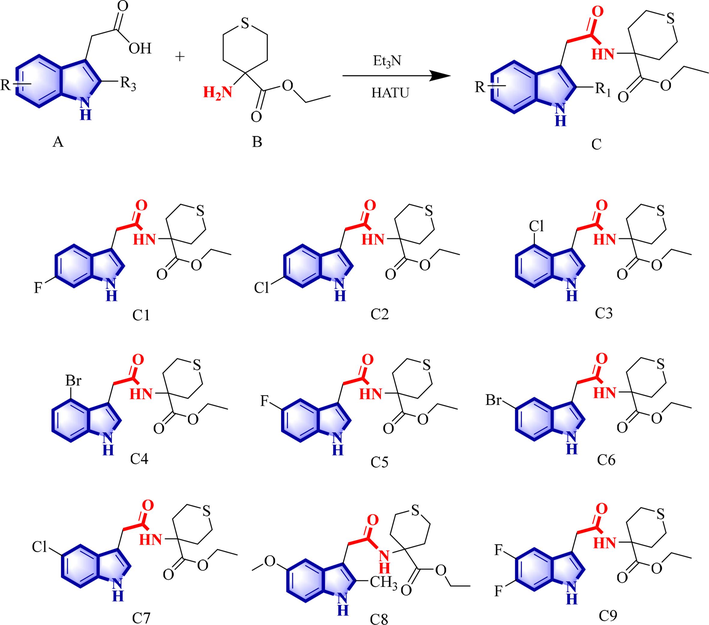
The synthetic route of target compound C and the structures of C1–C9.
2.3 Bio-activity
Different concentrations of compound solutions were prepared using DMSO as the solvent. The medium containing 10 μM IAA served as the positive control, while the medium containing a 0.1 % DMSO solution was used as the blank control. The DMSO content in the compound-treated group was consistent with that of the blank control group. A. thaliana was used for the study and the activity was tested at 100 µM for plant root growth. The experimental procedures were referenced from methods in the literature (Bai et al., 2021; Fendrych et al., 2018). A. thaliana plants were transplanted into the medium containing the compounds in groups of 6 plants, and 3 replicates were done for each compound. The original root length was marked, and the root length was measured after 10 days of growth. Each experiment was repeated three times.
Culture medium configuration. 1/2 Murashige & Skoog (MS) culture medium containing 0.8 % agar powder and with a pH of 5.8 was prepared. It was sterilized at 120 °C for 20 min. After sterilization, the culture medium was poured into 100 mm × 100 mm square culture dishes and allowed to cool and solidify.
Seed disinfection and cultivation. A. Thaliana seeds were first soaked in 70 % ethanol for 5 min., followed by a wash with 2.6 % sodium hypochlorite for 10 min. They were then rinsed 5–6 times with sterilized secondary water before being evenly distributed on the culture medium. Once sealed, the seeds were placed horizontally in an artificial climate box for 10 days to allow their true leaves to grow. The artificial climate box was programmed to a temperature of 21 °C, a humidity level of 67 % relative humidity, 16 h of light, and 8 h of darkness.
Root length test. A certain concentration of the compound was added to the culture medium and then poured into culture dishes, and left to solidify. Selected A. thaliana seedlings with uniform root length were transferred to culture dishes, and the initial root length was labeled. After sealing, they were placed vertically in an incubator and observed for growth. After 10 days, the root growth was observed and its length was recorded.
2.4 Molecular docking
The crystal structure of TIR1 (PDB ID: 2P1P) was obtained from the RCSB Protein Database(Tan et al., 2007). Molecular Operating Environment (MOE 2019) software was used to perform molecular docking. The preparation of TIR1 prior to docking was based on methods described in the literature (Wang et al., 2024). Docking was carried out with designed compounds and IAA, and the results were analyzed using MOE and PyMOL software tools.
2.5 Transcriptome analysis, molecular docking and qRT-PCR validation
The whole A. thaliana after 10 days of compound treatment was used as samples, and three experimental groups were selected for transcriptome sequencing analysis of compounds C2 and C6 at concentrations of 50 µM and 12.5 µM, respectively. The sequencing was done by Sangon Biotech (Shanghai) Co. The corresponding gene sequences were found in the NCBI database (https://www.ncbi.nlm.nih.gov) based on the gene ID. Primer design and synthesis were performed by Sangon Biotech (Shanghai) Co. The primer sequences are listed in Table 1. Functional categories were assigned through BLAST searches of the nonredundant GenBank database and by KEGG Pathway (http://www.genome.jp/kegg/) and Gene Ontology analysis (http://www.geneontology.org/).
Gene symbol
Forward
Reverse
IAA14
TGGGGAGTTATGGAGCACAAGGG
CACCAACGAGCATCCAGTCACC
LAX3
CGCCGTCACAGTGGAGATAATGC
GCGGATGGTAGCGTTAGCGTTAG
AXR3
GGGCAAACATGGAGGAGAAGAAGG
CGCCGACGAGCATCCAATCAC
GH 3.1
CTACGCAGACACAAGCACGATCC
CCAAGGCAGCAACGAGTCAGAG
GH3.3
CACGAGCCCTAACGAAGCCATC
ACGGAGGAGACCAGAAGCGAAG
BRU6
GGGTTCCATAATTCCGCTCCACAG
CAACCTCGACGCATTCTCCACTG
Actin
GGACACCGTAGCGAATCAATCAC
CGCGCCACAAAGAAGAAGTTTCATTAGG
A. thaliana plants were collected after 10 days of treatment with compounds C2 and C6. The RNA was then extracted by grinding and mashing with liquid nitrogen. RNA was reverse transcribed to obtain cDNA, and actin was used as an internal reference gene for real-time fluorescence quantitative PCR. Three replicates were performed for each experiment and repeated three times. RNA was extracted using the Spin Column Plant Total RNA Purification Kit. Reverse transcription was performed using the M-MuLV First Strand cDNA Synthesis Kit. Detection was done using the SGExcel FastSYBR Mixture. Each experiment was repeated three times. The experimental data were processed using GraphPad Prism 9 software. The qRT-PCR experiments and calculations performed in this work followed the literature (Livak and Schmittgen, 2001). Details of the gene sequences for primer design were performed in the Supporting Information.
3 Results and discussion
3.1 Synthesis of target compounds
The synthetic route for the title compounds is shown in Scheme 1. Compound A and compound B were added to a round bottom flask and dissolved in acetonitrile. Triethylamine and the condensation agent HATU were added and the reaction was stopped after 3–4 h. The reaction solution was poured into water, stirred until a solid precipitated, filtered and dried to obtain the target compound C. The target compounds were structurally characterized using 1H NMR, 13C NMR, 19F NMR, FT-IR, and HRMS. The NMR, HR-MS, and FT-IR spectra of the target compounds can be found in the Supporting Information. The physical and chemical properties of the target compound are as follows:
Ethyl 4-(2-(6-fluoro-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C1). Yield 93.4 %; white powder; m.p. 134–136 °C. 1H NMR (500 MHz, DMSO) δ: 10.93 (s, 1H, NH), 8.24 (s, 1H, NH), 7.54 (dd, J = 8.7, 5.5 Hz, 1H, Ar—H), 7.17 (d, J = 2.3 Hz, 1H, CH), 7.11 (dd, J = 10.2, 2.3 Hz, 1H, Ar—H), 6.84 (ddd, J = 9.8, 8.7, 2.4 Hz, 1H, Ar—H), 3.97 (t, J = 7.1 Hz, 2H, CH2), 3.56 (s, 2H, CH2), 2.78–2.72 (m, 2H, CH2), 2.48–2.43 (m, 2H, CH2), 2.21 (d, J = 16.1 Hz, 2H, CH2), 1.94 (td, J = 11.1, 5.8 Hz, 2H, CH2), 1.02 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.80, 171.07, 160.26, 158.40, 136.40 (d, J = 12.7 Hz), 124.76, 124.59, 120.22 (d, J = 10.3 Hz), 109.64, 107.30, 107.10, 97.85, 97.65, 60.83, 57.48, 33.37, 32.65, 23.08, 14.36. 19F NMR (471 MHz, DMSO) δ: −122.20 (td, J = 9.9, 5.6 Hz). HR-MS (ESI): Calculated for C18H21FN2O3S [M + H]+: 365.13296, found: 365.13297; IR (KBr, cm−1): ν 3383.67, 3240.82 (N–H), 1713.4, 1640.14 (C⚌O) cm−1, 1553.60 (stretching vibration of indole skeleton).
Ethyl 4-(2-(6-chloro-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C2). Yield 92.4 %; white powder; m.p. 146–147 °C. 1H NMR (500 MHz, DMSO) δ: 11.00 (s, 1H, NH), 8.25 (s, 1H, NH), 7.56 (d, J = 8.5 Hz, 1H, Ar—H), 7.38 (d, J = 1.8 Hz, 1H, Ar—H), 7.22 (d, J = 2.3 Hz, 1H, Ar—H), 6.99 (dd, J = 8.4, 1.9 Hz, 1H, NH), 3.96 (t, J = 7.1 Hz, 2H, CH2), 3.56 (s, 2H, CH2), 2.76 (dd, J = 18.4, 6.9 Hz, 2H, CH2), 2.48–2.44 (m, 2H, CH2), 2.21 (d, J = 16.1 Hz, 2H, CH2), 1.96–1.91 (m, 2H, CH2), 1.02 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.78, 171.00, 136.95, 126.55, 126.20, 125.34, 120.62, 119.06, 111.44, 109.75, 60.84, 57.49, 33.36, 32.54, 23.08, 14.36. HR-MS (ESI): Calculated for C18H21ClN2O3S [M + H]+: 381.10341, found: 381.10342; IR (KBr, cm−1): ν 3291.01 (N–H), 1727.01, 1646.63 (C⚌O), 1544.29 (stretching vibration of indole skeleton).
Ethyl 4-(2-(4-chloro-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C3). Yield 94.3 %; white powder; m.p. 131–133 °C. 1H NMR (500 MHz, DMSO) δ 11.23 (s, 1H, NH), 8.15 (s, 1H, NH), 7.32 (dd, J = 8.0, 0.7 Hz, 1H, Ar—H), 7.24 (d, J = 2.3 Hz, 1H, Ar—H), 7.02 (t, J = 7.8 Hz, 1H, CH), 6.96 (dd, J = 7.5, 0.6 Hz, 1H, Ar—H), 4.03 (q, J = 7.1 Hz, 2H, CH2), 3.82 (s, 2H, CH2), 2.86–2.80 (m, 2H, CH2), 2.47 (t, J = 3.6 Hz, 2H, CH2), 2.24 (d, J = 16.0 Hz), 2H, CH2, 1.99–1.92 (m, 2H, CH2), 1.13 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ 173.89, 171.26, 138.23, 126.55, 125.20, 124.32, 122.20, 119.59, 111.22, 109.03, 60.87, 57.50, 33.46 (d, J = 5.8 Hz), 23.14, 14.51. HR-MS (ESI): Calculated for C18H21ClN2O3S [M + H]+: 381.10341, found: 381.10342; IR (KBr, cm−1): ν 3278.92 (N–H), 1724.77, 1647.00 (C⚌O), 1502.38 (stretching vibration of indole skeleton).
Ethyl 4-(2-(4-bromo-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C4). Yield 93.6 %; white powder; m.p. 130–132 °C. 1H NMR (500 MHz, DMSO) δ: 11.25 (s, 1H, NH), 8.15 (s, 1H, NH), 7.37 (dd, J = 7.9, 0.5 Hz, 1H, Ar—H), 7.26 (d, J = 2.4 Hz, 1H, Ar—H), 7.14 (dd, J = 7.5, 0.5 Hz, 1H, Ar—H), 6.96 (t, J = 7.8 Hz, 1H, CH), 4.04 (q, J = 7.1 Hz, 2H, CH2), 3.85 (s, 2H, CH2), 2.87–2.80 (m, 2H, CH2), 2.48 (dd, J = 8.6, 4.7 Hz, 2H, CH2), 2.27–2.22 (m, 2H, CH2), 1.99–1.93 (m, 2H, CH2), 1.14 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.89, 171.17, 138.05, 126.79, 123.02, 122.57, 113.47, 111.76, 109.61, 60.88, 57.52, 33.45 (d, J = 11.3 Hz), 23.19, 14.55. HR-MS (ESI): Calculated for C18H21BrN2O3S [M + H]+: 425.05290, found: 425.05290; IR (KBr, cm−1): ν 3200.97 (N–H), 1731.28, 1652.52 (C⚌O), 1509.19 (stretching vibration of indole skeleton).
Ethyl 4-(2-(5-fluoro-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C5). Yield 91.3 %; white powder; m.p. 137–138 °C. 1H NMR (500 MHz, DMSO) δ: 10.96 (s, 1H, NH), 8.26 (s, 1H, NH), 7.32 (dt, J = 9.9, 4.2 Hz, 1H, Ar—H), 7.24 (d, J = 2.3 Hz, 1H, NH), 6.90 (td, J = 9.2, 2.5 Hz, 2H, Ar—H), 3.96 (q, J = 7.1 Hz, 2H, CH2), 3.54 (s, 2H, CH2), 2.79–2.73 (m, 2H, CH2), 2.49–2.44 (m, 2H, CH2), 2.25–2.19 (m, 2H, CH2), 1.97–1.90 (m, 2H, CH2), 1.01 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.79, 171.07, 133.27, 127.94 (d, J = 10.0 Hz), 126.33, 112.72 (d, J = 9.8 Hz), 109.67 (d, J = 5.0 Hz), 109.43, 103.88, 60.81, 57.49, 33.37, 32.67, 23.08, 14.30. 19F NMR (471 MHz, DMSO) δ: −125.47 (dd, J = 24.2, 13.1 Hz). HR-MS (ESI): Calculated for C18H21FN2O3S [M + H]+: 365.13296, found: 365.13297; IR (KBr, cm−1): ν 3355.51 (N–H), 1712.69, 1640.88 (C = O), 1553.85 (stretching vibration of indole skeleton).
Ethyl 4-(2-(5-bromo-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C6). Yield 91.6 %; brown solid; m.p. 82–84 °C. 1H NMR (500 MHz, DMSO) δ: 11.07 (s, 1H, NH), 8.28 (s, 1H, NH), 7.77 (d, J = 1.8 Hz, 1H, Ar—H), 7.32 (s, 1H, NH), 7.23 (d, J = 2.3 Hz, 1H, Ar—H), 7.17 (dd, J = 8.6, 1.9 Hz, 1H, Ar—H), 3.98–3.94 (m, 2H, CH2), 3.55 (s, 2H, CH2), 2.80–2.74 (m, 2H, CH2), 2.49–2.44 (m, 2H, CH2), 2.24–2.19 (m, 2H, CH2), 1.96–1.90 (m, 2H, CH2), 0.99 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.78, 171.00, 135.30, 129.55, 125.95, 123.86, 121.77, 113.82, 111.51, 109.26, 60.82, 57.50, 33.37, 32.56, 23.10, 14.34. HR-MS (ESI): Calculated for C18H21BrN2O3S [M + H]+: 425.05290, found: 425.05290; IR (KBr, cm−1): ν 3287.29 (N–H), 1723.20, 1649.20 (C⚌O), 1513.27 (stretching vibration of indole skeleton).
Ethyl 4-(2-(5-chloro-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C7). Yield 89.6 %; brown solid; m.p. 112–114 °C. 1H NMR (500 MHz, DMSO) δ: 11.06 (s, 1H, NH), 8.28 (s, 1H, NH), 7.62 (d, J = 2.0 Hz, 1H, Ar—H), 7.35 (d, J = 8.6 Hz, 1H, Ar—H), 7.25 (d, J = 2.3 Hz, 1H, NH), 7.05 (dd, J = 8.6, 2.1 Hz, 1H, Ar—H), 3.95 (q, J = 7.1 Hz, 2H, CH2), 3.56 (s, 2H, CH2), 2.81–2.74 (m, 2H, CH2), 2.49–2.40 (m, 2H, CH2), 2.22 (d, J = 15.9 Hz, 2H, CH2), 1.97–1.90 (m, 2H, CH2), 0.99 (t, J = 7.1 Hz, 2H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.78, 171.02, 135.07, 128.85, 126.11, 123.52, 121.35, 118.72, 113.34, 109.35, 60.81, 57.50, 33.37, 32.57, 23.09, 14.31. HR-MS (ESI): Calculated for C18H21ClN2O3S [M + H]+: 381.10341, found: 381.10342; IR (KBr, cm−1): ν 3395.66 (N–H), 1721.77, 1641.11 (C⚌O), 1538.26 (stretching vibration of indole skeleton).
Ethyl 4-(2-(5-methoxy-2-methyl-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C8). Yield 94.6 %; brown powder; m.p. 181–182 °C. 1H NMR (500 MHz, DMSO) δ: 10.59 (s, 1H, NH), 8.20 (s, 1H, NH), 7.09 (t, J = 6.0 Hz, 2H, Ar—H), 6.60 (dd, J = 8.6, 2.5 Hz, 1H, Ar—H), 3.97 (q, J = 7.1 Hz, 2H, CH2), 3.75 (s, 3H, CH3), 3.47 (s, 2H, CH2), 2.76 (dd, J = 19.2, 7.4 Hz, 2H, CH2), 2.44–2.40 (m, 2H, CH2), 2.31 (s, 3H, CH3), 2.22 (d, J = 15.0 Hz, 2H, CH2), 1.92–1.86 (m, 2H, CH2), 1.04 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.88, 171.42, 153.44, 134.15, 130.55, 129.21, 111.34, 110.19, 105.44, 100.69, 60.81, 57.46, 55.71, 33.25, 31.66, 23.00, 14.37, 12.04. HR-MS (ESI): Calculated for C20H26N2O4S [M + H]+: 391.16860, found: 391.16860; IR (KBr, cm−1): ν 3255.10 (N–H), 1734.59, 1663.12 (C⚌O), 1521.28 (stretching vibration of indole skeleton).
Ethyl 4-(2-(5,6-dibromo-1H-indol-3-yl) acetamido) tetrahydro-2H-thiopyran-4-carboxylate (C9). Yield 91.5 %; light brown powder; m.p. 159–160 °C. 1H NMR (500 MHz, DMSO) δ: 11.03 (s, IH, NH), 8.27 (s, 1H, NH), 7.52 (dd, J = 11.5, 8.1 Hz, 1H, Ar—H), 7.34 (dd, J = 11.3, 7.0 Hz, 1H, Ar—H), 7.24 (d, J = 2.2 Hz, 1H, Ar—H), 3.95 (q, J = 7.1 Hz, 2H, CH2), 3.54 (s, 2H, CH2), 2.79–2.72 (m, 2H, CH2), 2.49–2.44 (m, 2H, CH2), 2.25–2.19 (m, 2H, CH2), 1.97–1.90 (m, 2H, CH2), 1.00 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (126 MHz, DMSO) δ: 173.76, 170.93, 131.54 (d, J = 10.6 Hz), 126.20, 123.11 (d, J = 7.9 Hz), 109.93, 105.89 (d, J = 18.8 Hz), 99.59, 60.82, 57.50, 33.35, 32.63, 23.06, 14.27. 19F NMR (471 MHz, DMSO) δ: −145.57 to −145.89 (m), −149.27 (ddd, J = 17.3, 11.1, 5.7 Hz). HR-MS (ESI): Calculated for C18H20F2N2O3S [M + H]+: 383.12354, found: 383.12355; IR (KBr, cm−1): ν 3338.93 (N—H), 1709.31, 1642.05 (C⚌O), 1521.28 (stretching vibration of indole skeleton).
3.2 The activity for inhibition of root length
The inhibition of A. thaliana root length was tested in a stepwise manner (Table 2). Initially, all compounds were tested at a concentration of 100 µM. It was found that all nine compounds exhibited inhibitory activity on the root length of A. thaliana. Specifically, compounds C2, C3, C4, C6, and C7 exhibited showed over 90 % inhibition of root growth at 100 µM, which was comparable to the inhibition rate of IAA at 10 µM. To further investigate the effects of these compounds, the concentration of C2, C3, C4, C6, and C7 was reduced to 50 µM and tested. At this concentration, compounds C2, C4, and C6 demonstrated inhibition rates of 72.07 %, 75.99 %, and 78.56 %, respectively. Compounds C2, C3, and C7 are indoles substituted with chlorine at the 6th, 4th, and 5th positions, respectively. On the other hand, compounds C4 and C6 are indoles with bromine substitutions at the 4th and 5th positions. Meanwhile, compounds C1, C5, and C9 contain fluorine substitutions, and C8 is an indole acetamide with a methoxy substitution. The results indicate that chlorine and bromine substitutions in indole derivatives exhibit higher activity compared to fluorine and methoxy substitutions. The data in the table is the average value of three repetitions. ±standard error. aThe average of triplicate. bPositive control in 10 µM.
Compound
Inhibition rate (%)
100 µM
50 µM
25 µM
12.5 µM
C1
74.18 ± 3.79
/
/
/
C2
100.00 ± 0.00
72.07 ± 2.52
43.48 ± 4.13
34.38 ± 3.21
C3
94.94 ± 1.53
41.51 ± 3.05
/
/
C4
100.00 ± 0.00
75.99 ± 4.66
9.04 ± 2.94
/
C5
71.49 ± 4.09
/
/
/
C6
100.00 ± 0.00
78.56 ± 2.63
36.87 ± 4.24
14.73 ± 2.58
C7
100.00 ± 0.00
50.18 ± 4.83
−0.27 ± 4.09
/
C8
46.94 ± 4.32
/
/
/
C9
60.31 ± 4.79
/
/
/
IAAb
100 ± 0.00
100 ± 0.00
100 ± 0.00
100 ± 0.00
Compounds C2 and C6 were particularly effective in inhibiting the growth of A. thaliana and significantly impacting its lateral root growth (Fig. 2). The primary root length of A. thaliana treated with 10 µM IAA stopped growing and showed lateral root and hair root development. C2 and C6 had similar effects at 50 µM, and their inhibition of root length decreased with decreasing concentration. The fact that IAA can enhance root growth at low concentrations while inhibiting root growth at high concentrations is widely recognized. The effects of these compounds, which have structures based on the modified structure of IAA, suggest a possible correlation with the mechanism of auxin action.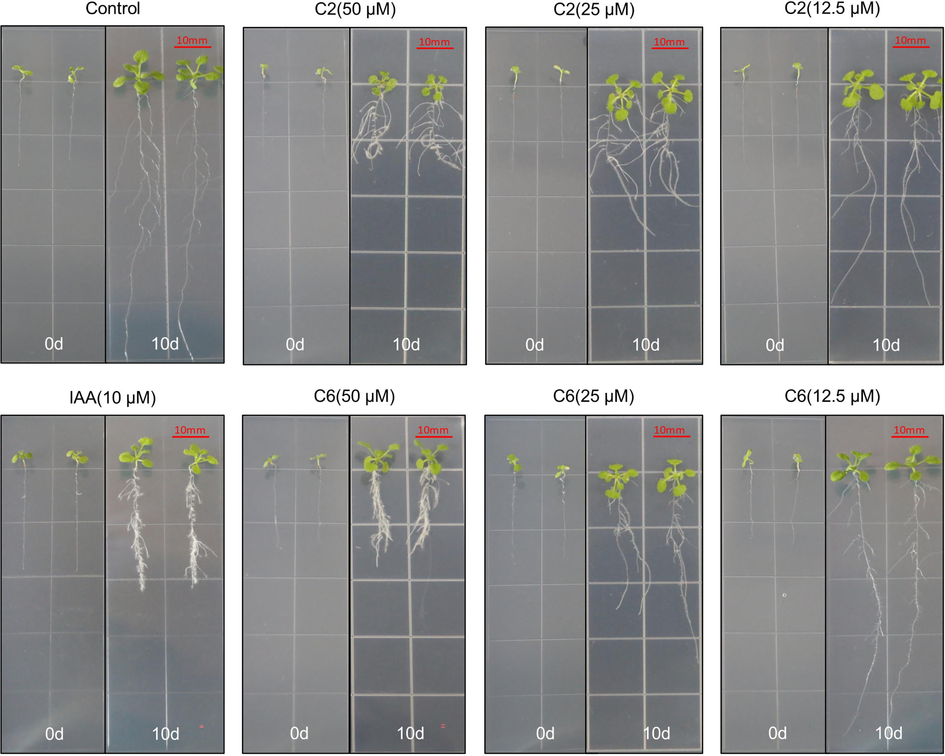
Effects of compounds C6 and C2 on root growth of A. thaliana before (0 d) and after (10 d) treatment.
3.3 Molecular docking analysis
The auxin receptor TIR1 was used to perform molecular docking. IAA was re-docked and its position was almost consistent with the original ligand, indicating the reliability of the software environment for docking (Fig. 3A). The results in Table 3 showed that modifying the side chain of the indole ring improved the ligand's binding potential. The final scores (S) for C6 and C2 were −7.1679 and −7.1467, respectively. C6 had the lowest conformationally optimized energy score (E_refine) of −40.2284, followed by C2. These two key indicators suggest that C6 and C2 have the best binding potential based on molecular docking for TIR1. S: The final score. Rmsd_refine: The mean square deviation between the laying refinement and after-refinement pose. E_conf: Energy conformer. E_place: Score for the ligand replacement phase. E_score1: The score of the first rescoring stage of generated poses. E_refine: The score of the refinement stage which use either the explicit molecular mechanics force field method or the grid-based energetics method. E_score2: The score of the final pose rescored using one of several scoring schemes (Behazin and Ebrahimi, 2018; Wahba et al., 2024).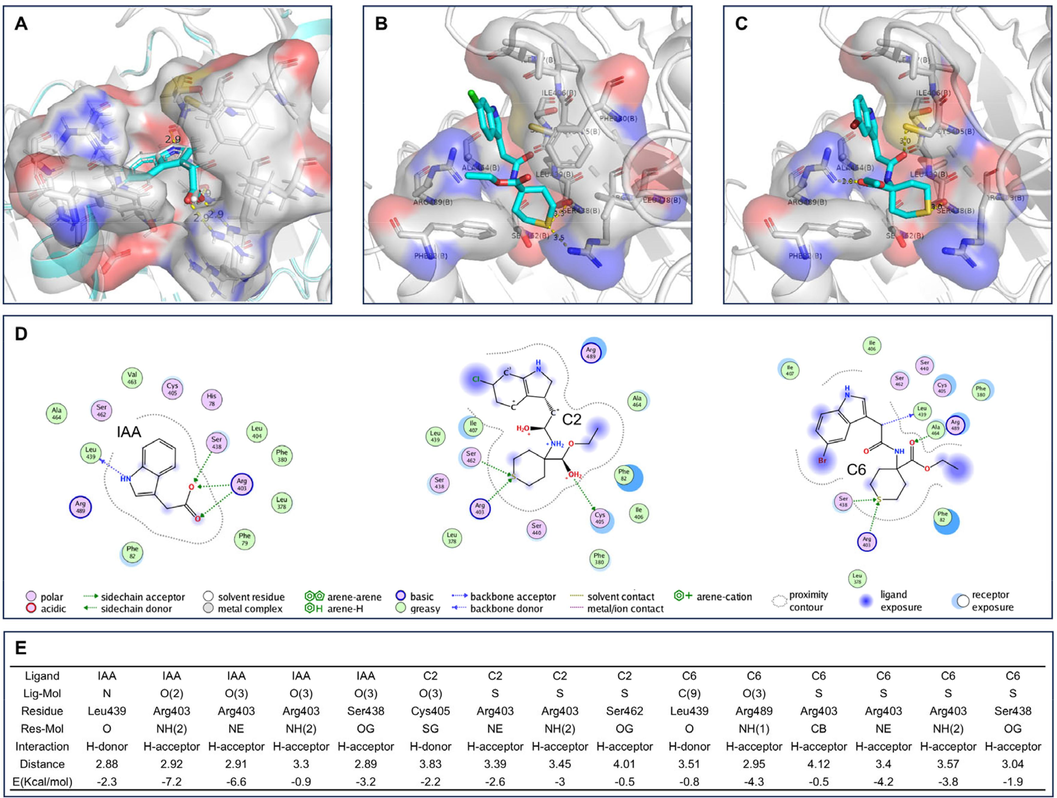
Visualization of Molecular Docking results for TIR1. (A) The original protein 2P1P (including IAA) is shown in white, and the redocking IAA is shown in cyan. (B) TIR1 with C2 docked into the binding pocket. (C) TIR1 with C6 docked into the binding pocket. (D) The 2D interactions of IAA, C2 and C6 with TIR1, respectively. (E) The results of interaction between ligands and TIR1.
Ligand
S
Rmsd_refine
E_conf
E_place
E_score1
E_refine
E_score2
IAA
−6.1992
1.8976
−53.9035
−58.7221
−11.6119
−37.5524
−6.1992
C1
−7.0980
2.3739
31.3360
−66.6915
−10.3555
−38.1116
−7.0980
C2
−7.1567
2.4197
28.2746
−56.3484
−9.1215
−38.8626
−7.1567
C3
−6.9773
0.8448
47.7374
−67.4063
−10.2848
−36.3680
−6.9773
C4
−6.9244
1.0847
56.6324
−53.0436
−9.0128
−34.7657
−6.9244
C5
−7.0944
1.5874
34.4199
−48.9477
−9.0196
−36.0892
−7.0944
C6
−7.1679
2.7296
25.7069
−70.9308
−9.4579
−40.2284
−7.1679
C7
−6.9384
2.3898
30.4306
−51.3474
−9.0779
−34.0082
−6.9384
C8
−6.8703
2.0527
21.0983
−44.7233
−9.7485
−33.6516
−6.8703
C9
−6.7764
1.3983
47.9787
−51.6302
−10.5733
−28.3215
−6.7764
The 2D interactions between ligands and TIR1 demonstrate the effect of structural modifications of IAA on TIR1 (Fig. 3D). In C2 and C6, sulfur atoms replace the carboxyl group of IAA and form hydrogen interactions with Arg and Ser. Arg403, Ser438, and Leu439 interact with IAA, while Arg403, Cys405, and Leu489 interact with C2. The amino acid residues that form hydrogen interactions with C6 are Arg403, Ser438, Leu439, and Arg489. In general, the lower the energy, the more stable it is. The hydrogen interaction distances of C2 and C6 are greater than those of IAA, resulting in their higher energy compared to IAA (Fig. 3E). Additionally, the indole rings of C2 and C6 are pushed to the outside of the pocket (Fig. 3B and C) and occupy the amino acid positions of AUX/IAA7 in the bound state with TIR1, which would result in AUX/IAA7 being pushed away from C2 and C6, making AUX/IAA7 less closely related to TIR1. These factors may be the reason why A. thaliana is less sensitive to C2 and C6 than to IAA.
3.4 Gene response analysis
To further explore the potential mechanisms of action of C2 and C6, a transcriptome analysis was performed on A. thaliana that treated with these compounds. Consistent with the results observed in Fig. 2, high concentrations of the compounds inhibited A. thaliana roots more strongly than low concentrations, resulting in a greater number of differentially expressed genes (DEGs). Treatment with 50 μM of compound C2 resulted in the up-regulation of 4784 genes and the down-regulation of 2208 genes (Fig. 4A). Treatment with 12.5 μM of compound C2 led to the up-regulation of 789 genes and the down-regulation of 1859 genes (Fig. 4B). Treatment with 50 μM of compound C6 resulted in the up-regulation of 3289 genes and the down-regulation of 1552 genes (Fig. 4C). Treatment with 12.5 μM of compound C6 led to the up-regulation of 184 genes and the down-regulation of 938 genes (Fig. 4D).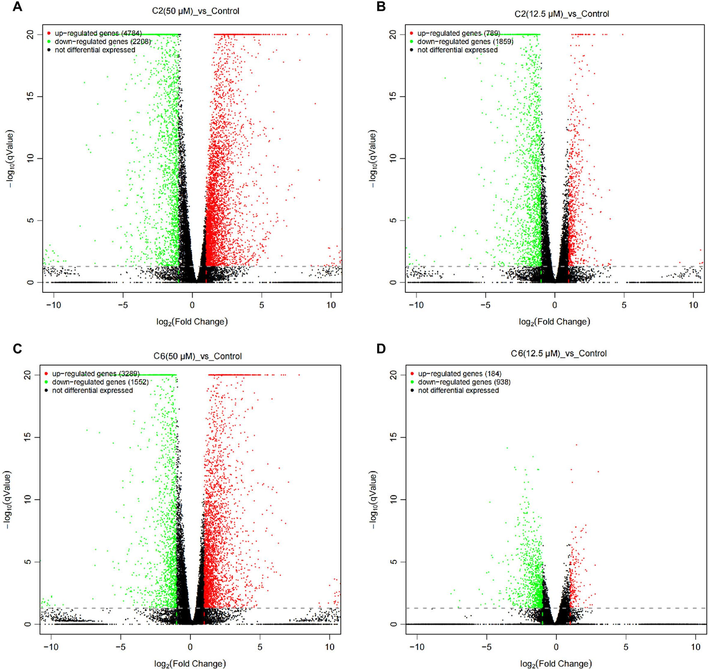
Volcano maps of differentially expressed genes (DEGs) between the control group and the treatment groups (C2 and C6). The DEGs were obtained by comparing the C2 and C6 treatments with control, and the criteria for the DEGs were log2 (fold change) ≥ 1 and false discovery rate < 0.05. (A) Comparison between compound C2 at 50 μΜ treatment and control. (B) Comparison between compound C2 at 12.5 μΜ treatment and control. (C) Comparison between compound C6 at 50 μΜ treatment and control. (D) Comparison between compound C6 at 12.5 μΜ treatment and control.
In general, we would assume that most of the genes induced by a low concentration should be part of the genes induced by a high concentration. However, the number of uniformly regulated genes in high and low concentrations was not overwhelmingly dominant (Fig. 5A and C, Fig. 6A and C). Out of the 184 genes up-regulated at a low C6 concentration, 112 were also up-regulated at a high C6 concentration, while only 319 of the 938 down-regulated genes were down-regulated at a high C6 concentration. The number of DEGs after C2 treatment was higher than that of C6. Out of the 789 genes up-regulated at a low concentration of C2, 514 genes were also up-regulated at a high concentration, and 1048 of the 1859 down-regulated genes were also down-regulated at high concentrations.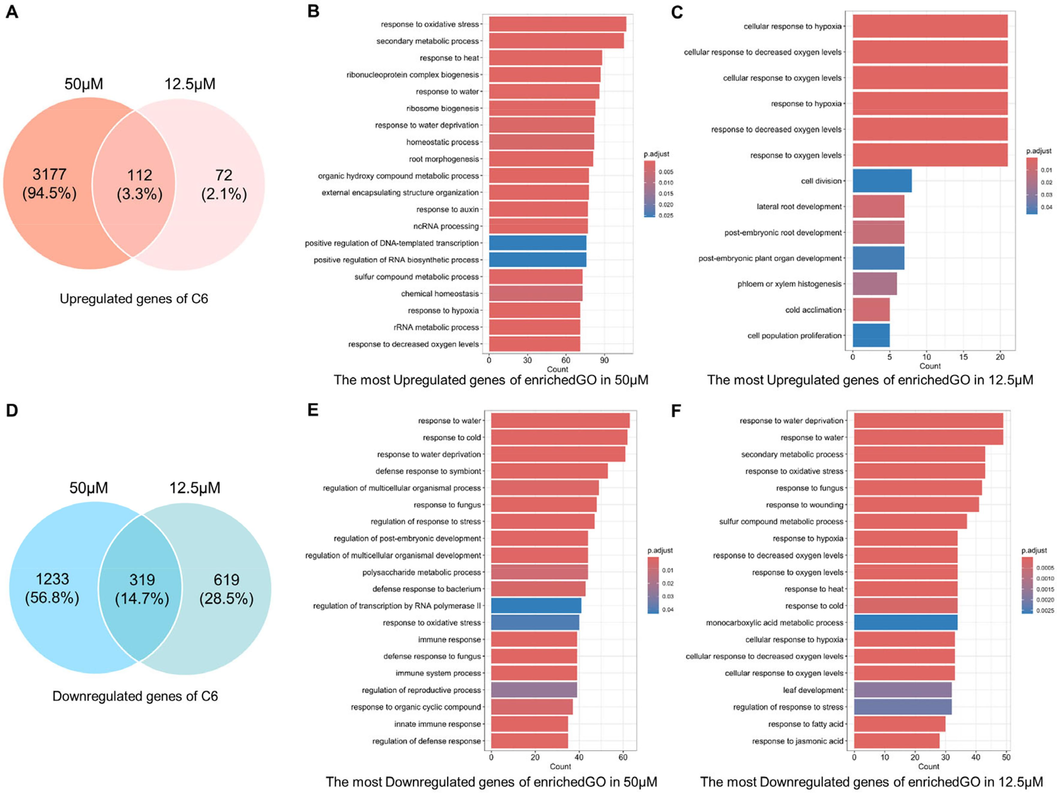
The similarity and differences in the transcriptional profiles in the treatment of C6. (A) and (D) The comparison of DEGs at high and low concentrations of C6. (B) and (E) The Biological Process results of Gene Ontology (GO) enrichment for C6 in 50 μΜ. (C) and (F) The Biological Process results of Gene Ontology (GO) enrichment for C6 in 12.5 μΜ.
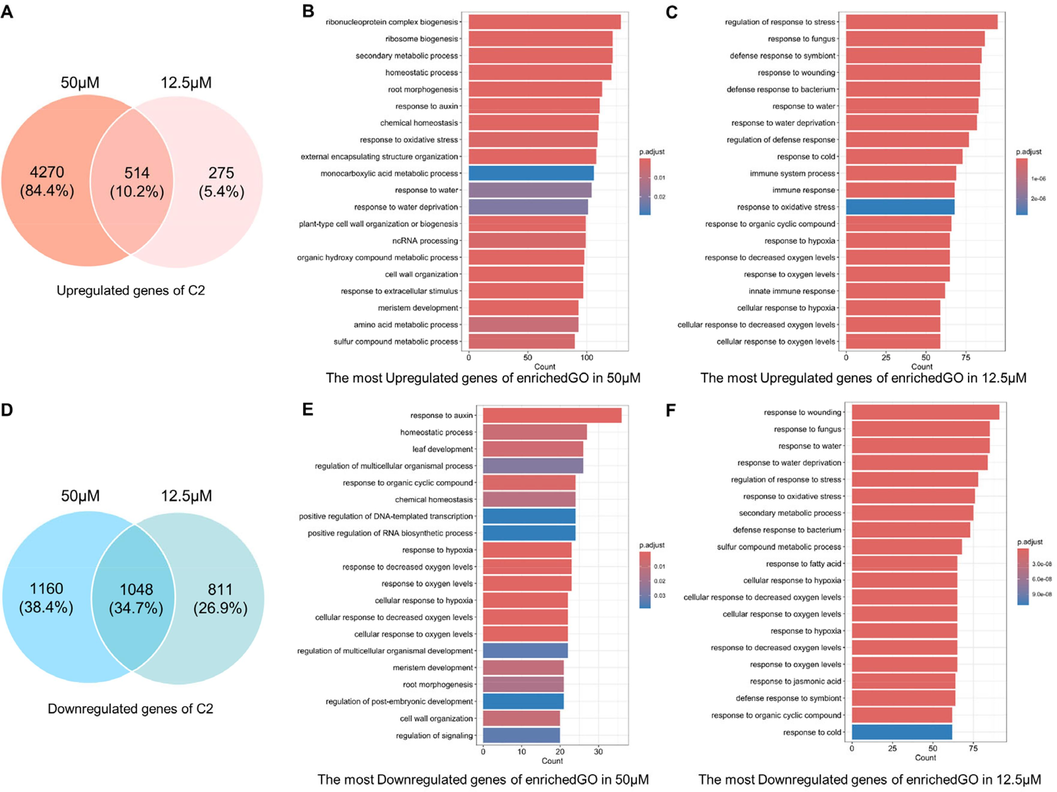
The similarity and differences in the transcriptional profiles in the treatment of C2. (A) and (D) The comparison of DEGs at high and low concentrations of C2. (B) and (E) The Biological Process results of Gene Ontology (GO) enrichment for C2 in 50 μΜ. (C) and (F) The Biological Process results of Gene Ontology (GO) enrichment for C2 in 12.5 μΜ.
The biological processes enriched in GO were used to further reveal the changes within A. thaliana caused by compound treatment. As shown in Fig. 5B and E, the up-regulated DEGs by C6 at 50 μΜ are enriched in multiple processes that respond to environmental changes, such as oxygen, temperature, and water. Changes in secondary metabolites, RNA and proteins are bound to occur. The responses of roots and auxin are important because they match the phenotype in Fig. 2. Corresponding to these, the down-regulated DEGs are mainly concentrated in plant resistance to biotic and abiotic stresses. As an active factor regulating plant growth, auxin can antagonize plant stress response (Zhao et al., 2024). These pieces of evidence suggest that the A. thaliana reaction treated with C6 at 50 μΜ is closely related to auxin. No dramatic root changes are observed in A. thaliana treated with lower concentrations of C6 (Fig. 2), so the number of up-regulated DEGs is much smaller (Fig. 5C and F). Significantly, the down-regulated DEGs were still heavily enriched in plant response to stress. This means that C6, as a potential chemical signal, is still sensed by plants and initiates inhibition of stress responses. A. thaliana treated with C2 at 50 μΜ induces a more pronounced auxin response and root development (Fig. 6B and E), while a low concentration of C2 is more prominent in stimulating plant stress responses (Fig. 6C and F).
The details of differentially expressed genes (DEGs) in response to auxin are presented in Table 4. The results revealed the presence of well-known genes such as LAX3, AXR3, and IAA14, as well as auxin metabolism-related genes GH3.1, GH3.3, and BRU6, all of which are associated with lateral root growth. The plant hormone IAA primarily regulates the expression of members of the Aux/IAA family of transcriptional regulators. The transcriptional regulation of auxin-responsive genes relies on two related families of transcriptional regulators: auxin-responsive factors (ARFs) and auxin/indole-3-acetic acid (Aux/IAAs). Specifically, ARF7 and ARF19 have been found to directly activate A. thaliana LBD/ASL genes, which play a crucial role in lateral root formation.
Gene ID (symbol)
C2 effect
C6 effect
Gene function
50 μM
12.5 μM
50 μM
12.5 μM
AT1G77690-LAX3
–
–
Up
–
Auxin influx carrier; promotes lateral root germination
AT4G14550-IAA14
Up
–
Up
–
Participate in lateral root development
AT1G04250-AXR3
Up
–
Up
–
Involved in auxin signaling
AT4G32280-IAA29
Down
–
Down
–
Auxin induced protein
AT2G01200-IAA32
Down
–
–
–
Belongs to the auxin-inducible gene family
AT2G14960-GH3.1
Up
–
Up
–
Encodes a protein similar to IAA-amine synthase
AT2G23170-GH3.3
Up
–
–
–
Encodes an IAA-amino synthetase
AT4G37390-BRU6
Up
–
–
–
Encodes an IAA-amino synthetase
AT4G03400-DFL2
Down
Down
Down
–
Encoding GH3-related genes
AT1G48660-F11I4.15
–
–
Up
–
Auxin-responsive GH3 family proteins
AT4G31320-SAUR37
Up
–
Up
–
Auxin responsive gene
AT3G09870-SAUR48
–
Up
Up
Up
Auxin responsive gene
AT2G21210-SAUR6
–
–
Up
–
Auxin responsive gene
AT2G37030-SAUR46
–
–
Up
Up
Auxin responsive gene
AT3G12830-SAUR72
–
–
Up
Up
Auxin responsive gene
AT2G28085-SAUR42
Down
–
Down
–
Auxin responsive gene
AT5G18050-SAUR22
Down
–
Down
–
Auxin responsive gene
AT4G22620-SAUR34
–
–
Down
Down
Auxin responsive gene
3.5 QRT-PCR analysis
The QRT-PCR validation of genes associated with lateral root development (Fig. 7). The qRT-PCR analysis results depicted in Fig. 7A indicate that compounds C2 and C6 significantly increased the expression of the IAA14 gene at both concentrations of 50 µM and 12.5 µM. IAA14 is a key factor in the initiation of lateral root growth in A. thaliana and also regulates the maturation stage of lateral root development (Guseman et al., 2015; Zhang et al., 2023). As shown in Fig. 8, the expression of GATA23 (a GATA transcription factor) and LBD16 (a Lateral Organ Binding Domain), which play important roles in lateral root growth, is influenced by IAA28 and IAA14, respectively (Lavenus et al., 2013; Li et al., 2022). SLR/IAA14 is a key regulator of growth hormone-regulated growth and development, particularly in lateral root formation (Fukaki et al., 2002). In slr-mutants, the stabilized mutant IAA14 protein inactivates ARF7/ARF19 function, thereby blocking lateral root formation in these mutants (Fukaki et al., 2005). It is evident that IAA14 interacts with other regulatory factors to govern lateral root growth and development.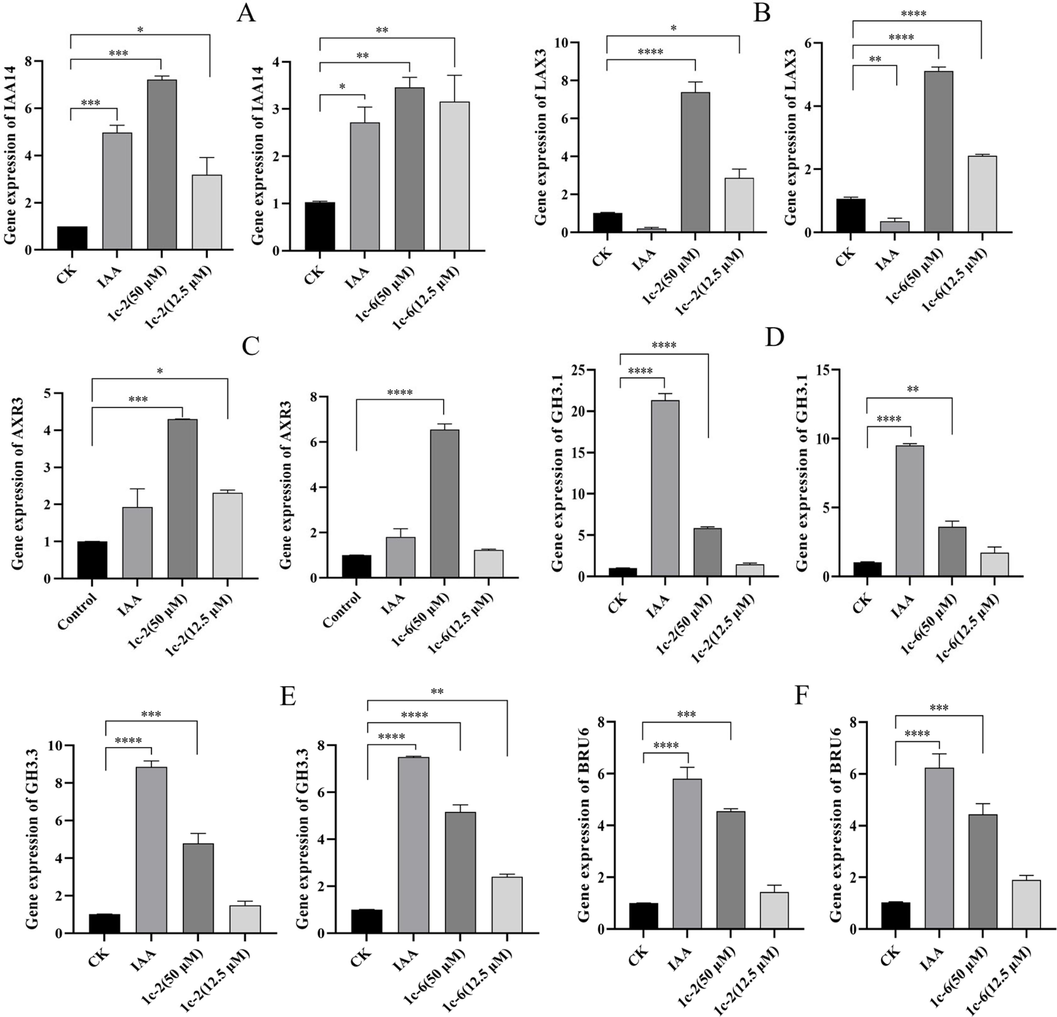
QRT-PCR expression analysis of auxin pathway-related genes. P indicates significant difference (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
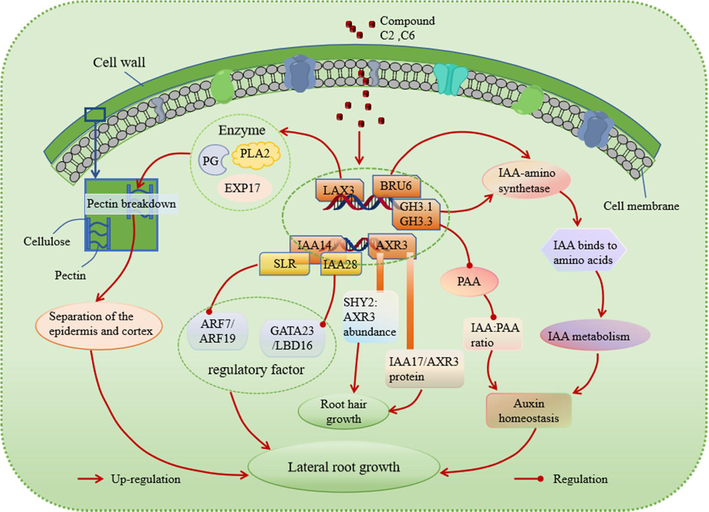
The possible signalling pathway regulated by compound C2 and C6.
The regulation of root growth and development is dependent on the LAX3 gene, which controls the influx of auxin. Notably, compounds C2 and C6 were discovered to markedly enhance the expression of the LAX3 gene (Fig. 7B). As shown in Fig. 8, LAX3 is specifically expressed near lateral root primordia and regulates the expression of cell wall remodeling proteins such as Pectin Lyase 2 (PLA 2), polygalacturonase (PG), and EXPASIN17 (EXP17). These proteins are involved in breaking down demethylated pectin in the cell wall (Cosgrove, 2000; Marín-Rodríguez et al., 2002). Increased LAX3 activity promotes the induction of cell wall remodeling enzymes, which may aid in cell separation before the development of lateral root primordia. LAX3 also facilitates the emergence of lateral roots by promoting the separation of the epidermis and cortex (Swarup et al., 2008).
The AXR3 gene was found to significantly up-regulate compounds C2 and C6 (Fig. 7C). The alleles AXR3-1 and AXR3-3 are associated with IAA-excessive phenotypes such as reduced elongation and a reduced number of root hairs (Leyser et al., 1996). As shown in Fig. 8, root hair initiation is controlled by the relative abundance of SHY2 (SHORT HYPOCOTYL 2) and AXR3 in the cell. A stabilizing mutation in AXR3/IAA17 inhibits root hair development and elongation (Knox et al., 2003). The IAA17/AXR3 protein participates in root development, and the accumulation of its mutant variant, AXR3-1, which cannot bind auxin, leads to a severe root growth phenotype and agravitropism (Kubalová et al., 2024). These findings illustrate the involvement of the AXR3 gene in regulating root hair development.
There are 19 GH3 genes in A. thaliana, which play a crucial role in regulating plant development and growth hormone balance. Studies have shown that changes in the expression of these genes can have a significant impact on plant growth and development (Guo et al., 2022; Kong et al., 2022). Interestingly, it has been observed that the application of 10 µM of auxin can lead to an increase in the expression of GH3.1 and GH3.3 genes, while compounds C2 and C6 have similar effects to growth factors (Fig. 7D and 7E). Moreover, mutants lacking all eight GH3 genes exhibit distinct phenotypes such as short roots, dense root hairs, and long hypocotyls and petioles (Guo et al., 2022). As shown in Fig. 8, the levels of phenylacetic acid (PAA) in A. thaliana have been linked to lateral root formation. GH3 growth hormone amide synthase plays a role in regulating the ratio of IAA to PAA during plant development (Aoi et al., 2020). Furthermore, members of the GH3 gene family encode acylamide synthetases that bind IAA to amino acids. Compounds C2 and C6 have been shown to up-regulate the BRU6 gene (Fig. 7F) (Casanova-Sáez et al., 2022). The GH3.1, GH3.3 and BRU6 genes all encode IAA-amino synthases that catalyze the binding of IAA and amino acids to ensure local IAA homeostasis, thereby regulating plant growth (Zhao, 2018).
4 Conclusion
Nine N-thianyl indole acetamide derivatives were designed, synthesized, and tested for their effects on regulating root growth. The results indicated that N-thianyl indole acetamide derivatives showed a good inhibitory effect on the root growth of A. thaliana, with some compounds exhibiting a 100 % inhibition rate at 100 µM. Compounds C2 and C6 not only inhibited main root growth but also promoted the increase of lateral roots. They regulated the growth of A. thaliana lateral roots by regulating the expression of lateral root growth and development-related genes IAA14, LAX3, and AXR3 in the auxin signaling pathway. Furthermore, they could also enhance the expression of IAA-amino synthetase-related genes GH3.1, GH3.3, and BRU6, catalyzing the combination of IAA and amino acids, ensuring the homeostasis of local auxin, and thereby regulating plant root growth.
5 Consent for publication
All authors consent to participate in the manuscript publication.
CRediT authorship contribution statement
Li Lei: Writing – original draft, Methodology, Investigation, Data curation. Xu Tang: Writing – review & editing, Validation. Wei Sun: Writing – review & editing, Validation. Anjing Liao: Writing – review & editing, Validation. Jian Wu: Funding acquisition, Formal analysis, Conceptualization.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2023YFD1700602), the Technology Achievement Transformation (general project) of Guizhou Province (Qiankehezhicheng[2024] the general 083, Qiankehechengguo [2022] the general 063), the Program of Introducing Talents to Chinese Universities (No. D20023), the Central Government Guides Local Science and Technology Development Fund Projects (Qiankehezhongyindi (2023) 001), Key Agricultural Technology R&D Projects of the Xinjiang Production and Construction Corps (NYHXGG, 2023AA602).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spectral and biological evaluation of a synthetic antimicrobial peptide derived from 1-aminocyclohexane carboxylic acid. Bioorg Med Chem. 2015;23:1341-1347.
- [CrossRef] [Google Scholar]
- GH3 auxin-amido synthetases alter the ratio of indole-3-acetic acid and phenylacetic acid in arabidopsis. Plant Cell Physiol. 2020;61:596-605.
- [CrossRef] [Google Scholar]
- Modulation of Arabidopsis root growth by specialized triterpenes. New Phytol. 2021;230:228-243.
- [CrossRef] [Google Scholar]
- The physicochemical properties and tyrosinase inhibitory activity of ectoine and its analogues: A theoretical study. Comput Theor Chem. 2018;1130:6-14.
- [CrossRef] [Google Scholar]
- A Survey of the Role of Noncovalent Sulfur Interactions in Drug Design. J Med Chem. 2015;58:4383-4438.
- [CrossRef] [Google Scholar]
- Inactivation of the entire Arabidopsis group II GH3s confers tolerance to salinity and water deficit. New Phytol. 2022;235:263-275.
- [CrossRef] [Google Scholar]
- Clauss, A., Glaess, C., Marciniak, G., Nave, J.F., Vivet, B., 2010. Quinazoline-2,4(1H,3H)-dione derivatives, their preparation and use as phosphodiesterase, especially PDE7, inhibitors for treating inflammation, diabetes, psychiatric, neurological and cardiovascular diseases. WO2010116088.
- Small molecule probes of ABA biosynthesis and signaling. Plant Cell Physiol. 2018;59:1490-1499.
- [CrossRef] [Google Scholar]
- Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat Plants. 2018;4:453-459.
- [CrossRef] [Google Scholar]
- Route to novel auxin: auxin chemical space toward biological correlation carriers. Chem Rev. 2010;110:4690-4708.
- [CrossRef] [Google Scholar]
- Inspired by nature: isostere concepts in plant hormone chemistry. J Agric Food Chem. 2023;71:18141-18168.
- [CrossRef] [Google Scholar]
- Fu, J., Karur, S., Madera, A.M., Pecchi, S., Sweeney, Z.K., Tjandra, M., Yifru, A., 2014. Preparation of hydroxamic acid derivatives as LpxC inhibitors useful for the treatment of bacterial infection. WO2014160649.
- Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002;29:153-168.
- [CrossRef] [Google Scholar]
- Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 2005;44:382-395.
- [CrossRef] [Google Scholar]
- Local conjugation of auxin by the GH3 amido synthetases is required for normal development of roots and flowers in Arabidopsis. Biochem Biophys Res Commun. 2022;589:16-22.
- [CrossRef] [Google Scholar]
- Auxin-induced degradation dynamics set the pace for lateral root development. Development. 2015;142:905-909.
- [CrossRef] [Google Scholar]
- Isolation and characterization of chemical compounds from fruit pulp of cassia fistula and their antimicrobial activity. J Drug Del Therap. 2018;8
- [CrossRef] [Google Scholar]
- Kast, J., Keil, M., Kolassa, D., Schirmer, U., Wuerzer, B., Meyer, N., Rademacher, W., Jung, J., 1988. Tetrahydro(thio)pyran-2,4-dione derivatives, procedure for their preparation, and their use as herbicides and plant growth regulators. DE3701298.
- AXR3 and SHY2 interact to regulate root hair development. Development. 2003;130:5769-5777.
- [CrossRef] [Google Scholar]
- Functional antagonism of WRI1 and TCP20 modulates GH3.3 expression to maintain auxin homeostasis in roots. Plants. 2022;11:454
- [CrossRef] [Google Scholar]
- Kontani, T., Miyata, J., Hamaguchi, W., Kawano, T., Kamikawa, A., Suzuki, H., Sudo, K., 2005. Preparation of tetrahydro-2H-thiopyran-4-carboxamides as anti-herpesvirus agents. US20050032855.
- Auxin co-receptor IAA17/AXR3 controls cell elongation in Arabidopsis thaliana root solely by modulation of nuclear auxin pathway. New Phytol. 2024;241:2448-2463.
- [CrossRef] [Google Scholar]
- Synthesis, antimicrobial and anticonvulsant screening of small library of tetrahydro-2H-thiopyran-4-yl based thiazoles and selenazoles. J Enzyme Inhib Med Chem. 2016;31:24-39.
- [CrossRef] [Google Scholar]
- Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450-458.
- [CrossRef] [Google Scholar]
- Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403-413.
- [CrossRef] [Google Scholar]
- PHB3 regulates lateral root primordia formation via NO-mediated degradation of AUXIN/INDOLE-3-ACETIC ACID proteins. J Exp Bot. 2022;73:4034-4045.
- [CrossRef] [Google Scholar]
- Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408.
- [CrossRef] [Google Scholar]
- Pectate lyases, cell wall degradation and fruit softening. J Exp Bot. 2002;53:2115-2119.
- [CrossRef] [Google Scholar]
- The importance of sulfur-containing motifs in drug design and discovery. Expert Opin Drug Discov. 2022;17:501-512.
- [CrossRef] [Google Scholar]
- Structural studies on monohalogenated derivatives of the phytohormone indole-3-acetic acid (auxin) Acta Crystallogr Sect B. 1996;52:332-343.
- [CrossRef] [Google Scholar]
- Directionality of hydrogen bonds to sulfur and oxygen. J Am Chem Soc. 1996;118:2726-2733.
- [CrossRef] [Google Scholar]
- Molecular requirements for auxin action–I. Halogenated indoles and indoleacetic acid. Phytochemistry. 1965;4:229-243.
- [CrossRef] [Google Scholar]
- Plant growth regulators: backgrounds and uses in plant production. J Plant Growth Regul. 2015;34:845-872.
- [CrossRef] [Google Scholar]
- A review on scopes, methods and rationale of integrative approach in siddha medicine with biomedicine. Int J Pharm Pharm Sci 2020:6-11.
- [CrossRef] [Google Scholar]
- The role of indole derivative in the growth of plants: a review. Front Plant Sci. 2023;13:1120613
- [CrossRef] [Google Scholar]
- Indole derivatives as agrochemicals: an overview. Chin Chem Lett. 2024;35:109005
- [CrossRef] [Google Scholar]
- The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 2008;10:946-954.
- [CrossRef] [Google Scholar]
- Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640-645.
- [CrossRef] [Google Scholar]
- Auxin activity of some indole derivatives. Plant Physiol. 1958;33:311-321.
- [CrossRef] [Google Scholar]
- Dynamic control of plant water use using designed ABA receptor agonists. Science. 2019;366
- [CrossRef] [Google Scholar]
- Electrochemical sensing of strontium ions cyclic voltammetry and investigation of rosemary extract’s effects on their behavior: Antibacterial properties of rosemary extract and molecular docking analysis for potential COVID-19 therapeutic benefits. Microchem J. 2024;200:110398.
- [CrossRef] [Google Scholar]
- Plant antiviral compounds containing pyrazolo [3,4-d] pyrimidine based on the systemin receptor model. Arab J Chem. 2024;17:105849
- [CrossRef] [Google Scholar]
- Novel ferulic amide Ac6c derivatives: design, synthesis, and their antipest activity. J Agric Food Chem. 2021;69:10082-10092.
- [CrossRef] [Google Scholar]
- Genetic regulation of lateral root development. Plant Signal Behav. 2023;18:2081397.
- [CrossRef] [Google Scholar]
- Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol.. 2010;61:49-64.
- [CrossRef] [Google Scholar]
- Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol. 2018;69:417-435.
- [CrossRef] [Google Scholar]
- Chemical driving the subtype selectivity of phytohormone receptors is beneficial for crop productivity. J Agric Food Chem. 2024;72:16583-16593.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.106082.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







