Translate this page into:
Synergetic effect of ZnIn2S4 nanosheets with metal-organic framework molding heterostructure for efficient visible- light driven photocatalytic reduction of Cr(VI)
⁎Corresponding author. bilal.fcc288794@qq.com (Muhammad Bilal Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The photocatalytic reduction of toxic Cr(VI), to green Cr(III) by visible light, is highly required. Metal-organic frameworks have been waged more and more devotion in the field of environmental remediation. Diversification along with functionalization is still thought-provoking and crucial for the progress of metal-organic framework (MOF)-based high activity materials. Herein, a succession of UiO-66-NH2@ZnIn2S4 composites with varying amount of UiO-66-NH2 is prepared by the facile solvothermal technique. Synergetic effect for Cr(VI) reduction is assessed under the influence of visible light (λ > 420 nm). UiO-66-NH2 octahedron is detained by ZnIn2S4 nanoflakes. The obvious enhancement in activity is observed which is credited to the well-suited energy band construction and close interaction between the interface of ZnIn2S4 and UiO-66-NH2, which leads to effective transfer and separation of photogenerated carriers. Synergistic effect could be evidently understood from the PL and UV -spectroscopy, after molding into heterostructure of UiO-66-NH2@ZnIn2S4. In addition, UiO-66-NH2@ZnIn2S4 composites exhibited good stability in photocatalytic reduction. Consequently, this UiO-66-NH2 constructed composite has high potential in the field of environmental remediation.
Keywords
Synergetic effect of ZnIn2S4 nanosheets
Photocatalytic Cr(VI) reduction
Visible light
Solvothermal approaches
Solar light harvestation
Varying amount of UiO-66-NH2
1 Introduction
A boost in expanding water pollution due to the expeditious growth of urbanization and industrialization resulted in a deliberate environmental issue throughout the globe (Bai et al., 2018; Hussain et al., 2020; Liu et al., 2020; Wang et al., 2019; Zhang et al., 2018). Hexavalent chromium (VI), which is highly soluble, toxic and carcinogenic in heavy metal pollutants, poses a serious threat to ecosystem (Liu et al., 2020; Zhang et al., 2014). Therefore, in the early phase, lots of approaches along with skills have been entrenched to eliminate wastewater Cr(VI), including ion-exchange method (Xing et al., 2007), membrane separation (Divrikli et al., 2007), chemical precipitation and adsorption (Xie et al., 2017). Nevertheless, the conventional means have the disadvantages of high cost, high energy utilization, poor performance and low removal rate, which hinder their comprehensive applications (Zhao et al., 2017). As an effective, green and efficient method, the photocatalytic reduction has been emerged as an effective strategy in the remediation of Cr(VI) pollutants as compared to traditional methods (Padhi et al., 2017).
The benefits of reduction by photocatalysis contain high efficacy, little cost, no production of pollution at the secondary level and direct utilization of the energy from the solar origin (Guo et al., 2019a; Guo et al., 2019b; Zhang et al., 2011b). Contrary to, single component photocatalyst, composite photocatalyst have the capability to heighten the efficacy of photocatalysis, chiefly due to the heterojunctions which contribute to the separation of electrons as well as the holes through the interface transfer of charge (Liu et al., 2015; Zhang et al., 2011a; Zhang et al., 2014). For instance, Tian and his colleagues prepared Mn3O4@ZnO/Mn3O4 heterojunction and reduced Cr(VI) by photocatalysis. The new photocatalyst has good Cr(VI) activity (200 mL, 10 mg/L) and demonstrated complete reduction after 70 min (Zhao et al., 2017). The magnetic and rectifiable TiO2/Fe3O4 heterostructures have been successfully synthesized by sounak' group. In the aerobic atmosphere, the photocatalytic ability of Cr(VI) photoreduction is stronger than that of bulk TiO2. Undoubtedly TiO2/Fe3O4 junction has the ability to promote the charge generation and hide the charge recombination mechanisms (Challagulla et al., 2016). That is why a jillion studied the utilization of a series of composite photocatalysts to reduce Cr(VI) by photocatalysis (Shen et al., 2013a).
Metal-organic frameworks (MOFs) are kind of materials having pores with the crystal structure of three-dimensions made up of metal units and organic ligands. As a consequence of its broadening particular surface area, exclusive structure having adjustable pore size, MOFs have a wide application prospect in the fields of gas adsorption, separation, chemical sensing, drug delivery and catalysis (Xiao et al., 2016). The current advancement in research shows that some MOFs have excellent properties as semiconductors and can be utilized for photocatalysis (Zhao et al., 2017). A large number of MOFs-based photocatalysts have applications in the elimination of organic pollutants (Shi et al., 2015), hydrogen production(Wu et al., 2017; Yang et al., 2016), CO2 reduction (Fu et al., 2012) organic transformations (Li et al., 2014) and especially in the reduction of Cr(VI) (Shen et al., 2013b) which is more described at present. As compared to the traditional semiconductor photocatalyst, MOF-based photocatalyst has greater application value, owing to the significant adjustments of metal-oxygen clusters and bridging organic connectors that can tolerate appropriate tuning and reasonable molecular design of photocatalyst, which is very reasonable (Yang et al., 2016).
In addition, the specific porosity of MOFs makes the substrate then products diffuse by accessible framework structure (Furukawa et al., 2014). Although MOF is believed to be a quickly developing attraction, the MOFs-based derivatives used for photocatalytic activity are still in the initial phase of research (Vermoortele et al., 2013). After photoexcitation, MOFs can perform electron-hole recombination at a very high rate like all single component photocatalysts (Zhao et al., 2017). The metal, ligand substitution and deposition of noble metals can be utilized to enhance the efficacy of photocatalysis of MOFs (He et al., 2016). In addition, the combination of MOFs and collector semiconductor materials to develop heterostructure is also a possible way of boosting the photocarriers separation and improving the efficiency of photocatalysis (Chambers et al., 2017). Lately, the synthesis of semiconductor @ MOF heterostructures like TiO2@UiO-66-NH2 (Dennis T. Lee et al., 2017), ZnIn2S4@MIL-125(Ti) (Liu et al., 2018), MoS2 @ UiO-66@CdS (Shen et al., 2015), UiO-66-NH2@C3N4 (Shen et al., 2014), ZnO@ZIF-8 (Wang et al., 2016), BiVO4@MIL-101 (Xu et al., 2015), BiOBr@UiO-66 (Tong et al., 2018), CdS@UiO-66-NH2 (Shen et al., 2013a) and Cd0.2Zn0.8S @UiO-66-NH2 (Su et al., 2017) expressed countless benefits because of synergistic effect. ZnIn2S4, a chalcogenide compound, has a suitable band gap, ranging from 2.34 eV to 2.48 eV, which is in good agreement with visible light absorption. However, to the best of our knowledge, no work related to UiO-66-NH2@ ZnIn2S4 has been done before.
Herein, we firstly reported the successful integration of ZnIn2S4 with UiO-66-NH2 via a new facile solvothermal method along with good morphology as a visible-light photocatalyst for reduction of Cr(VI) with efficient performance. The UiO-66-NH2@ ZnIn2S4 composite with different concentrations of UiO-66-NH2 has been effectively optimized. The synergistic effect could be clearly understood from the PL and UV -spectroscopy, after molding into heterostructure of UiO-66-NH2@ZnIn2S4, optimum amount of UiO-66-NH2 have obtained with enhanced band gap resulted in more visible light absorption and reduced recombination of electron and hole. The UiO-66-NH2@ZnIn2S4 has shown excellent photocatalytic performance for the reduction of Cr(VI) under the irradiation of visible light. The outstanding performance towards the photocatalytic reduction of Cr(VI) is due to the synergistic effect of ZnIn2S4 and UiO-66-NH2 which cause the efficient electron-hole separation and suppression in recombination. 20UN@ZIS shows excellent efficiency among these composites because of the high solubility and adsorption capacity of Cr(VI) metal ions in water. Moreover, the UiO-66-NH2@ZnIn2S4 composite exhibited excellent stability and recyclability which plays a vital role in wastewater treatment. It is hoped that these types of photocatalyst can expose a novel space for new stratagem of MOFs based nano-composites in the future.
2 Experimental
2.1 Materials
N, N-dimethylformamide (DMF, 99.9%), sodium sulfate (Na2SO4, 99.9%) were purchased from Sinopharm Chemical Reagents Co. Ltd. Zirconium (IV) chloride (ZrCl4, 99.9% metals basis), and diphenyl carbazide (DPC, 98%) were bought from Shanghai Macklin Biochemical Co. Ltd. Ethanol and Ethylene glycol (, 99.9%) were attained from Xilong Incorporated Chemical Industry Co. Ltd., 2-Aminoterephthalic acid [H2NC6H3-1,4-(CO2H)2, 98%] was bought from Beijing HWRK Chem Co., Ltd., Milli-Q H2O purification system provided the pure H2O water for its use in experiments. No further purification was required for the use of these analytical grade chemicals.
2.2 Preparation of UiO-66-NH2
Particularly, 5 mL DMF solution of ZrCl4 (9 mM) and 5 mL DMF solution of NH2-BDC (8 mM) were taken in glass bottle separately and mixed in third glass vial along with the addition of 1.2 mL of acetic acid. The sample was heated at 120 °C overnight without any stirring. Finally, the obtained product was first centrifuged and then washed several times first with DMF then with water and lastly with ethanol. The UiO-66-NH2 attained was activated at 50 °C overnight in an oven just to remove the solvent. UiO-66 was synthesized according to the same procedure as described above.
2.3 Preparation of UiO-66-NH2@ZnIn2S4
The UiO-66-NH2@ZnIn2S4 composites were synthesized by the facile solvothermal method. As a description, the specific concentration of UiO-66-NH2 octahedral was ultrasonically dissolved into 15 mL of DMF and 5 mL EG cooperatively. Later ZnCl2 (0.102 g), InCl3·4H2O (0.331 g) and thioacetamide (TAA) (0.150 g) were further mixed and stirred for 2 h. The resulting final solution was heated at 120 °C in oil for 12 h. After cooling the solid product washed mechanically with H2O and ethanol, respectively. Then it was dried at 75 °C overnight. For comparison, the pure ZnIn2S4 was also synthesized by the same solvothermal process without the addition of UiO-66-NH2. Similarly, UiO-66-NH2 was also synthesized by following the method mentioned in the literature (Xiao et al., 2016).
2.4 Characterization
TEM (JEM-1400) was used to characterize the products. For the measurement of XRD, D8 Focus X-ray with Cu Kα radiation (λ = 0.15418 nm) was used and the sample was put on a silicon substrate. UV–Vis-NIR spectrophotometer (Shimadzu UV-3101PC) was used to measure the DRS. PL lifetime and PL photoluminescence were measured on Transient State Fluorescence and Edinburgh FLS920 Multifunction Steady State.
2.5 Photoelectrochemical measurements
Solartron analytical electrochemical analyzer (ModuLab XM) was used for photoelectrochemical measurements, which is a three-electrode system, in which Ag/AgCl (KCl, 3 M) acted as reference electrode and Pt foil as a counter electrode. 10 mg sample dipped in ethanol was put on the surface of dry ITO glass substrate which acted as working electrodes. To dry the sample for EIS and TPR, substrate was put into air for ten minutes and later heated at 80 0C for just five minutes. Consequently, all the electrodes were dipped into a quartz cell having 0.3 M Na2SO4 aqueous solution. Bias potential of 0.6 V was applied to measure the TPR for 60 s on/off chopped lighting. Alternating current voltage was applied to measure the EIS with an amplitude of 10 mV and the range of frequency was from 4 MHz to 100 MHz.
2.6 Photocatalytic activity measurements
300 W Xe arc lamp with a 420 nm cutoff filter was used to investigate the activity of the following samples UiO-66-NH2, UiO-66-NH2@ZnIn2S4 and ZnIn2S4 for the Cr(VI) reduction. To obtain absorption-desorption equilibrium before illumination, the suspension was stirred magnetically for 30 min in the dark at room temperature. For Cr(VI) reduction, the source chosen was Potassium dichromate. The reduction was done in an apparatus containing 20 mg catalyst at 30 °C and 40 mL of 50 mg/L Cr(VI) solution. Diphenyl carbazide (DPC) method was used to investigate the Cr (VI) contents in the solution. 200 L of the reaction solution was dissolved with 1.8 mL of 0.2 M H2SO4 in a 2 mL tube. Then, 0.40 L from newly prepared 0.25% (w/v) DPC in acetone was supplementary added into the above solution. To make sure the color development, the solution was shacked for thirty seconds and purple color formed having absorption at 540 nm was observed. By dividing C over C0 the photocatalytic efficiency was determined where C means left concentration of Cr(VI) and C0 the starting concentration of Cr(VI). While making use of performance of composite, the stability and reusability of the composite were determined. At least four-time same performance was observed from the composite. The photocatalyst was separated after each cycle and was washed, dried and reused.
3 Results and discussion
3.1 X-ray characterization
X-ray diffraction was used to determine the phase structure of samples. As shown in (Fig. 1), there were diffraction peaks at 2 θ = 21.1°, 27.7°, 47.5°, 52.4° and 56.4°. These peaks were related to (0 0 6), (1 0 2), (1 1 0), (1 1 6) and (0 2 2) crystal planes of hexagonal ZnIn2S4 (JCPDS No.65-2023) (Liu et al., 2018). UiO-66-NH2 showed the same diffraction peaks as reported in the literature (Kandiah et al., 2010). For the UiO-66-NH2@ZnIn2S4 sample, all peaks of the original material coexisted uniformly in the 20UN@ZIS composite.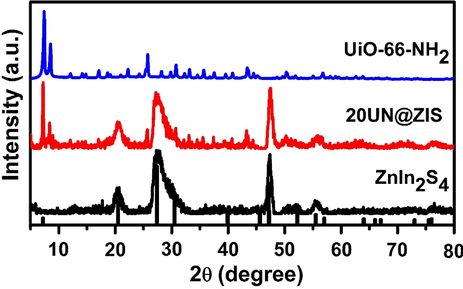
The powder X-ray diffraction (PXRD) pattern of UiO-66-NH2, UiO-66-NH2@ ZnIn2S4- core-shell NPs and ZnIn2S4.
3.2 Morphological characterization
UiO-66-NH2 demonstrated octahedral morphology with sharp edge and flat surface (Fig. 2g). The average diameter of octahedron was about 150 nm, which provided a good environment for the assembly of Photocatalysts. The original ZnIn2S4 had the morphology of microspheres, each of which was composed of many nanoflakes, cross-linked to each other. Low and high magnification TEM images of pure ZnIn2S4 are shown in (Fig. 2a). ZnIn2S4 microspheres grew uniformly and compactly on the surface of UiO-66-NH2. After hybridizing with UiO-66-NH2, ZnIn2S4 microspheres were consistently dispersed on external of UiO-66-NH2 octahedron, and UiO-66-NH2 was completely encapsulated by ZnIn2S4 sheets. Composite structures of the two layers were evidently observed. Visually, ZnIn2S4 nanosheets formed the outer layer connected to the surface of UiO-66-NH2 giving sphere-like morphology keeping the UiO-66-NH2 in inner layer. In addition, ZnIn2S4 nanosheets were connected with each other and conducive to transferring the electrons between both materials. A series of different contents of UiO-66-NH2, in the UiO-66-NH2@ZnIn2S4, were prepared (Fig. 2). By changing the concentration of UiO-66-NH2 from 10% to 50%, in weight, the morphology of the composite changed slightly. When it reached 50%, the formation process was stopped, as shown in (Fig. 2f). And it can be proved by studying their performance (Fig. S1).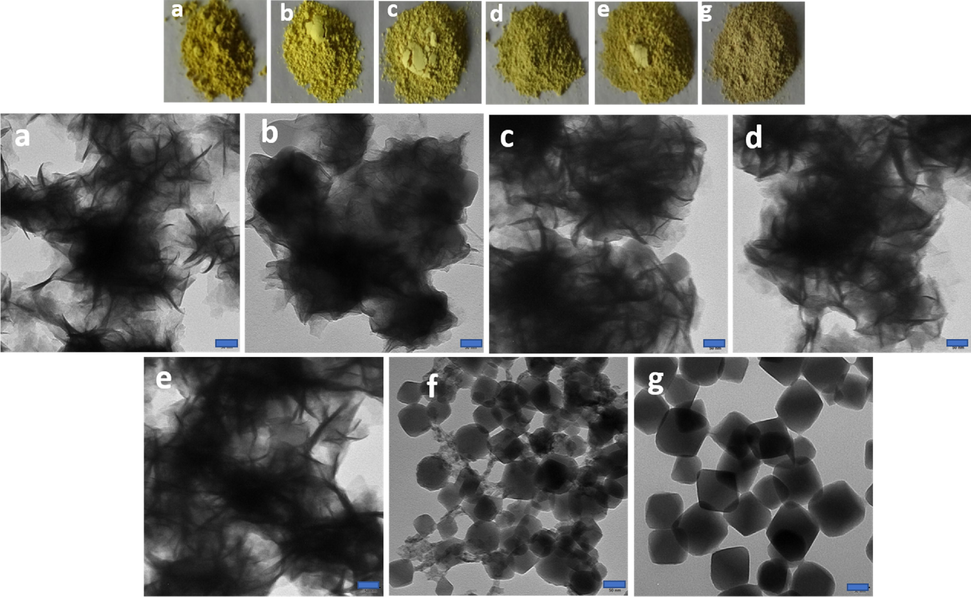
TEM images of (a) ZnIn2S4, (b) 10UiO-66-NH2@ZnIn2S4-NPs (c) 20UiO-66-NH2@ZnIn2S4-NPs (d) 30UiO-66-NH2@ZnIn2S4-NPs (e) 40UiO-66-NH2@ZnIn2S4-NPs (f) 50UiO-66-NH2@ZnIn2S4-NPs and (g) UiO-66-NH2 -NPs. Scale bar for each image is 50 nm.
3.3 Optical characterization
UV–Vis diffuse reflectance spectroscopy was used to measure the absorption characteristics of pristine UiO-66-NH2, ZnIn2S4 and the composite with the specific concentration of UiO-66-NH2 Fig. 3(a). Pure UiO-66-NH2 had an absorption edge in the visible region (Shen et al., 2013b). Pure ZnIn2S4 had an absorption edge at 600 nm, related to intrinsic band gap absorption (Liu et al., 2018). Comparatively UiO-66-NH2@ZnIn2S4 composite showed a longer absorption edge than the pure UiO-66-NH2. Red-shift specified more absorption of visible light along with more production of electron and hole pairs by the composite. The band gap energy (Eg) of the sample can be obtained from the graph of the relationship between (αhV)1/2 and photon energy (hv). The (Eg) values UiO-66-NH2, 20UN@ZIS and ZnIn2S4 were estimated to be 2.75, 2.42 and 2.29 EV, respectively (Fig. 3b) (Shen et al., 2013b; Zhang et al., 2018). In addition, according to the principle of light reflection spectrum, the light reflectivity on the surface of high-density composite 20UN@ZIS is generally lower than the original ZnIn2S4. Specifically, the surface of the composite can improve the light absorption efficiency of 20UN@ZIS, which is verified by the light absorption calculation of UV–Vis diffuse reflectance spectrum Fig. 3(b). 20UN@ZIS demonstrated enhanced light absorption in the visible light region. It is confirmed that 20UN@ZIS has a higher light absorption capacity than the original ZnIn2S4, which is of great significance to improve photocatalytic efficiency.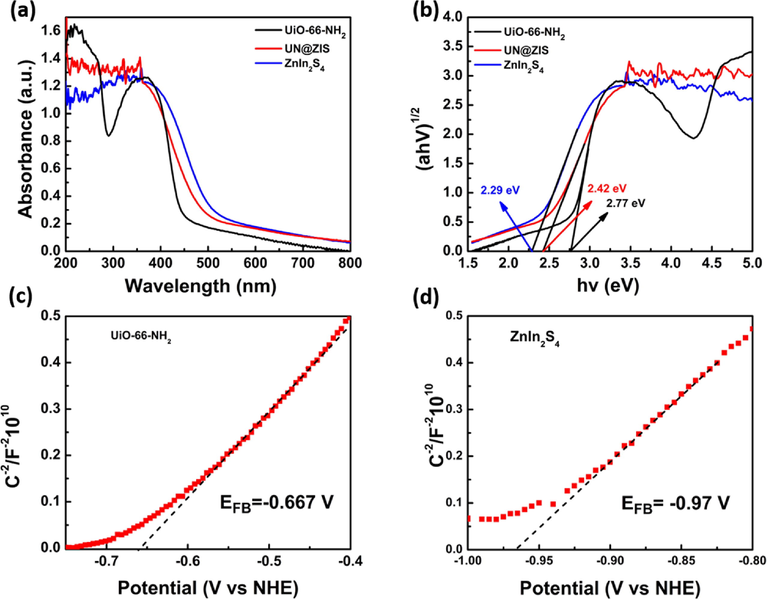
(a) UV–Vis diffuse reflectance spectra (DRS), and (b) band-gap values assessed by a correlated curve of (ahv)1/2 set against photon energy plots of the as-synthesized UiO-66-NH2@ZnIn2S4-NPs core-shell NPs. Mott-Schottky plot for (c) UiO-66-NH2 and (d) ZnIn2S4 nanosheets electrode in saturated Na2SO4 electrolyte solution (0.1 M, pH = 6.8) vs NHE.
The band gap of 20UN@ZIS calculated by square root absorption method was 2.42 eV, which was higher than the original ZnIn2S4 (2.29 eV), fell in between two semiconductors. It indicated the formation of heterostructure magnificently. Meanwhile, the recombination of electrons and holes was suppressed due to the core-shell heterostructure. In sense, its performance was significantly enhanced as compared to pristine UiO-66-NH2 and ZnIn2S4.
The flat band potential of UiO-66-NH2 was also derived from the mott-schotky plots which was about −0.82 V vs. Ag/AgCl (i.e. −0.72 V vs. NHE). The bottom of many semiconductors is n-type if it is more negative by 0.1 V as compared to the flat band potential. The CB of UiO-66-NH2 was estimated to be −0.667 V vs. NHE. Similarly, the (EVB) value of UiO-66-NH2 was calculated to be +2.02 V vs. NHE as shown in Fig. 3(c).
The calculated EVB and ECB values of ZnIn2S4 are +1.32 eV and −0.97 eV, respectively.
A typical n-type mott-Schottky plot of ZnIn2S4 and UiO-66-NH2 was measured at frequency range of 1000 to 2000 Hz as presented in Fig. 3(d).
3.4 Photocatalytic activity
The photocatalytic activity of Cr(VI) in aqueous solution was investigated under the influence of visible light. Fig. 4(a) shows the reduction efficacy of Cr(VI) of UiO-66-NH2@ZnIn2S4. It was observed that Cr(VI) absorption decreased steadily at 540 nm and disappeared completely after 30 min. Every time, the color of the reaction solution changed from pink to colorless (inset Fig. 4(a)), indicated that Cr(VI) was reduced to Cr(III) during photocatalytic reduction. The UiO-66-NH2 as a photocatalyst, exhibited lower performance of Cr (VI) reduction compared with ZnIn2S4 (Fig. 4b).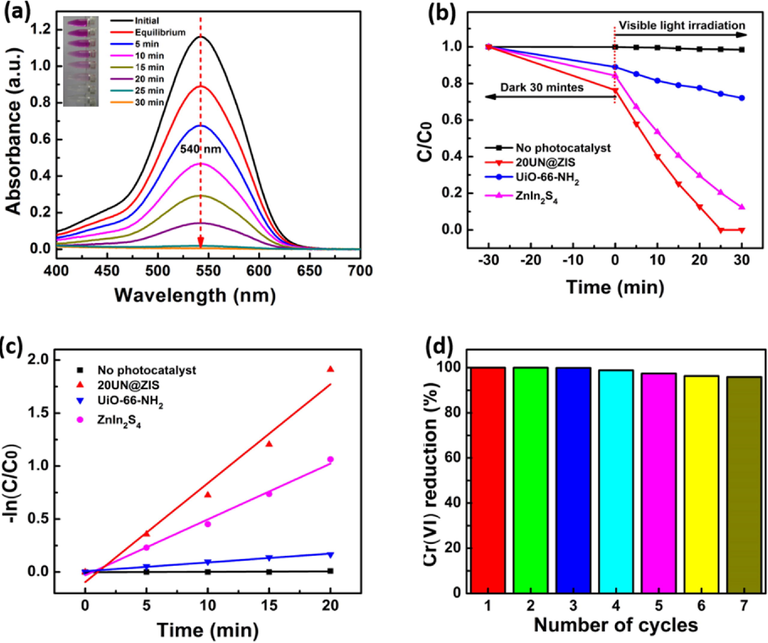
(a) UV–Vis spectra along with color (inset) change of Cr(VI) aqueous solution in the existence of UiO-66-NH2@ZnIn2S4 core-shell NPs under the visible irradiation with a 300 W Xenon lamp at room temperature. (b) The reduction consequences (C/C0) of several photocatalysts on Cr(VI) under the similar condition, (c) The matching kinetic plots for the reduction of Cr(VI). (d) The various reduction results of Cr(VI) with UiO-66-NH2@ ZnIn2S4 core-shell NPs.
The photocatalytic activity of ZnIn2S4 with Cr(VI) concentration of 50 mg/L, UiO-66-NH2 and ZnIn2S4 were in accordance with the literature (Shen et al., 2014; Zhang et al., 2018). After coupling with UiO-66-NH2, the photocatalytic activity of ZnIn2S4 was significantly improved. The reduction of Cr(VI) in UiO-66-NH2@ZnIn2S4 composite increased with the heightening the content of UiO-66-NH2 and reached the maximum value when UiO-66-NH2 content was left 20 wt%. However, a further increase in the content of UiO-66-NH2 resulted in a decrease in Cr(VI) (Fig. S1). This may be due to the following factors: I- with the increase of UiO-66-NH2, the related contents of ZnIn2S4 decreased, which is the most favorable active substance in the reaction. II- excess UiO-66-NH2 may be converted into the composite midpoint of the charge, which can be confirmed by the photoluminescence results (Fig. 7(a)), which might be the reason for the decrease of Cr(VI) reduction. Therefore, choosing the appropriate amount of UiO-66-NH2 is the key to obtain the best Cr(VI) reduction rate by using UiO-66-NH2@ZnIn2S4 hybrid material. The results indicated that the order of the regularization rate is basically the same as that of the invention, and the 20UN@ZIS sample still showed the best efficiency (Table 1). In particular, the photocatalytic reduction rate of Cr(VI) by 20UN@ ZIS was higher than that by ZnIn2S4 based photocatalysts previously reported. For example, UiO-66-NH2 (Shen et al., 2014),MIL-88(B)–NH2(Fe)(Shi et al., 2015), UiO-66-NH2@grephene (Shen et al., 2014), ZnO@ZIF-8 (Wang et al., 2016), Pd@UiO-66-NH2(Shen et al., 2013c), CdS@ ZnIn2S4 (Zhang et al., 2018) and so on. Fig. 4(c) shows the relationship between ln (C/C0) and irradiation time when Cr (VI) is reduced by several catalysts. The results show that the reaction rate was close to the first-order kinetics, that is, ln (C/C0) = KT, where k is the first-order kinetics constant. The K value determined in advance by the corresponding linear curve slope pointed that the reaction rate constant of Cr(VI) reduction was 0.93 min−1 on the core–shell NPs of UiO-66-NH2@ ZnIn2S4, which was 1.78 and 11.20 higher than that of ZnIn2S4 (0.052 min−1) and UiO-66-NH2 (0.0083 min−1), respectively. Furthermore, 20UN@ZIS still maintained its high activity after several cycles of reduction reactions, which is very important in practical applications (Fig. 4d). .
Sample
Sample (mg)
K2Cr2O7 (mg/L)
volume (mL)
Time (min)
References
20UN@ZIS
20 mg
50 mg/L
40 mL
30
This study
ZnIn2S4/CdS
50 mg
50 mg/L
50 mL
30
(Zhang et al., 2018)
TiO2/ZnIn2S4
40 mg
50 mg/L
40 mL
60
(Li et al., 2020)
CNFs/ZnIn2S4
40 mg
50 mg/L
40 mL
60
(Qiu et al., 2019)
Bi2S3/BiOCl@ZnIn2S4
40 mg
50 mg/L
40 mL
60
(Qiu et al., 2020)
CaIn2S4/ZnIn2S4 un
50 mg
20 mg/L
50 mL
30
(Xu et al., 2018)
The physical mixture of UiO-66-NH2 and ZnIn2S4 was passed through catalytic reduction just to confirm the effect of the composite. As shown in Fig. 5(a) the lower performance was observed by the mixture represented by PM-20UN@ZIS, compared with original composite 20UN@ZIS. Improvement in photocatalytic activity suggested that heterostructure was responsible for indorsing the electron transfer and parting. The effect of scavenger can be seen in Fig. 5(a). The rapid increase in the reduction of Cr(VI) was observed when citric acid was added. Under five minutes light illumination, the reduction of PM-20UN@ZIS and 20UN@ZIS + 0.5 mL citric acid were 31.7% and 75.5% respectively Fig. 5(c). The simplest approach to mechanism is that the adsorbed citric acid on the surface of the catalyst experienced oxidation by holes, meanwhile suppressing the recombination. The changes in the spectra of UV–vis absorption for the relevant materials are illustrated in Fig. 5(b) and (d).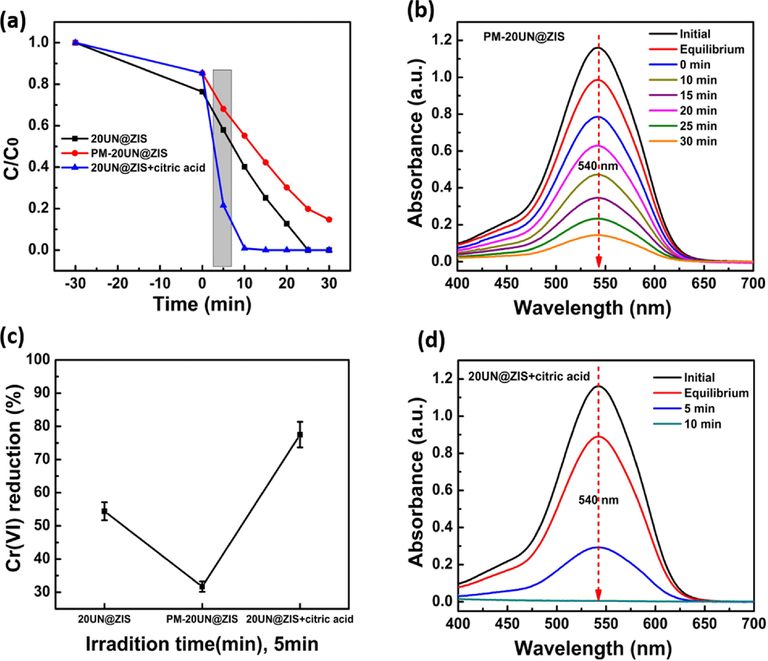
(a), (c) Assessment of the Photocatalytic Cr(VI) reduction efficacies over 20UN@ZIS, PM-20UN@ZIS and 20UN@ZIS + 0.5 mL citric acid. (b), (d) UV–vis absorption spectra of Cr(VI) solution treated with PM-20UN@ZIS and 20UN@ZIS + 0.5 mL citric acid.
TEM notes showed that after many reduction tests, the morphology of the core-shell NPs of UiO-66-NH2@ZnIn2S4 was almost unaffected. In particular, the high density in the sphere can be clearly observed on the surface of the recovered UiO-66-NH2@ZnIn2S4 core-shell NPs which reflects their outstanding stability in the photocatalytic reaction. In addition, according to the XRD and TEM results shown in Fig. 6(a), it was found that 20UN@ZIS can still maintain its original integrity after several photocatalytic cycles.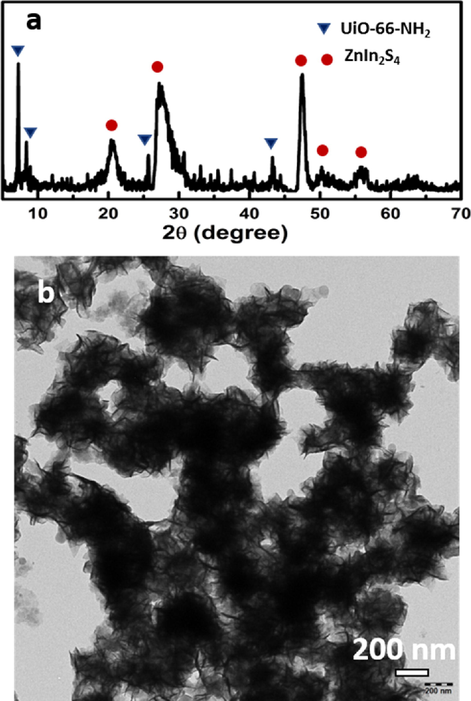
(a) The powder X-ray diffraction (PXRD) pattern (b) TEM images of UiO-66-NH2@ZnIn2S4-NPs core-shell NPs.
3.5 Photoluminescent characterization
PL can provide valuable information about the recombination and capture of charge carriers in semiconductors. Fig. 7(a) shows a comparison of the (PL) of various samples observed at an excitation wavelength of 310 nm. It can be seen that pure ZnIn2S4 had the highest emission peak near 445 nm. For 20NM@ZIS composite, the fluorescence peak was similar to the original ZnIn2S4, but an obvious decline in the intensity of the fluorescence peak was observed (Liu et al., 2015; Long et al., 2012). The obvious decline in the intensity of this fluorescence curve indicated a decline in the recombination rate of photogenerated e- and h+ in 20NM@ZIS system. In addition, the order of photoluminescence intensity was ZnIn2S4 > 40NM@ZIS > 30NM@ZIS > 10NM@ZIS > 20NM@ZIS, which is in good agreement with the actual results of photocatalytic activity.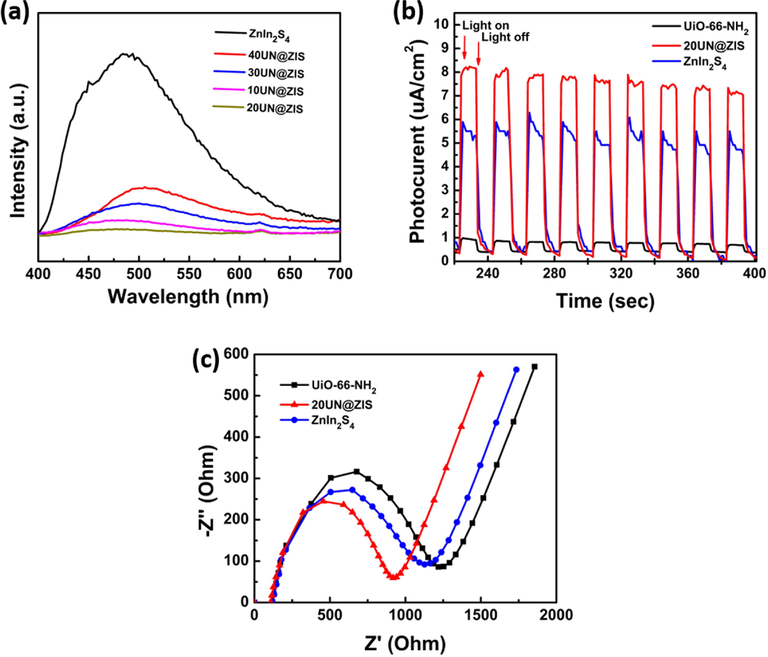
(a) Photoluminescence spectra (PL), (b) transient photocurrent response, and (c) electrochemical impedance (EIS) spectra of UiO-66-NH2@ZnIn2S4 core-shell NPs.
3.6 Transient photocurrent characterization
The photoelectrochemical properties can further test the charge separation ability. Fig. 7(b) shows a transient photo-current response of UiO-66-NH2, ZnIn2S4, and NH@ ZIS 40 under visible light. The original sample of UiO-66-NH2 presented a small photocurrent density, which can be attributed to the rapid recombination of photogenerated electron and hole in UiO-66-NH2. The observed transient photocurrent response of ZnIn2S4 was according to the literature. As compared to ZnIn2S4 and UiO-66-NH2, 20UN@ZIS composite exhibited higher photocurrent intensity. These results confirmed that under the influence of light, the composite had a stronger ability to produce and transmit light-excited carriers(Shen et al., 2013c; Zhang et al., 2018).
3.7 Electrochemical impedance
In order to further validate the above-mentioned results and to study the development of interfacial charge motion, electrochemical impedance spectroscopy (EIS) was used. As compared to the diameters of ZnIn2S4 and UiO-66-NH2 separately, it can be perceived that the Nyquist semicircle diameter of 20UN@ZIS composite was declined obviously, demonstrating the lower resistance of the composite and promoting the electron holes separation as well corresponding reduction Fig. 7 (c). On this basis, scheme 1 clarifies the photocatalytic activity mechanism of 20UN@ ZIS photocatalyst.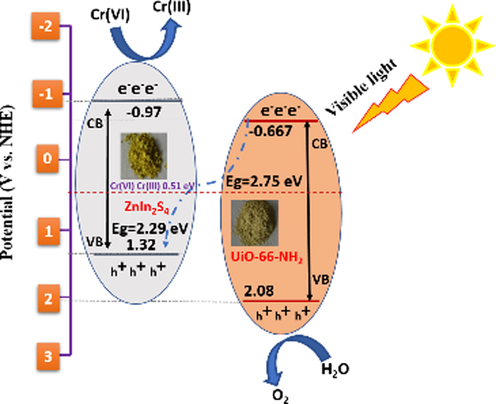
Schematic illustration of Cr(VI) reduction mechanism over 20UN@ZIS core–shell NPs composite under the influence of visible light irradiation.
ZnIn2S4 has both conduction band (CB) and valance band (VB) higher than that of UiO-66-NH2. When the composite was excited under visible light, both components generated electrons and holes, respectively as shown in Scheme 1 (Ge et al., 2019). In the interim, electron from the UiO-66-NH2 CB transferred rapidly to the VB of ZnIn2S4 followed the route of direct Z-scheme. After coupling ZnIn2S4 with UiO-66-NH2 photocatalyst, the photo-excited electrons in the CB of UiO-66-NH2 recombined with the photogenerated holes in the VB of ZnIn2S4 photocatalyst, reserving the electrons in the CB of ZnIn2S4 photocatalyst and holes in the VB of UiO-66-NH2. This charge transfer route endows there served electrons and holes with high reduction and oxidation abilities (Liu et al., 2018; Long et al., 2012).
4 Conclusions
In conclusion, UiO-66-NH2@ZnIn2S4 photocatalyst with high visible light driven activity was successfully synthesized by a facile solvothermal approach. A sequence of UiO-66-NH2@ZnIn2S4 nano-composite with the variable content of UiO-66-NH2 was prepared. Enhancement in the reduction activity of Cr(VI) was observed under the irradiation of visible light. The optimal content of UiO-66-NH2 which exhibited high reduction activity and could participate distinctly in the reduction activity is 20%. The momentous improvement of photocatalytic performance of UiO-66-NH2@ZnIn2S4 is credited to the subsequent two factors: (i) with the increase of UiO-66-NH2, the related contents of ZnIn2S4 decreased, which is the most favorable active substance in the reaction; (ii) excess UiO-66-NH2 may be converted into the composite midpoint of the charge, which was confirmed by the photoluminescence results. In addition, owing to having good reusability and stability, this work climaxes the intrinsic inspiration of MOFs on the recital of Cr(VI) reduction and also provides important guidance for making efficient photocatalyst with full utilization of the definite role of metal-organic frameworks.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synergy removal of Cr (VI) and organic pollutants over RP-MoS2/rGO photocatalyst. Appl. Catal. B: Environ.. 2018;239:204-213.
- [Google Scholar]
- Acrylate-based polymerizable sol-gel synthesis of magnetically recoverable TiO2 supported Fe3O4 for Cr(VI) photoreduction in aerobic atmosphere. ACS Sustainable Chem. Eng.. 2016;4(3):974-982.
- [Google Scholar]
- Maximizing the photocatalytic activity of metal-organic frameworks with aminated-functionalized linkers: substoichiometric effects in MIL-125-NH2. J. Am. Chem. Soc.. 2017;139(24):8222-8228.
- [Google Scholar]
- Preconcentration of Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II) ions in environmental samples by membrane filtration prior to their flame atomic absorption spectrometric determinations. J. Hazard Mater.. 2007;145(3):459-464.
- [Google Scholar]
- An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. Engl.. 2012;51(14):3364-3367.
- [Google Scholar]
- Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc.. 2014;136(11):4369-4381.
- [Google Scholar]
- S-scheme heterojunction TiO2/CdS nanocomposite nanofiber as H2-production photocatalyst. ChemCatChem. 2019;11(24):6301-6309.
- [Google Scholar]
- Fabrication of pn CuBi2O4/MoS2 heterojunction with nanosheets-on-microrods structure for enhanced photocatalytic activity towards tetracycline degradation. Appl. Surf. Sci.. 2019;491:88-94.
- [Google Scholar]
- 2D/2D Z-scheme heterojunction of CuInS2/g-C3N4 for enhanced visible-light-driven photocatalytic activity towards the degradation of tetracycline. Sep. Purif. Technol.. 2019;210:608-615.
- [Google Scholar]
- Controlled growth of a metal–organic framework on gold nanoparticles. CrystEngComm. 2016;18(28):5262-5266.
- [Google Scholar]
- The synthesis of a BiOCl x Br 1–x nanostructure photocatalyst with high surface area for the enhanced visible-light photocatalytic reduction of Cr (vi) RSC Adv.. 2020;10(8):4763-4771.
- [Google Scholar]
- Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater.. 2010;22(24):6632-6640.
- [Google Scholar]
- UiO-66-NH2 Metal−Organic Framework (MOF) nucleation on TiO2, ZnO, and Al2O3 atomic layer deposition-treated polymer fibers: role of metal oxide on MOF growth and catalytic hydrolysis of chemical warfare agent simulantsf. ACS Appl. Mater. Interfaces. 2017;9:44847-44855.
- [Google Scholar]
- Tandem catalysis by palladium nanoclusters encapsulated in metal-organic frameworks. ACS Catal.. 2014;4(10):3490-3497.
- [Google Scholar]
- Fabrication of TiO2 embedded ZnIn2S4 nanosheets for efficient Cr(VI) reduction. Mater. Res. Bull.. 2020;122:110671
- [Google Scholar]
- Synergistic improvement of Cr(VI) reduction and RhB degradation using RP/g-C3N4 photocatalyst under visible light irradiation. Arabian J. Chem.. 2020;13(2):3836-3848.
- [Google Scholar]
- Fabrication of ZnIn2S4–g-C3N4 sheet-on-sheet nanocomposites for efficient visible-light photocatalytic H2-evolution and degradation of organic pollutants. RSC Adv.. 2015;5(119):97951-97961.
- [Google Scholar]
- Construction of heterostructured ZnIn2S4@NH2-MIL-125(Ti) nanocomposites for visible-light-driven H2 production. Appl. Catal. B: Environ.. 2018;221:433-442.
- [Google Scholar]
- Amine-functionalized zirconium metal–organic framework as efficient visible-light photocatalyst for aerobic organic transformations. Chem. Commun.. 2012;48(95):11656.
- [Google Scholar]
- Green synthesis of Fe3O4/RGO nanocomposite with enhanced photocatalytic performance for Cr(VI) reduction, phenol degradation, and antibacterial activity. ACS Sustain. Chem. Eng.. 2017;5(11):10551-10562.
- [Google Scholar]
- Facile construction of three-dimensional netted ZnIn2S4 by cellulose nanofibrils for efficiently photocatalytic reduction of Cr(VI) Chem. Eng. J.. 2019;375:121990
- [Google Scholar]
- Bismuth sulfide bridged hierarchical Bi2S3/BiOCl@ZnIn2S4 for efficient photocatalytic Cr(VI) reduction. J. Hazard. Mater.. 2020;389:121858
- [Google Scholar]
- Multifunctional NH2-mediated zirconium metal-organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(VI) Dalton Trans.. 2013;42(37):13649-13657.
- [Google Scholar]
- Highly dispersed palladium nanoparticles anchored on UiO-66(NH(2)) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst. Nanoscale. 2013;5(19):9374-9382.
- [Google Scholar]
- CdS-decorated UiO–66(NH2) nanocomposites fabricated by a facile photodeposition process: an efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A. 2013;1(37):11473.
- [Google Scholar]
- Electrostatically derived self-assembly of NH 2-mediated zirconium MOFs with graphene for photocatalytic reduction of Cr (vi) RSC Adv.. 2014;4:2546-2549.
- [Google Scholar]
- Noble-metal-free MoS2 co-catalyst decorated UiO-66/CdS hybrids for efficient photocatalytic H2 production. Appl. Catal. B: Environ.. 2015;166–167:445-453.
- [Google Scholar]
- An Amine-Functionalized Iron(III) metal-organic framework as efficient visible-light photocatalyst for Cr(VI) reduction. Adv. Sci. (Weinh). 2015;2(3):1500006.
- [Google Scholar]
- Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B: Environ.. 2017;200:448-457.
- [Google Scholar]
- BiOCl/UiO-66 composite with enhanced performance for photo-assisted degradation of dye from water. Appl. Organomet. Chem.. 2018;32(2):e4049
- [Google Scholar]
- Synthesis modulation as a tool to increase the catalytic activity of metal-organic frameworks: the unique case of UiO-66(Zr) J. Am. Chem. Soc.. 2013;135(31):11465-11468.
- [Google Scholar]
- Integration of adsorption and photosensitivity capabilities into a cationic multivariate metal-organic framework for enhanced visible-light photoreduction reaction. Appl. Catal. B: Environ.. 2019;253:323-330.
- [Google Scholar]
- Rapid construction of ZnO@ZIF-8 heterostructures with size-selective photocatalysis properties. ACS Appl. Mater. Interfaces. 2016;8(14):9080-9087.
- [Google Scholar]
- Highly efficient photocatalytic hydrogen production from pure water via a photoactive metal–organic framework and its PDMS@MOF. J. Mater. Chem. A. 2017;5(17):7833-7838.
- [Google Scholar]
- Boosting photocatalytic hydrogen production of a metal–organic framework decorated with platinum nanoparticles: The platinum location matters. Angew. Chem. Int. Ed.. 2016;55(32):9389-9393.
- [Google Scholar]
- One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: Reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J.. 2017;308:791-797.
- [Google Scholar]
- Electrically regenerated ion exchange for removal and recovery of Cr (VI) from wastewater. Environ. Sci. Technol.. 2007;41:1439-1443.
- [Google Scholar]
- Facile synthesis of novel CaIn(2)S(4)/ZnIn(2)S(4) composites with efficient performance for photocatalytic reduction of Cr(VI) under simulated sunlight irradiation. Nanomaterials (Basel). 2018;8(7)
- [Google Scholar]
- BiVO4/MIL-101 composite having the synergistically enhanced visible light photocatalytic activity. RSC Adv.. 2015;5(54):43473-43479.
- [Google Scholar]
- Porous molybdenum phosphide nano-octahedrons derived from confined phosphorization in UIO-66 for efficient hydrogen evolution. Angew. Chem. Int. Ed. Engl.. 2016;55(41):12854-12858.
- [Google Scholar]
- Preparation of ZnIn2S4 nanosheet-coated CdS nanorod heterostructures for efficient photocatalytic reduction of Cr(VI) Appl. Catal. B: Environ.. 2018;232:164-174.
- [Google Scholar]
- Size-tunable hydrothermal synthesis of SnS2 nanocrystals with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI) Environ. Sci. Technol.. 2011;45(21):9324-9331.
- [Google Scholar]
- High-performance visible-light-driven SnS(2)/SnO(2) nanocomposite photocatalyst prepared via in situ hydrothermal oxidation of SnS(2) nanoparticles. ACS Appl. Mater. Interfaces. 2011;3(5):1528-1537.
- [Google Scholar]
- One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(VI) Appl. Catal. B: Environ. 2014;144:730-738.
- [Google Scholar]
- Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc.. 2017;139(24):8078-8081.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.04.029.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







