Translate this page into:
Identification of active chemical constituents of Asplenium ruprechtii Sa. Kurata based on in vitro neuroprotective activity evaluation
⁎Corresponding authors at: Department of Pharmaceutical Engineering, School of Chemistry and Chemical Engineering, North Minzu University, Yinchuan 750021, PR China (C.-L. Li). Institute of Traditional Chinese Medicine, Shandong Academy of Pharmaceutical Sciences, Jinan 250101, PR China (F. Wang). chlong@nun.edu.cn (Chong-Long Li), wangfang_84@163.com (Fang Wang), wu.xiuli2005@163.com (Xiu-Li Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mining structural novel and bioactive natural products from traditional Chinese drugs and folk medicines have attracted attention from pharmacologists and pharmaceutical chemists for a long time. In this study, the chemical constituents of Asplenium ruprechtii Sa. Kurata was systematically studied based on the evaluation of neuroprotective activity on hydrogen peroxide (H2O2)-induced damage of SH-SY5Y cell lines. Sixteen chemical monomers (1–16), including two new structures of 9,19-cycloartane-triterpenoid saponins (1,2), were discovered. Their chemical structures were established based on extensive spectroscopic data analysis, including the one- and two-dimensional (1D and 2D) nuclear magnetic resonance (NMR) data and high-resolution electrospray ionization mass spectrometry (HRESIMS). All monomer compounds were docked with an “active pocket” at the N-terminal of the GluN2B protein, where compounds 13–16 were found to possess high binding scores, which warrant further study on the molecular mechanism. Our findings provide insight and research techniques for discovering new drugs based on neuroprotective activity.
Keywords
Asplenium ruprechtii
Neuroprotective activity
Chemical constituents
9,19-Cycloartane-triterpenoid saponins
Structural determination
1 Introduction
Screening novel structures and bioactive entities have become one of the most important methods for discovering clinical drugs. Statistics show that over 50% of clinical drugs come directly from natural products or their derivatives (Huang et al., 2019; Jamzivar et al., 2019; Treml et al., 2020). Traditional Chinese medicine and folk drugs have become important sources for drug development due to their long history (thousands of years) of safe use and systematic medication experience. With the development of modern separation methods and technologies, thousands of compounds have been separated from natural resources annually, including hundreds of new structural entities (Caputo et al., 2020). Because there are few compounds with good biological bioactivity, systematic and in-depth study of biological activities remains essential. Separation of compounds without the guidance of biological activity increases the probability of repeated discovery. Each year, over 500 compounds are reported repeatedly either in the same or different species (Newman and Cragg, 2020). Therefore, the construction of activity-mediated separation of bioactive molecules has become one of the hotspots in natural products research.
Asplenium ruprechtii Sa. Kurata, previously named Comptosorus sibiricus rupr., is widely distributed in the north of China, Korea, Japan, and the Far East of Russia. The whole plant has been used to treat severe endometrorrhagia, bleeding wound, and Buerger’s disease in clinical practice or folk use (Ching, 1978; Christenhusz et al., 2011; Christenhusz and Chase, 2014; Hasebe et al., 1995; Lin and Ronald, 2013). The early research, led by Shenyang Pharmaceutical University, showed that the plant that was collected from Northeast China was rich in triterpenoid saponin, flavonoid glycoside, and phenolic and organic acids (41K of Shenyang College of Pharmacy, 1977; Yang et al., 2012). Our previous study on the activity of total flavonoids from the large polar parts of A. ruprechtii collected in Henan province of China on thromboocclusive vasculitis model rats showed that total flavonoids dilated peripheral blood vessels and could block α receptors and excitatory β receptors, relieve vasospasm of skin and mucous membrane, and improve the overall blood circulation of diseased limbs (Liang et al., 2011). In addition, we discovered over 40 compounds in continuous chemical constituent studies, including a novel skeleton of C-stiryl iridoid glycoside and five new cycloartane glycosides (Liang et al., 2017; Noh and Ismail, 2020; Vieira et al., 2016; Wang et al., 2020; Wang et al., 2019a, 2019b). However, these compounds could not fully represent the clinical efficacy of A. ruprechtii.
N-methyl-D-aspartic acid (NMDA) receptors are a class of ionic glutamatergic receptors, which control various glutamate synaptic communications (Zhou et al., 2013). GluN2B NMDA receptor in synapses can maintain active continuous cell communication. Multiple animal experimental evidence suggests that the NMDA receptor of GluN2B (GluN2B-NMDA receptor) induces excitotoxic cell death and amyloid-beta (Aβ) synaptic dysfunction (Li et al., 2010; Li et al., 2008; Liu et al., 2020). Therefore, the inhibition of the GluN2B-NMDA receptor is considered as a potential therapeutic strategy for Alzheimer’s disease to provide neuroprotection and improve cognitive function. We established a method to evaluate the neuroprotective biological activity of extracts from traditional Chinese medicine and folk medicine using hydrogen peroxide (H2O2)-induced damage of SH-SY5Y cell lines as a model and found potential bioactivity in A. ruprechtii extract. Based on in vitro neuroprotective activity evaluation, the chemical constituents were investigated. Sixteen monomers (1–16, Fig. 1) were discovered and structurally characterized using extensive analysis of one- and two-dimensional (1D and 2D) nuclear magnetic resonance (NMR) data and high-resolution electrospray ionization mass spectrometry (HRESIMS). Herein, we report biological activity-mediated separation and structural identification and characterization.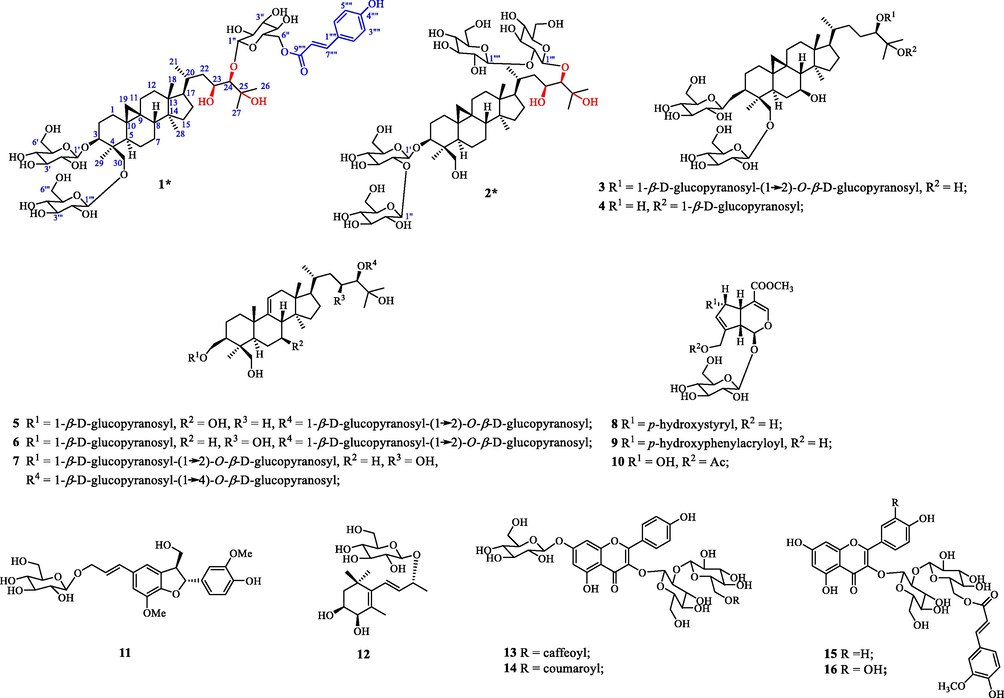
Chemical structures of compounds 1–16. New compounds 1 and 2 are assigned by asterisks (*).
2 Materials and methods
2.1 Chemicals and material
A. ruprechtii Sa. Kurata samples were collected in April 2017 from Yuzhou City, Henan Province, China. Plant identity was verified by Professor Qi Guo of Shandong Academy of Pharmaceutical Sciences, where a voucher specimen (No. 20160425) was deposited.
Anton Paar MCP 300 modular circular polarimeter (Anton Paar GmbH, Germany) was used to measure optical rotations. The NMR spectra of compounds 1 to 16 were acquired at 600 or 400 MHz for 1H and 150 or 100 MHz for 13C, respectively, using Bruker Avance AVIII-600 (or 400) spectrometer, and the references were standardized by solvent peaks. The HRESIMS data were measured using the LTQ Orbitrap XL instrument (Thermo Scientific, SanJose, CA, USA), while the high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) data were acquired using the Agilent Q-TOF 6530 instrument (Agilent Technologies, Santa Clara, CA, USA). Silica gel (200–300 mesh, Qingdao Marine Chemical Inc. Qingdao, China) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden) were used for column chromatography (CC). Semi-preparative HPLC separation was performed with the Shimadzu LC-10AT instrument with a refractive index detector (Shodex RI-201H; Shimadzu Corp., Tokyo, Japan), using a Shim-pack VP-ODS column (250 mm × 20 mm, 5 μm; Shimadzu Corp., Tokyo, Japan). The thin-layer chromatography (TLC) was performed with glass precoated silica gel GF254 plates (Qingdao Marine Chemical Inc.). Spots were visualized under ultraviolet (UV) light or by spraying with 7% sulphuric acid (H2SO4) in 95% ethanol alcohol (EtOH) followed by heating. Unless otherwise noted, all chemicals were obtained from commercially available sources and were used without further purification.
2.2 Neuroprotective activity assay
2.2.1 SH-SY5Y cell culture
Human neuroblastoma SH-SY5Y cells were grown in T-75 flasks and incubated at 37 °C and 5% carbon dioxide (CO2) in a humidified incubator. The completed media was composed of Dulbecco’s Modified Eagle Medium (DMEM) + Ham’s F-12 (1:1, Sigma Aldrich). When cells reached 80–90% confluency, they were subcultured. First, the culture medium was discarded and washed two times with phosphate-buffered saline (PBS) without calcium and magnesium ions. Then, 1.0 mL digestive solution (0.25% trypsin-0.53 mM EDTA) was added into the culture bottle, which was turned upside down and incubated at 37 °C for three minutes to preheat. The culture bottle was then over and allowed trypsin contact with the cell surface for about 30 s. Thereafter, the digestion of the cells was observed under the inverted microscope. Once most of the cells (over 80%) become round, they were quickly taken back to the operating table and a small amount of complete medium was added to terminate digestion. About 8.0 mL/bottle of the complete medium was added and then divided it into a new culture bottle.
2.2.2 Cell viability
SH-SY5Y cells were seeded at a density of 1 × 104 cells per well in clear 96-well cell culture plates. Different concentrations of H2O2 (50–250 µM) were freshly prepared before each experiment from a 30% stock solution and incubated at 37 °C with 5% CO2 for 90 min.
The color rendering method was after Zhang et al. (2007). After 90 min exposure to H2O2, 10 µL of MTT (5 mg/mL in PBS) was added to each well and the cells were incubated for 60 min. After obtaining the supernatant, 60 µL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the precipitate. The solution was measured at λ = 570 nm using a SpectraMax 190 Microplate Reader (Molecular Devices, San Jose, CA, USA). To the test group, samples were added in concentrations of 50 μg/mL for the crude extract and separation fragments and 10-5 M for the monomer compounds; a blank control was used for the control group.
2.3 Cytotoxicity assay
The cytotoxicity assay was performed following Zhang et al. (2008). The human cancer cell lines (HL-60 and HepG2) were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Life Technologies, Grand Island, NY, USA), supplemented with 10% of heat-inactivated bovine serum (Sijiqing Biomaterial, Hangzhou, China), 2 g/L of sodium bicarbonate (NaHCO3), and 100 units/mL of both penicillin and streptomycin in humidified 5% CO2 at 37 °C. Briefly, cells were seeded in 96-well tissue culture plates for 24 h, and then 100 μL of cell culture medium containing the test compounds dissolved in DMSO was added to the cells. The cells were re-incubated with CO2 for another 72 h with the various concentrations of the compounds. About 20 μL of cell counting kit-8 (CCK-8) solution (Dojindo Molecular Technologies, Japan) was added into the mixture and incubated for 2 h. The optical density at 450 nm was measured on a BioRad 550 instrument (BioRadLaboratories, Hercules, CA, USA). All compounds were tested at five concentrations (50, 5, 0.5, 0.05, and 0.005 μM/mL) in triplicate, with Sorafeni used as a positive reference.
2.4 Extraction and isolation
The air-dried whole herbs of A. ruprechtii (17.4 kg) were extracted with 11.0 L of 95% EtOH at room temperature for 3 × 48 h. The ethanol extract was evaporated under reduced pressure to yield a dark brown residue (528.6 g), which was suspended in water (H2O) (1.5 L) and partitioned with ethyl acetate (EtOAc, 6 × 1 L). The aqueous phase (GSJ-M) was applied to an AB-8 macroporous adsorbent resin (1000 g) column and eluted successively with H2O (GSJ-M-A), 30% EtOH (GSJ-M-B), 50% EtOH (GSJ-M-C), and 95% EtOH (GSJ-M-D) (5000 mL each), to yield four corresponding fractions GSJ-M-A-D. After removing the solvent under reduced pressure, fraction GSJ-M-C (73.9 g) was separated by CC over MCI gel CHP 20P (1 L), with successive elution using H2O (2 L), 15% EtOH (3 L), 30% EtOH (3 L), 70% EtOH (3 L), and 95% EtOH (2 L), to give fractions GSJ-M- C1 to GSJ-M-C5. Fraction GSJ-M-C4 (10.3 g) was subjected to CC over silica gel, eluting with a gradient of increasing 95% EtOH concentration (0–50%) in EtOAc, to yield fractions GSJ-M-C4-1 to GSJ-M-C4-3 based on TLC analysis. Fraction GSJ-M-C4-3 (3.6 g) was separated via reverse-phase medium-pressure liquid chromatography (RP-MPLC) eluting with a gradient of MeOH (0–100%) in H2O, and then purified by RP-HPLC (27% MeOH in H2O) to give 1 (33.3 mg), 2 (14.3 mg), and 10 (103.4 mg), respectively. Fraction GSJ-M-C4-2 (7.5 g) was subjected over Sephadex LH-20 (CH2Cl2/MeOH, 1:1), and then purified by RP-HPLC (25% MeOH in H2O) to obtain compounds 13 (5.72 mg), 14 (2.49 mg), 15 (33.5 mg), and 16 (173.3 mg), respectively. The isolation and purification progress for compounds 3 to 7 see Wang et al. (2020) and 8, 9, 11, 12 see Wang et al. (2019a, 2019b).
2.5 Docking procedure
Molecular docking was implemented using the surflex-docking package of Sybyl-X 2.1. A cocrystal structure of the heterodimer of the GluN1b-GluN2B NMDA receptor in which amino-terminal domains bind to allosteric inhibitor 93-31 (6E7U) was obtained from the Protein Data Bank (PDB). Before docking, 6E7U was prepared by removing water and magnesium ions and extracting the ligand. The addition of hydrogen and charges and treatment of the terminal residues were also performed on 6E7U. Then “protomol” was generated using the ligand-based mode and an appropriate binding pocket was formed. The reliability of the surflex-docking was validated by re-docking the original ligand into the binding pocket. Next, all the candidate compounds were docked into the binding pocket and 20 possible docked conformations were obtained with different scores.
2.5.1 Results and discussion
The whole plants of A. ruprechtii were extracted with 95% EtOH and the low polarity of the fat-soluble fraction and plant pigment was removed by extraction with acetic ether. Residual organic solvents in the aqueous phase were removed under reduced pressure and the water mixture was fragmented through AB-8 macroporous adsorbent resin.
All intermediate fragments were evaluated for their potential neuroprotective effect on cell viability of H2O2-induced apoptosis in SH-SY5Y. We found that after elution with 50% EtOH, the viability of SH-SY5Y cell lines was 77.10%, which was 15.5% higher than the negative control (Table 1). This implied that compounds in 50% EtOH elution might possess neuroprotective activity. Therefore, the chemical compositions were systematically investigated, which yielded 16 monomers (Fig. 1). The structures of compounds 1 to 16 were determined by extensive spectroscopic data analysis. Structural characterization of compounds 2 to 7 and 8, 9, 11, 12 have been reported in Wang et al. (2020) and Wang et al. (2019a, 2019b), respectively. In this paper, we reported the structural determination of new (1 and 2) and known (10 and 13–16) compounds (see Table 2).
Fragments
H2O2 damage model
Cell viability and deviation
Blank
100.00 ± 8.79
–
Model
61.98 ± 3.52###
GSJ-M
63.80 ± 7.88
GSJ-M-A
53.44 ± 6.54*
GSJ-M-B
77.22 ± 6.74*
GSJ-M-C
77.10 ± 8.21*
GSJ-M-D
59.25 ± 6.33*
No.
1H NMR, δ in ppm, coupling constant in Hz
13C NMR
1
2
1
2
1
2.06, 1.15(each 1H, m)
1.26,0.94 (each 1H, m)
32.60
31.62
2
2.54,2.03 (each 1H, m)
2.35, 1.84 (each 1H, m)
29.97
29.76
3
3.67 (1H,dd, 12.0, 4.8)
3.63 (1H, dd, 11.4, 4.8)
89.41
90.81
4
–
–
44.86
44.12
5
1.39 (1H, m)
1.18 (1H, m)
48.27
47.38
6
1.68, 1.28 (each 1H, m)
1.46,1.38 (each 1H, m)
22.71
21.54
7
1.32, 1.27 (each 1H, m)
0.87 (2H, m)
29.37
26.36
8
1.39(1H, m)
1.23 (1H, m)
48.38
48.22
11
1.64, 1.88 (each 1H, m)
1.38, 0.83 (each 1H, m)
26.67
26.35
12
1.24, 1.16(each 1H, m)
1.11(2H, m)
35.63
35.59
13
–
–
45.36
45.32
14
–
–
48.74
48.64
15
1.67 (2H,m)
1.51 (2H,m)
33.07
33.10
16
2.14,1.49 (each 1H, m)
2.04, 1.40 (each 1H, m)
28.16
28.22
17
1.71(1H, m)
1.57 (1H, m)
53.52
53.45
18
0.93 (3H, s)
0.85 (3H, s)
18.41
18.23
19
0.22, 0.79 (each 1H, brs)
0.09, 0.01 (each 1H, brs)
29.34
29.53
20
1.62 (1H, m)
1.97 (1H, m)
32.52
32.46
21
1.10 (3H, d, 6.0)
1.00 (3H, d, 6.0)
18.05
18.06
22
2.43, 1.30 (each 1H, m)
2.33 (1H, dd, 14.4, 11.4); 1.21 (1H, m)
42.66
42.67
23
4.26 (1H, m)
4.15 (1H, m)
67.45
67.43
24
3.80 (1H, brs)
3.67 (1H, brs)
93.66
93.72
25
–
–
73.17
73.21
26
1.76(3H, s)
1.63 (3H, s)
26.26
26.26
27
1.59(3H, s)
1.47 (3H, s)
26.76
26.68
28
0.93 (3H, s)
0.78 (3H, s)
19.41
19.27
29
0.90 (3H, s)
1.23 (3H, s)
20.31
20.51
30
4.68(1H, d, 10.2); 4.28(1H, m)
4.31, 3.30 (each 1H, brs)
70.84
63.51
1′
4.96 (1H, d,7.8) 3-1b
4.67 (1H, d,7.8) 3-1b
107.16
104.2
2′
4.12(1H, m)
4.02(1H, m)
75.77
80.11
3′
4.25 (1H, m)
3.68(1H, m)
78.35
76.27
4′
4.18(1H, m)
4.17 (1H, m)
71.25
71.45
5′
3.98 (1H, m)
4.10 (1H, m)
78.21
78.29
6′
4.54, 4.38 (each 1H, m)
4.36, 4.16 (each 1H, m)
62.48
62.09
1″
5.20(1H, d, 7.8) 24-1d
5.05(1H, d,7.8) 3-2c
106.80
104.66
2″
4.10(1H, m)
3.96 (1H, m)
75.30
74.67
3″
4.27 (1H, m)
4.24(1H, m)
78.32
78.32
4″
3.97(1H, m)
4.17(1H, m)
71.57
71.48
5″
4.17 (1H, m)
3.84(1H, m)
78.01
78.30
6″
5.08 (2H, m)
4.40, 4.27 (each 1H, m)
64.38
62.07
1‴
4.78(1H, d,7.8) 30-1f
5.03(1H, d,7.8) 24-1d
105.41
106.79
2‴
4.01(1H, m)
4.16 (1H, m)
75.34
80.43
3‴
4.18 (1H, m)
4.15(1H, m)
78.31
76.73
4‴
4.18(1H, m)
4.18(1H, m)
71.65
71.57
5‴
3.79(1H, m)
4.01(1H, m)
77.89
78.03
6‴
4.46 (2H, m)
4.34, 4.26 (each 1H, m)
62.58
61.65
1‴′
–
5.40(1H, d,7.8) 24-2e
133.66
104.11
2‴′
7.62 (1H, d, 8.4)
3.96 (1H, m)
130.53
75.83
3‴′
7.18 (1H, d, 8.4)
4.25(1H, m)
116.66
78.31
4‴′
–
4.20(1H, m)
161.26
71.60
5‴′
7.18 (1H, d, 8.4)
4.01(1H, m)
116.66
78.10
6‴′
7.62 (1H, d, 8.4)
4.40, 4.27 (each 1H, m)
130.53
61.47
7‴′
8.00 (1H, d, 15.6)
–
145.08
–
8‴′
6.73 (1H, d, 15.6)
–
115.05
–
9‴′
–
–
167.27
–
2.6 Structural characterization of compounds 1, 2, 10, and 13–16
Compound 1 was obtained as colorless gum with the specific rotation of [α]D20 + 14.29 (c 0.28, CH3 OH). Compound 1 had a molecular formula of C57H88O22 as determined by the negative HRESIMS at m/z 1123.5679 [M−H]− and 1159.5445 [M+Cl]−, with different values of 1.38 and 1.40 ppm compared to those of the calculated quasi-molecular ion peaks at [M−H]− (1123.5694 Da) and [M+Cl]− (1159.5461 Da). The chemical structure of 1 was fully discussed based on the NMR analysis. In the 1H NMR spectrum (in pyridine‑d5), resonances at the low field region of 5.0 to 8.5 ppm corresponded to characteristic protons of a moiety of trans-p-hydroxyphenylacryloyl (p-coumaroyl) at δH 7.62 (2H, d, J = 8.4 Hz, H-2‴′, 6‴′), 7.18 (2H, d, J = 8.4 Hz, H-3‴′, 5‴′), 8.00 (1H, d, J = 15.6 Hz, H-7‴′), and 6.73 (1H, d, J = 15.6 Hz, H-8‴′). In addition, resonance signals at δH 0.19 and 0.79 (each 1H, brs) of methylene (CH2) should be attributed to characteristic cyclopropane methylene protons (CH2-19), the specific three-member ring of cycloartane triterpenoid that has been previously discovered in A. ruprechtii (Wang et al., 2020). This speculation was supported by the existence of five single methyl signals at 0.93 (3H, s), 1.76 (3H, s), 1.59 (3H, s), 0.93 (3H, s), and 0.90 (3H, s) and a doublet one at 1.10 (3H, d, 6.0). The 13C and DEPT NMR data showed that 1 has other 24 carbons of the cycloartane triterpenoid skeleton, including 10 methylene, 7 methine, and 6 quaternary carbon. Furthermore, three glycosyl groups were supported by anomeric protons at δH 4.96 (1H, d, 7.8), 5.20 (1H, d, 7.8), and 4.78 (1H, d, 7.8) and corresponding carbons at 107.16, 106.80, and 105.41 ppm.
By comparing the NMR data of 1 to that of 3β,30-O-β-D-glucopyranosyl-24-O-[β-d-glucopyranosyl-(1 → 2)-O-β-d-glucopyranosyl]-7β,25-dihydroxycycloartane (aspleniumside A) (Wang et al., 2020), reported in our previous studies, we found that O-CH-7 in aspleniumside A was reduced to a CH2 group in 1 and CH2-23 in aspleniumside A was oxidized to CH-OH. These results were also supported by 2D NMR spectra including 1H-1H COSY, qHMQC, and heteronuclear multiple bond correlation (HMBC) (Fig. 2). In the 1H-1H COSY, the cross-peaks of H2-15/H2-16/H-17/H-20(H3-21)/H2-22/H-23(O)/H-24(O) verified the existence of CH(OH)-23. The glycosyl groups were shown to connect with C-3, C-24, and C-30, respectively, based on HMBC correlations of H-1′ with C-3, H-1″ with C-24, and H-1‴ with C-30. Additionally, the HMBC correlation of H-6″ to C-9‴′ indicated that the p-coumaroyl moiety was connected with C-6″ forming an ester linkage. The glycosyl groups in 1 were determined by acid-catalyzed hydrolysis. The co-TLC of the hydrolysate with a positive standard of D-glycoside in the development solvents of CHCl3:CH3OH:H2O of 7:3:1 suggested that all glycosyl groups in 1 were β-D-glucopyranosyl as other cycloartane-triterpenoid saponins that were previously isolated in the same species (Li et al., 2010; Li et al., 2008; Li et al., 2006a, 2006b; Wang et al., 2020; Zhang et al., 2008).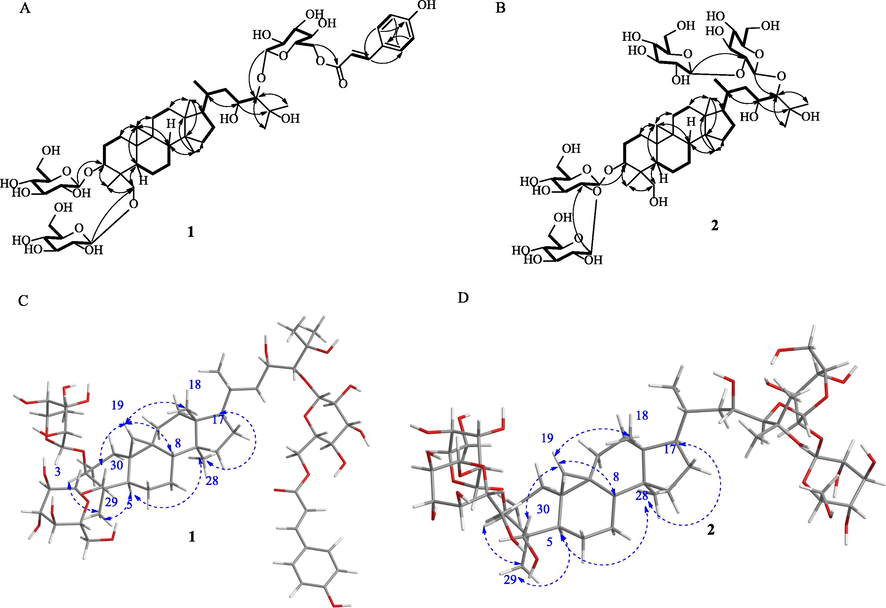
Key 2D NMR correlations for determining the structures of 1 and 2. 1H-1H COSY (thick bonds) and HMBC correlations (solid arrows) were exhibited as A (1) and B (2), while the key NOESY correlations (blue dash arrows) were provided as C (1) and D (2), respectively.
The relative configuration of 1 was determined by 2D NOESY analysis (Fig. 2). The correlations of H2-19 with H2-30 and H-8 with H3-18 indicated that these protons bear the same orientation. The NOESY correlations of H-3 with H-5, H-5 with H3-28, and H3-29 and H3-28 with H-17 suggested that they were orientated on the other side. Therefore, compound 1 was determined as 3β, 30-O-β-D-glucopyranosyl-24-O-[6″-O-p-coumaroyl-β-D-glucopyranosyl]-23, 25-dihydroxycycloartane, assigned as aspleniumside H.
The physicochemical properties of compound 2 resembled that of 1 as a colorless gum with a specific rotation of [α]D20 + 17.54 (c 0.34, CH3OH). The molecular formula C54H92O25 was deduced by the negative HRESIMS ions at m/z 1139.5822 [M−H]− (calculated as C54H93O25 of 1139.5855 Da by a difference of 2.89 ppm) and m/z 1175.5596 [M+H]− (calculated as C54H92O25Cl of 1175.5622 Da by a difference of 2.19 ppm), together with the NMR data (Table 1). The 1H NMR of 2 was very similar to that of 1 except the lack of a set of signals in the low field corresponding to the trans-p-hydroxyphenylacryloyl group. The resonance signals at 0.09 and 0.01 (each 1H, brs) were attributed to CH2 and six CH3 signals at 0.85 (3H, s), 1.00 (3H, d, 6.0), 1.63 (3H, s), 1.47 (3H, s), 0.78 (3H, s), and 1.23 (3H, s), together with the glycosyl signals in a range of 3.0 to 5.5 ppm, indicating that 2 was also a member of cycloartane-triterpenoid saponins. The glycosyl groups were determined as two β-D-glucopyranosyl-(1 → 2)-O-β-D-glucopyranosyl group linked to C-3 and C-24, respectively, by HMBC correlations (Fig. 2) of H-1′ with C-3, H-1″ with C-2′, H-1‴ with C-24, and H-1‴′ with C-2‴. The 1H-1H COSY cross-peaks of H2-15/H2-16/H-17/H-20(H3-21)/H2-22/H-23/H-24 verified the existence of O-bearing carbon of C-23. Therefore, compound 2 was determined as 3β, 24-di-O-[β-D-glucopyranosyl-(1 → 2)-O-β-D-glucopyranosyl]-23, 25, 30-trihydroxycycloartane, assigned as aspleniumside I.
The known compounds were determined as 10-O-acetyl-6-hydroxyadoxoside (10) (Su et al., 2005), kaempferol-3-O-[(6-O-E-caffeoyl)-β-D-glucopyranosyl]-(1 → 2)-β-D-glucopyranosyl-7-O-β-D-glucopyranoside (13) (Fan et al., 2012), kaempferol-3-O-[(6-O-p-coumaroyl)-β-D-glucopyranosyl]-(1 → 2)-β-D-glucopyranosyl-7-O-β-D-glucopyranoside (14) (Li et al., 2006a, 2006b), kaempferol-3-O-[(6-O-E-feruloyl)-β-D-glucopyranosyl]-(1 → 2)-β-D-galacopyranoside (15) (Li et al., 2006a, 2006b), and quercetin-3-O-[(6-O-E-feruloyl)-β-D-glucopyranosyl]-(1 → 2)-β-D-glucopyranoside (16) (Li et al., 2006a, 2006b), by comparison of their NMR data to those in the literature.
2.7 Neuroprotective activity assay of the monomers
The neuroprotective activity of the monomers was determined using H2O2-induced damage of SH-SY5Y cell lines as a model at a concentration level of 10-5 M. Compounds 10 and 13–16 showed protective activities with 1.09 to 1.26 times of cell viability compared to the negative control. Compounds 1 to 7 showed some toxic effects in terms of lower cell viability (Table 3).
Fragments
H2O2 damage model
Cell viability and deviation
blank
100.00 ± 8.79
–
model
61.98 ± 3.52###
1
38.82 ± 3.48
2
33.44 ± 7.46*
3
32.22 ± 6.70*
4
47.10 ± 7.21*
5
52.25 ± 4.30*
6
58.43 ± 6.76*
7
54.22 ± 3.30*
8
60.20 ± 3.20*
9
58.24 ± 5.56*
10
56.43 ± 3.14*
11
57.12 ± 4.46*
12
56.24 ± 4.68*
13
56.43 ± 3.14*
14
75.63 ± 5.44*
15
71.61 ± 7.94*
16
66.54 ± 6.32*
2.8 Cytotoxicity assay
Cytotoxicity effects of compounds 3–7 on the tumor cells have previously been reported by our group. Herein, compound 2 exhibited cytotoxic activity against HL-60 and HepG2 cells with the half-maximal inhibitory concentration (IC50) value of 60.24 ± 4.26 and 40.15 ± 2.23 μM, respectively, and >100 μM for compound 1, while the positive control of Sorafeni showed cytotoxic activity against HL-60 and HepG2 cells with an IC50 value of 10.61 ± 0.43 and 13.43 ± 1.12 μM, respectively, indicating that 2 has a weak anti-tumor activity.
2.9 Docking of compounds 1–16 to the N-terminal of the GluN2B protein
The N-terminal of the GluN2B protein has been considered to be the “active pocket” in the literature. In this study, the neuroprotective activity of all monomer compounds was evaluated using the autodocking method. Stable conformation of 1–16 was yielded by the Chem3D software using the default MM2 algorithm. The binding effect between small molecular ligands and macromolecular protein was determined by the total score (Table S1). The higher binding scores of 13–16 were identical to their in vitro neuroprotective activity data, indicating that binding to the GluN2B protein is one of the important mechanisms that should be considered.
Modern medicinal-oriented natural products research has broken numerous difficulties and bottlenecks, especially in the improvement of modern analytical methods and techniques. This has facilitated the establishment of structures of ultra-trace chemical components in natural resources using combined chromatographic analyses, such as HPLC-MS/MS, HPLC-NMR-MS/MS, etc. Several new compounds and skeletons have been discovered and reported (Jiang et al., 2017; Meng et al., 2016; Jiang et al., 2018; Shi et al., 2016; Wu et al., 2019). However, due to the limitation of the number of compounds and the lack of tracking during the isolation progress, systematic evaluation of most important biological activities has been impossible. Therefore, the establishment of novel bioassay-guided techniques in the process of screening bioactive compounds remains a hot topic among organic chemists and pharmacologists.
Traditional Chinese medicines and ancient folk medicine in India and other countries with a long history have been used clinically and proved effective. However, their active and effective chemicals have always been a mystery, attracting scientific attention. Systematically expounding the chemical compositions based on bioactivity evaluation may be advantageous in the discovery of active chemical molecules, hence, saving time and resources during research and development of effective drugs. In this study, the evaluation of neuroprotective activity was employed in the screening of new bioactive compounds in a folk medicine plant, A. ruprechtii, leading to the discovery of 16 compounds. We attempted to validate these compounds using the in vitro neuroprotective activity evaluation and docking to the “active pocket” of the GluN2B protein that was considered to be related to the neuroprotective activity. This yielded four flavonoid glycosides (13–16), which exhibited biological activities.
3 Conclusions
Based on neuroprotective activity evaluation, the chemical compositions of a thromboangitis obliterans-treating folk medicine (A. ruprechtii) were investigated, leading to the isolation of 16 compounds, including two new cycloartane-triterpenoid saponins (1 and 2). Their structures were determined based on the extensive analysis of the NMR data. In vitro evaluation demonstrated that compounds 13 to 16 had potential neuroprotective activity against H2O2-induced damage in SH-SY5Y cell lines and the higher docking binding scores to the GluN2B protein. Overall, our findings demonstrate the importance of neuroprotective activity evaluation as an effective technique in the characterization of bioactive compounds from medicinal plants.
Contributors’ Statement
Extraction and separation: X. Lu, J. Chen, and X. Ma; Cytotoxicity test: D. Zhang; Cell culture: F. Wang; Cell viability assay: F. Wang; Docking experiments: S. Cao; Project supervision: Z. Jiang; Structural analysis: Y. Ke, X. Guo, and H. Liu; NMR spectroscopy: C. Li and X. Wu.
Acknowledgements
This work was co-funded by the Key R&D projects in Ningxia province [Grant numbers: 2020BFG02006 and 2019BEB04028); the ‘2019 Western Light’ Program for Scholars in Western China [Grant number: XAB2019AW05); the Scientific Research Start-up Project for Recruitment Talents of North Minzu University in 2019 [Grant number: 113159150]; Jinan innovation team project 2019 [Grant number: 2019GXRC039]; the Natural Science Foundation of Ningxia Province [Grant numbers: 2020AAC03248 and 2020AAC03206]; and the National Natural Science Foundation of China [Grant number: 81603006].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 41K of Shenyang College of Pharmacy, 1977. Dilatation of blood vessels and toxicities of Camptosorus sibiricus Rupr. J. Shenyang Pharm. Univ. 7, 34–37.
- LEGO MINDSTORMS fraction collector: A low-cost tool for a preparative high-performance liquid chromatography system. Anal. Chem.. 2020;92:1687-1690.
- [Google Scholar]
- The Chinese fern families and genera: systematic arrangement and historical origin. J. Syst. Evol.. 1978;16:1-19.
- [Google Scholar]
- A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa. 2011;19:7-54.
- [Google Scholar]
- Chemical constituents of Asplenium ruta-muraria L. Nat. Prod. Lett.. 2012;26:1413-1418.
- [Google Scholar]
- Natural products for treating colorectal cancer: a mechanistic review. Biomed. Pharmacother.. 2019;117:109142
- [Google Scholar]
- Unraveling the importance of molecules of natural origin in antifungal drug development through targeting ergosterol biosynthesis pathway. Iran J. Microbiol.. 2019;11:448-459.
- [Google Scholar]
- Three structurally-related impurities in norvancomycin drug substance. J. Antibiot (Tokyo). 2017;70:158-165.
- [Google Scholar]
- Structure-based manual screening and automatic networking for systematically exploring sansanmycin analogues using high-performance liquid chromatography-tandem mass spectroscopy. J. Pharm. Biom. Anal.. 2018;158:94-105.
- [Google Scholar]
- New cycloartane glycosides from Camptosorus Sibiricus Rupr. J. Asian Nat. Prod. Res.. 2008;10:119-124.
- [Google Scholar]
- Flavonoids from Camptosorus Sibiricus Rupr. J Asian Nat. Prod. Res.. 2006;8:167-171.
- [Google Scholar]
- A new cycloartane glycoside from Camptosorus sibiricus Rupr. Nat. Prod. Res.. 2006;20:1041-1045.
- [Google Scholar]
- New triterpene glycosides from Camptosorus Sibiricus. Nat. Prod. Commun.. 2010;5:1557-1560.
- [Google Scholar]
- Chemical constituents of the water extract from Camptosorus sibiricus Rupr. J. Shenyang Pharm. Univ.. 2017;34:541-552.
- [Google Scholar]
- Improving effect of total flavonoids from Camptosorus sibiricus on rats with thromboangiitis obliterans. Chin. Tradit. Herb. Drugs. 2011;26:481-484.
- [Google Scholar]
- Lin, Y.X., Ronald, V., 2013. Flora of China. Science Press & the Missouri Botanical Garden Press 2, 16.
- A comprehensive description of GluN2B-selective N-methyl-D-aspartate (NMDA) receptor antagonists. Eur. J. Med. Chem.. 2020;200:112447
- [Google Scholar]
- Napelline-type C20-diterpenoid alkaloid iminiums from an aqueous extract of “fu zi”: Solvent-/base-/acid-dependent transformation and equilibration between alcohol iminium and aza acetal forms. Chin. Chem. Lett.. 2016;27:993-1003.
- [Google Scholar]
- Natural products as sources of new drugs over nearly four decades. J. Nat. Prod.. 2020;83:770-803.
- [Google Scholar]
- A review on chronic pain in rheumatoid arthritis: a focus on activation of NR2B subunit of N-methyl-D-aspartate receptors. Malays J. Med. Sci.. 2020;27:6-21.
- [Google Scholar]
- Improving the N-terminal diversity of sansanmycin through mutasynthesis. Microb. Cell. Fact.. 2016;15:1-15.
- [Google Scholar]
- Chemical constituents of the fruits of Morinda citrifolia (Noni) and their antioxidant activity. J. Nat. Prod.. 2005;68:592-595.
- [Google Scholar]
- Natural products-derived chemicals: breaking barriers to novel anti-HSV drug development. Viruses. 2020;12:154.
- [Google Scholar]
- Multiple domains in the C-terminus of NMDA receptor GluN2B subunit contribute to neuronal death following in vitro ischemia. Neurobiol. Dis.. 2016;89:223-234.
- [Google Scholar]
- An anti-inflammatory C-stiryl iridoid from Camptosorus Sibiricus Rupr. Fitoterapia. 2019;134:378-381.
- [Google Scholar]
- Structural determination and in vitro tumor cytotoxicity evaluation of five new cycloartane glycosides from Asplenium ruprechtii Sa. Kurata. Bioorg. Chem.. 2020;102:104085
- [Google Scholar]
- Cytotoxic 9,19-cycloartane type triterpenoid glycosides from the roots of Actaea dahurica. Phytochemistry. 2019;160:48-55.
- [Google Scholar]
- Aconicatisulfonines A and B, analgesic zwitterionic C20-diterpenoid alkaloids with a rearranged atisane skeleton from Aconitum carmichaelii. Org. Lett.. 2019;21:6850-6854.
- [Google Scholar]
- Research progress in chemical constituents and pharmacological activities of Camptosorus sibiricus Rupr. Modern. Chin. Med.. 2012;14:18-22.
- [Google Scholar]
- Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol.. 2007;564:18-25.
- [Google Scholar]
- New cycloartane glycosides from Camptosorus sibiricus Rupr. J. Asian Nat. Prod. Res.. 2008;10:1069-1074.
- [Google Scholar]
- Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J. Biol. Chem.. 2013;288:24151-24159.
- [Google Scholar]
Appendix A
Supplementary matrial
The (-)-HRESIMS, 1H and 13C NMR, DEPT, COSY, gHMQC, HMBC, and NOESY spectra for compounds 1 and 2; and the 1H and (or) 13C NMR for compounds 10 and 13-16 are provided in Appendix A. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.10.011.
Appendix A
Supplementary matrial
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







