Translate this page into:
Adsorption performance and stability of the modified straws and their extracts of cellulose, lignin, and hemicellulose for Pb2+: pH effect
⁎Corresponding author at: No. 693 Xiongchu Avenue, Hongshan District, Wuhan, Hubei 430074, China. yujunxia_1979@163.com (Jun-xia Yu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Adsorption performance and stability of the carboxyl groups modified straws and their extracts of cellulose, lignin, and hemicellulose for Pb2+ were investigated, and the optimum pH range for Pb2+ adsorption was determined by considering both the stability and capacity of the modified biosorbents for the first time. Results showed that adsorption capacity and stability of the straws and extracts were both improved significantly after modification. Adsorption capacities of the modified straws and extracts followed the order: modified hemicellulose > modified lignin, modified straw > modified cellulose, while stability of them followed the reverse order. In the optimum pH range from 4.0 to 5.0, modified rape and cotton straw showed better stability than the modified maize straw, and total organic carbon (TOC) values determined from the two modified straws and extracts were lower than 5.0 mg L−1 even after adsorption for 30 days, which reached the drinking water standard in China.
Keywords
Straw
Cellulose
Lignin
Hemicellulose
Adsorption
1 Introduction
With the development of the industry, large amount of heavy metal wastewater was produced. Due to the unique biological toxicity and easy accumulation, heavy metals, especially Pb2+, would pose significant harm to ecosystem and human health even at low concentration (Dong et al., 2016).
To remove Pb2+ from aqueous solution, many technologies including chemical precipitation, membrane filtration, extraction, ion-exchange, adsorption, and biosorption had been used (Ding et al., 2018). Among these technologies, biosorption, using the waste straws as biosorbents, had attracted much attention due to the abundant resource and low cost (Joseph et al., 2019; Zheng et al., 2012). China is a big agricultural country, and annual output of straw is over 900 million tons. Parts of them were discarded directly, which not only waste the resources but also pollute the environment. Utilization of these waste straws had important economic and environmental benefits. Waste straws were mainly consisted of cellulose, lignin, and hemicellulose. Cellulose is the source of cell wall strength and the main structural component working as a framework substance, which was a homopolymer consisted of glucose with β-1,4-glucosidic linkages shown in Fig. 1a (Yoon et al., 2014). Hemicellulose interacted with cellulose and lignin through hydrogen bond and covalent band, respectively, and it was a heterogeneous polysaccharide, which was mainly composed of D-xylose, D-glucose, D-mannose, D-galactose, D-glucuronic acid, L-arabinose, 4-O-methyl-d-glucuronic acid, and D-galacturonic acid shown in Fig. 1b (Xiao et al., 2001). Lignin is a binder of the plant cell and it's often embedded with hemicellulose and cellulose, which was a complex phenolic polymer formed by coumarin, terpineol and sinapyl alcohol shown in Fig. 1c. It had been reported that both the raw agricultural wastes and their main components had poor adsorption capacity and stability for heavy metals, and organic matter would release from the raw materials during the sorption process, which resulting the unsatisfied results in treating wastewater (Sun et al., 2018; Zheng et al., 2012).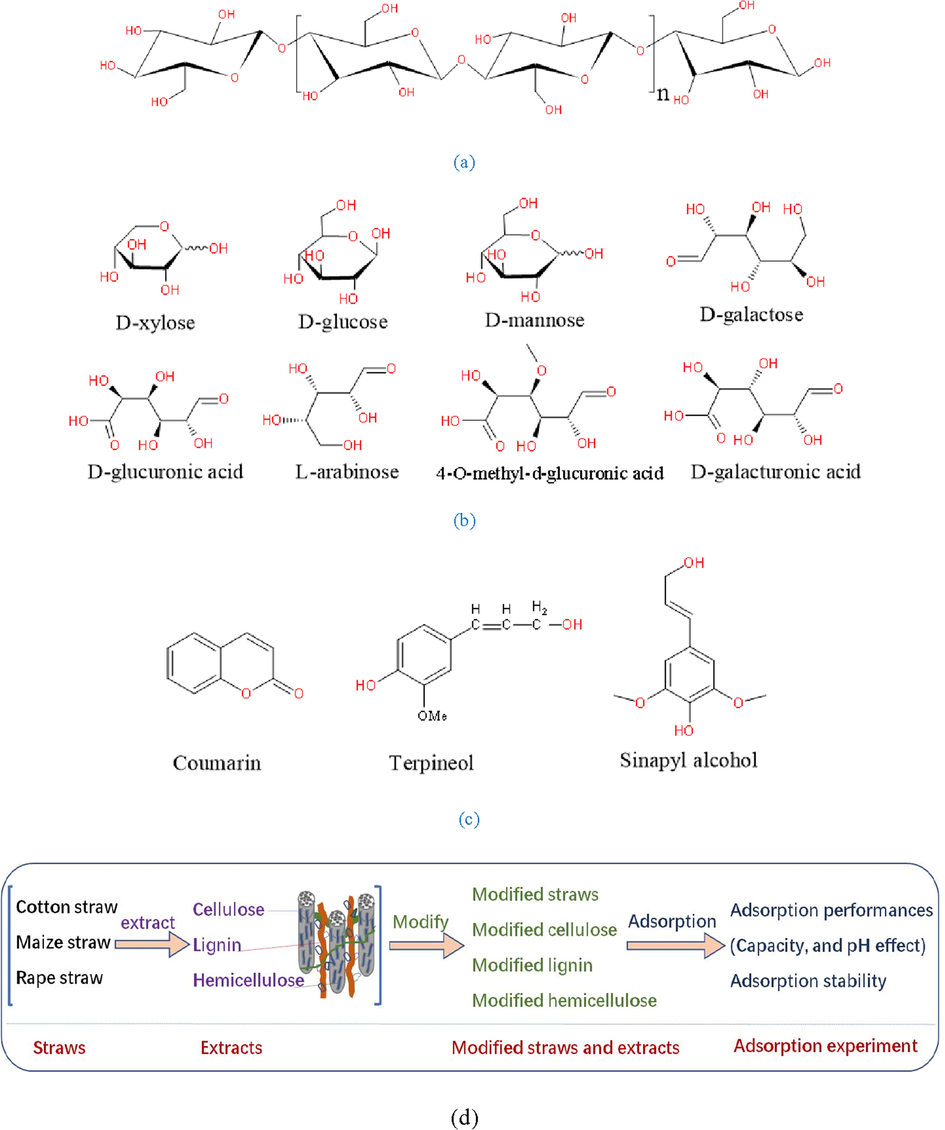
Structure information of (a) cellulose, (c) hemicellulose, (b) lignin, and (d) flowchart of the experiment.
Literatures had reported that surface modification by carboxyl groups could improve the adsorption capacity of the biosorbents for heavy metal ions (Moyo et al., 2017; Wang and Wang, 2018). Moyo et al. (2017) had reported that maximum amount of Pb2+ adsorbed on sugarcane bagasse increased from 59.25 to 306.33 mg g−1 after surface modification by carboxyl groups from EDTA dianhydride. Tang et al. (2018) had reported that adsorption capacity of bagasse for Pb2+ increased from 60.5 to 241.7 mg g−1 after modification by carboxyl groups from pyromellitic dianhydride. Júnior et al. (2009) had found that cellulose adsorption capacity for Pb2+ increased to 205.9 mg g−1 after surface modification by carboxyl groups from succinic acid. These results illustrated that surface modification could improve the adsorption capacity of the biosorbents. However, to our knowledge, surface modification effect on the stability of the biosorbents had rarely been investigated. Kumar et al. (2011) had pointed out that stability of the biosorbents is an important factor that needed to be considered in practical use since organic matter might release into aqueous solution during the sorption process. Anawar and Strezov (2019) also had emphasized that stability of the biosorbents should be determined before use. It was necessary to investigate the effects of surface modification on the stability of the biosorbents. Since the straw mainly consisted of cellulose, lignin and hemicellulose (Efthalia et al., 2012; Lu et al., 2011; Wang et al., 2016), surface modification on the adsorption capacity and stability of them should also be determined and compared with the raw materials.
Additionally, solution pH was one of the main factors that affected the heavy metal adsorption and the biosorbent performance, and the optimum pH should be determined by both considering the adsorption capacity and stability of the biosorbent (Dao et al., 2004; Tang et al., 2018). However, the optimum pH was often determined just from the adsorption capacity without considering the stability of the biosorbents, and it was necessary to choose the optimum pH range by both considering the two factors.
In this study, rape, maize, and cotton straw were chosen as the raw straw because they were typical crops and grown in large quantities in China. Cellulose, lignin, and hemicellulose were extracted from the three waste straws by three different extraction methods. Surface modification of the raw straws and the extracted cellulose, lignin and hemicellulose were carried out by using pyromellitic dianhydride as the carboxyl resource, and scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR) and energy dispersive X-Ray spectroscopy (EDX) were used to characterize these straws and extracts before and after modification. Adsorption capacity and stability of the modified and unmodified straws and the extracts were investigated and compared under different solution pH, and the optimum pH range for heavy metal ions adsorption were determined. Additionally, Pb2+ adsorption mechanism on the modified biosorbents was investigated by X-ray photoelectron spectroscopy (XPS).
2 Materials and methods
2.1 Materials
Rape, maize, and cotton straws were obtained from HuiFeng straw agricultural products deep processing company (Jiangsu province, China), and it was grinded and sieved to 100–200 mesh before use. Reagents of pyromellitic dianhydride, lead nitrate, hydrochloric acid, sodium hydroxide, hydrogen peroxide, glacial acetic acid, ethylenediamine tetraacetic acid disodium salt, and toluene with analytical grade were purchased from Sinopharm Chemical Reagent (Shanghai, China) and used without further purification. The commercial cellulose, lignin (Dealkaline), and hemicellulose were purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China).
2.2 Extraction of cellulose from rape, maize, and cotton straws
Cellulose was extracted from these three straws under acid conditions (Idrissi et al., 2013). 2.0 g of the straw and 30 mL of mixed acid solution (5 wt% of nitric acid and 80 wt% of acetic acid) were added into a round bottom flask, and reflux at 120 ℃ for 30 min. After that, the obtained extract was washed by hot water until the filtrate became colorless and transparent, and then it was freeze-dried and stored before use.
2.3 Extraction of lignin from rape, maize, and cotton straws
Lignin was extracted from the straws of rape, maize, and cotton straw by a two-step sulfuric acid hydrolysis method according to Sluiter et al. (2008) and Angelini et al. (2016) with some modification, and the extracted lignin was acid insoluble lignin. Briefly, 2.0 g of the straw was added into 30 mL of sulphuric acid (60 wt%). After stirring at room temperature for 1.5 h, 500 mL distilled water was added and reflux at 120 ℃ for another 4 h. Then the extracted lignin was washed with distilled water to neutral and freeze-dried before use.
2.4 Extraction of hemicellulose from rape, maize, and cotton straws
Hemicellulose was extracted from rape, maize, and cotton straws according to Sun et al. (2000) and Xiao et al. (2001). 2.0 g of the raw straw and 50 mL of mixture solution of toluene and ethanol with volume ratio of 4:1 were added into Soxhlet extractor and reacted at 85 ℃ for 3 h, and then soaked in 1 wt% of NaOH solution under ultrasonic condition for 2 h. After dewaxing treatment, the obtained solid was washed to neutral and added into 30 mL mixed solution of NaOH (2 mol L−1) and H2O2 (1 wt%). After ultrasonic dispersion for 10 min, the mixtures were reacted at 55 ℃ for another 10 h. Then the mixture was filtered, and the obtained solid was washed with 150 mL of NaOH (2 mol L−1). The washing liquor and the filtrate were collected and mixed, pH of it was adjusted to 4.5 by HCl. Solid hemicellulose was obtained after treated by 2 times volume of absolute ethanol, and it was freeze-dried after being washed and centrifugated.
2.5 Surface modification of the straws and the extracted cellulose, lignin, and hemicellulose
Surface modification of the straws and the extracts were carried out according to our previous study (Yu et al., 2019a; Yu et al., 2019b). Briefly, 1.0 g of pyromellitic dianhydride, 40.00 mL of dimethylformamide, and 1.0 g of the raw straw or the extracts were added into a round bottom flask with condensing plant. After stirring at 60 ℃ under close system for 4 h, the obtained modified biosorbents were washed in order with dimethylformamide and distilled water for five times, and then dried in a reduced pressure vacuum oven (DZF-6050, Shanghai, China) at 45 ℃ for 12 h.
2.6 Characterization
To determine the changes in morphology and the functional groups on the biosorbents surface after modification, the modified and unmodified straws and extracts were characterized by SEM (Hitachi S4800, Japan) with EDX (Bruker QUANTAX, Germany) and FTIR (Thermo Nicolet Corporation, America), respectively. In FTIR analysis, about 1 mg of sample and 100 mg KBr was mixed completely and pressed into disks for determination, and the wavenumber range of 400–4000 cm−1 was recorded. To clarify the adsorption mechanism, XPS (Thermo Fisher Scientific Esca Lab 250Xi, America) of the modified hemicellulose before and after Pb2+ adsorption was carried out.
2.7 Adsorption and desorption experiments
2.7.1 Adsorption isotherm of Pb2+ on modified and unmodified straws and extracts
In this experiment, 0.0040 g of the modified and unmodified biosorbent was added into 40.00 mL of Pb(NO3)2 solution with different initial concentration at pH 4.5. After adsorption on a shaker (200 rpm) at room temperature for 50 min, Pb2+ concentration before and after adsorption were tested by atomic absorption spectrum (AAS, iCE 3500, ThermoFisher, America). Adsorption capacity of Pb2+ were calculated according to Eq. (1), where qe (mg g−1) is the amount of Pb2+ adsorbed on the biosorbents at equilibrium, v (mL) is the volume of the solution, C0 (mg L−1) and Ce (mg L−1) is the initial and equilibrium concentration of the Pb2+, respectively, and m (g) is the weight of the biosorbents.
2.7.2 Adsorption kinetic of Pb2+ on modified straws and extracts
In this experiment, 0.0080 g of the modified straws and extracts was added into 80.00 mL of Pb(NO3)2 solution (100.00 mg L−1) with pH 4.5. Samples were collected at different time and Pb2+ adsorption amount on different modified straws and extracts were calculated.
2.7.3 Effects of pH on Pb2+adsorption on the modified and unmodified straws and extracts
In this experiment, 0.0040 g of the modified and unmodified biosorbent was added into 40.00 mL of 100.00 mg L−1 Pb(NO3)2 solution with different pH. After adsorption on a shaker (200 rpm) at room temperature for 50 min, Pb2+ concentration before and after adsorption were tested by AAS and amounts of Pb2+ adsorbed were determined.
2.7.4 Effects of pH on the organic matter release from the modified and unmodified straws and the extracts
In this experiment, 0.0040 g of the modified and unmodified biosorbent was added into 40.00 mL of Pb(NO3)2 solution (100.00 mg L−1) with pH 4.0, 5.0, and 6.0. After adsorption for 50 min, amounts of organic matter released from the modified and unmodified straws and the extracts were decided through determination of the TOC values (TOC-L, Shimadzu, Japan) of the solution.
2.7.5 Effects of time on the organic matter release from the modified and unmodified straws and the extracts
In this experiment, 0.0150 g of the modified and unmodified bio-sorbent was added into 150.00 mL of Pb(NO3)2 solution (100 mg L−1) with pH 4.5, amounts of organic matter released from the modified and unmodified straws and extracts at different sorption time were determined by TOC analyzer.
2.7.6 Desorption of Pb2+ from the modified straws and extracts
To determine the desorption efficiency of Pb2+ from the saturated modified straws and extracts, 0.01 mol L−1 of EDTA-2Na was used as eluent. Briefly, 0.0040 g of the Pb2+ saturated modified biosorbent was added into 20.00 mL of EDTA-2Na solution. After desorption on a shaker (200 rpm) at room temperature for 50 min, Pb2+ concentration in the eluent were tested by AAS and desorption efficiency was determined.
Flow chart of all the experiment was shown in Fig. 1.
3 Results and discussion
3.1 Characterization of the raw straws
SEM images in Figs. 2a-4a shows that the three raw straws of rape, maize and cotton were all block structure with comparative compact surface, which illustrated that cellulose, hemicellulose and lignin interacted closely. Fig. 5a shows the FTIR of the three raw straws. Peaks at 3340 and 2900 cm−1 were ascribed to the stretching vibration of O-H and C-H, respectively. Peaks at 1720 cm−1 was correspond to the C = O stretching vibration. Bands at about 1630 cm−1 was assigned to aromatic skeleton vibrations in lignin, and that from 1300 to 1500 cm−1 was due to the vibration of C-H and C-C etc. The prominent band at 1030 cm−1 is correspond to the C–O, C–C stretching or C–OH bending in cellulose, lignin, and hemicellulose.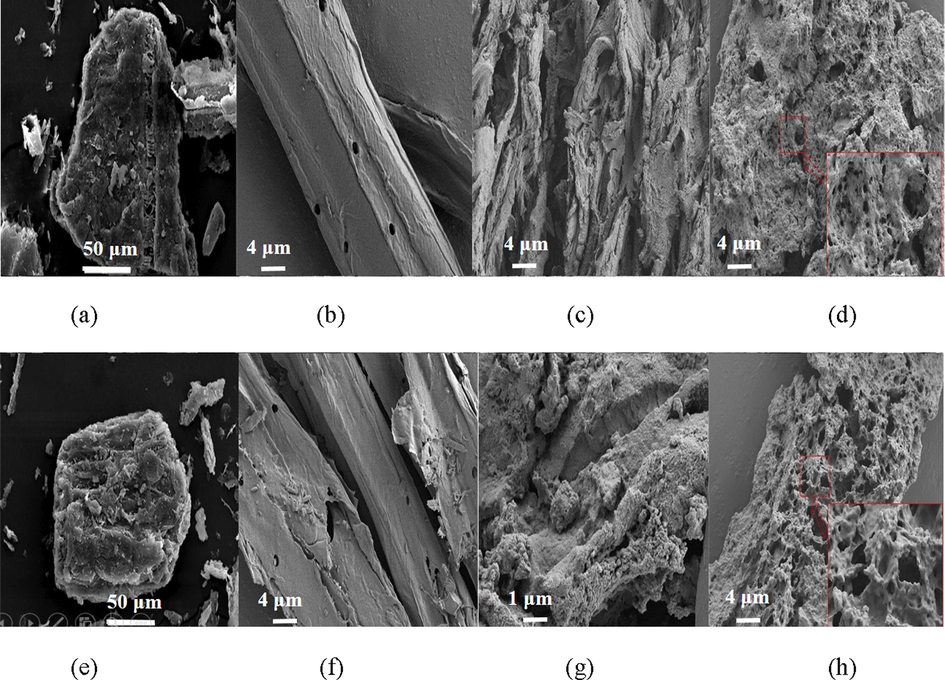
SEM images of raw (a) rape straw, the extracted (b) cellulose, (c) lignin and (d) hemicellulose and modified (e) rape straw, (f) cellulose, (g) lignin, and (h) hemicellulose.
3.2 Extraction of cellulose from the rape, maize, and cotton straw
Acid or alkaline could break the physical interactions including Van deer Waals and hydrogen bonds, and further facilitates the delignification and elimination of non-cellulosic components. Literatures had reported that cellulose could be extracted from the straw in acid or alkaline medium (Vignon et al., 2004). In this work, cellulose was extracted from rape, maize, and cotton straw in acetic acid medium by using nitric acid as the catalyst. Idrissi et al. (2013) had reported that the oxidation of lignin and removal of the residue coagulations of the hemicelluloses and lignin from cellulosic fibers would become easy in the presence of nitric acid. Results showed that extraction yield of cellulose from rape, maize, and cotton straw were 52.11, 37.82, and 62.35%, respectively. SEM of cellulose extracted from the three straws were shown in Figs. 2b–4b, and it was observed that the extracted three cellulose all kept intact structure. Different from the block structure of the raw straw, cellulose extracted from rape and cotton straw were sticks haped with multi-hole, while that from maize straw was rod and sheet-like with compact structure. These phenomena demonstrated that lignin and hemicellulose were removed and cellulose skeleton was present. Fig. 5b showed the FTIR spectra of the cellulose extracted from the three straws. It was observed that similar spectra were obtained for the three celluloses extracted from the different straws. Peaks at 3332 and 2900 cm−1 corresponded to stretching vibration of O-H and C-H bond, respectively, and peaks at 1645 cm−1 was ascribed to the naturally adsorbed water (Pastorova et al., 1994). Bonds around 1440 and 1338 cm−1 were assigned to the bending vibration of C-H and O-H, respectively, and those around 1330 and 1260 cm−1 were allotted to the characteristic vibration of the cellulose skeleton. Peaks at 1160 and 1073 cm−1 were assigned to the vibration of C-O and C-O-C bond, whereas peak at about 896 cm−1 was the characteristic vibration of the b-glucidic bond (Sun et al., 2002). Similar cellulose spectra were reported by Liu et al. and Ma et al. (Liu et al., 2013; Ma et al., 2012). FTIR spectrum of the commercial product showed similar absorption bands at 3335, 2900, 1645, 1430, 1316, 1200, 1160, 1030, and 896 cm−1, indicating the similarity of the extracted cellulose with the purchased cellulose.
SEM images of raw (a) maize straw, the extracted (b) cellulose, (c) lignin and (d) hemicellulose and modified (e) maize straw, (f) cellulose, (g) lignin, and (h) hemicellulose.
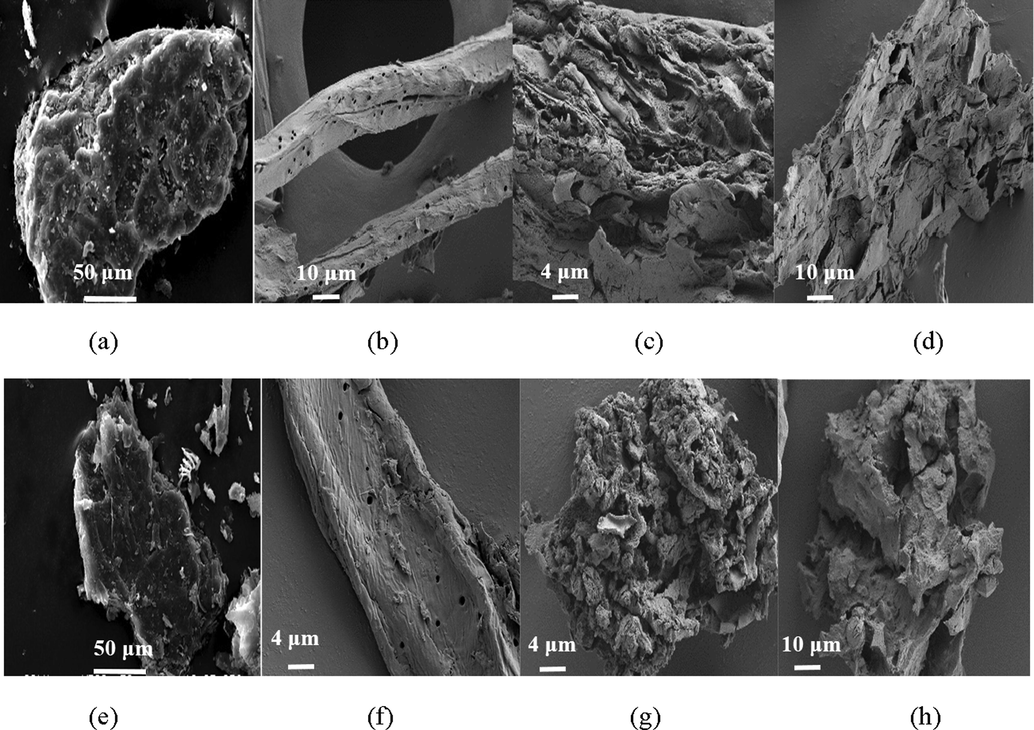
SEM images of raw (a) cotton straw, the extracted (b) cellulose, (c) lignin and (d) hemicellulose and modified (e) cotton straw, (f) cellulose, (g) lignin, and (h) hemicellulose.
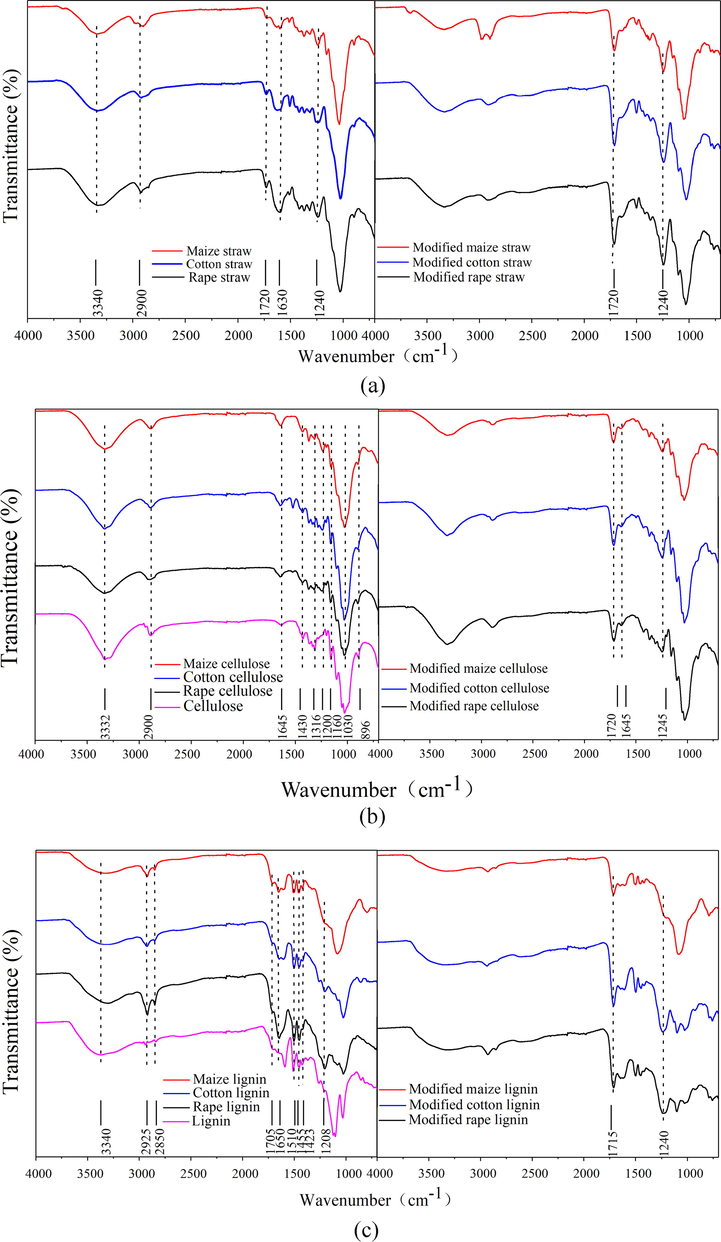
FTIR spectra of modified and unmodified (a) straws and extracted (b) cellulose (c) lignin, and (d) hemicellulose.
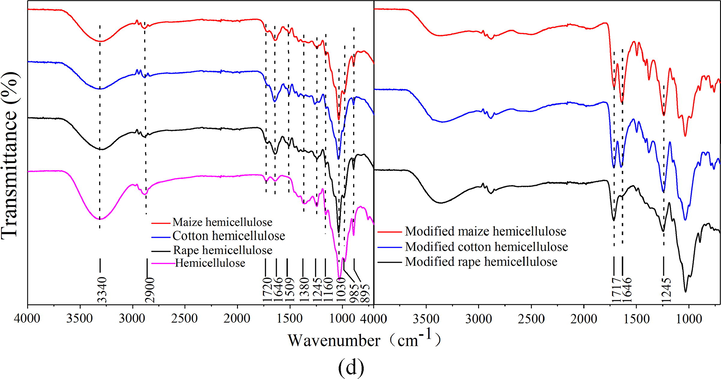
FTIR spectra of modified and unmodified (a) straws and extracted (b) cellulose (c) lignin, and (d) hemicellulose.
3.3 Extraction of lignin from the rape, maize, and cotton straw
Lignin was extracted from the three straws by a two-step sulfuric acid hydrolysis method, and the obtained lignin was acid-insoluble lignin. Similar methods were adopted by Savy and Piccolo (2014) and Lin and Dence (1992). Results showed that extraction yield of lignin from rape, maize, and cotton straw were 20.32, 16.13, and 20.14%, respectively. SEM of the extracted lignin was shown in Figs. 2c–4c, and it was observed that irregularly shaped heterogeneous lignin with loose and fluffy block structure was obtained. These results demonstrated the efficient hydrolysis of the polysaccharides in hemicellulose and cellulose by the sulfuric acid treatment, and lignin was remained. Similar morphology had been reported by Angelini et al. (2016) and Donohoe et al. (2008). Fig. 5c showed the FTIR of the extracted lignin, and it was observed that spectra of lignin extracted from the three straws all exhibited similar spectrum with that of the purchased lignin, indicating the similarity of the extracted lignin with the commercial lignin. Peaks at about 3340 cm−1 were assigned to the O-H stretching vibration, while those at 2925 and 2850 cm−1 were due to the C-H bond vibration. The small band at 1705 cm−1 was due to the unconjugated ketone or carbonyl stretching, while that at 1650 cm−1 was attributed to carbonyl stretching conjugated with aromatic rings (Angelini et al., 2016; Xiao et al., 2001). Peaks at 1630, 1510, and 1423 cm−1 were assigned to the aromatic skeleton vibrations and that at 1455 cm−1 was the indicative of C-H bending vibration in the methoxy groups (Jahan et al., 2007). In the low wave number range, peaks at 1208 and 1040 cm−1 were correspond to the alkyl aromatic ethers (Ar–O) of lignin (Angelini et al., 2014). Similar FTIR spectrum was reported by Jonglertjunya et al. (2014) and Zeng et al. (2012).
3.4 Extraction of hemicellulose from the rape, maize, and cotton straw
Hemicellulose was a branched polymer with lower molecular weight, and it was extracted from the straws under alkaline condition. In this condition, hemicellulose was liberated from lignocelluloses through the alkaline hydrolysis of ester linkages and followed by dissolved into aqueous medium. Similar method was used to extract hemicellulose from sugarcane bagasse and rice straw (Sun et al., 2004; Sun et al., 2000). Results showed that extraction yield of hemicellulose from rape, maize, and cotton straw were 14.96, 27.85, and 13.58%, respectively. SEM of the extracted hemicellulose was shown in Figs. 2d–4d, and it was observed that amorphous insoluble macromolecular compounds were obtained. Due to the different structure of the raw materials, hemicellulose extracted from rape showed a multi-porous structure while that extracted from maize and cotton showed a more compact structure. Fig. 5d showed FTIR of the hemicellulose extracted from the rape, maize, and cotton straw. As can be seen, the obtained three hemicelluloses showed similar spectrum with the commercial hemicellulose. Peaks at 3340 and 2900 cm−1 corresponded to the O-H and C-H stretching vibration, respectively. Peaks at 1720 cm−1 was ascribed to the C = O stretching vibration in carboxyl groups. The absorption at 1646 cm−1 was associated with the absorbed water, which was also observed in the spectrum of the cellulose. The bands from 1200 to 1500 cm−1 were assigned to the C-H, OH or CH2 bending. The prominent band at 1030 cm−1 correspond to the C–O, C–C stretching or C–OH bending in hemicelluloses, and the weak and sharp peak at 896 cm−1 was ascribed to the ring or the C1 group frequency, and it was the characteristic peak of β-glycosidic linkages between the sugar units (Bian et al., 2012; Fang et al., 1999). Similar hemicellulose spectra were reported by Xiao et al. (2001) and Sun et al. (2004) extracted from the other straw.
3.5 Modification of the raw straws and the extracts of cellulose, lignin, and hemicellulose
Surface modification was occurred through the alcoholysis reaction between anhydride and the hydroxyl groups on the straw and the extracts surface (Tang et al., 2018; Yu et al., 2015). SEM images shown in Figs. 2e-f–4e-f illustrated that morphology of the straws and the extracts did not change after modification, demonstrating that the surface modification reaction was a mild reaction and did not change the materials structure. BET specific surface area of the modified straw and cellulose, lignin, and hemicellulose was determined to be 1.52, 0.51, 0.62, and 12.61 m2/g for rape, 0.75, 0.88, 20.15, and 6.42 m2/g for maize, and 1.60, 0.68, 1.75, and 9.63 m2/g for cotton, respectively, illustrating that the modified straws and the extracts had small specific surface area. FTIR shown in Fig. 5a-d depicted that an obvious new peak at about 1710 cm−1, assigned to C = O stretching vibration, was present in the spectra of all the modified straw and extracts, and peaks corresponding to stretching vibration of C-O at 1240 cm−1 intensified after the modification reaction, which certified the reaction between anhydride and hydroxyl groups. The appearance of asymmetric stretching vibration of carboxyl groups at about 1645 and 1380 cm−1 further demonstrate the success of the surface modification (Zhu et al., 2017). To further determine the changes occurred on the biosorbents surface, EDX analysis of the straws and extracts before and after modification was carried out and results were shown in Table 1. It was observed that oxygen content of the straws and extracts all increased after modification, and hemicellulose showed the largest increase, followed by lignin and straw, which demonstrating that more carboxyl groups were grafted onto the hemicellulose surface after modification, followed by lignin, and the raw straw.
Element
Straw
Modified straw
Cellulose
Modified cellulose
Lignin
Modified lignin
Hemicellulose
Modified hemicellulose
Rape
C
37.81 ± 1.21
37.91 ± 1.77
35.18 ± 1.66
34.47 ± 1.39
49.92 ± 1.55
44.56 ± 2.74
36.71 ± 2.55
34.97 ± 1.24
O
53.96 ± 2.14
61.36 ± 2.56
63.58 ± 2.53
64.37 ± 2.04
47.84 ± 2.51
53.42 ± 2.58
53.28 ± 1.42
63.51 ± 1.42
Maize
C
37.60 ± 2.02
36.24 ± 1.85
34.47 ± 2.66
32.64 ± 1.79
40.56 ± 2.96
36.15 ± 2.13
45.83 ± 2.16
35.99 ± 2.11
O
54.66 ± 2.15
63.11 ± 1.65
64.93 ± 1.38
66.74 ± 1.34
58.48 ± 1.04
62.94 ± 1.09
48.14 ± 2.49
61.02 ± 1.21
Cotton
C
40.07 ± 1.34
40.71 ± 2.04
33.65 ± 2.54
33.19 ± 2.41
42.54 ± 1.01
42.51 ± 1.14
54.94 ± 2.03
34.79 ± 2.15
O
52.05 ± 1.02
58.46 ± 1.12
65.49 ± 2.09
65.76 ± 1.98
55.71 ± 2.34
55.68 ± 2.61
37.03 ± 1.57
60.46 ± 1.04
3.6 Adsorption and desorption of Pb2+ on modified and unmodified straws and the extracts
Adsorption isotherm of Pb2+ on modified and unmodified straw and the extracts of cellulose, lignin, and hemicellulose were carried out under different initial concentration at pH 4.5. Similar adsorption systems were reported by Fadzil et al. (2016) and Wang et al. (2019). Fig. 6 showed the adsorption isotherm of Pb2+ on the modified and unmodified straws and extracts. It was observed that the three raw straws and the same extracts showed similar adsorption capacity for Pb2+. Adsorption capacity (qe) was 54.37, 47.58 and 58.41 mg g−1 for the three raw straws of rape, maize, and cotton. For the three extracts from rape, maize, and cotton, qe was 29.43, 32.68 and 38.26 mg g−1 for the cellulose, 82.08, 66.61 and 73.59 mg g−1 for the lignin, and 104.25, 69.68 and 76.07 mg g−1 for the hemicellulose, respectively. From the results above, it could be concluded that adsorption capacity of the straws and three extracts followed the order: hemicellulose > lignin, straw > cellulose. Hemicellulose showed the highest adsorption capacity for Pb2+ might due to the presence of negatively charged carboxyl groups on its molecular structure, followed by lignin with easily hydrolyzed phenolic hydroxyl group. After modification, adsorption capacity of these biosorbents increased significantly. For example, maximum amount of Pb2+ adsorbed on the modified rape, maize and cotton straws increased to 178.26, 171.58 and 156.78 mg g−1, and that on the modified cellulose, lignin and hemicellulose extracted from the above three straws increased to 123.31, 146.73 and 140.24 mg g−1, 167.17, 222.53 and 201.92 mg g−1, and 330.97, 276.69 and 244.73 mg g−1, respectively. These results illustrated that adsorption capacity of the modified straw and extracts followed the order: modified hemicellulose > modified lignin, modified straw > modified cellulose, which was in the same order with the unmodified one. Above results also showed that the same extracts from the different straws had different adsorption capacity toward Pb2+ due to their different structure. For example, modified cellulose extracted from the cotton had higher adsorption capacity than that from maize and rape, modified lignin extracted from the maize had higher adsorption capacity than that from rape and cotton, while modified hemicellulose extracted from rape had higher capacity than that from cotton and maize. Among the modified straws and extracts, the modified rape hemicellulose showed the highest adsorption capacity for Pb2+ (330.97 mg g−1) due to the spongy structure and the large amount of the increased carboxyl groups, which could be seen from Fig. 2h and Table 1. To clarify the adsorption mechanism on the modified straws and extracts, XPS analysis was determined. Fig. 7 shows Pb4f and O1s XPS of the representative modified hemicellulose before and after Pb2+ adsorption. It was observed that peaks of Pb4f were observed on Fig. 7a, c and e, which demonstrated that Pb2+ was adsorbed on the modified biosorbents (Yu et al., 2007). Fig. 7b, d and f shows that O1s spectra of the modified hemicellulose could be splited into three peaks of COOH, C-OH, and C-O-C. After Pb2+ loaded, peaks of C-OH and COOH shifted from 532.37 and 533.14 eV to higher binding energy of 532.38 and 533.19 eV for rape, from 532.28 and 533.08 eV to 532.33 and 533.15 eV for maize, and from 532.36 and 533.11 eV to 532.45 and 533.21 eV for cotton straw. These phenomena demonstrated that carboxyl and hydroxyl groups were involved in Pb2+ adsorption on the modified biosorbents through electrostatic attraction and surface complex (Liu et al., 2019).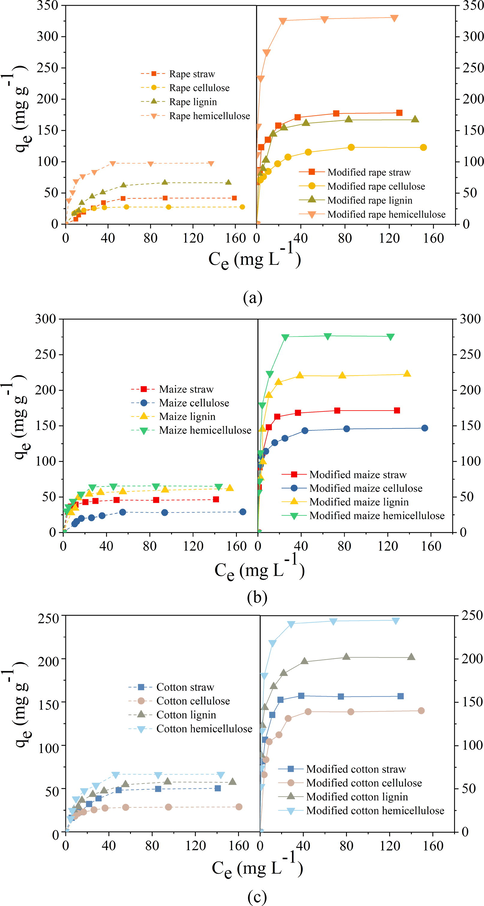
Adsorption isotherms of modified and unmodified (a) rape straw and its extracts, (b) maize straw and its extracts, and (c) cotton straw and its extracts for Pb2+.
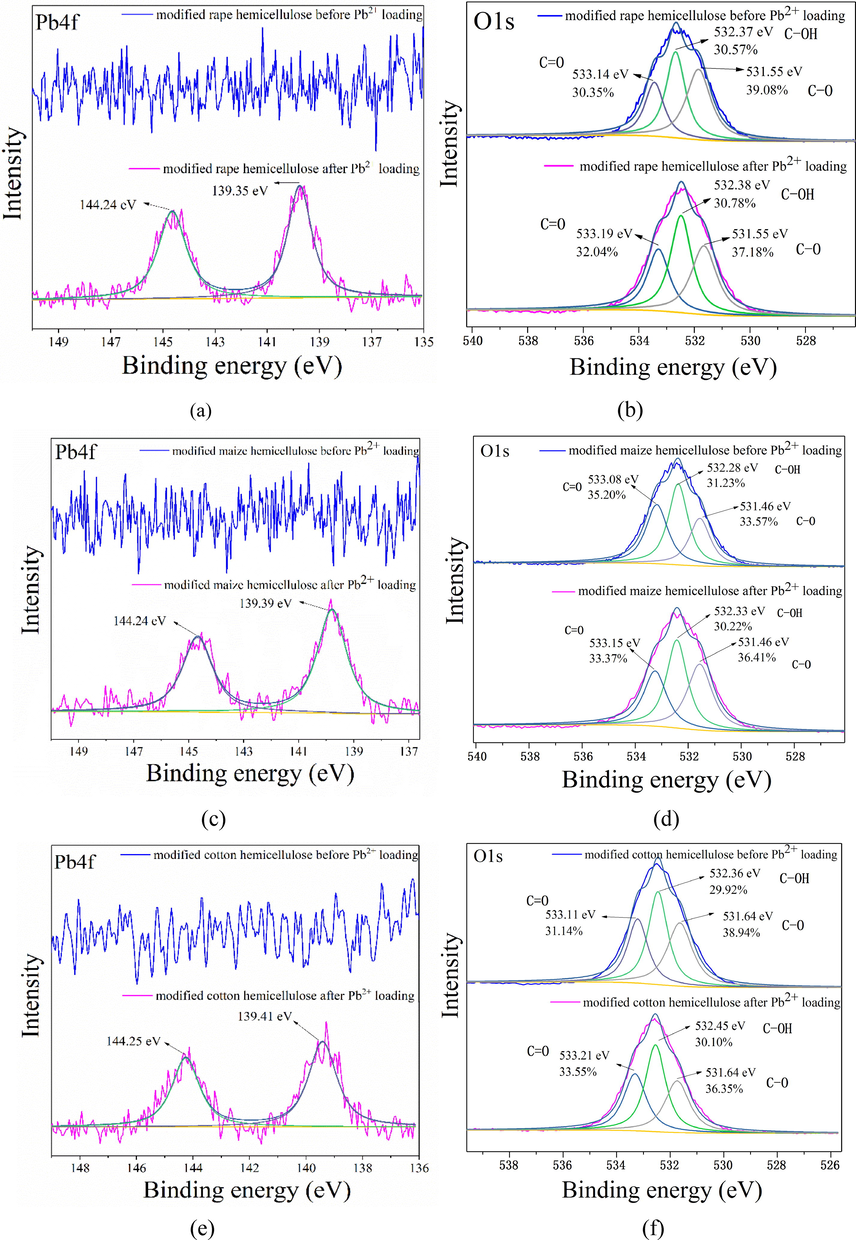
XPS spectra of the modified hemicellulose before and after Pb2+ adsorption.
To better understand the adsorption performance of Pb2+ on the modified and unmodified straws and extracts, the isotherm curves were fitted to Langmuir, Freundlich and Temkin model (Eq. (2)–(4)), where qm (mg g−1) is the maximum amount of Pb2+ on the biosorbents, KL, KF, 1/n, AT, and bT are the adsorption constants.
Results shown in Tables S1-3 illustrated that Langmuir model fitted the curves better than Freundlich and Temkin model, demonstrating that adsorption of Pb2+ on modified and unmodified straw and their extracts were all a mono-layer adsorption. According to the Langmuir model, the predicted adsorption capacity for the modified straw, cellulose, lignin, and hemicellulose was 182.15, 122.79, 172.31 and 320.46 mg g−1 for rape, 174.53, 143.85, 211.88 and 292.68 mg g−1 for maize, and 156.99, 160.00, 199.31 and 257.18 mg g−1 for cotton, respectively, which was very close to the experimental values.
Fig. S1 shows the adsorption kinetic of Pb2+ on the modified straw and extracts. It was observed that the adsorption rate was fast, and the process could complete within 50 min. It was also found that Pb2+ adsorption on the modified biosorbents all predicted well by the pseudo-second order model, and adsorption rate of the modified hemicellulose was the fastest.
To test the desorption efficiency of the adsorbed Pb2+, experiments were carried out by using 0.01 mol L−1 of EDTA-2Na as the eluent. Results showed that desorption capacity for the modified straw, extracted cellulose, lignin, and hemicellulose was 173.04, 117.82, 162.14 and 299.30 mg g−1 for rape, 167.72, 135.51, 195.78 and 270.73 mg g−1 for maize, and 150.86, 150.88, 184.36 and 239.18 mg g−1 for cotton, respectively. Desorption efficiency for the modified straw and the three extracts was 95.0, 96.0, 94.1, and 93.4% for rape, 96.1, 94.2, 92.4, and 92.5% for maize, and 96.1, 94.3, 92.5, and 93.0% for cotton. Above results illustrated that the adsorbed Pb2+ could be desorbed efficiently from the modified straws and extracts, similar results were reported by Chen et al. (2017).
3.7 Effects of pH on adsorption capacity and stability of the modified and unmodified straws and their extracts
pH is an important factor that affects the adsorption performance, it not only controls the surface property of the biosorbents but also affects the species of the metal ions (Júnior et al., 2009). Fig. 8a-c showed the effect of pH on Pb2+ adsorption on the modified and unmodified straws and extracts. It was observed that adsorption capacity of the modified straw and the extracts were all higher than the unmodified one in the whole investigated pH range. For the modified sorbents, Pb2+ adsorption amount increased significantly with the pH increase from 1.0 to 4.0, and then it varied a little with the continuous increase to pH 6.0. The optimum pH range for the modified straws and the extracts was from 4.0 to 6.0, and in this range the biosorbents kept at high adsorption capacity toward Pb2+. High adsorption capacity at high solution pH could be mainly ascribed to the dissociation of carboxyl groups, which made the sorbent surface more negatively charged.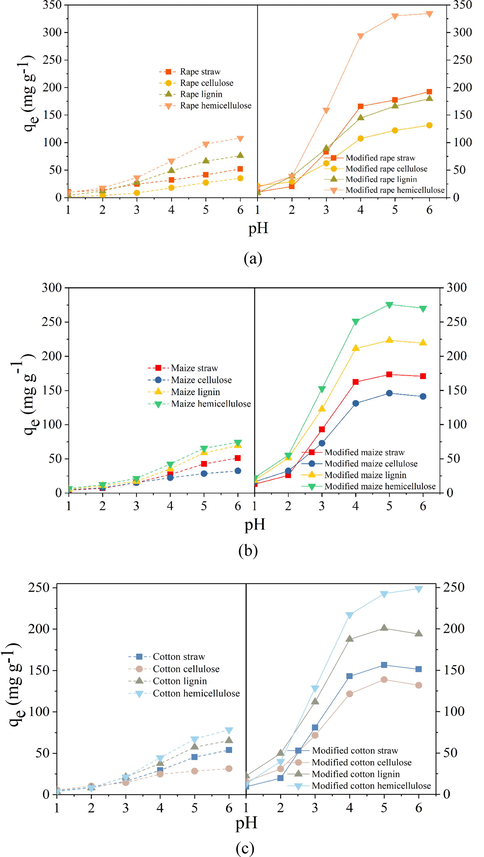
Effects of pH on Pb2+ adsorption on unmodified and modified (a) rape straw and its extracts, (b) maize straw and its extracts, and (c) cotton straw and its extracts.
Since the optimum pH range for Pb2+ absorption was from 4.0 to 6.0, stability of the modified and unmodified straws and extracts in this pH range was determined by testing the amount of organic matter released during the sorption process (Kumar et al., 2011). Fig. 9a-c showed that TOC vales determined from the modified biosorbents were all lower than that from the unmodified biosorbents in the test pH range, which demonstrated that stability of the biosorbents were also improved after surface modification. On the other hand, it also could be seen that TOC values determined at pH 4.0 and 5.0 were much lower than that obtained at pH 6.0, illustrating that the optimum pH range for the biosorbents was from 4.0 to 5.0 from the point of stability. At pH 5.0, TOC values determined from the modified straws and cellulose, lignin and hemicellulose were 4.27, 3.90, 3.90 and 5.10 mg L−1 for rape (Fig. 9a), 5.50, 4.04, 6.01 and 5.66 mg L−1 for maize (Fig. 9b), and 4.10, 1.81, 3.52, 4.81 mg L−1 for cotton (Fig. 9c). Most of the determined TOC values were lower than 5.00 mg L−1, which was the drinking water standard in China.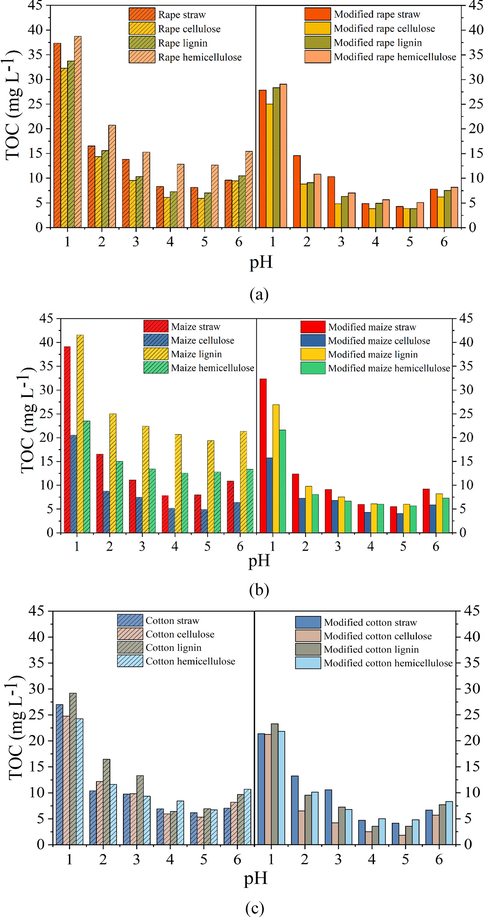
Effects of pH on organic matter release from unmodified and modified (a) rape straw and its extracts, (b) maize straw and its extracts, and (c) cotton straw and its extracts.
3.8 Effects of time on stability of the modified and unmodified straws and their extracts
To further test the stability of the modified straws and extracts, organic matter released in the optimum pH range were tested at different sorption time and results shown in Fig. 10a-c. Within the tested 30 days, amounts of organic matter released from the raw straw, hemicellulose, lignin and cellulose increased steadily from 6.27, 10.29, 5.55 and 3.56 mg L−1 to 8.21, 13.09, 7.02 and 5.61 mg L−1 for rape (Fig. 10a), from 5.36, 9.89, 16.47 and 2.65 mg L−1 to 8.27, 12.32, 20.75 and 5.02 mg L−1 for maize (Fig. 10b), and from 5.84, 5.94, 5.09 and 4.34 mg L−1 to 6.64, 6.45, 6.95 and 5.14 mg L−1 for cotton (Fig. 10c). According to the values of TOC, it was not difficult to find that cotton straw showed the better stability than rape and maize straw, and extracted cellulose showed better stability than the hemicellulose and lignin. After modification, amounts of organic matter released from the straw and extracts decreased obviously, and more importantly, organic matter release was mainly occurred at the first 10 days. TOC values determined from the modified straw, hemicellulose, lignin and cellulose was 4.13, 5.23, 4.77 and 3.37 mg L−1 for rape (Fig. 10a), 6.08, 5.85, 5.49 and 3.95 mg L−1 for maize (Fig. 9b), and 4.41, 4.62, 3.55 and 1.79 mg L−1 for cotton (Fig. 10c) even after adsorption for 30 days. These results illustrated that stability of the modified straws and the three extracts followed the order: modified cellulose > modified lignin, modified straw > modified hemicellulose. Among the three modified straws, modified rape and cotton showed better stability than the modified maize, TOC values of the modified rape straw, cotton straw and their extracts were all lower than 5.0 mg L−1 even after adsorption for 30 days, which reached the drinking water standard in China, demonstrating the safety of using these biosorbents.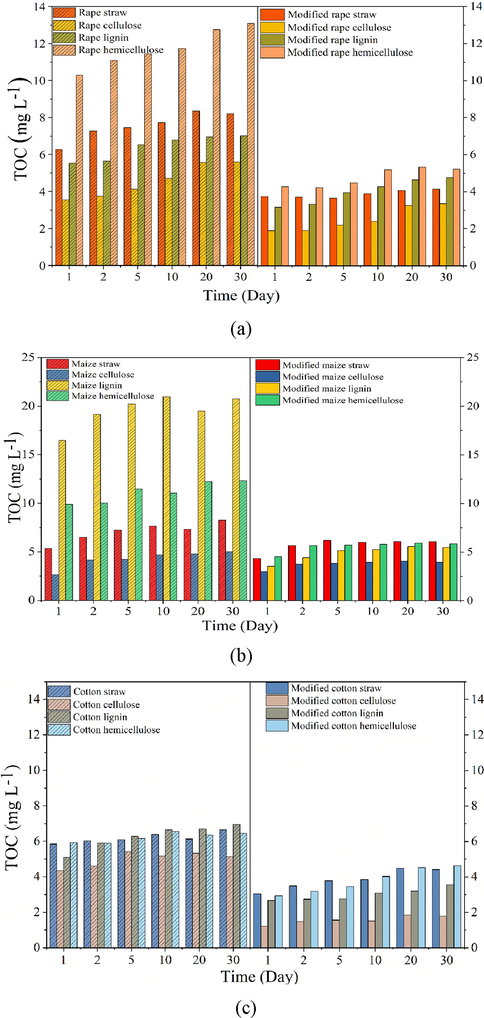
Effects of adsorption time on organic matter release from unmodified and modified (a) rape straw and its extracts, (b) maize straw and its extracts, and (c) cotton straw and its extracts.
4 Conclusion
Cellulose, lignin, and hemicellulose were extracted from rape, maize and cotton straws, and the raw straws and the extracts were surface modified by carboxyl groups. Results showed that the same kinds of straw, cellulose, lignin, and hemicellulose showed similar adsorption capacity toward Pb2+ due to the similar structure, and it followed the order of hemicellulose > lignin, straw > cellulose. After modification, adsorption capacity of the straw and extracts all increased significantly, and that of the modified straw and extracts showed the same sequence with the unmodified one. pH experiments showed that adsorption capacities of the modified straws and extracts were all higher than the unmodified one in the investigated range, and the optimum pH range was from 4.0 to 5.0. Stability experimental results showed that organic matter released from the modified biosorbents were much lower than that unmodified one, and it followed the order: modified cellulose < modified lignin, modified straw < modified hemicellulose. Totally, surface modification could both improve the adsorption capacity and stability of the straw and the extracts of cellulose, lignin, and hemicellulose. Among the modified straws and extracts, modified cellulose showed the best stability while hemicellulose showed the highest adsorption capacity, and that of the modified straws lied between the extracts. Investigation and comparison of the surface modification effect on the adsorption capacity and stability of the waste agricultural straws and its extracts would provide useful information for choosing the biosorbents with good performance in practical waste water treatment.
Acknowledgments
The work is funded by National Natural Science Foundation of China (No. 21978226), the Key Project of Chinese Ministry of Education (No. 213024A) and the Program for Excellent Young Scientific and Technological Innovation Team of Hubei Provincial Department of Education, China (No. T201506).
References
- Anawar H.M., Strezov, V., 2019. Synthesis of biosorbents from natural/agricultural biomass wastes and sustainable green technology for treatment of nanoparticle metals in municipal and industrial wastewater. In: Emerging and nanomaterial contaminants in wastewater, 83-104. doi:10.1016/b978-0-12-814673-6.00004-8.
- From biowaste to bioresource: Effect of a lignocellulosic filler on the properties of poly(3-hydroxybutyrate) Int. J. Biol. Macromol.. 2014;71:163-173.
- [Google Scholar]
- Acid-insoluble lignin and holocellulose from a lignocellulosic biowaste: Bio-fillers in poly(3-hydroxybutyrate) Eur. Polym. J.. 2016;76:63-76.
- [Google Scholar]
- Isolation of hemicelluloses from sugarcane bagasse at different temperatures: Structure and properties. Carbohydr. Polym.. 2012;88(2):638-645.
- [Google Scholar]
- Selective adsorption and recycle of Cu2+ from aqueous solution by modified sugarcane bagasse under dynamic condition. Environ. Sci. Pollut. Res.. 2017;24(10):9202-9209.
- [Google Scholar]
- Cellulose/chitin beads for adsorption of heavy metals in aqueous solution. Water Res.. 2004;38(11):2643-2650.
- [CrossRef] [Google Scholar]
- A wheat straw cellulose-based hydrogel for Cu (II) removal and preparation copper nanocomposite for reductive degradation of chloramphenicol. Carbohydr. Polym.. 2018;190:12-22.
- [Google Scholar]
- Efficient and selective adsorption of multi-metal ions using sulfonated cellulose as adsorbent. Carbohydr. Polym.. 2016;151:230-236.
- [Google Scholar]
- Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng.. 2008;101(5):913-925.
- [Google Scholar]
- Wet oxidation pretreatment of rape straw for ethanol production. Biomass Bioenergy. 2012;39:94-105.
- [Google Scholar]
- Adsorption of lead(II) onto organic acid modified rubber leaf powder: Batch and column studies. Process Saf. Environ. Prot.. 2016;100:1-8.
- [Google Scholar]
- Comparative study of hemicelluloses from wheat straw by alkali and hydrogen peroxide extractions. Polym. Degrad. Stab.. 1999;66(3):423-432.
- [Google Scholar]
- Physicochemical characterization of celluloses extracted from esparto “stipa tenacissima” of eastern morocco. J Appl Polym Sci. 2013;128:537-548.
- [CrossRef] [Google Scholar]
- Characterization of lignin isolated from some nonwood available in Bangladesh. Bioresour. Technol.. 2007;98(2):465-469.
- [Google Scholar]
- Properties of lignin extracted from sugarcane bagasse and its efficacy in maintaining postharvest quality of limes during storage. LWT - Food Sci. Technol.. 2014;57(1):116-125.
- [Google Scholar]
- Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere. 2019;229:142-159.
- [Google Scholar]
- Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD) Carbohydr. Polym.. 2009;77(3):643-650.
- [Google Scholar]
- Application of agro-based biomasses for zinc removal from wastewater - a review. CLEAN - Soil, Air, Water. 2011;39:641-652.
- [CrossRef] [Google Scholar]
- Lin, S.Y., Dence, C.W., 1992. Methods in lignin chemistry. Springer.doi:10.1007/978-3-642-74065-7
- Synthesis of wheat straw cellulose-g-poly (potassium acrylate)/PVA semi-IPNs superabsorbent resin. Carbohydr. Polym.. 2013;94(1):539-546.
- [Google Scholar]
- Performance of Pb(II) removal by an activated carbon supported nanoscale zero-valent iron composite at ultralow iron content. J. Hazard. Mater.. 2019;361:37-48.
- [Google Scholar]
- Microwave pretreatment of rape straw for bioethanol production: Focus on energy efficiency. Bioresour. Technol.. 2011;102(17):7937-7940.
- [Google Scholar]
- High-intensity ultrasound irradiated modification of sugarcane bagasse cellulose in an ionic liquid. Ind. Crops Prod.. 2012;35(1):135-139.
- [Google Scholar]
- Biosorption of lead(II) by chemically modified Mangifera indica seed shells: Adsorbent preparation, characterization and performance assessment. Process Saf. Environ. Prot.. 2017;111:40-51.
- [Google Scholar]
- Cellulose char structure: a combined analytical Py-GC-MS, FTIR, and NMR study. Carbohydr. Res.. 1994;262(1):27-47.
- [Google Scholar]
- Physical–chemical characteristics of lignins separated from biomasses for second-generation ethanol. Biomass Bioenergy. 2014;62:58-67.
- [CrossRef] [Google Scholar]
- Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., et al. 2008. Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory (NREL) Golden, CO.
- Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym.. 2004;56:195-204.
- [CrossRef] [Google Scholar]
- Adsorption of mercury ions from wastewater aqueous solution by amide functionalized cellulose from sugarcane bagasse. Int. J. Biol. Macromol.. 2018;108:1199-1206.
- [Google Scholar]
- Comparative study of hemicelluloses from rice straw by alkali and hydrogen peroxide treatments. Carbohydr. Polym.. 2000;42(2):111-122.
- [Google Scholar]
- Sun, R.C., Sun, X.F., Liu, G.Q., Fowler, P., Tomkinson, J., 2002. Structural and physicochemical characterization of hemicelluloses isolated by alkaline peroxide from barley straw. Polym. Int., vol. 51, 117-124. doi:10.1002/pi.815.
- Novel combined method of biosorption and chemical precipitation for recovery of Pb2+ from wastewater. Environ. Sci. Pollut. Res.. 2018;25(28):28705-28712.
- [Google Scholar]
- Arabinan–cellulose composite in opuntia ficus-indica prickly pear spines. Carbohydr. Res.. 2004;339:123-131.
- [CrossRef] [Google Scholar]
- Carboxyl functionalized Cinnamomum camphora for removal of heavy metals from synthetic wastewater-contribution to sustainability in agroforestry. J. Cleaner Prod.. 2018;184:921-928.
- [Google Scholar]
- Effect of pyrolysis conditions on levoglucosan yield from cotton straw and optimization of levoglucosan extraction from bio-oil. J. Anal. Appl. Pyrol.. 2016;122:294-303.
- [Google Scholar]
- Comparative studies on Pb(II) biosorption with three spongy microbe-based biosorbents: High performance, selectivity and application. J. Hazard. Mater.. 2019;373:39-49.
- [Google Scholar]
- Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab.. 2001;74(2):307-319.
- [Google Scholar]
- The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy. 2014;77:19-24.
- [Google Scholar]
- A simple method to prepare poly(amic acid)-modified biomass for enhancement of lead and cadmium adsorption. Biochem. Eng. J.. 2007;33(2):126-133.
- [Google Scholar]
- Simultaneous removal of cationic and anionic dyes by the mixed sorbent of magnetic and non-magnetic modified sugarcane bagasse. J. Colloid Interface Sci.. 2015;451:153-160.
- [Google Scholar]
- Separation of Pb2+ from Mg2+ by modified sugarcane bagasse under batch and column conditions: Effect of initial concentration ratio. Arab. J. Chem.. 2019;12:4438-4445.
- [CrossRef] [Google Scholar]
- Removal of cationic dyes by modified waste biosorbent under continuous model: Competitive adsorption and kinetics. Arab. J. Chem.. 2019;12:2044-2051.
- [CrossRef] [Google Scholar]
- The delignification effects of white-rot fungal pretreatment on thermal characteristics of moso bamboo. Bioresour. Technol.. 2012;114:437-442.
- [CrossRef] [Google Scholar]
- Preparation of cellulose derived from corn stalk and its application for cadmium ion adsorption from aqueous solution. Carbohydr. Polym.. 2012;90:1008-1015.
- [CrossRef] [Google Scholar]
- Effects of co-ion initial concentration ratio on removal of Pb2+from aqueous solution by modified sugarcane bagasse. Korean J. Chem. Eng.. 2017;34:1721-1727.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.10.024.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







