Translate this page into:
Quantum chemical study of thiaozole derivatives as corrosion inhibitors based on density functional theory
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Quantum chemical and theoretical calculations were carried out in the present study of some thiaozole derivatives. Relationship between electronic parameters of thiaozole derivatives 5-benzylidene-2,4-dioxo tetrahydro1,3-thiazole (5-BDT) 5-(4′-isopropylbenzylidene)-2,4-dioxotetrahydro-1,3-thiazole (5IPBDT), 5-(3′-thenylidene)-2,4-dioxotetrahydro-1,3-thiazole (5-TDT) and 5-(3′,4′dimetoxybenzylidene)-2,4-dioxotetrahydro-1,3-thiazole (5-MBDT) and corrosion inhibition efficiency have been investigated by the Hartree-Fock (HF) and Becke, 3-parameter, Lee-Yang-Parr (B3LYP), M06-2X method with 3-21G, 6-31G, and sdd basis set. All calculations have been performed using the Gaussian 09W suite of programs. The properties most relevant to their potential action as corrosion inhibitors: EHOMO, ELUMO, ΔE (HOMO-LUMO energy gap), electronegativity (χ), chemical potential (μ), chemical hardness (η), electrophilicity (ω), nucleophilicity (ε), global softness (σ) and proton affinity (PA) have been studied.
Keywords
DFT
Thiaozole derivatives
Quantum chemical studies
Corrosion Inhibition
Metal
1 Introduction
Corrosion inhibitory properties of derivatives consisting of five-ring atom groups containing heteroatoms such as nitrogen and sulfur are compared with theoretical methods. Previous studies have shown that the most important features of a good inhibitor are a good electron donor, high purity at low cost (Kaya et al., 2016; Tüzün, 2014; Tüzün, 2019). It appears that Thiaozole and its derivatives 5-benzylidene-2,4-dioxo tetrahydro1,3-thiazole (5-BDT) 5-(4′-isopropylbenzylidene)-2,4-dioxotetrahydro-1,3-thiazole (5IPBDT), 5-(3′-thenylidene)-2,4-dioxotetrahydro-1,3-thiazole (5-TDT) and 5-(3′,4′dimetoxybenzylidene)-2,4-dioxotetrahydro-1,3-thiazole (5-MBDT) have these properties. The corrosion inhibition of copper in acidic solution by Thiaozole and its derivatives as inhibitor has been studied experimentally by Shaban et al. (2015) and they find that 5-IPBDT showed higher inhibition efficiency. Theoretical studies have shown that the molecular structure of inhibitors is very important to be a good inhibitor. According to the HSAB (hard and soft Acid-Base) theorem (Pearson, 1997; Pearson, 1963; Kovačević and Kokalj, 2011), molecules containing sulfur in the structure are in the class of soft bases. Soft chemical species do not show resistance to electron cloud polarization or deformation. As a result, thiaozole and its derivatives give electrons to metal atoms more easily.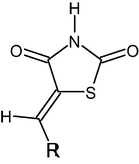
Inhibitor
R
Inhibitor
R
5-BDT
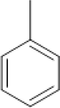
5-MBDT
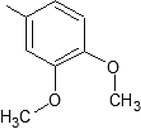
5-IPBDT
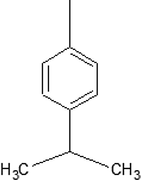
5-TDT
2 Methods
The theoretical methods used to compare the biological and chemical activities of molecules have become more widespread today. There are many programs used to obtain numerical values of chemical activities of molecules. Many parameters and data are obtained with these programs. In this study, these programs whose names are GaussView 5.0.8, Gaussian09 AS64L-G09RevD.01, ChemDraw Professional 15.1, Chemcraft V1.8 package programs were used in the preparation of molecules (Kovačević and Kokalj, 2011; Dennington et al., 2016; Frisch et al., 2009; PerkinElmer, 2012; Chemissan, 2016). These studied molecules were performed by the Hartree-Fock (HF) (Vautherin and Brink, 1972; Becke, 1993) and Becke, 3-parameter, Lee-Yang-Parr (B3LYP) (Wiberg, 2004; Becke, 1993), M06-2X (Hohenstein et al. 2008) method with 3-21G, 6-31G, and sdd basis set. As a result of the calculations made on these basis sets, many parameters can be obtained. HOMO that is Highest Occupied Molecular Orbital and LUMO that is Lowest Unoccupied Molecular Orbital values are used to obtain information about the activities of molecules by using these methods. There are many parameters obtained from quantum chemical calculations and these are called quantum chemical parameters such as EHOMO, ELUMO, ΔE (HOMO-LUMO energy gap), electronegativity (χ), chemical potential (μ), chemical hardness (η), electrophilicity (ω), nucleophilicity (ε), global softness (σ) and proton affinity (PA) (Bilgiçli et al., 2020; Tüzün, 2020; Günsel et al., 2020a; Günsel et al., 2020b; Günsel et al., 2019a, 2019b).
Ionization energy (I) and electron affinity (A) of studied molecules are calculated with HOMO and LUMO energy that are interested Electronegativity, global softness and chemical hardness obtaining the following equations (Brus,1983).
As it is well known that global softness is defined as the inverse of the chemical hardness.
The global electrophilicity index (Parr and Yang,1989) (ω) that is investigated by Parr et al., is the inverse of nucleophilicity and are given in equality (8). Electrophilicity and nucleophilicity are used for the prediction organic and inorganic reaction mechanisms. Nucleophilicity (ε) is defined as the inverse of the electrophilicity in Eq. (9).
3 Results and discussion
Many previous experimental and theoretical studies have shown that molecules with hetero atoms in their structure are more likely to be good inhibitors. Therefore, both experimental studies and theoretical studies have influenced each other very much. It is seen that the experimental studies conducted are more in terms of both time and material. Therefore, theoretical studies have become widespread and have guided experimental studies (Tüzün and Kaya, 2018). The studied molecules are optimized using the gaussian software program. With these calculations, many quantum chemical parameters are obtained. By using these parameters, the corrosion inhibition values of the molecules are compared. All parameters obtained from the quantum chemical calculations are given in Table 1. (see Tables 2, 3, 4, 5).
EHOMO
ELUMO
I
A
ΔE
η
σ
χ
Pİ
ω
ε
dipol
Energy
B3LYP/3–21 g LEVEL
Thiaozole
−6,963
−2,061
6,963
2,061
4,901
2,451
0,408
4,512
−4,512
4,153
0,241
1,428
−20507,636
5BDT
−6,356
−2,278
6,356
2,278
4,078
2,039
0,490
4,317
−4,317
4,570
0,219
2,525
−26758,646
5IPBDT
−5,828
−2,053
5,828
2,053
3,775
1,888
0,530
3,941
−3,941
4,113
0,243
4,694
−32955,536
5MBDT
−6,193
−2,184
6,193
2,184
4,008
2,004
0,499
4,188
−4,188
4,377
0,228
3,288
−29948,631
5TDT
−6,325
−2,247
6,325
2,247
4,078
2,039
0,490
4,286
−4,286
4,504
0,222
1,727
−35445,772
B3LYP/6–31 g LEVEL
Thiaozole
−7,206
−2,446
7,206
2,446
4,761
2,380
0,420
4,826
−4,826
4,892
0,204
1,895
−20611,193
5BDT
−6,492
−2,533
6,492
2,533
3,959
1,980
0,505
4,512
−4,512
5,143
0,194
3,609
−26895,371
5IPBDT
−5,848
−2,335
5,848
2,335
3,513
1,757
0,569
4,092
−4,092
4,765
0,210
4,049
−33124,218
5MBDT
−6,323
−2,435
6,323
2,435
3,887
1,944
0,514
4,379
−4,379
4,933
0,203
4,414
−30101,924
5TDT
−6,473
−2,515
6,473
2,515
3,958
1,979
0,505
4,494
−4,494
5,102
0,196
2,733
−35623,987
B3LYP/SDD LEVEL
Thiaozole
−7,412
−2,747
7,412
2,747
4,665
2,333
0,429
5,079
−5,079
5,530
0,181
2,084
−20612,884
5BDT
−6,685
−2,802
6,685
2,802
3,883
1,941
0,515
4,743
−4,743
5,795
0,173
3,968
−26897,476
5IPBDT
−6,214
−2,613
6,214
2,613
3,601
1,800
0,555
4,413
−4,413
5,410
0,185
6,083
−33127,661
5MBDT
−6,490
−2,683
6,490
2,683
3,807
1,903
0,525
4,587
−4,587
5,527
0,181
4,983
−30104,290
5TDT
−6,665
−2,766
6,665
2,766
3,900
1,950
0,513
4,716
−4,716
5,702
0,175
3,197
−35626,061
HF/3–21 g LEVEL
Thiaozole
−10,013
1,983
10,013
−1,983
11,997
5,998
0,167
4,015
−4,015
1,344
0,744
2,246
−20433,552
5BDT
−8,868
1,331
8,868
−1,331
10,199
5,100
0,196
3,768
−3,768
1,392
0,718
3,567
−26643,058
5IPBDT
−8,386
1,368
8,386
−1,368
9,755
4,877
0,205
3,509
−3,509
1,262
0,792
2,482
−32804,842
5MBDT
−8,630
1,422
8,630
−1,422
10,052
5,026
0,199
3,604
−3,604
1,292
0,774
4,256
−29809,846
5TDT
−8,974
1,389
8,974
−1,389
10,362
5,181
0,193
3,792
−3,792
1,388
0,721
2,188
−35326,025
HF/6–31 g LEVEL
Thiaozole
−10,187
1,557
10,187
−1,557
11,744
5,872
0,170
4,315
−4,315
1,585
0,631
2,805
−20535,602
5BDT
−8,915
1,070
8,915
−1,070
9,984
4,992
0,200
3,922
−3,922
1,541
0,649
4,544
−26777,852
5IPBDT
−8,443
1,125
8,443
−1,125
9,567
4,784
0,209
3,659
−3,659
1,399
0,715
5,519
−32970,777
5MBDT
−8,676
1,163
8,676
−1,163
9,839
4,919
0,203
3,756
−3,756
1,434
0,697
5,288
−29960,859
5TDT
−9,037
1,111
9,037
−1,111
10,149
5,074
0,197
3,963
−3,963
1,548
0,646
3,075
−35501,936
HF/SDD LEVEL
Thiaozole
−10,322
1,209
10,322
−1,209
11,531
5,766
0,173
4,556
−4,556
1,800
0,555
2,862
−20536,918
5BDT
−9,069
0,775
9,069
−0,775
9,844
4,922
0,203
4,147
−4,147
1,747
0,572
4,574
−26779,589
5IPBDT
−8,673
0,836
8,673
−0,836
9,509
4,755
0,210
3,918
−3,918
1,615
0,619
5,295
−32973,792
5MBDT
−8,810
0,904
8,810
−0,904
9,714
4,857
0,206
3,953
−3,953
1,609
0,622
5,474
−29962,962
5TDT
−9,189
0,864
9,189
−0,864
10,053
5,026
0,199
4,162
−4,162
1,723
0,580
3,234
−35503,414
M062X/3–21 g LEVEL
Thiaozole
−8,426
−0,960
8,426
0,960
7,467
3,733
0,268
4,693
−4,693
2,950
0,339
1,590
−20502,460
5BDT
−7,710
−1,360
7,710
1,360
6,350
3,175
0,315
4,535
−4,535
3,238
0,309
2,577
−26750,850
5IPBDT
−7,494
−1,314
7,494
1,314
6,179
3,090
0,324
4,404
−4,404
3,138
0,319
3,290
−32945,182
5MBDT
−7,523
−1,275
7,523
1,275
6,249
3,124
0,320
4,399
−4,399
3,097
0,323
3,281
−29939,201
5TDT
−7,664
−1,288
7,664
1,288
6,375
3,188
0,314
4,476
−4,476
3,143
0,318
1,755
−35437,959
M062X/6–31 g LEVEL
Thiaozole
−8,622
−1,331
8,622
1,331
7,291
3,645
0,274
4,976
−4,976
3,397
0,294
1,980
−20606,232
5BDT
−7,801
−1,602
7,801
1,602
6,199
3,100
0,323
4,701
−4,701
3,565
0,280
3,433
−26887,913
5IPBDT
−7,615
−1,582
7,615
1,582
6,033
3,017
0,331
4,598
−4,598
3,504
0,285
4,005
−33114,198
5MBDT
−7,612
−1,521
7,612
1,521
6,091
3,046
0,328
4,566
−4,566
3,423
0,292
4,155
−30092,866
5TDT
−7,768
−1,547
7,768
1,547
6,221
3,110
0,321
4,658
−4,658
3,487
0,287
2,533
−35616,519
M062X/SDD LEVEL
Thiaozole
−8,830
−1,651
8,830
1,651
7,180
3,590
0,279
5,240
−5,240
3,825
0,261
2,204
−20608,124
5BDT
−8,002
−1,889
8,002
1,889
6,113
3,056
0,327
4,946
−4,946
4,001
0,250
3,805
−26890,351
5IPBDT
−7,473
−1,721
7,473
1,721
5,752
2,876
0,348
4,597
−4,597
3,674
0,272
6,795
−33118,076
5MBDT
−7,775
−1,771
7,775
1,771
6,004
3,002
0,333
4,773
−4,773
3,794
0,264
4,757
−30095,593
5TDT
−7,972
−1,814
7,972
1,814
6,157
3,079
0,325
4,893
−4,893
3,888
0,257
3,022
−35618,945
Metal
EHOMO
ELUMO
I
A
η
χ
Cu
−7,618
1,445
7,618
−1,445
4,532
3,087
Thiaozole
5BDT
5IPBDT
5MBDT
5TDT
ΔN
−6,388
−3,976
−2,664
−3,161
−4,207
Molecule
Metal atom
Complexes
ΔG
Thiaozole
−20535.60198
−44567.32281
−85661.09865
–22.57189
5BDT
−26777.85175
−44567.32281
−58862.34902
39260.67729
5IPBDT
−32970.77710
−44567.32281
−71247.20940
39261.66760
5MBDT
−29960.85913
−44567.32281
−65228.37103
39260.67005
5TDT
−35501.93574
−44567.32281
−65228.44227
50342.75203
Molecule
Metal atom
Complexes
ΔH
Thiaozole
−754.62497
−1637.78717
−85659.15033
–23.23539
5BDT
−984.00859
−1637.78717
−58859.76381
39259.94182
5IPBDT
−1211.58066
−1637.78717
−71243.93821
39260.96786
5MBDT
−1100.97225
−1637.78717
−65225.24781
39259.99442
5TDT
−1304.61227
−1637.78717
−65225.32561
50342.65826
The two most important parameters among these quantum chemical parameters are the HOMO and LUMO of the molecules. The HOMO energy value of the molecules indicates the ability of the molecule to donate electron to metal atoms. indicates whether the molecules are a good corrosion inhibitor (Günsel et al., 2019a, 2019b). The molecule has the highest HOMO energy value and is a good corrosion inhibitor. Because the molecule with the highest value is likely to give electrons to metal atoms. As a result, the probability of the molecule becoming a good inhibitor increases as the probability of giving electrons to the metal atoms of the molecule increases (Kaya et al. 2016). The inhibition efficiency of organic and inorganic inhibitor increases with increasing electron donating ability at the metal surface. On the other hand, the LUMO energy value of the molecule indicates the ability of the molecule to accept electrons (Günsel et al., 2019a, 2019b; Obi-Egbedi et al., 2011; Bhawsar et al., 2018). The lowest energy value of this parameter of the molecule increases the probability of accepting electrons from the metal atom. accordingly, the molecule becomes a better corrosion inhibitor as the probability of accepting electrons increases. It should be well known that molecules can be explained by the HOMO and LUMO energy values to be a good corrosion inhibitor. As a result of calculations, the best inhibitor is 5IPBDT according to the HOMO energy value of the inhibitors. If the best inhibitor is ranked according to the HOMO energy value: Thiaozole < 5TDT < 5BDT < 5MBDT < 5IPBDT. The HOMO, LUMO, and ESP shapes of all inhibitors studied are given in Fig. 1.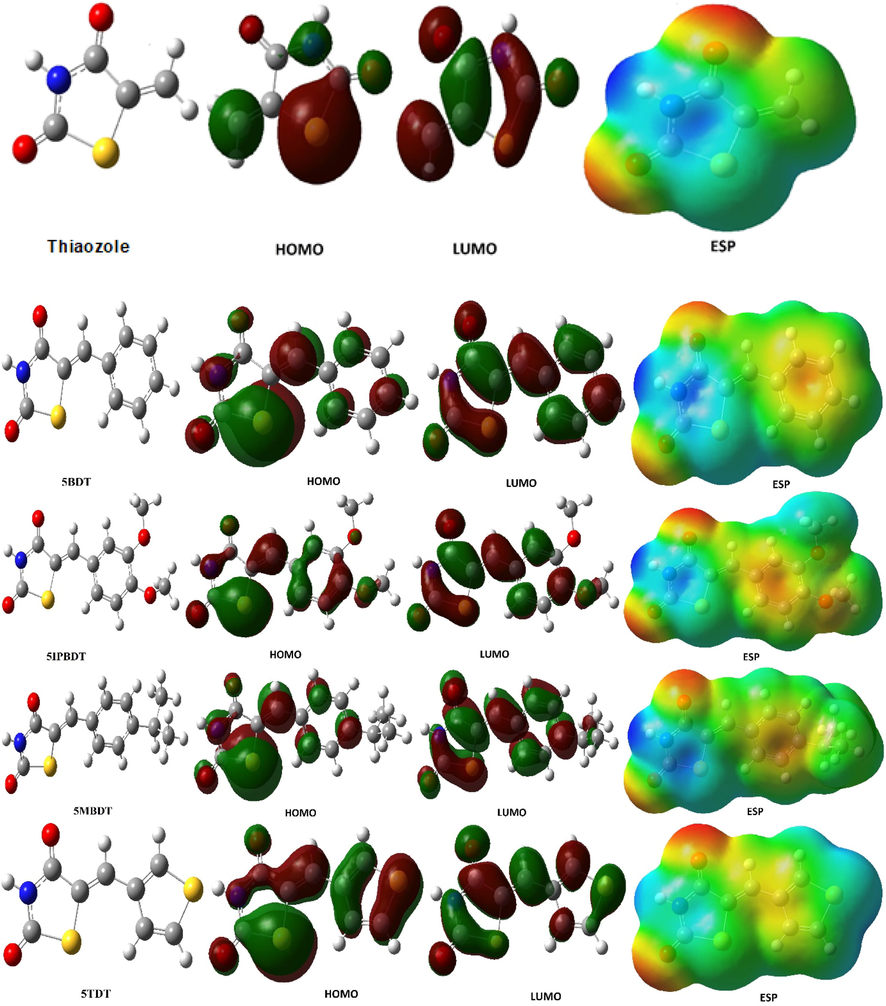
Optimization structures of inhibitors, HOMO, LUMO, and ESP shapes representation.
With the theoretical calculations, the interactions between metal and molecules are examined in more detail. A good corrosion inhibitor must have a strong Lewis base. Because this contains a pair of lean electrons on the base. The electronegativity value of the strong inhibitor gives information about the electron transfer that takes place between the metal and the inhibitor (Verma et al., 2018). The electronegativity value of the inhibitors is used to explain the reactivity in organic and inorganic chemistry. The electronegativity of inhibitors is another important parameter used to explain the inhibitory properties. This parameter shows the power of inhibitors to attract bond electrons in the gas phase. It is understood by the theoretical calculations that the metal atom or the inhibitor will give electrons. From the equation given below, it is clearly seen that as the inhibitor's electronegativity values decrease, the electron transfer value between the metal and the inhibitor increases, according to Sanderson’s electronegativity equalization (10) (Kaya et al., 2016; Bratsch, 1984).
where and are electronegativity of metal and ligand, respectively. and are chemical hardness of ligand wand metal, respectively.
Various quantum chemical parameters of the copper metal atom were calculated using the HF/6-31g basis set. The neutral state of copper metal was calculated. However, these parameters were substituted at equalization 10 and the ΔN value was calculated. As a result of the calculations, it is seen that the electron density is high on the oxygen atom bound to the carbonyl carbon in the Thiaozole ring (Kaya et al., 2016). This is shown in the ESP forms of inhibitors. The red colored region is electron-rich. The blue colored region is electron-poor (Tüzün, 2014). Therefore, these inhibitors are expected to bond with these oxygens when binding with metal atoms. Using Eq. (10), the value of electron transfer between this oxygen atom and the copper metal atom is calculated.
The ΔN values calculated for these five inhibitors can be both negative and positive. If the value of the calculated ΔN of the inhibitors is negative, the copper metal atom supplies the inhibitor with electrons. If the value of the calculated ΔN of the inhibitors is positive, it should be well known that there is an electron transfer from the inhibitor to the copper metal atom (Kaya et al., 2016; Bratsch, 1984). The calculated ΔN value of the inhibitors shows that the whole ΔN value is negative and the copper metal atom has been shown to give electrons to the inhibitors. As a result of the calculations of the ΔN value, it was seen that the most electron transfer value was seen in Thiaozole. On the other hand, the least electron transfer value was seen in 5IPBDT. It is seen that the order of ΔN values and the order of most inhibitors are similar.
An interaction occurs between the copper metal atom of Thiaozole and its derivatives. As a result of this interaction, a chemical event occurs between them. In order to explain these events theoretically, enthalpy and the Gibbs free energy values were calculated. Enthalpy that is symbolized by H, is the sum of all kinds of energies stored in the structure of matter. Gibbs free energy is a thermodynamic variable obtained by subtracting the product of entropy and absolute temperature from enthalpy. In the calculation of these two terms, the following Eq. (11) is used.
where is the energy of the metal complex, is the energy of the Thiaozole and its derivatives and is energy of the metal ion.
The Gibbs free energy value can have three different values. In the first, if this value is greater than zero, this interaction does not happen spontaneously. Secondly, this value is equal to zero, the system is in equilibrium. in the latter, this value is less than zero, the interaction is automatic. On the other hand, the enthalpy value being less than zero indicates that heat is given to the environment during interaction. The reaction enthalpy can be positive or negative depending on the process. In endothermic events (the system takes heat from the environment) the ΔH value is positive (ie ΔH > 0), in exothermic events (the system releases heat to the environment) ΔH is negative (ie ΔH < 0).
In the light of the explanations above, it is seen that the Gibbs free energy values are negative only for thiaozole. In this case it was observed only for Thiaozole. This is not the case for others. On the other hand, when the enthalpy values are examined, it appears that the gene is only negative for Thiaozole. This is not the case for others.
4 Conclusion
As a result of the theoretical calculations, many parameters were obtained. As a result of the calculations made, many theoretical parameters for molecules have been calculated. HOMO and LUMO values of the molecules are at the top of these parameters. However, when the ΔN values of the molecules against the copper atom are calculated, the electron values taken into the molecules are calculated. It was seen that the least electron transfer was at 5IPBDT with −2,664 values. Apart from these, ΔG and ΔH values were calculated. The Thiaozole molecule was calculated to be both ΔG with a value of –22.57189 and ΔH with a value of –23.23539. These values of the Thiaozole molecule are both the most negative and the only molecule that is spontaneous. Given the numerical value of these parameters, all five inhibitors can be good inhibitors against copper metal. but to compare among themselves, the best inhibitor is 5IPBDT. It should be well known that the best inhibitor has been shown to have fewer electron transfer values. The numerical values obtained as a result of the theoretical calculations show that both the EHOMO value and the ΔN value for thiaozole are more negative than the values of other molecules. When the experimental results were compared with these calculations, it was seen that only 5-IPBDT activity was similar. The reason for the difference in sequencing of other molecules is due to too much experimental input in experimental processes. but the theory is done in a completely isolated and pure environment.
Acknowledgments
This research was made possible by TUBITAK ULAKBIM, High Performance and Grid Computing Center (TR-Grid e-Infrastructure).
Declaration of Competing Interest
None.
References
- Density-functional thermochemistry. III. The role of exact Exchange. J. Chem. Phys.. 1993;98(7):5648-5652.
- [Google Scholar]
- Quantum Chemical Assessment of Two Natural Compounds: Vasicine and Vasicinone as Green Corrosion Inhibitors. Int. J. Electrochem. Sci.. 2018;13:3200-3209.
- [Google Scholar]
- The new ball-type zinc phthalocyanine with SS bridge, Synthesis, computational and photophysicochemical properties. J. Photochem. Photobiol., A. 2020;389:112287
- [Google Scholar]
- A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys.. 1983;79:5566-5571.
- [Google Scholar]
- Chemissan, 2016. Version 4.43 package.
- GaussView, Version 6. Shawnee Mission, KS: Semichem Inc.; 2016.
- Gaussian 09, Revision D.01. Wallingford, CT: Gaussian Inc; 2009.
- Comparison of spectroscopic, electronic, theoretical, optical and surface morphological properties of functional manganese (III) phthalocyanine compounds for various conditions. J. Mol. Struct.. 2019;1193:247-264.
- [Google Scholar]
- Synthesis of non-peripherally tetra-substituted copper (ii) phthalocyanines: characterization, optical and surface properties, fabrication and photo-electrical properties of a photosensitive diode. Dalton Trans.. 2019;48(39):14839-14852.
- [Google Scholar]
- Optoelectronic parameters of peripherally tetra-substituted copper (ii) phthalocyanines and fabrication of a photoconductive diode for various conditions. New J. Chem.. 2020;44(2):369-380.
- [Google Scholar]
- Novel biologically active metallophthalocyanines as promising antioxidant-antibacterial agents: Synthesis, characterization and computational properties. J. Mol. Struct.. 2020;1200:127127
- [Google Scholar]
- Assessment of the performance of the M05–2X and M06–2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput.. 2008;4(12):1996-2000.
- [Google Scholar]
- Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study. J. Taiwan Inst. Chem. Eng.. 2016;58:528-553.
- [Google Scholar]
- Analysis of molecular electronic structure of imidazole-and benzimidazole-based inhibitors: a simple recipe for qualitative estimation of chemical hardness. Corros. Sci.. 2011;53(3):909-921.
- [Google Scholar]
- Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some phenanthroline derivatives on mild steel surface. Int. J. Electrochem. Sci.. 2011;6(5649):5675.
- [Google Scholar]
- Density functional theory of atoms and molecules. Oxford: Oxford University Press; 1989.
- Pearson, R.G., 1997. Chemical hardness: applications from molecules to solids.
- PerkinElmer, 2012 ChemBioDraw Ultra Version (13.0.0.3015), CambridgeSoft Waltham, MA, USA.
- Piezogravimetric investigation of heterocyclic compounds as potential inhibitors against copper corrosion in acidic media. Int. J. Corros. Scale Inhib.. 2015;4(4):328-337.
- [Google Scholar]
- Selectivity of Salicylaldoxime and its Derivatives. J. New Results Sci.. 2014;3(5):67-85.
- [Google Scholar]
- Investigation of Benzimidazole Derivates as Corrosion Inhibitor by DFT. Cumhuriyet Sci. J.. 2019;40(2):396-405.
- [Google Scholar]
- Investi̇gati̇on of pyrazoly derivatives schi̇ff base li̇gands and thei̇r metal complexes used as anti-cancer drug. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2020;227:117663
- [Google Scholar]
- Investigation of DNA–RNA molecules for the efficiency and activity of corrosion inhibition by DFT and molecular docking. J. Bio-and Tribo-Corros.. 2018;4(4):69.
- [Google Scholar]
- Hartree-Fock Calculations with Skyrme's Interaction. I. Spherical Nuclei. Phys. Rev. C. 1972;5:626-647.
- [Google Scholar]
- Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: Gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies. Appl. Surf. Sci.. 2018;457:134-149.
- [Google Scholar]
- Basis set effects on calculated geometries: 6-311++ G** vs. aug-cc-pVDZ. J. Comput. Chem.. 2004;25(11):1342-1346.
- [Google Scholar]







