Translate this page into:
Targeting PI3K/Akt/Nrf2 pathway by glabridin alleviates acetaminophen-induced hepatic injury in rats
⁎Corresponding author. njsxl2000@163.com (Xiaolei Shi) njsxl2001@sina.com (Xiaolei Shi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acetaminophen (APAP) induced liver injury is a leading cause for drug-induced liver damage. Glabridin is an isoflavonoid that is reported to possess anti-inflammatory and oxidative stress preventing effects. The aim of this study was to evaluate the protective effects of glabridin against APAP-induced liver injury in rats. Rats were pre-administered with glabridin (30 mg/kg, p.o.; 7 days) followed by single dose of APAP (500 mg/kg, i.p.; 7th day) one hour after glabridin administration. The resulting levels of liver enzymes (AST, ALT, ALP), lipid peroxidation, antioxidant enzymes (CAT, GPx, SOD, GST), reduced glutathione (GSH), inflammatory mediators (TNF-α, IL-6, IL-1β), and apoptosis regulator proteins (casp-3, casp-9, Bcl2, Bax) were determined. Oxidative stress, inflammation and apoptosis triggered due to APAP-induced liver injury were significantly alleviated (p < 0.05) by glabridin. Western blot analysis showed the regulatory effect of glabridin on phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and Nrf2/HO-1 protein expressions against APAP-induced toxicity. Histopathological (H&E) data provided evidence for the protective effect of glabridin on rat liver tissue from injury caused by APAP. In conclusion, the protective effect of glabridin on APAP-induced liver injury was exerted by activating the PI3K/Akt/Nrf2/HO-1 pathway, inhibiting oxidative stress, inflammation, and apoptosis.
Keywords
Glabridin
Apoptosis
Inflammation
Oxidative stress
Liver injury
1 Introduction
Acetaminophen (APAP) is a common analgesic and antipyretic drug which could be obtained over the counter and is widely used. This drug has been reported to cause liver injury and toxicity and has been a leading cause for drug-induced acute hepatic damage and liver failure in many countries (Elshamy et al., 2020). Numerous studies using animal models have demonstrated acute hepatotoxicity upon administration of APAP. The underlying pathogenesis of APAP-induced liver toxicity and injury is not fully elucidated (Kuznietsova et al., 2019). Hence, the mechanisms involved in the pathological events of acute liver injury triggered by APAP are hypothesized to include oxidative stress, inflammation and apoptosis (Neag et al., 2020; Güvenç et al., 2019). The involvement of inflammatory and apoptosis pathways such as nuclear factor-kappa β (NF-kβ) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) have been proven in the pathogenesis of acute liver injury (Abdel-Hamid et al., 2020; Lodhi et al., 2020). Nuclear factor erythroid 2-related factor (Nrf2) is the regulator of cellular antioxidant defense genes, which triggers the activation of downstream genes for antioxidant enzymes like heme oxygenase-1 (HO-1) (Cai et al., 2019). The activation of Nrf2/HO-1 was shown in previous studies using animal models in liver protective mechanism against chemically induced liver injury (Ge et al., 2019; Wang et al, 2016). The activation and regulation of these pathways can possibly prevent the pathogenesis of APAP-induced acute liver injury.
Glabridin is an isoflavonoid that could be found as the major active compound in licorice extract. Licorice extract from the dried roots of Glycyrrhiza species is a common ingredient in Traditional Chinese Medicine practice to treat a number of diseases. Licorice extract has been reported with hepatoprotective and anti-inflammatory activities in carbon-tetrachloride (CCl4)-induced oxidative damage and alcohol induced fatty liver (Huo et al., 2011; Jung et al., 2016). Glabridin being the active ingredient of licorice have also been reported with various pharmacological properties including anti-melanogenesis, anti-cancer, anti-obesity, cardioprotective, and anti-inflammatory effects (Yokota et al., 1998; Simmler et al., 2013; Parlar et al., 2020; Tamir et al., 2000; Huang et al., 2019; Chen et al., 2016; Ahn et al., 2013). Recently, it has been reported that glabridin exerted liver protective effects in diabetic rats against collagen deposits and destruction of hepatocytes (Komolkriengkrai et al., 2019). But there are no reports on the liver protective effects of glabridin against APAP-induced acute liver injury and the mechanisms involved in the prevention of liver damage. Therefore, this study was aimed to discover the role of glabridin in preventing APAP-induced liver injury in rats and its effect on inflammatory and apoptosis signaling mechanisms involved.
2 Materials and methods
2.1 Experimental protocol
Male Sprague-Dawley rats (150–180 g), 4–6 weeks old, were housed in a group of six (n = 6) rats in plastic cages, under 12-hour light/dark cycle at 24 ± 2 °C and acclimatized for a week prior experiment. The animals were given free access to standard food pellet and water ad libitum. The animal studies were performed according to the guidelines stipulated by ethical board of Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China according to the international standards and protocols of National Institute of Health (approval no: 2020A0067390; NIH publication no. 85–23, revised 1985).
Rats were placed in three groups for the experimental procedure; Group I (Normal control group); Group II (APAP 500 mg/kg alone treated model); Group III (glabridin 30 mg/kg + APAP). The dose of glabridin was selected based on preliminary study results. The dose and exposure time of APAP was selected based on the study by Ge et al. (2019) and Mohamed et al. (2020). Glabridin was orally administered in 0.5% tween 80 aqueous solution in a single dose for seven days for rats of Group III, whereas rats of Group I and II received oral administration of 0.5% tween 80 aqueous solution in a single dose for seven days. All animals except normal control rats (Group I) were injected (i.p.) with single dose of APAP on the 7th day, an hour after administration with glabridin or tween 80. After 24 h, the animals were euthanized under anesthesia with 3% isoflurane, liver tissues and blood samples were collected. The liver samples were homogenized in phosphate buffered saline (PBS, pH 7.4) and centrifuged (12 000 g, 10 min, 4 °C). Blood serum was obtained by centrifugation of blood samples (1000 g, 4 °C, 10 min), and stored at −80 °C for further biochemical analysis. A portion of liver from all groups were fixed in 10% neutral formaldehyde.
2.2 Biochemical analysis for liver enzymes and pro-inflammatory markers
Serum alanine aminotransferase (ALT) (cat no. AL7930), aspartate aminotransferase (AST) (cat no. AS7938), and alkaline phosphatase (ALP) (cat no. AP7927) levels were determined using serum liver marker colorimetric assay kits (Biological Technology Company Ltd., Shanghai, China) following standard methods provided by manufacturer. Serum levels of pro-inflammatory cytokines TNF-α (cat no. SRTA00), IL-1β (cat no. SRLB00), and IL-6 (cat no. SR6000B) were analyzed with commercial ELISA assay diagnostic kits (R&D Systems, Minneapolis, US) following the manufacturer’s protocols. The measurement units were standardized for all samples given as units per liter.
2.3 Biochemical analysis of liver oxidative stress markers
Supernatant of liver tissues were used for the determination of lipid peroxidation (MDA formation), reduced glutathione (GSH), antioxidant enzymes (GPx, CAT, GST, SOD) levels in APAP-induced liver toxicity and the effect of glabridin. The oxidative stress markers were determined using standard colorimetric assay kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) following protocols provided by manufacturer. The measurement units for MDA and GSH were given as nmol / mg protein, whereas the measurement units for GPx, SOD, CAT, and GST were given as units / mg protein.
2.4 Western blot analysis
Liver tissues were homogenized in RIPA buffer with protease inhibitor cocktail, centrifuged (12 000 g, 10 min, 4 °C). Supernatants obtained were used for protein concentration measurement using BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). Equal amounts of sample proteins were separated through SDS-PAGE, transferred onto PVDF membrane, blocked with 5% defatted milk, and incubated with specific primary antibodies for Bax (cat no. BS2538)(1:500), Bcl2 (cat no. BS70205)(1:500), casp-3 (cat no. BS61583)(1:500), casp-9 (cat no. BS6444)(1:500), PI3K (cat no. BS60314)(1:500), PI3K (cat no. phospho-Tyr467) (cat no. AP0152)(1:500), Akt (cat no. BS1978)(1:500), Akt (cat no. phospho-Ser473) (cat no. BS9913M)(1:1000), nuclear Nrf2 (cat no. BS1258)(1:1000), HO-1 (cat no. BS6626)(1:1000), β-actin (cat no. AP0060)(1:5000) followed by HRP-conjugated secondary antibodies, anti-rabbit (cat no. BS13278)(1:5000) (Bioworld Technologies, Nanjing, China). Immunoblots were visualized using enhanced chemiluminescent substrate (ECL, Thermo Scientific, USA) and quantified by Image J software (NIH, USA).
2.5 Histopathological and immunohistochemical analysis
The liver sections fixed in formaldehyde were dehydrated in automated tissue processor, embedded in paraffin wax, sectioned to 5 µm thickness, and stained with hematoxylin and eosin (H&E) to be visualized under light microscope with digital camera facilities (Olympus BX-60, Tokyo, Japan). Pathological changes were observed, verified and scored by a blinded pathologist unaware of the experimental groups.
For immunohistochemical analysis, paraffin embedded liver tissue sections were deparaffinized and rehydrated in a gradient of alcohol and xylene solutions. Antigen retrieval was performed using citrate buffer (pH 6.0) then the tissue sections were washed with PBS, incubated with primary antibodies rabbit polyclonal anti-4-HNE (cat no. ab46545)(1:1000), anti-8-OHdG (cat no. ab145595)(1:1000), anti-PGE2 (cat no. ab2318)(1:500), followed by secondary antibodies at room temperature. The tissue sections were stained with diaminobenzidine (DAB)(Dako Rabbit Envision Kit, cat no. K401011) for detection of antibody attachments and counterstained with hematoxylin. Color development in each tissue was visualized under microscope with photographic facility (Olympus BX-60) at 100 × magnification and quantified using optical density reading by Image J software (NIH, USA).
2.6 Statistical analysis
Data are given as mean ± SD of six animals analyzed by GraphPad Prism software 6.0 (ISI, San Diego, USA). Differences between groups were analyzed by one-way ANOVA followed by Bonferonni multiple comparison test. Significant difference was acknowledged at p < 0.05.
3 Results
3.1 Effect of glabridin on APAP-induced liver enzymes
Liver enzymes AST, ALT, and ALP were significantly increased in the serum of APAP-induced rats in contrast to the normal rats (p < 0.05). Levels of AST, ALT and ALP were elevated in the APAP-alone treated group by two folds approximately (p < 0.05) as compared to the normal group. The levels of serum ALT, AST, and ALP in glabridin pretreated rats were significantly decreased compared to the APAP-induced rats (p < 0.05). The results are displayed in Fig. 1. The levels of AST in glabridin treated group was reduced about 43%, levels of ALT about 32%, and ALP about 38%, compared to APAP alone treated group.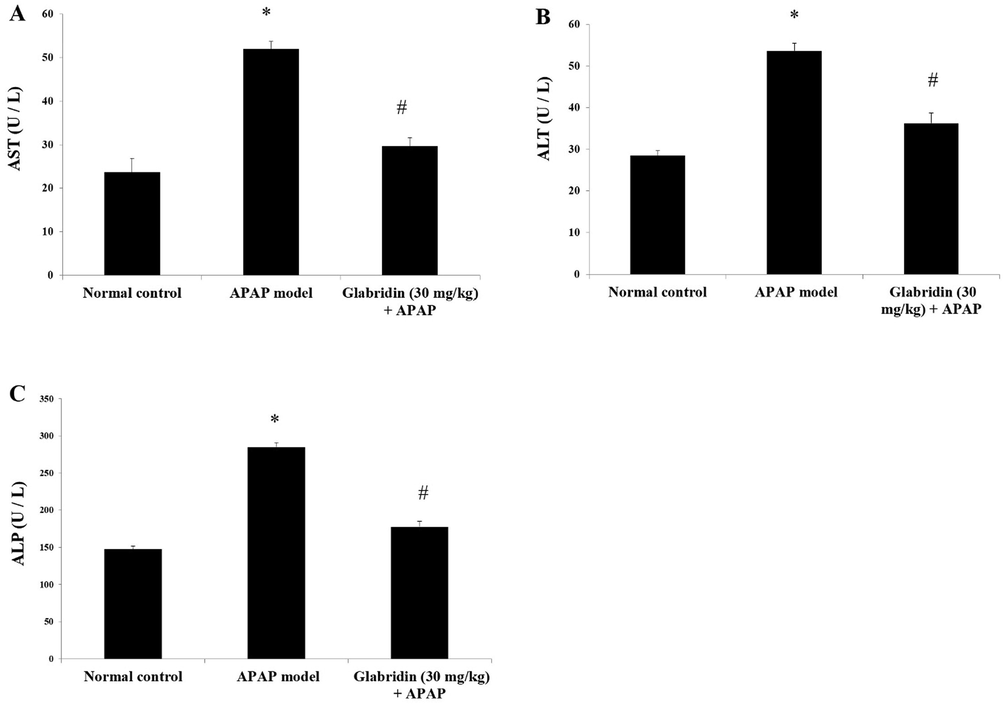
Effect of glabridin on liver enzymes (A) AST, (B) ALT and (C) ALP in APAP-induced rats. Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II.
3.2 Effect of glabridin on APAP-induced inflammatory cytokines
Serum levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in APAP-induced rats were found to have significantly elevated by 117%, 59%, and 61% (p < 0.05) in the ELISA test results as compared to the results of normal control rats. The levels of TNF-α, IL-1β, and IL-6 were significantly suppressed in serum levels of glabridin pretreated group (p < 0.05). The results are shown in Fig. 2. The percentage of reduction of TNF-α in glabridin treated group was 50%, IL-1β was reduced by 27% in glabridin pretreatment, and IL-6 was reduced by 34% in glabridin treated group compared to APAP-induced group.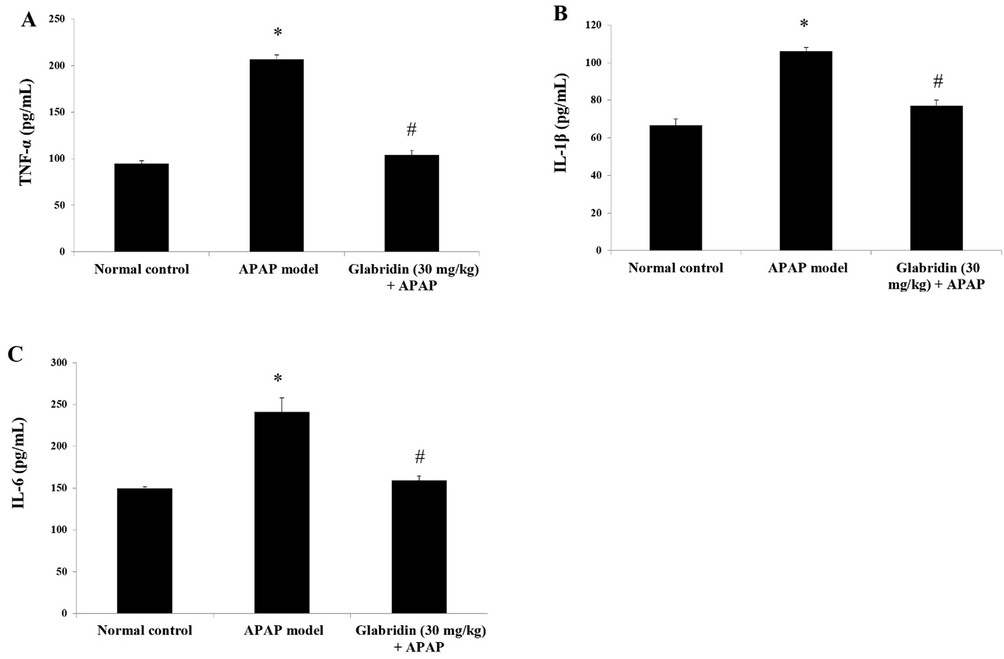
Effect of glabridin on APAP-induced pro-inflammatory cytokines (A) TNF-α, (B) IL-1β and (C) IL-6 in rats. Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II.
3.3 Effect of glabridin on APAP-induced oxidative stress markers
Oxidative stress markers GSH, SOD, CAT, GPx, and GST were significantly reduced by 34%, 48%, 36%, 46%, and 29% (p < 0.05) and the MDA level was increased by 111% (p < 0.05) in liver of APAP-induced rats against normal control rats. It was also evident that glabridin preserved the activities of antioxidant enzymes SOD, GPx, CAT, and GST by 74%, 75%, 51%, and 38% (p < 0.05). Moreover, glabridin treatment elevated GSH level by 39% (p < 0.05), whereas significantly reduced MDA level by 51% (p < 0.05) compared to the APAP-induced rats. The results are shown in Fig. 3.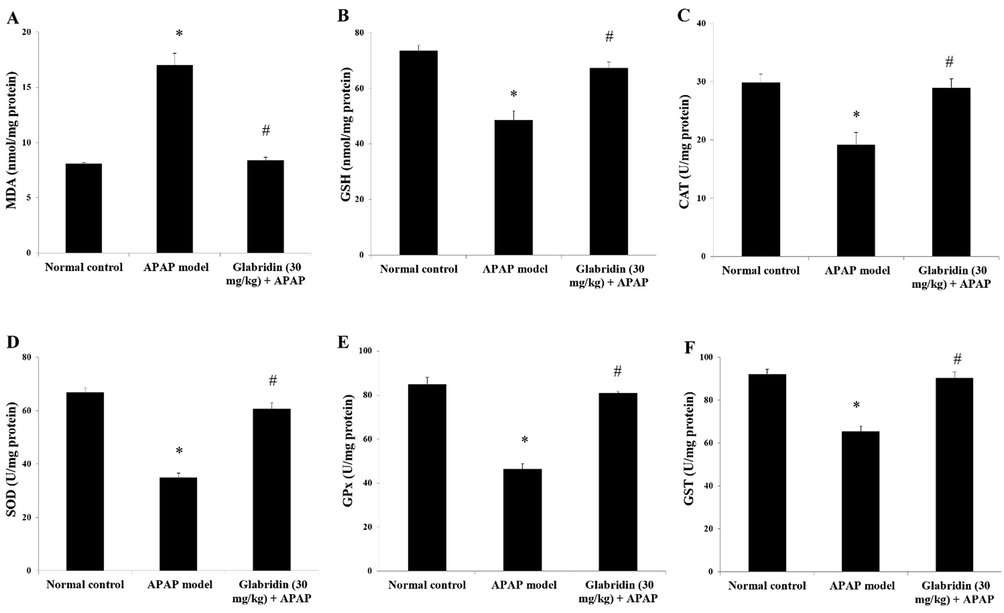
Effect of glabridin on APAP-induced (A) MDA, (B) GSH and antioxidant enzymes (C) CAT, (D) SOD, (E) GPx, (F) GST in rats. Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II.
3.4 Effect of glabridin on APAP-induced protein expressions
The western blot analysis results showed suppressed levels in protein expressions of phosphorylated PI3K, phosphorylated Akt, nuclear Nrf2 and HO-1 approximately by 0.5 folds (p < 0.05) in APAP-induced rats compared to normal control rats. The protein expressions of phosphorylated PI3K, phosphorylated Akt, nuclear Nrf2 and HO-1 were approximately increased by 1.5 – 2 folds (p < 0.05) in glabridin pretreated group compared to the APAP-induced group. The results are demonstrated in Fig. 4.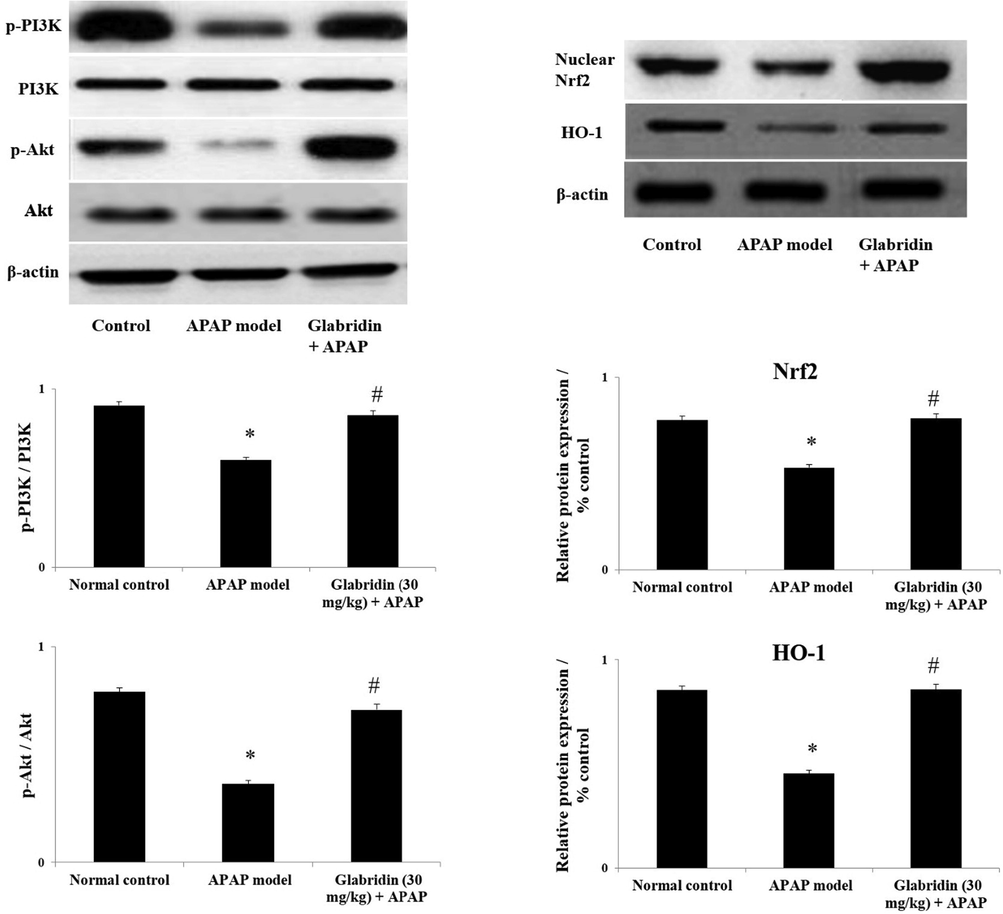
Effect of glabridin on PI3K/Akt and Nrf2 signaling pathways in APAP-induced rats. Protein expressions of PI3K, Akt, nuclear Nrf2 and HO-1 were measured using western blot analysis and β-actin was used as loading control. Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II.
3.5 Effect of glabridin on APAP-induced immunohistochemical modulation
Immunohistochemical analysis of inflammatory biomarkers (PGE2, 4-HNE) and DNA oxidative damage biomarker (8-OHdG) in APAP-induced rat liver showed increased expression of the biomarkers in contrast to the normal control rats. The area percentage of PGE2, 4-HNE, and 8-OHdG expressions in APAP-alone treated group were significantly increased by approximately 5 – 6 folds (p < 0.05). The pretreatment of glabridin significantly prevented the expression of PGE2, 4-HNE, and 8-OHdG biomarkers by approximately 0.5 folds (p < 0.05) as compared to the APAP alone treated rats. The results are shown in Fig. 5.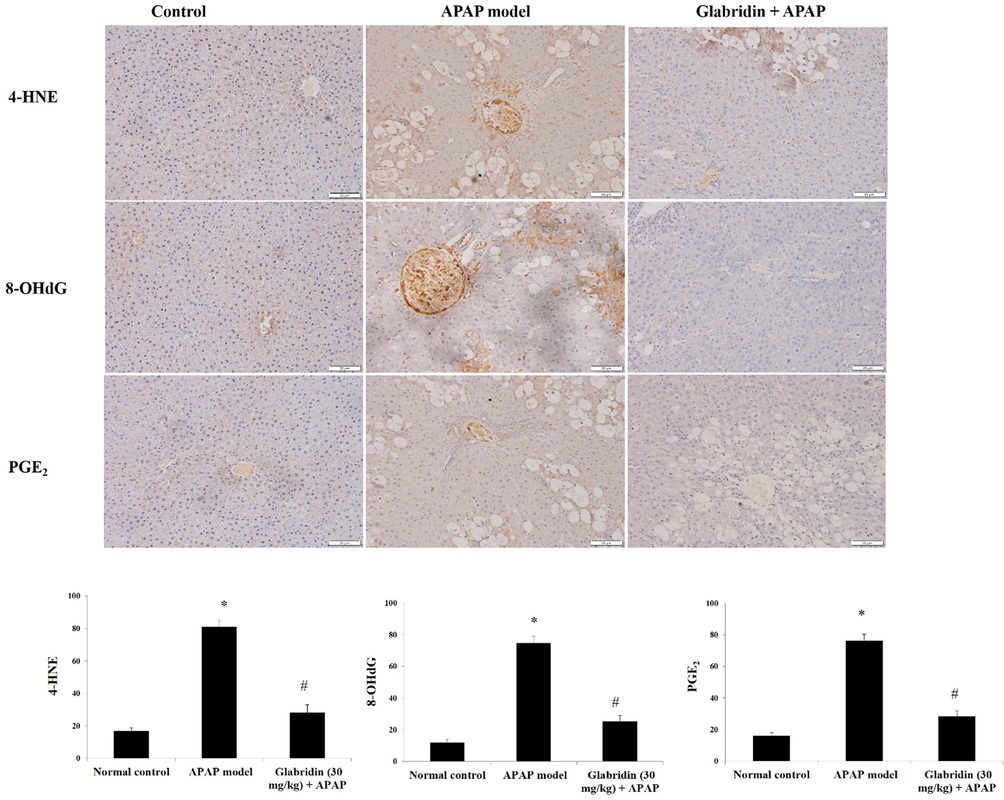
Effect of glabridin on 4-HNE, 8-OHdG, and PGE2 immunohistochemical expressions in APAP-induced rats. Data were shown as mean ± SD (n = 6). Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II. Representation of staining percentage was done based on the quantification using optical density of positively stained areas at 100 × magnification.
3.6 Effect of glabridin on APAP-induced apoptosis and anti-apoptosis proteins
Protein expression levels of apoptosis markers casp-3, casp-9, Bax, and anti-apoptotic Bcl2 were determined through western blot analysis. The expressions of cleaved casp-3, cleaved casp-9, and Bax were significantly increased approximately by 1.5 folds for both caspases and 3 folds for Bax (p < 0.05) in APAP-induced rats whereas Bcl2 was significantly reduced by 0.5 fold (p < 0.05), against normal control group. The protein expressions of cleaved casp-3, cleaved casp-9, and Bax were suppressed approximately by 1 fold for both caspases and 0.5 fold for Bax (p < 0.05) whereas Bcl2 was increased approximately by 2 folds (p < 0.05) in glabridin treated group in contrast with the APAP-induced group. The results are expressed in Fig. 6.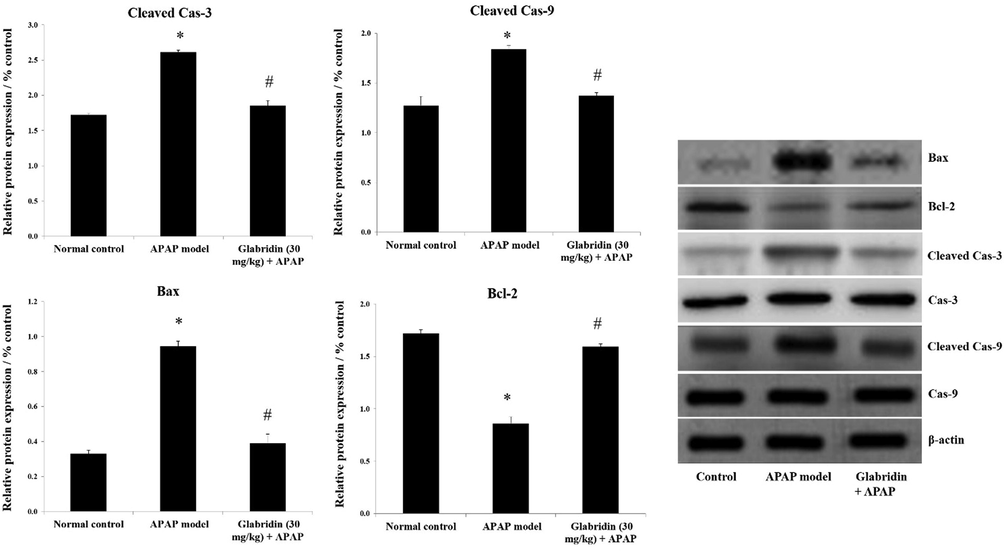
Effect of glabridin on apoptosis regulators in APAP-induced rats. Protein expressions of caspase-3, caspase-9, Bcl2 and Bax were measured using western blot analysis and β-actin was used as loading control. Data were shown as mean ± SD (n = 6). Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II.
3.7 Effect of glabridin on APAP-induced histopathological changes
The effect of APAP on hepatocellular morphology was observed in H&E staining of the liver sections. Normal arrangements of hepatocytes with distinct sinusoids, intact nucleus, healthy cell structures were observed in the normal group rats. In disparity, heavy loss of cellular boundaries, infiltration of inflammatory cells, dilated sinusoids, and vacuolated cytoplasm were observed in APAP-induced rats. Pretreatment with glabridin significantly preserved the hepatocyte arrangements, sinusoid spaces, limited the inflammatory cells infiltration, and minimized the structural damage to hepatocytes as shown in Fig. 7.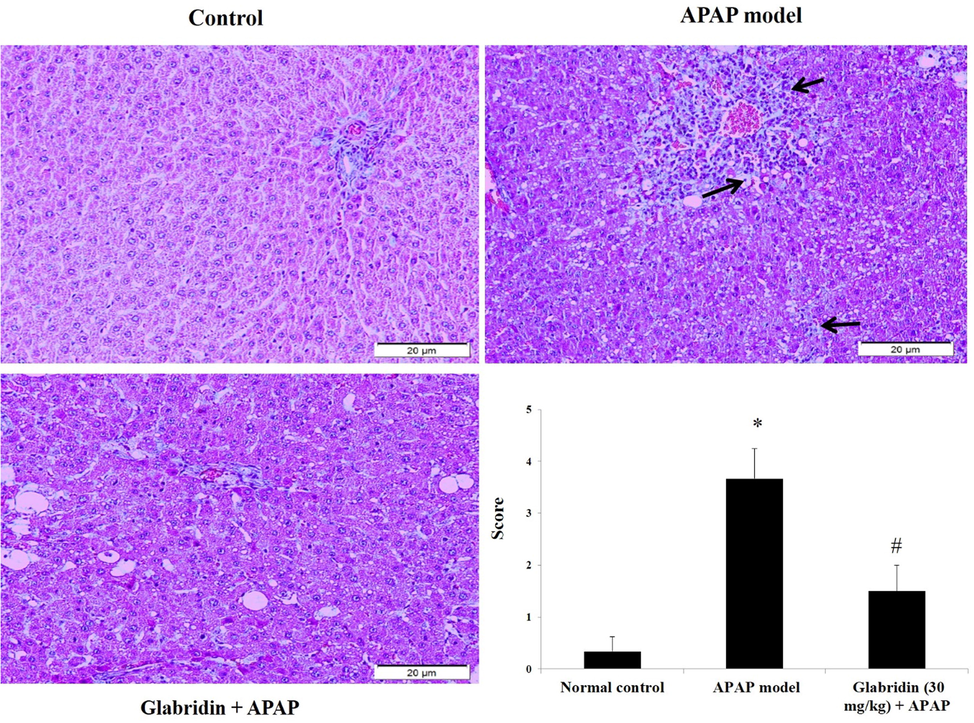
Effect of glabridin on histopathological changes in APAP-induced rats. Injury scores were given by a blinded pathologist on a scale of 0–4; 0 = no changes, 1 = mild changes, 2 = moderate pathological changes, 3 = bad injury and pathological changes, 4 = severe injury and inflammation. Black arrows indicate damaged hepatocytes due to necrosis/apoptosis, infiltration of inflammatory cells, and dilated sinusoids. Data were shown as mean ± SD (n = 6). Data were shown as mean ± SD (n = 6). ‘*’ represents statistical difference (p < 0.05) from normal group I, whereas ‘#’ represents statistical difference (p < 0.05) from APAP-induced group II. Liver tissues were stained with H&E for pathological verification and viewed at 100× magnification.
4 Discussion
Liver is an important organ for metabolism and storage of functional nutrients. Moreover, liver is a vital organ for detoxification of xenobiotics. Drug induced acute liver injury is an alarming condition in many countries around the world. The biotransformation of xenobiotic drugs of synthetic origin in liver by the isoforms of cytochrome P450, forms reactive oxygen species (ROS) which turns deleterious to the hepatocytes (Gnanaraj et al., 2017). APAP associated liver injury has been widely described to be initiated by free radicals or in other words due to the pathogenesis of oxidative stress during biotransformation of the drug. As for the treatment of oxidative stress triggered conditions, antioxidants have become the prioritized choice (Zheng et al., 2017). Bioactive compounds of natural origin have been reported to prevent the progression of ROS related diseases in many occasions (Hasanein & Sharifi, 2017). Glabridin has been reported with antioxidant and anti-inflammatory effects, which supports the efficiency of the compound in this study (Yokota et al., 1998; Simmler et al., 2013; Parlar et al., 2020). It has also been reported that glabridin did not exert toxicity or mortality at 400 mg/kg in acute toxicity test using rodents (Parlar et al., 2020), therefore it is assumed that glabridin alone at 30 mg/kg will not exert toxic effects to the rats. In this study, glabridin exerted significant antioxidant effects by replenishing GSH, restoring antioxidant enzyme activities, and preventing lipid peroxidation against oxidative stress induced by APAP. ROS causing lipid peroxidation leads to the formation of 4-HNE which subsequently triggers the inflammatory reactions and other related pathways. 4-HNE and PGE2 are biomarkers and mediators of inflammation, were clearly expressed in the immunohistochemical results of APAP-induced rats. This can be related to the prevalence of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the serum ELISA test results of APAP-induced rats. These findings are supported by past research that APAP triggers oxidative stress and inflammation experimental models (Mohamed et al., 2020; Mohammadi et al., 2019).
Genes encoding the transcription of inflammatory mediators are regulated by the NF-kβ pathway (Wang et al., 2019). Pretreatment with glabridin remarkably prevented the activation of inflammatory response by suppressing the pro-inflammatory cytokines, reducing the inflammatory biomarkers 4-HNE and PGE2, and reducing lipid peroxidation in APAP-induced rats. APAP-induced oxidative stress also causes cellular DNA damage, confirmed by the heavy expression of oxidative DNA damage biomarker 8-OHdG in the immunohistochemical results. Oxidative DNA damage triggers apoptosis pathways to prevent the progression of cellular injury and ROS from attacking healthy neighboring cells (Leng et al., 2018). The histopathological results further evidence the prevention of membrane lipid peroxidation of hepatocytes by glabridin with preserved cellular boundaries and sinusoids. Liver enzymes AST, ALT, and ALP are normally leaked into the bloodstream when liver tissues are structurally damaged. Presence of high concentrations of liver enzymes in the blood serum indicates excessive liver damage, similar to the findings in APAP alone treated group. Glabridin significantly prevented the structural damage of hepatocytes as shown in the histopathological results, hence the leakage of liver enzymes into the blood was minimized. Presence of inflammatory cells in the histopathological slides of APAP-induced rats indicates inflammatory reaction which supports the ELISA results.
Apoptosis reaction was initiated by APAP in the acute liver injury model in rats (Shu et al., 2020). Pro-apoptotic proteins, casp-3, casp-9, and Bax were upregulated in the APAP-induced rats, whereas the anti-apoptotic Bcl2 was suppressed. Pretreatment of glabridin reversed the effect of APAP by inhibiting the apoptosis reactions triggered by upregulating anti-apoptotic Bcl2 protein and suppressing pro-apoptotic casp-3, casp-9, and Bax proteins. Apoptosis signals in hepatic cells are triggered by the stress levels of organelles like mitochondria, fragmented DNA due to oxidative stress, and activation of apoptosis pathways (Ghosh et al., 2010). The anti-apoptotic signaling protein expressions of PI3K/Akt were suppressed in the western blot results of APAP-induced rats. Pretreatment of glabridin significantly reversed the apoptosis reaction by elevating the protein expressions of PI3K/Akt. These findings were similarly reported by other researchers stating the importance of this regulatory pathway in preventing APAP-induced acute liver injury (Wang et al., 2019; Leng et al., 2018; Yan et al., 2018).
Another important pathway in the prevention of APAP-induced oxidative stress and inflammation is the Nrf2/HO-1. Nfr2 and its downstream gene HO-1 have crucial role in the regulation of antioxidant enzymes and act as defense mechanism to inhibit oxidative stress and inflammatory response (Chen et al., 2019). The Nrf2-ARE pathway has been indicated as potential therapeutic target in the pathophysiology of liver damage through prevention of oxidative stress and inflammation. Activation of Nrf2-ARE pathway regulates specific genes for antioxidant defense such as glutamate-cysteine ligase, sufiredoxin-1, glutathione peroxidase-2, besides activating the stress response HO-1 gene that possesses anti-inflammatory and antioxidant effects thus providing protection against ROS and oxidative stress (Shin et al., 2013; Xu et al., 2019). Apparently, Nrf2 pathway regulates glutathione metabolism and antioxidant response through the activation of Trx1-PI3K/Akt-HIF1-HO-1/Cyclin D1, hence preventing liver damage by oxidative stress and inflammation (Xu et al., 2019; Ahmed et al., 2017). These are common regulators of PI3K/Akt in cell proliferation that has downstream signaling towards Nrf2/HO-1 to hinder oxidative damage and inflammation. It is learned that activation of Keap1/Nrf2-ARE pathway negatively regulate the pro-inflammatory cytokines, inflammatory mediators, their releasing factors and apoptosis mediators, therefore directly or indirectly inhibits the NF-kβ and MAPK pathways during the initial stages of inflammation (Ahmed et al., 2017; Moreno et al., 2020). It was shown in the western blot results that the protein expressions of phosphorylated PI3K/Akt, nuclear Nrf2 and HO-1 were reduced in APAP-induced rats but glabridin was able to upregulate the expressions of the proteins. The hypothesis involving pathophysiology of liver injury triggered by APAP as explained by Neag et al. (2020) and Güvenç et al. (2019) is supported by the findings of this study. APAP treated group exhibited the inactivation of PI3K/Akt/Nrf2/HO-1 pathway, instead the NF-kβ pathway could have been activated since the expression of pro-inflammatory cytokines and oxidative stress markers were significantly elevated compared to the normal control group. The increased expression of GSH, antioxidant enzymes, and suppression of pro-inflammatory cytokines in glabridin treated group evidences the connection of the increased protein expression of PI3K/Akt/Nrf2/HO-1. These results are in harmony with the findings of other researchers (Ge et al., 2019; Wang et al, 2016). Therefore, it can be said the APAP-induced acute liver injury in rats was prevented by the activation of PI3K/Akt/Nrf2/HO-1 signaling pathways. However, further pharmacological research on the curative mechanism of glabridin against drug-induced liver damage is necessary to understand the underlying pathways and specific genes involved in the hepatoprotective action of glabridin.
5 Conclusion
The concluding remarks for this study is glabridin has shown effective liver protection against APAP-induced acute liver injury. The preventive measure exerted by glabridin is evidenced by the activation of PI3K/Akt/Nrf2/HO-1 signaling pathway to hinder oxidative stress, apoptosis and inflammation. Increased activity of GSH / antioxidant enzymes, suppressed pro-inflammatory cytokines, and inhibition of apoptosis inducing proteins, shows that glabridin potentially activated the Keap1/Nrf2-ARE pathway thus inhibiting the activation of NF-kβ / MAPK pathway. Moreover, the histopathological and immunohistochemical results demonstrate the extent of liver protection of glabridin against APAP-induced liver injury. Therefore, this study proves the effectiveness of glabridin as a potential candidate for enhancement of liver ailments. Further studies are needed to ascertain the therapeutic efficacy of glabridin to be developed as a pharmaceutical supplement.
Funding declaration (Funding source)
This research was funded by Nanjing Medical Science and Technique Development Foundation (Grant No. JQX16023, QRX17130).
Author contribution
HM wrote the manuscript and contributed in conceptualization of the study; HR, JW, XY, and XW performed experimental analysis, data collection and statistical analysis; XS edited and finalized the manuscript and provided the experimental protocols.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- C-peptide corrects hepatocellular dysfunction in a rat model of type 1 diabetes. J. Physiol. Biochem.. 2020;76(3):417-425.
- [CrossRef] [Google Scholar]
- Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta. 2017;1863(2):585-597.
- [CrossRef] [Google Scholar]
- Anti-obesity effects of glabridin-rich supercritical carbon dioxide extract of licorice in high-fat-fed obese mice. Food Chem. Toxicol.. 2013;51:439-445.
- [CrossRef] [Google Scholar]
- 7,8–Dihydroxyflavone activates Nrf2/HO–1 signaling pathways and protects against osteoarthritis. Experimental and Therapeutic Medicine. 2019;18:1677-1684.
- [CrossRef] [Google Scholar]
- Inhibitory mechanisms of glabridin on tyrosinase. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2016;168:111-117.
- [CrossRef] [Google Scholar]
- Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Research & Therapy. 2019;21:300.
- [CrossRef] [Google Scholar]
- Shoot aqueous extract of Manihot esculenta Crantz (cassava) acts as a protective agent against paracetamol-induced liver injury. Nat. Prod. Res.. 2020;1–5
- [CrossRef] [Google Scholar]
- Tempol protects against acetaminophen induced acute hepatotoxicity by inhibiting oxidative stress and apoptosis. Front. Physiol.. 2019;10:660.
- [CrossRef] [Google Scholar]
- Arjunolic acid, a triterpenoid saponin, prevents acetaminophen (APAP)-induced liver and hepatocyte injury via the inhibition of APAP bioactivation and JNK-mediated mitochondrial protection. Free Radical Biol. Med.. 2010;48(4):535-553.
- [CrossRef] [Google Scholar]
- Hepatoprotective mechanism of Lygodium microphyllum (Cav.) R.Br. through ultrastructural signaling prevention against carbon tetrachloride (CCl4)-mediated oxidative stress. Biomed. Pharmacother.. 2017;92:1010-1022.
- [CrossRef] [Google Scholar]
- Nobiletin attenuates acetaminophen-induced hepatorenal toxicity in rats. J. Biochem. Mol. Toxicol.. 2019;34(2):E22427.
- [CrossRef] [Google Scholar]
- Effects of rosmarinic acid on acetaminophen-induced hepatotoxicity in male Wistar rats. Pharm. Biol.. 2017;55(1):1809-1816.
- [CrossRef] [Google Scholar]
- Glabridin prevents doxorubicin-induced cardiotoxicity through gut microbiota modulation and colonic macrophage polarization in mice. Front. Pharmacol.. 2019;10:107.
- [CrossRef] [Google Scholar]
- Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int. J. Mol. Sci.. 2011;12(10):6529-6543.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. BMC Complementary and Alternative Medicine. 2016;16:19.
- [CrossRef] [Google Scholar]
- Effect of glabridin on collagen deposition in liver and amelioration of hepatocyte destruction in diabetes rats. Experimental and Therapeutic Medicine. 2019;18:1164-1174.
- [CrossRef] [Google Scholar]
- Water-soluble pristine C60 fullerene attenuates acetaminophen-induced liver injury. BioImpacts. 2019;9(4):227-237.
- [CrossRef] [Google Scholar]
- NF-κB and AMPK/PI3K/Akt signaling pathways are involved in the protective effects of Platycodon grandiflorum saponins against acetaminophen-induced acute hepatotoxicity in mice. Phytotherapy Research. 2018;32(11):2235-2246.
- [CrossRef] [Google Scholar]
- Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. Journal of Traditional and Complementary Medicine. 2020;10(1):60-69.
- [CrossRef] [Google Scholar]
- Apigenin alleviated acetaminophen-induced hepatotoxicity in low protein-fed rats: Targeting oxidative stress, STAT3, and apoptosis signals. J. Biochem. Mol. Toxicol.. 2020;34(5):E22472.
- [CrossRef] [Google Scholar]
- Chrysin effect in prevention of acetaminophen-induced hepatotoxicity in rat. Chem. Res. Toxicol.. 2019;32(11):2329-2337.
- [CrossRef] [Google Scholar]
- Roles of Nrf2 in liver diseases: Molecular, pharmacological, and epigenetic aspects. Antioxidants. 2020;9:980.
- [CrossRef] [Google Scholar]
- Probiotic Bacillus spores protect against acetaminophen induced acute liver injury in rats. Nutrients. 2020;12(3):632.
- [CrossRef] [Google Scholar]
- Glabridin alleviates inflammation and nociception in rodents by activating BKCa channels and reducing NO levels. Biol. Pharm. Bull.. 2020;43(5):884-897.
- [CrossRef] [Google Scholar]
- Role of the Nrf-ARE pathway in liver diseases. Oxid. Med. Cell. Longevity. 2013;2013:763257
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of Citrus aurantium L. against APAP-induced liver injury by regulating liver lipid metabolism and apoptosis. International Journal of Biological Sciences. 2020;16(5):752-765.
- [CrossRef] [Google Scholar]
- Phytochemistry and biological properties of glabridin. Fitoterapia. 2013;90:160-184.
- [CrossRef] [Google Scholar]
- Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res.. 2000;60(20):5704-5709.
- [Google Scholar]
- Tanshinone IIA protects against acetaminophen-induced hepatotoxicity via activating the Nrf2 pathway. Phytomedicine. 2016;23:589-596.
- [CrossRef] [Google Scholar]
- Maltol improves APAP-induced hepatotoxicity by inhibiting oxidative stress and inflammation response via NF-κB and PI3K/Akt signal pathways. Antioxidants. 2019;8(9):395.
- [CrossRef] [Google Scholar]
- The role of Nrf2 in liver disease: Novel molecular mechanisms and therapeutic approaches. Frontiers of Pharmacology. 2019;9:1428.
- [CrossRef] [Google Scholar]
- Dietary alpha-mangostin provides protective effects against acetaminophen-induced hepatotoxicity in mice via Akt/mTOR-mediated inhibition of autophagy and apoptosis. Int. J. Mol. Sci.. 2018;19:1335.
- [CrossRef] [Google Scholar]
- The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res.. 1998;11(6):355-361.
- [CrossRef] [Google Scholar]
- Portulaca oleracea L. alleviates liver injury in streptozotocin-induced diabetic mice. Drug Design, Development and Therapy. 2017;12:47-55.
- [CrossRef] [Google Scholar]







