Translate this page into:
Pre-esterification of high acidity animal fats to produce biodiesel: A kinetic study
⁎Corresponding author. jencinar@unex.es (J.M. Encinar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Biodiesel is generally produced through transesterification with base catalysts, using vegetable oils as raw materials. These vegetable oils can be expensive, being replaced for other alternatives like animal fats. However, animal fats usually present high free fatty acid (FFA) levels, which make the use of base catalysts difficult. Pre-esterification of these raw materials is an effective pre-treatment to reduce FFA levels as free fatty acids react with methanol to produce methyl esters and water. In this research work, the esterification of animal fats was studied, obtaining kinetic parameters through a simplified model. This model, with 1.5 reaction order with respect to FFA, only takes into account the direct reaction of esterification chemical equilibrium, proving that the reaction rate of the inverse reaction is negligible. The reaction rate coefficient, which presents a linear correlation between methanol and the catalyst (H2SO4), was adjusted to temperature through the Arrhenius equation, obtaining the activation energy and the pre-exponential factor. A thorough experimentation has been carried out in order to determine the influence of catalyst concentration, methanol/fat ratio and reaction times corresponding to esterification and transesterification. Reaction times for esterification and transesterification longer than 120 min were enough to obtain biodiesel yields above 97%.
Keywords
Animal fats
Free fatty acids
Biodiesel
Esterification
Kinetic study
1 Introduction
The use of biodiesel as an alternative for fossil fuels has been a reality for decades. Traditional raw materials to produce biodiesel have been vegetable oils, like jatropha, rapeseed, safflower or sunflower, among others, which have been widely studied (Chhetri et al., 2008; Encinar et al., 2020, 2019; Knothe and Razon, 2017; Marjanović et al., 2010; Nogales-Delgado et al., 2019; Sánchez et al., 2019; Voloshin et al., 2016). Apart from frying used oils, which are wastes that are difficult to manage, vegetable oils can be expensive due to the production, collection and extraction process, and there might be concerns about the competition between food and biofuel production, among other economic and environmental issues (Coronado et al., 2009; Knothe and Razon, 2017; Popp et al., 2016). That is the reason why the use of wastes from vegetable oils (like frying oils) or animal fats have resulted in interesting raw materials for biodiesel production, due to their low (or zero) cost and the valorization of contaminating wastes (Adewale et al., 2014; Ramos et al., 2019; Toldrá-Reig et al., 2020). However, animal fats show a high free fatty acid (FAA) content, and therefore, hydrolysis and oxidation reactions can take place in these substances (Banković-Ilić et al., 2014; Demirbas, 2007).
Transesterification reaction between triglycerides and methanol is the most common way to produce fatty acid methyl esters (that is, biodiesel). Homogeneous base catalysts like sodium or potassium hydroxide or methoxide are the most popular catalysts for this process (Athar and Zaidi, 2020).
Under these circumstances, the presence of high-acidity raw materials implies the reaction between free fatty acids and base catalysts, producing soap and the subsequent catalyst loss for biodiesel production (Alajmi et al., 2018). Therefore, pre-treatments are necessary when acidity is high in the raw materials used for biodiesel production. Among the existing options, acid esterification with methanol is the most popular. Thus, methanol reacts with free fatty acids to produce an ester (biodiesel) and water (Demirbas, 2007). Once the pre-treatment is carried out, the remaining triglycerides can react with methanol through acid or base transesterification, to obtain about 100% conversions (Pisarello et al., 2010).
Whereas transesterification process has been widely studied, including different raw materials and operating conditions (Aransiola et al., 2014; Rathore et al., 2016; Singh et al., 2020), esterification as a pre-treatment for biodiesel production has not been completely covered, with most studies dealing with pure free fatty acids (Bart et al., 1994; Goto et al., 1991) or oils that were artificially acidified (Berrios et al., 2007; Marchetti et al., 2011; Pisarello et al., 2010).
Concerning kinetics, several studies have covered the kinetic modeling of the esterification of fatty acids. For instance, different raw materials or wastes, like sunflower oil (Pisarello et al., 2010) or olive pomace (Che et al., 2012), with high acidity, were studied for this purpose. Also, different catalyst were used, and the kinetics of the process was assessed, like for the use of layered zinc laureate and zinc stearate (de Paiva et al., 2015), La3+/ZnO-TiO2 as a photocatalyst (Guo et al., 2021), or hydrochloric acid (Su, 2013). Even the use of microwaves was studied from a kinetic point of view, as in the case of the esterification of free fatty acids from Ceiba pentandra oil seed (Lieu et al., 2016). However, to the best of our knowledge, studies that deal with the pre-esterification of animal fats, including a thorough kinetic study, were scarce.
In this research work, a thorough study was carried out, including a kinetic model for high acidity animal fat esterification. The chemical reactions were done with methanol and sulfuric acid (as alcohol and catalyst, respectively) using a wide range of experimental conditions that include different catalyst and methanol concentrations and different temperatures. This model was applied to animal fats with different fatty acid content. Finally, after esterification, the resulting raw material was tested, carrying out a base transesterification and assessing the characteristics and properties of the biodiesel obtained in order to compare with the equivalent product without pre-treatment.
2 Materials and methods
Animal fats were supplied by a company from Mérida (Extremadura, Spain), called “Extremeña de Grasas, S. A.”. The fats were made up of non-edible fatty wastes from slaughterhouses. The studies about esterification and the influence of temperature and catalyst concentration were carried out with animal fat samples with a free fatty acid (FFA) content of 10.7%, using different reaction temperatures (from 35 to 65 °C), methanol/oil ratios (from 3:1 to 18:1) and different catalyst concentrations (from 0.1 to 2.0% H2SO4).
Also, sulfuric acid esterification, a secondary reaction that can take place during esterification was studied by using a method that was similar to the one described in the literature (Pisarello et al., 2010). According to this method, 250 g of biodiesel was introduced in the reactor, with an acid value less than 0.3 mgKOH·g−1, instead of fats. Once the reaction temperature was reached, the methanol-sulfuric acid mixture was added, following the reaction by taking samples at certain reaction times and measuring acid value. Under these conditions (without esterification), the changes in acid value were only due to the alkylation of sulfuric acid with methanol. Consequently, if the acid value obtained for these conditions (without fats) is subtracted from the acid value obtained for the high acidity fats, the result will provide the change in acid value due entirely to esterification of free fatty acids.
For transesterification studies, additional fat samples were used, with FFA contents of 4.9 and 13.5%. The transesterification reaction had the following conditions: reaction time, 65 °C; catalyst concentration (KOH); methanol/fat ratio, 6:1; reaction time, from 30 to 120 min. Thus, Methanol (95% v/v), KOH and sulfuric acid (98% w/w) were supplied by Panreac (Barcelona, Spain). Ethanol and diethyl ether, used for acid value determination, were analytical grade and supplied by Panreac. For the transesterification experiments that were carried out to compare biodiesel samples (obtained by different conditions), chromatographic standards (fatty acid methyl esters, FAMEs) were used, which were analytical grade and supplied by Sigma-Aldrich (San Luis, USA). Details about the procedure followed and the facilities used can be found in previous works (Encinar et al., 2011).
The esterification reactions were carried out in a 500-ml spherical reactor, equipped with a thermostat, a magnetic stirrer, a condensate system and sample collection. First, 250 g of animal fats was pre-heated in the reactor, to the reaction temperature. When that temperature was reached, a mixture of methanol and sulfuric acid was added, considering that event the initial time of the experiment. At certain time intervals, 1-ml samples were taken to obtain the acid value. This parameter was determined at different reaction times, according to the UNE-EN 14104:2003 standard, by adding 25 ml of ethanol 96°, 25 ml of diethyl ether and phenolphthalein, using a solution of KOH 0.1 N for tritation (UNE-EN 14104:2003, 2003). The experiments were done with a methanol/fat ratio of 6:1, which was a suitable mole ratio to carry out the esterification of fats, as established elsewhere (Encinar et al., 2011).
Finally, a kinetic study about the esterification of free fatty acids from animal fats was carried out, in order to develop a kinetic model for real animal fat transesterification. Thus, a model that considered only the esterification process (with a first-reaction order for the direct reaction and a second reaction order for the reverse reaction) was selected, as explained in following sections. In order to avoid the effect of the catalyst on the kinetic data, the samples collected were pre-washed with water to remove the surplus sulfuric acid and methanol. For the results obtained, the adjustment was obtained by least squares adjustments, showing the coefficient of determination (R2) as a measure of the variation explained by the kinetic model.
3 Results and discussion
In an esterification process of high acidity fats, apart from the esterification reaction, secondary reactions take place, which should be taken into account in the development of a kinetic study. Generally, high acidity raw materials are complex mixtures that, apart from triglycerides and free fatty acids, contain other compounds such as phospholipids, pigments and other insaponifiable compounds. In the literature (Pisarello et al., 2010), these possible secondary reactions were assessed. These reactions, apart from esterification of free fatty acids, include transesterification of tri-, di- and monoglycerides, hydrolysis of glycerides and alkylation of sulfuric acid with methanol. Among the latter, alkylation was the reaction with the greatest impact on the kinetics of the process. In this present work, the variation of acid value due to sulfuric acid alkylation was assessed. Fig. 1 shows the results obtained. The acid value corresponding to free fatty acid esterification was obtained according to the difference between the abovementioned procedure in Materials and Method section (A. V. FFA calculated) and also experimentally through sample collection and prewash with water to remove the surplus sulfuric acid and methanol (A. V. FFA). The coincidence of the two curves points out that it is possible to calculate the free fatty acid concentration during the reaction by using the acid value of the reaction medium and the corresponding and the acid value corresponding to sulfuric acid alkylation.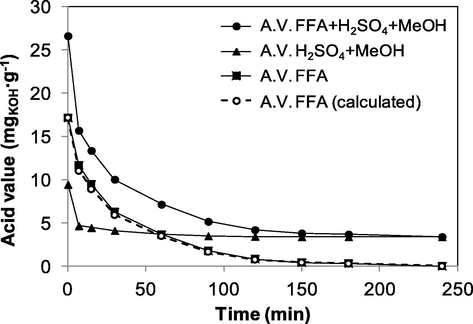
Variation of acid value for different samples. Reaction conditions: catalyst amount = 0.5 wt%, methanol:fats molar ratio = 6:1, reaction temperature = 65 °C.
3.1 Kinetic model
Although there might be secondary reactions, FFA concentration during esterification can be suitably adjusted to a simple kinetic model where the main esterification reaction is considered (Bart et al., 1994; Berrios et al., 2007; Marchetti et al., 2011). In this work, whose aim was the development of a kinetic model for real animal fat esterification, the esterification reaction was only considered, admitting that it is a reversible reaction. The reaction is expressed by Eq. (1), where FFA represents the free fatty acids and FAME are fatty acid methyl esters.
As the reaction is carried out with excess methanol, it can be assumed that the concentration of this reagent remains constant. Under these circumstances, a model was proposed, where the direct reaction was considered a first order reaction and the reverse reaction as a second order one. This model, represented by Eq. (2), was used by Berrios et al.(Berrios et al., 2007) for the esterification of sunflower oil with FFA addition. In this equation, all the concentrations were expressed in mol L−1 and k1 andk2are the pseudo-kinetic constants for the direct and reverse reaction, respectively. In these constants, methanol concentration (k1) and the contribution of sulfuric acid as a catalyst (k1 and k2) are included.
Eq. (2) can be simplified, considering that initial FAME and H2O concentration are zero. On the other hand, as the reaction represents a 1:1 stoichiometry for all its components, the concentrations of all the compounds at a certain reaction time can be written as a function of the initial concentration of free fatty acids ([FFA]O) and conversion (X). These concentrations are given by Eqs. (3)–(5).
Taking into account these premises, Eq. (2) becomes Eq. (6):
The adjustment of experimental data to Eq. (6) has been carried out by applying a differentialmethod of analysis of data through statistics software (MicroMath Scientist). However, the adjustment was not satisfactory, especially for long reaction times. For this reason, a correction was made, assuming a reaction order of M with respect to fatty acid for the direct reaction. Consequently, Eq. (6) becomes Eq. (7).
Fig. 2 shows the adjustment of experimental data to Eq. (7) for an experiment. Data were adjusted with great accuracy, obtainingk1, k2 and M values of 0.0770, 0.0002 and 1.50, respectively. According to these results, k1was 400 times greater than the constant for the reverse reaction, k2. This way, a kinetic model that only considers the direct reaction, like the one represented in Eq. (8), should be taken into account. On the other hand, concerning Eq. (2), it should be noted that the inclusion of water in the reverse reaction is questionable, as water is mainly present in methanol phase, due to the heterogeneous nature of this reaction. As a consequence, only considering the direct reaction can better fit the experimental data.
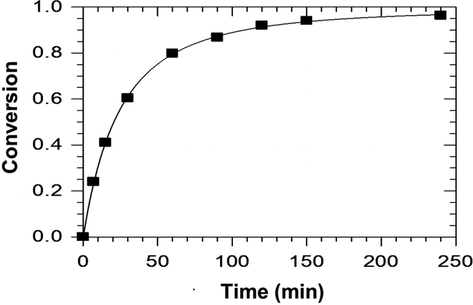
Adjustment of experimental data to Eq. (7) (Catalyst concentration, 0.5% H2SO4; MeOH/fat ratio, 6:1; T, 65 °C).
Following the same procedure (as explained above), Eq. (8) was adjusted. The adjustment provided a R2 value of 0.99995 and a kinetic constant k1value of 0.0770, that is, the kinetic constant was identical to the one obtained previously. Thus, for further experimentation, the direct reaction was only considered, which allowed to work with the integral method, which facilitates the adjustment of experimental data. The integration of Eq. (8) results in Eq. (9), that is, the equation that will be used to study the rest of experiments.
3.1.1 Influence of catalyst concentration
The influence of catalyst concentration on the esterification process has been tested by carrying out four experiments with H2SO4 concentrations between 0.1 and 2.0% with respect to animal fats. These concentrations, expressed in mol·L−1, corresponded to the following values: 0.007, 0.035, 0.071 and 0.142, respectively. In these experiments, MeOH/fat mole ratio was 6:1, and temperature was 65 °C. Fig. 3 shows the evolution of acid value for these experiments. As it can be seen, the catalyst concentration of 0.1% (0.007 mol·L-1) was not enough to produce the esterification reaction, and therefore the acid value was kept constant throughout the experiment. In the rest of the cases, a considerable decrease in acid value was observed, especially during the first minutes of reaction. Afterwards, an asymptotic trend was reached after two hours. Considering the above, three experiments (0.5, 1.0 and 2.0%) were considered for the kinetic study.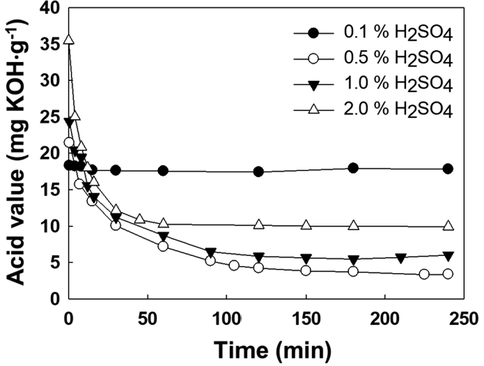
Acid value evolution with catalyst concentration (MeOH/fat mole ratio, 6:1; T, 65 °C).
The results corresponding to the previous experiments were adjusted to the proposed kinetic model (Eq. (9)). For this purpose, the real acid value corresponding to the free fatty acids in the reaction medium was determined, that is, the acidity corresponding to the catalyst was removed. Table 1 shows the results obtained. It can be observed that the kinetic constant increased as the catalyst concentration was higher, which suggests that k1 includes the contribution of the catalyst. Other studies pointed out the same trend as the catalyst concentration increased (Su, 2013). In order to determine this dependence, the linear model represented by Eq. (10) was proposed, where [H2SO4] is the catalyst concentration, expressed in mol·L−1, k’ is the kinetic constant regarding the catalytic contribution, expressed in L1.5·mol−1.5·min−1, and k’’ is the kinetic constant for the non-catalytic contribution, expressed in L0.5·mol−0.5·min−1.
[H2SO4], mol·L−1
k1, L0.5·min−1·mol−0.5
R2
0.035
0.078
0.99
0.071
0.137
0.98
0.142
0.298
0.98
According to Eq. (10), the graphical representation of k1 vs [H2SO4] should be a straight line whose slope and intercept are k’ and k’’, respectively (see Fig. 4). It can be observed a good adjustment, and the value of the intercept (k’’) was small, which points out the poor contribution of the non-catalytic process to the global transesterification reaction.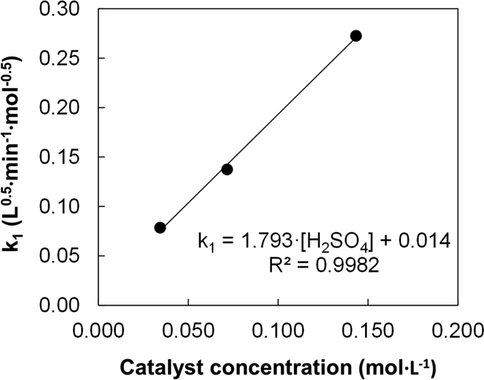
Dependence of k1 with catalyst concentration (MeOH/fat mole ratio, 6:1; T, 65 °C).
3.1.2 Influence of methanol/fat ratio
The influence of this variable was studied through four experiments with different MeOH/fat ratios: 3:1, 6:1, 12:1 and 18:1. Thus, the methanol concentrationsin the reaction medium for these experienceswere 3.25, 5.75, 9.35 and 11.80 mol·L−1, respectively. Also, its concentration in volume was 13.14, 23.24, 37.74 and 47.57% v/v, respectively. For these experiments, the catalyst concentration was 0.035 mol·L−1and the reaction temperature was 65 °C. Fig. 5 shows the evolution of the acid value under these circumstances. The reaction rate increased with methanol concentration and, as a consequence, a decrease in acid value was observed. Apart from the reaction rate, methanol concentration affected the chemical balance, promoting the ester generation as its concentration increased. On the other hand, the higher the amount of methanol, the higher dilution for water was observed, affecting the development of the reaction to a lesser degree (Gerpen et al., 2010; Ghadge and Raheman, 2005).![Acid value evolution with MeOH/fat mole ratio ([H2SO4], 0.035 mol·L−1; T, 65 °C).](/content/184/2021/14/4/img/10.1016_j.arabjc.2021.103048-fig5.png)
Acid value evolution with MeOH/fat mole ratio ([H2SO4], 0.035 mol·L−1; T, 65 °C).
Considering these results, 6:1 mol ratio seemed to be the optimum one, as similar balance values were obtained compared to 12:1 and 18:1, reducing the subsequent costs related to methanol recovery.
As in the previous case, the experimental data were adjusted to Eq. (9). Table 2 shows the values for k1and R2. As it can be observed, k1increased with methanol concentration, as k1 includes methanol concentration (according to the proposed model). The same behavior was observed in the case of free fatty acid content in olive pomace, where % v/v of methanol increased from 15 to 40, showing k values from 0.0104 to 0.0261 (Che et al., 2012). Other authors have observed the same trend when methanol molar ratio increased, as in the case of free fatty acid esterification with hydrochloric acid (Su, 2013). In this table, k1/[MeOH]O values are also included, which should be constant according to the model. These constant values were kept for all the experiments except for the one with 3:1 mol ratio, where the constant concentration for methanol deviated from reality due to the low methanol concentration. For this reason, as previously indicated, methanol concentration was 6:1 or higher, except for the abovementioned experiment.
MeOH/fat ratio
k1, L0.5.min−1.mol−0.5
k1/[MeOH]O L1.5.mol−1.5.min−1
R2
3:1
0.021
0.008
0.97
6:1
0.078
0.016
0.99
12:1
18:10.120
0.1650.015
0.0160.99
0.99
Taking into account the results obtained in the study about the influence of catalyst concentration and methanol/fat mole ratio, k1could be expressed according to Eq. (11), where k represents the real kinetic constant.
3.1.3 Influence of reaction temperature
The influence of temperature was carried out through four experiments whose reaction temperatures were between 35 and 65 °C. For these experiments, catalyst concentration was 0.035 mol·L−1 and methanol/fat ratio was 6:1. Fig. 6 shows the evolution of acid value depending on the reaction temperature. The typical behavior of an endothermic reaction was observed, as the conversion increased with temperature, that is, in accordance with Le Chatelier’s principle, the chemical balance changed towards product generation as temperature increased. On the other hand, as it was observed for the slopes of the curves, the reaction rate increased with temperature. Other authors observed a similar behavior in the literature (Marchetti and Errazu, 2008; Suresh et al., 2017). Indeed, similar temperature ranges were covered in other studies for esterification of free fatty acids, observing a considerable increase in the reaction rate (from 2.3·10−3 to 1.84·10−2) (Su, 2013).![Acid value evolution with temperature ([H2SO4], 0.035 mol L−1; MeOH/fat ratio, 6:1).](/content/184/2021/14/4/img/10.1016_j.arabjc.2021.103048-fig6.png)
Acid value evolution with temperature ([H2SO4], 0.035 mol L−1; MeOH/fat ratio, 6:1).
As in previous sections, the experimental data were adjusted to Eq. (9). The values for k1 and R2 are included in Table 3. As expected, k1increased with the reaction temperature. As this constant included several dependencies, the values for the real kinetic constant k is shown (obtained by applying Eq. (11)), following an increasing trend with temperature.
Temperature, °C
k1, L0.5.min−1.mol−0.5
k, L2.5.mol−2.5.min−1
R2
35
0.016
0.093
0.98
45
0.028
0.160
0.98
55
650.052
0.0780.296
0.4470.99
0.99
The dependence between the kinetic constant and temperature is usually expressed by the Arrhenius equation, whose logarithmic regression is given by Eq. (12), where Ea is the activation energy (kJ·mol−1), R is the ideal gas constant (kJ·mol−1·K−1), T is absolute temperature (K) and Ao is the pre-exponential factor, whose units depend on k.
The representation of Eq. (12) should be a straight line, whose slope and intercept allow the determination of the activation energy and pre-exponential factor, respectively. Fig. 7 shows the abovementioned representation and Table 4 shows the results of the adjustment. The activation energy value fell within the range of values obtained by other authors in the literature, for FFA mixtures with sunflower, palm oil waste and frying oils (Aranda et al., 2008; Berrios et al., 2010; 2007). On the other hand, the activation energy was also similar to the values obtained by other authors for free fatty acids from Ceiba pentandra seed oil (Lieu et al., 2016).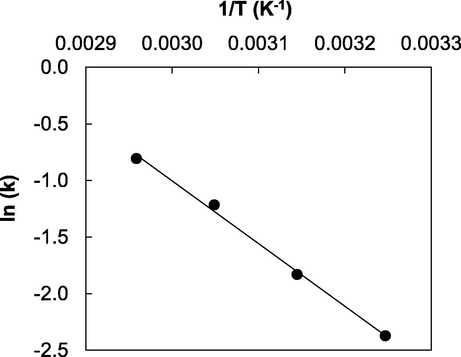
Graphic representation of Arrhenius equation.
AO, L2.5 mol−2.5 min−1
Ea, kJ mol−1
R2
6.106
46.04
0.99
3.2 Reaction time. Two-stage process (esterification and transesterification)
As it was pointed out previously, the esterification stage had the aim of reducing the initial acidity of the sample so that the basic transesterification can be carried out with a high yield in fatty acid methyl esters. The efficiency of the base catalyst used in this second stage greatly depends on the acidity decrease obtained during the esterification stage. Table 5 shows a comparison among different situations (with different reaction times for these stages). The reaction conditions were chosen according to previous works in the literature (Encinar et al., 2011) and the experimental data of this research work.
Run
Esterification time*, min
Final acid value, mgKOH·g−1
Transesterification time**, min
FAME yield, %
1
0
18.84
120
–
2
240
1.08
120
97.3
3
4120
1202.04
2.11120
6097.2
95.7
5
120
1.93
30
92.5
As expected, the absence of esterification prevented the transesterification in the second stage. In this case, such high free acidity provoked the neutralization of the base catalyst (KOH), and transesterification did not take place. The esterification time reduction from 240 to 120 min (Runs 2 and 3) provoked a slight increase in final acid value. Nevertheless, these negligible values, although in the worst-case situation, did not influence on the effectiveness of transesterification and the yield was practically the same for Runs 2 and 3. Concerning Runs 4 and 5, a comparison between different transesterification times was carried out. Compared to Runs 2 and 3, a slight decrease in FAME yield was observed, especially in the case of Run 5 where FAME yield was 92.5%. Thus, according to the above, it can be assumed that esterification and transesterification times of 120 min are enough to obtain high FAME yields.
4 Conclusion
High methyl ester content biodiesel was obtained by a two–step process from high acidity animal fats. The first step was an acid esterification and it was followed by basic transesterification. The experimental conditions were: 0.1–2.0% H2SO4, 35–65 °C, and MeOH:fat ratio from 3:1 to 18:1. As a result, 0.5% H2SO4 allowed the reduction of acid value in animal fats to 2.11 mgKOH·g−1, using 6:1 as MeOH:fat molar ratio, 65 °C and 120 min. These fats were converted into biodiesel (with 97.2% of fatty acid methyl ester content) by basic transesterification with 0.5% KOH, 6:1 MeOH:fat ratio, at 65 °C and 120 min.
The experimental data pointed out that, although there were secondary reactions, it was possible to establish a kinetic model to estimate the free fatty acid content in animal fats during esterification with methanol. With this kinetic model, a reaction order of 1.5 with respect to free fatty acids was obtained. As methanol was in excess, its concentration was included in the pseudo constant rate, which shows a linear evolution with catalyst concentration and methanol/fat ratio. The activation energy obtained was 46.04 kJ·mol−1, which was similar to other values included in the literature. The time required to decrease free fatty acid concentration in animal fats was inferred from the kinetic model and the subsequent kinetic constants, with the initial concentration as the only factor required.
Concerning further research, the simultaneous effects of sulfuric acid, methanol and free fatty acid concentration should be taken into account to assess the possibility of additive or synergistic effects.
Acknowledgements
The authors would like to thank the “Junta de Extremadura” (Ayudas para la realización de actividades de investigación y desarrollo tecnológico, de divulgación de transferencia de conocimiento por los Grupos de Investigación de Extremadura) and the FEDER “Fondos Europeos de Desarrollo Regional (Una manera de hacer Europa)” for the financial support received (GR 18150 and IB18028).
Declaration of Competing Interest
The author declare that there is no conflict of interest.
References
- Rheological, thermal, and physicochemical characterization of animal fat wastes for use in biodiesel production. Energy Technol.. 2014;2:634-642.
- [CrossRef] [Google Scholar]
- Recent trends in biodiesel production from commonly used animal fats. Int. J. Energy Res.. 2018;42:885-902.
- [CrossRef] [Google Scholar]
- Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids. Catal. Lett.. 2008;122:20-25.
- [CrossRef] [Google Scholar]
- A review of current technology for biodiesel production: state of the art. Biomass Bioenergy. 2014;61:276-297.
- [CrossRef] [Google Scholar]
- A review of the feedstocks, catalysts and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng.. 2020;8:104523
- [CrossRef] [Google Scholar]
- Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev.. 2014;32:238-254.
- [CrossRef] [Google Scholar]
- Kinetics of esterification of levulinic acid with n-butanol by homogeneous catalysis. Ind. Eng. Chem. Res.. 1994;33:21-25.
- [CrossRef] [Google Scholar]
- Study of esterification and transesterification in biodiesel production from used frying oils in a closed system. Chem. Eng. J.. 2010;160:473-479.
- [CrossRef] [Google Scholar]
- A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel. 2007;86:2383-2388.
- [CrossRef] [Google Scholar]
- Exploring a promising feedstock for biodiesel production in Mediterranean countries: a study on free fatty acid esterification of olive pomace oil. Biomass Bioenergy. 2012;36:427-431.
- [CrossRef] [Google Scholar]
- Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci.. 2008;9:169-180.
- [CrossRef] [Google Scholar]
- Determination of ecological efficiency in internal combustion engines: the use of biodiesel. Appl. Therm. Eng.. 2009;29:1887-1892.
- [CrossRef] [Google Scholar]
- Esterification of fatty acids with ethanol over layered zinc laurate and zinc stearate – kinetic modeling. Fuel. 2015;153:445-454.
- [CrossRef] [Google Scholar]
- Progress and recent trends in biofuels. Prog. Energy Combust. Sci.. 2007;33:1-18.
- [CrossRef] [Google Scholar]
- Encinar, J.M., Sánchez, N., Martínez, G., García, L., 2011. Study of biodiesel production from animal fats with high free fatty acid content. 102, 10907–10914. https://doi.org/10.1016/j.biortech.2011.09.068.
- Sunflower oil transesterification with methanol using immobilized lipase enzymes. Bioprocess Biosyst. Eng.. 2019;42:157-166.
- [CrossRef] [Google Scholar]
- Biodiesel and biolubricant production from different vegetable oils through transesterification. Eng. Reports.. 2020;1–10
- [CrossRef] [Google Scholar]
- Gerpen, J. Van, Shanks, B., Pruszko, R., Clements, D., Knothe, G., 2010. Biodiesel production technologies. US Department of Energy. National Renewable Energy Laboratory. 1–166.
- Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy. 2005;28:601-605.
- [CrossRef] [Google Scholar]
- Kinetics of the esterification of palmitic acid with isobutyl alcohol. Int. J. Chem. Kinet.. 1991;23:17-26.
- [CrossRef] [Google Scholar]
- Process optimization of biodiesel production from waste cooking oil by esterification of free fatty acids using La3+/ZnO-TiO2 photocatalyst. Energy Convers. Manage.. 2021;229:113745
- [CrossRef] [Google Scholar]
- Kinetic study on microwave-assisted esterification of free fatty acids derived from Ceiba pentandra Seed Oil. Bioresour. Technol.. 2016;211:248-256.
- [CrossRef] [Google Scholar]
- Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenergy. 2008;32:892-895.
- [CrossRef] [Google Scholar]
- Production of biodiesel from acid oil using sulfuric acid as catalyst: Kinetics study. Int. J. Low-Carbon Technol.. 2011;6:38-43.
- [CrossRef] [Google Scholar]
- Kinetics of the base-catalyzed sunflower oil ethanolysis. Fuel. 2010;89:665-671.
- [CrossRef] [Google Scholar]
- Safflower biodiesel: improvement of its oxidative stability by using BHA and TBHQ. Energies.. 2019;12(1940):19-22.
- [Google Scholar]
- Esterification with ethanol to produce biodiesel from high acidity raw materials: kinetic studies and analysis of secondary reactions. Fuel Process. Technol.. 2010;91:1005-1014.
- [CrossRef] [Google Scholar]
- Biofuels and their co-products as livestock feed: global economic and environmental implications. Molecules. 2016;21:1-26.
- [CrossRef] [Google Scholar]
- Biodiesel production processes and sustainable raw materials. Energies. 2019;12:1940.
- [CrossRef] [Google Scholar]
- Processing of vegetable oil for biofuel production through conventional and non-conventional routes. Energy Sustain. Dev.. 2016;31:24-49.
- [CrossRef] [Google Scholar]
- Biodiesel production from castor oil by two-step catalytic transesterification: optimization of the process and economic assessment. Catalysts. 2019;9(10):864.
- [CrossRef] [Google Scholar]
- A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel. 2020;262:116553
- [CrossRef] [Google Scholar]
- Kinetic study of free fatty acid esterification reaction catalyzed by recoverable and reusable hydrochloric acid. Bioresour. Technol.. 2013;130:522-528.
- [CrossRef] [Google Scholar]
- Esterification of free fatty acids in non-edible oils using partially sulfonated polystyrene for biodiesel feedstock. Ind. Crops Prod.. 2017;95:66-74.
- [CrossRef] [Google Scholar]
- Trends in biodiesel production from animal fatwaste. Appl. Sci.. 2020;10:3644.
- [CrossRef] [Google Scholar]
- UNE-EN 14104:2003, 2003. Oil and fat derivatives. Fatty Acid Methyl Esters (FAME). Determination of acid value.Spanish Association of Standardisation-UNE.
- Review: Biofuel production from plant and algal biomass. Int. J. Hydrogen Energy.. 2016;41(39):17257-17273.
- [CrossRef] [Google Scholar]







