Translate this page into:
Degradation of perfluorosurfactant in aqueous solution using non-thermal plasma generated by nano-second pulse corona discharge reactor
⁎Corresponding author at: Department of Chemistry, College of Science, University of Sulaimani, Qlyasan Street, Sulaimani City 46001, Kurdistan Region, Iraq. kosar.hamaaziz@univsul.edu.iq (Kosar Hikmat Hama Aziz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Perfluorosurfactants (PFS) are global environmental pollutants and are considered as a new anthropogenic type of carcinogenic contaminant that remains untreated by conventional wastewater treatment plants. These organic surfactants are typically refractory, non-biodegradable, and pose great threats to environmental safety and human health. The gradual increase in PFS product consumption by various industries resulted in their abundant occurrence in the ecosystem. Non-thermal plasma-based advanced oxidation processes with powerful oxidation ability appeared to be applicable for the degradation of refractory organic pollutants and enhancing biodegradability. In this work, the decomposition of Perfluorooctanesulfonic acid (PFOS) in water by the application of nano-second pulse corona discharge under oxygen was examined. Working corona discharge under oxygen, argon, and helium has no effects on the decomposition rate. The energy yields at 50% conversion (G50) of about 1 mg L−1 initial PFOS concentration was estimated as 22 mg/kWh under argon or oxygen and 27 mg/kWh under helium. The energy yield G50 for initial concentrations of 7.5 and 18.5 µg L−1 in helium and oxygen was estimated as 0.20 and 0.33 mg/kWh, respectively. A considerable difference in the generation of short perfluoroalkyl acids by-products and fluoride ions was observed when corona discharge was conducted under oxygen and much more of these by-products are formed. A synergistic effect was observed in the presence of other surface-active compounds. Degradation efficiency was enhanced by 10% after the addition of surface-active sulfobetaine into the medium following the same experimental procedure.
Keywords
Non-thermal plasma
Perfluorooctanesulfonic acid
Sulfobetaine
Nano-pulse corona discharge
1 Introduction
Perfluorooctanesulfonic acid (PFOS) is a well-known per-and polyfluoroalkyl substance that consists of a long hydrophilic perfluorinated carbon chain and a hydrophilic sulfonate functional group. These unique different amphiphilic properties allow PFOS to have excellent properties of lowering the surface tension of aqueous solution and enable them to be widely used in various industrial applications such as surface treatment of paper, fabric protection, optoelectronic industries, electroplating industries, and firefighting foams (Lu et al., 2020; Du et al., 2014). PFOS have high chemical and thermal stability and are non-flammable, and recalcitrant towards the majority of oxidizing and reducing species due to the strong bonding energy of C-F in fluorocarbon (C–F, 485 kJ/mol), high redox potential (F to F−, E° = 3.6 V) and perfect orbitals overlap (2s and 2p) (Lu et al., 2020; Trojanowicz et al., 2018). These properties make PFOS non-biodegradable pollutants that cannot be removed by conventional wastewater treatment technologies. As a result, PFOS can be accumulating in the aquatic environment and be converted to carcinogenic and harmful contaminants (Gao et al., 2021; Nguyen et al., 2020). Their widespread application may produce long-term and extensive harm to animals and human health such as damages of organs, immune systems disorder, and even carcinogenic effects, which have been confirmed in many reports (Fujii et al., 2007; Liu et al., 2012; Groffen et al., 2018). Thus, they have already been classified as persistent organic pollutants. Because of their resilience to chemical and biological degradation, these chemicals are found in surface waters and nature and accumulated in the food chain (Houde et al., 2006). Therefore, water treatment techniques with good performance and high efficiency for the removal of PFOS are urgently needed.
Currently, numerous treatment technologies have been developed and applied for removing different organic pollutants such as surfactants, pesticides, organic dyes, and pharmaceutical residues from water. Advanced oxidation processes (AOPs) have been the most significant ones that can decompose most organic pollutants efficiently via the generation of highly reactive and non-selective hydroxyl radicals (Aziz, 2019; Aziz et al., 2018, 2019; Lado Ribeiro et al., 2019). Great scientific interest is associated with the application of non-thermal plasma-based AOPs in the remediation of environmental pollution, especially in wastewater treatment. The advantages of these methods are short processing time, no addition of chemicals, low operational temperature, high energy efficiency, environmental compatibility, and efficient removal of recalcitrant organic pollutants (Liu et al., 2018; Ceriani et al., 2018; Magureanu et al., 2015; Aziz et al., 2018). The degradation processes are mostly due to their ability to produce an in-situ variety of powerful oxidizing species such as ozone, hydrogen peroxide, atomic oxygen, hydroperoxy radicals, superoxide anions, UV-light, and mainly hydroxyl radicals. The synergistic effect can be observed when non-thermal plasma is applied in combination with other degradation systems such as persulfate activator in the presence of ferrous ions (Shang et al., 2019; Shang et al., 2017). Ozonation and UV-based photolytic treatment methods have proven to be ineffective for the degradation and mineralization of PFOS due to the presence of strong carbon-fluoride bonds (Trojanowicz et al., 2018). Among the new techniques that can be applied for the removal of PFOS such as persulfate activation (Liu et al., 2012); sonolysis (Campbell et al., 2009), and photocatalysis (Wang et al., 2017; Chen et al., 2016), non-thermal plasma has shown the highest removal rate and promising method in the removal of PFOS in comparison with other techniques (Nzeribe et al., 2019). The application of non-thermal plasma for the removal of surfactants based on various discharge generators has been investigated in many reports (Lee et al., 2020; Mahyar et al., 2019; Jovicic et al., 2018). Saleem et al. have investigated the removal of perfluorooctanoic acid using three reactor designs and implementing different plasma regimes. The results based on the obtained energy yields and removal rate have shown the self-pulsing streamer discharge was found to be the most powerful technique (Saleem et al., 2020). Singh et al. have used a slow treatment reactor based on a non-thermal plasma treatment technique to increase by-product generation and propose a degradation pathway for perfluorinated alkyl Substances as well as detection the produced cyclic perfluoroalkanes in the gaseous phase of the plasma treatment process (Singh et al., 2019). The efficiency of plasma-based water treatment for perfluorooctanoic acid transformation and defluorination was evaluated under two sets of parameters, high removal rate by using high input power and high removal efficiency by using far lower input power (Stratton et al., 2017). Pilot-scale enhanced contact plasma discharge reactors under argon have been investigated for the treatment of real wastewater with low concentrations of up to a few hundred of ng L-1 (Singh et al., 2019; Singh et al., 2020). Among different configuration designs of non-thermal plasma reactors, nano-pulse corona discharge is found to be a suitable and efficient technique to degrade PFOSs. (Mahyar et al., 2019).
This study aims to demonstrate the effectiveness of non-thermal plasma generated in a nano-pulse corona discharge reactor under oxygen for the degradation of PFOS in water with realistic concentrations (10 µg PFOS per liter). In addition, the effect of a special Fe (II) containing surface-active sulfobetaine on the enhancement of the concentration of PFOS in the plasma zone aiming to increase the degradation efficiency has been investigated. Furthermore, the presence of iron (II) in the sulfobetaine composition may induce additional Fenton oxidation with generated hydrogen peroxide during the plasma discharge and enhance the degradation efficiency and kinetics.
2 Material and methods
2.1 Materials
PFOS (95%) was obtained from Riedel-de Haën. Deionized water (≤0.1 µS/cm) was used to prepare a stock solution of PFOS. All chemicals and reagents were used as received. The synthetic Fe (II) containing surface-active sulfobetaine was prepared by Dr. Horst Seibt (IONYS AG, Berlin, Germany). Deionized water was used to prepare other reagents.
2.2 Experiments
The nano-pulse corona discharge reactor has been designed as illustrated in Fig. 1. This design has been successfully used for the degradation of chloroacetic acid under different gas atmospheres (Aziz et al., 2019). This reactor design was also applied in our published work for the degradation of perfluorosurfactants in aqueous solutions (Mahyar et al., 2019). The high voltage electrodes are made of a circuit board equipped with ca. 2000 tinned brass pins, each with 1.3 mm diameter and 7 mm length, directed toward the water surface. When the reactor was loaded with 5 L of polluted water at a flow rate of 1 L/min, the gap between brass pins and the water surface was 4 mm. The ground electrode is made of a stainless steel sieve fixed inside the solution at the bottom of the reactor (Fig. 1). A FPG 30-2NM20 nano-pulse generator (FID GmbH, Burbach, Germany) was used to generate the high voltage nano-second pulses. It produces 20-ns pulses with positive polarity and a peak voltage of up to 20 kV and a pulse repetition rate of up to 2 kHz. The power of the corona discharge was measured via current and voltage waveforms. The voltage waveform was measured by a passive high voltage probe (P6015A, Tektronix, Newark, USA) and the current waveform by a current transformer (Stangenes, Palo Alto, USA) model 2–0.1 W, output volts/ampere 0.1) in combination with an oscilloscope (DPO 202B, Tektronix). Typical waveforms of pulse voltage and discharge current for the pulsed corona discharge reactor under He atmosphere at 18 kV are shown in Fig. 2. The residence time can be controlled by changing the flow rate. The pollutants and carrier gas are subjected to the plasma discharge zone for a longer time at higher residence time, leading to an increase in removal efficiency. In all experiments, the liquid flow rate of 1 L min−1 was selected for water recycling inside the reactor. Mass flow controller at 30 L h−1 in a continuous mode was used to saturate the atmosphere and control the gas flow rate of desired gas inside the reactor. The nanosecond pulse corona reactor was operated at 15–18 kV and 2 kHz yielding power of 85–110 W using He and 100–130 W for Ar and O2.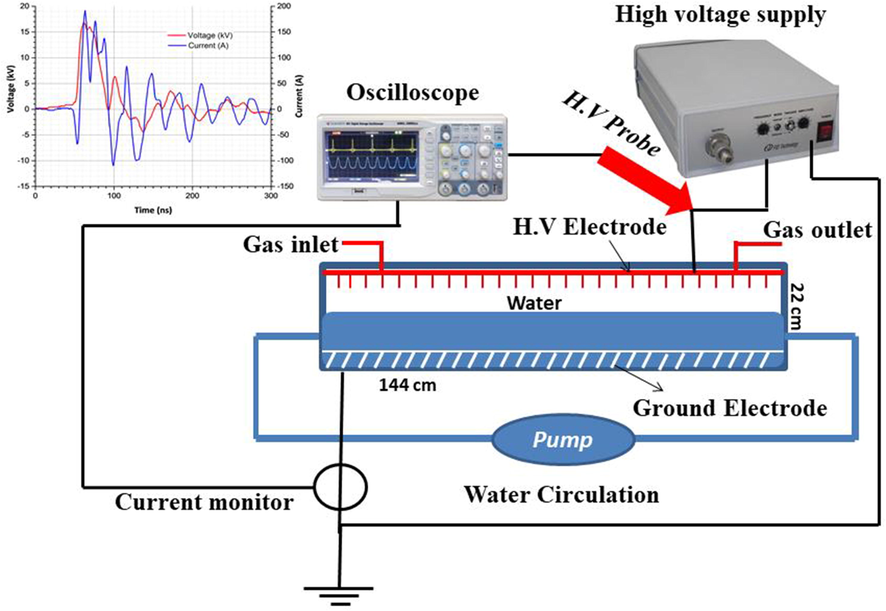
Schematic diagram of nano-pulse corona discharge reactor.
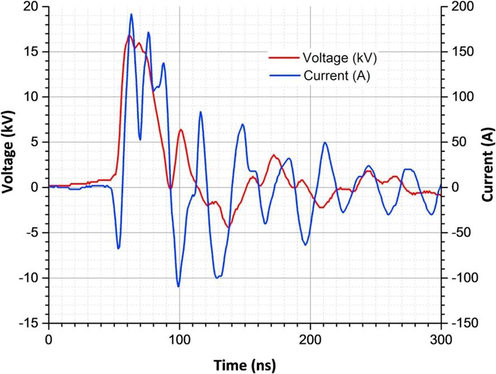
Typical waveforms of pulse voltage and discharge current for the pulse corona discharge reactor under He atmosphere at 18 kV.
The solution of five liters with an initial concentration of 10 µg L−1 or 1 mg L−1 PFOS and pH of 5.5 was used in experiments. The polluted water was recycled in the reactor at a flow rate of 1 L min−1 during the treatment using a gear pump (Ismatec Reglo-z Digital, Germany). Each experiment was conducted in two replicates and the results reported were averaged.
2.3 Analysis
HPLC-MS with the condition described in (DIN 38407-F42:2011–03 method) was applied for the determination of initial PFOS concentrations and their decomposition by-products perfluoroalkyl acids. The identification and quantification of the degradation products were performed based on the retention times and peak areas. Where necessary, solid-phase extraction was used as a preconcentration procedure to enhance the concentration of degradation products and purified before the measurements using ion-exchange polymer (Oasis Wax, Waters). HPLC analyses were performed using analytical columns containing polar modified reversed-phase materials (Zorbax Eclipse XDB-C18, Agilent), and a mixture of methanol/water solution as a mobile phase.
To reduce the effect of by-product intermediate that may not be ignored at decomposition higher than 50% on the value of energy yield. The energy yield (G50) in mg/kWh at 50% degradation of target pollutants was estimated using equation (1) (Aziz et al., 2019).
The ion chromatography method was used to measure the released fluoride ions concentration from the decomposition of PFOS. The system consists of a Dionex DX 500 with a CD20 conductivity detector connected to an IonPac TAC-LP1 (4 × 35 mm) anion concentrator column, an IonPac AG14 (4 × 50 mm) guard column, and an IonPac AS14 (4 × 250 mm) analytical column. The mobile phase consisting of NaHCO3 (1.0 mM) and Na2CO3 (3.5 mM) at a flow rate of 1.2 mL/min was used. For each analysis, a sample volume of 1 mL was injected into the IonPac TAC-LP1 column.
3 Results and discussion
3.1 Degradation of Perfluorooctanesulfonic acid (PFOS)
Figs. 3 and 4 show the degradation efficiency of PFOS at different initial concentrations as a function of treatment time by nano-second pulse corona discharge under different gas atmospheres. Removal of perfluorosurfactants in water was carried out in helium and argon atmosphere in our previous work by different non-thermal plasma reactors with concentrations ≥1 mg PFOS/L (Aziz et al., 2017). In the present work, the degradation of ≤1 mg PFOS/L, as well as more realistic concentrations (about 10 µg PFOS /L) from water under oxygen, helium, and argon atmosphere, were examined. The results have shown in Figs. 3 and 4 proved that the nature of the gas atmosphere had no significant effect on the degradation efficiency of PFOS. However, there is a significant difference in the formation of shorter perfluoroalkyl acids as by-products.![Relative concentration profiles of PFOS degradation ([PFOS]° = 18.5 µg L−1 under O₂ and 7.5 µg L−1 under helium, respectively. Solution volume: 5 L and plasma power 100 W).](/content/184/2021/14/10/img/10.1016_j.arabjc.2021.103366-fig3.png)
Relative concentration profiles of PFOS degradation ([PFOS]° = 18.5 µg L−1 under O₂ and 7.5 µg L−1 under helium, respectively. Solution volume: 5 L and plasma power 100 W).
![Relative concentration profiles of PFOS degradation ([PFOS]° = 1 mg L−1, Solution volume: 5 L and plasma power 100 W).](/content/184/2021/14/10/img/10.1016_j.arabjc.2021.103366-fig4.png)
Relative concentration profiles of PFOS degradation ([PFOS]° = 1 mg L−1, Solution volume: 5 L and plasma power 100 W).
Fig. 5 demonstrates the variation of intermediate by-products concentration as a function of time under He, Ar, and O2 for the treatment of PFOS by nano-second pulse discharge reactor. The results illustrate that in the presence of oxygen atmosphere, a significant amount of much more of these low-chain by-products are formed and its decomposition pathway is different from that of argon and helium. The main perfluoroalkyl acids (PFAS) identified by LC-MS were perfluorooctanoic acid (PFOA) perfluoroheptanoic acid (PFHpA) perfluorohexanonic acid (PFHxA), perfluoropentanoic acid (PFPnA), and perfluorobutanonic acid (PFBuA). The variation of produced by-products concentrations as a function of time under He, Ar, and O2 during the treatment of PFOS is shown in Fig. 5. Estimation of energy yield at 50% degradation of initial pollutants (G50) can be used to compare the efficiency of various reactor designs for the application of non-thermal plasma under different gas conditions for the removal of contaminants.![The concentration of generated by-products during PFOS degradation ([PFOS]° = 1 mg L−1. Solution volume: 5 L and plasma power 100 W).](/content/184/2021/14/10/img/10.1016_j.arabjc.2021.103366-fig5.png)
The concentration of generated by-products during PFOS degradation ([PFOS]° = 1 mg L−1. Solution volume: 5 L and plasma power 100 W).
Defluorination is an important step for the decomposition of PFOS, due to the high stability of C-F bonds (Huang and Jaffé, 2019; Liu et al., 2020). Fig. 6 depicts the defluorination efficiencies of PFOS as a function of treatment time under He, Ar, and O2. From the results shown in Fig. 6 f a rapid and highly efficient defluorination was observed under an oxygen atmosphere. The high-efficient defluorination of PFOS reduced their toxicities.
Formation of fluoride ion during the degradation of PFOS under different gas atmosphere (initial concentration 1 mg/L).
The value of energy yield at 50% conversion of PFOS (G50) was calculated according to Equation (1). This value was chosen for two reasons, at G50 the degradation of intermediate by-products by generated reactive species may be negligible and the value of G50 can be used for comparing the efficiency of various non-thermal plasma reactor designs. The value of the observed rate constant (Table 1) which stands for the first-order removal rate constant (min−1) was calculated from the degradation profile. The results of energy yield at G50 for removal of PFOS was estimated from equation (1) at a different initial concentration under He, Ar, and O₂ was shown in Table 1.
Plasma gas
[PFOS]0
Power (W)
K (min−1)
G50 (mg/kWh)
He
0.75 mg L−1
85
0.014
27
Ar
0.73 mg L−1
112
0.016
22
O2
0.653 mg L−1
103
0.016
22
O2
18.5 µg L−1
106
0.0088
0.33
He
7.5 µg L−1
110
0.0135
0.20
3.2 Addition of sulfobetaine to enhance the degradation efficiency
The addition of surface-active sulfobetaine is an effective approach to enhance the concentration of PFOS in the plasma-liquid interface where contaminants are degraded. Singh et al. have used a bench-scale plasma reactor for the removal of perfluoroalkyl substances from landfill leachate samples (Singh et al., 2021). The short-chain perfluoroalkyl acids were treated by the addition of cationic surfactant after removing long-chain perfluoroalkyl acids. The authors have hypothesized that the electrostatic interaction between the positively charged functional group of the cationic surfactant and negatively charged carboxylate/sulfonate group of perfluoroalkyl acids combined with hydrophobic interactions between the molecule’s tails allows the combined molecules to be transported to the plasma-liquid interface which increases the removal efficiency. In this work, a special surface-active sulfobetaine (Seibt et al., 1991) contains substituents of iron (II)-sulfonate containing iron (II) has been investigated to enhance the concentration of PFOS on the plasma-liquid interface and improving the degradation efficiency. The sulfobetaine is a stable complex and because of the good surface activity of this special sulfobetain, iron(II) is enriched in the liquid/gas interface. To this end, 100 mL of this special sulfobetaine (sulfobetaine sulfonate Fe (II) lactate, each 25 mL equivalent to about 10 mg Fe (II) L-1) was added to the solution of PFOS. Our previous study was proved that the reaction of the degradation process mostly takes place at the water gas–liquid interface of the plasma zone (Aziz et al., 2018). The degradation of PFOS after 90 min treatment was increased from 80% to 90% after the addition of surface-active sulfobetaine. It is thinkable a piggyback-mechanism for instance between sulfobetain and PFSA. As a result there is an anhancement of the diffusion rate of PFSA to the solution interface where the degradation takes place. It has been confirmed that the generated reactive radicals were scavenged in the presence of the same surface-active sulfobetaine during the treatment of chloroacetic acid by nano-second pulse corona discharge reactor. The results have shown that the degradation of chloroacetic acid was completely prevented in the bulk solution (Aziz et al., 2018). This surface-active sulfobetaine diffuses much faster to the surface than other usual surface-active substances like PFOS; therefore, it was supposed that it may act as a carrier to transport the PFOS to the surface of the water. Homogeneous and heterogeneous Fenton oxidation is one of the most popular processes for improving the biodegradability and mineralization of recalcitrant organic pollutants which is conducted in the presence of hydrogen peroxide and iron (II). It is well known that a significant amount of hydrogen peroxide is generated during plasma discharge especially under Ar and He atmosphere (Aziz et al., 2018). Therefore, the presence of iron (II) in the composition of sulfobetaine may induce additional Fenton oxidation (R1-R3) with produced hydrogen peroxide as an additional source of OH radicals.
4 Conclusions
The efficiency of a nano-pulse corona discharge for the degradation of hardly-degradable PFOS pollutants in water under oxygen has been successfully examined and compared with argon and helium atmosphere. Nano-pulse corona discharge showed excellent efficiency for the removal of recalcitrant Perfluorosurfactant and is a promising technology for the remediation of wastewater. The nature and amount of plasma active species depend on the composition of the gas atmosphere. The results of energy yield for PFOS degradation at G50 was 22 under (Ar, O₂) and 27 mg/kWh under He atmosphere. However, the quantity and the nature of shorter perfluoroalkyl acid degradation products are significantly different. The addition of surface-active sulfobetaine was showed a significant enhancement in the degradation efficiency from 80% to 90% PFOS degradation after 90 min treatment. The increase in degradation efficiency with the addition of surface-active sulfobetaine can be explained by increased PFOS concentration in the plasma zone at the plasma-liquid interface and simultaneously initiate additional Fenton reaction which is induced by the Fe (II) present in the composition of sulfobetaine and generated hydrogen peroxide during the discharge, resulting in increased reactive species generation and, as a result, improved degradation efficiency. Finally, based on the findings, nano-pulse corona discharge was found to be an effective and promising approach for refractory organic pollutant degradation. Due to rapid and efficient defluorination under an oxygen atmosphere, the residual toxicities were also significantly reduced.
Acknowledgments
The German Federal Ministry of Economic Affairs and Energy provided funding for this project (BMWi, Grant Number ZF4296101CR6). The nano-pulse generator was generously provided by Vladimir Efanov and FID GmbH. The authors acknowledge the help of the University of Sulaimani in Kurdistan-Iraq for this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere. 2019;228:377-383.
- [Google Scholar]
- Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma. Chem. Eng. J.. 2017;313:1033-1041.
- [Google Scholar]
- Comparative study on 2, 4-dichlorophenoxyacetic acid and 2, 4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. Mater.. 2018;343:107-115.
- [Google Scholar]
- Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. PROCESS SAF ENVIRON. 2018;113:319-329.
- [Google Scholar]
- Removal of dichloroacetic acid from aqueous solution using non-thermal plasma generated by dielectric barrier discharge and nano-pulse corona discharge. Sep. Purif. Technol. 2019
- [Google Scholar]
- Perfluorinated surfactant chain-length effects on sonochemical kinetics. J. Phys. Chem. A. 2009;113:9834-9842.
- [Google Scholar]
- Complete mineralization of organic pollutants in water by treatment with air non-thermal plasma. Chem. Eng. J.. 2018;337:567-575.
- [Google Scholar]
- Decomposition of perfluorooctanoic acid by ultraviolet light irradiation with Pb-modified titanium dioxide. J. Hazard. Mater.. 2016;303:111-118.
- [Google Scholar]
- Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—a review. J. Hazard. Mater.. 2014;274:443-454.
- [Google Scholar]
- New POPs in the water environment: distribution, bioaccumulation and treatment of perfluorinated compounds–a review paper. J. Water Supply: Res. Technol.-AQUA. 2007;56:313-326.
- [Google Scholar]
- Direct regeneration of ion exchange resins with sulfate radical-based advanced oxidation for enabling a cyclic adsorption–regeneration treatment approach to aqueous perfluorooctanoic acid (PFOA) Chem. Eng. J.. 2021;405:126698
- [Google Scholar]
- Distribution of perfluorinated compounds (PFASs) in the aquatic environment of the industrially polluted Vaal River, South Africa. Sci. Total Environ.. 2018;627:1334-1344.
- [Google Scholar]
- Biological monitoring of polyfluoroalkyl substances: a review. Environ. Sci. Technol.. 2006;40:3463-3473.
- [Google Scholar]
- Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol.. 2019;53:11410-11419.
- [Google Scholar]
- Degradation of low concentrated perfluorinated compounds (PFCS) from water samples using non-thermal atmospheric plasma (NTAP) Energies. 2018;11:1290.
- [Google Scholar]
- Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J.. 2019;363:155-173.
- [Google Scholar]
- Corona discharge plasma-based degradation of simulated residual linear alkylbenzene sulphonate and dodecyl benzene sulfonate surfactants. Int. J. Environ. Sci. Technol.. 2020;17(2):1171-1178.
- [Google Scholar]
- Effect of temperature on oxidative transformation of perfluorooctanoic acid (PFOA) by persulfate activation in water. Sep. Purif. Technol.. 2012;91:46-51.
- [Google Scholar]
- Influence of different solvents on the preparation and photocatalytic property of BiOCl toward decontamination of phenol and perfluorooctanoic acid. Chem. Phys. Lett.. 2020;748:137401
- [Google Scholar]
- Degradation of aniline in aqueous solution using non-thermal plasma generated in microbubbles. Chem. Eng. J.. 2018;345:679-687.
- [Google Scholar]
- Treatment train approaches for the remediation of per-and polyfluoroalkyl substances (PFAS): a critical review. J. Hazard. Mater.. 2020;386:121963.
- [CrossRef] [Google Scholar]
- Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res.. 2015;81:124-136.
- [Google Scholar]
- Development and application of different non-thermal plasma reactors for the removal of perfluorosurfactants in water: a comparative study. Plasma Chem. Plasma Process. 2019:1-14.
- [Google Scholar]
- Photocatalytic remediation of persistent organic pollutants (POPs): a review. Arabian J. Chem. 2020
- [Google Scholar]
- Physico-chemical processes for the treatment of per-and polyfluoroalkyl substances (PFAS): a review. Crit. Rev. Environ. Sci. Technol.. 2019;49(10):866-915.
- [Google Scholar]
- Comparative performance assessment of plasma reactors for the treatment of PFOA; reactor design, kinetics, mineralization and energy yield. Chem. Eng. J.. 2020;382:123031.
- [CrossRef] [Google Scholar]
- Synergetic degradation of Acid Orange 7 (AO7) dye by DBD plasma and persulfate. Chem. Eng. J.. 2017;311:378-384.
- [Google Scholar]
- Degradation of p-nitrophenol by DBD plasma/Fe2+/persulfate oxidation process. Sep. Purif. Technol.. 2019;218:106-112.
- [Google Scholar]
- Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol.. 2019;53:2731-2738.
- [Google Scholar]
- Rapid removal of poly-and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol.. 2019;53:11375-11382.
- [Google Scholar]
- Removal of poly-and per-fluorinated compounds from ion exchange regenerant still bottom samples in a plasma reactor. Environ. Sci. Technol.. 2020;54:13973-13980.
- [Google Scholar]
- Treatment of PFAS-containing landfill leachate using an enhanced contact plasma reactor. J. Hazard. Mater.. 2021;408:124452
- [Google Scholar]
- Plasma-based water treatment: efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol.. 2017;51:1643-1648.
- [Google Scholar]
- Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)–A review of recent advances. Chem. Eng. J.. 2018;336:170-199.
- [Google Scholar]
- Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: a critical review. Chem. Eng. J.. 2017;328:927-942.
- [Google Scholar]







