Translate this page into:
Scoping insight on antiviral drugs against COVID-19
⁎Corresponding author at: Dept of Pharmacology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia. Profahmedali@Gmail.com (Ahmed S. Ali) asali@kau.edu.sa (Ahmed S. Ali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
COVID-19 is an ongoing viral pandemic produced by SARS-CoV-2. In light of in vitro efficacy, several medications were repurposed for its management. During clinical use, many of these medications produced inconsistent results or had varying limitations.
Objective
The purpose of this literature review is to explain the variable efficacy or limitations of Lopinavir/Ritonavir, Remdesivir, Hydroxychloroquine, and Favipiravir in clinical settings.
Method
A study of the literature on the pharmacodynamics (PD), pharmacokinetics (PK), safety profile, and clinical trials through academic databases using relevant search terms.
Results & discussion
The efficacy of an antiviral drug against COVID-19 is associated with its ability to achieve therapeutic concentration in the lung and intestinal tissues. This efficacy depends on the PK properties, particularly protein binding, volume of distribution, and half-life. The PK and PD of the model drugs need to be integrated to predict their limitations.
Conclusion
Current antiviral drugs have varying pharmacological constraints that may associate with limited efficacy, especially in severe COVID-19 patients, or safety concerns.
Keywords
SARS-CoV-2
Pharmacokinetics
Pharmacodynamics
Remdesivir
Lopinavir
Hydroxychloroquine
Favipiravir
1 Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by a highly infectious respiratory virus, SARS-CoV-2. It resulted in significant human and economic losses. About 181 million cases had been confirmed as of June 29, 2021, and 3.92 million verified deaths (Roser, et al., 2021; Ciotti, 2019; Chakraborty and Maity, 2020; Yang et al., 2020). Drug repurposing is the process of providing new uses for currently approved drugs. Some repurposed FDA-approved drugs were subjected to in vitro testing and showed promising results against SARS-CoV-2 (Touret et al., 2020; Saul and Einav, 2020). However, clinical trials of most antiviral drugs demonstrated inconsistent results or limitations (Siordia et al., 2020; Jomah et al., 2020). Limited publications were concerned with integrating pharmacokinetics/pharmacodynamics (PK/PD) parameters to predict their efficacy in clinical settings (Zeitlinger, 2020; Arshad et al., 2020). For an antiviral drug to be effective in treating COVID-19, it must achieve sufficient concentrations that suppress viral replication in multiple sites, primarily the cells of the upper and lower respiratory tract (Zhang et al., 2020). Wang; et al. suggested antiviral drugs, which showed activity against the virus in vitro and have high lung distributions, might benefit COVID-19 patients by reducing viral load (Table 1). The low concentration of unbound lopinavir (LPV) in the lung tissues limits its efficacy in COVID-19 patients (Wang and Chen, 2020).
Antiviral Drugs
EC50 [μM]a
Distribution to lung & other tissues.
Lung/plasma drug concentration
Ability to reduce viral load in COVID-19b
Hydroxychloroquine
4.51
Lung & adrenal gland
51
Yes
Favipiravir
61.88–100
Lung & intestine
0.2
Yes
Lopinavir
26.1
Lung, stomach & intestine
0.5c
No
Remdesivir
0.11–0.77
Unknown
?
No
The present review aims to provide a deeper understanding of the reasons behind variable efficacy or limitations of some antiviral drugs through the integration of their PK/PD and safety profiles. Four drugs were selected: Remdesivir (RDV), Lopinavir/ritonavir (LPV/r), Hydroxychloroquine (HCQ), and Favipiravir (FPV).
2 Remdesivir
2.1 Overview
RDV (Veklury) is a broad-spectrum antiviral drug RNA polymerase inhibitor (Fig. 1). It was initially developed in the United States to treat hepatitis C, but it was directed toward Ebola viral infection treatment. It is currently the first FDA approved antiviral drug against COVID-19. Only intravenous injection formulation is available, which needs patients to be hospitalized for administration. It is a prodrug that is expected to enable better intracellular delivery of an adenosine analog (GS-441524) monophosphate, which is then biotransformed into the active triphosphate intracellular metabolite (GS-443902) (Fig. 2) (Eastman et al., 2020; Amirian and Levy, 2020; Jorgensen et al., 2020).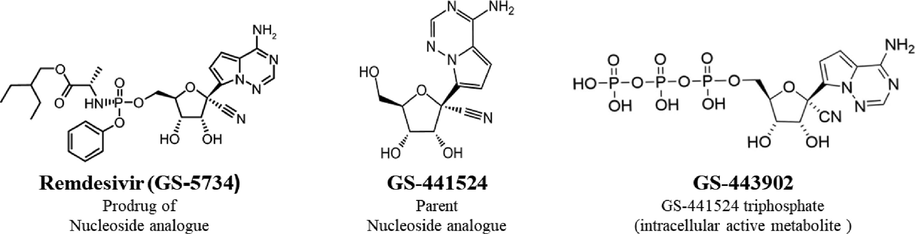
Structure of RDV; the parent nucleoside analogue and final active intracellular metabolite.
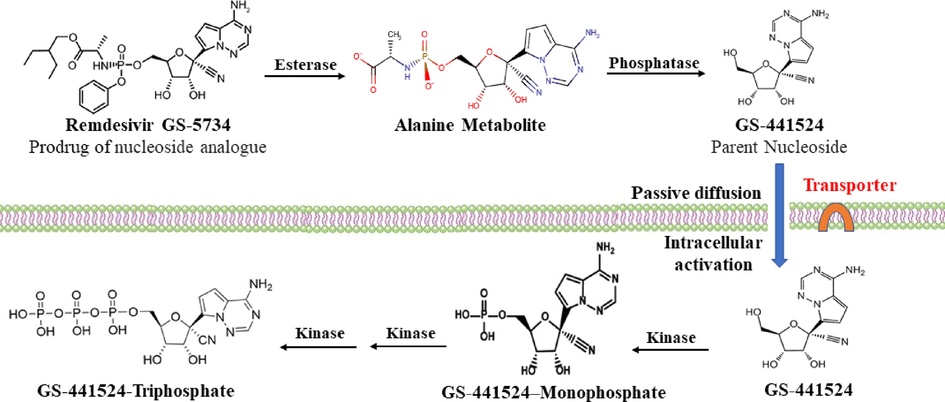
In vivo bioactivation pathway of RDV (Yan and Muller, 2020), In the presence of serum enzymes, the phosphate prodrugs are hydrolyzed prematurely to the nucleoside. GS-441524, which after access to the cells activated to the triphosphate. Other pathway (not shown) involves, access of RDV into the cells, its metabolism to GS-441524 monophosphate, then to GS-441524 triphosphate.
2.2 Pharmacodynamics
RDV is an adenosine analog prodrug; within the cells, it is transformed into its active triphosphate metabolite that competes endogenous adenosine triphosphate to inhibit SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) ultimately inhibiting viral replication (Remdesivir, 2021).
Studies in VeroE6 cells demonstrated selectivity and high potency against SARS-CoV-2 demonstrated by its IC50 of 0.77 µM and an IC90 of 1.760 µM (Yang et al., 2020). However, a higher IC50 value of 23.15 µM was reported (Choy, 2020).
2.3 Pharmacokinetics
In monkeys, RDV demonstrated widespread tissue distribution and transformation into the final active metabolite (GS-443902) in both peripheral blood mononuclear cells and respiratory tissues (Deb, 2021). Animal PK studies revealed that renal and biliary excretion were the primary routes of elimination (Pardo, 2020). PK parameters of RDV and its major metabolite in humans are summarized in Table 2 (Remdesivir, 2021; Gordon et al., 2020; Singh et al., 2020; Ko, 2020). On day 1, healthy volunteers received 200 mg RDV IV over 30 min, followed by 100 mg IV daily over 30 min on days 2 to 5. On the first day, AUC24 was presented, and on the fifth day, AUC tau was presented for GS-441524 and RDV, respectively. T1/2 = half-life; AUC = area under the concentration–time curve.
Parameter
RDV
GS-441524
1st day
5th day
1st day
5th day
Cmax (µg/ml)
5.44
2.61
0.15
0.14
AUC (h* µg/ml)
2.92
1.56
2.24
2.23
T1/2 h, mean (range)
0.98 (0.82–1.03)a
0.89
N/A
25.3 (24.1–30.3)a
Free fraction (%)
6–12
98
RDV should be given by slow IV infusion, over 60 min. At the end of the infusion, peak concentration of RDV in the blood was reached; however, disappeared after 1 h. due to its rapid metabolism and distribution (Humeniuk, 2021). RDV has a high ability to bind to plasma proteins (88–93.6% bound). In contrast, the plasm protein binding of GS-441524 is as low as 2%. RDV is extensively metabolized in the liver and blood. The nucleoside metabolite GS-441524 is mainly excreted in the urine and most of the dose recovered in urine was as GS-441524 (48.6%) and about 10% of the RDV dose was recovered in the urine unchanged.
The intracellular activation of RDV was suggested or demeonstrated to involve several steps that end up by forming the final active nucleoside triphosphate (GS-443902) (Fig. 2).
The following data (Fig. 3) were based on RDV PK study in two severe COVID-19 patients, one of them has moderate renal dysfunction. On the first day, RDV was given as 200 mg loading dose then 100 mg daily. On days 3 through 9, blood samples were taken (after the end of IV infusion) immediately (C0), 1 h. (C1), and 24 h. (C24). RDV serum concentration reached a peak (C0), then began to decline to almost undetectable after 1 h. In contrast, the plasma concentrations of the metabolite GS-441524 peaked at C1 and remained measurable until the next dose.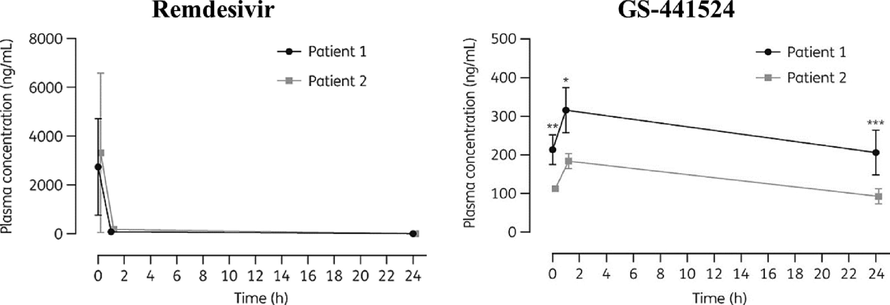
RDV PK in critically ill patients after several doses of IV RDV. Left RDV, Right GS-441524 Patient 1 with renal impairment and Patient 2 without renal impairment; mean SD estimated 3–9 days after RDV initiation).
2.4 Clinical trials
Some early reports and meta-analyses suggested that RDV is not sufficient on its own for the management of COVID-19 in hospitalized patients (Alexander, 2020; Piscoya, 2020; Nasir, 2020). Later several meta-analyses suggested favorable benefit-risk profiles for RDV compared with placebo effects (Davies et al., 2020; Shrestha, 2021; Musa, 2020; Lai et al., 20212021; Frediansyah et al., 2021; Bansal, 2020). A recent meta-analysis concluded that RDV attenuates disease progression, leading to lower odds of mechanical ventilation/extracorporeal membrane oxygenation (MV/ECMO) and greater odds of hospital discharge for COVID-19 patients. However, RDV does not affect the odds of mortality (Reddy Vegivinti et al., 2021).
On the other hand, the WHO Solidarity Trial, which included 11,330 in-patients with COVID-19 who were randomized to receive HCQ (n = 954), LPV (n = 1411), RDV (n = 2750), interferon beta-1a regimens (n = 2063), or none of these drugs (n = 4088), found that all these investigated drugs had little to no effect on overall mortality (Consortium and W.S.T., 2021). However, RDV becomes the 1st FDA approved drug for the management of COVID-19 (Aleem and Kothadia, 2021).
2.5 Safety concern
Many adverse effects were reported for RDV during COVID-19 clinical trials, severe bradycardia being of particular concern, which is consistent with RDV's PD properties, (Touafchia, 2021), changes in ECG, anaphylaxis, infusion-related reactions, nephrotoxicity, and hepatic toxicity (Bistrovic and Lucijanic, 2021).
2.6 GS-441524 as an alternative to RDV
The oral bioavailability of GS-441524 in beagle dogs was investigated, and it was discovered that plasma concentrations up to 24-fold greater than the EC50 against SARS-CoV-2 could be maintained readily and safely. These findings support the development of GS-441524 as an oral COVID-19 treatment (Yan, 2021). Furthermore, GS-441524 effectively inhibited SARS-CoV-2 infection in mouse models (Li, 2021).
GS-441524 is a potent inhibitor of SARS-CoV-2 infected cells, with EC50 values on par with RDV (EC50 = 0.47–1.09 M), according to cell-based research (Pruijssers, 2020).
Moreover, there is evidence of fast metabolism of RDV in the blood into the parent molecule, nucleoside analog GS-441524. Moreover, the enzymes required for RDV metabolism and activation were more expressed in liver and kidney cells than in type ll pneumocytes in the lungs. Given these emerging data, it seemed logical to speculate that the anti-COVID-19 effect of RDV in vivo is mainly mediated through its parent compound GS-441524. Yan and Muller concluded that GS-441524 is thought to be superior to RDV in the management of COVID-19.
In addition, GS-441524 has a simplified synthesis method, easier to formulate as IV or inhalation (Yan and Muller, 2020). Moreover, RDV solution contains 6% sulfobutylated beta-cyclodextrin, which is likely to accumulate in patients with severe renal impairment (Remdesivir, 2021).
3 Lopinavir/ritonavir
3.1 Overview
LPV is an antiretroviral protease inhibitor (Fig. 4) indicated for the treatment of HIV-1 infection and has been repurposed to manage COVID-19. It is available as a fixed combination with ritonavir, a potent inhibitor of cytochrome P450-3A4, which allows LPV to be effective orally (Drożdżal, 2020; FURUTA et al., 2017).
Chemical structure of repurposed antiviral drugs.
3.2 Pharmacodynamics
LPV has been found to inhibits SARS-CoV-2 replication by binding to viral main protease 3C-like protease (3CLpro) with an EC50 of 16.7 µg/ml; however, other values have been reported (Vargas et al., 2020).
3.3 Pharmacokinetics
When administered alone, LPV has a low oral bioavailability of 25%; therefore, it is only given in combination with ritonavir, which increases its bioavailability by slowing its metabolism allowing therapeutic LPV concentrations to be obtained. After oral dosing, the maximal plasma concentrations of LPV/r are attained about 4.4 h; when taken with a meal, bioavailability increases (130% in the case of solution, 20% in case of tablets). The Vd after an oral dose is about 16.9 L (DRug-Bank(b). Lopinvair, 2021).
LPV is 98% bound to plasma proteins. Both alpha-1-acid glycoprotein and albumin are involved. LPV is mainly metabolized by hepatic CYP3A isozymes. Biotransformation is reduced and plasma levels of the active antiviral drug are enhanced when concomitantly taken with ritonavir, a potent inhibitor of CYP3A enzymes (DRug-Bank(b). Lopinvair, 2021).
3.4 Clinical trials
A meta-analysis concluded no significant advantage of LPV/r in alleviating symptoms of COVID-19 (Tobaiqy et al., 2020). Moreover, the WHO published results from the Solidarity Trial showed that LPV was not significantly different from control in reducing mortality or hospitalization. Similar results were confirmed by the Randomized Evaluation of COVID-19 Repurposed Antiviral Drugs for COVID-19 Therapy (RECOVERY) trial. Most protocols are now against its use in the management of COVID-19.
3.5 Limitations
At a dose of LPV/r 400 /100 mg twice orally, the steady-state peak (4 h. post-dose) and trough (before next dose) LPV concentration was about 18 μg/mL. Recall that its free level (unbound) was only about 1% (protein binding > 98%). Thus, the therapeutic free drug level (active form) is not achievable (Fig 5). Other limitations include its low Vd (about 17 L) and short half-life (about 7 h). As a result, only a tiny fraction of the drug is available to enter the target lung cell. Indeed, a study by Cattaneo et al. suggested that the protein-adjusted IC90 values of LPV required to inhibit SARS-CoV-2 replication in plasma were 200-fold higher than the concentrations measured in blood samples obtained from COVID-19 patients (Cattaneo et al., 2021). This explanation for the ineffectiveness of the drug was the subject of several published studies.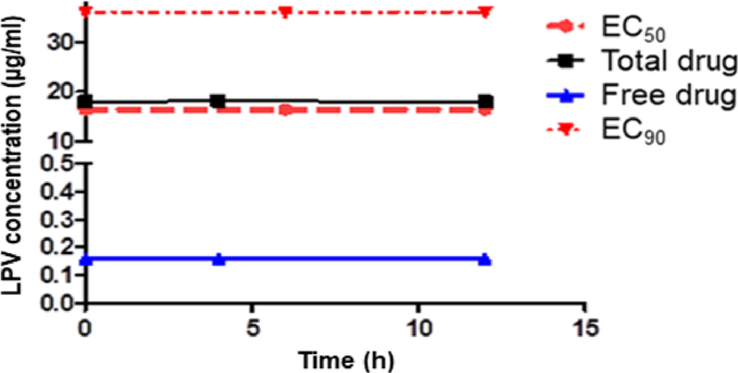
Total and unbound LPV median peak and trough levels in COVID −19 patients. LPV/r 400/100 mg twice daily (Gregoire et al., 2020).
Another critical limitation is that ritonavir, a potent inhibitor of cytochrome P450-3A4 is given in combination with LPV; therefore, a long list of interactions with other medications must be considered in COVID-19 patients (Sanders, 2020).
4 Favipiravir
4.1 Overview
FPV (T-705) is a modified pyrazine analogue (Fig. 4). It is a broad-spectrum antiviral RdRp inhibitor. It was developed in Japan and approved by the Pharmaceuticals and Medical Devices Agency (PMDA) to treat influenza. Also, it has been included in COVID-19 treatment guidelines in many countries (Chen et al., 2021; Cai et al., 2020).
4.2 Pharmacodynamics
FPV is a modified nucleoside analog that targets RdRp enzymes. It is a prodrug that undergoes intracellular activation to favipiravir-ribofuranosyl-5′-triphosphate (T-705-RTP) that binds to and inhibits RdRp, consequently inhibiting viral transcription and replication (Joshi et al., 2021). Wang, et al. reported low potency against SARS-CoV-2 (EC50 61.88 µM) (Wang et al., 2020). However, other in vitro studies showed different EC50 values.
4.3 Pharmacokinetic
The drug is administered orally with a bioavailability of about 97%. FPV has non-linear PK demonstrated as a decrease in drug concentration after chronic administration. This may be explained by the auto-induction of certain CYP450 enzymes responsible for its metabolism (Madelain et al., 2016). Moreover, there is an ethnic variation in FPV’s disposition (Hayden and Shindo, 2019; Nguyen, 2017).
4.4 Clinical trials
FPV accelerates viral clearance by seven days and contributes to clinical improvement in about fourteen days. Particularly in patients with mild to moderate disease (Agrawal et al., 2020; Chen, 2020; Manabe et al., 2021). FVP treatment results in considerable clinical and radiological improvements compared to standard care, with no significant differences in viral clearance, need for oxygen, or side effect profiles. A study reported favorable outcomes when compared with umifenovir or LPV/r (Kaur, 2020). A randomized controlled trial in non-severe COVID-19 patients demonstrated that on day 7, FPV provided a better clinical recovery rate and was more effective than umifenovir in alleviating fever and cough.
4.5 Limitations
FPV is still under evaluation. Its PK/PD profile suggests potential effectiveness, at least, in mild and moderate cases of COVID-19. However, a PK study in critically ill COVID-19 patients who received the recommended dose of FPV demonstrated a low trough level (1 µg/mL) (Mohammad., 2020). Recall low potency against SARS-CoV-2 with EC50 61.88 µM (Wang et al., 2020). The lung-to-tissue level of FPV was estimated to be about 50% of that in the blood. These PK data suggest moderate drug access to lung tissues (Irie, 2020). The drug did not show life-threatening adverse effects in clinical trials, but it has some adverse effects including a rise in serum uric acid, liver enzymes, diarrhea, nausea, vomiting, and tachycardia (Kaur, 2020). FPV may be teratogenic and has a long list of potential drug-drug interactions [Drug67].
5 Hydroxychloroquine
5.1 Overview
HCQ is a 4-aminoquinoline, similar to chloroquine (CQ) (Fig. 4) (Drug-Bank(c). Hyroxychloroquine, 2021). It is a low-cost drug that has been used to prevent and treat malaria and manage rheumatoid arthritis, lupus, and porphyria, among other conditions. (Johnson and Charnley, 1979; Browning, 2014; Filipova, 2021). It is usually taken orally as HCQ sulfate. The drug received extensive interest and debate due to its potential activity against COVID-19 (Saghir, 2021). Many African countries have already approved the use of HCQ or CQ to treat COVID-19 at the national level (Belayneh, 2020).
5.2 Pharmacodynamics
In vitro studies showed that HCQ is more potent than CQ against SARS-CoV-2 (EC50 of 0.72 μM for HCQ and 5.47 μM for CQ). As a result, HCQ was one of the earliest drugs to be tested against COVID-19. Regardless of the antiviral activity, HCQ has immunomodulatory effects (Hassan, 2019; Schrezenmeier and Dörner, 2020), which provided the basis of their utility to prevent cytokine release syndrome (CRS) (Hughes, 2018). Interestingly, HCQ has been suggested as a valuable drug for prophylaxis against lung thrombosis (Kravvariti, 2020).
5.3 Pharmacokinetics
Absorption of HCQ after oral administration is good but extensively variable (∼70%; range: 25 to 100%). HCQ is considered a lysosomotropic drug that accumulates intracellularly at concentrations up to 1000-fold higher than the extracellular concentration. The rise in endosomal pH mediated by HCQ blocks virus/cell fusion. The elevated Golgi apparatus pH impairs the terminal glycosylation of the angiotensin-converting enzyme 2 (ACE2) receptor and reduces its binding affinities to SARS-CoV-2 spike protein (Al-Bari, 2017; Shittu and Afolami, 2020). This accumulation in lysosomes is likely to explain the considerable very high volume of distribution of HCQ (Vd = 70 L/kg). The lung/plasma ratio of HCQ was suggested to be high, at least 50. HCQ has a long elimination half-life of about 40 to 50 days. These PK/PD characteristics explain the potential efficacy of HCQ against the novel virus (Derendorf, 2020; Zhou, 2020). HCQ is metabolized in the liver through CYP 2C3, 2D6, 2C8, 3A4, and 3A5 into active and inactive metabolites. Therefore, the genetic polymorphism of these enzymes would affect its blood level. About 20% of HCQ dose is excreted in urine as unchanged drugs; hence renal function is likely to affect its clearance (U.S. FDA, 2020). HCQ has a narrow therapeutic range and moderate protein binding (about 50%), primarily with albumin (Furst, 1996).
5.4 Clinical trials
Million et al. reported the efficacy of HCQ/Azithromycin (AZ) in the early management of COVID-19. The study was conducted retrospectively and involved 1061 COVID-19 patients (Million, 2020). Yao et al. suggested optimized dosing regimens of HCQ for the treatment of SARS-CoV-2 based on the integration of its PD and physiologically based PK (PBPK) modeling and simulation. The authors simulated different dosing regimens and demonstrated that the ratios of HCQ lung concentration to the EC50 value would be approximately 20 to 170. These findings theoretically support the role of HCQ in the management of the COVID-19 (Yao et al., 20202020). Moreover, a cohort study in Saudi Arabia reported that HCQ had a modest effect on hospital length of stay in the ICU compared with standard treatment. Similar publications provided a similar impression of the potential benefit of HCQ in the management of COVID-19 (Gautret, 2020; Almazrou et al., 2020; Sahraei and Aminoquinolines against coronavirus disease, , 2019; Gao et al., 2020; Sarma et al., 2020).
According to a systemic review, HCQ was found beneficial in hospitalized COVID-19 patients when given early in the outpatient setting. HCQ is consistently effective against COVID-19, has not caused disease deterioration, and is well tolerated (Prodromos and Rumschlag, 2020)
In contrast, the WHO published the Solidarity Trial results showing that HCQ was not significantly different from control in reducing mortality or hospitalization (Pan, 2021). This was in line with RECOVERY trial that concluded: “there is no benefit of using HCQ in COVID-19 patients” (Horby et al., 2020).
5.5 Limitations
An in vitro study suggested that HCQ suppresses trained immunity, which is may be counterproductive to the antiviral innate immune response to SARS-CoV-2 (Rother, 2020). Lung acidosis that can be induced by severe COVID-19 is likely to reduce access of the weakly basic drug to lung tissues (Liu et al., 2016). Hence, HCQ has marked reduction in cellular uptake in severely ill patients (Geary et al., 1990). Consequently, Ali et al. suggested that HCQ is not likely to provide a potent antiviral effect in severe cases of COVID-19. If indicated, it should be given as early as possible to optimize its use (Ali et al., 2020). Another limitation of HCQ is potential QT prolongation and ventricular arrhythmia. Unfortunately, there has been no dose–response relationship study to accurately predict the association of HCQ drug level with cardiac toxicities (Horby et al., 2020; Javelot, 2020; Juurlink, 2020). Moreover, the drug showed extreme variability in drug levels in COVID-19 patients, as shown in Fig. 6.![PK of HCQ in COVID-19. Peak level after loading dose of 200 mg TID extremely variable [0.28–0.62], mean 0.5 ug/ml. Achievement of the assumed therapeutic level [1–2 ug/ ml] was delayed (Chakraborty and Maity, 2020; Yang et al., 2020; Touret et al., 2020; Saul and Einav, 2020; Siordia et al., 2020) mean 4 days. Potentially toxic level > 2 ug/ml was observed in some patients after 5 days (Painvin et al., 2020).](/content/184/2021/14/10/img/10.1016_j.arabjc.2021.103385-fig6.png)
PK of HCQ in COVID-19. Peak level after loading dose of 200 mg TID extremely variable [0.28–0.62], mean 0.5 ug/ml. Achievement of the assumed therapeutic level [1–2 ug/ ml] was delayed (Chakraborty and Maity, 2020; Yang et al., 2020; Touret et al., 2020; Saul and Einav, 2020; Siordia et al., 2020) mean 4 days. Potentially toxic level > 2 ug/ml was observed in some patients after 5 days (Painvin et al., 2020).
6 Impact of pathophysiological changes, drug interactions and comorbidity
To ensure optimal use of medications in management of a disease; the integration of their PK/PD and side effects should be extended to include other variables such as genetic, chronic diseases, drug interactions, immunological status etc. A complex disease-drug-drug interaction is expected in severe COVID-19 (Kumar and Trivedi, 2021). Pathophysiological changes induced by severe COVID-19 include, hyperinflammation, severe hypoxia, acute respiratory distress syndrome (ARDS), encephalopathy, myocardial injury, heart failure, coagulation dysfunction and acute kidney injury (Polak et al., 2020) are likely to affect drug transporters, and its PK (Deb and Arrighi, 2021). For example, COVID-19 induced hypoxia and inflammation can reduce the intracellular transport of RDV main metabolite GS-441524 and its activation to GS-441524 monophosphate (Rasmussen, 2021).
COVID-19 associated complications also predispose the patients for drug induced toxicities (Nardo et al., 2021). For example, hypokalemia predisposes the patient to tachyarrhythmias, the cytokine storm is also known to prolong QT intervals (Kochav, 2020). This may explain the higher incidence of cardiac toxicity of antiviral drugs such as HCQ.
Pharmacotherapy of COVID-19 in patients with pre-existing comorbidities, especially elderly, is highly challenging due to the use of multiple medications with great potential for drug-drug interactions (Baburaj et al., 2021; Hodge et al., 20202020; Lemaitre et al., 2020; Rezaee, 2021). The PK properties (e.g., induction or inhibition of cytochrome P450 (CYP) isoenzymes, competition in renal elimination) as well as the PD properties (e.g., QT prolongation) are largely responsible for such drug–drug interactions. In addition to these interactions, COVID-19 patients have a significant pathophysiological changes, which can alter the PK of the medications (e.g., downregulation of CYP isoenzymes, organ failure, modification of plasma protein binding) (Morgan, 2009; Ofotokun et al., 2011).
Dosing of drugs used to treat COVID-19 in patients with renal or hepatic impairment requires great attention. Fortunately guidelines for dose adjustment and precautions are available in the drug monograph and some publications (Marra, 2020). For example, RDV should only be used in adults and children with an eGFR of less than 30 mL/min if the possible benefit justifies the potential danger (Adamsick et al., 2020).
Based on the genetics of COVID-19 patients, pharmacogenetics could explain the inter-individual variability in medication response. Variants in genes encoding drug-metabolizing enzymes, transporters, or receptors have been identified, and they may provide the information needed to develop a tailored therapy that optimize the use of pharmacotherapy for COVID-19 (Takahashi et al., 2020; Babayeva and Loewy, 2020; Zubiaur et al., 2020).
7 Inhaled formulations
Using a nebulizer with an inhaled nanoparticle formulation to deliver medications directly to the primary site of infection may allow for more targeted and accessible delivery in hospitalized and non-hospitalized patients, as well as potentially decrease systemic exposure to the drug. Inhaled nanoformulations of RDV is under development (Sahakijpijarn, 2021; Sun, 2020; CLINICAL-Trial.gov. Pharmacokinetics of Inhaled Nanoparticle Formulation of Remdesivir (GS-5734) and NA-831., 2021). Inhaled HCQ is also under investigation and showing promising results (Albariqi et al., 2021; Kavanagh, 2020; Klimke, 2020; Tai et al., 2021).
8 Summary and conclusion
Table 3 summarizes the PK/PD data of four repurposed antiviral medications. Even though all these medications displayed potency in-vitro against SARS-CoV-2, these preliminary findings do not always imply a consistent favorable clinical outcome in the treatment of COVID-19. A thorough assessment of the pharmacological profile from all angles, examining many factors impacting medication PK/PD and adverse effects such as drug interactions, genetics, chronic conditions, and so on, would allow a prior prediction of their usefulness or limitations in COVID-19 management. Developing an inhalable drug delivery system to optimize the use of these medications in management of respiratory viral infections is recommended.
Repurposed drug
Antiviral mode of action
EC50
EC90
Lung/plasma ratio
Ion trapping
Impact of acidosis
Remdesivir (Wang et al., 2020)
Viral RNA polymerase inhibitor.
0.77 μM
1.76 μM
ND
Active metabolites
–
Lopinavir/Ritonavir (Cattaneo, 2020)
HIV protease inhibitor and a boost of other protease inhibitors.
16.7 μg/mL.
23.4 μM
0.5
No
–
Favipiravir (Driouich, 2021)
Viral RNA polymerase inhibitor
61.88 μM
52.5 µg/mL
0.2
No
–
Hydroxychloroquine (Gendrot, 2020)
Inhibition of pH-dependent steps in viral replication (Savarino et al., 2003)
E 1.5 μM.
3.0 μM
51
Yes
Reduced access to lung cells
9 Disclaimer
What was mentioned in this paper is a scientific view and information relating to general principles which should not be construed as specific instructions to change or criticize any protocols. But rather an academic discussion to push further research and development of these drugs for better achievements.
Acknowledgement
The authors acknowledge Miss Asmaa A. Ali, 6th year medical students at Ibn Sina National college for medical studies for designing the figures of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Remdesivir in patients with acute or chronic kidney disease and COVID-19. J. Am. Soc. Nephrol.. 2020;31(7):1384-1386.
- [Google Scholar]
- Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76(4):370-376.
- [Google Scholar]
- Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect.. 2017;5(1):e00293
- [Google Scholar]
- Inhalable Hydroxychloroquine Powders for Potential Treatment of COVID-19. J. Aerosol. Med. Pulm Drug Deliv.. 2021;34(1):20-31.
- [Google Scholar]
- Aleem, A., Kothadia, J., Remdesivir, in StatPearls. 2021, StatPearls Publishing.
- Alexander, P.E., et al., Remdesivir use in patients with coronavirus COVID-19 disease: a systematic review and meta-analysis of the Chinese Lancet trial with the NIH trial. medRxiv, 2020: p. 2020.05.23.20110932.
- Optimizing the Use of Hydroxychloroquine in the Management of COVID-19 Given Its Pharmacological Profile. J. Pharmaceut. Res. Int. 2020:29-43.
- [Google Scholar]
- Comparing the impact of Hydroxychloroquine based regimens and standard treatment on COVID-19 patient outcomes: A retrospective cohort study. Saudi Pharmaceut. J.. 2020;28(12):1877-1882.
- [Google Scholar]
- Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128
- [Google Scholar]
- Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin. Pharmacol. Ther.. 2020;108(4):775-790.
- [Google Scholar]
- Repurposing drugs for COVID-19: Pharmacokinetics and pharmacogenomics of chloroquine and hydroxychloroquine. Pharmacogenomics Pers. Med.. 2020;13:531.
- [Google Scholar]
- Potential Drug Interactions of Repurposed COVID-19 Drugs with Lung Cancer Pharmacotherapies. Arch. Med. Res.. 2021;52(3):261-269.
- [Google Scholar]
- Mortality Benefit of Remdesivir in COVID-19: A Systematic Review and Meta-Analysis. Front. Med. (Lausanne). 2020;7:606429
- [Google Scholar]
- Off-Label Use of Chloroquine and Hydroxychloroquine for COVID-19 Treatment in Africa Against WHO Recommendation. Res. Rep. Trop. Med.. 2020;11:61-72.
- [Google Scholar]
- Remdesivir might induce changes in electrocardiogram beyond bradycardia in patients with coronavirus disease 2019—The pilot study. J. Med. Virol.. 2021;n/a(n/a)
- [Google Scholar]
- Pharmacology of chloroquine and hydroxychloroquine. In: Hydroxychloroquine and chloroquine retinopathy. Springer; 2014. p. :35-63.
- [Google Scholar]
- Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6(10):1192-1198.
- [Google Scholar]
- Prediction of lopinavir/ritonavir effectiveness in COVID-19 patients: a recall of basic pharmacology concepts. Eur. J. Clin. Pharmacol.. 2021;77(5):791-792.
- [Google Scholar]
- COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ.. 2020;728:138882
- [Google Scholar]
- Chen, P.J., Chao, C.M., Lai, C.C., Clinical efficacy and safety of favipiravir in the treatment of COVID-19 patients. J. Infect., 2021. 82, 5, p. 199-200.
- Chen, C., et al., Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv, 2020: p. 2020.03.17.20037432.
- Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res.. 2020;178:104786
- [Google Scholar]
- CLINICAL-Trial.gov. Pharmacokinetics of Inhaled Nanoparticle Formulation of Remdesivir (GS-5734) and NA-831. 2021 [cited 2021 15 July]; Available from: https://clinicaltrials.gov/ct2/show/NCT04480333.
- Consortium, W.S.T., Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. New England journal of medicine, 2021. 384, 6, p. 497–511.
- Remdesivir in Treatment of COVID-19: A Systematic Benefit-Risk Assessment. Drug Saf. 2020;43(7):645-656.
- [Google Scholar]
- ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential. Pharmaceuticals. 2021;14(7):655.
- [Google Scholar]
- Potential effects of COVID-19 on cytochrome P450-mediated drug metabolism and disposition in infected patients. Eur. J. Drug Metab. Pharmacokinet. 2021:1-19.
- [Google Scholar]
- Excessive lysosomal ion-trapping of hydroxychloroquine and azithromycin. Int. J. Antimicrob. Agents 2020106007
- [Google Scholar]
- Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat. Commun.. 2021;12(1):1735.
- [Google Scholar]
- FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat.. 2020;53:100719
- [Google Scholar]
- DRug-Bank(b). Lopinvair 2021 [cited 2021 12 July]; Available from: https://go.drugbank.com/drugs/DB01601.
- Drug-Bank(c). Hyroxychloroquine 2021 [cited 2021 15 July]; Available from: https://go.drugbank.com/drugs/DB01611.
- Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci.. 2020;6(5):672-683.
- [Google Scholar]
- In Search for the Truth about Hydroxychloroquine Prophylaxis of Covid-19. Health Sci. J.. 2021;15(5):1-7.
- [Google Scholar]
- Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin. Epidemiol. Glob. Health. 2021;9:123-127.
- [Google Scholar]
- Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(Suppl 1):S11-S15.
- [Google Scholar]
- Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad., Ser. B. 2017;93(7):449-463.
- [Google Scholar]
- Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020;14(1):72-73.
- [Google Scholar]
- U.S. FDA. (2020). Emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients [Fact Sheet]. Retrieved from https://www.fda.gov/media/136534/download.
- Gautret, P., et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents, 2020. 56, 1, p. 105949.
- Kinetic modelling of the response of Plasmodium falciparum to chloroquine and its experimental testing in vitro: implications for mechanism of action of and resistance to the drug. Biochem. Pharmacol.. 1990;40(4):685-691.
- [Google Scholar]
- Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Travel Med. Infect. Dis.. 2020;37:101873
- [Google Scholar]
- The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem.. 2020;295(15):4773-4779.
- [Google Scholar]
- Gregoire, M., et al., Lopinavir pharmacokinetics in COVID-19 patients. J. Antimicrob. Chemotherapy, 2020. 75: p. 2702–2704.
- Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med.. 2020;382(24):2327-2336.
- [Google Scholar]
- Anti-malarial and cytokine-modulating effects of andrographolide in a murine model of malarial infection. Trop. Biomed.. 2019;36(3):776-791.
- [Google Scholar]
- Influenza virus polymerase inhibitors in clinical development. Curr. Opin. Infect. Dis.. 2019;32(2):176-186.
- [Google Scholar]
- Hodge, C., et al., Drug interactions: a review of the unseen danger of experimental COVID-19 therapies. J. Antimicrob. Chemother., 2020. 75, 12, p. 3417-3424.
- Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2020;396(10259):1345-1352.
- [Google Scholar]
- Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of Remdesivir, a SARS-CoV-2 Replication Inhibitor. Clin. Pharmacokinet. 2021:1-15.
- [Google Scholar]
- Pharmacokinetics of Favipiravir in critically ill patients with COVID-19. Clin. Transl. Sci.. 2020;13(5):880-885.
- [Google Scholar]
- COVID-19 and (hydroxy)chloroquine-azithromycin combination: Should we take the risk for our patients? Br. J. Clin. Pharmacol.. 2020;86(6):1176-1177.
- [Google Scholar]
- Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin. Orthop. Relat. Res.. 1979;144:174-177.
- [Google Scholar]
- Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review. J. Infect. Publ. Health. 2020;13(9):1187-1195.
- [Google Scholar]
- Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy: J. Hum. Pharmacol. Drug Therapy. 2020;40(7):659-671.
- [Google Scholar]
- Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis.. 2021;102:501-508.
- [Google Scholar]
- Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450-E453.
- [Google Scholar]
- Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect. Drug Resistance. 2020;13:4427.
- [Google Scholar]
- Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19. Med. Hypotheses. 2020;143:110110
- [Google Scholar]
- Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med. Hypotheses. 2020;142:109783
- [Google Scholar]
- Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020;55(4):105933
- [Google Scholar]
- Cardiac arrhythmias in COVID-19 infection. Circulation: Arrhythmia Electrophysiol.. 2020;13(6):e008719
- [Google Scholar]
- The effect of hydroxychloroquine on thrombosis prevention and antiphospholipid antibody levels in primary antiphospholipid syndrome: a pilot open label randomized prospective study. Autoimmun. Rev.. 2020;19(4):102491
- [Google Scholar]
- Disease-drug and drug-drug interaction in COVID-19: risk and assessment. Biomed. Pharmacother. 2021111642
- [Google Scholar]
- Lai, C.-C., et al., Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J. Antimicrob. Chemother., 2021. 76, 8, p. 1962–1968.
- Lemaitre, F., et al., Potential drug-drug interactions associated with drugs currently proposed for COVID-19 treatment in patients receiving other treatments. Fundam. Clin. Pharmacol., 2020. 34, 5, p. 530-547.
- Remdesivir Metabolite GS-441524 Effectively Inhibits SARS-CoV-2 Infection in Mouse Models. J. Med. Chem. 2021
- [Google Scholar]
- The Influence of Virus Infection on the Extracellular pH of the Host Cell Detected on Cell Membrane. Front. Microbiol.. 2016;7
- [CrossRef] [Google Scholar]
- Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet.. 2016;55(8):907-923.
- [Google Scholar]
- Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect. Dis.. 2021;21(1)
- [CrossRef] [Google Scholar]
- Marra, F., et al., Recommendations for dosing of repurposed COVID-19 medications in patients with renal and hepatic impairment. Drugs in R&D, 2020: p. 1–19.
- Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille France. Travel Med. Infect. Dis.. 2020;35:101738
- [Google Scholar]
- Mohammad reza, M., Morteza, A.-Z., 2020. Safety and effectiveness of favipiravir for novel coronavirus (COVID-19): a rapid review of available evidence. Health Technol. Assess. Action, 4, 1.
- Impact of infectious and inflammatory disease on cytochrome P450–mediated drug metabolism and pharmacokinetics. Clin. Pharmacol. Ther.. 2009;85(4):434-438.
- [Google Scholar]
- Remdesivir for the Treatment of COVID-19: A Systematic Review of the Literature. West J. Emerg. Med.. 2020;21(4):737-741.
- [Google Scholar]
- Pathophysiological mechanisms of liver injury in COVID-19. Liver Int.. 2021;41(1):20-32.
- [Google Scholar]
- Use of Remdesivir in the Management of COVID-19: A Systematic Review on Current Evidences. Mymensingh Med. J.. 2020;29(2):481-487.
- [Google Scholar]
- Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl. Trop. Dis.. 2017;11(2):e0005389
- [Google Scholar]
- Ofotokun, I., et al., 2011. Immune activation mediated change in alpha‐1‐acid glycoprotein: impact on total and free lopinavir plasma exposure. J. Clin. Pharmacol., 51, 11, p. 1539–1548.
- Hydroxychloroquine pharmacokinetic in COVID-19 critically ill patients: an observational cohort study. Intensive Care Med.. 2020;46(9):1772-1773.
- [Google Scholar]
- Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med.. 2021;384(6):497-511.
- [Google Scholar]
- Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. PLoS ONE. 2020;15(12):e0243705
- [Google Scholar]
- A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol.. 2020;33(11):2128-2138.
- [Google Scholar]
- Hydroxychloroquine is effective, and consistently so when provided early, for COVID-19: a systematic review. New Microbes New Infect.. 2020;38:100776
- [Google Scholar]
- Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell reports. 2020;32(3):107940
- [Google Scholar]
- Cellular Uptake and Intracellular Phosphorylation of GS-441524: Implications for Its Effectiveness against COVID-19. Viruses. 2021;13(7):1369.
- [Google Scholar]
- Remdesivir therapy in patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. Surg. (Lond.). 2021;62:43-48.
- [Google Scholar]
- UPTODATE. Remdesivir: Drug information. 2021 25 April 2021]; Available from: https://0o112ngb4-y-https-www-uptodate-com.kau.proxy.deepknowledge.io/contents/remdesivir-drug-information.
- Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview. Pharmacol. Res. Perspect.. 2021;9(1):e00705
- [Google Scholar]
- Roser, M., et al. Coronavirus pandemic (COVID-19). Our world in data 2021 [cited 2021 29 June]; Available from: https://ourworldindata.org/coronavirus.
- Hydroxychloroquine Inhibits the trained innate immune response to interferons. Cell Reports Med.. 2020;1(9):100146
- [Google Scholar]
- Chloroquine and hydroxychloroquine for the prevention and treatment of COVID-19: A fiction, hope or hype? An updated review. Ther. Clin. Risk Manag.. 2021;17:371.
- [Google Scholar]
- In vivo pharmacokinetic study of remdesivir dry powder for inhalation in hamsters. Int. J. Pharmaceut.: X. 2021;3:100073
- [Google Scholar]
- Sahraei, Z., et al., Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int. J. Antimicrob. Agents, 2020. 105945.
- Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824-1836.
- [Google Scholar]
- Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis. J. Med. Virol.. 2020;92(7):776-785.
- [Google Scholar]
- Old drugs for a new virus: repurposed approaches for combating COVID-19. ACS Infect. Dis.. 2020;6(9):2304-2318.
- [Google Scholar]
- Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet. Infect. Dis. 2003;3(11):722-727.
- [Google Scholar]
- Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020:1-12.
- [Google Scholar]
- Improving the efficacy of Chloroquine and Hydroxychloroquine against SARS-CoV-2 may require Zinc additives - A better synergy for future COVID-19 clinical trials. Infez Med. 2020;28(2):192-197.
- [Google Scholar]
- Remdesivir: A potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. 2021;264:118663
- [Google Scholar]
- Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metabolic Syndrome. 2020;14(4):641-648.
- [Google Scholar]
- Systematic and Statistical Review of Coronavirus Disease 19 Treatment Trials. SN Comprehensive Clin. Med.. 2020;2(8):1120-1131.
- [Google Scholar]
- Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional benefit. AAPS J.. 2020;22:1-6.
- [Google Scholar]
- A Strategy to Treat COVID-19 Disease With Targeted Delivery of Inhalable Liposomal Hydroxychloroquine: A Preclinical Pharmacokinetic Study. Clin. Transl. Sci.. 2021;14(1):132-136.
- [Google Scholar]
- Tobaiqy, M., Alhumaid, S., Mutair, A.A., 2020. Efficacy and Safety of Lopinavir/Ritonavir for Treatment of COVID-19: A Systematic Review and Meta-Analysis. medRxiv, p. 2020.06.16.20133298.
- Touafchia, A., et al., 2021. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns. Clinical Microbiology and Infection, 27, 5, p. 791. e5-791. e8.
- In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Lopinavir/ritonavir for the treatment of SARS, MERS and COVID-19: a systematic review. Eur. Rev. Med. Pharmacol. Sci.. 2020;24(16):8592-8605.
- [Google Scholar]
- Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res.. 2020;30(3):269-271.
- [Google Scholar]
- Lung tissue distribution of drugs as a key factor for COVID-19 treatment. Br. J. Pharmacol.. 2020;177(21):4995.
- [Google Scholar]
- Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID-19 treatment. Eur. J. Pharmacol.. 2020;889:173634
- [Google Scholar]
- Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment. ACS Med. Chem. Lett.. 2020;11(7):1361-1366.
- [Google Scholar]
- Yan, V.C., et al., Pharmacokinetics of Orally Administered GS-441524 in Dogs. bioRxiv, 2021: p. 2021.02.04.429674.
- Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int. J. Biol. Sci.. 2020;16(10):1708-1717.
- [Google Scholar]
- Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat. Med.. 2020;8(5):475-481.
- [Google Scholar]
- Yao, X., et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical infectious diseases, 2020. 71(15): p. 732-739.
- Pharmacokinetics/Pharmacodynamics of Antiviral Agents Used to Treat SARS-CoV-2 and Their Potential Interaction with Drugs and Other Supportive Measures: A Comprehensive Review by the PK/PD of Anti-Infectives Study Group of the European Society of Antimicrobial Agents. Clin Pharmacokinet 2020:1-22.
- [Google Scholar]
- Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging Microbes Infect.. 2020;9(1):386-389.
- [Google Scholar]
- Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies. J. Exp. Med.. 2020;217(10)
- [Google Scholar]
- Important Pharmacogenetic Information for Drugs Prescribed During the SARS-CoV-2 Infection (COVID-19) Clin. Transl. Sci.. 2020;13(6):1023-1033.
- [Google Scholar]







