Translate this page into:
Antiproliferative effects of levan polysaccharide against colorectal cancer cells mediated through oxidative stress-stimulated HOTAIR/Akt signaling pathway: In vitro
⁎Corresponding authors at: Department of Oncology, Shaanxi Cancer Hospital, NO. 309 Yanta West Road, Xi 'an 710061, China (Z. Zhao). baiqian@zzu.edu.cn (Qian Bai), zzhao.oncology@yahoo.com (Zheng Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The overexpression of lncRNA HOTAIR can result in cancer progression through upregulation of the Akt signaling pathway. We aimed to evaluate the anticancer effects of levan polysaccharide from Erwinia herbicola on colorectal cancer cells (HT-29) and explore the role of the ROS-mediated HOTAIR/Akt signaling pathway. The HT-29 cancer cells were treated with levan either alone or along with N-acetyl-L-cysteine (NAC), as a potential antioxidant for 24 hrs and different assays including MTT, LDH, ROS, apoptosis, SOD activity, CAT activity, caspase-3 activity, qRT-PCR, and western blot analysis were performed. It was shown that levan treatment induces apparent reduction in cell viability, SOD and CAT activity, HOTAIR expression, the ratio of pAkt protein level and a significant increase in the ROS level, apoptosis induction, and caspase-3 activity, which were effectively suppressed by NAC co-treatment. These data indicated the effective antiproliferative effect of levan on colorectal cancer cells, in which downregulation of ROS-mediated HOTAIR/Akt plays an important role.
Keywords
Levan polysaccharide
Colorectal cancer
Akt
lncRNA
HOTAIR
Signaling
1 Introduction
Colorectal cancer is known as the third most common cancer worldwide (Favoriti et al., 2016). Over the past decade, some agents, including natural plant-based materials, which contain large amounts of biologically active substances, have led to the safety, recovery, and survival of patients with colorectal cancer (Redondo-Blanco et al., 2017; DeLuca et al., 2018; Rejhová et al., 2018; Forster et al., 2013; Benarba and Pandiella, 2018; Gavrilas et al., 2016).
Finding natural polymers with desirable properties for use in biomedicine is one of the important axes of research in the world (Redondo-Blanco et al., 2017; DeLuca et al., 2018; Rejhová et al., 2018; Forster et al., 2013; Benarba and Pandiella, 2018; Gavrilas et al., 2016; Ranjbari et al., 2017). Polysaccharides are one of the types of natural polymers that have various applications in various industries as well as in the field of medicine (Matsuda et al., 2003; Lu et al., 2017; Mendhulkar et al., 2020). Due to their abundance, biodegradability, naturalness and renewability, they are a good option to replace some substandard materials and applications that require optimal biocompatibility (Xie et al., 2020). The enormous structural diversity of polysaccharides in nature has given rise to a wide range of anticancer applications (Xie et al., 2020). For this reason, identifying the structure of polysaccharides is necessary to justify and improve their properties and is considered one of the sciences of interest to researchers today. Identification of the structure of water-soluble non-cellulose polysaccharides, due to their great structural diversity and to determine their medicinal properties and effects, is widely used in research and articles around the world (Xie et al., 2020; Gamal-Eldeen et al., 2020).
Levan is known as one of fructose polysaccharides, which is commonly synthesized by various microorganisms and some plants. It has been widely reported that levan can show potential anticancer properties (Yoo et al., 2004; Sarilmiser and Oner, 2014; Koşarsoy Ağçeli and Cihangir, 2020). For example, it has been shown that levan induced potential selective anticancer effects against hepatocellular carcinoma cells (Abdel-Fattah et al., 2012). Furthermore, it has been indicated that levan polysaccharide increases anticancer and pro-apoptotic impacts in breast cancer cells via production of reactive oxygen species (ROS) (Queiroz et al., 2017).
Although the anticancer properties of levan have been reported in different studies, the signaling pathways involved in the antiproliferative effect of this compound on cancer cells, especially colorectal cancer, have not been well examined. Overexpression of long noncoding RNAs (lncRNAs) HOX antisense intergenic RNA (HOTAIR) was demonstrated to promote human cancer proliferation and drug resistance through modulation of the Akt signaling pathway (Yan et al., 2016; Yu et al., 2017; Pan et al., 2019). For example, it has been shown that HOTAIR can control proliferation, apoptosis, and migration of breast cancer cells through the Akt signaling pathway (Li et al., 2019). Also, it has been reported that genetic variants in lncRNA HOTAIR can promote the risk of colorectal cancer (Xue et al., 2015) or downregulation of LncRNA HOTAIR prevents colorectal cancer cell proliferation (Lin et al., 2018).
Therefore, the main novelty of this work is to examine the association between the anticancer properties of levan against colorectal cell line and inactivation of lncRNA HOTAIR/Akt signaling pathway.
2 Materials and methods
2.1 Materials
Levan from Erwinia herbicola, 2, 7-dichlorofluorescin diacetate (H2-DCFH-DA), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), antibiotics, and Annexin V FITC iodide were purchased from Sigma-Aldrich (St. Louis, Missouri, United States).
2.2 Cell culture and treatment
HT-29 cells were subcultured in DMEM culture medium supplemented with FBS (10%) and 1% antibiotics at CO2 (5%) at 37 °C. The cells at 85% confluence were then subcultured into different plates based on designed assays. The stock solution of levan (5 mg/mL) was prepared in double distilled water and diluted for the experimental assays and added to cells for 24 hrs. Control cells were not incubated with levan. For assessing the role of oxidative stress in levan-stimulated cytotoxicity, the cells were co-incubated with N-acetyl-L-cysteine (NAC) as a well-known antioxidant with a concentration of 20 µM for 24 hrs.
2.3 MTT assay
The reduction of MTT into formazan crystals in HT-29 cells was assessed to explore the toxicity of levan. HT-29 cells (5 × 103 per well) were cultured and exposed to increasing concentrations of levan (0, 0.1, 0.5, 10, 50 and 100 mg/mL) for 24 hrs. The culture media was then removed and replaced by fresh culture ones (200 µL) containing MTT solution (5 mg/mL) and incubated for 4 h at 37 °C. The formed crystals were then solubilized in DMSO and the absorbance of the samples was read at 570 nm with a microplate reader.
2.4 Lactate dehydrogenase enzyme (LDH) assay
The cells were treated with increasing concentrations of levan for 24 hrs. Then, the culture media were removed and LDH assay was done by using LDH assay kit (601170 Cayman chemical kit, Netherland) following the manufacturer’s protocol.
2.5 Evaluation of intracellular ROS
Generation of intracellular ROS in HT-29 cells after exposure to IC50 concentration of levan for 24 hrs was investigated based on H2-DCFH-DA staining method. Briefly, 1 × 106 cells were exposed to IC50 concentration of levan for 24 hrs, washed, added by DCFH-DA (10 µM) at 37 °C for 45 min, and washed. Finally, the fluorescence intensity was read by flow cytometry.
2.6 Apoptosis assay
Apoptosis assay was done using Annexin V apoptosis kit (BD Bioscience, San Jose, CA, USA) following the manufacturer’s protocol. Briefly, 1 × 106 cells were exposed to IC50 concentration of levan for 24 hrs, collected and used for staining with Annexin V. The samples were then analyzed by flow cytometry.
2.7 Enzyme assay
After treatment of the cells with IC50 concentration of levan for 24 hrs, cell lysates were prepared in RIPA buffer supplemented with protease and phosphatase inhibitors. The activity of superoxide dismutase (SOD, Cat# ab65354, UK), catalase (CAT, Cat# ab83464, UK), and caspase-3 (Cat# ab39401, UK) was then assessed following the manufacturer’s protocols.
2.8 qRT-PCR analysis of HOTAIR
After treatment of the cells with IC50 concentration of levan for 24 hrs, total RNA was extracted using RNA purification kit (RNB100, Sigma, USA) and cDNA was synthesized using cDNA synthesis kit (11117831001, Sigma, USA) following the manufacturer’s protocol. HOTAIR levels were determined using LightCycler® 480 probes master (Roche Life Science) with the specific primers (Table 1) and the values were normalized to β-actin. Other experimental set up was done based on the previous studies (Livak and Schmittgen, 2001; Zinovieva et al., 2018).
Gene
Forward primer
Reverse primer
HOTAIR
5′-ACGGAACCCATGGACTCATA-3′
5′-TTGGGGAAGCATTTTCTGAC-3′
β-actin
5′- TGACAGGATGCAGAAGGAGA-3′
5′-TAGAGCCACCAATCCACACA-3′
2.9 Western blot analysis
After treatment of the cells with IC50 concentration of levan for 24 hrs, cell lysates were prepared and equal amounts of proteins were assessed by SDS-polyacrylamide gels (12%) and then transferred on to a nitrocellulose membrane for western immunoblotting with specific monoclonal antibodies, namely anti-pAkt and anti- β-actin was used as a loading control. The signals were detected by chemiluminescence.
2.10 Statistical analysis
All data were compared as the average of three independent experiments. One way ANOVA followed by Dunn’s test was used to determine the differences between groups and P ≤ 0.05 was considered a significant difference.
3 Results
3.1 Cytotoxicity assay
The cytotoxic effect of levan on HT-29 cells was explored by MTT and LDH assays and the resultant data were shown in Fig. 1a and b, respectively. It was shown that the levan stimulated cell mortality (Fig. 1a) in a concentration dependent fashion. However, it was determined that levan did not induce a significant effect on the level of LDH release in cell culture media (Fig. 1b), indicating that the levan-stimulated antiproliferative effects against HT-29 cells are not mediated by membrane damage. This data may be due to the hydrophilic nature of levan polysaccharide, which limits its interaction with biological membranes (Peng et al., 2019).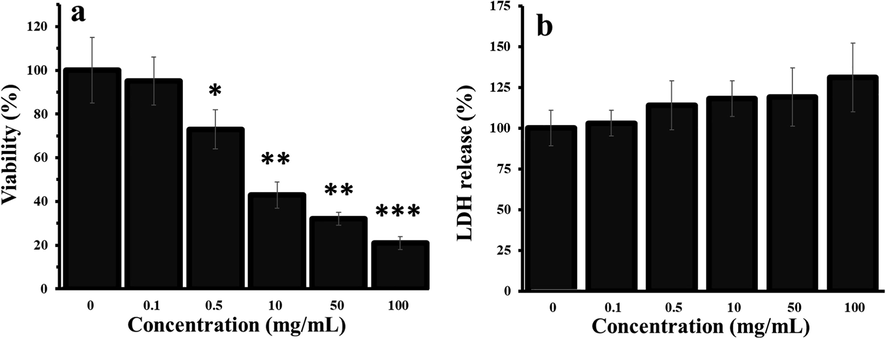
Antiproliferative effects of levan on HT-29 cells for 24 hrs, as explored by (a) MTT and (b) LDH assays. *P < 0.05, ** P < 0.01, *** P < 0.001 vs. control. (The data were obtained based on the methods described in sections 2–3 and 2–4). The cells without any treatment (Concentration of ‘0′ mg/ml) were used as control. Abbreviation: LDH: lactate dehydrogenase.
Thus, it was deduced that levan induced a significant antiproliferative effect against HT-29 cells evidenced by MTT. Also, it was determined that the IC50 concentration of levan against HT-29 cells was about 8.39 ± 1.21 mg/mL.
3.2 ROS and apoptosis assays
Generation of intracellular ROS and subsequent apoptosis can play an essential role in antiproliferative effects of anticancer drugs (Zaidieh et al., 2019; Kim et al., 2019). The generation of high levels of ROS results in inhibition of uncontrolled cell proliferation and induction of cancer. Therefore, many therapeutic platforms are developed to produce a high level of ROS to prevent cancer cell proliferation (Zaidieh et al., 2019; Kim et al., 2019; Vallejo et al., 2017).
To assess whether the anticancer effects of levan in HT-29 cells were associated with generation of high level of ROS and subsequent apoptosis induction, the ROS and apoptosis assays were assessed by flow cytometry. The DCF intensity as a marker of ROS was evaluated in the presence of levan with or without NAC, as a potential antioxidant agent. It was shown that the DCF fluorescence intensity of cells treated with levan is significantly more than control and levan/NAC-treated cells (Fig. 2a). Quantitative analysis of ROS assay showed that the DCF fluorescence intensity values were 85.09, 249.01 and 779.19 for control, levan/NAC- and levan-treated cells, respectively (Fig. 2b). The staining with Annexin V was carried out to explore to assay the apoptosis induction by levan. The flow cytometry analysis indicated that levan increased the Annexin V fluorescence intensity of cells which was reduced after co-incubation of cells with NAC (Fig. 2c). The quantitative assay showed that levan significantly increased the apoptotic cell fractions from 13.21% up to 33.08%, whereas co-incubation of cells reduced the percentage of Anenxin-V+ cells down to 17.01% (Fig. 2d).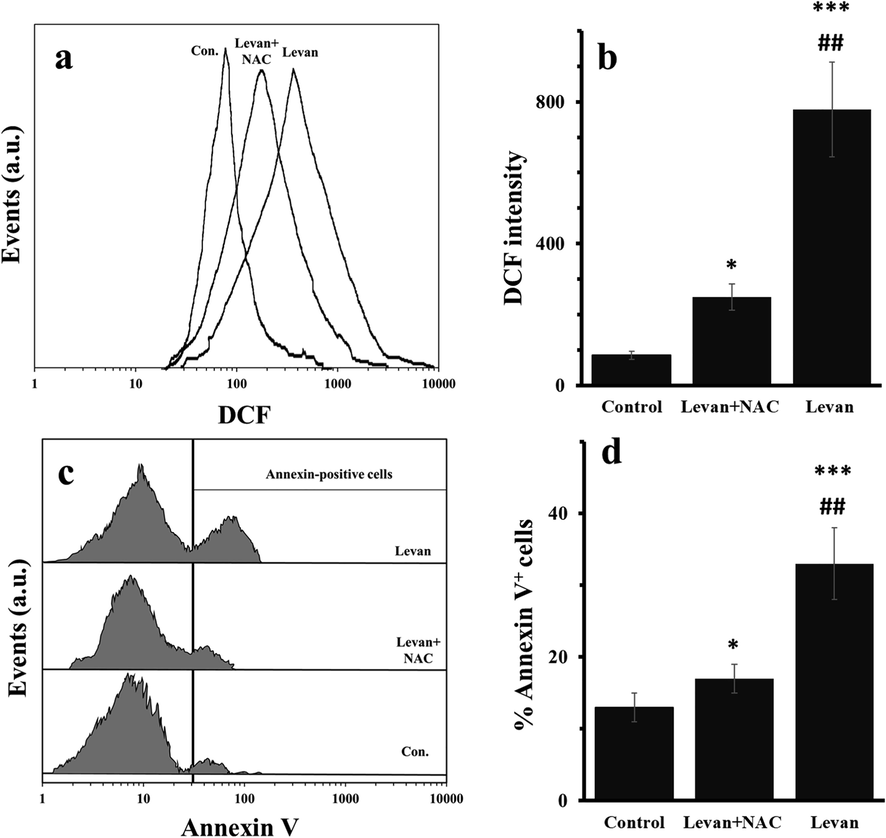
Generation of ROS and induction of apoptosis in HT-29 cells treated with either levan (8.97 mg/mL) or combination of levan (8.39 mg/mL) and NAC (20 µM) for 24 hrs. Representative histogram of flow cytometry analysis of ROS by DCFH-DA (a). Quantitative analysis of generation of ROS (b). Representative histograms of flow cytometry analysis of apoptosis induction measured by Annexin-V staining (c). Quantitative analysis of Annexin-V+ cells (d). *P < 0.05, *** P < 0.001 vs. control. ##P < 0.01 vs. Levan + NAC-treated cells. (The data were obtained based on the methods described in sections 2–5 and 2–6). Abbreviation: DCF; dichlorofluorescein.
3.3 The activity assays of SOD, CAT and caspase-3
Apoptosis regulation is stimulated by the deactivation of antioxidant enzymes and activation of caspases-3 (Santos et al., 2017). The activity of SOD (Fig. 3a) and CAT (Fig. 3b) and caspase-3 (Fig. 3c) was assessed in the HT-29 cells treated with either levan or levan/NAC. It was observed that the activity of SOD (Fig. 3a) and CAT (Fig. 3b) was reduced, whereas the activity of caspase-3 (Fig. 3c) was increased after incubation of cells with levan for 24 hrs. Afterwards, it was determined that co-incubation of levan-treated treated cells with NAC resulted in the recovery of antioxidant enzymes and reduction of caspase-3 activity.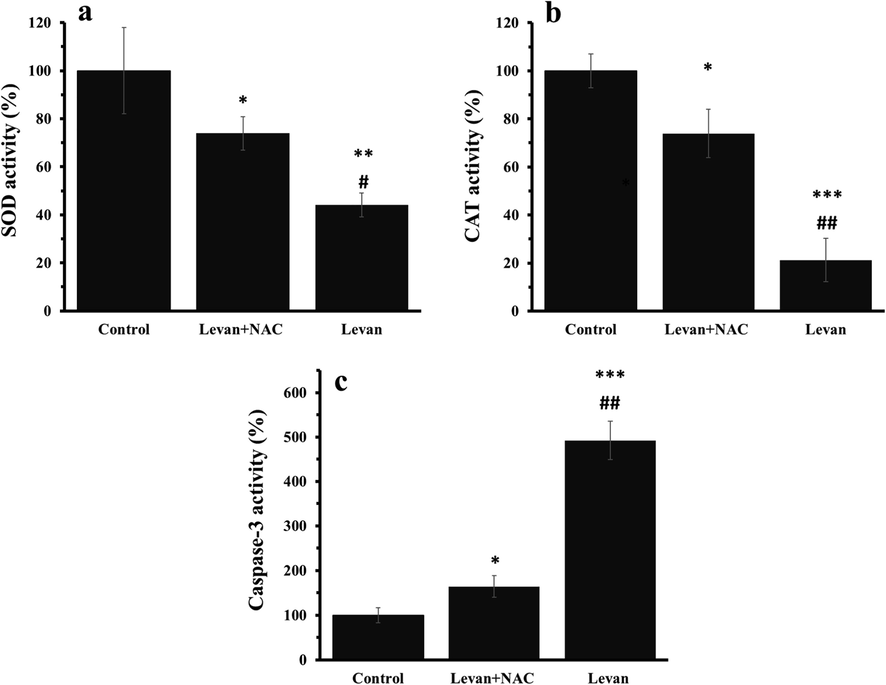
The effect of either levan (8.39 mg/mL) or combination of levan (8.39 mg/mL) and NAC (20 µM) on the activity of antioxidant and apoptotic enzymes in HT-29 cells after 24 hrs. SOD activity (a), CAT activity (b), caspase-3 activity (c). *P < 0.05, ** P < 0.01, *** P < 0.001 vs. control. #P < 0.01, ##P < 0.01 vs. Levan + NAC- treated cells. (The data obtained based on the methods described in section 2–7). Abbreviations: SOD; superoxide dismutase, CAT: Catalase.
3.4 HOTAIR expression
qRT-PCR analysis was done to assess the levels of the HOTAIR in HT-29 cells, and the outcomes indicated a significant down-regulation of HOTAIR in the levan-treated cells, which were significantly up-regulated by NAC co-incubation (Fig. 4).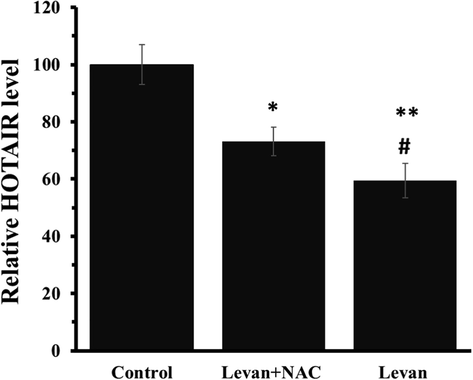
The effect of either levan (8.39 mg/mL) or combination of levan (8.39 mg/mL) and NAC (20 µM) on the relative HOTAIR level in HT-29 cells after 24 hrs (c). *P < 0.05, ** P < 0.01 vs. control. #P < 0.05 vs. Levan + NAC-treated cells. (The data obtained based on the methods described in section 2–8). Abbreviation: HOTAIR; HOX Transcript Antisense RNA.
3.5 The pAkt protein level
Due to the vital role of the HOTAIR signaling pathway in the control of the Akt, we examined the impact of levan on Akt activation in the protein level. Fig. 5a indicated that the level of pAkt in HT-29 cells significantly downregulated after incubation of cells with levan. However, co-treatment with NAC effectively prevented downregulation of pAkt in HT-29 cells (Fig. 5a). Moreover, the quantification of western blot analysis indicated that the relative low ratio of pAkt protein level in levan- treated cells was recovered after co-incubation of cells with NAC (Fig. 5b).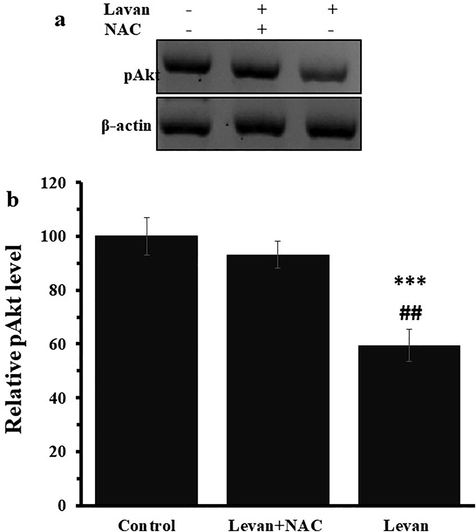
The effect of either levan (8.39 mg/mL) or combination of levan (8.39 mg/mL) and NAC (20 µM) on the relative pAkt protein level in HT-29 cells after 24 hrs. Representative western blot analysis (a), Quantification of western blot analysis (b). *** P < 0.001 vs. control. ##P < 0.01 vs. Levan + NAC-treated cells. (The data obtained based on the methods described in section 2–9).
4 Discussion
Levan [β-(2,6)-linked fructose polymer] polysaccharides have demonstrated promising anticancer effects (Abdel-Fattah et al., 2012; Queiroz et al., 2017), however the effective mechanism underlying the toxicity of levan on colorectal cancer cells is not well-assessed.
Since, HT-29 cell line is a primary tumor of human colon tissue and serves as a typical model of human colon cancer cells with the ability to express carcinoembryonic antigen and form into the differentiated adenocarcinoma consistent with colonic primary, grade I, in this study, a HT-29 cell line was used to assess the toxicity and mechanism of levan-induced apoptosis.
The data revealed that incubation of HT-29 cells with levan could cause a significant cell mortality in a dose dependent manner, produce high amount of ROS, induce cell apoptosis, deactivate antioxidant enzymes and downregulate the expression level of lncRNA HOTAIR and pAkt protein.
The MTT outcome indicated that levan potentially reduced cell proliferation based on a dose-dependent way. Nevertheless, the impact of 0.1 mg/ml levan on HT-29 cell viability was not statistically remarkable compared to the negative control cells. This data indicates that colorectal cancer cells are resistant to the cytotoxicity induced by levan at low concentrations. This concentration-dependent cytotoxicity of levan has been also reported by Abdel (Abdel-Fattah et al., 2012) and Queiroz (Queiroz et al., 2017) on HepG2 and MCF-7 cell lines. It was also shown that levan polysaccharide does not induce a significant injury on the membrane integrity as evidenced by LDH release, indicating the weak interaction of hydrophilic levan polysaccharide with hydrophobic cell membrane. In other words, the LDH assay showed that membrane integrity was not damaged after incubation with levan polysaccharide, whereas the cell mortality assessed by MTT assay was revealed to be significant in the same experimental set up, determining an intracellular target for the induced cytotoxicity (Lemarchand et al., 2006). Indeed, it has been reported that polysaccharide shell can mitigate both opsonization by proteins in the cell culture medium and hydrophobic interactions between the encapsulated particles and the cell membrane (Lemarchand et al., 2006).
In order to investigate whether the levan-induced cytotoxicity against HT-29 cells was associated with the through generation of ROS-triggered apoptosis, ROS assay, antioxidant enzyme assay, apoptosis assay and caspase-3 activity assay was done. The data displayed that levan polysaccharide could stimulate ROS generation through inactivation of SOD and CAT which result in apoptosis. It was also shown that NAC, a potent antioxidant, can mitigate these cytotoxic effects of levan against HT-29 cells. This anticancer effect of levan which induced its cytotoxic effect through oxidation was similar to Queiroz’ research outcomes (Queiroz et al., 2017).
LncRNAs are known as one of the potential regulators in cell proliferation. It has been suggested that HOTAIR could be an effective biomarker for screening colorectal carcinoma (Zhao et al., 2015). Indeed, HOTAIR can regulate colorectal cell growth, apoptosis and tumor growth (Lu et al., 2018). HOTAIR has been confirmed as the oncogene in colorectal cancer development, which could be screened for colorectal progression (Hajjari and Salavaty, 2015). Activation of the AKT pathway involves a wide range of cancers and can be considered as a targeting therapeutic signaling. It has been reported that HOTAIR upregulation exerts its regulatory effect by activating the AKT pathway (Yu et al., 2017; Li et al., 2019; Chen et al., 2015).
To explore the signaling pathway involved in anticancer properties of levan against colorectal cancer cells, the lcRNA HOTAIR/AKT signaling was assessed. The expression level of lcRNA HOTAIR and phosphorylated protein levels of the Akt cascade were significantly inhibited by incubation of cells with levan, while co-incubation of cells with levan and NAC reversed the phosphorylated degree.
Indeed, a number of studies confirms that lncRNAs including HOTAIR are one the key agents in regulating most cancer hallmarks mediated by their interaction with biological macromolecules such as DNA, RNA or proteins and peptides (Tang and Hann, 2018). ROS can control every phase of tumorigenesis by serving as second messengers in tumor cells (Krstić et al., 2015). The interplay between the production of ROS and lncRNAs has been also revealed to control the regulation of multiple mRNAs and the downregulation of a number of signaling pathways involved in cancer progression, demonstrating a probable mechanistic relationship among HOTAIR, ROS and cancer proliferation (Sosa et al., 2013; Liu et al., 2019; Chen et al., 2021). Indeed, it has been indicated that dioscin (Ma et al., 2016) and caveolin (Liu et al., 2018) inhibit the proliferation of gastric and cancer cells, respectively through downregulation of lncRNA HOTAIR. Furthermore, it has been indicated that LncRNA HOTAIR confers drug resistance in different cancer cells including breast (Xue et al., 2016), prostate (Zhang et al., 2015), lung (Guo et al., 2018), gastric (Yan et al., 2016), and colorectal (Li et al., 2017).
In this paper, we provided data that showed the essential role of HOTAIR and the interplay between ROS and HOTAIR in downregulating cancer cell proliferation, which may expand our knowledge of HOTAIR in cancer therapy from a new perspective.
Therefore, it can be speculated that levan as a polysaccharide with potential anticancer effect (Khan et al., 2019) may mitigate the HT-29 cell proliferation by lcRNA HOTAIR, which may downregulate the downstream Akt kinase function, and subsequent cascades activated by AKT. As a result, the inactivation of the AKT pathway eventually leads to ROS-mediated apoptosis. Therefore, levan can be introduced as a potential anticancer agent which may be used for the development of anticancer platforms.
5 Conclusion
The present study demonstrated that levan can be used as a potential anticancer drug against colorectal cancer cells. It probably can be indicated that levan increased the rate of apoptosis and oxidative stress in colorectal cancer cells which result in ROS-mediated deactivation of the HOTAIR /Akt signaling pathway. In the future studies, in addition to exploring the anticancer effects of levan in vivo, the anticancer effects of various species of levan with different degrees of branching can be examined.
Acknowledgement
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







