Translate this page into:
Fluorinated azole anticancer drugs: Synthesis, elaborated structure elucidation and docking studies
⁎Corresponding authors at: Department of Chemistry, Faculty of Science, Cairo University, Giza 12613, Egypt (T. A. Farghaly). thoraya-f@cu.edu.eg (Thoraya A. Farghaly), mrgenidi@uqu.edu.sa (Mohamed R. Shaaban)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present article deals with the synthesis of novel nano-sized fluorinated thiazoles and studying their anticancer potentiality. The targeted azoles could be accessed via trifluoro-methylated thiosemicarbazone (3) prepared by reaction of with thiosemicarbazide in acidic solution of ethanol. The latter a fluorinated building block (3) have been reacted with appropriate derivatives of a-halo compounds namely, N-aryl 2-oxopropane-hydrazonoyl chlorides 4a-f using dioxane containing TEA as base catalyst. Also, the reaction between N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) and chloroacetonitrile 8 under the same experimental conditions furnished the corresponding amino thiazole derivative 11. In the same manner the base catalyzed cyclocondensation reaction between N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) and phenacyl bromide derivatives 12a-d afforded the corresponding thiazoles 13a-d in good yield. The structure of all synthesized thiazole derivatives as well as their mechanistic pathways were studied based on spectral data analysis and physical characteristics. The nanosized products were confirmed by using XRD analysis. Moreover, twelve samples were submitted for evaluation of their cytotoxicity activities against MDA-MB-231 (breast cancer cell) using colorimetric MTT assay, in comparison with Cisplatin standard drug. Two nano-sized thiosemicarbazone derivative 3 and the thiazole derivative 7c showed potent activity with IC50 = 7.7 and 2.97 µg/ml, respectively in compared with the IC50 = 4.33 µg/ml of cisplatin. The nanosized thiazole derivative 7c was more potent than cisplatin. Also, two thiazole derivatives 13b and 7b showed good activity with IC50 = 13.4 and 14.9 µg/ml. In addition, the molecular docking studies have been achieved using 4hy0, (X-chromosome-linked- inhibitor of apoptosis protein; (XIAP)).
Keywords
Thiazoles
Fluorinated compounds
Hydrazonoyl chlorides
Breast cancer
Docking study
1 Introduction
Cancers of all kinds are considered one of the most terrible diseases in the world, as they cause the death of more than 80% of patients annually. However, it causes many mental illnesses, especially in women with breast cancer after undergoing a mastectomy. To overcome the dangerous psychological symptoms of cancer, scientists are doing their best to design and manufacture new anti-cancer drugs, especially for breast cancer. Azole rings are known for their effectiveness against cancer (Al-Said et al., 2011; Mahmoud et al., 2020; Ahmad et al., 2018), bacteria (Al-Hussain et al., 2021; Muhammad et al., 2021), fungi (Ahmad et al., 2018) and many incurable diseases (Ahmad et al., 2018).
Among the azoles, thiazole ring has attracted significant attention in medicinal chemistry for the discovery and development of biologically active compounds (Ankali et al., 2021; Cordeiro and Kachroo, 2020; Mermer, 2020; Oliveira et al., 2020; Qazi et al., 2021), particularly, anticancer agents (Sunkari and Eppakayala, 2021; Ansari et al., 2020; Al-Hussain et al., 2020). It is found in many marine or naturally occurring compounds (Blunt et al., 2018; Li et al., 2018; Williamson et al., 1999) which has biological activity. The efficacy of derivatives containing thiazoles as antimicrobials (Eryılmaz et al., 2020), antineoplastic (Popsavin et al., 2007), anti-diabetes (Chen et al 2013), and others has been proven. Fig. 1 contains the structures of commercially available active drugs incorporated with the thiazole moiety. On the other hand, heterocyclic compounds combined with fluorine atoms have shown important and valuable results in the field of modern organic compound synthesis and the development of biologically relevant drugs used in the construction of pharmaceuticals. Furthermore, several fluorinated compounds have been synthesized as functional materials and spectroscopic methods have been used to investigate docking mechanisms in biological systems (Kirsch, 2013; Fluorine, 2014; Fluorinated, 2009; Reddy, 2015; Fluorine, 2009; Nosovaet al., 2018). By searching the literature, we found that more than 20% of the known drugs and 30% of the common agricultural chemicals on the market are with fluorine atoms (Liu et al., 2015). The trifluoromethyl moiety has bioisosteric characteristics of several groups and has a unique impact in its molecules as excellent metabolic stability, high electronegativity, and lipophilicity (Zhou et al., 2016; Fujiwara and O’Hagan, 2014). This group (CF3) can significantly alter the susceptibility of small molecules to lipids and increase their ability to penetrate the blood-brain barrier, which can lead to better absorption within the body and more desirable transport (Filler et al., 1993).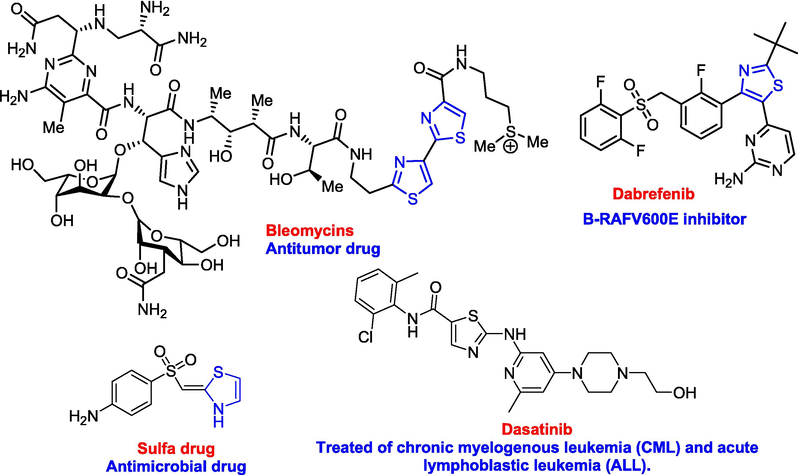
Some commercial drugs containing thiazole ring.
Moreover, nano-size-heterocyclic compounds characterized with effective biological activities due to their small size that allow to penetrate the cell membrane of the viruses or the microbes to DNA easily than the bulky groups. From our observation of all of the above and a complement to our research activities in field of synthesis bioactive heterocyclic compounds (Shaaban et al., 2022; Alhasani et al., 2022; Muhammad et al., 2019; Alsaedi et al., 2019; Dawood et al., 2019; Althagafi et al., 2019; Edrees and Farghaly, 2017; Muhammad et al., 2017), we focused herein to synthesis a series of nano-sized thiazoles carrying in its skeleton a trifluoromethyl group to investigate their activity against breast cancer. Additionally, a docking study of the most promising compounds will be conducted to predict their binding modes within the active site of 4hy0.
2 Results and discussion
2.1 Chemistry
One of the most common and versatile precursors in in heterocyclic synthesis are thiosemicarbazones, they are extensively used in in construction of valuable thiazole derivatives under mild reaction conditions (Mahmoud et al., 2020; Al-Soliemy et al., 2021). The required starting material N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) was prepared via reaction of N-(4-acetylphenyl)-2,2,2-trifluoroacetamide 1 as a fluorinated building block with thiosemicarbazide 2 in ethanol under reflux in the presence of a catalytic amount of hydrochloric acid (Scheme 1). The structure of the thiosemicarbazone 3 was confirmed by all possible data from spectral and elemental analyses. For example, the 1H NMR spectrum showed signals at δ 11.31, 10.19, 8.26, and 7.94 ppm attributed to four NH protons. In addition to one singlet signal with integration = 3H for methyl CH3 group at δ 2.30 and two doublet signals in the rang of δ 7.68–7.97 for the aromatic protons (Fig. 2). It is important to noted that the two protons of amino group are nonequivalent due to the intramolecular-H bond of one H with =N group (Zhang et al., 2009). Mass spectrum of the thiosemicarbazone derivative 3 showed the expected molecular ion peak at m/z = 304. In addition, the absorption bands for NH, NH2, C=O and C=N were appeared in its IR spectrum at 3410, 3349, 3269, 1704, 1606 cm−1, respectively. In addition to the other vibrational frequencies at 1537, 1183, 832 cm−1.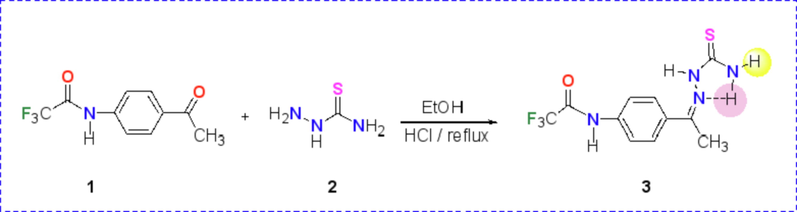
Synthesis of thiosemicarbazone derivative 3.
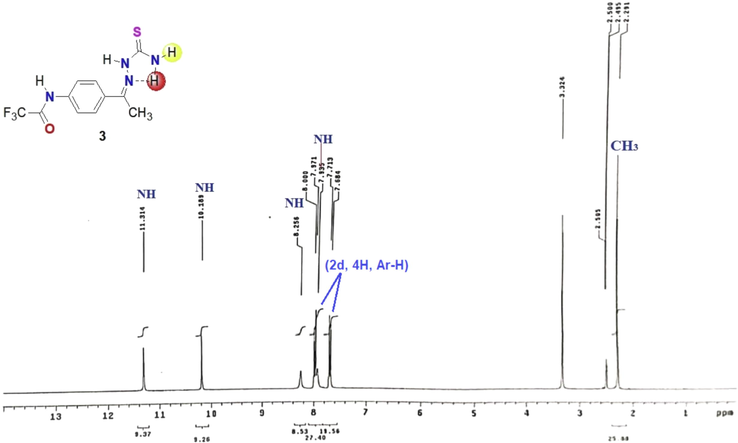
The 1H NMR spectrum of thiosemicarbazone derivative 3.
The synthetic utility of the thiosemicarbazone 3 have been explored in the synthesis of targeted thiazole derivatives containing trifluoroacetamido group via reaction between N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) and N-aryl 2-oxopropane-hydrazonoyl chlorides 4a-f as depicted in Scheme 2. The thiazole derivatives 7a-f were achieved through the substitution and cyclization reaction of thiosemicarbazone derivative 3 with 2-oxo-N-arylpropanehydrazonoyl chloride 4a-f in dioxane under reflux and in the presence of TEA as a catalyst. The structure of these products 7a-f were proved based on their spectral and elemental analyses. For example, the 1H NMR spectrum of thiazole derivative 7b (Fig. 3) showed the characteristic three methyl group singlet signals at δ = 2.29, 2.49 and 2.58 ppm in addition to two NH’s singlet signals at δ = 10.47 and 11.42 ppm and the characteristic protons of the two aromatic rings as multiples in the range at δ = 6.78–7.78 with J = 8.7 Hz. Also, all IR spectra of derivatives 7a-f were free from the characteristic carbonyl groups absorption bands of the starting hydrazonoyl halide substrate, as instance, the characteristic bands of 7b appeared at νmax 3285 (br. 2NH), 3050 (sp2 CH), 2922 (sp3 C-H), 1727 (C=O), 1608 (C=N), 1493, 1290, 1188, 1159, 1017 cm−1.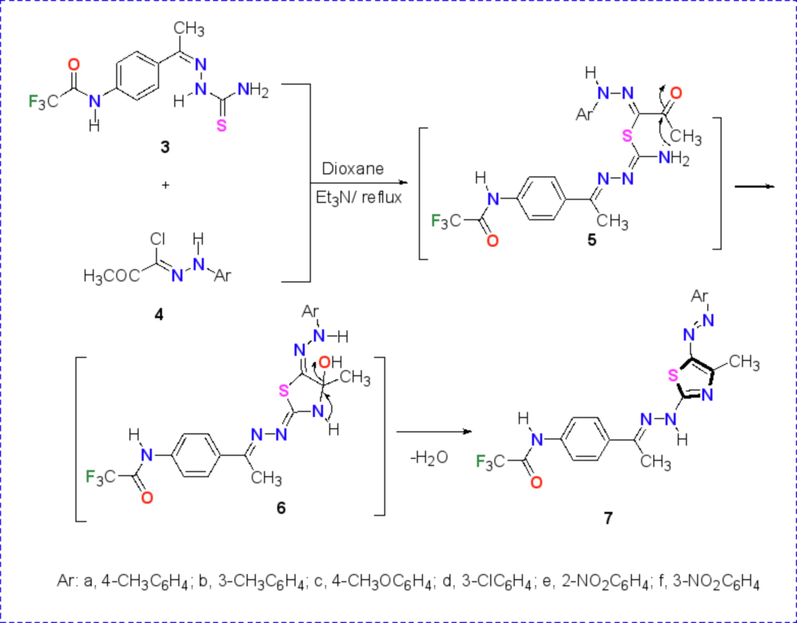
Synthesis of thiazole derivatives 7a-f.
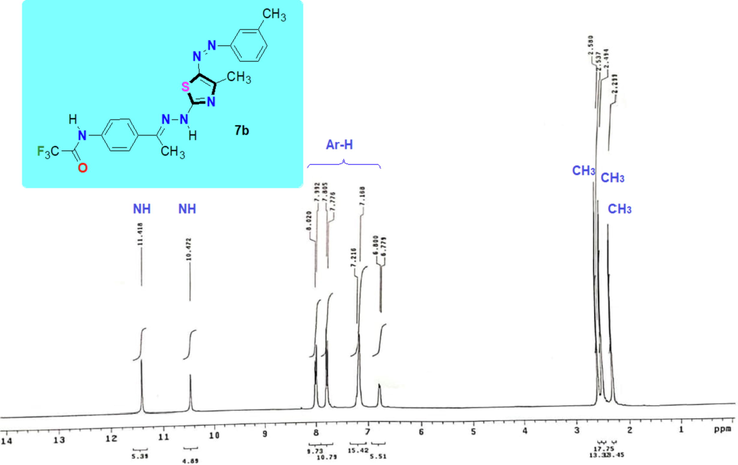
The 1H NMR spectrum of Thiazole derivatives 7b.
Two intermediates 5 and 6 are involved in this reaction through the initial S-alkylation of the thiosemicarbazone 3 by the hydrazonoyl chlorides 4a-f followed by cyclization through nucleophilic condensation reaction as shown in Scheme 2.
The reactivity of carbothioamide 3 towards α-halo nitriles was examined to achieve the synthesis of novel amino thiazole derivative incorporated a trifluoroacetamido group as shown in Scheme 3. Thus, the reaction between N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) and chloroacetonitrile 8 in the presence of a basic catalyst (TEA) in refluxing dioxan furnished the corresponding amino thiazole derivative 11. The structure of the synthesized amino thiazole has been elucidated via the recorded spectral δata and elemental analyses. 1H NMR spectrum of amino-thiazole derivative 11 was recorded in DMSO‑d6 and revealed four singlet signals for CH3, NH2, CH2 and NH at δ = 2.55, 3.95, 4.45 and 10.18 ppm. The IR spectrum of amino-thiazole derivative 11 showed absorption bands at 3385, 3164 and 3112 cm−1 due to the NH2 and NH groups.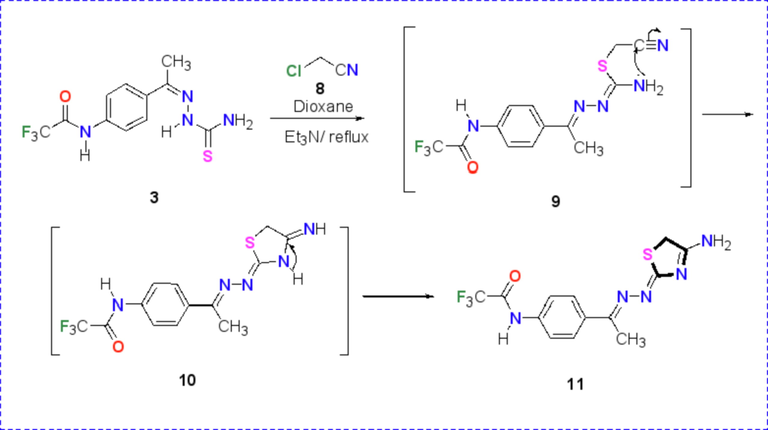
Synthesis of amino-thiazole derivative 11.
In the same manner the base catalyzed cyclocondensation reaction between N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) and phenacyl bromide derivatives 12a-d afforded the corresponding thiazoles 13a-d as depicted in Scheme 4. The structures of the reaction products have been confirmed by spectroscopic data as well as elemental analysis.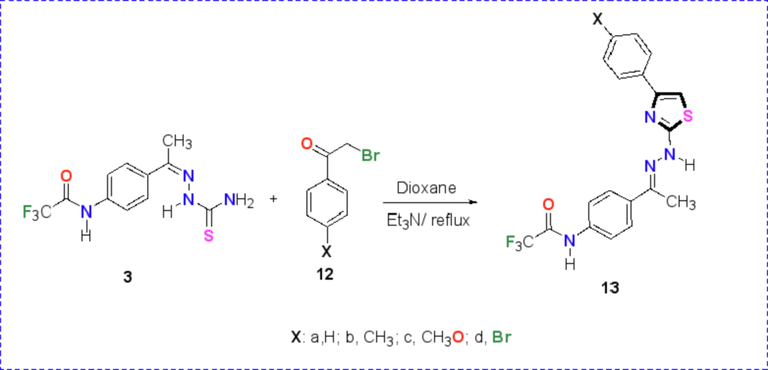
Synthesis of thiazole derivatives 13a-d.
2.2 X-Ray diffraction studies of the synthesized derivatives
XRD scanning was recorded for four selected derivatives 3, 7c, 11 and 13d using Cu/Kα1 radiation-source at 2θ ranging from 0° to 90° (Fig. 4& Fig. 5). The starting thiosemicarbazone 3 and thiazole derivative 7c found in crystalline feature while the other two thiazole derivatives 11 and 13d are found as amorphous shape. Through FWHM method, the size of the crystals was estimated by Bragg and Scherrer equations (Cullity and Stock, 2001) for the two crystalline derivatives. The size of derivatives 3 and 7c are in the nanometer range that equal 36.59 and 27.99 nm.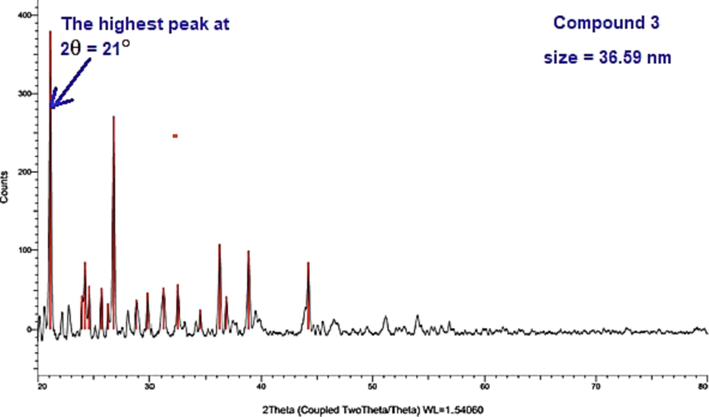
XRD of compound 3.
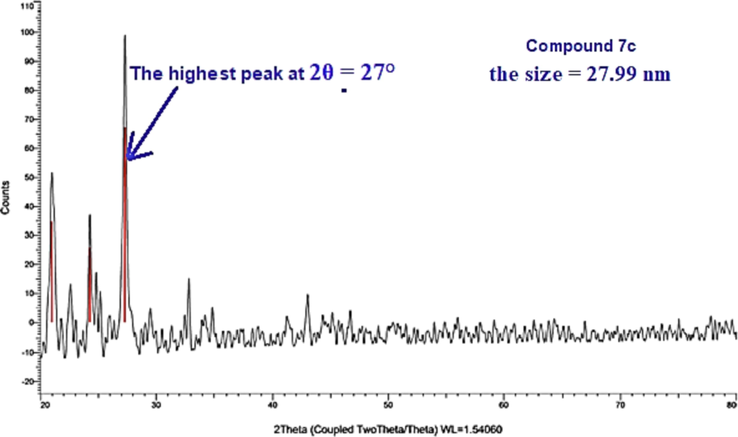
XRD of compound 7c.
2.3 Biological screening of the novel thiazole derivatives
Twelve samples were submitted for evaluation of their cytotoxicity activities against MDA-MB-231 (breast cancer cell) using colorimetric MTT assay, in comparison with Cisplatin standard drug. All drug concentration were plotted against the cell viability to get the survival curve (cited in supplementary file). The 50% inhibitory concentration (IC50) was obtained and the anti-proliferative activity was expressed as the mean IC50 of three independent experiments (μM) ± standard deviation from three replicates. The results were tabulated in Table 1. From the results of activity, we noted that the most active derivatives are the two nano-sized thiosemicarbazone derivative 3 and the thiazole derivative 7c with IC50 = 7.7 and 2.97 µg/ml, respectively in compared with the IC50 = 4.33 µg/ml of cisplatin. In detailed, For the thiazole derivatives 7a-f, the methoxy derivative 7c revealed good anticancer activity. The thiazole derivative carrying Cl at position 3 of aromatic ring 7d showed moderate activity with IC50 value of 27.2 µg/ml. On the other hand, compounds 7a and 7f were the least active derivatives in this series showing IC50 of 97.1 and 366 µg/ml, respectively. Concerning the other thiazole series 13a-d, derivative 13b showed good activity. While thiazoles 13a and 13c displayed moderate cytotoxicity (IC50 = 28.8 and 55.8 µg/ml, respectively). The bromo-thiazole derivatives 13d exhibited weak activity with IC50 value of 408 µg/ml. Finally. the nanosized thiazole derivative 7c was more potent than cisplatin. Also, two thiazole derivatives 13b and 7b showed good activity with IC50 = 13.4 and 14.9 µg/ml.
Sample Code
IC50 values (µg/ml)
MDA-MB-231
3
7.7 ± 0.41
7a
97.1 ± 4.9
7b
14.9 ± 0.97
7c
2.97 ± 0.32
7d
27.2 ± 1.7
7e
51.8 ± 3.4
7f
366 ± 21.4
11
45.8 ± 2.8
13a
28.8 ± 1.9
13b
13.4 ± 0.85
13c
55.8 ± 3.8
13d
408 ± 19.8
Cisplatin
4.33 ± 0.12
2.4 Docking study for selected derivatives
The use of theoretical programs to predict the biological activity of compounds before starting to synthesize is considered a scientific trend that benefits the drug manufacturing system. As it reduces time and effort and helps design drugs with functional groups of high efficiency. One of those programs is molecular docking, which has become a widespread tool for drug discovery (Gümüş et al, 2022; Mahmudov et al., 2022). To foretell the binding mode of thiosemicarbazone derivative 3 and the thiazole derivatives 7b, 7c, 7d, 11, 13a and 13b into the active site of the tumor cell MDA-MB-231, a docking study of these selected derivatives was executed using MOE (2014.0901) (Molecular Operating Environment software). The molecular docking studies have been achieved using 4hy0, (X-chromosome-linked- inhibitor of apoptosis protein; (XIAP)). XIAP is a member of IAP family which has important role in blocking cell death. Its inhibitor leads to apoptosis induction and thus can be used as a target in cancer treatment. The results are presented in Table 2 and Figs. 6-9. Redocking of the co-crystallized ligand (3S,7R,8AR)-2-{(2S)-2-(4,4-difluorocyclohexyl)-2-[(N-methyl-L-alanyl)amino]acetyl}-N-[(4R)-3,4-dihydro-2H-chromen-4-YL]-7-ethoxyocta-hydropyrrolo[1,2-a]pyrazine-3-carboxamide) was first done for validation. It revealed docking score = -5.2142 kcal/mol. Noticeably, all tested derivatives were involved in interactions with 4hy0-protein with docking score ranging from −5.6099 to −4.2056 kcal/mol. The most reactive derivative 7c showed the highest docking score −5.6099 kcal/mol; this result was coincident with the in vitro experimental results. Table 2 and Fig. 7 and Fig. 8 indicated that there are several types of interaction as H-acceptor, H-donor pi-H. The essential part for the interaction in derivative 7c were the thiazole ring and the trifluoromethyl groups which involved in the interaction with the 4hy0-protein THR271 and LYS299 residue, respectively. The docking score for compound 7c is larger than that for the co-crystallized ligand. The low value of docking score of thiosemicarbazide 3 in compared with all tested thiazole derivatives 7b, 7c, 7d, 11, 13a and 13b can be attributed to the lack of the thiazole ring.
Compd.
Ligand moiety
Receptor site
Interacting residues (Type of interaction)
Distance (oA)
E (kcal/mol)
Docking score (kcal/mol)
3
N 11
O THR 271 (A)
H-donor
2.99
−6.0
−4.2056
7b
N 2
6-ring
6-ringO GLY 273 (A)
CA THR 271 (A)
N GLY 295 (A)H-donor
pi-H
pi-H3.30
4.02
4.45−1.3
−0.6
−0.9−5.0713
7c
F 50
5-ringNZ LYS 299 (A)
CA THR 271 (A)H-acceptor
pi-H2.97
4.02−1.3
−0.7−5.6099
7d
–
–
–
–
–
−5.0313
11
N 20
O 31N GLY 273 (A)
N GLY 295 (A)H-acceptor
H-acceptor3.35
3.35−1.2
−0.9−4.6352
13a
O 29
N GLY 295 (A)
H-acceptor
3.34
−0.6
−5.0236
13b
–
–
–
–
–
−5.0880
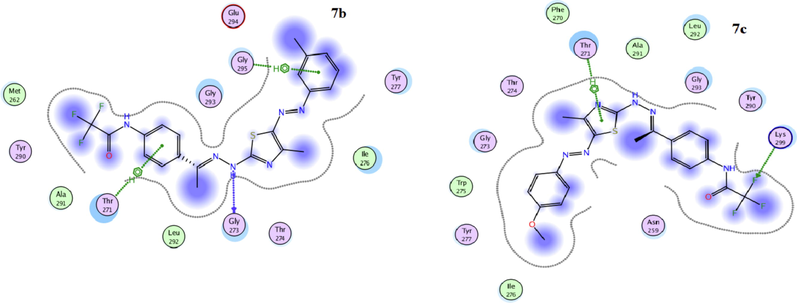
The 2D docked model of compounds 7b and 7c into the active site of 4hy0.
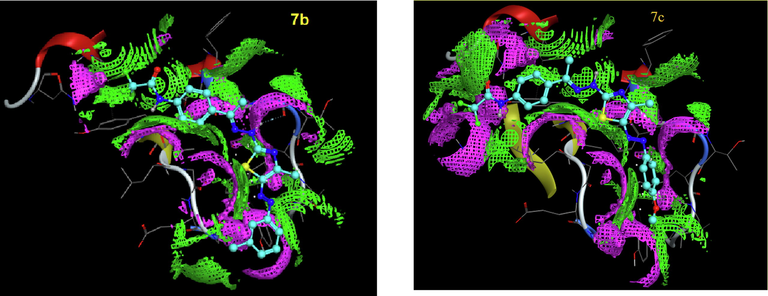
The contact performance of compounds 7b and 7c into the active site of 4hy0, respectively.
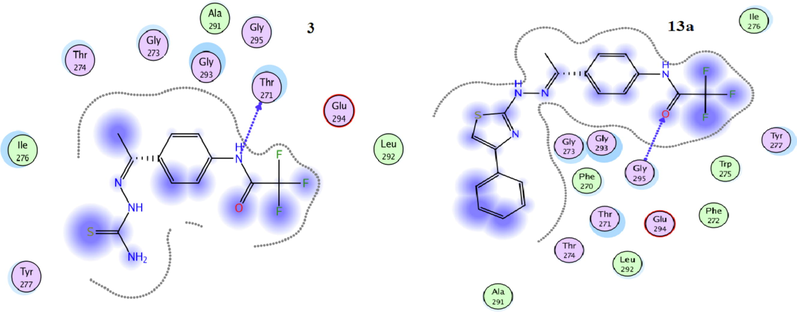
The 2D docked model of compounds 3 and 13a into the active site of 4hy0.
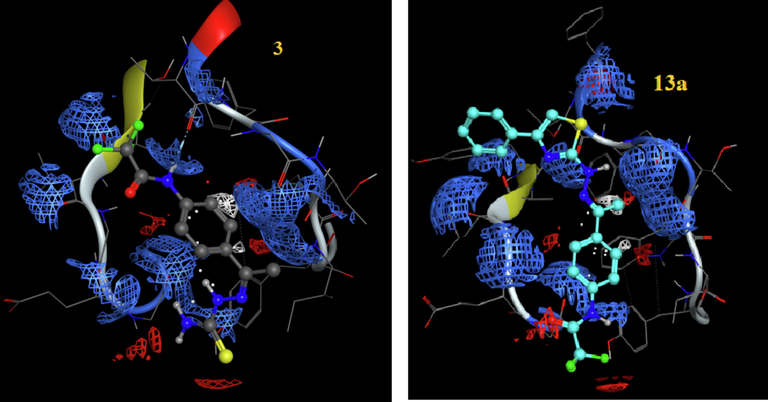
The electrostatic map of compounds 3 and 13a into the active site of 4hy0, respectively.
3 Conclusion
In conclusion, it is evident that this study has shown a convenient pathway to access novel nano-sized fluorinated thiazoles using a mild reaction condition. Thus, the trifluoro-methylated thiosemicarbazone (3) have been reacted with appropriate derivatives of a-halo compounds namely, N-aryl 2-oxopropane-hydrazonoyl chlorides 4a-f, chloroacetonitrile 8 and phenacyl bromide derivatives 12a-d in dioxane containing catalytic amount of triethylamine afforded the corresponding thiazoles 13a-d in good yield. The structure elucidation of the targeted compounds could be achieved by spectral data analysis and physical tools. Evaluation of the cytotoxicity activities of the synthesized products against MDA-MB-231 (breast cancer cell) using colorimetric MTT assay, in comparison with Cisplatin standard drug have been established. Some nano-sized products showed a fruitful antitumor activity specially when compared with the reference drug. The molecular docking studies have concluded the obtained biological results efficiently.
4 Experimental
4.1 Chemistry
The supplementary file contains all characterization and pictures for each device that have been utilized to record the spectral data for the new synthesized derivatives (S1).
4.1.1 Synthesis of N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3)
Previously [20,23], we described the synthesis of related thiosemicarbazone derivatives as follows: condensation reaction under reflux of acetyl derivative 1 (0.005 mol) with thiosemicarbazide 2 (0.005 mol) in 20 mL ethanol after the solution was boiled, drops of conc. HCl was added. Through the reflux of the reaction, the yellow solid of the thiosemicarbazone derivative 3 was precipitated after ten min., then the reflux was completed for one hour. The yellow solid was collected and crystallized from ethanol as white solid, (84% yield), mp 210–212 °C; IR (KBr) νmax 3410, 3349, 3269 (NH and NH2), 3181 (sp2 C-H), 1704 (C = O), 1606 (C = N), 1537, 1183, 832 cm−1; 1H NMR (DMSO‑d6) δ 2.30 (s, 3H, CH3), 7.68 (d, J = 9 Hz, 2H, Ar-H), 7.94 (s, 1H, NH), 7.97 (d, J = 9 Hz, 2H, Ar-H), 8.26 (s, 1H, NH), 10.19 (s, 1H, NH), 11.31 (s, 1H, NH); MS m/z (%) 304 (M+, 11), 298 (39), 256 (25), 255 (31), 235 (73), 205 (21), 167 (38), 135 (34), 101 (28), 97 (62), 82 (26), 78 (34), 77 (20). Anal. Calcd. for C11H11F3N4OS (304.29): C, 43.42; H, 3.64; N, 18.41. Found: C, 43.38; H, 3.47; N, 18.31%.
4.1.2 Synthesis of thiazole derivatives 7a-f, 11 and 13a-d
A mixture of N-(4-(1-(2-carbamothioylhydrazineylidene)ethyl)phenyl)-2,2,2-trifluoroacetamide (3) and α-haloketones 4a-f or 12a-d or chloro-acetonitrile 8 (0.0025 mol of each) in 100 mL round flask dissolved on hot in dioxane (20 mL). Then, Et3N (0.35 mL) was added and the whole mixture was refluxed for 5 h. After the reactions were completed via monitoring with TLC, the colored solids were collected and purified through crystallization from dioxane to give thiazole derivatives 7a-f, 13a-d and 11, respectively.
4.1.2.1 2,2,2-Trifluoro-N-(4-{1-[(4-methyl-5-p-tolylazo-thiazol-2-yl)-hydrazono]-ethyl}-phenyl)-acetamide (7a)
Red solid, (86% yield), mp 238–240 °C; IR (KBr) νmax 3400, 3307 (2NH), 2938 (sp3 C-H), 1704 (C = O), 1599 (C=N), 1536, 1503, 1375, 1298, 1236, 1181, 1034, 830 cm−1; 1H NMR (CDCl3) δ 2.36 (s, 3H, CH3), 2.52 (s, 3H, CH3), 2.68 (s, 3H, CH3), 7.17–8.12 (m, 8H, Ar-H), 10.42 (s, 1H, NH), 11.21 (s, 1H, NH); MS m/z (%) 460 (M+, 43), 434 (23), 390 (39), 382 (53), 368 (55), 360 (52), 344 (56), 334 (60), 319 (70), 299 (64), 229 (52), 168 (100), 128 (32), 96 (33), 76 (29). Anal. Calcd. for C21H19F3N6OS (460.48): C, 54.77; H, 4.16; N, 18.25. Found: C, 54.59; H, 4.02; N, 18.09%.
4.1.2.2 2,2,2-Trifluoro-N-(4-{1-[(4-methyl-5-m-tolylazo-thiazol-2-yl)-hydrazono]-ethyl}-phenyl)-acetamide (7b)
Orange solid, (83% yield), mp 212–214 °C; IR (KBr) νmax 3285 (br. 2NH), 3050 (sp2 CH), 2922 (sp3 C-H), 1727 (C=O), 1608 (C=N), 1493, 1290, 1188, 1159, 1017 cm−1; 1H NMR (CDCl3) δ 2.29 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.58 (s, 3H, CH3), 6.78–7.22 (m, 4H, Ar-H),7.78 (d, J = 8.7 Hz, 2H, Ar-H), 7.99 (d, J = 8.7 Hz, 2H, Ar-H), 10.47 (s, 1H, NH), 11.42 (s, 1H, NH); MS m/z (%) 460 (M+, 26), 447 (91), 426 (47), 423 (100), 408 (41), 361 (54), 334 (66), 223 (44), 172 (51), 90 (15). Anal. Calcd. for C21H19F3N6OS (460.48): C, 54.77; H, 4.16; N, 18.25. Found: C, 54.65; H, 4.02; N, 18.19%.
4.1.2.3 2,2,2-Trifluoro-N-[4-(1-{[5-(4-methoxy-phenylazo)-4-methyl-thiazol-2-yl]-hydrazono}-ethyl)-phenyl]-acetamide (7c)
Red crystals, (82% yield), mp 203–204 °C; IR (KBr) νmax 3441 (br. 2NH), 1708 (C=O), 1599 (C=N), 1374, 1293, 1265, 1192, 1161 cm−1; 1H NMR (CDCl3) δ 2.47 (s, 3H, CH3), 2.57 (s, 3H, CH3), 3.75 (s, 3H, OCH3), 6.93 (d, J = 7.8 Hz, 2H, Ar-H), 7.34 (d, J = 7.8 Hz, 2H, Ar-H), 7.77 (d, J = 8.7 Hz, 2H, Ar-H), 7.97 (d, J = 8.7 Hz, 2H, Ar-H), 10.0 (s, 1H, NH), 11.44 (s, 1H, NH); MS m/z (%) 476 (M+, 15), 469 (81), 459 (50), 442 (51), 416 (58), 404 (40), 369 (23), 347 (50), 328 (47), 320 (100), 314 (77), 290 (42), 274 (60), 269 (57), 154 (36), 76 (35). Anal. Calcd. for C21H19F3N6O2S (476.47): C, 52.94; H, 4.02; N, 17.64. Found: C, 52.86; H, 3.97; N, 17.48%.
4.1.2.4 N-[4-(1-{[5-(3-Chloro-phenylazo)-4-methyl-thiazol-2-yl]-hydrazono}-ethyl)-phenyl]-2,2,2-trifluoro-acetamide (7d)
Orange solid, (86% yield), mp 237–238 °C; IR (KBr) νmax 3410, 3287 (2NH), 1706 (C=O), 1595 (C=N), 1595, 1511, 1244, 1169 cm−1; 1H NMR (DMS-d6) δ 2.50 (s, 3H, CH3), 2.59 (s, 3H, CH3), 6.98–7.35 (m, 4H, Ar-H), 7.78 (d, J = 8.7 Hz, 2H, Ar-H), 8.0 (d, J = 8.7 Hz, 2H, Ar-H), 10.61 (s, 1H, NH), 11.44 (s, 1H, NH); MS m/z (%) 480 (M+, 48), 475 (53), 449 (39), 432 (40), 424 (100), 376 (47), 328 (39), 271 (29), 198 (38), 150 (37), 131 (43), 77 (18). Anal. Calcd. for C20H16F3N6OS (480.89): C, 49.95; H, 3.35; N, 17.48. Found: C, 49.83; H, 3.26; N, 17.35%.
4.1.2.5 2,2,2-Trifluoro-N-[4-(1-{[4-methyl-5-(2-nitro-phenylazo)-thiazol-2-yl]-hydrazono}-ethyl)-phenyl]-acetamide (7e)
Red solid, (80% yield), mp 212–214 °C; IR (KBr) νmax 3287 (br. 2NH), 1703 (C=O), 1609 (C=N), 1535, 1350, 1248, 1162 cm−1; 1H NMR (CDCl3) δ 2.59 (s, 3H, CH3), 2.70 (s, 3H, CH3), 7.02–8.22 (m, 8H, Ar-H), 10.65 (s, 1H, NH), 11.80 (s, 1H, NH); MS m/z (%) 491 (M+, 34), 455 (57), 424 (100), 390 (68), 371 (57), 306 (66), 276 (50), 108 (66), 104 (70). Anal. Calcd. for C20H16F3N7O3S (491.45): C, 48.88; H, 3.28; N, 19.95. Found: C, 48.74; H, 3.16; N, 19.87%.
4.1.2.6 2,2,2-Trifluoro-N-[4-(1-{[4-methyl-5-(3-nitro-phenylazo)-thiazol-2-yl]-hydrazono}-ethyl)-phenyl]-acetamide (7f)
Red solid, (78% yield), mp 210–212 °C; IR (KBr) νmax 3353 (br. 2NH), 3058 (sp2 C-H), 1713 (C=O), 1598 (C=N), 1514, 1439, 1321, 1157 cm−1; 1H NMR (CDCl3) δ 2.29 (s, 3H, CH3), 2.63 (s, 3H, CH3), 6.66–7.83 (m, 8H, Ar-H), 10.76 (s, 1H, NH), 11.24 (s, 1H, NH); MS m/z (%) 491 (M+, 26), 451 (66), 366 (41), 280 (54), 266 (100), 247 (87), 190 (56), 171 (33), 124 (99), 109 (69), 76 (21). Anal. Calcd. for C20H16F3N7O3S (491.45): C, 48.88; H, 3.28; N, 19.95. Found: C, 48.69; H, 3.09; N, 19.78%.
4.1.2.7 N-(4-{1-[(4-Amino-5H-thiazol-2-ylidene)-hydrazono]-ethyl}-phenyl)-2,2,2-trifluoro-acetamide (11)
Buff solid, (88% yield), mp 190–192 °C; IR (KBr) νmax 3385, 3164, 3112 (NH2, NH), 1649 (C=O), 1600 (C=N), 1530, 1485, 1441, 1247, 1188, 1155, 1031 cm−1; 1H NMR (DMS-d6) δ 2.55 (s, 3H, CH3), 3.95 (s, 2H, NH2), 4.45 (s, 2H, CH2), 7.69–8.30 (m, 4H, Ar-H), 10.18 (s, 1H, NH); MS m/z (%) 343 (M+, 36), 335 (70), 315 (45), 300 (99), 283 (71), 267 (64), 192 (44), 144 (100), 122 (31), 88 (50), 75 (42). Anal. Calcd. for C13H12F3N5OS (343.33): C, 45.48; H, 3.52; N, 20.40. Found: C, 45.31; H, 3.43; N, 20.28%.
4.1.2.8 2,2,2-Trifluoro-N-(4-{1-[(4-phenyl-thiazol-2-yl)-hydrazono]-ethyl}-phenyl)-acetamide (13a)
White solid, (83% yield), mp 270–272 °C; IR (KBr) νmax 3459, 3113 (2NH), 3072 (sp2 CH), 1702 (C=O), 1619, 1587 (C=N), 1514, 1414, 1367, 1291, 1255, 1208, 1159 cm−1; 1H NMR (CDCl3) δ 2.45 (s, 3H, CH3), 7.37–8.33 (m, 10H, Ar-H and Thiazole-H), 11.01 (s, 1H, NH), 11.40 (s, 1H, NH); MS m/z (%) 404 (M+, 20), 391 (21), 355 (34), 319 (57), 284 (48), 260 (46), 240 (74), 1227 (100), 109 (36). Anal. Calcd. for C19H15F3N4OS (404.41): C, 56.43; H, 3.74; N, 13.85. Found: C, 56.28; H, 3.69; N, 13.77%.
4.1.2.9 2,2,2-Trifluoro-N-(4-{1-[(4-p-tolyl-thiazol-2-yl)-hydrazono]-ethyl}-phenyl)-acetamide (13b)
White solid, (86% yield), mp 255–256 °C; IR (KBr) νmax 3391, 3310 (2NH), 2924 (sp3 CH), 1708 (C=O), 1607 (C=N), 1544, 1287, 1157 cm−1; MS m/z (%) 418 (M+, 61), 392 (61), 382 (53), 347 (38), 278 (38), 243 (100), 202 (35), 182 (38), 166 (51), 133 (42), 127 (61), 81 (37). Anal. Calcd. for C20H17F3N4OS (418.44): C, 57.41; H, 4.10; N, 13.39. Found: C, 57.39; H, 4.01; N, 13.28%.
4.1.2.10 2,2,2-Trifluoro-N-[4-(1-{[4-(4-methoxy-phenyl)-thiazol-2-yl]-hydrazono}-ethyl)-phenyl]-acetamide (13c)
White solid, (80% yield), mp 240–242 °C; IR (KBr) νmax 3400 (br. 2NH), 1714 (C=O), 1612 (C=N), 1541 1252, 1157, 1090 cm−1; 1H NMR (CDCl3) δ 2.36 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 6.86–8.10 (m, 8H, Ar-H), 7.44 (s, 1H, Thiazole-H), 11.34 (s, 1H, NH), 11.91 (s, 1H, NH); MS m/z (%) 434 (M+, 27), 427 (68), 416 (46), 390 (37), 340 (22), 304 (30), 282 (100), 227 (54), 150 (43), 128 (41), 86 (30), 80 (32). Anal. Calcd. for C20H17F3N4O2S (434.43): C, 55.29; H, 3.94; N, 12.90. Found: C, 55.17; H, 3.79; N, 12.82%.
4.1.2.11 N-[4-(1-{[4-(4-Bromo-phenyl)-thiazol-2-yl]-hydrazono}-ethyl)-phenyl]-2,2,2-trifluoro-acetamide (13d)
Pale yellow solid, (88% yield), mp 232–234 °C; IR (KBr) νmax 3444 (br. 2NH), 3005 (sp2 CH), 1716 (C=O), 1597 (C=N), 1510, 1337, 1246, 1154, 1111 cm−1; MS m/z (%) 482 (M+, 21), 389 (27), 359 (21), 262 (58), 241 (30), 174 (48), 161 (100), 136 (41), 103 (36), 99 (60), 85 (32), 77 (32). Anal. Calcd. for C19H14BrF3N4OS (483.30): C, 47.22; H, 2.92; N, 11.59. Found: C, 47.07; H, 2.83; N, 11.41%.
4.1.3 Antitumor assay
MDA-MB-231 (breast cancer cell) was obtained VACSERA Tissue Culture Unit. and the used chemicals as DMSO, crystal violet and trypan blue dye were supplied from Sigma Aldrich company (St. Louis, Mo., USA). The detailed method used for the propagation and evaluation the cytotoxicity was followed the reported procedure (Mosmann, 1983).
4.1.4 Molecular docking studies
Molecular docking analysis was done by using MOE-Dock 2014 software (Molecular, 2014). Chemical structures of the thiosemicarbazone 3 and thiazole derivatives 7b, 7c, 7d, 11, 13a and 13b were drawn by MOE-builder and minimized through the program force field MMFF94x. Then, the protein was prepared, hydrogen atoms were added and undesirable water molecules were removed. After that, docking of the 3D conformers was done; using rescoring 1(London dG) and rescoring 2 (GBVI/WSA dG). “Ligand Interactions” tool was utilized for the visualization of the 2D protein-ligand interactions showing the different formed interactions.
Ethics approval and consent to participate
Not applicable.
Human and animal rights
No Animals/Humans were used for studies that are the basis of this research.
Availability of data and materials
The data supporting the findings of the article is available at the corresponding author.
Funding
The study was funded by the Deanship scientific research, Taif University, KSA, [Research project number: 1-441-144].
Acknowledgement
All authors would like to thank Deanship of Scientific Research at Taif University for the financial support to this research [Research project number: 1-441-144].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmad, Khan, Baig, Imran, Gupta, Role of Azoles in Cancer Prevention and Treatment: Present and Future Perspectives. Anticancer Agents Med Chem., 18(1), 46-56.
- Role of Azoles in Cancer Prevention and Treatment: Present and Future Perspectives. Anti-Cancer Agents Med. Chem.. 2018;18:46-56.
- [Google Scholar]
- Mono- and bimetallic complexes of pyrazolone based ligand: Synthesis, characterization, antitumor and molecular docking studies. J. Mol. Str.. 2022;1249:131607.
- [Google Scholar]
- Discovery of novel indolyl-1,2,4-triazole hybrids as potent vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors with potential anti-renal cancer activity. Bioorg. Chem.. 2020;105:104330.
- [Google Scholar]
- Fluorinated hydrazonoyl chlorides as precursors for synthesis of antimicrobial azoles. J. Heterocyclic Chem.. 2021;58:589-602.
- [Google Scholar]
- Alsaedi, A.M.R., Farghaly, T.A., Shaaban, M.R., 2019. Synthesis and Antimicrobial Evaluation of Novel Pyrazolopyrimidines Incorporated with Mono- and Diphenylsulfonyl Groups. Molecules, 24, 4009; doi:10.3390/molecules, 24214009.
- Anti-breast cancer activity of some novel 1,2-dihydropyridine, thiophene and thiazole derivatives. Eur. J. Med. Chem.. 2011;46:137-141.
- [Google Scholar]
- Synthesis of thiazolyl-N-phenylmorpholine derivatives and their biological activities. Med. Chem.. 2021;17:790-805.
- [Google Scholar]
- Novel Nano-sized bis-indoline Derivatives as Antitumor Agents. J. Heterocyclic Chem.. 2019;56:391-399.
- [Google Scholar]
- Synthesis and Molecular Docking of novel 1,3-Thiazole Derived 1,2,3-Triazoles and In vivo Biological Evaluation for their Anti anxiety and Anti inflammatory Activity. J. Mol. Struct.. 2021;1236:130357.
- [Google Scholar]
- New thiazole-2(3H)-thiones containing 4-(3,4,5-trimethoxyphenyl) moiety as anticancer agents. Eur. J. Med. Chem.. 2020;185:111784.
- [Google Scholar]
- Design, synthesis, and biological evaluation of novel 2-ethyl-5-phenylthiazole-4-carboxamide derivatives as protein tyrosine phosphatase 1B inhibitors with improved cellular efficacy. Eur. J. Med. Chem.. 2013;69:399-412.
- [Google Scholar]
- Synthesis and biological evaluation of anti-tubercular activity of Schiff bases of 2-Amino thiazoles. Bioorg. Med. Chem. Lett.. 2020;2020(30):127655.
- [Google Scholar]
- Elements of X-Ray Diffraction. New Jersey: Prentice Hall; 2001.
- ZnO Nanoparticles catalyst in Synthesis of Bioactive Fused Pyrimidines as Anti-breast Cancer Agents Targeting VEGFR-2. Med. Chem.. 2019;15(3):277-286.
- [Google Scholar]
- Edrees, M.M., Farghaly, T.A., 2017. Synthesis and Antitumor activity of benzo[6'',7'']cyclohepta [1'',2'':4',5']pyrido[2',3'-d][1,2,4]triazolo[4,3-a]pyrimidin-5-ones, Arabian J. Chem., 10, 1613–S1618.
- Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg. Chem.. 2020;95:103476.
- [Google Scholar]
- Filler R., Kobayashi Y., Yagupolskii L.M., eds. Organofluorine compounds in medicinal chemistry and biomedical applications. Amsterdam: Elsevier; 1993.
- Fluorinated Heterocycles. (ACS Symp. Ser. Vol. 1003). (Eds A. Gakh, K. L. Kirk). (Washington, DC: American Chemical Society, 2009). P. 384.
- Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications. (Ed. V.A.Petrov). Wiley, New York, 2009.
- Fluorine in Medicinal Chemistry and Chemical Biology. (Ed. I.Ojima). (New York: Wiley-Blackwell, 2009).
- Fluorine in Heterocyclic Chemistry. Vols 1, 2. (Ed. V. Nenajdenko). (Heidelberg: Springer, 2014).
- J. Fluorine Chem.. 2014;167:16.
- Discovery of sulfadrug-pyrrole conjugates as carbonic anhydrase and acetylcholinesterase inhibitors. Arch Pharm.. 2022;355(1):e2100242.
- [CrossRef] [Google Scholar]
- Modern Fluoroorganic Chemistry. Synthesis, Reactivity, Applications. (2nd Completely Revised and Enlarged Edn). Weinheim: Wiley-VCH; 2013.
- Novel Natural Compounds from Endophytic Fungi with Anticancer Activity. Eur. J. Med. Chem.. 2018;156:316-343.
- [CrossRef] [Google Scholar]
- Chem. Rev.. 2015;115:683.
- Novel 2-indolinone thiazole hybrids as sunitinib analogues: Design, synthesis, and potent VEGFR-2 inhibition with potential anti-renal cancer activity. Eur. J. Med. Chem.. 2020;208:112752.
- [Google Scholar]
- Mahmudov, I., Demir, Sert, Abdullayev, Sujayev, Alwasel, Gulcin, Synthesis and inhibition profiles of N-benzyl- and N-allyl aniline derivatives against carbonic anhydrase and acetylcholinesterase – a molecular docking study. , 15, 103645.
- Design, synthesize and antiurease activity of novel thiazole derivatives: machine learning, molecular docking and biological investigation. J. Mol. Struct.. 2020;1222:128860.
- [Google Scholar]
- Molecular Operating Environment (MOE) 2014.09, Chemical Computing Group Inc., 1010 Sherbrooke Street West, Suite 910, Montréal, H3A 2R7, Canada, http://www.chemcomp.com.
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [Google Scholar]
- Anti-inflammatory, analgesic and anti-ulcerogenic activities of novel bis-thiadiazoles, bis-thiazoles and bis-formazanes. Med. Chem.. 2017;13:226-238.
- [Google Scholar]
- Synthesis of Novel Bis-pyrazole Derivatives as Antimicrobial Agents Mini-reviews in Medicinal. Chemistry. 2019;19:1276-1290.
- [Google Scholar]
- Synthesis of antimicrobial azoloazines and molecular docking for inhibiting COVID-19. J. Heterocyclic Chem.. 2021;58:1286-1301.
- [CrossRef] [Google Scholar]
- J. Fluorine Chem.. 2018;212:51.
- Study of in vitro biological activity of thiazoles on Leishmania (Leishmania) infantum. J. Global Antimicrobial Resistance. 2020;22:414-421.
- [Google Scholar]
- Synthesis and Antitumour Activity of New Tiazofurin Analogues Bearing a 2,3-Anhydro Functionality in the Furanose Ring. Bioorg. Med. Chem. Lett.. 2007;17:4123-4127.
- [CrossRef] [Google Scholar]
- Semicarbazones, thiosemicarbazone, thiazole and oxazole analogues as monoamine oxidase inhibitors: Synthesis, characterization, biological evaluation, molecular docking, and kinetic studies. Bioorg. Chem.. 2021;2021(115):105209.
- [Google Scholar]
- Organofluorine Compounds in Biology and Medicine. Amsterdam: Elsevier; 2015.
- Microwaves assisted synthesis of antitumor agents of novel azoles, azines, and azoloazines pendant to phenyl sulfone moiety and molecular docking for VEGFR-2 kinase. J. Mol. Str.. 2022;1249:131657.
- [Google Scholar]
- Design, synthesis and anticancer evaluation of chalcone based thieno[2,3-d]thiazoles as anticancer agents. Chem. Data Collect.. 2021;34:100742.
- [Google Scholar]
- Biosynthesis of the Marine Cyanobacterial Metabolite Barbamide. 2: Elucidation of the Origin of the Thiazole Ring by Application of a New GHNMBC Experiment. Tetrahedron Lett. 1999;40:5175-5178.
- [CrossRef] [Google Scholar]
- Acta Crystallogr. Sect. E. Struct. Rep.. 2009;65:02244.
- Zhou, Y., Wang, J., Gu, Z., Wang, S., Zhu, W., Aceñ, J., Soloshonok, V.A., Izawa, K., Liu, H., 2016. Chem. Rev., 116, 422.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103782.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







