Translate this page into:
CS@Cu2O and magnetic Fe3O4@SiO2-pAMBA-CS-Cu2O as heterogeneous catalysts for CuAAC click reaction
⁎Corresponding author. shjavan@iust.ac.ir (Shahrzad Javanshir)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The recovery of agricultural and food wastes are one of the main areas of current research for optimal biowaste management to reduce greenhouse gas (GHG) emissions that are generated when it is not properly treated. Corn silk (CS) as biowaste from the agricultural sector is a rich source of natural compounds especially polysaccharides. We present here a chemical activation method to convert CS to values added heterogeneous catalyst for the synthesis of triazoles compounds via copper catalyzed azide–alkyne cycloaddition (CuAAC) reaction. For this purpose, cuprous oxide coated CS (CS@Cu2O) and multifunctional Fe3O4@SiO2–para-aminomethyl benzoic acid–CS–Cu2O composite (denoted as Fe3O4@SiO2‐pAMBA-CS‐Cu2O) were fabricated. Different analytical techniques have been used to describe the size, crystal structure, elemental composition and other physical properties of the fabricated catalysts. These heterogeneous catalysts showed excellent catalytic activities for the synthesis of 1,4-disubstituted-1,2,3-triazoles via click reaction in H2O at 70 °C under base and external-reductant-free conditions. The magnetic properties of the catalyst allowed easy separation after reaction by simply applying an external magnet. Other advantages of this work are the recyclability of the catalyst, the absence of reducing agent and base, besides utilisation of bio wastes for the production of heterogeneous catalyst.
Keywords
Click chemistry
Bio-waste
Corn Silk
Heterogeneous catalysts
Magnetic nanoparticles
Triazole
1 Introduction
Growth of human population contributes to increasing demands for agricultural products and related products of food industry (Ravindran et al., 2021), (Usmani et al., 2020). In general, Bio-wastes emanate from 3 specific sources which are agricultural, municipal and industrial wastes (Ravindran et al., 2021). Bio-wastes originates from agricultural sources, mostly consists of livestock manure and crop residues. Agricultural and food industries generate enormous liquid and solid wastes which inability to effectively convert these materials into valuable substances can lead to environmental damage (Ravindran et al., 2021). This issue has led chemists to promote routs for reducing the amount of the pollutants and turn them into more valuable materials (Sun et al., 2021), (Athinarayanan et al., 2019).Scheme 1.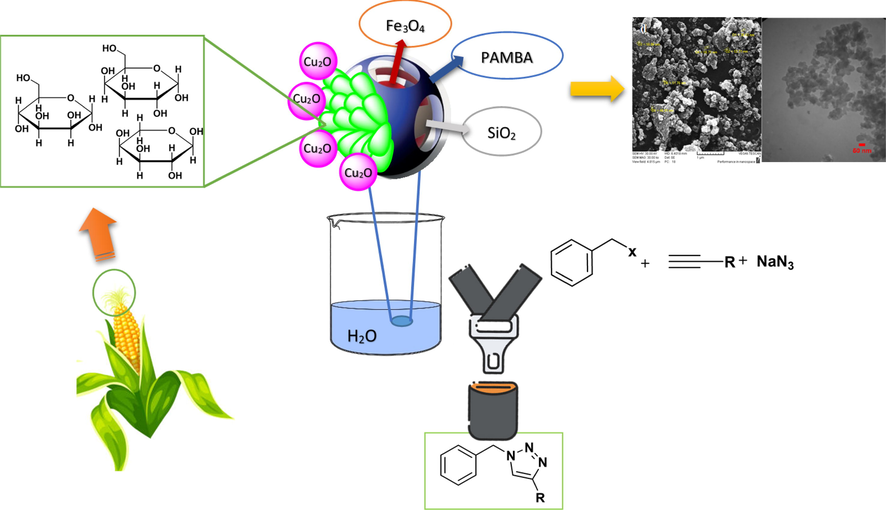
Graphical scheme of the reaction.
Corn is the one of the most versatile and widely consumed agricultural products around the world. CS, also known as Maydis Stigma or Zea Mays, is the waste of the corn. In fact, CS, the long shiny fibers at the top of an ear of corn, is one of the agricultural by-product (Rahman and Rosli, 2014). This Bio-waste is used as traditional medicine for treatment of diabetes and diuresis in China (Jia et al., 2021). It also has health benefits such as diuretic, antioxidant, anti-obesity, anti-coagulation, anti-tumor and it might alter blood sugar levels so used to treat diabetes, and help reduce inflammation (Hasanudin et al., 2012), (Liu et al., 2011), (Ren et al., 2013). This Bio-waste is a rich source of bioactive compounds such as proteins, carbohydrates, vitamins, minerals, fiber, and steroids such as sterols and stigma sterols, alkaloids, tannins and saponins (Jia et al., 2021; Hasanudin et al., 2012; Liu et al., 2011; Ren et al., 2013). In studies of the CS components has been shown that carbohydrates (67.3%) constitute of the bulk of its structure (Jia et al., 2021; Haslina et al., 2017). For this reason, this natural compound can be widely used as a carbohydrate structure that chemical modification of this bio-composite can be another effective way for the advancement of chemical biology and drug development (Choi et al., 2020). On the other hand, due to the presence of natural reducing compounds such as ascorbic acid in the composition of CS (Rahman and Rosli, 2014) CS can also act as a reducing agent (Kumar et al., 2020).
Click chemistry first was reported by Sharples in 2001, to describe an effective method for the synthesis of various compounds especially various pharmaceuticals. One of the most important categories of click reactions is the CuAAC reaction between terminal alkynes and azides in the presence of a copper catalyst(I) to form 1,4-disubstituted 1,2,3-triazoles (Kolb et al., 2001; Bahsis et al., 2018; Finn and Fokin, 2010; Wang et al., 2015) that have applications in the various fields such as pharmaceutical industry, dyes, agriculture, corrosion inhibitors, optical brighteners and biological activity like hypotensive medications, anti-tuberculosis, anti-allergic, anti-bacterial, anti-HIV and Src-kinase inhibitors (Zirak and Garegeshlagi, 2018; Vibhute et al., 2018; Aghbash et al., 2019; Pagliai et al., 2006; Dolatkhah et al., 2018; Choi et al., 2020). Several example of 1,2,3-triazole compounds have anticancer property such as carboxy amido triazole (CAI), tert-butyl dimethyl silyl spiroamino oxathiole dioxide(TSAO), b-lactum antibiotic Tazobactum and Cefatrizine (Fig. 1) (Agalave et al., 2011; Mohammadi et al., 2018).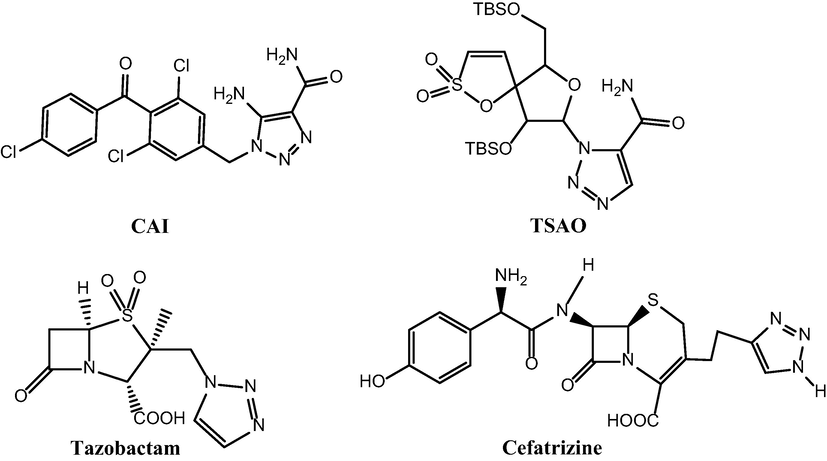
Anticancer compounds containing 1, 2, 3-triazole.
Considerable research has focused on using bio-based materials to reduce costs, create cost-effective and environmentally friendly materials, and numerous reports have documented the use of natural compounds as catalysts. Among the reports on the use of natural substances as catalyst for triazoles synthesis, the use of CeI/Cu (Hamzavi et al., 2020), CuICC(Ghosh et al., 2020), GO-Fe3O4@CuO(Jain et al., 2020), L‐Proline‐MCM‐41‐CuCl(Zhao et al., 2020), γ-Fe2O3@Sh@Cu2O (Norouzi and Javanshir, 2020), Whey protein (Garg et al., 2021), CuBr/[DBU]OAc (Garg et al., 2020) and Cu2O@PS(Dolatkhah et al., 2019) can be pointed out. These documents, along with articles which use magnetic core as based of the heterogeneous catalyst, emphasize on the easier use and green synthesis procedure (Hassankhani et al., 2021; Nourmohammadi et al., 2021). All these issues led us to design a project in which CS bio-waste was used as a support and reducing agent to prepare CS@Cu2O and Fe3O4@SiO2‐pAMBA-CS‐Cu2O as heterogeneous catalysts for the synthesis of triazole derivatives
2 Methods
2.1 Materials
All chemicals were purchased from Merck, Aldrich or Fluka were used without further purification. All of them were analytical grade. Fourier-transform infrared (FT-IR) spectroscopy spectra were recorded in KBr on Bruker FT-IR spectrometer and are reported in wave numbers (cm−1). All melting points were measured on a capillary melting point apparatus.1H NMR spectra were performed by a Bruker Avance DPX 300. Scanning electron microscopy (SEM) was recorded on a VEG//TESCAN 100EM10C-KV and transmission electron microscopy (TEM) was recorded on a Zeiss EM900. The surface area of catalyst was recorded Brunauer-Emmett-Teller (BET) Surface Area & Porosity Analyzer. Energy-dispersive X-ray (EDX) spectroscopy was recorded on a VEG//TESCAN-XMU. X-ray diffraction (XRD) pattern was recorded on a Bruker AXS D8-advance X-ray diffractometer using Cu Kα radiation (λ = 1.5418A°). Magnetic measurements were performed using a vibrating sample magnetometer (VSM) analysis. The metal loading was detected by an inductively coupled plasma-atomic emission spectrometer (ICP).
X-ray photoelectron spectroscopy (XPS) spectra are obtained using the Thermofisher Scientific K-Alpha XPS spectrometer. Thermal Gravimetric Analysis (TGA) and Differential Thermogravimetric (DTG) were recorded on a STA 504 BÄHR Thermoanalyse GmbH (Hüllhorst, Germany).
2.2 Preparation of CS@Cu2O
The collected corn silks were washed with distilled water several times to remove impurities, dried in an oven at 60 °C, and powdered in a ball-mill. The ball-milled CS (1 g) and EtOH (30 mL) were mixed in a round-bottom flask and stirred at room temperature for 30 min. Then 0.5 g of Cu(OAc)2 was added to the mixture under vigorous stirring for overnight. The catalyst was then centrifuged and washed with water, ethanol, and acetone and dried in the oven at 70 °C.
2.3 Preparation of Fe3O4 nanoparticles
Fe3O4 nanoparticles was synthesized according to our previous method (Dolatkhah et al., 2018). In a typical procedure, a solution with a 2:1 ratio of Fe2+/Fe3+ from FeCl2·4H2O and FeCl3·6H2O in deionized water was prepared. After 2 h of stirring under N2 atmosphere, the participation was performed by dropwise addition of NaOH solution (1 M) until pH was adjusted to 10. The magnetic nanoparticle was separated by an external magnetic field, then washed three times with deionized water and ethanol. Eventually, Fe3O4 particles dried for 10 h in an oven at 65 °C.
2.4 Preparation of Fe3O4@SiO2-pAMBA-CS-Cu2O
First, 1 g of Fe3O4 magnetite in 50 mL of water were added in a round-bottom flask and stirred at room temperature for 30 min. Then, 5 mL of ammonia solution and 50 mL EtOH was added to the mixture. In the next step, a mixture of 1.5 mL TEOS and 10 mL ethanol was added into the suspension drop by drop under vigorous stirring for 24 h. The obtained precipitate Fe3O4@SiO2 was collected with a magnet, washed several times with H2O and EtOH and dried.
1 g of Fe3O4@SiO2 in 100 mL of water were dissolved in a round-bottom flask. Then, 0.3 g of para-aminomethyl benzoic acid was added to the mixture. The mixture was stirred vigorously for 12 h under reflux conditions the obtained precipitate Fe3O4@SiO2-pAMBA was Exit with a magnet, washed with H2O and EtOH and dried at 50 °C.
1 g of Fe3O4@SiO2-pAMBA in 50 mL of EtOH were mixed for 30 min at room temperature. Then, 0.2 g of powder CS was added to the mixture and the mixture was stirred vigorously for 6 h under reflux conditions. Finally, the obtained precipitate Fe3O4@SiO2-pAMBA-CS was magnetically separated, washed several times with H2O and EtOH and dried at 50 °C for 12 h.
1 g of Fe3O4@SiO2-pAMBA-CS in 30 mL of EtOH were dispersed in a round-bottom flask and stirred at room temperature for 30 min. Then, 0.5 g of Cu(OAc)2 was added to the mixture under vigorous stirring for overnight (Scheme 2). The final catalyst was washed with distilled water, then dried at room temperature.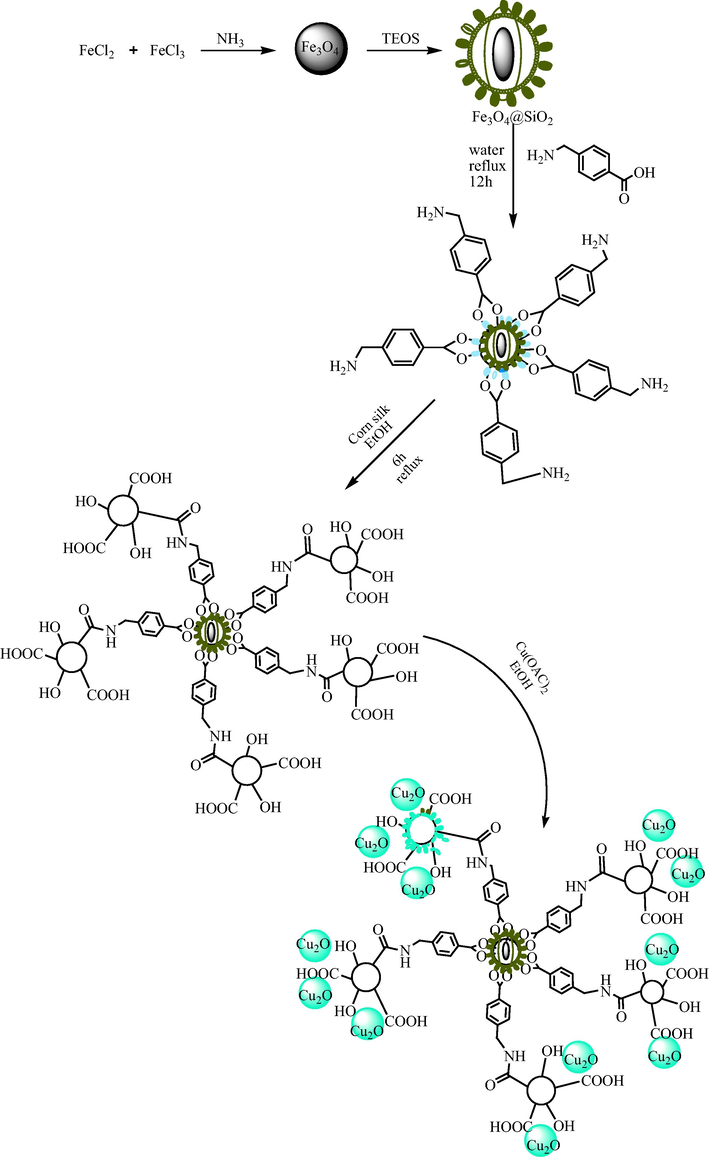
Synthesis of Fe3O4@SiO2-pAMBA-CS-Cu2O.
2.5 General procedure for click reactions
NaN3 (1.2 mmol), alkyne (1.2 mmol) and benzyl halide (1 mmol) were added to a suspension of CS@Cu2O (7 mol% Cu, 0.05 g catalyst) or Fe3O4@SiO2-pAMBA-CS-Cu2O (7 mol% Cu, 0.06 g catalyst) in H2O (2 mL). The reaction mixture was stirred at 70 °C and monitored by TLC (EtOAc:n-hexane (1:5). After completion of the reaction, the catalyst was easily removed from reaction mixture and the filtrate was extracted with chloroform (2 × 2 mL). The organic solvents were removed under vacuum and the pure product was obtained by recrystallization with CHCl3: n-hexane (1:3). All of the Click products are known compound and were reported previously.
3 Results and discussions
3.1 Characterization of the catalyst
The catalysts were characterized by diverse physicochemical techniques such as FT-IR, XRD, SEM, TEM, EDX, VSM, BET, XPS and ICP.
3.1.1 FT-IR investigation
The FT-IR spectra of CS, CS@Cu2O, and recycled CS@Cu2O after five runs in click reaction are shown in Fig. 2. The wide peak at 3400 cm−1 indicates the presence of an acidic group in the CS. The peaks around 1654 cm−1 and 1049 cm−1 belongs to C⚌O and C—O bond respectively. The slight shift from 1654 cm−1 in CS to 1643 cm−1 in CS@Cu2O may be due to the chelation of copper on the surface of CS.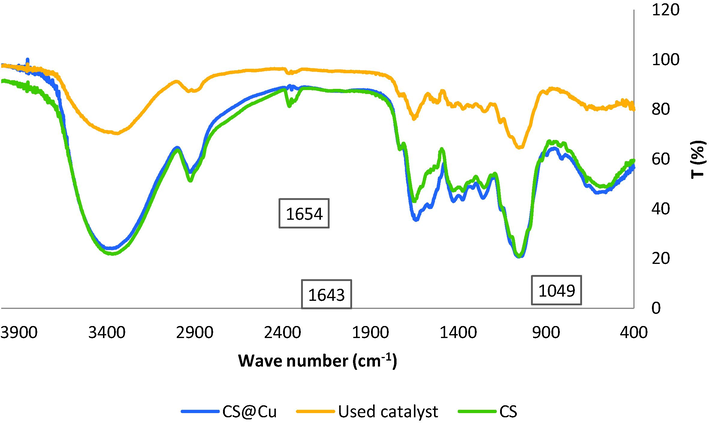
FT-IR spectra of CS (a), CS@Cu2O (b), CS@Cu2O after 5 times use (c).
The FT-IR spectra of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-pAMBA-CS, Fe3O4@SiO2-pAMBA-CS-Cu2O and recycled Fe3O4@SiO2-pAMBA-CS-Cu2O after five runs are shown in Fig. 3. The FT-IR spectrum of Fe3O4 displays an intense absorption band at 588 cm−1 attributed to the typical Fe–O vibrations of the magnetite structure whereas a broad band at 3340 cm−1 is relevant to the surface OH groups. The absorption band appeared at 1030 cm−1 in Fe3O4@SiO2 structure can be attributed to Si–O(Zandipak et al., 2020). The wide peak at around 3400 cm−1 indicates the presence of acidic group in the magnetic CS. The bands at 1654 cm−1 in the IR spectrum of Fe3O4@SiO2-pAMBA-CS-Cu2O were assigned to carbonyl groups. The slight shift from 1090 cm−1 in Fe3O4@SiO2-pAMBA-CS to 1085 cm−1 in Fe3O4@SiO2-pAMBA-CS-Cu2O may be due to the chelated-copper on the surface of Fe3O4@SiO2-pAMBA-CS.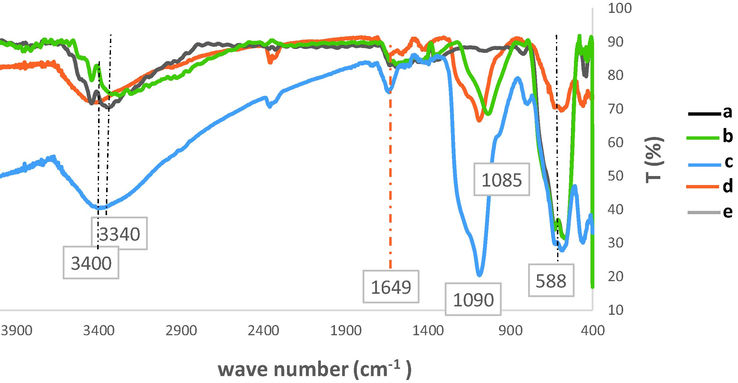
FT-IR spectra of Fe3O4 (a), Fe3O4@SiO2 (b), Fe3O4@SiO2-pAMBA-CS (c), Fe3O4@SiO2-pAMBA-CS-Cu2O (d), Fe3O4@SiO2-pAMBA-CS-Cu2O after 5 run in click reaction (e).
3.1.2 Morphology study
The XRD pattern of the CS@Cu2O shown in Fig. 4a exhibited a diffraction peak at 2θ = 20°, which revealed the formation of amorphous copper oxide (Faeghi et al., 2018). The diffraction peak under 2θ = 30° belongs to CS which is amorphous organic material (Wang et al., 2014; Norouzi and Javanshir, 2020).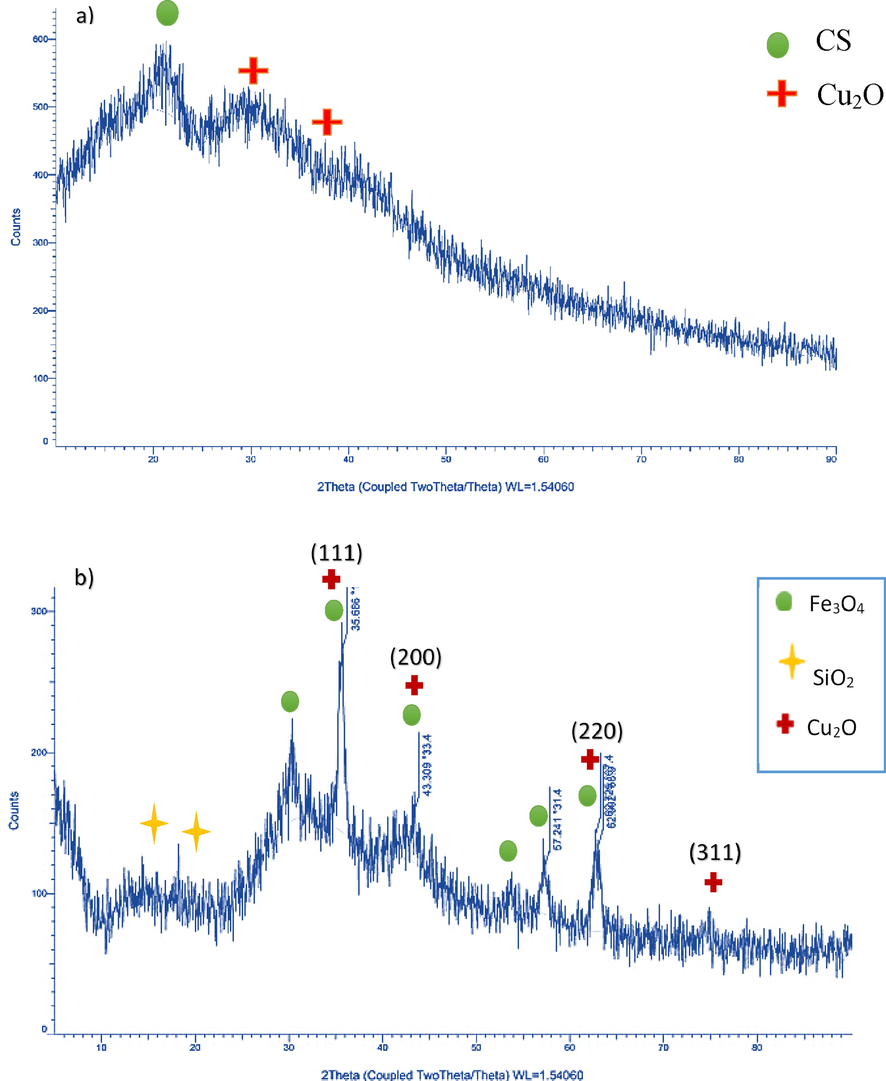
XRD pattern of CS@Cu2O (a), Fe3O4@SiO2-pAMBA-CS-Cu2O (b).
In the XRD pattern of the Fe3O4@SiO2-pAMBA-CS-Cu2O, the diffractions at 2θ = 35.6°, 43.3°, 62.7°, and 74.8° can be assigned to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) lattice planes of Cu2O, in accordance with Cu2O standard data (JCPDS card NO. 05–0667). The diffractions at 2θ = 30.3°, 35.6°, 43.3°, 53°, 57.1° and 62.9° can be assigned to the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) crystalline planes of cubic lattices structure of Fe3O4 respectively (JCPDS No. 85–1436). Also, the peaks at around 2θ = 14.4 and 18.2° in the XRD pattern is due to the amorphous silica shell on the surface of the magnetite nanoparticles. (Fig. 4b).
Comparing the EDX analysis of CS@Cu2O clearly shows the presence of Cu, C, O and N elements in the structure of this material (Fig. 5a). Also, the presence of Cu, C, O, N, Fe and Si elements in the catalyst structure have been confirmed from the EDX analysis of Fe3O4@SiO2-pAMBA-CS-Cu2O (Fig. 5b).
EDX and map analysis of CS@Cu2O (a), Fe3O4@SiO2-pAMBA-CS-Cu2O (b)The morphology and size of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-pAMBA-CS, Fe3O4@SiO2-pAMBA-CS-Cu2O and CS@Cu2O NPs were investigated using SEM analysis (Fig. 6a–e). The SEM images of Fe3O4@SiO2-pAMBA-CS-Cu2O show the formation of spherical particles in size around 38–56 nm (Fig. 6d).
The TEM image of the prepared CS@Cu2O is shown in Fig. 7a and b. The TEM images shown two regions with different size and electron density which are related to CS@Cu2O nanoparticles and Cu.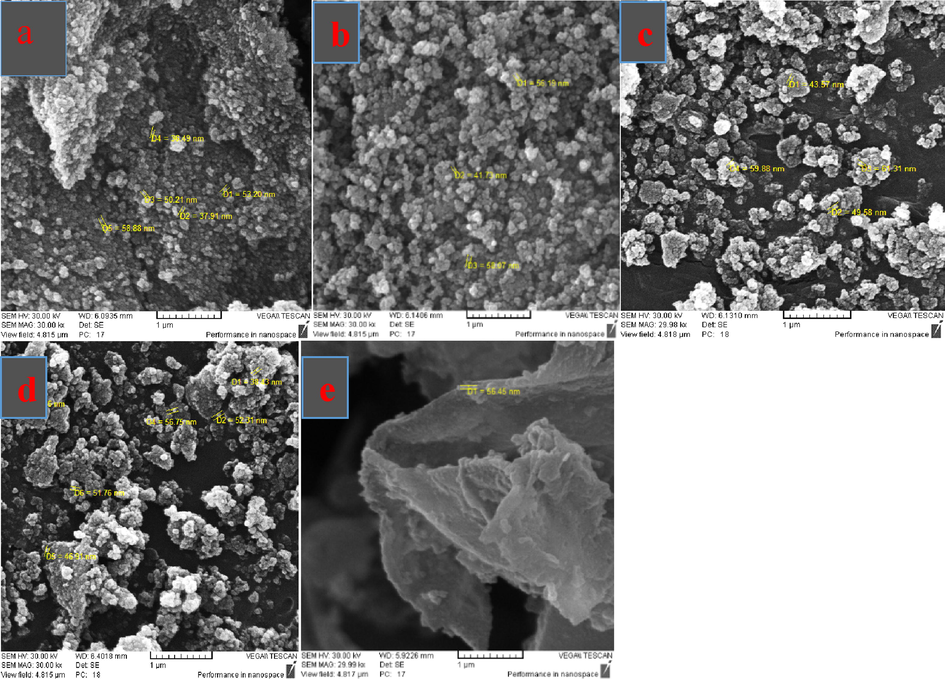
SEM images of Fe3O4 (a), Fe3O4@SiO2 (b), Fe3O4@SiO2-pAMBA-CS (c), Fe3O4@SiO2-pAMBA-CS-Cu2O (d), CS@Cu2O (e).
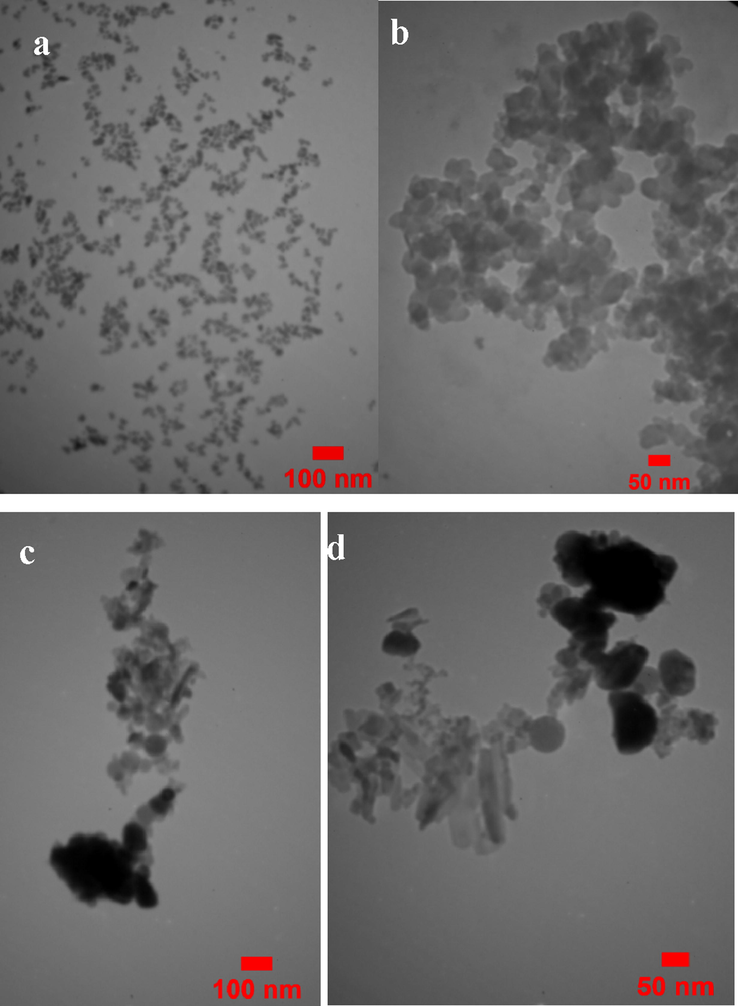
TEM images of CS@Cu2O (a, b), Fe3O4@SiO2-pAMBA-CS-Cu2O (c, d).
The TEM image of the prepared nano‐Fe3O4@SiO2‐pAMBA-CS‐Cu2O is shown in Fig. 7c, and d. The TEM image shows the presence of three regions with different size and electron density which dense regions represent to Fe3O4@SiO2 nanoparticles and Cu and a less dense region related to Natural base of CS (Sabaqian et al., 2017).
3.1.3 Magnetic properties
The magnetic properties of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-pAMBA-CS and Fe3O4@SiO2-pAMBA-CS-Cu2O were investigated using VSM analysis. The magnetization curves recorded at room temperature are shown in Fig. 8. The saturation magnetization of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-pAMBA-CS and Fe3O4@SiO2-pAMBA-CS-Cu2O are 63.9, 61.49, 57.76 and 50.35 emu.g−1, respectively. The catalyst has a good magnetic property and is easily removed using an external magnet after the reaction.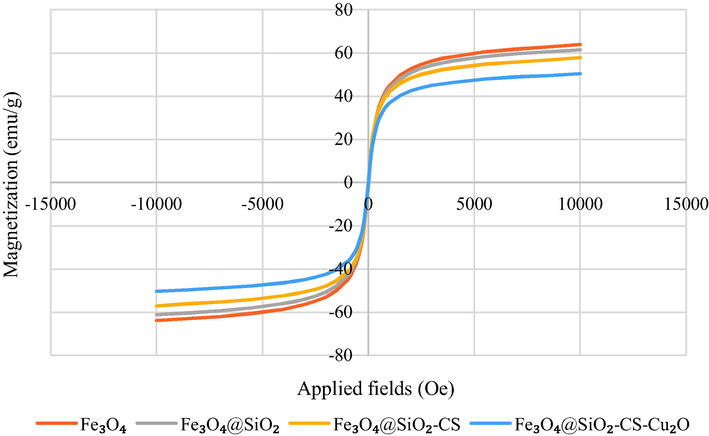
VSM analysis for Fe3O4 (a), Fe3O4@SiO2 (b), Fe3O4@SiO2-pAMBA-CS (c), and Fe3O4@SiO2-pAMBA-CS-Cu2O (d).
3.1.4 Adsorption study
The surface area and pore volume of synthesized catalysis were estimated from the N2 adsorption/desorption isotherms and T-plot (Fig. 9). The BET surface area and average pore diameter are 39.54 m2g−1 and 7.17 nm for CS@Cu2O, and 33.65 m2g−1 and 17.59 nm for Fe3O4@SiO2-pAMBA-CS-Cu2O, respectively. The volume of the single-point adsorption cavity is 0.07 cm3g−1 and 0.148 cm3g−1 for CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O, respectively. Also, the single-point cavity dissipation volume is 0.073 cm3g−1 and 0.128 cm3g−1 for CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O.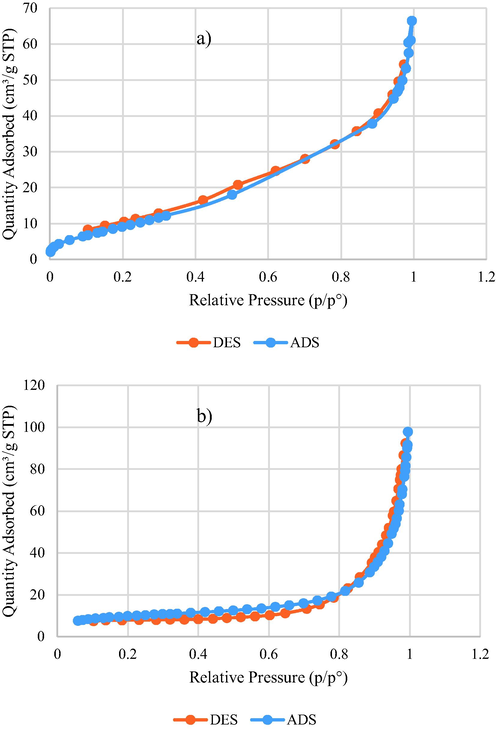
BET analysis of CS@Cu2O (a), Fe3O4@SiO2-pAMBA-CS-Cu2O (b).
In addition, as determined by ICP, the copper loading of CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O are 1.41 and 1.25 mmol.g−1, respectively.
3.1.5 XPS analysis
To determine the oxidation states of Cu in the prepared nanocomposite, XPS analysis was performed and the obtained results are depicted in Fig. S1. The Fine-scan XPS spectrum (Fig. S1a) demonstrates that Fe3O4@SiO2-pAMBA-CS-Cu2O contains Fe, Cu, O, Si and C elements. The peaks at around 104.5 eV in Fig. S1a correspond to SiO2 (Jensen et al., 2013). The high-resolution spectrum of Fe 2p shown in Fig. S1b exhibited two main peaks of Fe 2p3/2 at around 710.8 and 714.1 eV attributed to Fe2+ and Fe3+, respectively, demonstrating the existence of Fe3O4. The peak at 724.6 eV, corresponding to Fe 2p1/2 is also in accordance with the reported values for Fe3O4 (Huang et al., 2017). The characteristic peaks at 932.7 and 943.80 corresponding to Cu 2p3/2 and Cu2p1/2, respectively, demonstrate the existence of Cu2O (Vasquez, 1998).
3.1.6 TGA analysis
The thermal behavior of the catalyst was determined by TGA and DTG (Fig. S2). The TGA thermogram of Fe3O4@SiO2-pAMBA-CS-Cu2O shows two step weight loss steps over the temperature range of TG analysis. The first step, including a low amount of weight loss (5%) at T ∼ 120 °C, resulted from the release of both the physiosorbed and chemisorbed water, the second stage at about 280 °C to nearly 450 °C is attributed to the decomposition of the organic moiety in the nanocomposite including a weight loss (40%). The amount of organic compounds bound on the surface of the nanoparticles is predictable from the percentage of weight loss from the TGA curve.
3.2 Catalytic studies
The catalytic behavior of CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O were investigated in the click synthesis of 1,2,3-triazoles. The reaction of benzyl bromide, sodium azide and phenyl acetylene approved as a model reaction for carrying out in different conditions. The results are shown in Table 1 (Entries 1–18). This reaction was done by employing various solvents such as EtOH, toluene, EtOH-H2O, chloroform and H2O at 70 °C for 1 h (Entries 1–7). It was found that H2O was the most effective solvent for triazole synthesis. Then, other conditions like temperature, amount of catalyst and presence/absence of the base was examined in the model reaction (Entries 9–17). It was found that by increasing the amount of CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O from 0.5 to 7 mol%, the yield increased from 74% to 96%, and 69% to 93% respectively (Table 1, Entries 12–16). Further increase in catalyst amount had no profound effect on the yield of the desired product. The reaction has not progressed in the absence of catalyst (Entry 18). Accordingly, the best results are obtained when the reaction is performed in water in the presence of 7 mol% of the both catalysts at 70 °C (Table 1, Entry 16). Some of the catalyst components such as Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2-pAMBA-CS, CS, and Cu(OAc)2 were also examined under optimal conditions. However, in most cases the reaction efficiency was not improved (Table 2).
Entry
Solvent
T(°C)
Base
Cat. (mol%)
Isolated yield (%)[b]
Isolated yield (%)[c]
1
EtOH
70
K2CO3
5
71
69
2
Toluene
70
K2CO3
5
Trace
Trace
3
H2O:EtOH (1:1)
70
K2CO3
5
84
79
4
H2O:EtOH (2:1)
70
K2CO3
5
62
52
5
H2O:EtOH (1:2)
70
K2CO3
5
76
69
6
Chloroform
70
K2CO3
5
Trace
Trace
7
H2O
70
K2CO3
5
87
82
8
H2O
70
—
5
88
85
9
H2O
25
—
5
58
55
10
H2O
50
—
5
63
59
11
H2O
70
—
5
88
85
12
H2O
70
—
0.5
74
69
13
H2O
70
—
1
80
72
14
H2O
70
—
2.5
84
78
15
H2O
70
—
5
88
85
16
H2O
70
—
7
96
93
17
H2O
70
—
10
96
93
18
H2O
70
K2CO3
—
Trace
Trace
Entry
Catalyst
Time
Isolated yield
1
Fe3O4
360 min
—
2
Fe3O4@SiO2
360 min
—
3
Fe3O4@SiO2-CS
360 min
—
4
CS
360 min
Trace
5
Cu(OAc)2
360 min
Trace
6
CS@Cu2O
20 min
96
7
Fe3O4@SiO2-pAMBA-CS-Cu2O
20 min
93
Subsequently, the reaction among various benzyl halide derivatives, acetylene derivatives and NaN3 was investigated and the corresponding triazoles were obtained in good to excellent yields within relatively short times (Table 3).
Entry
R1
R2
X
Product
Time (min)[a]
Time (min)[b]
Isolated yield[a]
Isolated yield[b]
M.P. (°C)
Ref.
1
Ph
Ph
Br
3a
20
20
96
93
129–130
(Khalili and Rezaee, 2019)
2
Ph
Ph
Cl
3b
20
25
92
89
128–129
(Velpuri and Muralidharan, 2019)
3
Ph
4-Br-Ph
Br
3c
20
20
98
95
151–153
(Pasupuleti and Bez, 2019)
4
Ph
4-Me-Ph
Br
3d
20
20
91
89
110–112
(Bunev et al., 2016)
5
Ph
4-NO2-Ph
Br
3e
25
30
89
86
155–157
(Lai et al., 2018)
6
Ph
2-Cl-Ph
Cl
3f
30
35
88
84
83–86
(Zarchi and Nazem, 2014)
7
CH2OH
Ph
Br
3 g
25
30
90
90
74–76
(Khojastehnezhad et al., 2019)
8
CH2OH
Ph
Cl
3 h
30
35
86
85
77–79
(Bahri-Laleh et al., 2018)
9
CH2OH
4-Br-Ph
Br
3i
25
25
93
89
110–112
(Safa and Mousazadeh, 2016)
10
CH2OH
4-Me-Ph
Br
3j
25
25
87
84
91–93
(Dubrovina et al., 2013)
11
CH2OH
4-NO2-Ph
Br
3 k
30
30
84
79
122–123
(Apperley et al., 2017)
12
CH2OH
2-Cl-Ph
Cl
3 l
40
40
86
82
98–100
(Safa and Mousazadeh, 2016)
The catalytic activity of CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O were compared with other catalysts in the Click reaction of phenylacetylene, benzyl bromide and NaN3 (Table 4). The use of H2O as green solvent and natural base catalyst, easy isolation of product from the reaction mixture, absence of reducing agent and base, operational simplicity, high yield and reusability of catalyst are the merits of present method.
Entry
Catalyst
Catalyst loading
Time
T (°C)
Solvent
Yield
Refs.
1
bis-(MIM)(CuBr2)
5 mol%
1 h, 40 min
80
H2O/EtOH
91
(Dige et al., 2017)
2
Cu(I)‐AMPS
1 mol%
1 h
25
H2O
82
(Bahsis et al., 2019)
3
Mag-Cu
2 mol%
6 h
55
H2O/tBuOH
93
(Banan et al., 2017)
4
AA-Clin@Cu
0.06 g
24 h
25
H2O
94
(Gholinejad et al., 2019)
5
Fe3O4@LDH@cysteine–Cu(I)
0.02 g
25 min
75
Choline azide
90
(Pazoki et al., 2020)
6
Fe3O4@SiO2-PIA-Cu
0.01 g
12 h
70
H2O
95
(Zirak and Garegeshlagi, 2018)
7
CS@Cu2O
7 mol%
20 min
70
H2O
96
This work
8
Fe3O4@SiO2-pAMBA-CS-Cu2O
7 mol%
20 min
70
H2O
93
This work
Then, we examined the heterogeneous nature of the catalysts. The catalytically active particles were removed from the reaction by filtration after 5 min using a hot filtration. The reaction progress did not change after running the hot filtration (Fig. 10a, b).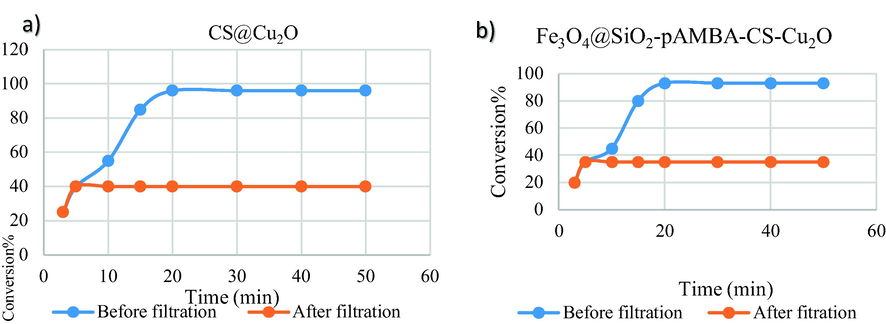
Hot filtration test of CS@Cu2O (a), Fe3O4@SiO2-pAMBA-CS-Cu2O (b).
The reusability of catalysts was investigated in the reaction of benzyl bromide, phenylacetylene, and NaN3. After completion of the reaction, the catalyst was recovered by an external magnet and washed several times with EtOH, and then re-used after drying it at 60 °C. The results show that the performance of the catalyst did not decrease significantly after recycling (Fig. 11a, b).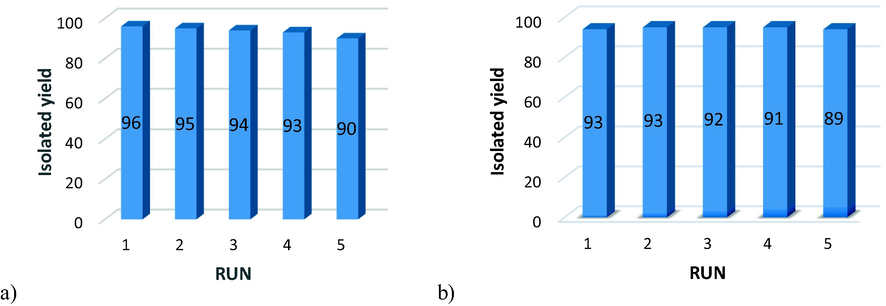
Recyclability of CS@Cu2O (a), Fe3O4@SiO2-pAMBA-CS-Cu2O (b).
A mechanism for the catalytic activity in the synthesis of 1-aryl-1,2,3-triazole derivatives is shown in Scheme 3. Initially, catalyst and acetylene are joined together and then alkyl azide is added. Then, an unusual six-membered copper metallacycle is formed and finally, the catalyst is removed and triazole is formed (Wang et al., 2016) Scheme 4.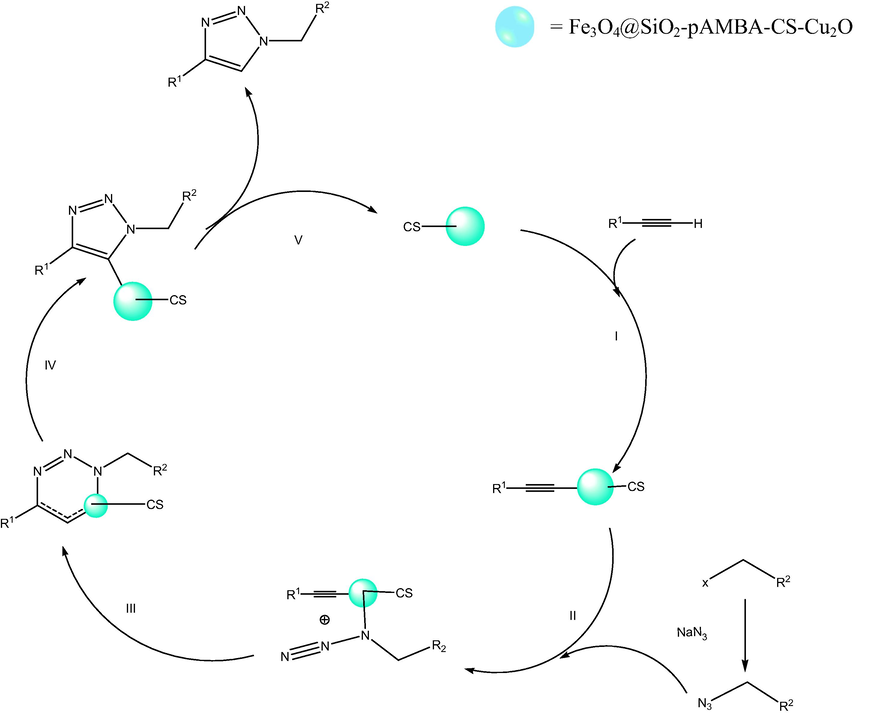
Suggested mechanism for model reaction.
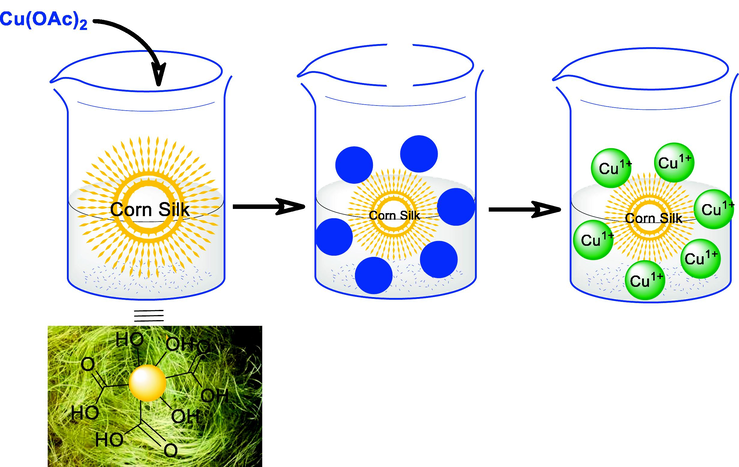
Proposed mechanism for the generation of Cu (I).
3.3 Selected characterization data
(1-(4-Methylbenzyl)-1H-1,2,3-triazol-4-yl) methanol (3j): IR (KBr): 653.7, 777.1, 835, 1012.4, 1121.8, 1222, 1330, 1446.3, 1515.7, 2944.7, 3249.4.
1H NMR (400 MHz, CDCl3, 25 °C, TMS): δ = 2.32 (3H, s), 2.99 (1H, s), 4.72 (2H, s), 5.44 (2H, s), 7.15 (4H, s,), 7.40 (1H, s).
4 Conclusion
Herein, we reported the synthesis of two eco-friendly heterogeneous catalyst, CS@Cu2O and Fe3O4@SiO2-pAMBA-CS-Cu2O, and their high catalytic performance for the synthesis of 1,2,3-triazole derivatives via a one-pot Huisgen 1,3-dipolar cycloaddition reaction in H2O at 70 °C. The existence of natural reducing agents such as the ascorbic acid in the CS structure allows it to act as a reducing agent and convert Cu(OAc)2 to Cu2O.
This approach for triazoles synthesis is distinguished by the exploitation and valorisation of CS biomass waste for the preparation of a green and recyclable catalyst, its high atom economy, low catalyst loading, simple operations under mild conditions, good product yields, the ease of catalyst-product separation, alongside absence of any reducing agent and base.
Funding Source Declaration
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. asian J.. 2011;6:2696-2718.
- [Google Scholar]
- Cu(I)-catalyzed alkyne-azide ’click’cycloaddition (CuAAc): a clean, efficient and mild synthesis of new 1,4-disubstituted 1H–1,2,3-triazole-linked 2-amino-4,8-dihydropyrano [3,2-b] pyran-3-carbonitrile-crystal. Res. Chem. Intermed.. 2019;45:2079-2094.
- [Google Scholar]
- Development of new scaffolds as reversible tissue transglutaminase inhibitors, with improved potency or resistance to glutathione addition. MedChemComm.. 2017;8:338-345.
- [Google Scholar]
- Phoenix dactylifera lignocellulosic biomass as precursor for nanostructure fabrication using integrated process. Int. J. Biol. Macromol.. 2019;134:1179-1186.
- [CrossRef] [Google Scholar]
- CuI-functionalized halloysite nanoclay as an efficient heterogeneous catalyst for promoting click reactions: Combination of experimental and computational chemistry. Appl. Organomet. Chem.. 2018;32:4283.
- [Google Scholar]
- Cellulose-copper as bio-supported recyclable catalyst for the clickable azide-alkyne [3+2] cycloaddition reaction in water. Int. J. Biol. Macromol.. 2018;119:849-856.
- [Google Scholar]
- A reusable polymer-supported copper(I) catalyst for triazole click reaction on water: An experimental and computational study. Appl. Organomet. Chem.. 2019;33:4669.
- [Google Scholar]
- Copper immobilized onto polymer-coated magnetic nanoparticles as recoverable catalyst for ‘click’reaction. Appl. Organomet. Chem.. 2017;31:3604.
- [Google Scholar]
- Copper(II) oxide nanowhiskers—A new efficient catalyst of azide–alkyne cycloaddition. Russ. J. Org. Chem.. 2016;52:1537-1539.
- [Google Scholar]
- Visible-Light-Induced Cysteine-Specific Bioconjugation: Biocompatible Thiol-Ene Click Chemistry. Angew. Chemie. 2020;59:22514-22522.
- [CrossRef] [Google Scholar]
- Dicationic 1, 3-Bis (1-methyl-1H-imidazol-3-ium) propane copper(I) dibromate: novel heterogeneous catalyst for 1, 3-dipolar cycloaddition. Catal. Letters. 2017;147:301-309.
- [Google Scholar]
- Magnetic Isinglass a nano-bio support for copper immoblization: Cu-IG@Fe3O4 a heterogeneous catalyst for triazoles synthesis. Chemistryselect. 2018;3:5486-5493.
- [Google Scholar]
- Peanut shell as a green biomolecule support for anchoring Cu2O: a biocatalyst for green synthesis of 1, 2, 3-triazoles under ultrasonic irradiation. BMC Chem.. 2019;13:97.
- [Google Scholar]
- New mono-and bidentate P-ligands using one-pot click-chemistry: synthesis and application in Rh-catalyzed hydroformylation. Tetrahedron. 2013;69:8809-8817.
- [Google Scholar]
- Faeghi, F., Javanshir, S., Molaei, S., 2018. Natural Polymer‐Based Copper/ Sandarac Resin Catalyzed Regioselective One‐Pot Synthesis of 1,4‐Disubstituted 1,2,3‐Triazoles under Ultrasonic Irradiation.
- A simple work-up-free, solvent-free approach to novel amino acid linked 1,4-disubstituted 1,2,3-triazoles as potent antituberculosis agents. ACS Omega. 2020;5:29830-29837.
- [CrossRef] [Google Scholar]
- Bio-waste Derived Catalytic Approach Towards NH-1,2,3-Triazole Synthesis. ChemistrySelect. 2021;6:7266-7270.
- [CrossRef] [Google Scholar]
- Clinochlore-Supported Copper Nanoparticles as Green and Efficient Catalyst for Room-Temperature Synthesis of 1, 2, 3-Triazoles in Water. Chemistryselect. 2019;4:3151-3160.
- [Google Scholar]
- A Cu(II)-inorganic Co-crystal as a versatile catalyst towards “click” chemistry for synthesis of 1,2,3-triazoles and beta-hydroxy-1,2,3-triazoles. Chemistryselect. 2020;5:75-82.
- [Google Scholar]
- preparation and characterization of a novel spherical cellulose-copper(II) oxide composite particles: as a heterogeneous catalyst for the click reaction. Mol. Divers.. 2020;24:201-209.
- [Google Scholar]
- Corn silk (Stigma maydis) in healthcare: a phytochemical and pharmacological review. Molecules. 2012;17:9697-9715.
- [Google Scholar]
- Chemical and phytochemical characteristics of local corn silk powder of three different varieties. Int. J. Adv. Sci. Eng. Inf. Technol.. 2017;7
- [Google Scholar]
- Sustainable design and novel synthesis of highly recyclable magnetic carbon containing aromatic sulfonic acid: Fe3O4@C/Ph—SO3H as green solid acid promoted regioselective synthesis of tetrazoloquinazolines. Appl. Organomet. Chem.. 2021;35:1-10.
- [CrossRef] [Google Scholar]
- Remarkable high-temperature Li-storage performance of few-layer graphene-anchored Fe3O4 nanocomposites as an anode. J. Mater. Chem. A. 2017;5:23035-23042.
- [CrossRef] [Google Scholar]
- Sonochemical decoration of graphene oxide with magnetic Fe3O4@CuO nanocomposite for efficient click synthesis of coumarin-sugar based bioconjugates and their cytotoxic activity. Catal. Lett.. 2020;150:1142-1154.
- [Google Scholar]
- Chemical structure and inhibition on α -glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohydr. Polym.. 2021;252:117185
- [CrossRef] [Google Scholar]
- Recent Advances of Visible-Light-Mediated Organic Transformations in Water. Green Chem.. 2021;23:232-248.
- [CrossRef] [Google Scholar]
- Impregnated copper ferrite on mesoporous graphitic carbon nitride: An efficient and reusable catalyst for promoting ligand-free click synthesis of diverse 1, 2, 3-triazoles and tetrazoles. Appl. Organomet. Chem.. 2019;33:5219.
- [Google Scholar]
- Synthesis, characterization, and investigation of catalytic activity of copper(II) porphyrin graphene oxide for azide–alkyne cycloaddition. Res. Chem. Intermed.. 2019;45:4473-4485.
- [Google Scholar]
- Click chemistry: diverse chemical function from a few good reactions. Angew. Chemie Int. Ed.. 2001;40:2004-2021.
- [Google Scholar]
- Andean sacha inchi (Plukenetiavolubilis l.) leaf-mediated synthesis of Cu2O nanoparticles: A low-cost approach. Bioengineering. 2020;7:1-10.
- [CrossRef] [Google Scholar]
- Bifunctional Solid Catalyst for Organic Reactions in Water: Simultaneous Anchoring of Acetylacetone Ligands and Amphiphilic Ionic Liquid “Tags” by Using a Dihydropyran Linker. Chem. asian J.. 2018;13:2529-2542.
- [Google Scholar]
- The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem.. 2011;126:261-269.
- [Google Scholar]
- Synthesis of nanomagnetic supported thiourea-copper(I) catalyst and its application in the synthesis of triazoles and benzamides. Appl. Organomet. Chem.. 2018;32:3933.
- [Google Scholar]
- Nutritional compositions aand antioxidative capacity of the silk obtained from immature and mature corn. J. King Saud Univ.. 2014;26:119-127.
- [Google Scholar]
- Magnetic γ-Fe2O3@Sh@Cu2O: an efficient solid-phase catalyst for reducing agent and base-free click synthesis of 1, 4-disubstituted-1, 2, 3-triazoles. BMC Chem.. 2020;14:1-16.
- [Google Scholar]
- Magnetic nanocomposite of crosslinked chitosan with 4,6-diacetylresorcinol for gold immobilization (Fe3O4@CS/DAR-Au) as a catalyst for an efficient one-pot synthesis of propargylamine. Mater. Today Commun.. 2021;29
- [Google Scholar]
- Rapid synthesis of triazole-modified resveratol analogues via click chemistry. J. Med. Chem.. 2006;49:467-470.
- [Google Scholar]
- CuI/L-proline catalyzed click reaction in glycerol for the synthesis of 1, 2, 3-triazoles. Tetrahedron Lett.. 2019;60:142-146.
- [Google Scholar]
- Synthesis and Characterization of Copper(I)-Cysteine Complex Supported on Magnetic Layered Double Hydroxide as an Efficient and Recyclable Catalyst System for Click Chemistry Using Choline Azide as Reagent and Reaction Medium. Catal. Letters. 2020;150:1186-1195.
- [Google Scholar]
- Cleaner production of agriculturally valuable benignant materials from industry generated bio-wastes : A review. Bioresour. Technol.. 2021;320:124281
- [CrossRef] [Google Scholar]
- Antioxidative activity of five flavones glycosides from corn silk (stigma maydis) Czech J. food Sci.. 2013;31:148-155.
- [Google Scholar]
- Copper (I) iodide supported on modified cellulose-based nano-magnetite composite as a biodegradable catalyst for the synthesis of 1, 2, 3-triazoles. Appl. Organomet. Chem.. 2017;31:3660.
- [Google Scholar]
- Synthesis of novel 1, 4-disubstituted 1, 2, 3-triazoles bearing organosilicon-sulfur groups via the click reaction sonocatalyzed by LaCuxMn1-xO3 nanoparticles. Synth. Commun.. 2016;46:1595-1604.
- [Google Scholar]
- Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol.. 2020;304:123003
- [CrossRef] [Google Scholar]
- Multicomponent click reaction catalyzed by organic surfactant-free copper sulfide (sf-CuS) nano/micro flowers. J. Organomet. Chem.. 2019;884:59-65.
- [Google Scholar]
- Synthesis of magnetically separable catalyst Cu-ACP-Am-Fe3O4@SiO2 for Huisgen 1,3-dipolar cycloaddition. Tetrahedron Lett.. 2018;59:3643-3652.
- [Google Scholar]
- Metal-catalyzed azide-alkyne “click” reactions: Mechanistic overview and recent trends. Coord. Chem. Rev.. 2016;316:1-20.
- [Google Scholar]
- Rice straw modified by click reaction for selective extraction of noble metal ions. Bioresour. Technol.. 2015;177:182-187.
- [CrossRef] [Google Scholar]
- Synthesis of CuxO (x = 1, 2)/ Amorphous Compounds by Dealloying and Spontaneous Oxidation Method. Mater. Res.. 2014;17:33-37.
- [Google Scholar]
- Synthesis and application of nanocomposite Fe3O4@SiO2@CTAB–SiO2 as a novel adsorbent for removal of cyclophosphamide from water samples. Sep. Sci. Technol.. 2020;55:456-470.
- [Google Scholar]
- One-pot three-component synthesis of 1, 4-disubstituted 1H–1, 2, 3-triazoles using green and recyclable cross-linked poly (4-vinylpyridine)-supported copper sulfate/sodium ascorbate in water/t-BuOH system. J. Iran. Chem. Soc.. 2014;11:1731-1742.
- [Google Scholar]
- Practical one-pot synthesis of 5-alkynyl-1,2,3-triazoles via heterogeneous copper(I)-catalyzed tandem three-component click/alkynylation reaction. Appl. Organomet. Chem.. 2020;34:5319.
- [Google Scholar]
- Picolinimidoamide-Cu(II) complex anchored on Fe3O4@SiO2 core-shell magnetic nanoparticles: an efficient reusable catalyst for click reaction. J. Coord. Chem.. 2018;71:1168-1179.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103838.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







