Translate this page into:
Searching for high performance asymmetrically substituted teterazine energetic materials based on 3-hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazine
⁎Corresponding authors. zqguo@nwu.edu.cn (Zhaoqi Guo), mahx@nwu.edu.cn (Haixia Ma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tetrazine compounds are promising candidates of high performance energetic materials (EMs). For the purpose of exploring high performance asymmetrically substituted teterazine explosives, 3-hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazine was selected as the precursor and a series of teterazine EMs were prepared based on three synthetic strategies. All the obtained compounds were fully characterized and the crystal structures of seven compounds were further confirmed by single crystal X-ray diffraction. The thermal dynamics and thermal safety parameters, the detonation properties and impact sensitivities were also obtained. The results revealed that compounds 15–17 show excellent energetic properties (detonation velocities > 9000 m·s−1, detonation pressures > 30 GPa) and have impact sensitivities (8–12 J) that comparable or lower than RDX. Compounds 9–13 are insensitive to impact and have detonation velocities range from 8452 to 8775 m·s−1. These compounds can be good candidates of high performance secondary explosives or insensitive EMs.
Keywords
Energetic materials
Tetrazine
Asymmetric substitution
Detonation performances
1 Introduction
With the increasing demand in the military and civilian fields, energetic materials (EMs) have been rapidly and extensively developed. High-performance EMs require a good balance between the energy and stability (Domasevitch et al., 2019; Song et al., 2020; Ma et al., 2020; Gao and Shreeve, 2011; Zhang et al., 2021). Among the reported EMs, nitrogen-rich heterocyclic compounds are very promising since they possess high heat of formation and large conjugated system that can enhance the molecular stability (Tang et al., 2020; Wu et al., 2020; Lin et al., 2018; Zhang et al., 2020; Yang et al., 2020). Scheme 1 Scheme 2.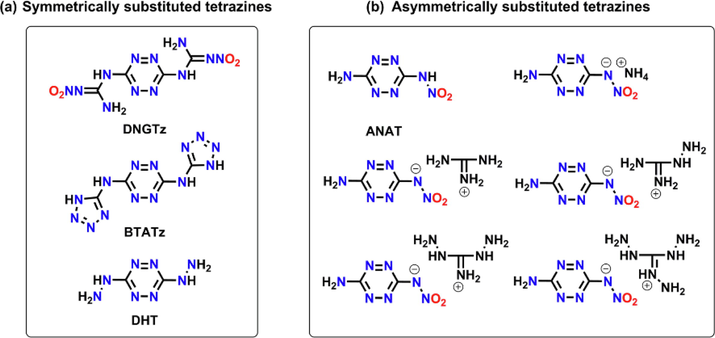
The structures of symmetrically substituted tetrazines (a) and asymmetrically substituted teterazines (b).
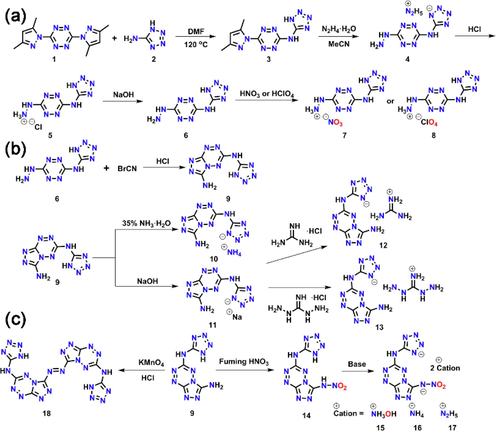
(a) Preparation of oxygen-rich salts of 6. (b) Preparation of fused ring compounds. (c) Preparation of the nitroamine and azo compounds.
Tetrazine rings, which show a high nitrogen content of 68.3%, are useful building blocks in constructing high energy density materials (Chen et al., 2018; Zhang et al., 2020; Snyder et al., 2019; Rudakov et al., 2021; Chen et al., 2020; Wei et al., 2014; Liu et al., 2019; Hu et al., 2018). There are three isomers for tetrazine compounds and the most commonly used tetrazine skeleton is 1,2,4,5-tetrazine, which was also called s-tetrazine. The electron-withdrawing effect of nitrogen atoms gives rise to an electron-deficient structure of tetrazine ring. As a result, the carbon atoms on the ring are easy to be attacked by nucleophiles to yield various tetrazine-based EMs. Representatives include 3,6-bis-nitroguanyl-1,2,4,5-tetrazine (DNGTz) (Chavez et al., 2004), 3,6-bis(1H-1,2,3,4-tetrazol-5-ylamino)-1,2,4,5-tetrazine (BTATz) (Chavez and Hiskey, 1999) and 3,6-dihydrazino-1,2,4,5-tetrazine (DHT) (Hiskey et al., 2001) et. al. The common feature of these compounds is that they are symmetrically substituted compounds, which means the energetic substituents on both sides of the tetrazine ring are the same. Over the past few decades, these symmetrically substituted tetrazines are reported a lot, asymmetrically substituted s-teterazine-based EMs (ASTEMs), which possess different energetic groups on the 3, 6 sites of the tetrazine ring, are rarely seen (Chen et al., 2018; Zhanget al., 2020; Snyder et al., 2019; Rudakov et al., 2021; Chen et al., 2020; Wei et al., 2014; Chavez et al., 2004). However, some reported ASTEMs also show comparable detonation performance with symmetrically substituted tetrazine explosives. Chavez and Hiskey synthesized 3-amino-6-nitroamino-tetrazine (ANAT) for the first time and its energetic salts were further studied by Gao et al (Chavez and Hiskey, 1999; Gao et al., 2006). These ASTEMs show calculated energetic properties comparable to tetryl (2,4,6-trinitrophenylmethylnitramine), PETN (pentaerythritol tetranitrate), TATB (1,3,5-triamino-2,4,6-trinitrobenzene) and RDX(hexahydro-1,3,5-trinitro-1,3,5-triazine). Hence, it’s very meaningful to further explore novel ASTEMs which have the potential as high performance EMs.
The reason why the ASTEMs are seldom seen is that the synthesis routes of ASTEMs are more complex and difficult compared with symmetrically substituted tetrazines. The most common and feasible strategy of constructing ASTEMs is to introduce different energetic moieties on the tetrazine ring successively through nucleophilic substitution reaction (NSR). The groups incorporated at the beginning will change the chemical environment of the tetrazine skeleton, making subsequent substitution reactions difficult or causing undesirable side reactions. For example, 3.6-dichloro-s-tetrazine is easy to convert to 3-amino-6-chloro-s-tetrazine within 20 min while the remaining leaving group was very difficult to displace (Chavez and Hiskey., 1999). Employing strong nucleophiles, such as hydrazine, can solve this problem to a certain extent. 3-hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazine (HTATz), which can be yielded by the NSR of hydrazine hydrate and 3-(1H-tetrazolyl-5-ylimino)-6-(3,5-dimethylpyrazol-1-yl)-s-tetrazine, behaves high thermal stability, a calculated density of 1.72 g·cm−3 and a detonation velocity of 8100 m·s−1 (Sinditskii et al., 2012). The nitrogen content of HTATz is up to 79% and shows the potential to be further modified to enhance the energy.
Given this background, in this work, HTATz was selected as the precursor to explore novel high-performance ASTEMs. Three strategies were designed and carried out. (1) Oxygen-rich ions (NO3–, ClO4-) were introduced based on the protonation and deprotonation reactions of hydrazine and tetrazole group to enhance the oxygen balance (OB) of obtained ASTEMs. (2) Nitroamine groups on a mono-ring system were used to improve the density and detonation property. However, this will greatly reduce the stability of the energetic compounds. Here, a fused cyclic framework was firstly incorporated into the structure of HTATz to enhance the conjugate effect thus improve the stability of the backbone (Chen et al., 2020; Tang et al., 2019; Xia et al., 2019; Hu et al., 2020; Tang et al., 2018) and then the nitrification process was implemented. (3) Azo linkage was incorporated to acquire high heats of formation (Qu and Babailov, 2018; Chinnam et al., 2020; Yount et al., 2020), which was hoped to obtain ASTEMs with high energetic performance.
Based on the above strategies, in this work, a series of ASTEMs were synthesized and fully characterized by multinuclear NMR, infrared spectra, elemental analyses and differential scanning calorimetry (DSC). Single crystal X-ray diffraction technology was also applied to obtained the crystal structures of ASTEMs. The thermal behaviours, thermal dynamics and thermal safety parameters were acquired. By calculating the energetic properties and determining the impact sensitivity, the application prospects of these ASTEMs were discussed.
2 Experimental section
Caution! Although no explosions were observed during the preparation and handling of all the compounds in this work, they must be synthesized only on a small scale and mechanical actions involving scratching or scraping should also be avoided. Eye protection, face shields and leather gloves must be worn.
2.1 Sample preparation
Materials and methods can be found in the supporting information. 3-Hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazine was synthesized according the literature (Sinditskii et al., 2012).
2.1.1 3-Hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazinium nitrate (7)
To 1 mL 20% nitric acid was added HTATz (0.195 g, 1 mmol). The slurry was stirred at room temperature for 1 h. The precipitate was filtered, washed with isopropyl alcohol and air dried to yield compound 7. Orange solid; 65% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 10.72 (s, 1H), 12.48 (s, 3H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 161.11, 159.05, 158.61 ppm; IR (KBr): ṽ = 3204, 3000, 2856, 2694, 1614, 1560, 1500, 1410, 1368, 1320, 1050, 966, 942, 804, 732, 565 cm−1; elemental analysis (%) for C3H6N12O3 (258.16): calcd C 13.96, H 2.34, N 65.11; found: C 13.68, H 2.20, N 65.32.
2.1.2 3-Hydrazino-6-(1H-1,2,3,4-tetrazol-5-ylimino)-s-tetrazinium perchlorate (8)
To 1 mL 20% perchloric acid was added HTATz (0.195 g, 1 mmol). The mixture was stirred at room temperature for 0.5 h. The slurry was filtered, washed with isopropyl alcohol and air dried to yield compound 8. Orange solid; 50% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 10.75 (s, 1H), 12.47 (s, 3H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 160.96, 159.14, 158.60 ppm; IR (KBr): ṽ = 3396, 3240, 2868, 2694, 1608, 1548, 1422, 1344, 1284, 1074, 1038, 948, 780, 727, 684, 630, 565 cm−1; elemental analysis (%) for C3H6N11O4Cl (295.60): calcd C 12.19, H 2.05, N 52.12; found: C 12.08, H 2.21, N 52.41.
2.1.3 3-Amino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (9)
To a suspension of hydrochloric acid (1 mol·L-1, 3 mL) and HTATz (0.195 g, 1 mmol) was added cyanogen bromide (0.1 g, 1 mmol). The mixture was then heated to 80 °C under stirring and kept for 4 h. A brown precipitate formed and it was then filtrated, washed with a large amount of water and dried to obtain compound 9. Brown solid; 60% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 7.64 (s, 1H), 2.55 (s, 3H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 150.32, 149.45, 149.22, 40.91 ppm; IR (KBr): ṽ = 3522, 3322, 2983, 1612, 1529, 1420, 1331, 1300, 1248, 1055, 1037, 941, 770 cm−1; elemental analysis (%) for C4H4N12 (237.19): calcd C 21.82, H 1.83, N 76.35; found C 21.71, H 1.99, N 76.51.
2.1.4 Ammonium 3-amino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (10)
To a suspension of compound 9 (0.220 g, 1 mmol) and methanol (5 mL) was added 25 % aqueous ammonia (0.154 g, 1.1 mmol). The mixture was stirred at room temperature for 2 h, filtrated, washed with ethanol and dried to obtain compound 10. Yellowish brown solid; 75% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 7.15 (br, 7H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 157.01, 154.39, 149.18, 148.56 ppm; IR (KBr): ṽ = 3032, 2912, 2821, 1621, 1559, 1444, 1391, 1252, 1156, 1050, 968, 901, 829, 733, 657, 618, 560 cm−1; elemental analysis (%) for C4H7N13 (237.19): calcd C 20.26, H 2.97, N 76.77; found: C 20.37, H 2.85, N 76.53.
2.1.5 Synthesis of energetic salts 12 and 13
Compound 9 (0.220 g, 1 mmol) was suspended in 8 mL methanol. A solution of sodium hydroxide (0.040 g) in water (2 mL) was added dropwise at room temperature. Then the mixture was heated to 60 °C and stirred for 0.5 h. Then guanidinium monohydrochloride (0.095 g, 1 mmol) or diaminoguanidinium monohydrochloride (0.126 g, 1 mmol) was slowly added. The mixture was kept at 60 °C for 4 h and then cooled to room temperature. The precipitate was filtered, washed with acetonitrile and air dried to obtain compound 12 or 13.
Guanidinium 3-amino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (12). Yellowish brown solid; 81% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 7.13 (s, 2H), 7.30 (s, 6H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 158.51, 157.28, 149.11, 148.46 ppm; IR (KBr): ṽ = 3565, 3421, 3095, 2754, 1635, 1554, 1472, 1391, 1252, 1137, 1050, 968, 906, 829, 733, 618 cm−1; elemental analysis (%) for C5H9N15 (279.23): calcd C 21.51, H 3.25, N 75.24; found: C 21.39, H 3.41, N 75.13.
Diaminoguanidinium 3-amino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (13). Yellowish brown solid; 84% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 4.61 (s, 4H), 7.12 (d, 4H), 8.74 (s, 2H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 160.25, 157.10, 149.13, 148.47, 89.99 ppm; IR (KBr): ṽ = 3354, 3305, 3109, 2797, 1645, 1611, 1549, 1492, 1463, 1391, 1252, 1180, 1151, 1045, 973, 896, 834, 738, 657, 618, 556 cm−1; elemental analysis (%) for C5H11N17 (309.26): calcd C 19.42, H 3.59, N 77.00; found: C 19.08, H 3.79, N 76.72.
2.1.6 3-Nitramino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (14)
Fuming nitric acid (6 mL) was cooled to −15 °C and compound 9 (0.660 g, 3 mmol) was added carefully. The mixture was stirred at −15 °C for 0.5 h, then 6 h at room temperature. The obtained solution was poured to crushed ice. The precipitate was filtered, washed with cold water and air dried to give compound 14. Yellow solid; 88% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 13.06 (s, 3H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 157.97, 152.98, 149.37, 148.81 ppm; elemental analysis (%) for C4H3N13O2 (265.16): calcd C 18.12, H 1.14, N 68.67; found: C 18.38, H 1.35, N 68.26.
2.1.7 Synthesis of energetic salts 15–17
Compound 14 (0.265 g, 1 mmol) was suspended in 3 mL ethanol. 50 % aqueous hydroxylamine (0.132 g, 2 mmol), 25 % aqueous ammonia (0.280 g, 2 mmol), 50 % hydrazine monohydride (0.200 g, 2 mmol) was slowly added under stirring to the slurry. The mixture was stirred at room temperature for 2 h. The precipitate was filtered, washed with ethanol and air dried to give compound 15–17.
Hydroxylammonium 3-nitramino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (15). Yellowish brown solid; 92% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 10.32 (s, 9H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ 156.03, 152.35, 148.82, 148.81 ppm; IR (KBr): ṽ = 2931, 2706, 1607, 1563, 1520, 1477, 1400, 1295, 1228, 1050, 1007, 882, 762, 733, 700, 657, 599 cm−1; elemental analysis (%) for C4H9N15O4 (331.22): calcd C 14.51, H 2.74, N 63.43; found: C 14.39, H 2.88, N 63.19.
Ammonium 3-nitramino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (16). Yellowish brown solid; 93% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 7.63 (s, 8H) ppm; 13C NMR (125 MHz, [D6]D2O) δ = 157.33, 152.39, 147.91, 147.77 ppm; IR (KBr): ṽ = 3181, 2778, 1611, 1559, 1520, 1453, 1396, 1290, 1237, 1131, 1055, 1007, 973, 906, 872, 825, 757, 733, 661, 603 cm−1; elemental analysis (%) for C4H9N15O2 (299.22): calcd C 16.06, H 3.03, N 70.22; found: C 16.18, H 2.87, N 70.41.
Hydrazinium 3-nitramino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (17). Yellowish brown solid; 93% yield; 1H NMR (500 MHz, [D6]DMSO) δ = 7.83 (s, 11H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ = 157.00, 152.63, 148.80, 148.77 ppm; IR (KBr): ṽ = 3335, 2725, 2624, 1607, 1559, 1515, 1477, 1391, 1295, 1233, 1103, 1040, 954, 820, 762, 724, 705, 657, 599 cm−1; elemental analysis (%) for C4H11N17O2 (329.25): calcd C 14.59, H 3.37, N 72.32; found: C 14.26, H 3.05, N 72.71.
2.1.8 3,3′-(Diazene-1,2-diyl)bis(N-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazin-6-amine) (18)
Compound 9 (0.220 g, 1 mmol) was added in concentrated hydrochloric acid (2 mL) and the mixture was cooled to −5 °C. A solution of KMnO4 (0.110 g, 0.69 mmol) in H2O (8 mL) was added dropwise under stirring. The slurry was heated to 45 °C for 4 h and then cooled to room temperature. The precipitate was filtered, washed with water and air dried to obtain compound 18. Yellow solid; 92% yield; 1H NMR (500 MHz, [D6]DMSO) δ 13.31 (s, 4H) ppm; 13C NMR (125 MHz, [D6]DMSO) δ 158.58, 154.33, 151.84, 151.80 ppm; IR (KBr): ṽ = 3483, 2802, 1621, 1539, 1501, 1434, 1362, 1261, 1131, 1040, 825, 757, 671, 609 cm−1; elemental analysis (%) for C8H4N24 (436.29): calcd C 22.02, H 0.92, N 77.05; found: C 22.18, H 1.08, N 77.31.
3 Results and discussion
3.1 Syntheses
HTATz (6) was obtained by employing the methods reported by Sinditskii et al. The hydrazinyl of HTATz is readily to react with 20% nitric or perchloric acid to yield its nitrate and perchlorate salts.
In order to incorporate a fused cyclic skeleton to enhance the stability, HTATz was treated with cyanogen bromide in 1 M hydrochloric acid at 80 °C, the fused ring tetrazine 3-amino-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine (9) can be obtained in good yield. When compound 9 was reacted with aqueous hydroxylamine or hydrazine monohydride, unidentified products were again formed. Fortunately, the ammonium salt (10) can be synthesized by the acid-base reaction and the guanidinium (12) or diaminoguanidinium salt (13) can be obtained by the metathesis reactions of guanidinium or diaminoguanidinium monohydrochloride, respectively.
Nitration of compound 9 was carried out using fuming nitric acid. Nitramino compound 14 was obtained as a yellow solid in 88% yield. 14 can further react with base to give hydroxylammonium, ammonium and hydrazinium salts 15–17.
Azo fused cyclic explosive 18 was formed smoothly as a yellow solid by acidic potassium permanganate solution as the oxidant.
3.2 Crystallography
The crystals of 6, 7, 9, 12, 13 and 18 were cultivated successfully while the crystal of 14 can’t be obtained due to its strong polarity. However, the crystal of its sodium salt can be prepared using water as the solvent. All the crystal structures were shown in Fig. 1. Crystallographic data are summarized in Table S1 and S2.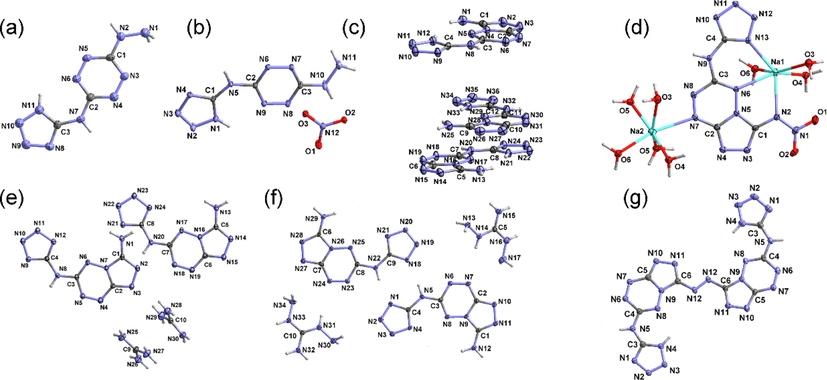
The crystal structures of 6·CH3OH (a), 7··2H2O (b), 9·4/3DMF (c), 12·5/2H2O (d), 13·3/2H2O (e), Na2·14··4H2O (f), 18·2DMSO·2CH3OH (g). The solvent molecules are hided.
HTATz (6) crystallizes in monoclinic P21/c space group (Fig. 1a) with good molecular planarity and the homogenization of the bond lengths revealing the existence of conjugate effect. Two adjacent HTATz molecules connect each other by hydrogen-bonding rings to form one-dimensional (1D) chains (Fig. 2a). While the methanol gives various hydrogen bonds to link the 1D chains to three-dimensional (3D) wave-like structure (Fig. 2b).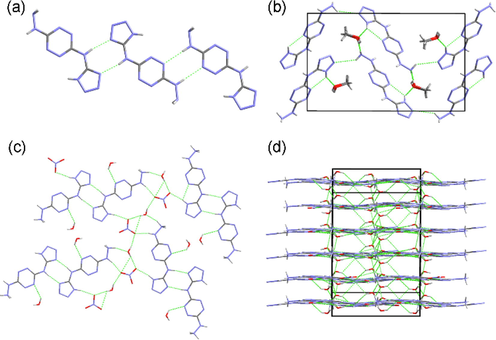
The 1D (a), 3D (b) structures of 5·CH3OH and the 2D (c) and 3D (d) structures of 7··2H2O.
Compound 7 crystallizes in monoclinic P21/n space group and the conjugate effect was also observed (Fig. 1b). The cations connect each other by “tail-to-tail” mode through hydrogen-bonding rings to form a dimer. These dimers accompany with water and nitrate anions to constitute a two-dimensional (2D) structure (Fig. 2c). The hydrogen bonds yielded by water molecules and the π···π contacts further connect the 2D structures into a 3D layered architecture (Fig. 2d).
The crystal of compound 8 can only be cultivated in the filtrate. Because there is excess perchloric acid left after the reaction, the obtained single-crystal were found to be the cocrystal of the water, perchlorate salt and perchloric acid (Fig. S1). The cell volume of 8·1/2HClO4·7/2H2O is up to 6117.8(14) Å3 and the R factors is high owing to the existence of twin crystal. The water molecules give rise to abundant hydrogen bonds to construct a 3D layered network (Fig. S2). The crystallographic data are not incorporated in Table S1 and S2 due to the poor quality.
Fused-ring compound 9 crystallizes in triclinic P-1 space group (Fig. 1c). The introduction of polycyclic skeleton not only enhances the conjugate effect but also improves the planarity of the compound. Each two molecules of 9 link together by “head-to-head” or “tail-to-tail” mode to compose a dimer (Fig. 3a). Similar to 7··2H2O, these dimers, together with solvent molecules, form 2D layers. These layers further construct a 3D layered structure (Fig. 3b).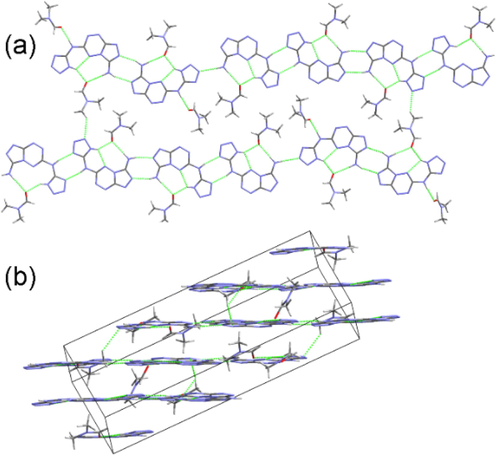
The 2D (a), 3D (b) structures of 9·4/3DMF.
The guanidinium salt 12 crystallizes in triclinic P-1 space group with two cations and two anions in the asymmetric unit (Fig. 1d). Benefitting from the large conjugate system, the cations exhibit good planarity. “Head-to-head” and “tail-to-tail” connecting modes are also found in 12·5/2H2O (Fig. 4a). The dihedral angles between the anion and the cation are 64.27 and 70.24°. All the hydrogen atoms of guanidinium cation take part in the formation of hydrogen bonds and these interactions connect all the ions and the water molecules into a 3D layered structure (Fig. 4b).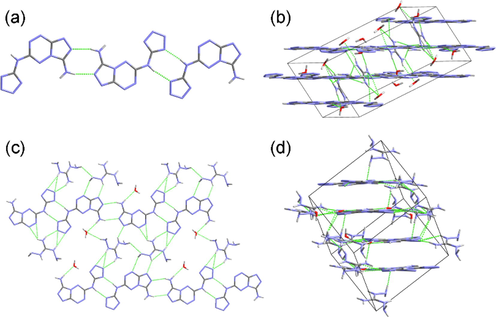
(a) The connecting modes in 12·5/2H2O. (b) The 3D structure of 12·5/2H2O. (c) The 2D layer of 13·3/2H2O. (d) The 3D structure of 13·3/2H2O.
The diaminoguanidinium salt 13 crystallizes in the same crystal system and space group with 12, i.e. triclinic P-1. The result shows that there are two pairs of ions in the asymmetric unit (Fig. 1e). The two anions link with each other through the hydrogen bonds yielded by the tetrazolyl moiety. The difference between 13 and 12 is that the dihedral angles between the anion and the cation in 13 are much smaller than those in 12, which are 13.61 and 28.94° respectively, demonstrating the better coplanarity of salt 13. The ions, together with the crystalline water molecules, constructing a 2D hydrogen-bonding layer (Fig. 4c). These layers further constitute a 3D architecture through interlayer hydrogen bonds and π···π interactions (Fig. 4d).
The sodium salt of compound 14 crystallizes in triclinic P-1 space group with two kinds of coordination modes. Every Na cation shows a six coordination structure, one coordinates with three aqua molecules and another coordinates with five water molecules. Two adjacent Na cations link with each other through two bridge water molecules to constitute a 2D metal–organic framework (MOF) (Fig. 5a). The hydrogen-bonding interactions produced by the coordination water further connect the 2D MOF into a 3D structure (Fig. 5b).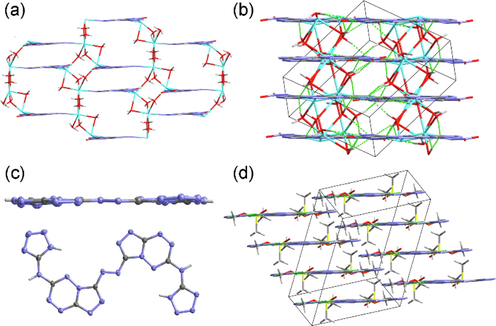
(a) The 2D metal–organic framework of Na2·14··4H2O. (b) The 3D structure of Na2·14··4H2O. (c) The molecular planarity of 18·2DMSO·2CH3OH. (d) The 3D structure of 18·2DMSO·2CH3OH.
Azo compound 18 also crystallizes in triclinic P-1 space group. The introduction of azo building block on the one hand connects two ASTEMs to a symmetrically substituted structure, on the other hand, the azo moiety also expands the conjugate system, which bring about the good molecular planarity of 18·2DMSO·2CH3OH (Fig. 5c). The crystalline DMSO molecules produce intermolecular hydrogen bonds to assist the formation of 1D chains. Although lack of hydrogen bond donor, 18·2DMSO·2CH3OH forms a layered structure in the 3D space by using π···π contacts (Fig. 5d).
3.3 Thermal behavior analysis
The thermal decomposition behaviors of compounds 6–10, 12–18 were determined by differential scanning calorimetry (DSC) at the heating rate of 5 oC·min−1. The DSC curves were drawn in Fig.S3-Fig.S14 and the thermal decomposition parameters are summarized in Table 3. All the compounds decompose directly without melting. The endothermic peaks for 6, 8, 10, 12, 13, 14, 18 are the processes of losing residual solvents. Besides, many compounds show multiple exothermic decomposition processes. This may due to the incorporation of different energetic groups and the asymmetric structure of ASTEMs.
Compound 6 decomposes at 176 °C with a desolvation process at 86 °C. The introduction of the oxygen-rich ions obviously has impacts on the thermal stability of 6. The nitrate shows lower decomposition temperature (Td) at 153 °C while the perchlorate has a better thermal stability (Td = 275 °C). The Td of compound 6 was improved to 189 °C (compound 9) after the incorporation of fused cyclic framework. Meanwhile, the broad exothermic peak indicates that the thermal decomposition process is not as intense as compound 6. Beyond that, the ammonium (10, Td = 247 °C), guanidinium (12, Td = 280 °C), diaminoguanidinium (13, Td = 221 °C) salt of 9 also shows good thermal stability, which demonstrate that a fused skeleton does help to enhance the thermal stability of ASTEMs. After nitrification, compound 14 shows a Td of 167 °C, which clearly reveal that the nitrification process will weaken the stability of EMs, even though a fused cyclic framework was firstly incorporated. The effect of fused ring structure on improving thermal stability is also affected by the energetic groups connected to it. The negative impact of nitrification process can be regulated by synthesizing energetic salts. The hydroxylammonium (15, Td = 168 °C), ammonium (16, Td = 206 °C), hydrazinium (17, Td = 178 °C) salt of 14 all decompose at higher temperatures than the neutral compound. This may be caused by the existence of more and stronger hydrogen bonds in dication salts. The azo compound 18 shows a Td at 240 °C with a broad decomposition process. Its Td is much higher than that of compound 9, which reveals that the introduction of azo building block can enhance the thermal stability of tetrazine EMs.
3.4 Thermal dynamics and thermal safety
Thermal safety parameters, including thermal ignition temperature (Tbe), self-accelerating decomposition temperature (TSADT), and critical temperature of thermal explosion (Tbp), are very important for the application of EMs. Generally, the higher these parameters of the EMs, the better their resistance to heat. Beyond that, the activation Gibbs free energy (ΔG≠), activation enthalpy (ΔH≠) and activation entropy (ΔS≠) can also assist the assessment of thermal safety of EMs (Liu et al., 2017).
For the purpose of obtain above mentioned parameters, the activation energy (E) and pre exponential factor (A) should be firstly calculated. Kissinger (equation (1)) and Ozawa (equation (2)) method are used to obtain E and A. Then, Tbe, TSADT, Tbp, ΔG≠, ΔH≠ and ΔS≠ can be further calculated by equation 3–8.
In the above equations, β, R, Tp are the heating rates, molar gas constant and the peak temperature respectively. Te0/p0 are temperatures when β approaches zero. Besides, h and kB are Planck and Boltzmann constants. For the obtained compounds that possess a stable baseline and a complete single exothermic peak for the first decomposition process, the thermal dynamics and thermal safety parameters were calculated and listed in Table 1–2. The Te and Tp at different heating rates are listed at Table S3. [a] Activation energy calculated by Ozawa method using peak temperature. [c] Activation energy calculated by Kissinger method using peak temperature. [d] Pre-exponential factor. [f] Activation energy calculated by Ozawa method using extrapolated temperature. [b][e][g] Linear correlation coefficient. [a] Decomposition temperature (onset, 5 oC·min−1). [b] Nitrogen content. [c] Density measured with a gas pycnometer (25 °C). [d] Heat of formation. [e] Detonation velocity (calculated with Explo5 v6.04). [f] Detonation pressure (calculated with Explo5 v6.04). [g] Impact sensitivity. [h ] Liu et al., 2019.
Compound
Eop[a] (kJ·mol−1)
rop[b]
Ek[c] (kJ·mol−1)
logAk[d] (s−1)
rk[e]
Eoe[f] (kJ·mol−1)
roe[g]
6
291.45
0.999
298.82
32.31
0.999
230.22
0.996
12
268.90
0.995
273.47
23.79
0.995
265.56
0.995
13
193.24
0.999
194.86
18.44
0.998
193.10
0.983
15
213.93
0.992
217.31
22.93
0.992
140.16
0.998
16
218.68
0.990
221.38
20.63
0.990
181.24
0.988
17
202.31
0.990
204.86
20.83
0.990
192.45
0.990
Compound
Tp0 (oC)
Te0 (oC)
Tbp (oC)
Tbe (oC)
TSADT (oC)
ΔS≠
(J·mol−1·K−1)ΔG≠
(kJ·mol−1)ΔH≠
(kJ·mol−1)
6
179.19
174.22
185.18
181.69
174.22
361.84
131.38
295.06
12
274.20
272.82
283.79
282.49
272.82
197.15
161.01
268.92
13
212.63
213.50
223.23
224.15
213.50
95.73
144.32
190.82
15
174.06
157.89
182.12
169.51
157.89
182.37
132.03
213.59
16
228.39
199.01
238.34
209.71
199.01
137.39
148.31
217.21
17
188.82
173.62
197.94
182.59
173.62
141.90
135.47
201.02
Compound
Td[a]
(oC)N[b]
(%)
ρ[c]
(g·cm−3)ΔHf[d]
(kJ·mol−1/ kJ·g−1)
D[e]
(m·s−1)
P[f]
(GPa)IS[g]
(J)
7
153
65.1
1.72
814.5/3.16
8865
30.9
8
8
275
52.1
1.76
858.2/2.90
8670
32.1
6
9
189
76.4
1.76
907.5/4.12
8452
26.1
36
10
247
76.8
1.71
939.9/3.96
8775
27.7
40
12
280
75.2
1.70
898.1/3.22
8504
25.3
40
13
221
77.0
1.67
1117.6/3.61
8772
27.2
36
14
167
68.7
1.80
927.8/3.50
8594
29.0
6
15
168
63.4
1.81
1015.7/3.07
9374
35.2
10
16
206
70.2
1.75
895.9/2.99
9022
30.4
10
17
178
72.3
1.75
1230.2/3.74
9488
33.9
8
18
240
77.1
1.79
2175.3/4.99
8472
26.9
10
HTATz (6)
176
79.0
1.72
623.0/3.19
8100
–
10
RDX[h]
204
37.8
1.80
70.3/0.36
8795
34.9
7.4
HMX[h]
280
37.8
1.90
105.0/0.25
9320
39.5
7
As can be seen, when polycyclic skeleton is incorporated, the thermal safety of ASTEMs are improved. The values of the thermal safety parameters of 12 and 13 are much higher than that of 6, which again demonstrates that fused ring moiety can effectively improve the thermal stability of ASTEMs. Nevertheless, the thermal safety parameters of fused ring tetrazines become lower when the nitrification process was carried out. The thermal safety of the ammonium salt 16 is the best among the three nitramine ionic compounds. The Tbp, Tbe and TSADT of 15 and 17 are lower than 200 °C while the Tbp, Tbe and TSADT of 16 are close to those of 13. Hence, it can be found that the incorporation of fused ring skeleton is helpful to improve the thermal safety of ASTEMs while the substituents on the fused ring and the types of ions also have an impact on thermal safety. The amino-substituted fused ring compound and the guanidinium, diaminoguanidinium and ammonium salts show better thermal safety.
3.5 Energetic property and sensitivity
The densities of 7–10, 12–18 were determined by using gas pycnometer (Table 3). After the formation of ionic compounds, the density of the nitrate salt (7) is the same with the precursor 6 and the density of the perchlorate salt (8) is improved to 1.76 g·cm−3. Besides, when polycyclic skeleton was incorporated, the density can also be improved. The nitrification of the fused ring tetrazine compounds and the introduction of azo building block are also the methods to increase the density. Compound 14 and 18 show densities of 1.80 and 1.79 g·cm−3 while the hydroxylammonium salt 15 possesses the highest density (1.81 g·cm−3) among all the synthesized compounds, which are comparable to that of RDX.
The heats of formation (ΔHf) of all the obtained ASTEMs were calculated by designing isodesmic reactions and the lattice enthalpies were obtained by using the methods that suggested by Jenkins et al, which can be found in the supporting information. The calculated results are listed in Table 3. All the compounds show high nitrogen contents except perchlorate salt 8 has the nitrogen content (NC) of 52.1% because of the incorporation of chlorine. The high NC thus bring about high ΔHf that higher than 800 kJ·mol−1 for all the synthesized ASTEMs. Compounds 7 and 8 not only have higher ΔHf than its precursor 6 but also possess better OB (6: −45.1%; 7: −18.6%; 8: −10.8%). Meanwhile, the incorporation of fused ring skeleton, the nitrification process and the introduction of azo moiety all yield a higher ΔHf comparing with compound 6. The azo compound 18 even shows a ΔHf of 2175.3 kJ·mol−1, demonstrating these methods are efficient ways to enhance the energy of ASTEMs.
Based on the measured density and the calculated ΔHf, the detonation velocity (D) and the detonation pressure (P) of 7–10, 12–18 were obtained by using Explo5 v6.04 (Suceska, 2017).As can be seen in Table 3, when oxygen-rich ions (NO3–, ClO4-) were introduced, the energy properties of compounds 7, 8 were improved comparing with 6 due to the optimization of the OB and ΔHf. The D of 7 and 8 are close to RDX while their P is higher than 30 GPa. The detonation properties of compounds 9–13 lie between 8452 and 8775 m·s−1 and 25.3–27.7 GPa. After nitrification, the D and P of these fused ring compounds are improved. Compounds 15–17 show higher D than RDX. Especially, the D of 17 is higher than that of HMX. Although the density of the hydroxylammonium salt 15 is higher than the hydrazinium salt 17, compound 17 possesses higher heat of formation and more gaseous products at the moment of detonation which make compound 17 shows higher detonation velocity. Surprisingly, although azo compound 18 has the highest ΔHf among all the synthesized ASTEMs, it possesses detonation properties of 8472 m·s−1 and 26.9 GPa, which are close to that of compound 9. This reveals that the azo moiety has a limited contribution to the detonation properties of ASTEMs.
The impact sensitivities (IS) of 7–10, 12–18 were also determined. The nitrate 7 (8 J) and the perchlorate salt 8 (6 J) show higher sensitivities than their precursor 6. When polycyclic skeleton was incorporated, the stability of ASTEMs was improved greatly. Compounds 9, 10, 12, 13 were all insensitive to impact, which may due to the enhanced molecular planarity and conjugation. This reveal the positive effects of fused ring skeleton in constructing low sensitive EMs. However, even though a polycyclic framework was incorporated firstly, the nitrification of the fused ring will still cause the increase of the IS. The results show that compound 14 (6 J) is sensitive to impact while the IS of 15 (10 J), 16 (10 J), 17 (8 J) are lower than that of 14, which may also due to the existence of more intermolecular hydrogen bonds in the dication salts. It is worth noting that compound 18 has an IS of 10 J and this is much lower than that of 9. This phenomenon reveal that the contribution of azo building block to energy is limited and it may bring high sensitivity to ASTEMs.
4 Conclusion
Twelve asymmetrically substituted s-teterazine-based energetic compounds were successfully obtained in this work. The oxygen-rich salts decompose between 153 and 275 °C and the introduction of fused ring and azo moieties were found to be capable of improving the thermal stability. Besides, the substituents on the fused ring and the types of ions have an impact on thermal safety. Compounds 15–17 show excellent energetic properties and have impact sensitivities that comparable or lower than RDX, which can be good candidates of high performance secondary explosives. The fused ring compounds 9–13 are insensitive to impact and show detonation velocities range from 8452 to 8775 m·s−1, which can be used as insensitive energetic materials. Compounds 7, 8, 14 have detonation velocities comparable to RDX while they are sensitive to impact. The properties of these compounds can be further optimized. For asymmetrically substituted teterazines, the contribution of azo building block to energy is limited and it may bring high sensitivity.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We gratefully acknowledge the financial support of National Natural Science Foundation of China (21673179, U1830134), Shaanxi Key Science and Technology Innovation Team Project (2022TD-33), and the Natural Science Foundation of Shaanxi Province (Grant No. 2020JZ-43).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel high-nitrogen materials based on nitroguanyl-substituted tetrazines. Org. Lett.. 2004;6(17):2889-2891.
- [Google Scholar]
- Crystal structures, thermal behavior analysis and thermal safety of two energetic salts of hydrazinyl-1,2,4,5-tetrazine with 3,5-dinitrosalicylic acid. Chem. Res. Chin. Univ.. 2018;34(6):959-964.
- [Google Scholar]
- Constructing a 3D-layered energetic metal-organic framework with the strong stacking interactions of hydrogen-bridged rings: the way to an insensitive high energy complex. CrystEngComm.. 2020;22(33):5436-5446.
- [Google Scholar]
- 5,6-Fused bicyclic tetrazolo-pyridazine energetic materials. Chem. Commun.. 2020;56(10):1493-1496.
- [Google Scholar]
- Azo- and methylene-bridged mixed azoles for stable and insensitive energetic applications. Dalton Trans.. 2020;49(33):11498-11503.
- [Google Scholar]
- Facile and selective polynitrations at the 4-pyrazolyl dual backbone: straightforward access to a series of high-density energetic materials. New J. Chem.. 2019;43(3):1305-1312.
- [Google Scholar]
- 3-Amino-6-nitroamino-tetrazine (ANAT)-based energetic salts. Chem. Commun.. 2006;38:4007-4009.
- [Google Scholar]
- Low-smoke pyrotechnic compositions.. 2001;US:6312537.
- Selecting suitable substituents for energetic materials based on a fused triazolo-[1,2,4,5]tetrazine ring. ACS Appl. Energy Mater.. 2020;3(6):5510-5516.
- [Google Scholar]
- Conjugated energetic salts based on fused rings: Insensitive and highly dense materials. J. Am. Chem. Soc.. 2018;140(44):15001-15007.
- [Google Scholar]
- Synthesis and properties of energetic ionic salts based on 3,6-bis (1 H-1,2,3,4-tetrazol-5-yl-amino)-s-tetrazine (BTATz) Chin J Energ Mater.. 2017;25(7):570-578.
- [Google Scholar]
- Stabilizing volatile azido in a 3D nitrogen-rich energetic metal-organic framework with excellent energetic performance. J. Solid State Chem.. 2018;265:42-49.
- [Google Scholar]
- Multipurpose [1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine-based energetic materials. J. Mater. Chem. A.. 2019;7(13):7875-7884.
- [Google Scholar]
- Pyrazol-triazole energetic hybrid with high thermal stability and decreased sensitivity: facile synthesis, characterization and promising performance. Chem. Eng. J.. 2020;379
- [Google Scholar]
- Azo-linked high-nitrogen energetic materials. J. Mater. Chem. A.. 2018;6(5):1915-1940.
- [Google Scholar]
- Synthesis and physicochemical properties of energetic 1,2,4,5-tetrazinyl derivatives of 5-nitro-2,4-dihydro-1,2,4-triazol-3-one. ChemistrySelect.. 2021;6(30):7654-7662.
- [Google Scholar]
- Thermal behavior and combustion mechanism of high-nitrogen energetic materials DHT and BTATz. Thermochim. Acta.. 2012;535:48-57.
- [Google Scholar]
- Suceska, M., EXPLO5 v6.04, Brodarski Institute, Zagreb, Croatia, 2017.
- Polycyclic N-oxides: high performing, low sensitivity energetic materials. Chem. Commun.. 2019;55(17):2461-2464.
- [Google Scholar]
- Melamine N-oxide based self-assembled energetic materials with balanced energy & sensitivity and enhanced combustion behavior. Chem. Eng. J.. 2020;395
- [Google Scholar]
- Energetic functionalized azido/nitro imidazole fused 1,2,3,4-tetrazine. Eur. J. Org. Chem.. 2018;19:1006-1012.
- [Google Scholar]
- Energetic and fluorescent azole-fused 4-amino-1,2,3-triazine-3-N-oxides. ACS Appl. Energy Mater.. 2019;2(12):8871-8877.
- [Google Scholar]
- A duo and a trio of triazoles as very thermostable and insensitive energetic materials. Inorg. Chem.. 2020;59(23):17766-17774.
- [Google Scholar]
- N-oxide 1,2,4,5-tetrazine-based high-performance energetic materials. Chem. Eur. J.. 2014;20(51):16943-16952.
- [Google Scholar]
- Coplanar fused heterocycle-based energetic materials. Propellants, Explos., Pyrotech.. 2020;45(4):536-545.
- [Google Scholar]
- Synthesis of thermally stable and insensitive energetic materials by incorporating the tetrazole functionality into a fused-ring 3,6-dinitropyrazolo-[4,3-c]pyrazole framework. ACS Appl. Mater. Interfaces.. 2019;11(49):45914-45921.
- [Google Scholar]
- New nitrogen-rich heterocyclic compounds to build 3D energetic metal complexes. CrystEngComm.. 2020;22(27):4573-4579.
- [Google Scholar]
- 4,4’,5,5’-Tetraamino-3,3’-azo-bis-1,2,4-triazole and the electrosynthesis of high-performing insensitive energetic materials. J. Mater. Chem. A.. 2020;8(37):19337-19347.
- [Google Scholar]
- A series of guanidine salts of 3,6-bis-nitroguanyl-1,2,4,5-tetrazine: green nitrogen-rich gas-generating agent. RSC Adv.. 2020;10(60):36287-36294.
- [Google Scholar]
- Studies on the synthesis and properties of nitramino compounds based on tetrazine backbones. Dalton Trans.. 2020;49(17):5590-5596.
- [Google Scholar]
- A high density and insensitive fused [1,2,3]triazolo-pyrimidine energetic material. Chem. Eng. J.. 2021;404
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103880.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







