Translate this page into:
UV/TiO2 photodegradation of metronidazole, ciprofloxacin and sulfamethoxazole in aqueous solution: An optimization and kinetic study
⁎Corresponding author at: Principal Scientific Officer, BCSIR, Dhaka, Bangladesh. dr.abdul.gafur.bcsir@gmail.com (Md. Abdul Gafur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
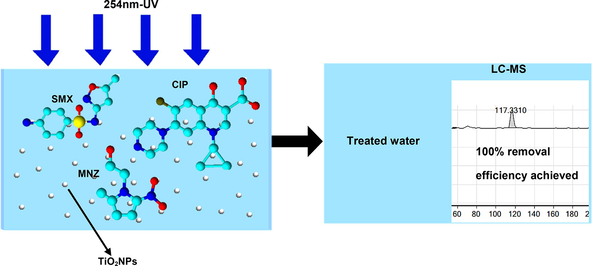
Abstract
Emerging pharmaceutical ingredients (APIs) like sulfamethoxazole (SMX), metronidazole (MNZ) and ciprofloxacin (CIP) are biopersistent and toxic to the environment and public health. In this study, UV/TiO2 photodegradation was applied in the degradation of SMX, MNZ and CIP individually and in a mixture. For a 5 mg/L SMX solution, about 97% of SMX was degraded within 360 min, which was reduced to 80% for 80 mg/L of SMX solution at the same TiO2 dosage and photodegradation time. The maximum removals of MNZ and CIP as individual components were 100% and 89%, respectively at 600 min of photodegradation reaction time. For binary mixtures, the highest removal (100%) was achieved for MNZ and CIP ([MNZ] = [CIP] = 40 mg/L) mixture at 120 min whereas the degradations were 97% and 96% for SMX and MNZ, and SMX and CIP binary mixtures, respectively, even after 600 min of experimental time at the same concentrations. For tertiary mixture, the maximum degradation 99% was observed for (SMX = CIP] = 20 mg/L and [MNZ] = [40 mg/L) at 600 min. The observed reaction rate was 0.01085 min−1 when SMX concentration was 5 mg/L, which decreased to 0.00501 min−1 for SMX concentration of 80 mg/L, indicating decreasing of reaction rate at higher concentration. The results indicate that the UV/TiO2 process is promising to apply for the treatment of pharmaceutical wastewaters.
Keywords
Ciprofloxacin
Degradation kinetics
Metronidazole
Sulfamethoxazole
Photodegradation
- SMX
-
Sulfamethoxazole
- MNZ
-
Metronidazole
- CIP
-
Ciprofloxacin
- AOP
-
Advanced oxidization process
- LC-MS
-
Liquid chromatography mass spectrometry
Abbreviations
1 Introduction
The industrial revolution is a blessing for human beings but the misuse and abuse of active pharmaceuticals ingredients, fertilizer, textile and industrial dye in the environment have accelerated the growing pollutants like diethyl phthalate, dimethyl phthalate, 2-4 dinitrophenol, etc. (Ahmadi et al., 2016; Azari et al., 2021a, 2021b, 2020; Yousefzadeh et al., 2017). Recently, the use of drugs has increased dramatically because of the spreading of various diseases (Kanakaraju et al., 2018). Thousands of different APIs (active pharmaceutical ingredients) are currently in use to treat or prevent human and animal diseases. In addition, APIs are extensively used as feed additives for livestock to promote the growth of animals (Carlsson et al., 2006). With the inadequate disposal of expired antibiotics and incomplete metabolism in the human and animal bodies, the concentration of antibiotics is increased in wastewaters day by day (Grenni et al., 2018; Malakootian and Ahmadian, 2019). Groundwater, surface water and river water are getting polluted by these drugs, which consequently affects the whole ecosystem of the contaminated region. For instance, Yantze river region, Guangzhon and Nairobi/Athi river basins (Kenya, East Africa) are regarded as contaminated hot spots from pharmaceutical ingredients (Bagnis et al., 2020; Leung et al., 2013). Among the different APIs, antibiotics are the main concern because of their biopersistent, toxic, carcinogenic and mutagenic nature (Gutierrez et al., 2013). The concentration of antibiotics in the surface waters is increasing day by day due to uncontrolled release and lack of proper treatment of hospital and municipal wastewaters before discharging. This causes multi-resistant bacteria to pose serious risks to human and veterinary health (Grenni et al., 2018). Antibiotics removal from wastewater is a great attraction for researchers due to their negative effects on various organisms and ecosystems. An effective treatment scheme for hospital and household wastewaters is urgently required for controlling the drug's concentration in the surface water bodies.
Hospital effluents contain 2–150 times higher concentrations of various drugs than in urban wastewater reported by Verlicch et al. (Verlicchi et al., 2010). Some antibiotics, like penicillin, are easily biodegraded, whereas others, like fluoroquinolones (e.g., ciprofloxacin), nitroimidazole (e.g., metronidazole), macrolides and tetracyclines, are significantly persistent. This leads to their remaining for an extended period in the environment, spreading wider and accumulating in higher concentrations. The input and presence of antibiotics and their fate in the environment are still of high interest because antibiotics pollution in rivers worldwide exceeds environmental safety thresholds (Khan et al., 2020). Metronidazole (MNZ, C6H9N3O3) is an imidazole antibiotic used for bacterial vaginosis and pelvic inflammatory disease (Neghi et al., 2019), ciprofloxacin (CIP, C17H18N3O3F) is a fluoroquinolone antibiotic mainly used for bacterial infections (Li et al., 2020), and sulfamethoxazole (SMX, C10H11N3O3S) is a sulfonamide antibiotic used for bronchitis and urinary tract infections (Xekoukoulotakis et al., 2011). They are widely used in both human and veterinary medicine and detected in aquatic environments like drinking water, ground/ surface water, soil sediments and wastewater from one to several ng L−1 (Neghi et al., 2019).
Recently, CIP, MNZ and SMX are shown as the most emerging pharmaceutical contaminants in Bangladesh. Anwar Hossain et al. found that the concentration of MNZ and SMX were distinctively higher in surface water of Bangladesh among nine APIs detected (Hossain et al., 2018; Hossain et al., 2017). Another study found that CIP is the highest concentration of antibiotics in Dhaka, Matlab sites and Rampura canal, Bangladesh. Susceptibility for select ESBL-p E. coli strains were tested and the study found that 85% of the strains were susceptible to CIP (Angeles et al., 2020). The bio persistent CIP, MNZ and SMX are almost ubiquitous in not only in surface water of Bangladesh but also globally.
Due to the potential adverse effects of antibiotics on human health, reliable techniques with stable removal effectiveness are highly desirable. Several treatment techniques including physical (e.g., membrane separation and adsorption) (Garcia-Ivars et al., 2017; Mahmoodi Meimand et al., 2019), chemical (Crini and Lichtfouse, 2019), biological (Martini et al., 2018) and advanced oxidization processes (AOPs) have been applied for the removal of antibiotics from water (Martini et al., 2018). Membrane separation and adsorption processes are not always effective to attain the discharge limits and are costly (Van der Bruggen et al., 2008). Chemical processes such as chlorination and ozonation produce secondary pollutants (halogenated byproducts) (Xu et al., 2002). Biological treatment cannot remove antibiotics completely from the water because of their toxic and biopersistent nature (Martini et al., 2018). AOPs including photo-Fenton, solar-photocatalytic and UV-based photocatalytic processes are promising techniques as a group of strong oxidizing agents such as superoxide radicals, peroxide radicals, persulfate radicals and hydroxyl radicals can be generated (Chen et al., 2021; Dharwadkar et al., 2021; Javid et al., 2020; Wu et al., 2020). These species are highly reactive and can degrade both biodegradable and nonbiodegradable organic pollutants from water (Shuchi et al., 2021; Tran et al., 2019). Recently, UV-based photocatalytic nanoparticles (NPs) have earned more attention to removing antibiotics from wastewater because it has the advantages of energy-saving without generating any secondary pollutants (Malakootian et al., 2020d, 2020a). Among the photocatalysts, TiO2 NPs are widely used in the treatment of organics in water because of their high photoactivity, high chemical stability, low cost, nontoxic, narrow bandgap, reusable and applicable for a wide range of pH (Al-Mamun et al., 2021).
However, most of the UV-based TiO2 photocatalysis process has been applied for a single compound. Individual studies of SMX, MNZ and CIP degradation have shown excellent performance (Farzadkia et al., 2015; Malakootian et al., 2019; Porcar-Santos et al., 2020; Silva et al., 2016). Impacts of operating parameters such pH, recirculatory flow, concentrations, catalyst dosage, UV light intensity and degradation kinetics have been studied well (Farzadkia et al., 2015; Salma et al., 2016). Competitive degradation of antibiotics in a mixture using photo- Fenton process (Perini et al., 2018) (mixture: ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine), solar simulator based Cu-modified TiO2 (Evgenidou et al., 2021) (mixture: isoniazid, metronidazole, sulfadiazine, sulfamethoxazole, trimethoprim, norfloxacin, moxifloxacin and lincomycin) and UV based TiO2 (metronidazole and amoxicillin) (Tran et al., 2019) process have been studied. Eleni Evgenidou et al used Pyrex glass reactor to remove a mixture of eight antibiotics and finally centrifuged to remove photocatalyst (0.8CuTiO2) (Evgenidou et al., 2021). To our best knowledge, no study proposed the degradation of SMX, MNZ and CIP in a mixture using UV-based TiO2 photocatalyst. In this context, the current study focused on UV-based TiO2 photocatalytic degradation of SMX, MNZ and CIP antibiotics individually and in mixtures, with various combinations to understand the degradation behaviors and reaction kinetics.
TiO2 can be applied through immobilization on glass and steel surfaces (Al-Mamun et al., 2021) or in an suspension in a photocatalytic reactor (Hasan Khan Neon and Islam, 2019). The photocatalytic activity of immobilized TiO2 NPs was decreased whether the activity was remain unchanged after five cycle experiments for suspended experiments (Farzadkia et al., 2015). Suspended TiO2 was settled from UV- TiO2 treated solutions after 24 h settling to avoid centrifugation for large scale applications. Therefore, the current study was carried out to investigate the photocatalytic performance of TiO2 NPs in aquatic suspension in the treatment of SMX, MNZ and CIP antibiotics under UV-irradiation. The major objectives of the study were: optimization of TiO2 NPs dosage, assessment of comparative degradations of SMX, MNZ and CIP antibiotics, study of degradation kinetics and assessment of competitive degradation of SMX, MNZ and CIP antibiotics in mixtures.
2 Materials and methods
2.1 Materials
The pharmaceutical metronidazole (MNZ), ciprofloxacin (CIP) and sulfamethoxazole (SMX) were obtained from Sigma-Aldrich, Chemie GmbH, Kappelwegl, Gemrany. TiO2 (anatase, 99.9%, 25 nm) was collected from Inframet Advanced Materials, USA, product# 22 N-0803A, Lot# TiO2N9140A3. Deionized water was collected from an ISO LAB (INARS, BCSIR, Dhaka, Bangladesh) and was used to prepare all stock solutions of antibiotics and used for dilutions for LC-MS analysis. The Nylon membrane 0.22 υm, 25 mm; PTFE; chrodisc by CHM was used for filtering the samples during the degradation. Formic acid, 98–100%, EssentQ® (Scharlau, Sentmenat, Spain) and acetonitrile, LC-MS (Scharlau, Sentmenat, Spain) were used for LC-MS analysis. Methanol and ethanol were purchased from Merck (HPLC grade, Germany). Ethanol was used for cleaning purpose and methanol was used as solvent to make standard sample for LC-MS analysis. All chemicals were used without any further purification. An Orbital sharer (JSOS-300, JSR, Rep. of Korea) was used for mixing purpose of all solutions.
2.2 Characterization of TiO2 NPs
The powder X-ray diffraction (XRD) patterns were done using D8 Advance, Bruker AXS, Germany XRD with Cu Kα radiation (λ = 1.5418 Å) at a scan rate of 2° min−1 from 10° to 90° following 40 kV of accelerating voltage and 40 mA of applied current.
2.3 Photocatalytic experiment
A simple photocatalytic reactor was used for the degradation of antibiotics and the schematic of the experimental set-up is shown in Fig. 1. Three (3) ultraviolet light, 8 W of each (254 nm) was used for the UV-irradiation source and a minimum distance (4.5 cm) was maintained between photocatalyst and radiation source so that the maximum fraction of UV light is utilized to generate a large number of hydroxyl radicals. All photodegradation experiments were performed in the same photoreactor so that it is easy to make an authentic comparison of the results.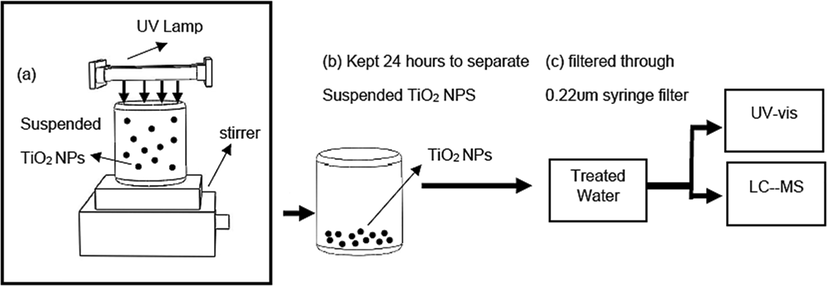
Experimental set-up representation.
A stock solution of SMX (80 mg/L) was prepared and stored in a refrigerator at 4 0C. To study the impact of TiO2 dosage on SMX under UV-irradiation, different concentrations of TiO2 were added in a predetermined volume of prepared SMX solution and stirred at 250 rpm. Experiments were carried out for selected TiO2 dosages of 0.5 g/L, 0.7 g/L and 1 g/L at room temperature for 6 h. The optimum dosage was determined based on SMX removal (0.7 g/L), and this optimum dosage was used for further studies. The impact of SMX concentration on UV-irradiation/TiO2 process performance was evaluated by changing SMX concentrations ranging from 5 to 80 mg/L. Then stock solutions for three antibiotics - metronidazole (MNZ), ciprofloxacin (CIP) and sulfamethoxazole (SMX) were prepared to study global degradation of MNZ, CIP and SMX mixtures. All stock solutions were kept in a refrigerator (at 4 0C) before use and all solutions are used at their inherent pH (MNZ = 6.54, SMX = 5.4 and CIP = 6.04) and without controlling temperature.
Before performing the photocatalytic experiment, an adsorption control experiment of TiO2 was performed under dark conditions. The solution loaded with TiO2 was stirred under dark for 60 min and a 5 mL solution was withdrawn using a syringe at distinct time intervals. The solutions were kept 24 h to settle down the suspended TiO2 Nps from solutions. The samples were then analyzed after filtration using a nylon membrane filter (pore size: 0.22 µm, dia: 25 mm; PTFE; chrodisc by CHM). The UV–vis absorption spectra of MNZ, CIP and SMX were measured using a UH4150 Spectrophotometer (PerkinElmer).
2.4 LC-MS/MS analysis
The LC-MS/MS instrument was of Agilent 6420 LC and TQ 1290, USA. A ZORBAX Eclipse Plus C18 rapid resolution HD 2.1 × 100 mm column, USA 1.8 µm was used for online extraction. The mobile phases consisted of A: 0.1% formic acid and B: 15% acetonitrile. Mobile phase C was used for cleaning the columns after separation.The ionization chamber was equipped with an electrospray ionization interface operated in positive-ion mode and the interface and total flow was 0.4 m/min. Quantification was performed using multiple reaction monitoring (MRM). MRM transitions, collision energies and dwell voltage fragmentor, and cell accelerator voltage are presented in Table S1. The whole LC-MS equipment was kept in a temperature-controlled environment at 27 °C. Samples were stored in the autosampler were kept at 4 °C before analysis and injected volume was 5 µl. The global degradation of the antibiotics was calculated from the initial and final area from the LC-MS analysis of three antibiotics.
2.5 Data analysis
The removal efficiency of antibiotics was calculated using the following equations:
(1)where C0 and Ct are the initial and the residual concentrations of the antibiotics at t time (mg/L), respectively.
The degradation kinetics of antibiotics in a batch system was explained by zero-order, pseudo-first-order, and second-order kinetic models. The kinetic models by photocatalytic degradation of antibiotics are presented by Eqs. (2)–(4).
Zero-order kinetics:
First-order kinetics:
Second-order kinetics:
3 Results and discussion
3.1 Characterization of TiO2
Fig. 2 shows the crystal phases of TiO2 nanoparticles using XRD analysis. The results matched with JCPDS 96-900-8217. The diffraction peaks occurring at 25.156°, 37.385°, 47.782°, 53.344°, 54.753°, 62.191, 68.052, 69.884 and 74.397° have been assigned to the (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 1 1), (2 0 4), (1 1 6), (2 2 0) and (2 1 5) lattice planes of pure tetragonal anatase phase of TiO2, respectively.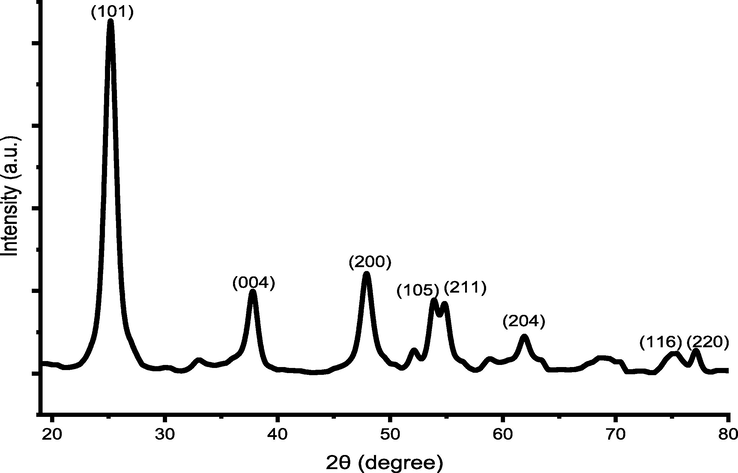
XRD patterns of TiO2 nanoparticles.
3.2 Photocatalytic activity analyses
3.2.1 Photodegradation analyses of SMX
Fig. 3a shows the percentage of removal efficiency and relative concentration (C/Co) of SMX with UV irradiation time for different doses of TiO2. The SMX removal efficiency was increased with UV irradiation time, whereas the relative concentration of SMX was decreased. The initial rate of SMX degradation was high, however, the degradation rate was decreased with experimental time increase. The figure also reveals the impact of photocatalyst TiO2 dosage on SMX degradation. For a 0.5 g/L TiO2 dosage in the reactor, the removal efficiency was 43% at 60 min of UV irradiation time and it increased to 53% for 0.7 g/L TiO2. The removal efficiency increased with increasing catalyst dosage because a large number of active sites were provided, which promoted the generation of hydroxyl radical and enhanced the degradation of SMX. Thus, the maximum removal efficiency reached 97 % for the 0.7 g/L TiO2 dosages (C/Co reached 0.1429) after 420 min of UV irradiation time. However, with further addition of TiO2 (1 g/L), the removal decreased to 14% at 60 min indicating an overdose of catalyst scavenged hydroxyl radicals (•OH) and inhibited the degradation of antibiotics (Wang and Zhuan, 2020). Excessive catalyst dosage would block incident UV light (Wang et al., 2010, 2012) and cause the loss of light energy through shielding, reflection and scattering of light by solid particles (Mourid et al., 2020). Therefore, the optimum catalyst dosage of 0.7 g/L TiO2 was selected for the rest of the studies to be carried out. Fig. 3b shows the effect of different concentrations of SMX on the photoreactor performance for the optimum dosage (0.7 g/L TiO2). SMX concentrations were varied for 5 mg/L, 40 mg/L and 80 mg/L. The figure represents the removal efficiency decreased with concentrations increased. Maximum 97%, 88% and 82% removal efficiencies were achieved within 480 min for 5 mg/L, 40 mg/L and 80 mg/L SMX, respectively. The degradation rate was 92% for 5 mg/L against 65% for 80 mg/L of SMX concentration (Fig. 3b) after 180 min indicating that antibiotic concentration has a great impact on degradation using UV/TiO2 process. Fig. S1 shows the evolution of the UV–vis absorption spectrum of SMX (5 mg/L) depending on the irradiation time. The top curve is for the initial time and SMX has a maximum absorbance peak at 270 nm. With the irradiation time, the intensity of the peak decreased and finally, it became flat after 360 min of experimental time, indicating the degradation of SMX under UV/TiO2 photocatalysis.![The effect of catalyst dosage and initial SMX concentration on the photocatalytic degradation of SMX. a) effect of catalyst dosage for, [SMX] = 80 mg/L and b) effect of initial SMX concentration for TiO2 = 0.7 g/L.](/content/184/2022/15/7/img/10.1016_j.arabjc.2022.103900-fig4.png)
The effect of catalyst dosage and initial SMX concentration on the photocatalytic degradation of SMX. a) effect of catalyst dosage for, [SMX] = 80 mg/L and b) effect of initial SMX concentration for TiO2 = 0.7 g/L.
UV-irradiation parameters, catalyst dosage and removal efficiencies of SMX, MNZ and CIP are summarized in Table 1. Previously, Ahmed et al. (Ahmed and Zahraa, 2014) and Abellán et al. (Abellán et al., 2007) used TiO2 as a photocatalyst to degrade SMX. According to Ahmed et al. and Abellán et al, the maximum removal efficiencies were 97% and 82% after 420 min (for 10 ppm) and 360 min (for 100 ppm) of UV irradiation time, respectively (Table 1). Their UV irradiation parameters are given in Table 1. Ahmed et al. (Ahmed and Zahraa, 2014) used TiO2 immobilized glass plate (4 g/L TiO2) in their study under UV-irradiation with 18 W lamp. A lower dosage (0.5 g/L TiO2) was applied in a suspended system (Abellán et al., 2007) but removal efficiency was lower than the present study as they used a higher concentration of SMX (100 ppm).
Antibiotics
Systems
Catalyst dosage (g/L)
Initial concentration (mg/L)
UV-irradiation parameters
Time (min)
Removal efficiency (%)
Reference
SMX
UV/TiO2
0.7
5
24 W
λ = 254 nm
Path length 4.5 cm360
97%
Present study
UV/TiO2
0.7
40
24 W
λ = 254 nm
Path length 4.5 cm480
92%
Present study
UV/TiO2
0.7
80
24 W
λ = 254 nm
Path length 4.5 cm480
86%
Present study
UV/TiO2 immobilized on glass plates
4
10
18 W
λ = 254 nm420
97%
(Ahmed and Zahraa, 2014)
Xenon lamp/TiO2
0.5
100
Xenon lamp (PHILIPS XOP-15-OF, 1000 W)
360
82%
(Abellán et al., 2007)
UV/TiO2
0.2
10
50 W xenon lamp
180
58%
(Zhang et al., 2017)
UV/TiO2/pBC
0.2
10
50 W xenon lamp
180
91%
(Zhang et al., 2017)
UV/biochar/TiO2
0.5
10
15 W
λ = 350 nm180
75%
(Kim and Kan, 2016)
MNZ
UV/TiO2
0.7
80
24 W
λ = 254 nm
Path length 4.5 cm600
100%
Present study
UV/ TiO2
2.5
0.1
32 W
λ = 254 nm120
100%
(Neghi and Kumar, 2017)
UV/TiO2
2.5
100
32 W
λ = 254 nm120
47%
(Neghi and Kumar, 2017)
UV/polymeric-TiO2
0.3
0.1
32 W
λ = 254 nm120
34%
(Neghi et al., 2019)
UV/polymeric-TiO2
0.3
10
32 W
λ = 254 nm120
18%
(Neghi et al., 2019)
UV/TiO2
0.5
80
125 W
λ = 254 nm120
99%
(Farzadkia et al., 2015)
CIP.
UV/TiO2
0.7
80
24 W
λ = 254 nm
Path length 4.5 cm600
89%
Present study
UV/TiO2 immobilized on glass plates
0.12
20
Irradiance 100Wm-2
λ = 365 nm480
75%
(Triquet et al., 2020)
UV/TiO2
0.12
20
Irradiance 100 Wm-2
λ = 365 nm60
75%
(Triquet et al., 2020)
UV/GMC-TiO2 composite
0.35
15
14 W
λ = 254 nm90
100%
(Zheng et al., 2018)
UV/TiO2 immobilized on glass plates
1
3
6 W
λ = 254 nm105
92%
(Malakootian et al., 2020c, p. 2)
Xenon lamp/TiO2 (hydrothermal)
0.25
160
500 W
Xenon lamp (CEL-LAX500)360
96%
(Gan et al., 2018)
3.2.2 Photodegradation analyses of MNZ and CIP
Photocatalytic degradation of MNZ (80 mg/L) was carried out using optimum TiO2 dosage (0.7 g/L) and the results are shown in Table 1 and Fig. 4. Fig. 4a depicts that as irradiation time increased, the percentage removal efficiency of MNZ increased. After 60 min of experimental time, the MNZ degradation was only 16% which reached 81% at 360 min and 100% at 600 min of experimental time. The C/Co tends to zero as observed in Fig. 4a. Similar results were found by other researchers (Neghi and Kumar, 2017). Neghi and Kumar, (2017) applied 2.5 g/L TiO2 dosage for different concentrations of MNZ, and the study revealed that (Neghi and Kumar, 2017) 100% removal of MNZ was observed for low concentration (0.1 mg/L MNZ) after 120 min, which decreased to 47% (maximum) for a higher concentration (100 mg/L) of MNZ. Neghi et al. (2019) applied lower photocatalyst dosage (0.3 g/L) for the the degradation of 10 mg/L MNZ solution and observed 18% degradation after 120 min indicating lower dosage was not effective for the removal of MNZ for a short period experiment. Fig. S2 shows the evolution of the UV–vis absorption spectrum of MNZ with irradiation time. The top curve represents the spectrum for the initial concentration of MNZ (80 mg/L) has a maximum peak intensity at 314 nm. The peak intensity of MNZ was decreased with time. After 600 min of experimental time, the peak intensity became flat indicating near 100% removal of MNZ.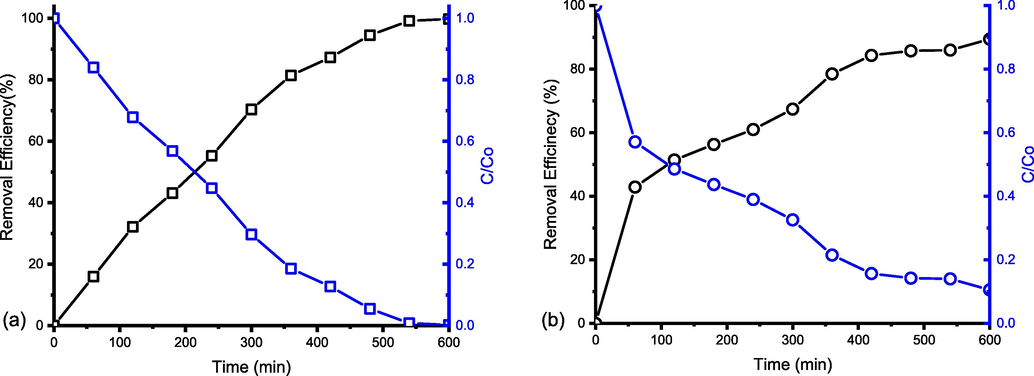
Photocatalytic degradations of MNZ and CIP for 80 mg/L using TiO2 of 0.7 g/L. a); percentage removal of MNZ concentration and b) percentage removal of CIP concentration.
Fig. 4b shows photocatalytic degradation (percentages removal and relative concentration) of CIP (80 mg/L) using the optimum TiO2 dosage of 0.7 g/L. A similar degradation trend was observed as obtained for SMX and MNZ degradations. The figure indicates that the removal efficiency increased with irradiation time. After 60 min of the experiment, the degradation was 43%, which increased to 78% at 360 min and 89% at 600 min. Very similar results were obtained by Triquet et al. (2020). Triquet et al. (2020) applied (TiO2 0.12 g/L) suspended TiO2 and coated TiO2 on the glass plate for the degradation of 20 mg/L CIP and the observed rate constant was higher for suspended TiO2. 75% CIP removal was observed for immobilized and suapended system after 480 min and 60 min, respectively. However, considering the separation of expensive TiO2 for a suspension system, TiO2 immobilized on glass plates system was considered as a promising technique. Fig. S3 shows the evolution of the UV–vis absorption spectrum of CIP degradation depending on the irradiation time. The top curve is for the initial concentration of CIP which has a maximum absorbance at 272 nm. With the passage of the experimental time, the absorbances peak was decreased.
3.3 Reaction kinetics study
Kinetic study is important to understand the degradation rate and design of a photocatalytic reactor. Fig. 5(a), (b) and (c) shows the zero order, 1st order and 2nd order kinetic curves of SMX (5&80 ppm), MNZ and CIP (80 ppm). The kinetics data of MNZ, SMX and CIP fitted best to the pseudo 1st order.The kinetics curve of SMX for 5 and 80 mg/L and MNZ and CIP for 80 mg/L for 0.7 g/L TiO2. The figure illustrates that the slope of 5 mg/L SMX is higher than the slope of 80 mg/L SMX, CIP and MNZ. To evaluate photocatalytic activity of TiO2 for MNZ, CIP and SMX observed rate constant (min−1) (Kobs) was calculated from the slope of -ln(C/Co) vs time curve. Fig. 5(b) shows the values of calculated rate constants for MNZ, CIP and SMX. The rate constants were 0.01085 min−1 and 0.00501 min−1 for 5 mg/L and 80 mg/L of SMX, respectively. For a higher SMX concentration, active sites of TiO2 were covered by the target molecule that retard the generation of the hydroxyl radicals (Ahmed and Zahraa, 2014; Xekoukoulotakis et al., 2011). Among SMX, MNZ and CIP, the reaction rate constant for MNZ was the highest. The lowest value of rate constant was observed for CIP.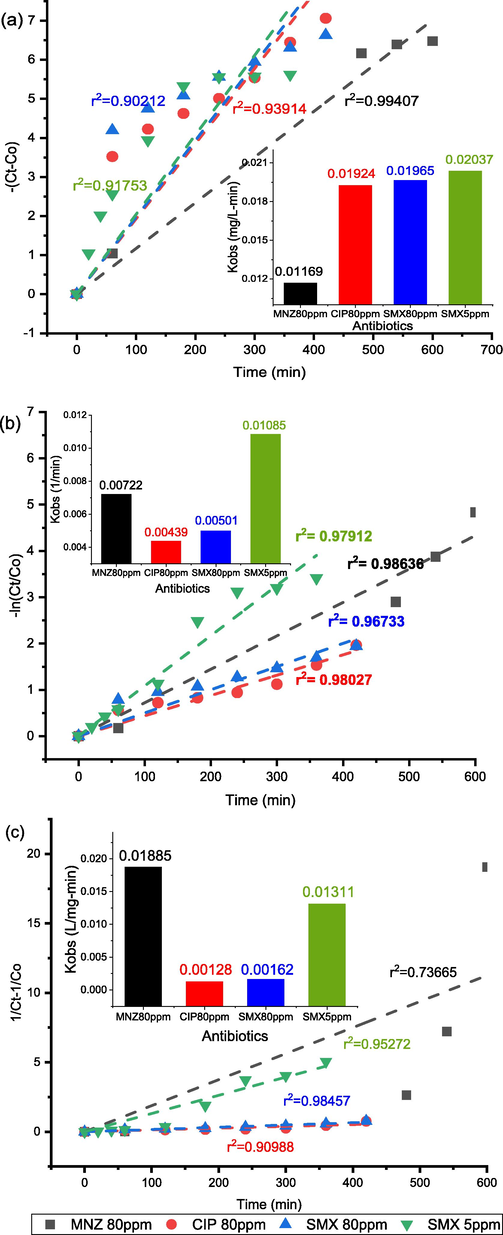
Kinetics curves of MNZ, CIP and SMX (TiO2 = 0.7 g/L) (a) zero order, (b) 1st order and (c) 2nd order ; inset: Kinetic parameters of different condition.
3.4 Competitive degradation of MNZ, CIP and SMX
The evolution of the UV–vis absorption spectrum of mixture antibiotics is shown in Fig. S4. As shown in Fig. S4, the peaks of SMX and CIP were observed at same wavelength and therefore, it was difficult to identify the competitive degradation of SMX and CIP. Therefore LC-MS analysis was performed to understand the competitive degradation of SMX and CIP under UV-irradiation.
The LC-MS analysis results of degradation of SMX, MNZ and CIP are represented in Fig. 6. Fig. 6(a), (b), (c) and (d) shows the competitive degradation of [MNZ] = [CIP] = 40 mg/L, [SMX] = [CIP] = 40 mg/L, [SMX] = [MNZ] = 40 mg/L, [MNZ] = 40 mg/L, [SMX] = [CIP] = 20 mg/L and [SMX] = 40 mg/L, [MNZ] = [CIP] = 20 mg/L respectively with LC-MS extracted ion chromatogram. Before degradation, maximum peaks of SMX, MNZ and CIP were found at 117, 85 and 70, respectively. The decrease of area proves that photocatalytic degradation of antibiotics took place with UV irradiation time. The mass spectra of SMX, MNZ and CIP are shown in Fig. 7. m/Z are 254 (Fig. 7(c)), 332 (Fig. 7(a)) and 172 (Fig. 7(b)) corresponding to the molecular weight [M + H] of CIP, MNZ and SMX. The product ions (Table S1) are related to the intermediate products which are formed during photodegradation.![Removal Efficiency vs time curve with LC-MS extracted ion chromatogram (a) [MNZ] = [CIP] = 40 mg/L, (b) [SMX] = [CIP] = 40 mg/L, (c) [SMX] = [MNZ] = 40 mg/L, (d) [MNZ] = 40 mg/L, [SMX] = [CIP] = 20 mg/L and (e) [SMX] = 40 mg/L, [MNZ] = [CIP] = 20 mg/L depending on the irradiation time (TiO2 = 0.7 g/L). Symbols:(■) SMX; (●) CIP; (▲) MNZ. (f) Global degradation of CIP, SMX and MNZ. (In mixture condition; TiO2 = 0.7 g/L).](/content/184/2022/15/7/img/10.1016_j.arabjc.2022.103900-fig7.png)
Removal Efficiency vs time curve with LC-MS extracted ion chromatogram (a) [MNZ] = [CIP] = 40 mg/L, (b) [SMX] = [CIP] = 40 mg/L, (c) [SMX] = [MNZ] = 40 mg/L, (d) [MNZ] = 40 mg/L, [SMX] = [CIP] = 20 mg/L and (e) [SMX] = 40 mg/L, [MNZ] = [CIP] = 20 mg/L depending on the irradiation time (TiO2 = 0.7 g/L). Symbols:(■) SMX; (●) CIP; (▲) MNZ. (f) Global degradation of CIP, SMX and MNZ. (In mixture condition; TiO2 = 0.7 g/L).
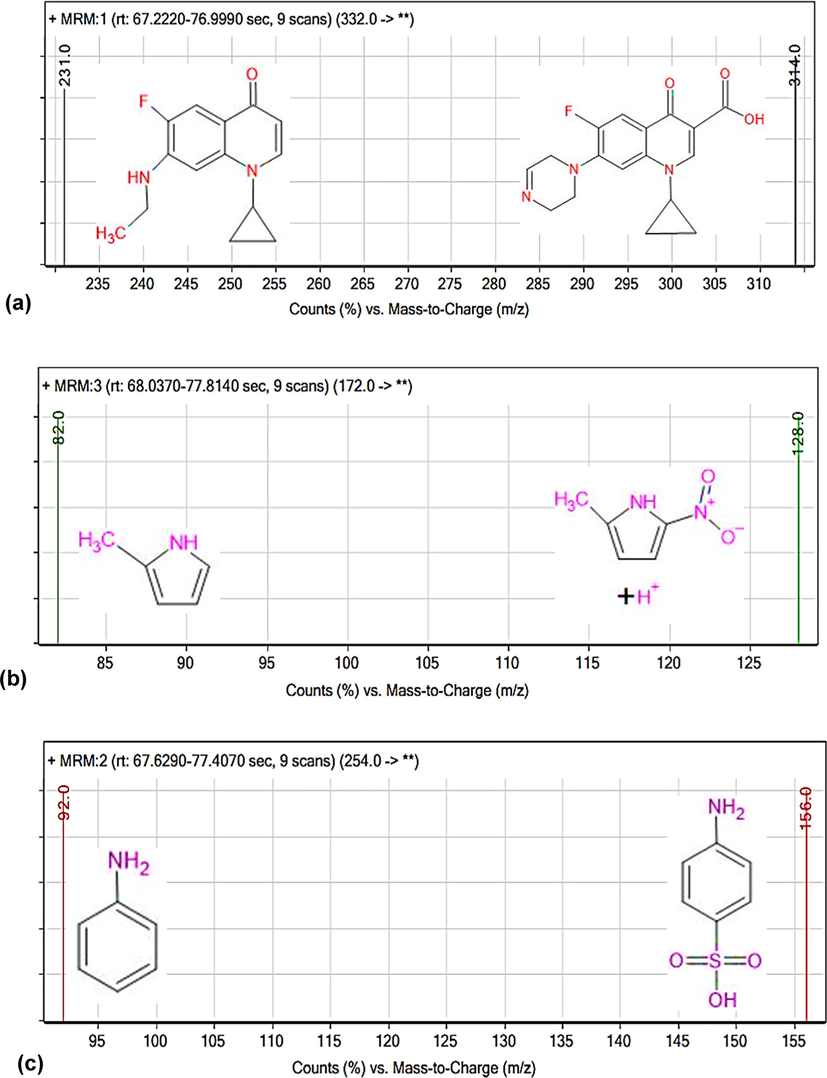
LC-MS mass spectra of generated ions of (a)CIP, (b)MNZ and (c)SMX.
Fig. 6a represents competitive degradation of [MNZ] = [CIP] = 40 mg/L. The photodegradation of both MNZ and CIP were faster and more than 90% of both MNZ (92%) and CIP (97%) were degraded within 120 min. The curve indicates CIP degradation was slight faster than MNZ antibiotics. In contrast, Fig. 6b indicates the degradation of SMX was slower than the degradation of CIP in a mixture of SMX and CIP ([SMX] = [CIP] = 40 mg/L). After 60 min, CIP and SMX degradations were 92% and 32% respectively, indicating the faster degradation of CIP as compared to SMX. Fig. 6c represents photodegradation results for SMX and MNZ mixture with a concentration of 40 mg/L for each antibiotics. The results indicate a very similar trend of degradation of both antibiotics when present at similar concentration ([SMX] = [MNZ] = 40 mg/L). Fig. 6d and 6e represents the degradation of tertiary mixtures of MNZ, SMX and CIP with concentrations [MNZ] = 40 mg/L, [SMX] = [CIP] = 20 mg/Land [SMX] = 40 mg/L, [MNZ] = [CIP] = 20 mg/L, respectively. As shown in Fig. 6d, the degradation trend was CIP > SMX > MNZ, which might be a reason of using higher concentration of MNZ in reaction mixture. The degradation trend in Fig. 6e was CIP > MNZ > SMX justified the use of higher concentration of SMX in the mixture.
Table 2 represents the LC-MS results for mixture of antibiotics. From Table 2 it can be concluded that 100% efficiency can be achieved within 30 min (Evgenidou et al., 2021; Perini et al., 2018), when the concentration of mixer of 8 antibiotics was 1 mg/L each for 0.1 g/L of Cu-TiO2 composite under 500 W UV light. In the current study, the maximum antibiotics degradation efficiency is 100% for an irradiation time of 120 min for [MNZ] = [CIP] = 40 mg/L. The degradation time is higher than that obtained in previous studies as we used a higher concentration of mixer antibiotics. Joao A. et al used the photo-Fenton UVC process (Perini et al., 2018). Due to the formation of solid sludge, as iron ions continuously lose during this process, Fenton has some economic and environmental drawbacks (Babuponnusami and Muthukumar, 2014). For the mixture of SMX, MNZ, CIP, 99% removal efficiency was achieved at 600 min and removal efficiency decreased to 79% when concentration for SMX was higher. The probable reason is the formation of 4-amino-N-(5-methyl-2-oxazolyl) benzenesulfonamide during photodegradation, which is an isomer of SMX (Fig. 8(a)). In contrast in the case of MNZ and CIP, unstable products, hydroxyethyl group (for MNZ) (Moore and Wilkins, 1990; Tong et al., 2011) and C13H12N2OF (for CIP) (Ferdig et al., 2005; Salma et al., 2016) are formed which are smaller than their parent ion (Fig. 8(b)(c)) and a faster degradation is observed. This reason is also satisfying for 100% degradation of MNZ and CIP in less time than other mixture antibiotics (Table 2).
Systems
pH
dosage (g/L)
[Antibiotics]0 mg/L
Photodegradation parameter
Time (min)
Global deg. (%)
Reference
pistachio shell powder coated with ZnO NPs
5
1 g/L
[Tetracycline] =
[Amoxicillin] =
[Ciprofloxacin] = 60adsorption
120
81%
(Mohammed et al., 2020)
Photo-Fenton UVC process
2.5
–
[Ciprofloxacin]=
[Amoxicillin] =
[Sulfathiazole]=
[Sulfamethazine] = 0.230 W
λ = 254 nm
Path length1.5 cm30
100%
(Perini et al., 2018)
UV/0.8CuTiO2
–
0.1
[Isoniazid]=
[Metronidazole]=
[Sulfadiazine]=
[Sulfamethoxazole]=
[Trimethoprim]=
[Norfloxacin] = [Moxifloxacin]=
[Lincomycin] = 1Irradiance
500 W m−2
λ > 300 nm30
100%
(Evgenidou et al., 2021)
Solar Photocatalysis/TiO2
7.5
0.5
[oxolinic acid] =
[oxytetracycline] = 20solar photocatalytic CPC reactor
30
100%
(Pereira et al., 2013, p. 2)
UV/TiO2
7
1.5
[Metronidazole] = [Amoxicillin] = 50
100 W
Irradiance
65 W m − 2
λ = 254 nm120
70%
(Tran et al., 2019)
UV/TiO2
–
0.7
[Metronidazole]=
[Ciprofloxacin] = 4024 W
λ = 254 nm
Path length 4.5 cm120
100%
Present study
UV/TiO2
–
0.7
[Sulfamethoxazole]=
[Ciprofloxacin] = 20
[Metronidazole] = 4024 W
λ = 254 nm
Path length 4.5 cm600
99%
Present study
UV/TiO2
–
0.7
[Sulfamethoxazole]
[Metronidazole] = 4024 W
λ = 254 nm
Path length 4.5 cm600
97%
Present study
UV/TiO2
–
0.7
[Sulfamethoxazole]=
[Ciprofloxacin] = 4024 W
λ = 254 nm
Path length 4.5 cm600
96%
Present study
UV/TiO2
–
0.7
[Sulfamethoxazole]=40
[Metronidazole]=
[Ciprofloxacin] = 2024 W
λ = 254 nm
Path length 4.5 cm540
79%
Present study
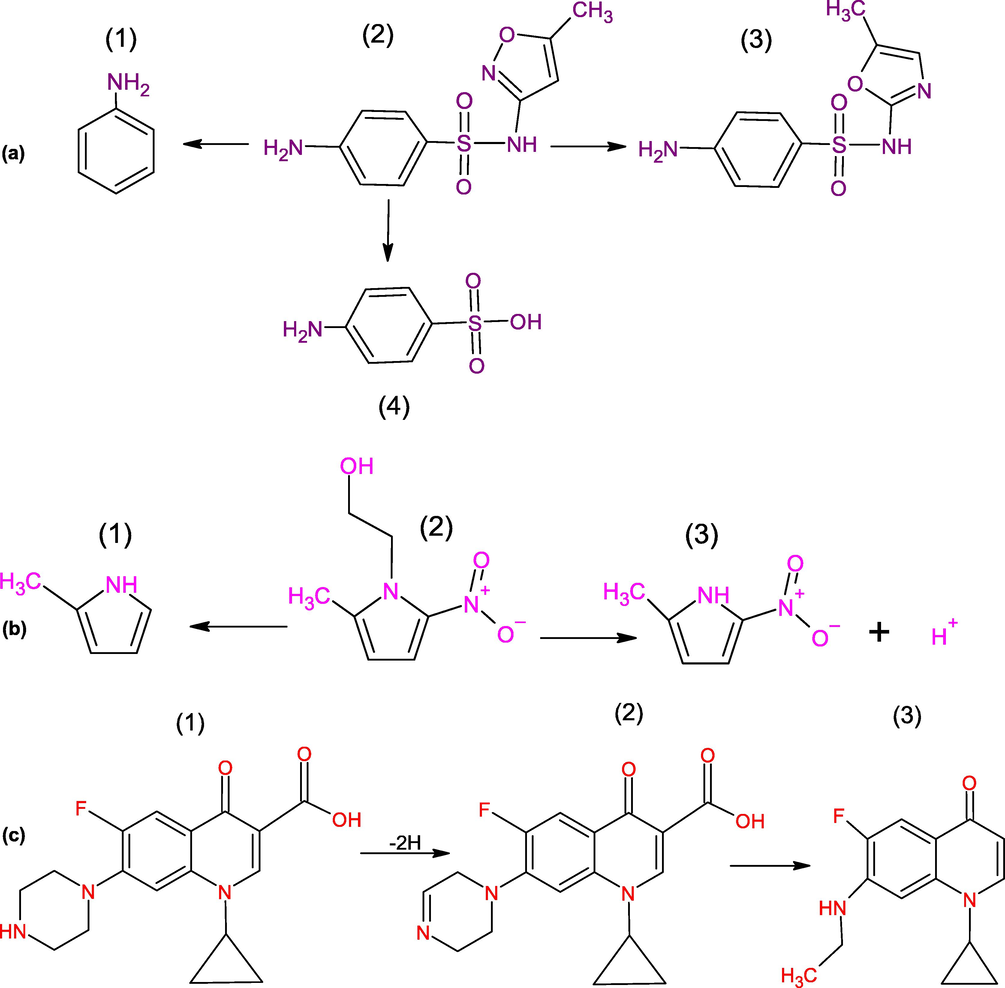
Reaction scheme for photolysis of (a)SMX, (b)MNZ and (c)CIP.
Initially, the photodegradation process was rapid due to highly reactive radicals OH• which can oxidize MNZ, CIP and SMX.TiO2 absorbs energy, they generate electrons (e-) with high reducing ability and holes (h+) with high oxidizing ability. These excited electrons(e-) reduced O2 hence generating superoxide radical (•O2−). While holes (h + ) migrate to the surface of the photo-catalysts, H2O will be oxidized to generate hydroxyl radical (•OH). MNZ, CIP and SMX could be decomposed by superoxide radical (•O2−) or hydroxyl radical (•OH). The mechanisms of photocatalytic oxidation are as following Eqs. (5)–(11) (Liu et al., 2018; Saadati et al., 2016).
Initially dissolved oxygen remains high in an aqueous solution. In other studies, it was found that initial dissolved oxygen was around 8 mg/L (Abellán et al., 2007), after the first hour of reaction it becomes 6 mg/L and is kept constant. Dissolved oxygen is highly electrophilic. It attracts electrons generated by the surface of TiO2 (Eq. (2)) and reduced the undesirable electron-hole recombination. As initially more dissolved oxygen remains, more reactive radicals OH• can produce to degrade antibiotics, that’s why degradation occurs faster at the beginning. Lack of adsorption and the negligible photolysis, abatement of degradation occurs after 60 min, similar results found in previous literature (Mourid et al., 2020; Porcar-Santos et al., 2020). The influence of inorganic ions on the degradation of mixture antibiotics demands further studies.
3.5 Degradation pathways of SMX, MNZ and CIP
SMX (4-amino-N-(5 -methyl-3-isoxazolyl)benzenesulfonamide) photodegradation undergoes several reaction pathways. Before the total disappearance of SMX, many of the intermediate products (at least five primary photoproducts) were identified. The proposed reaction scheme (Luo et al., 2018; Zhou and Moore, 1994) is shown in Fig. 8(a). The major intermediate product is 4-amino-N-(5-methyl-2-oxazolyl)benzenesulfonamide (3) which is an isomer of SMX along with other products like sulfanilic acid (4) and aniline (1) (Zhou and Moore, 1994). Aniline and sulfanilic acid have also been identified as photo products from other sulfonamides. They are fromed from free radicals following homolytic γ-fission and δ-fission of sulfonamides (Chignell et al., 1980).
During photodegradation of metronidazole (C6H9N3O3) as shown in Fig. 12(b), metronidazole C6H9N3O3 (2) produces two unstable intermediates: the hydroxyethyl group (3) (m/z 128) and radical cation at m/z 82 (1) (Moore and Wilkins, 1990; Tong et al., 2011). Loss of hydroxyethyl group, metronidazole produces nitroimidazole and this ion is corresponded to the cleavage of the hydroxyethyl moiety (Tong et al., 2011). Elimination of nitro group from nitroimidazole to generate the radical cation.
During photodegradation as shown in Fig. 13(c), two photoproducts C17H16N3O3F (2) and C13H12N2OF (3) were produced under photocatalytic oxidation (Ferdig et al., 2005; Salma et al., 2016). TiO2 nanoparticles absorbed UV light (254 nm) which actuated to direct photo-hole (h + ) oxidation and hydroxyl radical (An et al., 2010; Sturini et al., 2012) and interact with CIP (C17H18N3O3F) as mentioned in reaction mechanisms. It especially occurs in presence of TiO2, at the piperazing ring with preservation of the fluorine.
4 Conclusion
SMX, MNZ and CIP were successfully removed from aqueous solution using UV/TiO2 photodegradation technique at their inherent pH. 97% degradation of SMX was observed within 360 min for 5 mg/L SMX concentration which was 86% at 460 min for 80 mg/L SMX concentration. The optimum dosage of TiO2 was 0.7 g/L for SMX degradation. MNZ and CIP removals were 100% and 89%, respectively, at 600 min of photodegradation reaction. 100% degradation was observed at 120 min for MNZ and CIP binary mixture, whereas the degradations were 97% and 96% for SMX and MNZ, and SMX and CIP binary mixtures, respectively, even after 600 min of experimental time at the same concentrations. For tertiary mixture, the maximum removal 99% was observed for ([SMX = CIP] = 20 mg/L and [MNZ] = [40 mg/L]) at 600 min of experimental time. For mixture, it has been observed that the degradation was faster for lower concentration of SMX. The results indicate that the commercial TiO2 at low cost is promising under UV irradiation for the reclamation of pharmaceutical wastewaters.
CRediT authorship contribution statement
Surya Akter: Conceptualization, Data curation, Methodology, Validation, Writing – original draft. Md. Sahinoor Islam: Investigation, Methodology, Writing – review & editing. Md. Humayun Kabir: Formal analysis, Writing – review & editing. Md. Aftab Ali Shaikh: Project administration, Resources, Supervision. Md. Abdul Gafur: Project administration, Resources, Supervision, Conceptualization, Writing – review & editing.
Acknowledgements
The authors acknowledge the authority of Bangladesh Council of Scientific and Industrial Research, Ministry of S&T, Bangladesh for providing funding and testing facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl. Catal. B Environ.. 2007;74:233-241.
- [CrossRef] [Google Scholar]
- The performance of mesoporous magnetite zeolite nanocomposite in removing dimethyl phthalate from aquatic environments. Desalination Water Treat.. 2016;1–15
- [CrossRef] [Google Scholar]
- Ahmed, O., Zahraa, O., 2014. Degradation of sulfamethoxazole by photocatalysis using supported TiO2 8.
- Solar-TiO2 immobilized photocatalytic reactors performance assessment in the degradation of methyl orange dye in aqueous solution. Environ. Nanotechnol. Monit. Manag.. 2021;16:100514.
- [CrossRef] [Google Scholar]
- Mechanistic considerations for the advanced oxidation treatment of fluoroquinolone pharmaceutical compounds using TiO 2 heterogeneous catalysis. J. Phys. Chem. A. 2010;114:2569-2575.
- [CrossRef] [Google Scholar]
- Retrospective suspect screening reveals previously ignored antibiotics, antifungal compounds, and metabolites in Bangladesh surface waters. Sci. Total Environ.. 2020;712:136285
- [CrossRef] [Google Scholar]
- Magnetic multi-walled carbon nanotubes-loaded alginate for treatment of industrial dye manufacturing effluent: adsorption modelling and process optimisation by central composite face-central design. Int. J. Environ. Anal. Chem.. 2021;1–21
- [CrossRef] [Google Scholar]
- Integrated Fuzzy AHP-TOPSIS for selecting the best color removal process using carbon-based adsorbent materials: multi-criteria decision making vs. systematic review approaches and modeling of textile wastewater treatment in real conditions. Int. J. Environ. Anal. Chem.. 2020;1–16
- [CrossRef] [Google Scholar]
- The superior adsorption capacity of 2,4-Dinitrophenol under ultrasound-assisted magnetic adsorption system: Modeling and process optimization by central composite design. J. Hazard. Mater.. 2021;418:126348
- [CrossRef] [Google Scholar]
- A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng.. 2014;2:557-572.
- [CrossRef] [Google Scholar]
- Characterization of the Nairobi River catchment impact zone and occurrence of pharmaceuticals: Implications for an impact zone inclusive environmental risk assessment. Sci. Total Environ.. 2020;703:134925
- [CrossRef] [Google Scholar]
- Are pharmaceuticals potent environmental pollutants? Sci. Total Environ.. 2006;364:67-87.
- [CrossRef] [Google Scholar]
- Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater.. 2021;408:124461
- [CrossRef] [Google Scholar]
- Spectroscopic studies of cutaneous photosensitizing agents—I. Spin trapping of photolysis products from sulfanilamide, 4-aminobenzoic acid and related compounds. Photochem. Photobiol.. 1980;32:563-571.
- [CrossRef] [Google Scholar]
- Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett.. 2019;17:145-155.
- [CrossRef] [Google Scholar]
- Enhancement of LED based photocatalytic degradation of sulfolane by integration with oxidants and nanomaterials. Chemosphere. 2021;263:128124
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of a mixture of eight antibiotics using Cu-modified TiO2 photocatalysts: Kinetics, mineralization, antimicrobial activity elimination and disinfection. J. Environ. Chem. Eng.. 2021;9:105295
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of Metronidazole with illuminated TiO2 nanoparticles. J. Environ. Health Sci. Eng.. 2015;13:35.
- [CrossRef] [Google Scholar]
- Improved liquid chromatographic determination of nine currently used (fluoro)quinolones with fluorescence and mass spectrometric detection for environmental samples. J. Sep. Sci.. 2005;28:1448-1456.
- [CrossRef] [Google Scholar]
- Impact of post-processing modes of precursor on adsorption and photocatalytic capability of mesoporous TiO2 nanocrystallite aggregates towards ciprofloxacin removal. Chem. Eng. J.. 2018;349:1-16.
- [CrossRef] [Google Scholar]
- Nanofiltration as tertiary treatment method for removing trace pharmaceutically active compounds in wastewater from wastewater treatment plants. Water Res.. 2017;125:360-373.
- [CrossRef] [Google Scholar]
- Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J.. 2018;136:25-39.
- [CrossRef] [Google Scholar]
- β-lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat. Commun.. 2013;4:1610.
- [CrossRef] [Google Scholar]
- MoO3 and Ag co-synthesized TiO2 as a novel heterogeneous photocatalyst with enhanced visible-light-driven photocatalytic activity for methyl orange dye degradation. Environ. Nanotechnol. Monit. Manag.. 2019;12:100244.
- [CrossRef] [Google Scholar]
- Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res.. 2018;165:258-266.
- [CrossRef] [Google Scholar]
- Occurrence, distribution, ecological and resistance risks of antibiotics in surface water of finfish and shellfish aquaculture in Bangladesh. Chemosphere. 2017;188:329-336.
- [CrossRef] [Google Scholar]
- Ciprofloxacin removal from aqueous solutions by ozonation with calcium peroxide. Desalination Water Treat.. 2020;174:178-185.
- [CrossRef] [Google Scholar]
- Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manage.. 2018;219:189-207.
- [CrossRef] [Google Scholar]
- Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: A critical review. TrAC Trends Anal. Chem.. 2020;129:115921
- [CrossRef] [Google Scholar]
- Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manage.. 2016;180:94-101.
- [CrossRef] [Google Scholar]
- Pharmaceuticals in tap water: human health risk assessment and proposed monitoring framework in China. Environ. Health Perspect.. 2013;121:839-846.
- [CrossRef] [Google Scholar]
- Green synthesis of 3D tripyramid TiO2 architectures with assistance of aloe extracts for highly efficient photocatalytic degradation of antibiotic ciprofloxacin. Appl. Catal. B Environ.. 2020;260:118149
- [CrossRef] [Google Scholar]
- 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B Environ.. 2018;232:562-573.
- [CrossRef] [Google Scholar]
- UV direct photolysis of sulfamethoxazole and ibuprofen: An experimental and modelling study. J. Hazard. Mater.. 2018;343:132-139.
- [CrossRef] [Google Scholar]
- Adsorption of sulfur dioxide on clinoptilolite/nano iron oxide and natural clinoptilolite. Health Scope In Press 2019
- [CrossRef] [Google Scholar]
- Ciprofloxacin removal by electro-activated persulfate in aqueous solution using iron electrodes. Appl. Water Sci.. 2019;9:140.
- [CrossRef] [Google Scholar]
- Removal of metronidazole from wastewater by Fe/charcoal micro electrolysis fluidized bed reactor. J. Environ. Chem. Eng.. 2019;7:103457
- [CrossRef] [Google Scholar]
- A study on the photocatalytic degradation of p -Nitroaniline on glass plates by Thermo-Immobilized ZnO nanoparticle. Inorg. Nano-Met. Chem.. 2020;50:124-135.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of ciprofloxacin antibiotic by TiO 2 nanoparticles immobilized on a glass plate. Chem. Eng. Commun.. 2020;207:56-72.
- [CrossRef] [Google Scholar]
- Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. Prot.. 2020;134:292-307.
- [CrossRef] [Google Scholar]
- Sulfamethoxazole degradation by combination of advanced oxidation processes. J. Environ. Chem. Eng.. 2018;6:4054-4060.
- [CrossRef] [Google Scholar]
- Mohammed, A.A., Al-Musawi, T.J., Kareem, S.L., Zarrabi, M., Al-Ma’abreh, A.M., 2020. Simultaneous adsorption of tetracycline, amoxicillin, and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles. Arab. J. Chem. 13, 4629–4643. https://doi.org/10.1016/j.arabjc.2019.10.010.
- Common products from gamma-radiolysis and ultraviolet photolysis of metronidazole. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem.. 1990;36:547-550.
- [CrossRef] [Google Scholar]
- Development of a new recyclable nanocomoposite LDH-TiO2 for the degradation of antibiotic sulfamethoxazole under UVA radiation: An approach towards sunlight. J. Photochem. Photobiol. Chem.. 2020;396:112530
- [CrossRef] [Google Scholar]
- Neghi, N., Kumar, M., 2017. Performance Analysis of Photolytic, Photocatalytic, and Adsorption Systems in the Degradation of Metronidazole on the Perspective of Removal Rate and Energy Consumption. Water. Air. Soil Pollut. 228, 339. https://doi.org/10.1007/s11270-017-3532-0.
- Synthesis and application of stable, reusable TiO2 polymeric composites for photocatalytic removal of metronidazole: Removal kinetics and density functional analysis. Chem. Eng. J.. 2019;359:963-975.
- [CrossRef] [Google Scholar]
- Insights into solar TiO2-assisted photocatalytic oxidation of two antibiotics employed in aquatic animal production, oxolinic acid and oxytetracycline. Sci. Total Environ.. 2013;463–464:274-283.
- [CrossRef] [Google Scholar]
- Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation. Appl. Catal. B Environ.. 2018;224:761-771.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of sulfamethoxazole using TiO2 in simulated seawater: Evidence for direct formation of reactive halogen species and halogenated by-products. Sci. Total Environ.. 2020;736:139605
- [CrossRef] [Google Scholar]
- Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: A review. Crit. Rev. Environ. Sci. Technol.. 2016;46:757-782.
- [CrossRef] [Google Scholar]
- Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater.. 2016;313:49-59.
- [CrossRef] [Google Scholar]
- Heat-activated potassium persulfate treatment of Sudan Black B dye: Degradation kinetic and thermodynamic studies. J. Water Process Eng.. 2021;39:101690
- [CrossRef] [Google Scholar]
- Ciprofloxacin wastewater treated by UVA photocatalysis: contribution of irradiated TiO 2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016;6:95494-95503.
- [CrossRef] [Google Scholar]
- Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl. Catal. B Environ.. 2012;119–120:32-39.
- [CrossRef] [Google Scholar]
- Kinetic and mechanistic studies of the photolysis of metronidazole in simulated aqueous environmental matrices using a mass spectrometric approach. Anal. Bioanal. Chem.. 2011;399:421-428.
- [CrossRef] [Google Scholar]
- Removal of metronidazole and amoxicillin mixtures by UV/TiO2 photocatalysis: an insight into degradation pathways and performance improvement. Environ. Sci. Pollut. Res.. 2019;26:11846-11855.
- [CrossRef] [Google Scholar]
- TiO2 MOCVD coating for photocatalytic degradation of ciprofloxacin using 365 nm UV LEDs - kinetics and mechanisms. J. Environ. Chem. Eng.. 2020;8:104544
- [CrossRef] [Google Scholar]
- Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol.. 2008;63:251-263.
- [CrossRef] [Google Scholar]
- Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol.. 2010;389:416-428.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of metronidazole in aqueous solution by niobate K6Nb10.8O30. Wuhan Univ. J. Nat. Sci.. 2010;15:345-349.
- [CrossRef] [Google Scholar]
- Catalytic damage of bovine serum albumin by metronidazole under ultrasonic irradiation in the presence of nano-sized TiO2 powder. Russ. J. Phys. Chem. A. 2012;86:867-874.
- [CrossRef] [Google Scholar]
- Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ.. 2020;701:135023
- [CrossRef] [Google Scholar]
- Insights into mechanisms of UV/ferrate oxidation for degradation of phenolic pollutants: Role of superoxide radicals. Chemosphere. 2020;244:125490
- [CrossRef] [Google Scholar]
- Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal. Today. 2011;161:163-168.
- [CrossRef] [Google Scholar]
- Wastewater disinfection by ozone: main parameters for process design. Water Res.. 2002;36:1043-1055.
- [CrossRef] [Google Scholar]
- A comparative study of anaerobic fixed film baffled reactor and up-flow anaerobic fixed film fixed bed reactor for biological removal of diethyl phthalate from wastewater: a performance, kinetic, biogas, and metabolic pathway study. Biotechnol. Biofuels. 2017;10:139.
- [CrossRef] [Google Scholar]
- TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere. 2017;185:351-360.
- [CrossRef] [Google Scholar]
- Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci.. 2018;527:202-213.
- [CrossRef] [Google Scholar]
- Photochemical decomposition of sulfamethoxazole. Int. J. Pharm.. 1994;110:55-63.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103900.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







