Translate this page into:
Exploring the promoting mechanisms of bovine serum albumin, lignosulfonate, and polyethylene glycol for lignocellulose saccharification from perspective of molecular interactions with cellulase
⁎Corresponding authors. jiaqi.guo@njfu.edu.cn (Jiaqi Guo), junlong.song@njfu.edu.cn (Junlong Song)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Bovine serum albumin (BSA), polyethylene glycol (PEG) and lignosulfonate (LS) have been extensively employed as synergistic agents in lignocellulose saccharification. Nevertheless, the promoting mechanisms have not been fully understood and there are a number of controversial opinions existed. All attention has been paid to the interactions between respective additive and substrate. However, rarely attention has been paid to the interactions between additives and enzymes (cellulase from Trichoderma reesei in this investigation). This interaction is actually more important since cellulase interacts with the additives before it contacts with substrate. Therein, Quartz crystal microbalance with dissipation monitoring (QCM-D), surface plasma resonance (SPR) and small angel X-ray scattering (SAXS) were incorporated to study the interaction between enzyme and additives. The results showed synergistic agents have different interaction modes with cellulase. BSA and LS can form complexes with cellulase and the formed complexes prevent them from nonproductive binding by residue lignin; what’s more, the cellulase-BSA complexes improve the hydrolytic capability of pristine enzyme whereas cellulase-LS complexes reduce. PEG prevents the unproductive binding of cellulase to the residual lignin by forming a thin layer that actually acts as a steric hindrance to the residual lignin. This investigation helps us to understand the sophisticated interactions among the components in the complicated enzymatic system, especially the interactions between enzymes and synergistic agents. It will be helpful in the design and utilization of synergistic additives in the lignocellulose biorefinery process as well.
Keywords
Cellulase
Synergistic agents
Adsorption
Quartz crystal microbalance with dissipation monitoring (QCM-D)
Surface plasma resonance (SPR)

- BSA
-
Bovine serum albumin
- PEG
-
polyethylene glycol
- LS
-
lignosulfonate
- QCM-D
-
Quartz Crystal Microbalance with Dissipation monitoring
- SPR
-
Surface Plasmon Resonance
- SAXS
-
Small Angle X-ray Scattering
- GL
-
green liquor-pretreated lignocellulose
- MUA
-
Chemicals of 11-mercaptoundecanoic acid
- MPA
-
3-mercaptopropionic acid
- EDC
-
N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride
- NHS
-
N-hydroxysulfosuccinimide sodium salt
Abbreviations
1 Introduction
Lignocellulosic biomass are abundant and environmentally friendly on earth, it could be used to produced bioethanol which is an alternative energy with great development potential (Anwar et al., 2014). In recent years, lignocellulose has become a hot spot of modern biomass research. However, it is well known that enzymatic hydrolysis of lignocellulose is easily restricted due to the complex structure of lignocellulose. It is currently believed that the main reason affecting the hydrolysis of lignocellulose is cellulase’s non-productive adsorption onto the substrate lignin, thereby reducing the efficiency of cellulase (Rahikainen et al., 2013; Liu et al., 2016). Many studies have shown that adding some additives (or synergic agents) into the lignocellulose enzymatic hydrolysis system can enhance the enzymatic hydrolysis efficiency by reducing the non-productive adsorption of cellulase by lignin; among them, lignosulfonate (LS), polyethylene glycol (PEG) and Bovine Serum Albumin (BSA) are three typical synergic agents under extensive studies (Börjesson et al., 2007; Bin and Wyman 2010; Kapu et al., 2012; Cheng et al., 2016; Jin et al 2020).
LS is a by-product of Sulfite retreatment to Overcome Recalcitrance of Lignocelluloses (SPORL) pretreatment (with sulfite and dilute sulfuric acid) or sulfite pulping (Wang et al., 2013). Wang et al. (2013) and Zhou et al. (2013) found that LS inhibited cellulose saccharification of Whatman paper, but enhanced the saccharification of lignocellulosic substrates. They proposed that LS can bind to cellulase and the formed complex increases the amount of negative charges carried by cellulase, thereby increasing the electrostatic repulsion between cellulase and residual lignin in substrate, as a consequence reducing the non-productive adsorption of cellulase to substrate lignin. Zhou et al. (2013) reported that the level of enhancement was related to the molecular weight and degree of sulfonation of the lignin as well as the substrate lignin structure. By adding LS to the enzymatic hydrolysate of different pretreatment substrates, Wang et al. (2015c) found that the pretreatment method will affect the effect of LS. However, these studies were carried out in a very complex hydrolysis system, where both residual lignin and soluble lignin coexisted. As such, the action mechanism between cellulase and lignin cannot be clearly identified which part of lignin involved.
Adding PEG to the lignocellulose hydrolysis system can reduce the amount of enzymes used and increase the conversion rate of cellulose (Börjesson et al., 2007; Simone et al., 2011; Kapu et al., 2012). It is currently believed that PEG as a nonionic surfactant can promote enzymatic hydrolysis of cellulose. The main role of surfactants in substrate containing lignin is that lignin with adsorbed surfactants can inhibit the interaction between enzymes and lignin, which makes the efficiency of lignin-binding enzymes low (Eriksson et al., 2002). However, it was found that the efficiency of PEG is not always positively correlated with the lignin content (Kristensen et al., 2007). Therefore, some researchers believe that the reason why PEG can enhance the efficiency of enzymatic hydrolysis is due to increased cellulase activity (Yoon and Robyt 2005; Sipos et al., 2011) or the structural change of substrate induced by PEG addition (Li et al., 2012a; Li et al., 2019).
BSA is widely used in biochemical studies, and plays an active role in the enzymatic hydrolysis of cellulose (Mukasekuru et al., 2018; Ding et al., 2019). BSA can be used as a synergist for lignocellulose hydrolysis by reducing the non-specific adsorption of lignin to cellulose (Bin and Wyman 2010; Ding et al., 2019). Zhang et al. (2021) compared the effects of adding BSA on the lignocellulose conversion rate of acid-pretreated poplar wood, ethanol-washing acid-pretreated poplar wood, and delignified acid-pretreated poplar wood and they found that BSA acts as a synergistic agent by coating the substrate lignin, thereby blocking the binding site of the substrate lignin. Some researchers believe that in addition to preventing the non-productive adsorption of cellulase by lignin, BSA can also reduce the inactivation of exoglycanase, thereby improving the hydrolysis of microcrystalline cellulose (Kumar and Wyman 2009; Brethauer et al., 2011). While Wang et al. (2015a) thought both the stabilization effect of BSA to cellulase and its prevention of the nonproductive binding of cellulase were all important in promoting lignocellulose saccharification.
From the literature survey, it can be clearly seen that the emphasis was always put on the interaction between additives and substrate, rather than the interaction between cellulase and additives. Since both additive and cellulase are soluble, they actually should interact first when they mixed are together. Therefore, whether to form a complex between synergistic agent and cellulase is very critical in the discussion how they promote the enzymatic hydrolysis. Consequently, how synergistic agent interact with cellulase is very important to understand their role-played in enzymatic hydrolysis. Whereas in literature, the system was too complicated, e.g. in the LS promotion cases, both residual and soluble lignin were coexisted. Therefore, it was difficult to distinguish the exact roles residual and soluble lignin played. That was the reason why there are some controversies in the explanation of their promoting effects.
Quartz crystal microbalance with dissipation monitoring (QCM-D) and surface plasma resonance (SPR) are two commonly used noninvasive techniques to study interface effects in recent years (Yang et al., 2015; Ye et al., 2017; Das et al., 2019; Sengur-Tasdemir et al., 2019; Swiatek et al., 2019). QCM is made according to the principle of piezoelectric effect of quartz crystal. When the quality on the electrode chip changes, the electrical signal Δf output by the quartz crystal system will change accordingly. The quality change of the chip can be calculated based on the data of Δf obtained. The accuracy can reach nanogram level (Huang et al., 2017). Recently, some literatures have reported that QCM-D can be used to monitor the formation of thin films in real time and explore the properties of each film layer-by-layer adsorption of substances on the chip (Ju-Won et al., 2013; Deligöz and Tieke 2014; Deniz and Deligoz 2019). In this case, the viscoelastic Voigt model can be established to estimate the adsorption capacity of each layer more accurately (Maria et al., 2011). The viscous modulus, elastic modulus, and thickness of each film can be obtained by this model. SPR is a method to study intermolecular interactions by using optical properties. We usually study the information change of analyte by the angle change of SPR obtained. Small Angle X-ray scattering (SAXS) can be used to analyze the size and shape of molecules and obtain the aggregation information of proteins in solution (Bernado et al., 2007). In recent years, SAXS has also been applied to the structural research of wood or wood products (Nishiyama 2021).
In this investigation, we attempted to simply the system to study the interaction between the synergistic agents and cellulase alone, ignoring the influence of morphology and chemistry (e.g., residual lignin content, chemical difference, and distribution), using QCM-D and SPR techniques to monitor the interaction between cellulase that was immobilized onto the sensors of QCM-D and SPR and synergistic agents. In our previous work (Wang et al., 2021), we probed the cellulase biosensor formation in situ and in real time on gold chips. Based on the cellulase biosensor we monitored the interactions between cellulase and BSA/LS/PEG in situ and in real time. In addition, SAXS was used to study whether cellulase and additives form a complex and to evaluate the size of the complex. At the same time, the effect of adding these additives to the enzymatic hydrolysis system of pure cellulose (Avicel) and lignocellulose (green liquor-pretreated lignocellulose (GL) on the glucan conversion rate was investigated thoroughly.
2 Materials and methods
2.1 Materials
Chemicals of 11-mercaptoundecanoic acid (MUA, 98%), 3-mercaptopropionic acid (MPA, 98%), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC, 98%), N-hydroxysulfosuccinimide sodium salt (NHS, 98%) and bovine serum albumin (BSA, 96%) were purchased from Aladdin Corp. (Shanghai, China). Commercial soluble lignosulfonate (Reax 85A, with an averaged MW of 10,000) was supplied by Mead Westvaco (Atlanta, GA). Polyethylene glycol (PEG, with an averaged MW of 4000) was procured from Shenggong Bioengineering Corp. (Shanghai, China).
Lignocellulosic biomass material was a poplar pulp, which was pretreated with green liquor as described elsewhere (Wang et al., 2015b). Poplar chips were collected from the north of Jiangsu Province, China. The chips (1: 4 (w/v)) were first impregnated with the pretreatment solution (6.25% sodium sulfide (w/w), 25% sodium carbonate (w/w)) at 80 °C for 30 min. Then the temperature was increased to 160 °C at 2 °C/min and maintained for 1 h. The cellulase (Cellic®CTec2) for cellulose hydrolysis was provided by Novozymes Corp. (Franklinton, NC) and the activity determined by Whatman No. 1 filter paper was 180 FPU/mL through the standard method recommended by IUPAC Commission Biotechnology. The commercial cellulase Trichoderma reesei ATCC 26921 was purchased from Aladdin Company (Shanghai, China) for adsorption research. The SDS-PAGE of cellulase Trichoderma reesei ATCC 26921 and BSA are shown in Fig. 1. All other reagents were purchased from Nanjing Reagent Co., LTD (Nanjing, China).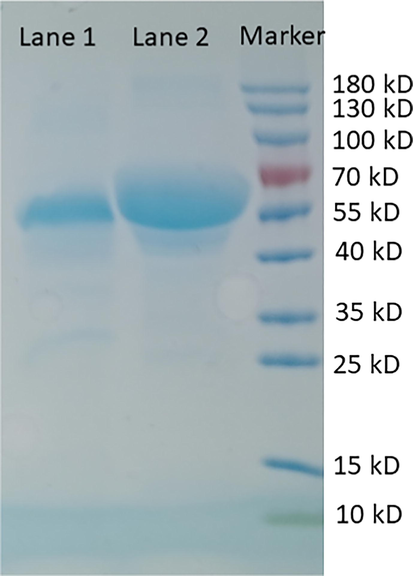
SDS-PAGE of cellulase Trichoderma reesei ATCC 26921 (Lane 1) and BSA (Lane 2).
QCM-D sensors with gold surface (Product number QSX 301) were supplied by Biolin Scientific Co. Ltd (Gothenburg, Sweden), while SPR sensors by BioNavis Co. Ltd. (Tampere, Finland).
The constant temperature shaker (air shaker, ZWY-2102) was supplied by Shanghai Zhicheng Analytical Instrument Manufacturing Company (Shanghai, China).
2.2 Real time interaction probing by QCM-D and SPR
The cleaning procedures of gold QCM-D and SPR chips and the in situ immobilization of cellulase on gold chips can refer to our previous work (Wang et al., 2021). Briefly, the chips were modified using a mixture of thiol-containing organic molecules (MUA and MPA), which resulted to form a mixed self-assembled monolayer with carboxylic groups as the exposed tail group. The cellulase then covalently bonds to the chips through a peptide bond.
Once a uniform cellulase layer was immobilized onto the gold chips of QCM or SPR sensors, an acetate buffer solution (pH = 4.8, 0.05 M) was flushed to the system and a stable baseline was obtained. Then the respective synergistic agent solution was introduced into the chambers of QCM-D or SPR and interacted with the immobilized cellulase until equilibrium. LS solution (Reax 85A) with a concentration of 4.5 mg/mL was pumped into the reaction tank at a rate of 0.100 mL/min. After adsorption equilibrium, the buffer solution was injected for rinsing. Similarly, the effects of BSA/PEG and cellulase were studied in the same way. PEG solution with a concentration of 4.5 mg/mL, whereas BSA had a concentration of 0.05 mg/mL (50 ppm). All synergistic agents were dissolved in the same buffer that was administered when the baseline was obtained.
2.3 SAXS measurements
X-ray small Angle scattering was used to study whether the complexes of cellulase and LS/PEG formed and what are the sizes of the complexes. This part of experiment was performed in Shanghai Light source center BL19U2 biological small Angle station. The sample moves at a speed of 10 μL/s in the capillary. The main technical indicators were beam intensity of 240 mA, energy of 12 keV, detector for Pilatus 1 M, sample to probe distance of 2.6 m, and the wavelength of 1.03 nm.
2.4 Enzymatic hydrolysis experiments
Enzymatic hydrolysis was performed at 2% (w/v) in 50 mL of pH = 4.8 and 0.05 M acetic acid - sodium acetate buffer in a corked conical flask at 50 °C and 180 rpm for 72 h. The substrate, buffer, synergic agent, and cellulase were added in turn. The cellulase loading was 20 FPU/g glucan. The addition amount of LS, PEG and BSA was 0.2 g/g (LS/glucan), 1 g/L and 5 g/L, respectively, based on the optimal dosage in literature (Lin et al., 2015; Wang et al., 2015c; Zhang et al., 2021). After enzymatic hydrolysis, the hydrolysate was boiled for 10 min to inactivate cellulase. After that, all the mixtures were filtered through a 0.22 μm aqueous phase membrane. The glucose content in hydrolysate was measured by liquid chromatography (Waters ACQUITY Arc, UAS). The mobile phase was 5 mM H2SO4, the flow rate was 0.6 mL/min, and the column temperature was 55 °C. All the data were averaged from the two groups and their errors were calculated.
3 Results and discussion
3.1 Interaction between BSA and cellulase
The Interactions study between cellulase (Cell) and BSA was conducted on a QCM-D E4 and a MP-SPR. The monitored curves are shown in Fig. 2. In Fig. 2a, the formation of self-assembled monolayer of MUA/MPA with carboxylic groups and the activation were carried out in the first 90 min. Once stable curves of frequency and dissipation value were obtained, the enzyme solution was injected into the system (point 1). Cellulase was covalently bond to the chip, evidencing by sharply decreased frequency (Δf). Δf was decreased by about 20 Hz. Then, the buffer solution was pumped in (Fig. 2a, point 2) for rinsing, and the frequency increased slightly. This showed that the enzyme was firmly attached to the gold chips. After the BSA solution was injected (Fig. 2a, point 3), the Δf dropped dramatically down by 20 Hz. The buffer was then pumped in to rinse the unbound and loosely bound BSA. This legend system applies to Figs. 2–4. The frequency of the chips went up slightly. Regarding to the thickness of adlayer on QCM sensors, both fitted thickness values derived from Sauerbrey equation (open symbols) and viscoelastic modeling (solid symbols) were plotted together in Fig. 2b for easy comparison. The immobilized cellulase thickness was calculated to be 8.9 and 8.2 nm, respectively, for before and after rinsing (Table 1). The thickness of BSA was 5.2 and 4.8 nm, respectively, for before and after rinsing (Table 1). Based on Sauerbrey estimation, all according values were less than half of the values based on viscoelastic modeling, indicating the layer formed was very soft and therefore the Sauerbrey estimation was substantially underestimated the layer thickness of cellulase and/or BSA (Song et al., 2009).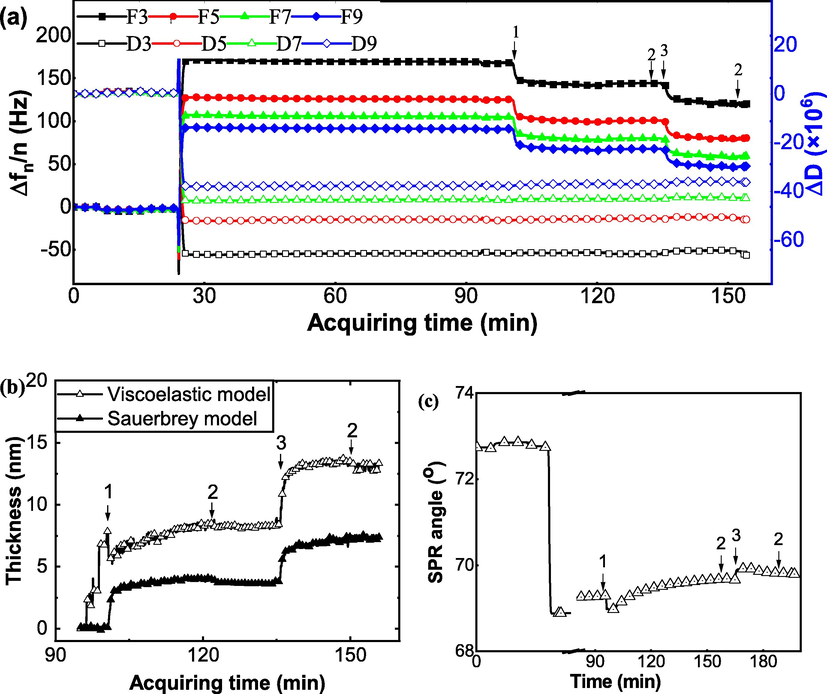
Interaction study between cellulase (Cell) and BSA. (a) Frequency change and energy dissipation for the interaction between cellulase and BSA was obtained on a QCM-D E4 equipment in-situ and in real time. (b) Fitted thickness of the layer on cellulase film with the addition of BSA. Open symbols represent the data extracted from viscoelastic modeling while solid symbols derived from Sauerbrey equation directly. (c) SPR signal change for the interaction between BSA and cellulase was obtained on a MP-SPR. Mark 1 for enzyme loading, 2 for buffer rinse, 3 for additive loading, and 4 for another additive loading.
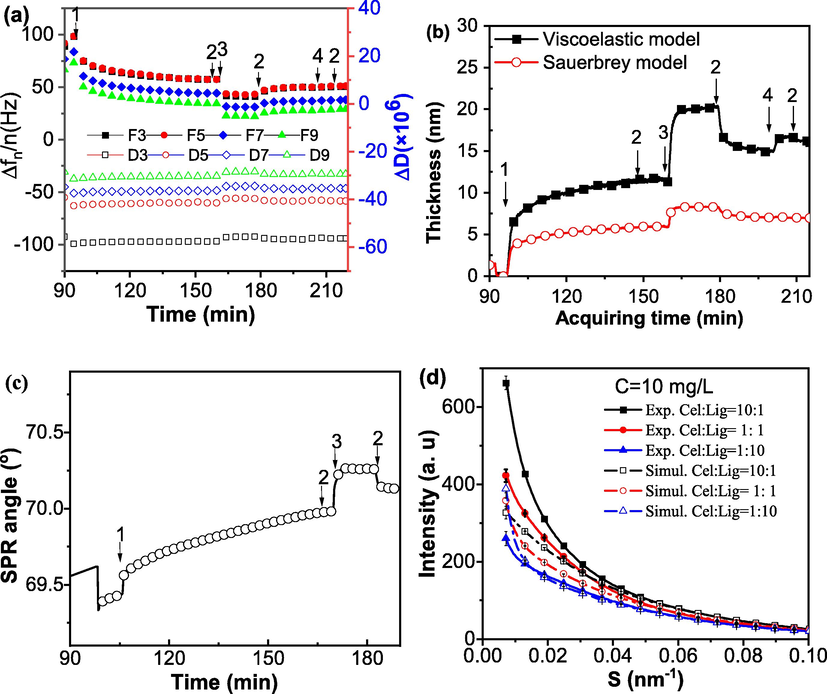
Interactions study between cellulase (Cell) and lignosulfonate (LS). (a) Frequency change and energy dissipation for the interaction between cellulase and LS was obtained on a QCM-D E4 equipment in-situ and in real time. (b) Fitted thickness of the layer on cellulase film with the addition of LS. Solid symbols and stars represent the data extracted from viscoelastic modeling while open symbols derived from Sauerbrey equation directly (c) SPR signal change for the interaction between LS and cellulase was obtained on a MP-SPR. (d) The small-angle X-ray scattering signal of the mixture of cellulase and LS in different proportion. (Cellulase and LS concentrations were both 10 mg/mL and dissolved in 0.05 M, pH 4.8 acetate buffer solution).
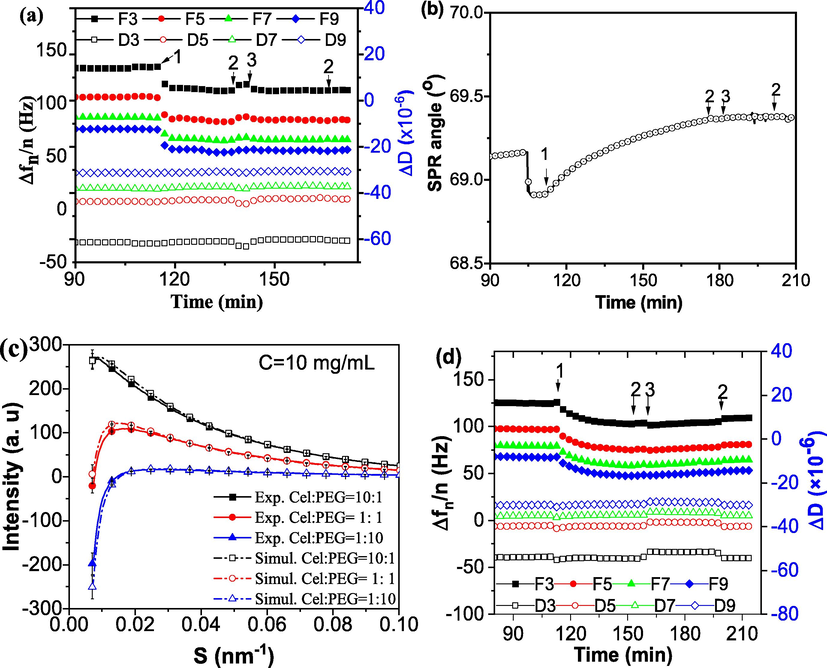
Interactions study between cellulase (Cell) and PEG. (a, d) Frequency change and energy dissipation for the interaction between cellulase and PEG was obtained on a QCM-D E4, and (b) SPR signal change for the interaction between PEG obtained on a MP-SPR. (c) The small-angle X-ray scattering signal of the mixture of cellulase and PEG in different proportion. Acetate buffer (0.05 M, pH 4.8) was used in a, b and c, while phosphate buffer (0.05 M, pH 7.5) was used in d.
Layer
Thickness (nm)
Coupled water
SPR
QCM
(%)
Cellulase: before rinsing
7.1
8.9
20.2
after rinsing
6.9
8.2
15.9
BSA: before rinsing
2.6
5.2
50.0
after rinsing
2.0
4.8
58.3
The same procedure was conducted on MP-SPR and the SPR angle changed with time is reported in Fig. 2c. The thickness of each layer based on MP-SPR was calculated and reported in Table 1. The adsorption thickness of BSA on cellulase was 2.0 nm after rinsing. The SPR data also showed that the thickness of cellulase layer was about 6.9 nm after rinsing. Since the molecular weight of cellulase is about 58 kDa, while that of BSA was 66.4 kDa. Thus, one BSA molecule can bind to four cellulase molecules to form a complex (BSA-Cell) in the planar adsorption as conducted in our system. One thing must be noted that the adsorption of BSA on immobilized cellulase is different than that in practice since there is only one interface is available for BSA adsorption while there is multiple direction for BSA binding in solution.
Since QCM-D detects the wet-mass of the adlayer on the surface, while SPR probes the dry-mass, the coupled water of cellulase layer and BSA layer could be derived based on the different thickness examined by QCM-D and SPR techniques and the results are reported in the last column of Table 1. It can be noted that the cellulase layer was much more compact while BSA much softer, since the former contained less than half of the coupled water as the latter.
3.2 Interactions between lignosulfonate and cellulase
Interactions study between cellulase (Cell) and lignosulfonate (LS) are present in Fig. 3. As stated previously, through the change of QCM frequency and SPR angle (Fig. 3a, 3c), it can be seen that LS can be adsorbed strongly and irreversibly on the cellulase film. The QCM frequency decreased by 15 Hz after LS solution was loaded. It can be seen from Fig. 3b that the thickness of the LS film adsorbed on the cellulase is about 8.3 nm, and the height of the stably adsorbed LS film after buffer cleaning is about 3.2 nm. According to SPR data (Table 2), the adsorption thickness of LS on cellulase was 0.73 nm after washing. In summary, cellulase and LS can form complexes (LS-Cell). This was consistent with the conclusions made by Wang et al. (2013). Through our adsorption experiments, it can be estimated that the formed complex (LS-Cell) composed of one LS and two cellulase molecules in the planar adsorption situation as conducted in our system.
Layer
Thickness (nm)
Coupled water
SPR
QCM
(%)
Cellulase: before rinsing
10.3
12.0
14.2
after rinsing
10.3
12.0
14.2
LS: before rinsing
1.29
8.3
84.5
after rinsing
0.73
3.2
77.2
The coupled water of cellulase layer and LS layer derived was reported in the last column of Table 2. The coupled water content in cellulase adlayer was about 14.2%, which agreed very well our results in the formation of BSA-Cell complex. However, the coupled water content of LS layer was about 80%, indicating LS was much extended in solution and the LS adlayer was very soft.
Fig. 3d showed that the experimental scattering intensity of the mixed solution of cellulase and LS was not the same as their theoretical scattering intensity without any interaction, and the experimental intensity was always greater than that of the theoretical one, indicating some complexes of LS-Cell formed. This is consistent with the QCM-D and SPR measurements addressed previously.
The pH of the buffer will affect the adsorption of LS on the cellulase film (Ju-Won et al., 2013). Our previous study (Wang et al., 2021) has shown that LS adsorbed more cellulase under high pH condition. Some researchers also found that with the increase of pH, the enzymatic hydrolysis efficiency of cellulose would increase (Wang et al., 2013). The cellulase carries some negative charge but the intensity is not very strong, since the zeta potential of cellulase is about −2.1 mV at pH 4.8. Therefore, according to the theory of electrostatic adsorption, it can form complex with LS likely at pH 4.8. Whereas at pH 7.5, cellulase carries a number of negative charges and its zeta potential can reach down to −30 mV (Wang et al., 2013) and therefore it is unlikely to form complex of LS-Cell in this situation. But in our previous research (Wang et al., 2021), the opposite result was observed. This indicates that electrostatic interaction is not the only factor that predominates the formation of complexes between cellulase and LS.
The above results showed that LS and cellulase could form complexes in different solutions, but with different structures, and the formation of this complex is not only related to electrostatic adsorption. This opinion is different from previous reports in the literature (Wang et al., 2013).
3.3 Interaction between PEG and cellulase
The interaction between PEG on cellulase film monitored by QCM-D is shown in Fig. 4a. After the PEG solution was injected, the frequency (Δf) basically had no fluctuation. There was no frequency change either after the buffer rinse. Accordingly, there was no fluctuation in dissipation values after PEG loading and rinsing. This suggested that PEG did not adsorb on cellulase film.
In order to verify the results of QCM, we used SPR to study the interaction between cellulase and PEG. It can be seen from Fig. 4b. Similarly, after PEG solution was injected, SPR angle remained unchanged.
At last, the SAXS signal of the mixture of cellulase and PEG in different proportion (10:1, 1:1 and 1:10) are plotted in Fig. 4c, along with the simulated signals with the volume ratio with cellulase and PEG. It can be found that the experimental scattering intensity of the mixed solution of PEG and enzyme was almost overlapped with the simulated scattering intensity assuming no interaction between PEG and cellulase.
Fig. 4d showed that pH did not affect the interaction between PEG and cellulase films. Although it is a fact that PEG can improve the enzymatic hydrolysis ability of lignocellulose (Wang et al., 2013). However, the above results all proved that PEG and cellulase did not combine to form complex. If look at the last phase of Fig. 3b, where PEG was loaded after LS forming. PEG actually had an adsorption thickness of about 0.2 nm on LS film (Fig. 3b, point 4). Even after rinsing, there was still about 0.1 nm PEG remained on LS film, indicating that PEG has a relative strong affinity to lignin or lignin-derived materials. This provides some evidences that PEG can be adsorbed on the surface of residual lignin in substrate, albeit this adsorption is not strong. The authors in other studies have similar findings (Lai et al., 2020).
3.4 Enzymatic efficiency assessment of enzymatic synergistic agents for Avicel and green liquor treated substrate
In this study, we aimed to elucidate the effect of addition on enzymatic hydrolysis reactions of pure microcrystalline cellulose, i.e., Avicel, and poplar pulp pretreated with green liquid containing lignin, to explore the mechanism of these addition in the process of cellulase hydrolysis.
Fig. 5 showed that by adding BSA the enzymatic hydrolysis efficiency of Avicel was increased from 46% to 69% while the efficiency of GL was increased from 69% to 83%. The addition of PEG increased the enzymatic hydrolysis efficiency of microcrystalline cellulose to 75% and the enzymatic hydrolysis efficiency of GL to 87%. Unlike them, the addition of LS inhibited the enzymatic hydrolysis of microcrystalline cellulose (35%), but it enhanced the enzymatic hydrolysis efficiency of GL (75%). In conclusion, BSA and PEG can be effective to increase the enzymatic hydrolysis efficiency for both pure cellulose and lignocellulose sample with residual lignin; while LS can also enhance the enzymatic hydrolysis efficiency of lignocellulose with residual lignin, but inhibits the enzymatic hydrolysis of pure cellulose. Similar results have been found in other studies (Wang et al., 2015c; Kumar and Wyman 2009).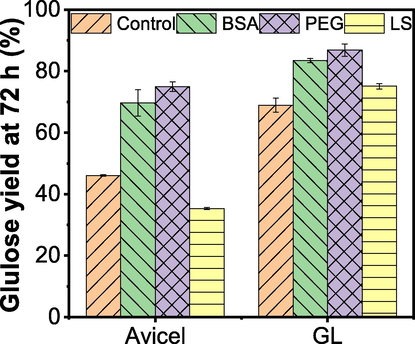
Effects of various additives on Avicel cellulose and poplar pulp enzymatic hydrolysis. The glucose conversion rates of Avicel and GL at 72 h were measured with or without the addition of synergistic agents, in which dosages of LS, PEG 6000, and BSA were 0.2, 1, and 5 g/g-substrate, respectively.
3.5 Proposed interaction modes
Based on our interaction study between cellulase and BSA/PEG/LS, and the saccharification promotion assessment for Avicel and green liquor treated substrate, the proposed interaction mechanisms of synergists of BSA/PEG/LS are presented in Fig. 6.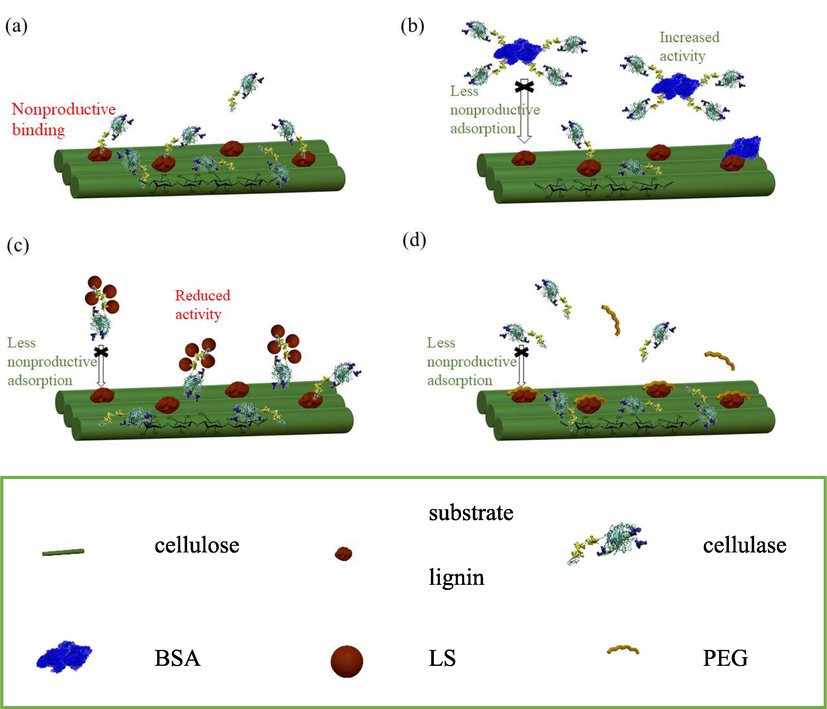
Proposed saccharification promotion mechanisms of (a) without synergetic agent addition, and with synergetic agent addition of (b) BSA, (c) LS and (d) PEG.
Without synergist, it has been widely recognized that during the enzymatic hydrolysis of cellulose, the substrate lignin (i.e. residual lignin) adsorbs cellulase unproductively, thus reducing the enzymatic hydrolysis efficiency of lignocellulose as illustrated in Fig. 6a (Rahikainen et al., 2013; Liu et al., 2016).
As illustrated in Fig. 6c, LS particles can bind with cellulase to form complexes through electrostatic interaction forces and other forces that not fully recognized and understood. Actually, the formed complex of LS-Cell has a lower enzymatic activity than their native one. It inhibits the enzymatic hydrolysis of cellulose in the absence of lignin. Nevertheless, in the situation where residual lignin is present in the substrate, the LS-Cell complex formed in the solution can eventually reduce the nonproductive adsorption of cellulase on residual lignin in substrate. Therefore, the lignocellulose saccharification promotion effect of LS for the enzymatic hydrolysis is very complicated. It should be balanced the benefits from the reduced non-productive binding to residual lignin and the costs of reduced enzymatic activity of LS-Cell complex. When there was non– or less residual lignin presence in the matrix of lignocellulose, the reduced enzymatic activity of LS-Cell complex cannot be overcome by the reduced non-productive binding, the total effect should be negative. While when the residual lignin in the lignocellulose matrix is substantial, the non-productive binding is dominant in the enzymatic hydrolysis. In this situation, the promotion effect of LS should be obvious since the gain in the nonproductive binding reduction can beat the loss in enzymatic activity. In practice, the pretreatment removes part of lignin from substrate, substantial residual lignin remains in the substrate. Therefore, LS can still be considered as a good synergistic agent in lignocellulose enzymatic hydrolysis.
As illustrated in Fig. 6d, unlike LS particles, PEG does not form complex with cellulase. It can enhance the activity of cellulase on one hand. On the other hand, PEG can adsorb on substrate lignin, preventing cellulase from adsorbing on substrate lignin nonproductively. This hypothesis was brought up by Lai et al. (2020) that PEG forms a physical barrier to reduce the adsorption of cellulase onto substrate lignin. It was confirmed by our interaction study that PEG does have a high affinity to lignin and it maybe form a physical barrier to prevent nonproductive binding of cellulase.
As illustrated in Fig. 6a, like LS, BSA does form BSA-Cell complex, whereas the BSA-Cell complex plays an active role in the enzymatic hydrolysis for microcrystalline cellulose. This can be attributed to that the BSA-Cell stabilizes cellulase activity (Simone et al., 2011). In sum, BSA can increase the sugar conversion rate of lignocellulosic biomass in two aspects, one is increased enzymatic activity induced by enzyme stabilization; the other is exhibited in nonspecific adsorption on residual lignin to occupy the binding sites for cellulase. It was found that with the increase of enzyme load, the improvement of enzymatic hydrolysis effect by adding BSA gradually decreased (Zhang et al., 2021). It supports the action mechanism brought up by us.
4 Conclusions
Although all three representatives of enzymatic hydrolysis synergistic agents can promote the enzymatic hydrolysis of lignocellulose, we found that LS, BSA and PEG have totally different action modes. LS and BSA can form complexes with cellulase, while the complexes formed with the former reduce the cellulase activity, as comparison, the latter enhances the cellulase activity. The complex of LS-Cell promotes the enzymatic hydrolysis of lignocellulose only in the presence of residual lignin of substrate due the electrostatic repulsion between LS-Cell complex and residual lignin. Unlike LS, BSA complex works both from its enhanced cellulase activity and reducing the nonspecific adsorption of cellulase and substrate lignin. Although PEG does not bind to cellulase, it can also enhance cellulase activity for pure cellulose due to its surfactant properties, while for lignocellulose containing residual lignin due to the reduced nonproductive adsorption of cellulase since it prefers to bind to residual lignin to form a steric physical barrier. This investigation shed some lights on the molecular mechanisms of how different enzymatic hydrolysis synergistic agents work from the perspective of their molecular interactions with enzymes. It will be helpful in the practical enzymatic hydrolysis process and in enzyme engineering.
CRediT authorship contribution statement
Peipei Wang: Investigation, Data curation, Methodology, Writing – original draft. Qingcheng Wang: Visualization. Tian Liu: Validation. Jiaqi Guo: Writing – review & editing. Yongcan Jin: Funding acquisition. Huining Xiao: Supervision. Junlong Song: Conceptualization, Data curation, Methodology, Funding acquisition, Writing – review & editing.
Acknowledgments
The authors thank the National Natural Science Foundation of China (Nos. 31730106 and 31770623) and the Priority Academic Program Development of Jiangsu Higher Education Institutions for supporting the work. We thank the guidance of Drs. Na Li, Yawen Li and Jinyou Lin from National Facility for Protein Science in Shanghai (NFPS) using the BL19U2 line station for SAXS data collection. We thank Prof. Yoshiharu Nishiyama of Univ. Grenoble Alpes, CNRS, CERMAV, France for his assistance in SAXS data analysis. The experimental assistance from the advanced analysis and test center of Nanjing Forestry University is gratefully acknowledged.
Availability of data and material
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci.. 2014;7(2):163-173.
- [Google Scholar]
- Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc.. 2007;129(17):5656-5664.
- [Google Scholar]
- BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotech. Bioeng.. 2010;94(4):611-617.
- [Google Scholar]
- Enhanced enzymatic conversion of softwood lignocellulose by poly(ethylene glycol) addition. Enzyme Microb Tech.. 2007;40(4):754-762.
- [Google Scholar]
- The effect of bovine serum albumin on batch and continuous enzymatic cellulose hydrolysis mixed by stirring or shaking. Bioresour. Technol.. 2011;102(10):6295-6298.
- [Google Scholar]
- Improving enzymatic hydrolysis of lignocellulosic substrates with pre-hydrolysates by adding cetyltrimethylammonium bromide to neutralize lignosulfonate. Bioresour. Technol.. 2016;216:968-975.
- [Google Scholar]
- Stereo-regulated Schiff base siloxane polymer coated QCM sensor for amine vapor detection. Mater. Chem. Phys.. 2019;226:214-219.
- [Google Scholar]
- QCM-D study of layer-by-layer assembly of polyelectrolyte blend films and their drug loading-release behavior. Colloid. Surf. A.. 2014;441:725-736.
- [Google Scholar]
- Flexible self-assembled polyelectrolyte thin films based on conjugated polymer: Quartz cristal microbalance dissipation (QCM-D) and cyclic voltammetry analysis. Colloid. Surf. A.. 2019;563:206-216.
- [Google Scholar]
- Synergy of hemicelluloses removal and bovine serum albumin blocking of lignin for enhanced enzymatic hydrolysis. Bioresour. Technol.. 2019;273:231-236.
- [Google Scholar]
- Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol.. 2002;31:353-364.
- [Google Scholar]
- Probing the interactions of organic molecules, nanomaterials, and microbes with solid surfaces using quartz crystal microbalances: methodology, advantages, and limitations. Environ. Sci.-Proc. IMP. 2017;19:793-811.
- [Google Scholar]
- Investigations of the effect of water-soluble lignin on enzymatic hydrolysis of lignocellulose. J. For. Eng.. 2020;5:12-19.
- [Google Scholar]
- Charge storage in polymer acid-doped polyaniline-based layer-by-layer electrodes. Acs Appl. Mater. Inter.. 2013;5(20):10127-10136.
- [Google Scholar]
- Surfactant-assisted pretreatment and enzymatic hydrolysis of spent mushroom compost for the production of sugars. Bioresour. Technol.. 2012;114:399-405.
- [Google Scholar]
- Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb. Technol.. 2007;40(4):888-895.
- [Google Scholar]
- Effect of additives on the digestibility of corn Stover solids following pretreatment by leading technologies. Biotechnol. Bioeng.. 2009;102(6):1544-1557.
- [Google Scholar]
- Incorporating lignin into polyethylene glycol enhanced its performance for promoting enzymatic hydrolysis of hardwood. ACS Appl. Mater. Inter.. 2020;8(4):1797-1804.
- [Google Scholar]
- Dissecting the effect of polyethylene glycol on the enzymatic hydrolysis of diverse lignocellulose. Int. J. Biol. Macromol.. 2019;131:676-681.
- [Google Scholar]
- The mechanism of poly(ethylene glycol) 4000 effect on enzymatic hydrolysis of lignocellulose. Colloid. Surf. A.. 2012;89:203-210.
- [Google Scholar]
- Lignin-based polyoxyethylene ether enhanced enzymatic hydrolysis of lignocelluloses by dispersing cellulase aggregates. Bioresour. Technol.. 2015;185:165-170.
- [Google Scholar]
- Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuel. Bioprod. Bior.. 2016;10:648-663.
- [Google Scholar]
- Layer-by-layer assemblies of chitosan and heparin: effect of solution ionic strength and pH. Langmuir.. 2011;27(12):7537-7548.
- [Google Scholar]
- Enhanced high-solids fed-batch enzymatic hydrolysis of sugar cane bagasse with accessory enzymes and additives at low cellulase loading. ACS Sustain. Chem. Eng.. 2018;6:12787-12796.
- [Google Scholar]
- Retrieving structural information from scattering and atteuation data of transparent wood and (nano)paper. J. Bioresour. Bioprod.. 2021;6:187-194.
- [Google Scholar]
- Cellulase-lignin interactions-The role of carbohydrate-binding module and pH in non-productive binding. Enzyme Microb Tech.. 2013;53(5):315-321.
- [Google Scholar]
- Characterization of aquaporin Z-incorporated proteoliposomes with QCM-D. Surf. Innov.. 2019;7(2):133-142.
- [Google Scholar]
- The effect of bovine serum albumin on batch and continuous enzymatic cellulose hydrolysis mixed by stirring or shaking. Bioresour. Technol.. 2011;102(10):6295-6298.
- [Google Scholar]
- Mechanism of the positive effect of poly(ethylene glycol) addition in enzymatic hydrolysis of steam pretreated lignocelluloses. C. R. Biol.. 2011;334(11):812-823.
- [Google Scholar]
- Tools to Probe Nanoscale Surface Phenomena in Cellulose Thin Films: Applications in the Area of Adsorption and Friction. John Wiley & Sons, Ltd.; 2009. p. :91-122.
- Adsorption of beta-lactoglobulin A on gold surface determined in situ by QCM-D measurements. Food Hydr.. 2019;91:48-56.
- [Google Scholar]
- The effect of nonenzymatic protein on lignocellulose enzymatic hydrolysis and simultaneous saccharification and fermentation. Appl. Biochem. Biotechnol.. 2015;175:287-299.
- [Google Scholar]
- In-situ and real-time probing cellulase biosensor formation and its interaction with lignosulfonate in varied media. Sensor Actuat B: Chem.. 2021;329:129114
- [Google Scholar]
- Effects of green liquor pretreatment on the chemical composition and enzymatic hydrolysis of several lignocellulosic biomasses. Bioresources.. 2015;10(1):709-720.
- [Google Scholar]
- Influence of lignin addition on the enzymatic digestibility of pretreated lignocellulosic biomasses. Bioresour. Technol.. 2015;181:7-12.
- [Google Scholar]
- Lignosulfonate-mediated cellulase adsorption: enhanced enzymatic saccharification of lignocellulose through weakening nonproductive binding to lignin. Biotechnol. Biofuels.. 2013;6(1):156.
- [Google Scholar]
- One-step purification and immobilization of his-tagged protein via Ni2+-functionalized Fe3O4@polydopamine magnetic nanoparticles. Biotechnol. Bioproc. E.. 2015;20:901-907.
- [Google Scholar]
- Polydopamine interconnected graphene quantum dots and gold nanoparticles for enzymeless H2O2 detection. J. Electroanalytical. Chem.. 2017;796(1):75-81.
- [Google Scholar]
- Activation and stabilization of 10 starch-degrading enzymes by Triton X-100, polyethylene glycols, and polyvinyl alcohols. Enzyme Microb Tech.. 2005;37(5):556-562.
- [Google Scholar]
- Impacts of degree of substitution of quaternary cellulose on the strength improvement of fiber networks. Int. J. Biol. Macromol.. 2021;181:41-44.
- [Google Scholar]
- Lignosulfonate to enhance enzymatic saccharification of lignocelluloses: role of molecular weight and substrate lignin. Ind. Eng. Chem. Res.. 2013;52(25):8464-8470.
- [Google Scholar]






