Translate this page into:
Determining β-lactam antibiotics in aquaculture products by modified QuECHERS combined with ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS)
⁎Corresponding author at: Ministry of Agriculture Laboratory of Quality & Safety Risky Assessment for Aquatic Product, Pearl River Fisheries Research Institute, Chinese Academic of Fishery Science, Guangzhou 510380, China. yin.yi@126.com (Yi Yin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
In this study, a multi-residue determination method of QuECHERS combined with UHPLC-MS/MS was developed to determine nine β-lactam antibiotics (penicillin G, amoxicillin, ampicillin, cephalexin, cefquinome, ceftiofur, cefazolin, cephapirin, cefuroxime) in aquaculture products. All the nine β-lactam antibiotics exhibited excellent linearity within the range of 2.0–200 ng/mL (cefuroxime: 5.0–200 ng/mL, r greater than 0.999), while the limits of detection (LODs) and quantification (LOQs) were 2.0–5.0 μg/kg and 5.0–10.0 μg/kg, respectively. The recoveries of this method in five aquaculture products (Penaeus Orientalis, Cyprinus Carpio, Channa Argus, Aristichthys Nobilis, and Ctenopharyngodon Idella) ranged from 85.4% to 113.3% and the intra-day and inter-day precisions (%RSDs) were less than 15%. The results suggested the feasibility of this method as a rapid, simple, and accurate approach for determining the residues of the β-lactam antibiotics in aquaculture products.
Keywords
QuECHERS
UHPLC-MS/MS
Aquaculture products
β-lactam antibiotic
1 Introduction
β-lactams antibiotics (β-LA), containing β-lactamide rings in their chemical structure, are a class of antibiotics dominated by penicillins and cephalosporins (Lara et al. 2012). The bactericidal mechanism of β-lactams antibiotics is characterized by the inhibition of cell wall sticky peptide synthetase hindering the cell wall sticky peptide synthesis, bacterial cell wall defects, and expansion and rupture of the bacterial cell through bacterial penicillin-binding proteins (PBPs) (Tong 2015). It is widely applied in clinics and animal husbandry due to its strong bactericidal activity, low toxicity, and broad indications (Casu et al. 2013). β-LA can disrupt the human intestinal flora balance and induce several allergic reactions. For instance, most people are sensitive to penicillin. Moreover, the residues of β-lactams antibiotics in animal food can enter the human body through the food chain and induce bacterial resistance and other risks (Benito-Peña et al. 2009; Feledziak et al. 2013). In view of the harmful effect of β-lactam antibiotics on human health, many countries, including the European Union, the United States, and China, have proposed relevant regulations on the maximum residue limits (MRLs) of β-lactam drugs in animal food. According to the European Union (Commission 2010), the MRL values of ceftiofur, cefaquinoline and penicillin G are 50–1000 µg/kg. Similarly, according to bulletin 235 of the Ministry of Agriculture of China and GB 31650–2019, National Standard for Food Safety Maximum Residue Limits for Veterinary Drugs in Food (China Food and Drug Administration 2019), the MRL values of amoxicillin and ampicillin in all food animals are 50 µg/kg. With the advancement of the aquaculture industry, the incidence of diseases has increased due to the high-density intensive aquaculture mode. Additionally, the overuse of drugs by aquaculturists, the clinical effects of human or animal drugs on aquaculture products should not be ignored. According to the EU Commission Regulation 37/2010, penicillins are β-LA licensed for aquaculture.
At present, the detection methods of β-lactam residues in food primarily focus on milk (Dorival-García et al.2016; Li et al. 2018; Souza et al.2021), livestock, poultry muscle tissues (such as pig, cattle, sheep, and chicken) (Chen et al. 2017; Macarov et al. 2012; Prez-Burgos et al. 2012; Rezende et al. 2012), eggs, and animal feeds (Benito-Peña et al. 2009; Dasenaki and Thomaidis 2015; Kantiani et al. 2009; Wang et al. 2021). The detection methods include the microbiological method (Souza et al. 2021), immunoassay (Benito-Peña et al. 2005), high-performance capillary electrophoresis (HPCE), and high-performance liquid chromatography (HPLC) (Benito-Peña et al. 2009; Lara et al. 2012). The pretreatment methods mainly include liquid–liquid extraction (LLE) (Adlnasab et al. 2012; Jank et al. 2012), solid-phase extraction (SPE) (Macarov et al.2012; Wang et al.2021; Yahaya et al.2015), and matrix-assisted solid-phase dispersion extraction (MSPD) (Wang et al. 2021; Yahaya et al. 2015; Karageorgou et al.2012). Among them, solid-phase extraction (SPE) is the most widely adopted method. Moreover, it is challenging to meet the requirements of multi-residue determination due to the lack of adequate studies on the determination methods of β-LA in aquaculture products (Arroyo-Manzanares et al. 2015; Dorival-García et al. 2016; Liu et al. 2014; Yahaya et al. 2015) and insufficient measured substances. Therefore, establishing a simple, fast, and accurate multi-residue determination method is highly necessary.
The QuEChERS method is a rapid, simple, economical, and eco-friendly pretreatment method, widely used in the detection of multiclass drug residues in food (Chen et al. 2017; Prez-Burgos et al. 2012; Shendy et al. 2016; Wang et al. 2015). However, its application in determining β-lactam from aquaculture products is limited. Therefore, QuECHERS combined with the UHPLC-MS/MS technique was developed in this study by optimizing the extraction and purification processes to determine nine β-lactam antibiotics residues in aquaculture products. Furthermore, the trueness, precision, and matrix effect of the method were verified. An accurate method for quantification of nine β-lactam antibiotics in aquaculture products was developed.
2 Materials and methods
2.1 Materials and reagents
Certified reference materials of penicillin G (purity of 99.5%), cephalexin (purity of 98.3%), ampicillin (purity of 99.0%), amoxicillin (purity of 99.5%), cephapirin (purity of 99.4%), cefuroxime (purity of 99.1%), cefazolin (purity of 98.5%), ceftiofur (purity of 99.8%), cefquinome (purity of 98.5%) and penicillin G-d7 (purity of 98.0%) were obtained from Dr. Ehrenstorfer (Augsburg, Germany) (Table.A.1). HPLC-grade acetonitrile (ACN) and methanol (MeOH) were purchased from Merck (Darmstadt, Germany). Acetic acid (AA) and formic acid (FA) of LC-MS grade were obtained from Fisher Scientific Inc. (Pittsburgh, PA, USA). The primary secondary amine (PSA, 40–63 µm), Clearnet alumina N (100–300 mesh), graphitized carbon black (GCB, 120–200 mesh), florisil (F), and C18 were purchased from ANPEL Laboratory Technologies Inc. (Shanghai, China). Anhydrous sodium sulfate (Na2SO4) was procured from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The ultrapure experimental water (≥18.2 MΩ) was produced by a Milli-Q purification system (Millipore, Bedford, USA).
The sample muscles of aquaculture products were purchased from the local supermarkets (Xilang, Guangzhou, China). The muscle homogenate was extracted from Penaeus Orientalis, Cyprinus Carpio, Channa Argus, Aristichthys Nobilis, Ctenopharyngodon Idella, and stored at −20 °C until analysis. All samples were freshwater aquaculture products. The blank muscle samples of Ctenopharyngodon Idella (nine β-lactam antibiotics not detected) used in the validation process were collected at the aquaculture base of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Science, where none of these antibiotics were used.
2.2 Preparation of standard solution
Stock standard solutions of nine β-lactam antibiotics and penicillin G-d7 (Internal standard) were prepared at 1.0 mg/mL in a mixture ACN:water (3:7,v/v), and stored at −20℃ in the dark for one month. The mixed intermediate solution was prepared at 10 μg/mL and stored at −20 ℃ in the dark.
2.3 Sample preparation
Approximately 2.0 ± 0.1 g of muscle samples were added to 15 mL centrifuge tubes and spiked 50 µL of the internal standards (1.0 µg/mL), after a vortex-mixing, 5 mL of 1% FA in ACN and 2.0 g Na2SO4 to each tube. Later, the tube was homogenized using a vortex oscillator (T25 Ultra-Turrax, IKA, Germany) for 1 min and then centrifuged at 5000 rpm for 5 min. The supernatant was transferred into a 10 mL plastic centrifuge tube containing 200 mg of C18 and 500 mg of Na2SO4. The mixture was vortexed for 1 min and centrifuged for 5 min at 5000 rpm, and the supernatant was transferred to another 10 mL glass tube. The extraction solution was dried with nitrogen at 45 ℃. After vaporizing, the residues were dissolved in 1 mL of the aqueous solution of 10% acetonitrile containing 0.1% formic acid and filtered through 0.22 µm nylon syringe filters before the UHPLC-MS/MS analysis.
2.4 The instrument conditions
2.4.1 Chromatographic conditions
The UHPLC-MS/MS system is comprised of an equity UHPLC liquid chromatography connected with Xevo TQD MS/MS triple quadrupole detector (Waters, Milford, USA). An Acquity ® UPLC® BEHTM C18 column (100 mm × 2.1 mm, with 1.7-µm particle size, Waters, Milford, USA) at the column temperature of 30 ℃ separated nine β-lactam antibiotics. The injection volume was 10 µL, mobile phase A consisted of 0.1% formic acid (v/v) in water, and mobile phase B consisted of 0.1% formic acid (v/v) in acetonitrile. The ratio of water: methanol solution (80:20 v/v) was used as a seal wash. The chromatographic conditions of the gradient for a total run time of 8.0 min are summarized as follows: 0–1.00 min, 10 %B; 1.00–3.00 min, 10–30% B; 3.0–5.0 min, 30–40% B; 5.00–5.01 min, 40–10% B; 5.01–8.00 min, 10% B at a flow rate of 0.4 mL/min (Table.A2).
2.4.2 Mass spectrometric conditions
Waters Xevo TQD mass spectrometer with an electro spray ionization (ESI) source (Waters, Milford, MA, USA) was connected to the UHPLC system through ESI interface and operated in the multi-reaction monitoring (MRM) positive ion mode (ESI + ). The capillary voltage was 0.5 Kv, the ion source and desolvation temperatures were 120 ℃ and 400 ℃, respectively. The nitrogen (N2) and argon (Ar) were used as the desolvation and collision gas at flow rates of 900 L/h and 20 L/h, respectively. The MRM-MS parameters of nine β-lactam antibiotics are summarized in Table 1. All data were acquired using MassLynx (Version4.1) software. * Quantitation ion.
Compounds
Retention time
(min)Precursor ion (m/z)
Daughter ion
(m/z)Dwell time
(ms)Cone voltage (V)
Collision energy (eV)
penicillin G
5.47
335
160*
30
62
22
176
30
62
26
amoxicillin
1.31
366
114*
30
22
18
208
30
22
12
ampicillin
3.25
350
160*
30
34
12
192
30
34
16
cephalexin
3.37
348
158*
30
32
12
174
30
32
16
cefquinome
3.02
529
134*
30
32
15
396
30
32
12
ceftiofur
4.97
524
241*
30
45
22
208
30
45
16
cefazolin
3.93
455
323*
30
45
15
158
30
45
10
cephapirin
1.94
424
292*
30
40
28
152
30
40
16
cefuroxime
4.16
447
386*
30
32
12
342
30
32
12
penicillin G-d7
5.36
374
160
30
62
22
2.5 Method validation
The QuEChERS and UHPLC-MS/MS techniques were employed to determine nine β-lactam antibiotics in aquaculture products. Validation parameters (Linearity, sensitivity, trueness, precision, and matrix effect) were evaluated according to the guidelines of SANTE/12682/2019, using blank samples (SANTE 2019). The information related to the blank sample is described in 2.1.
The sensitivity of the method was performed by determining the limit of detection (LOD) and limit of quantification (LOQ) of each antibiotic. The LOQ was the lowest concentration at which the method was validated, and the quantifier multiple reaction monitoring (MRM) transition of each compound was evaluated at S/N of ≥ 3; and ≥ 10, respectively.
The samples spiked with β-lactam antibiotics at LOQ, 2 × LOQ and 4 × LOQ (n = 6 at each level) in the muscle of P.orientalis, C.carpio, C.argus, A.nobilis, and C.idella were analyzed by the recovery experiments to validate the trueness and precision of the method. Intermediate precision (inter-day precision) was evaluated by the analysis of six spiked samples in the muscle of C.idella at 5.0 µg/kg in three different days. The analyses were carried out in triplicate. The results were expressed as the percentages of relative standard deviations (%RSD). The RSD within 20%, and the recoveries ranging from 70% to 120% of the nominal concentrations are the primary means to detect drug residues (Chun et al. 2005).
2.6 Matrix effects
Matrix effects (ME) refer to the direct or indirect effect of co-eluting substances in the matrix with response to the target analysis, leading to changes in the ionization efficiency of the analysis and enhancing or weakening the signal response of the target analysis (Chatterjee et al. 2016; Shin et al. 2018). Several studies have reported that high protein and fat contents in aquaculture products can easily cause matrix enhancement or suppression (Kruve et al. 2008). In this study, the matrix effect of nine β-lactam antibiotics in different aquaculture products was analyzed. The matrix effect was calculated by the following equation (Chatterjee et al. 2016; Guedes et al. 2016):
Where Sm is slope of the matrix-matched calibration curve, and Ss is the slope of the curve obtained by the standard solvent. The matrix effect can be ignored when its absolute value is less than 20%. When the absolute value of ME is between 20% and 50%, ME is considered medium. If the absolute values of ME are greater than 50%, the matrix effect exerts a significant influence (Chatterjee et al. 2016; Guedes et al. 2016).
2.7 Analyses of real samples
In this study, 20 samples were collected from freshwater aquaculture of C.argus in Guangdong Province. A pooled sample was prepared from at least 3 fishes. The homogenate of muscle was extracted and stored at −20℃ until analysis. The pretreatment method and chromatographic conditions established in this study were used to determine the residues of nine β-lactam antibiotics.
2.8 Data statistics
Data were collected by Waters MassLynx V4.1 software, and the qualitative and quantitative analyses of the test results were performed using the MassLynx V4.1 SCN843 data processing software. Statistical analyses were performed using Origin 2020 (Origin Lab, Northampton, MA, U.S.A.) and Excel 2016. Data were presented as mean ± standard deviation (SD).
3 Results and discussion
3.1 Optimization of instrument conditions
The UHPLC-MS/MS technique was developed for determining nine β-lactam residues in MRM mode. Approximately, 100 ng/mL of the mixed standard solution containing nine β-lactam antibiotics were injected into the mass spectrometry system, and the mass spectrum parameters, such as the decluttering voltage and collision energy, were optimized. The detailed optimized MRM parameters are summarized in Table 1. One parent ion, two daughter ions (Table 1), and the highest intense one was utilized for quantification, while the secondary strength ion was monitored for identification (Moreno-González and García-Campaña 2017).
Adding a certain amount of acid in the mobile phase can improve the ionization efficiency, peak shape, and separation effect in the positive ESI mode (Lopes et al. 2012; Moreno-González and García-Campaña 2017). Therefore, the ammonium acetate (5 mmol/L), formic acid, and acetic acid (0.1% in aqueous phase and organic phase) were optimized, while MeOH and ACN were evaluated as organic phases. The results indicated that the signal intensities of ACN as the organic mobile phase were higher than MeOH. The best ionization was observed with the addition of formic acid. Therefore, the percentages of FA were also optimized, and finally, 0.1% FA was employed as an additive in the aqueous and organic phases. Additionally, the gradient elution was optimized to achieve the best separation and peak efficiencies. At the beginning of the gradient, a high proportion of the aqueous phase (90%) was used to accelerate the elution of hydrophilic interfering substances. Additionally, a high percentage of organic phase was set before the end of the gradient to flush the column and resist interference caused by target residues (Table.A1). The ion chromatograms extracted from a standard solution at 50 ng/mL for nine β-lactam antibiotics (quantification transition was shown) are depicted in Fig.A1.
3.2 Optimization of mobile phase in chromatography
The composition of the dissolving agent is associated with the elution effect and response strength of the target compounds. Moreover, these agents play a significant role in obtaining a larger response strength during detection by dissolving the agents as much as possible. Therefore, the composition of the dissolving solution was optimized in this study. The response strengths of the target compounds in 10% acetonitrile–water, 30% acetonitrile–water, 50% acetonitrile–water, 10% ACN-water (containing 0.1% FA), and 10% ACN-water (containing 0.3 %FA) were compared. The peak area of 50 ng/mL β-lactam antibiotics was relatively high in 10% ACN and 10% ACN containing 0.1% FA (Fig. 1), indicating excellent elution ability of β -lactam antibiotics. The ampicillin, ceftiofur, and cefuroxime exhibited the highest response strength in 10% ACN aqueous (containing 0.1% FA). Therefore, 10% ACN solution with 0.1% FA was used as the dissolving solution in this study.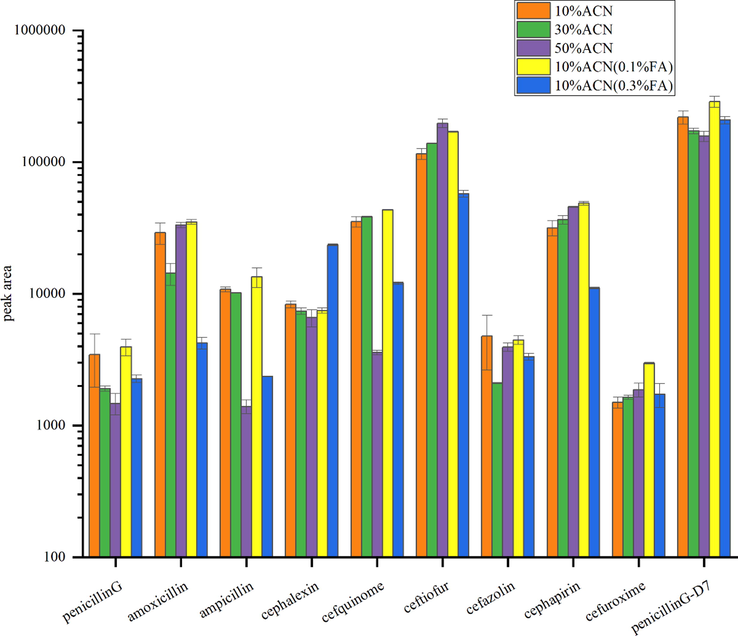
Peak area of β-lactam antibiotics in different solving liquid(50 ng/mL).
3.3 Optimization of extraction solvent
The selection of extraction solvent has significant importance in detecting the extraction effect of the target analytes in the matrix. The commonly used extraction agents for antibiotic extraction are methanol, acetonitrile, and acidified acetonitrile (Dasenaki and Thomaidis 2015). Herein, 100 ng of the β-lactam antibiotic standard mixed solution was mixed with a blank sample of grass carp and the recoveries of methanol, acetonitrile, 1% formic acid acetonitrile, and 1% acetate acid acetonitrile were compared as extraction agents. The spiked recovery results of each compound in these extraction agents are shown in Fig. 2. The recoveries of penicillin, cefquinome, and amoxicillin were lower than 80% upon using methanol as an extraction agent. The recoveries of cephalexin and cefquinome in pure acetonitrile were lower than 70%, while the recovery of ampicillin in acetonitrile with acetic acid was lower than 60%).When 1.0% FA-ACN was used as an extraction agent, the spiked recoveries of nine β-LA ranged from 86.9% to 111.7%, meeting the requirements of drug residue analysis.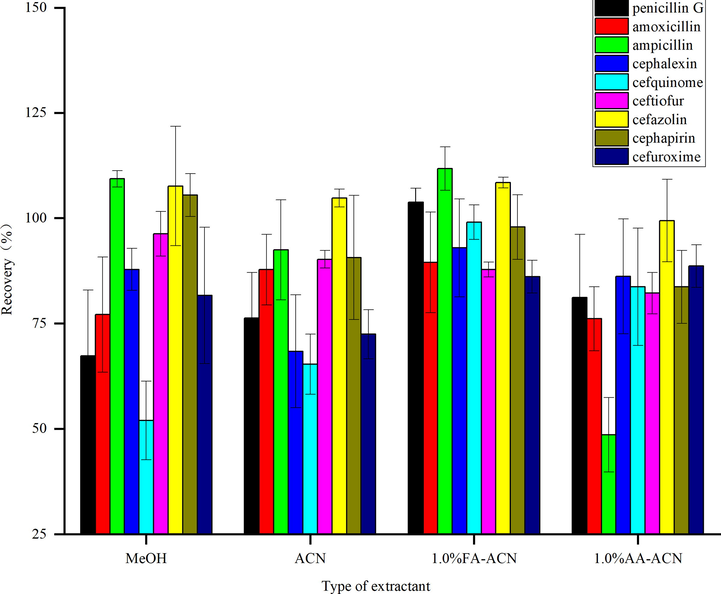
The effect of extractants on recoveries of β-lactam antibiotics (n = 3).
3.4 Selection of a purifying agent
Aquaculture products contain many impurities, such as protein and fat, which could easily induce target based matrix interference. Therefore, purifying the sample and reducing the interference through the impurity peak method are highly necessitated to reduce the target-based matrix co-extract interference. PSA, GCB, C18, neutral alumina and Florisil are the commonly used purifying agents in the QuEChERS method. The PSA is primarily used to remove organic acids, sugars, and fatty acids, while C18 is used for fat and cholesterol removal. GCB can effectively adsorb pigment and neutral alumina can remove fat and pigments. Florian silica is a highly polar and highly active compound, and can adsorb nonpolar and moderately polar compounds (Chatterjee et al. 2016; Ruiz-Medina et al. 2012). The type and dosage of the purifying agents were optimized by the matrix labeling method. 100 ng of β-LA mixed with the standard solution was added into the blank grass carp matrix, extracted with 1.0% formic acid acetonitrile, and purified with 100 mg of five sorbents, respectively. The recovery rates of each adsorbent were compared, and each adsorption step was repeated three times. The results showed that the four adsorbents except for C18 had strong adsorbents for β-LA and the spiked recoveries were less than 70%. When C18 was used as the adsorbent, the spiked recoveries of nine β-lactam antibiotics ranged from 90% to 130% (Fig. 3a). Therefore, C18 was selected as the purifying agent in this method. The dosages of C18 were optimized at the concentrations of 50, 100, 200, 300 and 500 mg, and the recovery rate and purification effect were compared to obtain a better purification effect. When the dosage of the purifying agent was 200 mg, the spiked recoveries of 9 β-lactam antibiotics ranged from 87.44% to 106.22%(Fig. 3b) without any impurity peak interference, meeting the requirements of drug residue detection. Therefore, 200 mg C18 was selected as the adsorbent for QuEChERS in this study.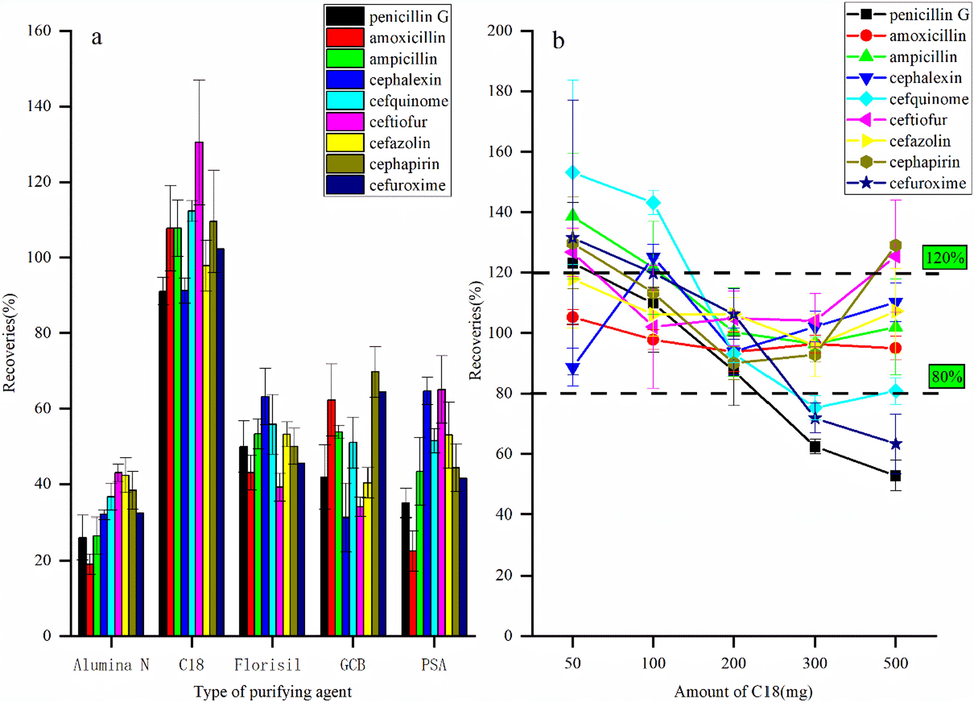
Effect of adsorbent type (a) and dosage (b) on spiked recovery rate (n = 3).
3.5 Method validation
3.5.1 Selectivity, sensibility and linearity
The matrix-matched calibration standard curve was plotted using the mixed standard solutions of blank matrix extract prepared by the series dilution method at the concentrations of 2.0, 5.0, 10, 20, 50, 100, 150 and 200 ng/mL. The chromatographic conditions established in this study were used for determination, and a standard curve was plotted with the ratio of the peak area of the target to the internal standard as Y-coordinate and the concentration as X-coordinate. The results showed that the linear range of 9 β-lactam was between 2.0 and 200 ng/mL (cefuroxime: 5.0–200 ng/mL), and the correlation coefficient (r) was greater than 0.999 (Table 2). The LODs and LOQs are listed in Table 2. The LOD and LOQ of the nine β-lactam antibiotics were measured in the spiked samples and calculated by considering the value 3 and 10 times more than the background noise, respectively (SANTE 2019). The LOD and LOQ of the nine β-lactam antibiotics were in the range of 2.0–5.0 µg/kg and 5.0–10 µg/kg, respectively (Table2).
Herbicides
The range of linearity (ng/mL)
Regression equations
Correlation coefficient(r)
LODa
(µg/kg)LOQb
(µg/kg)
penicillin G
2.0–200
y = 0.126843x0.026551
0.9996
2.0
5.0
amoxicillin
2.0–200
y = 0.068784x + 3.503905 × 10-4
0.9993
2.0
5.0
ampicillin
2.0–200
y = 0.355569x + 0.040544
0.9992
2.0
5.0
cephalexin
2.0–200
y = 0.241674x-0.028225
0.9995
2.0
5.0
cefquinome
2.0–200
y = 3.807627x-0.216908
0.9997
2.0
5.0
ceftiofur
2.0–200
y = 3.224660x-0.224095
0.9997
2.0
5.0
cefazolin
2.0–200
y = 0.278485x-0.014464
0.9992
2.0
5.0
cephapirin
2.0–200
y = 0.129537x-0.004699
0.9994
2.0
5.0
cefuroxime
5.0–200
y = 0.157906x-0.014075
0.9992
5.0
10
3.5.2 Recovery and precision
The feasibility of the method was evaluated by intermediary precision (inter-day) and repeatability (intra-day) as depicted in Table 3. The recoveries and precision of the method in the blank samples of P.orientalis, C.carpio, C.argus, A.nobilis, and C.idella were determined by adding 5, 10, and 20 μg/kg (5, 10, and 20 μg/kg for cefuroxime), respectively. The precision was determined with six replicates at each level and expressed as RSD As depicted in Table3, the average recoveries of the 9 β-lactam antibiotics were in the range of 85.4–113.3%. The RSDs of the antibiotics within the tested concentrations ranged from 0.68 %to 14.9%. Satisfied recoveries (70–120%) and precision (RSD ≤ 20%) were obtained according to the criteria established by SANTE/12682/2019 (SANTE 2019).
Compounds
Addition levels/μg·kg−1
Penaeus 0rientalis
Cyprinus carpio
Channa argus
Aristichthys nobilis
Ctenopharyngodon idella
Inter-day Precision (%)
Recoveries/%
RSD/%
Recoveries/%
RSD/%
Recoveries/%
RSD/%
Recoveries/%
RSD/%
Recoveries/%
RSD/%
penicillin G
5.0
91.8
1.58
103.5
10.6
92.4
9.84
95.1
10.8
97.6
9.18
13.4
10
89.9
1.99
98.8
10.6
100.9
12.1
89.6
10.2
88.9
6.56
3.36
20
99.4
10.8
96.1
3.02
105.8
8.27
99.5
12.8
86.5
4.52
0.68
amoxicillin
5.0
95.3
10.7
101.4
7.39
91.3
7.66
86.9
7.89
92.9
1.16
8.35
10
97.8
8.13
101.2
7.39
86.5
10.4
87.5
8.00
88.4
6.79
4.76
20
92.6
10.3
99.3
1.48
89.0
9.38
100.4
10.3
89.2
9.16
2.20
ampicillin
5.0
96.1
3.02
91.7
8.53
97.8
14.1
94.0
7.36
99.6
8.12
9.26
10
95.0
4.34
96.9
6.75
101.6
13.6
95.4
6.09
92.3
11.5
12.7
20
86.7
4.19
104.7
5.14
104.5
0.75
85.4
9.84
88.6
5.63
13.8
cephalexin
5.0
88.4
6.81
98.3
13.8
99.9
11.6
94.1
13.3
96.9
10.1
5.71
10
88.5
6.87
93.3
6.18
91.6
10.3
97.5
14.9
91.9
7.61
4.17
20
95.5
7.12
101.4
9.00
95.5
11.4
99.4
9.60
93.42
3.67
13.1
cefquinome
5.0
94.5
10.2
100.1
7.69
97.6
9.23
92.7
11.8
87.3
1.41
12.9
10
101.9
11.2
102.5
4.01
107.7
9.63
91.8
12.0
88.4
2.41
11.5
20
106.7
4.62
111.3
13.4
110.8
6.68
103.4
12.9
99.6
8.85
3.98
ceftiofur
5.0
95.3
10.8
101.8
2.02
107.3
11.7
99.5
11.1
94.8
7.77
13.8
10
96.9
9.88
96.6
7.15
113.3
8.79
96.0
7.99
96.9
4.88
11.0
20
105.6
10.1
92.8
3.91
105.2
10.6
95.8
4.67
104.8
11.2
5.68
cefazolin
5.0
98.7
1.57
97.2
8.58
95.8
6.38
100.2
8.81
96.14
5.45
1.89
10
98.7
1.64
103.3
11.0
97.3
4.07
107.1
9.78
96.95
5.68
13.8
20
93.2
7.17
101.2
9.35
101.7
8.35
103.4
9.86
97.5
13.9
1.59
cephapirin
5.0
105.8
8.27
99.6
8.12
94.7
12.6
88.7
5.36
99.6
8.85
10.1
10
104.8
7.37
92.3
11.5
96.5
12.6
95.5
11.4
99.9
8.41
9.53
20
103.5
10.6
88.6
5.63
95.7
2.4
105.3
8.51
89.4
6.98
5.70
cefuroxime
10
94.3
12.1
91.7
8.53
99.3
1.48
91.9
7.63
102.0
9.21
6.38
20
85.6
4.12
96.9
6.75
97.1
2.56
95.7
5.42
102.6
9.53
3.93
40
89.5
4.52
104.7
5.14
92.9
1.16
95.2
1.81
102.5
4.01
1.54
3.5.3 Matrix effect
The matrix effect produced by the aquatic product matrix co-extract could affect the trueness and sensitivity of the method. Therefore, in this study, the matrix standard curve and solvent standard curve were measured under the established pretreatment method and chromatographic determination conditions. The matrix effect was calculated according to the formula in 2.5. The matrix effects of nine β-lactam antibiotics in five aquaculture products (P.orientalis, C.carpio, C.argus, A.nobilis, C.idella) were compared and analyzed and the results are depicted in Fig. 4.The results demonstrated that the substrate effect of β-lactam antibiotics was significantly affected by the substrate type of aquaculture products. The matrix effects of cefazolin, ceftiofur, and cephalexin were within ± 20%, indicating that the matrix effect of these three drugs in the five aquaculture products was within the acceptable range. Generally, the matrix effect of most β-lactam compounds falls between ± 20% and ± 50%. In addition, there is a moderate matrix effect. The matrix effect of cefuroxime in C.argus was less than −50%, showing strong matrix inhibitiory effect (de Sousa et al. 2012; Guedes et al. 2016). Therefore, it is recommended to adopt a matrix calibration curve while determining the actual samples to reduce the matrix effect on the trueness of test results.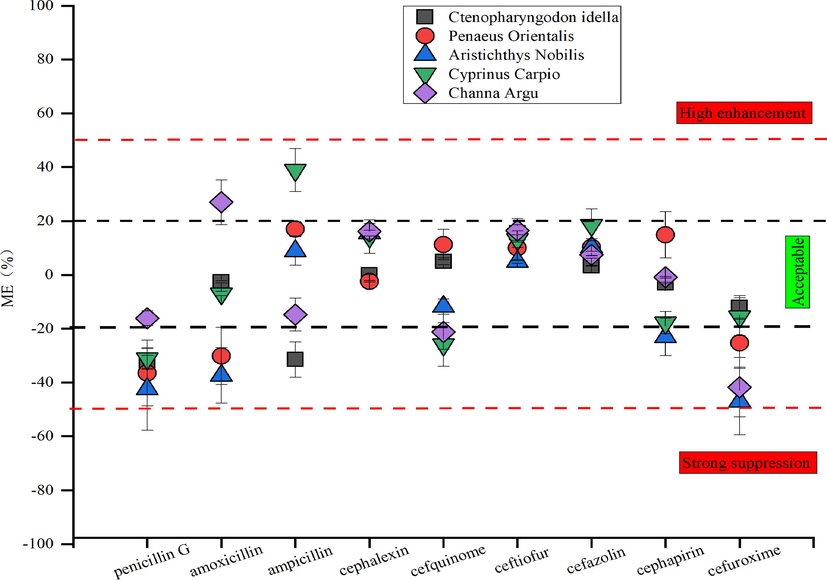
Matrix effects of nine β -lactam compounds in different aquatic product substrates (n = 3).
3.6 Analyses of actual samples
The established QuEChERS method was applied for the residue analysis of β-lactam antibiotics in 20C.argus samples obtained from the local market, and cefuroxime and cefazolin residues were detected (Table.A3). Cefuroxime was detected in 6 samples with the concentration ranging from 11.51 ± 1.03 µg/kg to 75.15 ± 3.61 µg/kg, and the residual concentration in the 4 samples was greater than the MRL value in the cattle muscle (20 µg/kg); while cefazolin was detected in 8 samples with the concentrations ranging from 15.21 ± 2.54 µg/kg to 38.12 ± 2.58 µg/kg.
4 Conclusions
A QuEChERS combined with UHPLC-MS/MS technique was developed to determine nine β-lactam antibiotic residues in aquaculture products, and the feasibility of the method was validated, following the international recommendations. The nine β-lactam antibiotics showed good linearity in the range of 2.0–200 ng/mL, and the correlation coefficient r was greater than 0.999. The LODs and LOQs of the nine β-lactam antibiotics ranged from 2.0 to 5.0 µg/kg and 5.0 to 10 µg/kg, respectively. The recoveries and precision were 85.4%-113.3% (n = 6) and 0.68%-14.9% (n = 6), respectively. The results validated the feasibility of the method for qualitative and quantitative analysis of β-lactam antibiotic residues in aquaculture products due to its simple, rapid, cost-effective, and eco-friendly nature, and the linearity, sensitivity, and precision of the method were in consistent with the requirements of veterinary drug residue analysis.
Author agreement
Lichun li: Conceptualization, Methodology, Software, Investigation, Writing - Original Draft;
Yi Yin: Funding acquisition,Supervision,Project administration; Writing-Review & Editing;Guangming Zheng: Supervision, Writing-Review & Editing, Formal analysis;
Shugui Liu: Resources, Investigation, Validation, Formal analysis;
Cheng Zhao: Resources, Investigation, Validation, Formal analysis;
Wenping Xie: Investigation, Validation, Writing-Review & Editing;
Lisha Ma: Investigation, Validation, Writing-Review & Editing;
Qi Shan: Software, Writing-Review & Editing;
Xiaoxin Dai: Writing-Review & Editing;
Linting Wei: Writing-Review & Editing.With kind regards;
JiaWei Lin: Writing-Review & Editing.With kind regards.
Acknowledgments
This research was supported by the National Quality and Safety Project of the Aquaculture products of China (Grant number:GJFP201700903), Science and Technology Program of Guangzhou, China (202102020983); Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams (2020KJ150) and China-ASEAN Maritime Cooperation Fund (CAMC-2018F).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A three phase dispersive liquid-liquid microextraction tech nique for the extraction of antibiotics in milk. Microchim. Acta. 2012;179:179-184.
- [CrossRef] [Google Scholar]
- Arroyo-Manzanares, N., Lara, F. J., Airado-Rodríguez, D., Gámiz-Gracia, L. & A, A. G. 2015. Determination of sulfonamides in serum by on-line solid-phase extraction coupled to liquid chromatography with photoinduced fluorescence detection. Talanta 138, 258-262. doi:10.1016/j.talanta.2015.03.012.
- Development of a Novel and Automated Fluorescent Immunoassay for the Analysis of β-Lactam Antibiotics. J. Agric. Food. Chem.. 2005;53:6635-6642.
- [CrossRef] [Google Scholar]
- Quantitative determination of penicillin V and amoxicillin in feed samples by pressurised liquid extraction and liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal.. 2009;49:289-294.
- [CrossRef] [Google Scholar]
- Can changes in renal function predict variations in β-lactam concentrations in septic patients? Int. J. Antimicrob. Agents. 2013;42:422-428.
- [CrossRef] [Google Scholar]
- Multiresidue analysis of multiclass pesticides and polyaromatic hydrocarbons in fatty fish by gas chromatography tandem mass spectrometry and evaluation of matrix effect. Food Chem.. 2016;196:1-8.
- [CrossRef] [Google Scholar]
- Quantification of 16 β-lactams in chicken muscle by QuEChERS extraction and UPLC-Q-Orbitrap-MS with parallel reaction monitoring. Journal of Pharmaceutical and Biomedical Analysis. 2017;145:525-530.
- [CrossRef] [Google Scholar]
- China Food and Drug Administration. 2019. National Food Safety Standard–Maximum Residue Limits for Pesticides in Food,
- Simultaneous Determination of the Novel Antithrombotic Agent, Acetylsalicylic Acid Maltol Ester (Aspalatone) and its Metabolites in Rat Plasma and Urine by HPLC. J. Liq. Chromatogr. Relat. Technol.. 2005;28:2403-2419.
- [CrossRef] [Google Scholar]
- Commission, E. 2010. COMMISSION DECISION (EU) No 37/2010 of December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin.
- Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta. 2015;880:103-121.
- [CrossRef] [Google Scholar]
- Evaluation of matrix effect on the GC response of eleven pesticides by PCA. Food Chem.. 2012;135:179-185.
- [CrossRef] [Google Scholar]
- Simultaneous determination of quinolone and β-lactam residues in raw cow milk samples using ultrasound-assisted extraction and dispersive-SPE prior to UHPLC MS/MS analysis. Food Control. 2016;382–393
- [CrossRef] [Google Scholar]
- An unprecedented reversible mode of action of β-lactams for the inhibition of human fatty acid amide hydrolase (hFAAH) Eur. J. Med. Chem.. 2013;60:101-111.
- [CrossRef] [Google Scholar]
- Matrix effect in guava multiresidue analysis by QuEChERS method and gas chromatography coupled to quadrupole mass spectrometry. Food Chem.. 2016;199:380-386.
- [CrossRef] [Google Scholar]
- β-lactam antibiotics residues analysis in bovine milk by LC-ESI-MS/MS: a simple and fast liquid–liquid extraction method. Food Additives & Contaminants. 2012;29:497-507.
- [CrossRef] [Google Scholar]
- Analytical methodologies for the detection of β-lactam antibiotics in milk and feed samples. TrAC, Trends Anal. Chem.. 2009;28:729-744.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted matrix solid phase dispersive extraction for the simultaneous analysis of β-lactams (four penicillins and eight cephalosporins) in milk by high performance liquid chromatography with photodiode array detection. J. Sep. Sci.. 2012;35:2599-2607.
- [CrossRef] [Google Scholar]
- Matrix effects in pesticide multi-residue analysis by liquid chromatography–mass spectrometry. J. Chromatogr. A. 2008;1187:58-66.
- [CrossRef] [Google Scholar]
- Lara, F. J., Olmo-Iruela, M. D., Cruces-Blanco, C., Quesada-Molina, C. & A, A. G. 2012. Advances in the determination of β-lactam antibiotics by liquid chromatography. Trac Trends in Analytical Chemistry 38. doi:10.1016/j.trac.2012.03.020.
- Multiclass analysis of 25 veterinary drugs in milk by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem.. 2018;257:259-264.
- [CrossRef] [Google Scholar]
- Solid phase extraction using magnetic core mesoporous shell microspheres with C18-modified interior pore-walls for residue analysis of cephalosporins in milk by LC-MS/MS. Food Chem.. 2014;150:206-212.
- [CrossRef] [Google Scholar]
- Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B. 2012;895–896:39-47.
- [CrossRef] [Google Scholar]
- Multi residue determination of the penicillins regulated by the European Union, in bovine, porcine and chicken muscle, by LC-MS/MS. Food Chem.. 2012;135:2612-2621.
- [CrossRef] [Google Scholar]
- Salting-out assisted liquid–liquid extraction coupled to ultra-high performance liquid chromatography–tandem mass spectrometry for the determination of tetracycline residues in infant foods. Food Chem.. 2017;221:1763-1769.
- [CrossRef] [Google Scholar]
- Quechers methodologies as an alternative to solid phase extraction (SPE) for the determination and characterization of residues of cephalosporins in beef muscle using LC-MS/MS. J. Chromatogr. B. 2012;899:57-65.
- [CrossRef] [Google Scholar]
- Optimisation and validation of a quantitative and confirmatory LC-MS method for multi-residue analyses of β-lactam and tetracycline antibiotics in bovine muscle. Food Additives & Contaminants: Part A. 2012;29:541-549.
- [CrossRef] [Google Scholar]
- Automated optosensor for the determination of carbaryl residues in vegetable edible oils and table olive extracts. J. Food Compos. Anal.. 2012;26:66-71.
- [CrossRef] [Google Scholar]
- SANTE. 2019. Guidance document on analytical quality control and method validation procedures for pesticides residues and analysis in food and feed (2019), from https://www.eurl-pesticides.eu/userfiles/file/EurlALL/ AqcGuidance_SANTE_2019_12682.pdf.
- Development and validation of a modified QuEChERS protocol coupled to LC–MS/MS for simultaneous determination of multi-class antibiotic residues in honey. Food Chem.. 2016;190:982-989.
- [CrossRef] [Google Scholar]
- Multi-residue Determination of Veterinary Drugs in Fishery Products Using Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods. 2018;11:1815-1831.
- [CrossRef] [Google Scholar]
- Development of a Methodology for the Simultaneous Analysis of Multiclass Contaminants in Milk. Food Anal. Methods. 2021;14:1075-1086.
- [CrossRef] [Google Scholar]
- Comparison of resistance to third-generation cephalosporins in Shigella between Europe-America and Asia-Africa from 1998 to 2012. Epidemiol. Infect.. 2015;143:2687-2699.
- [CrossRef] [Google Scholar]
- Multi-class analysis of veterinary drugs in eggs using dispersive-solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem.. 2021;334:127598
- [CrossRef] [Google Scholar]
- Development of a Method for the Analysis of Multiclass Antibiotic Residues in Milk Using QuEChERS and Liquid Chromatography-Tandem Mass Spectrometry. Foodborne Pathogens and Disease. 2015;12:693-703.
- [CrossRef] [Google Scholar]
- Dispersive Micro-Solid Phase Extraction Combined with High-Performance Liquid Chromatography for the Determination of Three Penicillins in Milk Samples. Food Anal. Methods. 2015;8:1079-1087.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103912.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







