Translate this page into:
Chemical profiling of Verbena officinalis and assessment of its anti-cryptosporidial activity in experimentally infected immunocompromised mice
⁎Corresponding author at: Medicinal Chemistry Department, Theodor Bilharz Research Institute, Kornaish El-Nile, Warrak El-Hadar, Imbaba (P.O. 30), Giza 12411, Egypt. m.ghareeb@tbri.gov.eg (Mosad A. Ghareeb)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
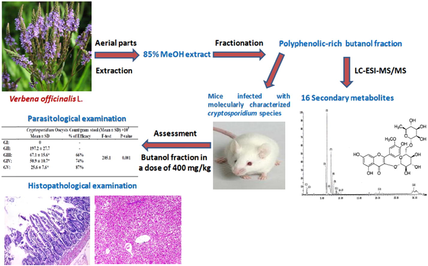
Verbena officinalis is an intrinsic supplier for biologically active metabolites comprising flavonoids, phenolic acids, organic acids, phenylethanoids, and coumarins. Verbena officinalis n-butanol extract was proved to have an effective anti-cryptosporidial activity in the infected immunocompromised mice exceeding the gold standard drug nitazoxanide. Verbena officinalis n-butanol extract was reported to have a promising anti-inflammatory effect, alleviating pathological changes in the small intestine and the liver of infected immunocompromised mice, exceeding the gold standard drug nitazoxanide. The combination of Verbena officinalis n-butanol extract, and nitazoxanide gave the best results.
Abstract
Cryptosporidiosis is a global zoonotic infection that causes water-borne epidemics of diarrhea. Nevertheless, there are few available therapies for cryptosporidiosis. However, the gold standard drug nitazoxanide (NTZ) has limited efficacy in malnourished and immunocompromised patients. Furthermore, Verbena officinalisL. is a herbal plant widely used in traditional medicine to cure several health disorders and is recognized to possess numerous therapeutic applications. In the present study, the phytochemical composition of aerial part extract from Verbena officinalis was investigated via LC-ESI-MS/MS.
Furthermore, the anti-cryptosporidial activity was also performed using an animal model. Fifty mice were divided into 5 groups; GI: non-infected (Negative control), GII: infected non treated (positive control), GIII: infected, treated with NTZ, GIV: infected, treated with V. officinalis n-butanol extract, GV: infected, treated with a combination of NTZ and V. officinalis. Parasitological examination revealed a highly significant difference (P-value < 0.001) between GIII, GIV, and GV compared to GII regarding the mean number of Cryptosporidium spp. oocyst in the stool. Moreover, GV showed the best efficacy with a percentage of 87%. Also, histopathological examination showed variable degrees of improvement in the villous broadening, and the inflammatory infiltrates in the small intestine with a reduction of hepatocyte degeneration and mononuclear infiltration in GIII, GIV, and GV compared to GII, with the best results seen in GV. Additionally, the chemical profiling of n-butanol extract identified 16 secondary metabolites comprising flavonoids, phenolic acids, phenylethanoids, and coumarins. In conclusion, V. officinalis is an intrinsic supplier of biologically active metabolites with outstanding anti-parasitic and possible anti-inflammatory effects.
Keywords
Verbena officinalis
Anti-cryptosporidial
Nitazoxanide
LC-ESI-MS/MS
Polyphenolics

- NTZ
-
Nitazoxanide
- LC-ESI-MS/MS
-
Liquid Chromatography Electrospray Ionization Mass Spectrometry
- FDA
-
Food and Drug Administration
- TBRI
-
Theodor Bilharz Research Institute
- PCR
-
Polymerase Chain Reaction
- PCR-RFLP
-
Polymerase Chain Reaction-Restriction Fragment Length Polymorphism
- DNA
-
Deoxyribonucleic Acid
- COWP
-
Cryptosporidium Oocyst Wall Protein
- UV
-
Ultraviolet
- dpi
-
day post-infection
- PBS
-
Phosphate-Buffered Saline
- MZN
-
Modified Zheil Nelsen
- H&E
-
Hematoxylin and Eosin
- SD
-
Standard Deviation
- ANOVA
-
Analysis of variance
- REC
-
Research Ethical Committee
- m/z
-
mass-to-charge
- Rt
-
Retention time
- Rha
-
Rhamnose
- Glu
-
Glucose
Abbreviations
1 Introduction
Cryptosporidium spp. is an important coccidian protozoan parasite that can infect the small intestinal microvilli region of epithelial cells in man and other mammals' alimentary tracts (Bayoumy et al., 2020). Critical complications were reported as a consequence of cryptosporidiosis in the immunodeficient patients, including severe diarrhea, which may lead to dehydration, electrolyte imbalance, and liver and lung problems (Guk et al., 2005). Unfortunately, despite the global burden of cryptosporidiosis, scanty therapies are still available. Serious infections can only be manipulated through supportive therapy like fluid and electrolyte replacement besides the specific treatment of nitazoxanide (NTZ), the only approved drug by the Food and Drug Administration (FDA) in the United States. Regrettably, NTZ has limited efficacy in malnourished children and seems to have no efficacy in immunodeficient patients (Abubakar et al., 2007a,b; Amadi et al., 2009; Cabada and White, 2010).
Parasitic infections have been treated by numerous medicinal herbs worldwide for hundreds of years (Behnke et al., 2008). The genus Verbena comprises nearly 250 species; these species are mostly present in tropical and subtropical regions like Canada, Southern Chile, Kashmir, Bhutan, Pakistan, India, Africa, and Europe (Ahmed et al., 2017). Verbena officinalis L. (V. officinalis) of the family Verbenaceae is an everlasting herb that has been used for many years ago in herbal medicine and as health supplements (Zhang et al., 2000; Mengiste et al., 2014). The plant has been used in folk medicine for the treatment of many health problems like inflammations (Calvo et al., 1998; Deepak and Handa, 2000a), Alzheimer's disease (Lai et al., 2006), renal dysfunction (Grases et al., 1994), and fungal infections (Casanova et al., 2008). Phytochemically, several classes of secondary metabolites, have been isolated or identified in different parts of the plant, including pentacyclic triterpenoids (Deepak and Handa, 2000b), iridoids (Deepak and Handa, 2000b; Müller et al., 2004; Tian et al., 2005; Rehecho et al., 2011; Shu et al., 2014), phenylpropanoid glycosides (Deepak and Handa, 2000b; Müller et al., 2004), steroidal saponins (Zhang et al., 2000), flavonoids (Müller et al., 2004; Tian et al., 2005; Chen et al., 2006; Rehecho et al., 2011), phenolic acid derivatives (Rehecho et al., 2011), and volatile constituents (Shamsardakani et al., 2010). Different parts of V. officinalis were reported to possess a broad spectrum of biological activities like antibacterial (Ahmed et al., 2017), anti-inflammatory (Speroni et al., 2007), antifungal (Casanova et al., 2008), antioxidant (Casanova et al., 2008; Rehecho et al., 2011), and hepatoprotective (Singh et al., 1998). Also, it was successfully tested as a novel anti-parasitic herbal treatment for Trichomonas vaginalis (Brandelli et al., 2013) and ascariasis (Teklay et al., 2013).
Despite its widespread use and many interesting medicinal properties, there is no available literature about the effect of V. officinalis against immunosuppressed mice infected with Cryptosporidium spp. Besides, the pharmacological and biological studies of the herb have not been sufficiently explored. Therefore, this study aims to make a breakthrough in chemical profiling and the anti-cryptosporidial effect of the V. officinalis n-butanol extract.
2 Materials and methods
2.1 Plant material
Aerial parts (600 g) of Verbena officinalis were collected from the Qalyubia governorate, Egypt, in March 2020. The plant was kindly identified and authenticated in the Herbarium of El-Orman Botanical Garden, Giza, Egypt. A voucher specimen was deposited at Medicinal Chemistry Department, TBRI, under accession number (V.o.ap.2020).
2.2 Extraction and fractionation
The collected materials were ground and extracted with 85% methanol (4 × 2 L) at room temperature. The combined extracts were filtered and evaporated under a vacuum using an evaporator at 40 °C. The crud extract (107.93 g) was defatted with petroleum ether (60–80 °C). The defatted methanolic extract underwent successive fractionation via dichloromethane, ethyl acetate, and n-butanol to afford 9.38, 10.63, 7.02, 26.9, and 38.74 g, respectively for petroleum ether, dichloromethane, ethyl acetate, n-butanol, and water fractions (Mohammed et al., 2019).
2.3 Preliminary phytochemical investigations
The obtained fractions were subjected to phytochemical investigations using preliminary phytochemical screening and two-dimensional paper chromatography (2D-PC) tests to assign the nature and distribution of secondary metabolites in each fraction (Mabry et al., 1970; Edeoga et al., 2005). The tests showed that the n-butanol fraction is the richest in secondary metabolites; among them are phenolic compounds. Accordingly, the n-butanol fraction was selected among all the tested fractions for further pharmacological and phytochemical examinations.
2.4 LC-ESI-MS/MS analysis
The chemical ingredients of the aerial parts' extract were identified using a Thermo Finnigan (Thermo electron Corporation, OK, USA), coupled with an LCQ Duo ion trap mass spectrometer with an ESI source in negative ionization mode (ThermoQuest Corporation, Austin, TX, USA) as previously described (Ghareeb et al., 2019).
2.5 Parasitological studies
2.5.1 Animals
Female, white Albino mice of CDI strain in which Cryptosporidium spp. have a complete successful cycle. This CD1 strain of mice has a uniform genotype that allows for easier and more credible interpretation of results, with a much lower liability to acquire and keep genetic abnormalities that allow for dangerous illness than the commonly used inbred strains. In addition, CD1 strain shows minimal evasion, struggling and aggressive behavior when caught and held (Aldinger et al., 2009; Arabo et al., 2014; Hsieh et al., 2017; Patil et al., 2009). Mice with an average age of 4–6 weeks old and an average weight of 20–25 g were obtained from Theodor Bilharz Research Institute's (TBRI) animal house. Animal experiments were performed in the Biological Unit of TBRI after the approval of the institutional ethical committee. All performed experiments on animals were conducted according to internationally valid guidelines. The animals were kept in suitable sanitary conditions away from direct sunlight in a well-ventilated plastic cage with clean wood-chip bedding in conditioned rooms (20–24 °C).
All the experiments on animals were carried out according to internationally valid guidelines after the approval of the Institutional Research Ethical Committee of the Theodor Bilharz Research Institute (TBRI-REC). The serial number of the protocol is PT (5 8 6).
2.5.2 Experimental design
Fifty laboratory-bred white albino female mice were used in this study. Mice were immunosuppressed and divided into 5 groups; 10 mice each:
Group I: non-infected (Negative control).
Group II: infected non-treated (Positive control).
Group III: infected and treated with NTZ.
Group IV: infected and treated with V. officinalis n-butanol extract.
Group V: infected and treated with a combination of V. officinalis n-butanol extract and NTZ.
2.5.3 Immunosuppression
Mice were given 250 ug/kg body weight (bw)/day of dexamethasone sodium phosphate through an oral route by gavages using an esophageal tube. Dexamethasone sodium phosphate was given daily for 2 weeks prior to oral inoculation with Cryptosporidium oocysts with weekly maintenance throughout the experiment for all groups (Tarazona et al., 1998).
2.5.4 Infection
C. parvum oocysts were obtained from the Animal Research Institute in Giza governorate, Egypt. Molecular identification and genotyping of C. parvum were performed through nested PCR and PCR-RFLP. Mice were orally infected with C. parvum oocysts via gavage using an esophageal tube (day 0). The infection dose for (GII-GV) was about 3 × 103C. parvum oocysts/mouse dissolved in 200 μl of PBS (Benamrouz et al., 2012; El-Wakil et al., 2021). Animals were put individually in special cages to collect the fecal samples. Fecal pellets were collected individually from each mouse, and the mean oocyst shedding per group was calculated one week after infection of the mice [7th-day post-infection (dpi)] to establish infection.
2.5.5 Drugs administration: Was conducted through oral gavage at seventh dpi
- GIII received NTZ in a dose of 100 mg/kg bw daily for 5 consequent days (Li et al., 2003). Nitazoxanide was obtained in the form of (nanazoxid) (100 mg/5 ml suspension) manufactured and provided by [Medizen Pharmaceutical industries for Utopia Pharmaceuticals].
The doses were calculated by extrapolation of human therapeutic doses to animal doses according to the table of (Barnes and Paget, 1965).
-
-
GIV received V. officinalis n-butanol extract in a dose of 400 mg/kg bw for 5 consequent days (Sisay et al., 2019).
Sisay et al. proved that both 80% methanol extracts of the roots (R-80ME) and the leaves of V. officinalis produced neither overt toxicity nor death during the 14 days observation period following oral administration of a single dose of 2000 mg/kg. The absence of mortality and any sign of overt toxicity up to 5 times the maximum effective dose of the extract suggested that the crude extracts have a wider safety margin and median lethal dose (LD50) >2000 mg/kg in the mice model.
-
-
GV received both (NTZ & V. officinalis n-butanol extract) for 5 consequent days.
2.5.6 Parasitological examination
-
-
Fecal pellets were collected at 12th dpi (the end of the treatment regimen) subjected to staining with modified Zheil Nelsen (MZN) stain according to (Henricksen and Pohlenz, 1981) using the oil immersion lens (x100).
-
-
The number of parasites was expressed per gram of feces (Benamrouz et al., 2012). The percentage of the efficacy of each drug was calculated using the following equation (Hosking et al., 1996):
2.5.7 Molecular identification of C. parvum
Molecular identification of the parasite up to genotyping was performed because the detection limits of conventional microscopy for Cryptosporidium smears with acid-fast stain have been reported to be as low as 50 000 to 500 000 oocysts per gram of human feces, resulting in low levels of infection or sporadic shedding possibly going unnoticed when conventional methods of detection are used (Weber et al., 1991).
DNA extraction: DNA extraction was performed from the stool specimens of the different study groups through QIAamp Fast DNA Stool Mini Kit (cat. no. 51604; Qiagen-Germany). The freeze/thaw cycle was allowed 5 times each for 5 min to rupture the oocyst wall. The DNA was isolated from the specimens by a fast spin-column technique; the PCR inhibitors were removed from DNA through Inhibit EX Buffer, guided by the manufacturer's instructions.
All primers specific to the C. parvum target genes used in this research were precisely illustrated according to previous reports. The primary PCR primers were BCOWPF (5′-ACCGCTTCTCAACAACCATCTTGTCCTC-3′) and BCOWPR (5′-CGCACCTGT TCCCACTCAATGTAAACCC-3′); while the nested PCR primers were Cry-15 (5′-GTAGATAATGGAAGAGATTGTG-3′) and Cry-9 (5′-GGACTGAAATACAGGC ATTATCTTG-3′) (Spano et al., 1997 a,b; Yu et al., 2009). The anticipated product sizes were 769-bp and 553-bp, consecutively. Extracted DNA (2.5 μl) in 1x PCR buffer was mixed with MgCl2 (1.5 mM), dNTP (250 μM), primers (10 pmoles), and Taq DNA polymerase (1.25 units) to reach a volume of 25 μl. Thirty cycles of denaturation were performed for the samples at 94 °C for 1 min, annealing at 65 °C for 1 min and extension at 72 °C for 10 min. The reaction mentioned above is called the extended COWP reaction. A 2.5 μl of the extended COWP product was used for the nested COWP reaction to amplify the 553-bp gene fragment (Spano et al., 1997b). Negative and positive controls were present in all PCR procedures.
Positive samples by nPCR were then subjected to digestion using RFLP with restriction enzyme cleavage Rsa I to detect Cryptosporidium genotypes. A mixture was produced, including 10 μl nested PCR product with 17 μl Nuclease-free water, 2 μl Green buffer and 1 μl RsaI Enzyme. The mixture's ingredients were exposed to gentle mixing and spinning for a few seconds. Incubation of samples was performed at 37 °C for 5 min. Checking the results was achieved by Agarose gel electrophoresis and UV light trans-illumination to visualize the digested fragments. Genotyping was detected by the digestion pattern; Genotype 1 was considered if Rsa I digestion resulted in 4 bands: 34 bp, 106 bp, 125 bp and 285 bp, and genotype 2 if Rsa I digestion resulted in 3 bands: 34 bp, 106 bp and 410 bp.
2.6 Histopathological examination
The sacrifice of all mice was performed 12 days post-infection (dpi) by euthanasia through decapitation (Boivin et al., 2016). The small intestine and liver specimens were obtained, then fixed in 10% buffered formalin solution, embedded in paraffin wax blocks that were sectioned and then stained in the pathology department, TBRI. Staining was performed using Hematoxylin and Eosin (H&E) to examine the pathological changes (Feldman and Wolfe, 2014). An expert histopathologist reported intestinal inflammation and hepatocyte degeneration through subjective qualitative evaluation.
2.7 Statistical analysis
Data were analyzed using Microsoft Excel 2016 and the statistical package for social science 'IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). Continuous normally distributed variables were represented as mean ± SD, with a 95% confidence interval. To compare the means of normally distributed variables between groups, the ANOVA test was performed and followed with Dunnett T3 as a post-hoc test. A P-value was set at 0.05, P > 0.05 non-significant, P-value < 0.05 significant and P-value < 0.001 highly significant (Peat and Barton, 2005).
3 Results
3.1 Molecular identification of C. parvum oocyst
The sample used for animal infection was positive in PCR reaction, and the nested PCR product size was about 553-bp. The restriction enzyme Rsa I digestion of nPCR product targeting COWP gene revealed the presence of Cryptosporidium parvum genotype 2. The genotype digestion products at 34, 106 and 410 bp are shown in Fig. 1.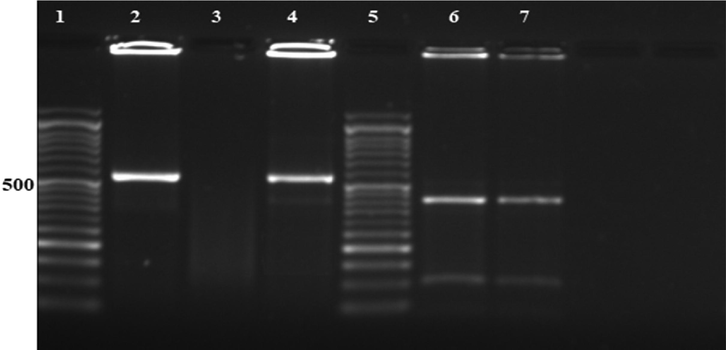
Agarose gel electrophoresis showing: Lane 1: 50 bp DNA molecular weight marker, Lane 2: Positive control, Lane 3: Negative control, Lane 4: Positive sample of nested PCR products targeting COWP gene of Cryptosporidium at 553 bp, Lane 5: 50 bp DNA molecular weight marker, Lane 6: RFLP products of positive control after digestion with RsaI endonuclease (C. parvum genotype 2 digestion products at 410, 106 and 34 (too small to be detected) bp. Lane 7: RFLP products of the positive sample after digestion with RsaI endonuclease (C. parvum genotype 2 digestion products at 410, 106 and 34 (too small to be detected) bp.
3.2 Parasitological examination
The parasitological investigations revealed that there is a highly significant difference (P-value < 0.001) between GIII, GIV, and GV when compared to the GII (infected non-treated positive control) regarding the mean number of Cryptosporidium spp. oocyst in the stool. GV showed the best efficacy with percentages of 87% (Table 1). Cryptosporidium oocysts Count/gram stool (Mean ± SD) × 103. *. The mean difference is significant at the 0.05 level.
GI:
GII:
GIII:
GIV:
GV:
Mean ± SD
0
197.2 ± 27.7
67.1 ± 15.6
50.9 ± 10.7
25.6 ± 7.6
% of Efficacy
-
-
66%
74%
87%
ANOVA
205.1 (P. value = 0.001**)
Post-Hoc test Dunnett T3
GII:
-
-
0.001**
0.001**
0.001**
GIII:
–
–
–
0.4
0.01*
GIV:
–
–
–
–
0.015*
GV:
–
–
–
–
–
3.3 Histopathological examination
GI: Histopathological sections of the small intestine showed normal villous architecture with an intact brush-border and normal mucin secreting pattern (Fig. 2a). Hepatic sections showed preserved hepatic lobular architecture (Fig. 3a).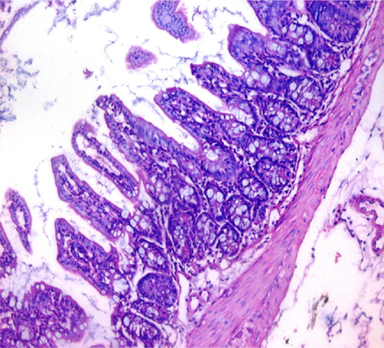
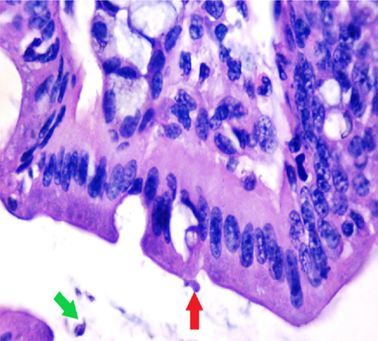
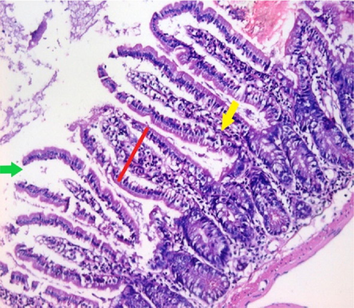
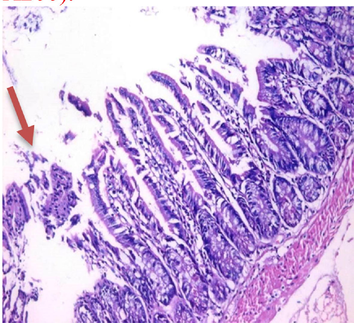
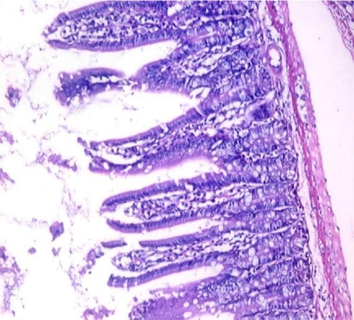
small intestinal (SI) sections from the experimental mice of the different study groups: Fig. 2a: section from SI of GI, Fig. 2b,c: section from SI of GII, Fig. 2d: section from SI of GIII, Fig. 2e: section from SI of GIV, Fig. 2f: section from SI of GV. Fig. 2a. Small intestinal sections demonstrate normal intestinal villi (H&E stain, X200). Fig. 2b. Small intestinal sections reveal significant villous broadening (red line). There was dense infiltration by mononuclear inflammatory cells (yellow arrows) and degeneration of the villous tip-regions (green arrows) (H&E stain, X200). Fig. 2c. Histopathological examination of sections from the small intestine shows many adherents (red arrow) and separate (green arrow) cryptosporidium oocysts. (H&E stain, X1000). Fig. 2d. GIII small intestinal sections reveal moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow) and focal degeneration of the villous tip regions (green arrow) (H&E stain, X200). Fig. 2e. Small intestinal sections show improved pathological changes in the villi with mild expansion by mononuclear inflammatory cells as shown by (red arrow). (H&E stain, X200). Fig. 2f. Small intestinal section demonstrates a nearly normal villous pattern (H&E stain, X200).
small intestinal (SI) sections from the experimental mice of the different study groups: Fig. 2a: section from SI of GI, Fig. 2b,c: section from SI of GII, Fig. 2d: section from SI of GIII, Fig. 2e: section from SI of GIV, Fig. 2f: section from SI of GV. Fig. 2a. Small intestinal sections demonstrate normal intestinal villi (H&E stain, X200). Fig. 2b. Small intestinal sections reveal significant villous broadening (red line). There was dense infiltration by mononuclear inflammatory cells (yellow arrows) and degeneration of the villous tip-regions (green arrows) (H&E stain, X200). Fig. 2c. Histopathological examination of sections from the small intestine shows many adherents (red arrow) and separate (green arrow) cryptosporidium oocysts. (H&E stain, X1000). Fig. 2d. GIII small intestinal sections reveal moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow) and focal degeneration of the villous tip regions (green arrow) (H&E stain, X200). Fig. 2e. Small intestinal sections show improved pathological changes in the villi with mild expansion by mononuclear inflammatory cells as shown by (red arrow). (H&E stain, X200). Fig. 2f. Small intestinal section demonstrates a nearly normal villous pattern (H&E stain, X200).
small intestinal (SI) sections from the experimental mice of the different study groups: Fig. 2a: section from SI of GI, Fig. 2b,c: section from SI of GII, Fig. 2d: section from SI of GIII, Fig. 2e: section from SI of GIV, Fig. 2f: section from SI of GV. Fig. 2a. Small intestinal sections demonstrate normal intestinal villi (H&E stain, X200). Fig. 2b. Small intestinal sections reveal significant villous broadening (red line). There was dense infiltration by mononuclear inflammatory cells (yellow arrows) and degeneration of the villous tip-regions (green arrows) (H&E stain, X200). Fig. 2c. Histopathological examination of sections from the small intestine shows many adherents (red arrow) and separate (green arrow) cryptosporidium oocysts. (H&E stain, X1000). Fig. 2d. GIII small intestinal sections reveal moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow) and focal degeneration of the villous tip regions (green arrow) (H&E stain, X200). Fig. 2e. Small intestinal sections show improved pathological changes in the villi with mild expansion by mononuclear inflammatory cells as shown by (red arrow). (H&E stain, X200). Fig. 2f. Small intestinal section demonstrates a nearly normal villous pattern (H&E stain, X200).
small intestinal (SI) sections from the experimental mice of the different study groups: Fig. 2a: section from SI of GI, Fig. 2b,c: section from SI of GII, Fig. 2d: section from SI of GIII, Fig. 2e: section from SI of GIV, Fig. 2f: section from SI of GV. Fig. 2a. Small intestinal sections demonstrate normal intestinal villi (H&E stain, X200). Fig. 2b. Small intestinal sections reveal significant villous broadening (red line). There was dense infiltration by mononuclear inflammatory cells (yellow arrows) and degeneration of the villous tip-regions (green arrows) (H&E stain, X200). Fig. 2c. Histopathological examination of sections from the small intestine shows many adherents (red arrow) and separate (green arrow) cryptosporidium oocysts. (H&E stain, X1000). Fig. 2d. GIII small intestinal sections reveal moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow) and focal degeneration of the villous tip regions (green arrow) (H&E stain, X200). Fig. 2e. Small intestinal sections show improved pathological changes in the villi with mild expansion by mononuclear inflammatory cells as shown by (red arrow). (H&E stain, X200). Fig. 2f. Small intestinal section demonstrates a nearly normal villous pattern (H&E stain, X200).
small intestinal (SI) sections from the experimental mice of the different study groups: Fig. 2a: section from SI of GI, Fig. 2b,c: section from SI of GII, Fig. 2d: section from SI of GIII, Fig. 2e: section from SI of GIV, Fig. 2f: section from SI of GV. Fig. 2a. Small intestinal sections demonstrate normal intestinal villi (H&E stain, X200). Fig. 2b. Small intestinal sections reveal significant villous broadening (red line). There was dense infiltration by mononuclear inflammatory cells (yellow arrows) and degeneration of the villous tip-regions (green arrows) (H&E stain, X200). Fig. 2c. Histopathological examination of sections from the small intestine shows many adherents (red arrow) and separate (green arrow) cryptosporidium oocysts. (H&E stain, X1000). Fig. 2d. GIII small intestinal sections reveal moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow) and focal degeneration of the villous tip regions (green arrow) (H&E stain, X200). Fig. 2e. Small intestinal sections show improved pathological changes in the villi with mild expansion by mononuclear inflammatory cells as shown by (red arrow). (H&E stain, X200). Fig. 2f. Small intestinal section demonstrates a nearly normal villous pattern (H&E stain, X200).
small intestinal (SI) sections from the experimental mice of the different study groups: Fig. 2a: section from SI of GI, Fig. 2b,c: section from SI of GII, Fig. 2d: section from SI of GIII, Fig. 2e: section from SI of GIV, Fig. 2f: section from SI of GV. Fig. 2a. Small intestinal sections demonstrate normal intestinal villi (H&E stain, X200). Fig. 2b. Small intestinal sections reveal significant villous broadening (red line). There was dense infiltration by mononuclear inflammatory cells (yellow arrows) and degeneration of the villous tip-regions (green arrows) (H&E stain, X200). Fig. 2c. Histopathological examination of sections from the small intestine shows many adherents (red arrow) and separate (green arrow) cryptosporidium oocysts. (H&E stain, X1000). Fig. 2d. GIII small intestinal sections reveal moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow) and focal degeneration of the villous tip regions (green arrow) (H&E stain, X200). Fig. 2e. Small intestinal sections show improved pathological changes in the villi with mild expansion by mononuclear inflammatory cells as shown by (red arrow). (H&E stain, X200). Fig. 2f. Small intestinal section demonstrates a nearly normal villous pattern (H&E stain, X200).
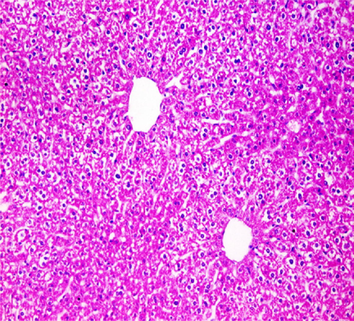
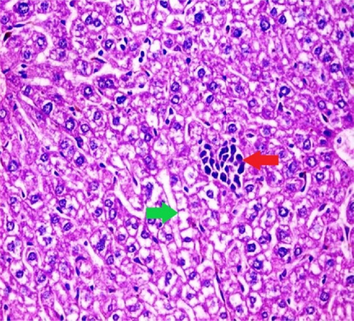
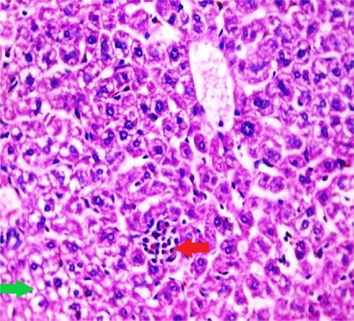
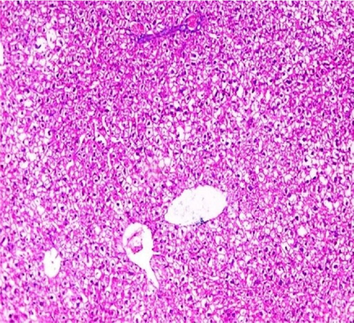
Liver sections from the experimental mice of the different study groups: Fig. 3a: section from the liver of GI, Fig. 3b: section from the liver of GII, Fig. 3c: section from the liver of GIII, Fig. 3d: section from the liver of GIV, Fig. 3e: section from the liver of GV. Fig. 3a. A section in the liver tissue of GI shows a normal hepatocyte pattern (H&E stain, X200). Fig. 3b. A section in liver tissue demonstrates moderate hepatocellular degeneration (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3c. A liver section of GIII reveals degenerated hepatocytes (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3d. A section in the liver tissue reveals mild hepatocellular degeneration (H&E stain, X200). Fig. 3e. A section in the liver tissue presents preserved hepatic lobular architecture (H&E stain, X200).
Liver sections from the experimental mice of the different study groups: Fig. 3a: section from the liver of GI, Fig. 3b: section from the liver of GII, Fig. 3c: section from the liver of GIII, Fig. 3d: section from the liver of GIV, Fig. 3e: section from the liver of GV. Fig. 3a. A section in the liver tissue of GI shows a normal hepatocyte pattern (H&E stain, X200). Fig. 3b. A section in liver tissue demonstrates moderate hepatocellular degeneration (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3c. A liver section of GIII reveals degenerated hepatocytes (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3d. A section in the liver tissue reveals mild hepatocellular degeneration (H&E stain, X200). Fig. 3e. A section in the liver tissue presents preserved hepatic lobular architecture (H&E stain, X200).
Liver sections from the experimental mice of the different study groups: Fig. 3a: section from the liver of GI, Fig. 3b: section from the liver of GII, Fig. 3c: section from the liver of GIII, Fig. 3d: section from the liver of GIV, Fig. 3e: section from the liver of GV. Fig. 3a. A section in the liver tissue of GI shows a normal hepatocyte pattern (H&E stain, X200). Fig. 3b. A section in liver tissue demonstrates moderate hepatocellular degeneration (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3c. A liver section of GIII reveals degenerated hepatocytes (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3d. A section in the liver tissue reveals mild hepatocellular degeneration (H&E stain, X200). Fig. 3e. A section in the liver tissue presents preserved hepatic lobular architecture (H&E stain, X200).
Liver sections from the experimental mice of the different study groups: Fig. 3a: section from the liver of GI, Fig. 3b: section from the liver of GII, Fig. 3c: section from the liver of GIII, Fig. 3d: section from the liver of GIV, Fig. 3e: section from the liver of GV. Fig. 3a. A section in the liver tissue of GI shows a normal hepatocyte pattern (H&E stain, X200). Fig. 3b. A section in liver tissue demonstrates moderate hepatocellular degeneration (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3c. A liver section of GIII reveals degenerated hepatocytes (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3d. A section in the liver tissue reveals mild hepatocellular degeneration (H&E stain, X200). Fig. 3e. A section in the liver tissue presents preserved hepatic lobular architecture (H&E stain, X200).
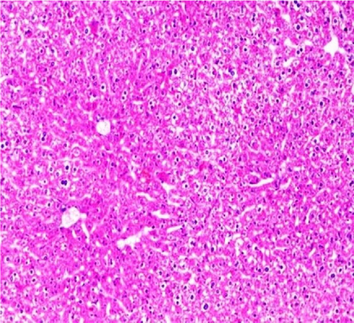
Liver sections from the experimental mice of the different study groups: Fig. 3a: section from the liver of GI, Fig. 3b: section from the liver of GII, Fig. 3c: section from the liver of GIII, Fig. 3d: section from the liver of GIV, Fig. 3e: section from the liver of GV. Fig. 3a. A section in the liver tissue of GI shows a normal hepatocyte pattern (H&E stain, X200). Fig. 3b. A section in liver tissue demonstrates moderate hepatocellular degeneration (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3c. A liver section of GIII reveals degenerated hepatocytes (green arrow) and focal mononuclear cellular infiltration (red arrow) (H&E stain, X400). Fig. 3d. A section in the liver tissue reveals mild hepatocellular degeneration (H&E stain, X200). Fig. 3e. A section in the liver tissue presents preserved hepatic lobular architecture (H&E stain, X200).
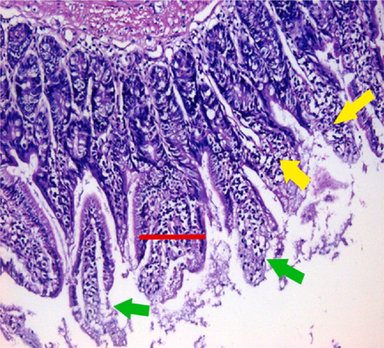
GII: Small intestinal sections revealed marked villous broadening with reduced villous height to crypt length ratio. There was dense infiltration by mononuclear inflammatory cells within the villous core, degeneration of the villous tip-regions and increased mucin production (Fig. 2b). Cryptosporidium oocysts appeared along the brush border of the villi and in the intestinal lumen as rounded to oval bodies, purple-stained and measuring 4–6 µm (Fig. 2c). Liver sections showed hepatocellular degeneration with moderate inflammatory cellular infiltration (Fig. 3b). GIII: Small intestinal sections showed moderate villous broadening, moderate infiltration with mononuclear inflammatory cells, with degeneration of the villous tip region (Fig. 2d). Liver sections revealed moderate hepatocellular degeneration and focal mononuclear cellular infiltration (Fig. 3c). GIV: Histopathological examination of sections from the small intestine revealed improved pathological changes in the form of mild broadening of villi with focal degeneration of the tip regions and mild infiltration by mononuclear inflammatory cells (Fig. 2e). Liver sections showed marked improvement with mild hepatocellular degeneration (Fig. 3d). GV: Histopathological examination of sections from the small intestine revealed improved pathological changes in the form of returning of the nearly normal villous pattern with occasional inflammatory cells (Fig. 2f). Liver sections revealed preserved hepatic architecture (Fig. 3e).
3.4 Identification and characterization of secondary metabolites of V. officinalis
To obtain the metabolite profile of the n-butanol fraction of V. officinalis aerial parts, LC-ESI-MS/MS analysis in negative ion mode (-ve mode) was performed. The chemical skeletons of the identified compounds were established using their MS fragmentation pattern and identified via using their molecular weights and published data on the plant (Table 2, Figs. 4 and 5). In total, sixteen compounds were detected and characterized in the investigated extract. These compounds were categorized as phenolic acids, organic acids, flavonoids (aglycones and glycosides), phenylethanoids, and coumarins. Flavonoid compounds predominate in the extract, such as eriodictyol-di-C-hexoside, quercetin 7-O-pentoside, isorhamnetin-3-O-rutinoside, and apigenin 7-O-glucuronide (Supplementary file 1S).
Peak ID
Rt (min)
m/z [M−H]-
M.wt.
M.F.
MS2 fragments (m/z)
Identified compounds
Chemical class
References
1
0.77
133
134
C4H5O5
115
Malic acid
Organic acids
Sobeh et al., 2018
2
0.98
179
180
C9H8O4
135, 107
Caffeic acid
Phenolic acids
Ghareeb et al., 2018a; Bakchiche et al., 2019
3
6.06
611
612
C27H31O16
491, 431, 401, 371
Eriodictyol-di-C-hexoside
Flavonoids
de Beer et al., 2012
4
6.15
449
450
C21H22O11
287, 151, 107
Eriodictyol 7-O-glucoside
Flavonoids
Friščić et al., 2016
5
6.33
433
434
C20H18O11
301, 271
Quercetin 7-O-pentoside
Flavonoids
Pascale et al., 2020
6
7.25
367
368
C17H20O9
191, 179, 161, 135, 117
Feruloylquinic acid
Phenolic acids
Abu-Reidah et al., 2013
7
7.42
623
624
C28H32O16
315, 300, 271
Isorhamnetin-3-O-rutinoside
Flavonoids
Al-Yousef et al., 2020
8
7.69
623
624
C29H36O15
461, 315
Verbascoside
Phenylethanoids
Friščić et al., 2016
9
8.04
445
446
C21H18O11
283, 269, 225, 175
Apigenin 7-O-glucuronide
Flavonoids
Friščić et al., 2016
10
8.71
621
622
C27H26O17
351, 269
Apigenin 7-O-diglucuronide
Flavonoids
Rehecho et al., 2011
11
8.85
301
302
C15H10O7
300, 271, 269, 255, 229, 179, 169, 151
Quercetin
Flavonoids
Ghareeb et al., 2018a
12
8.99
177
178
C9H6O4
147, 133
Esculetin
Coumarins
Ouyang et al., 2016
13
20.43
447
448
C21H20O11
285, 284, 255, 227, 151
Kaempferol-3-O-glucoside
Flavonoids
Ghareeb et al., 2018a
14
29.32
255
256
C15H12O4
213, 183, 172
Pinocembrin
Flavonoids
Simirgiotis et al., 2015; Ghareeb et al., 2018b
15
29.71
415
416
–
385, 251, 165, 147
Dihydro-p-coumaric acid derivative
Phenolic acids
Ghareeb et al., 2018c
16
29.75
461
462
C22H22O11
341, 299
Chrysoeriol 8-C-glucoside (Scoparin)
Flavonoids
Hassan et al., 2019
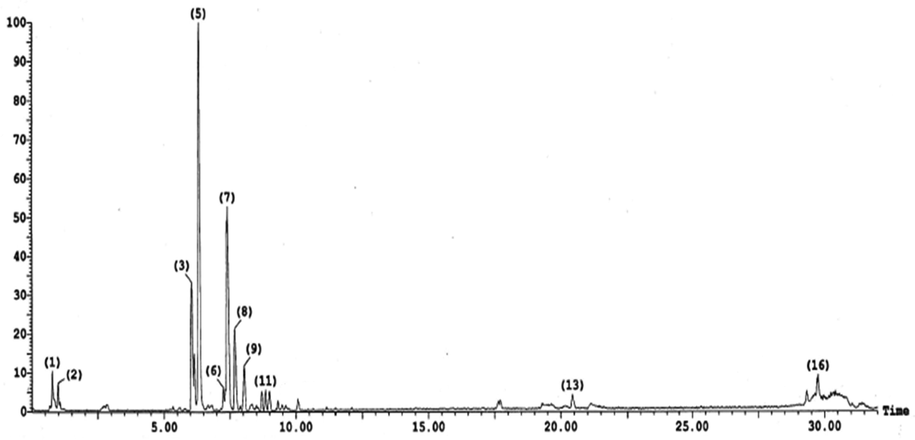
Negative LC-ESI-MS/MS profile of phenolic compounds in the n-butanol extract of V. officinalis aerial parts. Numbers at peaks refer to Table 2.
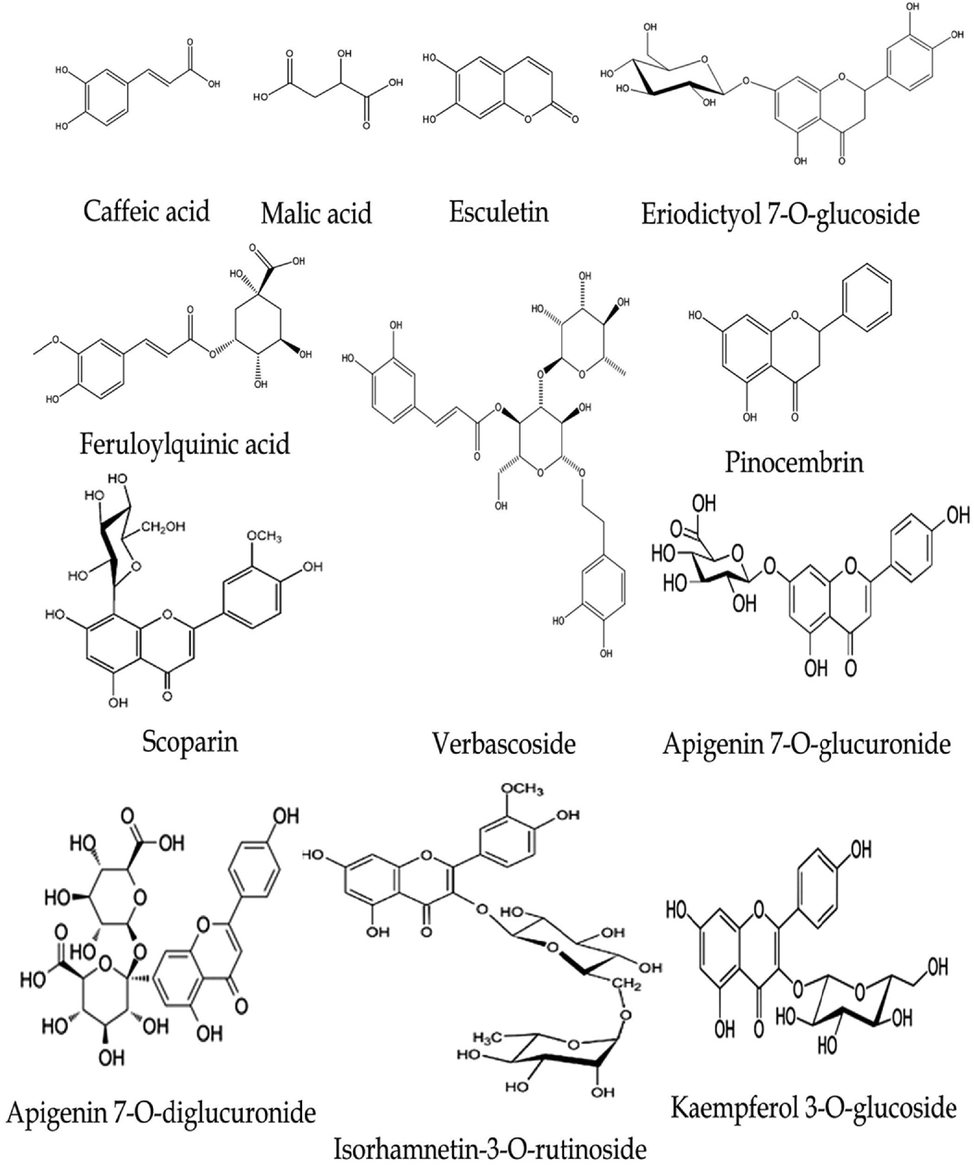
Chemical structures of some identified compounds in the n-butanol extract of V. officinalis.
4 Discussion
Medicinal plants have played a significant role in producing effective therapeutic agents for a long time ago. Nowadays, it is estimated that about 80% of people living in developing countries still depend on folk medicine for different encountered health problems. There is an increasing demand for herbal medicines, with a continuous rise in their popularity over time (Tiwari, 2008; Kumar et al., 2010; Olajuyigbe and Afolayan, 2012; Pathak and Das, 2013). Nitazoxanide is the only anti-cryptosporidial drug that has the approval of the U.S. Food and Drug Administration (FDA). It affects the parasite by inhibiting its anaerobic metabolism for energy production, facilitating its clearance (Gargala, 2008; Abaza et al., 2016).
According to the present study results, treatment with the gold standard drug against cryptosporidiosis, NTZ, recorded 66% efficacy in reducing the shed of oocyst in the stool of immunosuppressed infected mice. This was higher than a study that reported 50% efficacy of NTZ in reducing the shed of Cryptosporidium spp. oocyst in the stool of immunosuppressed infected mice (Oshiba et al., 2018). This may be due to the larger inoculum for infection used by Oshiba et al. (105C. parvum oocysts) in comparison to the infection inoculum of this study (3X103 C. parvum oocysts) with the same dose of NTZ treatment 100 mg/kg bw/day. Besides, NTZ treatment was administered 10 days after infection in the Oshiba et al. study, whereas in this study, it was administered 7 days after infection.
This drug has improved diarrhea and mortality rates in infected undernourished children. However, it is not efficient and thus ineffective for infected people with immunosuppression without competent immune responses. Regarding the broad burden of cryptosporidiosis, this lack of treatment choices should be considered an important public health problem (Gargala, 2008; Abaza et al., 2016).
Nitazoxanide (100 mg/5 ml suspension) was given orally at 100 mg/kg daily for five consequent days (Li et al., 2003). That was the cause for using V. officinalis extract for 5 days in this study. In the present study, GIV and GV received V. officinalis n-butanol extract in a dose of 400 mg/kg bw for 5 consequent days. Sisay et al. tried the antidiarrheal activities for the 80% methanol extracts of the roots (R-80ME) and the leaves (L-80ME) of V. officinalis in doses of 100, 200, and 400 mg/kg bw/ mice in castor oil-induced diarrhea in mice. The maximum in vivo antidiarrheal index was recorded at a dose of 400 mg/kg R-80ME. They also proved that both R-80ME and L-80ME of V. officinalis produced neither overt toxicity nor death for 2 weeks observation period after oral administration of a single dose of 2000 mg/kg. The lack of mortality and signs of overt toxicity up to 5 times the maximum effective dose of the extract anticipated that the crude extracts possess a wider safety margin and median lethal dose (LD50) >2000 mg/kg in the mice model (Sisay et al., 2019).
Treatment with V. officinalis recorded 74% efficacy in reducing the shed of Cryptosporidium spp. oocyst in the stool of immunosuppressed infected mice denotes a promising anti-parasitic activity of V. officinalis. The anti-parasitic properties of Verbena were also proved by a study that revealed optimal anti-T. vaginalis activity of Verbena sp. aqueous extract, inducing complete cytotoxicity. This was in congruence with Teklay et al., who proved that the extract or the juice of roots of V. officinalis could be used to treat ascariasis (Teklay et al., 2013). Treatment with the combination of V. officinalis and NTZ recorded the best results with 87% efficacy in reducing the shed of Cryptosporidium spp. oocyst in the stool of immunosuppressed infected mice. This could be considered a novel and effective combination for the treatment of cryptosporidiosis in immunosuppressed hosts that could minimize the side effects elaborated from using NTZ alone in high doses. Combination therapy also proved a good efficacy against cryptosporidiosis when platelet-rich plasma was given, with NTZ reporting 71.32% efficacy in reducing the shed of Cryptosporidium spp. oocyst in the stool of immunosuppressed infected rats (El-Kholy et al., 2021).
In the present study, histopathological examination of GIII (immunosuppressed, infected, and treated with NTZ) small intestinal tissue specimens showed moderate villous broadening, moderate infiltration by mononuclear inflammatory cells and focal degeneration of the villous tip regions. Livers sections of GIII showed moderate hepatocellular degeneration and focal mononuclear cellular infiltration. This was slightly different from Abdelmaksoud et al. study that showed significant improvement of the histopathological changes of the ileocaecal junction following cryptosporidiosis infection with NTZ treatment in the form of restoration of the normal villous architecture with preserved villous height to crypt length ratio. The mucosal lining was intact with the healing of the surface epithelium, preserved the villi's brush border, and minimal surface erosions. Goblet cells were preserved with no depletion and minimal inflammatory infiltration in the lamina propria. Liver sections showed preserved hepatic architecture with slightly congested sinusoids and central veins (Abdelmaksoud et al., 2020).
In the present study, histopathological examination of ileocaecal sections from GIV (immunosuppressed, infected, and treated with V. officinalis n-butanol extract) showed improved pathological changes in the form of mild broadening of villi with focal degeneration of the tip regions and mild expansion by mononuclear inflammatory cells. Liver sections showed mild hepatocellular degeneration. GV (immunosuppressed, infected, and treated with the combination of V. officinalis n-butanol extract and NTZ) tissue sections at the ileocaecal region showed improved pathological changes in returning the nearly normal villous pattern with occasional inflammatory cells. Liver sections of GV showed preserved hepatic lobular architecture. The possible anti-inflammatory effect of Verbena in the present study was in congruence with a study that confirmed the anti-inflammatory and analgesic effects of V. officinalis extract in different formulations (Calvo, 2006). The promising therapeutic effect of Verbena in the present study concurs with the studies that reported the medicinal effect of Verbena in treating the diarrheal diseases (Gedif and Hahn, 2003; Hedberg et al., 2006; Woldeab et al., 2018), abdominal pain, stomachache (Teklehaymanot and Giday, 2007; Parvez and Yadav, 2010; Calvo et al., 2013; Teklay et al., 2013), and dysentery (Gebeyehu et al., 2014; Molla et al., 2014). It is noteworthy that Guarrera et al. stated that the plasters of aerial parts of V. officinalis (sometimes with petrol) were used as hemostatic and antirheumatic agents in knees and elbows (Guarrera et al., 2005). Besides, the extract of aerial parts of V. officinalis was proved to have antipyretic, sedative (Ivanova et al., 2005), and anticancer effects in traditional medicine (Rigat et al., 2007). Also, the extract or the juice of roots of V. officinalis can be used effectively against tonsillitis. The leaf extract is also used to treat ear disease by applying some drops of the extract through the ear (Teklay et al., 2013) and controlling snake bite poisoning (Tolossa et al., 2013). A previous toxicological study conducted by Aliyu and his co-workers indicated that the acute toxic dose of the V. officinalis extract was higher than 5000 mg/kg (Aliyu et al., 2015). Moreover, Sisay et al. (2019) reported that the acute oral toxicity (LD50) of V. officinalis extracts was found to be > 2000 mg/kg. These findings suggested that the plant has a broader safety brim and is considered a good candidate for further examinations.
The results of the present study elucidated the promising importance of medicinal plants in the treatment of cryptosporidiosis in immunosuppressed hosts. This is supported by a study in which the Asafoetida herb successfully treated immunosuppressed mice infected with cryptosporidiosis (Abdelmaksoud et al., 2020).
5 Conclusion
V. officinalis is an extremely promising medicinal plant in treating cryptosporidiosis with a possible reduction of inflammatory changes in the small intestine. It is also effective in decreasing hepatocyte degeneration by restoring hepatic lobular architecture. It can be used alone or combined with the NTZ to obtain a better efficacy. Additionally, chemical investigation of the n-butanol fraction using the LC-ESI-MS/MS technique led to the identification of 16 secondary metabolites categorized as flavonoids, phenolic acids, phenylethanoids, and coumarins.
Further quantitative studies about the anti-inflammatory effects of V. officinalis n-butanol extract and using V. officinalis n-butanol extract in different doses at different times to treat immunosuppressed mice with cryptosporidiosis with parasitological assessment through the collection of fecal pellets daily throughout the study are highly recommended.
CRediT authorship contribution statement
Eman S. El-Wakil: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Maha A. El-Shazly: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Ayman M. El-Ashkar: Data curation, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review & editing. Tarek Aboushousha: Methodology, Visualization, Writing - original draft, Writing - review & editing. Mosad A. Ghareeb: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Acknowledgments
The authors would like to thank Dr. Trease Labib (National Gene Bank and Orman Botanical Garden Consultant) for her help in identifying and authenticating of the Verbena officinalis plant. We would also like to acknowledge the Animal Research Institute in Giza governorate, Egypt, for their sincere help and support by providing us with the Cryptosporidium spp. oocysts.
Authors' contributions
Eman S. El-Wakil: Designed the plan of anti-parasitic work, performed the parasitological practical part, and shared in writing and revising the manuscript. Tarek Aboushousha: Performed the histopathological study. El-Ashkar AM: Shared in designing the plan of anti-parasitic work, analyzing the data, writing, and revising the manuscript. Maha A. El-Shazly and Mosad A. Ghareeb: Conceived and designed the phytochemical study, searched for information, collected plant, performed extraction and fractionation processes, performed the phytochemical profiling of the plant, writing and revising the manuscript. The manuscript has been read and approved by all named authors. We further confirm that the order of authors listed has been approved by all of us.
Funding
There has been no financial support for this work that could have influenced its outcome.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Assessing the efficacy of nitazoxanide in treatment of cryptosporidiosis using pcr examination. J Egypt Soc Parasitol. 2016;46:683-692.
- [Google Scholar]
- Potential therapeutic and prophylactic effects of Asafoetida in murine cryptosporidiosis. J. Parasit. Dis.. 2020;44:646-653.
- [CrossRef] [Google Scholar]
- Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC-DAD-ESI-QTOF-MS. Food Chem.. 2013;141:2269-2277.
- [CrossRef] [Google Scholar]
- Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. 2007
- [CrossRef] [Google Scholar]
- Treatment of cryptosporidiosis in immunocompromised individuals: Systematic review and meta-analysis. Br. J. Clin. Pharmacol.. 2007;63:387-393.
- [CrossRef] [Google Scholar]
- Verbena officinalis a herb with promising broad spectrum antimicrobial potential. Cogent Chem.. 2017;3:1363342.
- [CrossRef] [Google Scholar]
- Median lethal dose (LD50) evaluation of some polyherbal formulations marketed in northern Nigeria. Int. J. Herb. Pharmacol. Res.. 2015;4:18-23.
- [Google Scholar]
- UPLC-ESI-MS/MS profile and antioxidant, cytotoxic, antidiabetic, and antiobesity activities of the aqueous extracts of three different Hibiscus species. J. Chem. 2020:1-17.
- [Google Scholar]
- Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PLoS ONE. 2009;4
- [CrossRef] [Google Scholar]
- High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: A randomised controlled trial. BMC Infect. Dis.. 2009;9
- [CrossRef] [Google Scholar]
- Temporal analysis of free exploration of an elevated plus-maze in mice. J. Exp. Psychol. Anim. Learn. Cogn.. 2014;40:457-466.
- [CrossRef] [Google Scholar]
- Identification, quantification, and antioxidant activity of hydroalcoholic extract of Artemisia campestris from Algeria. Turkish J. Pharm. Sci.. 2019;16:234-239.
- [CrossRef] [Google Scholar]
- In vitro Model for Cryptosporidium parvum infection. Al-Azhar Int Med J Artic. 2020;1:12-15.
- [Google Scholar]
- Developing novel anthelmintics from plant cysteine proteinases. Parasites Vectors. 2008;1
- [CrossRef] [Google Scholar]
- Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS ONE. 2012;7
- [CrossRef] [Google Scholar]
- Responses of male C57BL/6N mice to observing the euthanasia of other mice. J. Am. Assoc. Lab. Anim. Sci.. 2016;55:406-411.
- [Google Scholar]
- Remarkable anti- trichomonas vaginalis activity of plants traditionally used by the mbyá-guarani indigenous group in Brazil. Biomed Res. Int.. 2013;2013
- [CrossRef] [Google Scholar]
- Treatment of cryptosporidiosis: Do we know what we think we know? Curr. Opin. Infect. Dis. 2010
- [CrossRef] [Google Scholar]
- Anti-inflammatory and analgesic activity of the topical preparation of Verbena officinalis L. J Ethnopharmacol.. 2006;107:380-382.
- [Google Scholar]
- The pharmacological validation of medicinal plants used for digestive problems in Navarra. Spain. Eur J Integr Med.. 2013;5:537-546.
- [Google Scholar]
- Anti-inflammatory activity of leaf extract of Verbena officinalis L. Phytomedicine. 1998;5:465-467.
- [CrossRef] [Google Scholar]
- Antioxidant and antifungal activity of Verbena officinalis L. leaves. Plant Foods Hum. Nutr.. 2008;63:93-97.
- [CrossRef] [Google Scholar]
- Studies on chemical constituents of flavonoid from Verbena officinalis. Zhong Yao Cai.. 2006;29:677-679.
- [Google Scholar]
- Food ingredient extracts of Cyclopia subternata (Honeybush): Variation in phenolic composition and antioxidant capacity. Molecules. 2012;17:14602-14624.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother. 2000;14:463-465.
- [Google Scholar]
- Quantitative determination of the major constituents of Verbena officinalis using high performance thin layer chromatography and high pressure liquid chromatography. Phytochem. Anal.. 2000;11:351-355.
- [Google Scholar]
- The efficacy of platelet rich plasma as adjuvant therapy in the treatment of cryptosporidiosis in experimentally infected immunosuppressed rats. Parasitol. United J.. 2021;14(2):162-170.
- [Google Scholar]
- Evaluation of possible prophylactic and therapeutic effect of mefloquine on experimental cryptosporidiosis in immunocompromised mice. J. Parasit. Dis.. 2021;45:380-393.
- [CrossRef] [Google Scholar]
- Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol.. 2005;4:685-688.
- [CrossRef] [Google Scholar]
- Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol.. 2014;1180:31-43.
- [CrossRef] [Google Scholar]
- LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom.. 2016;51:1211-1236.
- [CrossRef] [Google Scholar]
- Drug treatment and novel drug target against Cryptosporidium. Parasite. 2008;15:275-281.
- [CrossRef] [Google Scholar]
- Ethnobotanical study of traditional medicinal plants and their conservation status in Mecha Wereda West Gojjam Zone of Ethiopia. Int J Pharm Heal. Care Res.. 2014;2:137-154.
- [Google Scholar]
- The use of medicinal plants in self-care in rural central Ethiopia. J. Ethnopharmacol.. 2003;87:155-161.
- [CrossRef] [Google Scholar]
- HPLCDAD-ESI-MS/MS characterization of bioactive secondary metabolites from Strelitzia nicolai leaf extracts and their antioxidant and anticancer activities in vitro. Phcog Res. 2018;10:368-378.
- [Google Scholar]
- HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharm. Pharmacol.. 2018;70:133-142.
- [CrossRef] [Google Scholar]
- Chemical profiling of polyphenolics in Eucalyptus globulus and evaluation of its hepato–renal protective potential against cyclophosphamide induced toxicity in mice. Antioxidants. 2019;8
- [CrossRef] [Google Scholar]
- HPLC-ESI-MS/MS profiling of polyphenolics of a leaf extract from Alpinia zerumbet (Zingiberaceae) and its anti-inflammatory, anti-nociceptive, and antipyretic activities in vivo. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy) J. Ethnopharmacol.. 2005;96:429-444.
- [CrossRef] [Google Scholar]
- Parasitic infections in HIV-infected patients who visited Seoul National University Hospital during the period 1995–2003. Korean J. Parasitol.. 2005;43:1-5.
- [CrossRef] [Google Scholar]
- Chemical composition and biological activities of the aqueous fraction of Parkinsonea aculeata L. growing in Saudi Arabia. Arab. J. Chem.. 2019;12:377-387.
- [Google Scholar]
- Flora of Ethiopia and Eritrea: gentianaceae to cyclocheilaceae. Addis Ababa, Ethiop: Addis Ababa Univ; 2006. p. :5.
- Staining of Cryptosporidium by a modified Ziehl-Neelsen technique. Acta. Vet. Scand. 1981;22:594-596.
- [Google Scholar]
- Multigeneric resistance to oxfendazole by nematodes in cattle. Vet. Rec.. 1996;138:67-68.
- [CrossRef] [Google Scholar]
- Outbred CD1 mice are as suitable as inbred C57BL/6J mice in performing social tasks. Neurosci. Lett.. 2017;637:142-147.
- [CrossRef] [Google Scholar]
- Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol.. 2005;96:145-150.
- [CrossRef] [Google Scholar]
- Pharmacological review on natural antidiarrhoel agents. Der Pharma Chem.. 2010;2:66-93.
- [Google Scholar]
- Novel neuroprotective effects of the aqueous extracts from Verbena officinalis Linn. Neuropharmacology. 2006;50:641-650.
- [CrossRef] [Google Scholar]
- Long-lasting anticryptosporidial activity of nitazoxanide in an immunosuppressed rat model. Folia Parasitol. (Praha). 2003;50:19-22.
- [Google Scholar]
- The systematic identification of flavonoids. Berlin, Heidelberg, New York: Springer-Verlag; 1970. p. :1-362.
- Mengiste B, Yesufn JM, G.B., 2014. In-vitro antibacterial activity and phytochemical analysis of leaf extract of Verbena officinalis. Int. J. Pharmacogn. 1, 774-779.
- Antibacterial and potential antidiabetic activities of flavone C-glycosides isolated from Beta vulgaris subspecies cicla L. var. flavescens (Amaranthaceae) cultivated in Egypt. Curr Pharm Biotechnol. 2019;20:595-604.
- [Google Scholar]
- Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci.. 2014;6:154-167.
- [Google Scholar]
- Analysis of the aerial parts of Verbena officinalis L. by micellar electrokinetic capillary chromatography. Chromatographia. 2004;60:193-197.
- [Google Scholar]
- Ethnobotanical survey of medicinal plants used in the treatment of gastrointestinal disorders in the Eastern Cape Province. South Africa. J Med Plants Res.. 2012;6:3415-3424.
- [Google Scholar]
- In vivo effect of pomegranate (Punica granatum) extracts versus Nitazoxanide drug on the ileum of experimentally infected mice with Cryptosporidium parvum oocysts. J. Am. Sci.. 2018;14:27-39.
- [CrossRef] [Google Scholar]
- Bioactive coumarins from the stems of Clausena emarginata. Chem. Biodivers.. 2016;13:1178-1185.
- [CrossRef] [Google Scholar]
- Ethnopharmacology of single herbal preparations of medicinal plants in Asendabo district, Jimma. Ethiopia. Indian J Tradit. Knowledge.. 2010;9:724-729.
- [Google Scholar]
- Profiling of quercetin glycosides and acyl glycosides in sun-dried peperoni di Senise peppers (Capsicum annuum L.) by a combination of LC-ESI(-)-MS/MS and polarity prediction in reversed-phase separations. Anal. Bioanal. Chem.. 2020;412:3005-3015.
- [CrossRef] [Google Scholar]
- Herbal medicine-a rational approach in health care system. Int J Herb. Med.. 2013;1:86-89.
- [Google Scholar]
- Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav. Brain Res.. 2009;198:58-68.
- [CrossRef] [Google Scholar]
- Peat, J., Barton, B., 2005. Medical statistics. A guide to data analysis and critical appraisal. Wiley-Blackwell, pp. 113–119.
- Rehecho, S., Hidalgo, O., Garcia-Ĩniguez de Cirano, M., Navarro, I., Astiasaran, I., Ansorena, D., Cavero, R., Calvo, M., 2011. Chemical composition, mineral content and antioxidant activity of Verbena officinalis L. LWT-Food Sci. Technol. 44, 875–882.
- Studies on pharmaceutical ethnobotany in the high river Ter valley (Pyrenees, Catalonia, Iberian Peninsula) J. Ethnopharmacol.. 2007;113:267-277.
- [CrossRef] [Google Scholar]
- Volatile constituents from the aerial part of Verbena officinalis L. (Vervain) Iran. J. Pharm.. 2010;2:39-42.
- [Google Scholar]
- Two new iridoids from Verbena officinalis L. Molecules. 2014;19:10473-10479.
- [CrossRef] [Google Scholar]
- Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules. 2015;20:11490-11507.
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia. 1998;69:135-140.
- [Google Scholar]
- Evaluation of the antispasmodic and antisecretory activities of the 80% methanol extracts of Verbena officinalis L: evidence from in vivo antidiarrheal study. J. Evidence-Based Integr. Med.. 2019;24
- [CrossRef] [Google Scholar]
- Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci. Rep.. 2018;8
- [CrossRef] [Google Scholar]
- Cloning of the entire COWP gene of Cryptosporidium parvum and ultrastructural localization of the protein during sexual parasite development. Parasitology. 1997;114(Pt 5):427-437.
- [CrossRef] [Google Scholar]
- PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol. Lett.. 1997;150:209-217.
- [CrossRef] [Google Scholar]
- Effects of differential extraction of Verbena officinalis on rat models of inflammation, cicatrization and gastric damage. Planta Med.. 2007;73:227-235.
- [CrossRef] [Google Scholar]
- C. parvum infection in experimentally infected mice: infection dynamics and effect of immunosuppression. Folia Parasitol. 1998;45(2):101-107.
- [Google Scholar]
- An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J. Ethnobiol. Ethnomed.. 2013;9
- [CrossRef] [Google Scholar]
- Ethnobotanical study of medicinal plants used by people in Zegie Peninsula. Northwestern Ethiopia. J. Ethnobiol. Ethnomed.. 2007;3
- [CrossRef] [Google Scholar]
- Studies on the chemical constitutents in herb of Verbena officinalis. Zhongguo Zhongyao Zazhi. 2005;30:268-269.
- [Google Scholar]
- Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in South Omo, Southern Ethiopia. J. Ethnobiol. Ethnomed.. 2013;9
- [CrossRef] [Google Scholar]
- Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J. Clin. Microbiol.. 1991;29:1323-1327.
- [CrossRef] [Google Scholar]
- Medicinal plants used for treatment of diarrhoeal related diseases in Ethiopia. Evid. Based. Complement. Alternat. Med.. 2018;2018:1-20.
- [CrossRef] [Google Scholar]
- Comparative sensitivity of PCR primer sets for detection of Cryptosporidium parvum. Korean J. Parasitol.. 2009;47:293-297.
- [CrossRef] [Google Scholar]
- Studies on chemical constituents of aerial parts of Verbena officinalis L. Zhongguo Zhong Yao Za Zhi.. 2000;25:676-678.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103945.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1






