Translate this page into:
Fabrication of ternary dual Z-Scheme AgI/ZnIn2S4/BiVO4 heterojunction photocatalyst with enhanced photocatalytic degradation of tetracycline under visible light
⁎Corresponding author. pzpzlxl@163.com (Changyu Lu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, a novel ternary AgI/ZnIn2S4/BiVO4(AZB) composite photocatalyst was successfully prepared by hydrothermal method and in-situ precipitation method. The as-synthesized samples were characterized by XRD, SEM, TEM, XPS and so on, and the photocatalytic activity was evaluated through photocatalytic degradation of tetracycline (TC) under visible light irradiation. When the molar ratio of Bi to Ag was 1:1, the degradation rate of TC can reach 91.44 % within 150 min. The AZB heterojunction demonstrated outstanding efficiency with the apparent reaction rate constants of 0.02118 min−1 for TC removal, was 4.68, 3.27 and 3.27 times higher than that of pure BiVO4, AgI and ZnIn2S4. Based on active species trapping experiments and ESR analysis, a dual Z-Scheme pathways among BiVO4, AgI and ZnIn2S4 for effective separation of photogenerated charges was recommended. This work provided a promising insight for the design of ternary dual Z-scheme heterojunction with multilevel electron transfer to present greater photo-absorption, charge separation, and photodegradation for environmental decontamination.
Keywords
Photocatalysis
AgI/ZnIn2S4/BiVO4
Dual Z-Scheme heterojunction
Tetracycline
1 Introduction
With economic development in recent years, environmental pollution, especially antibiotic pollution, have become increasingly serious (Li et al., 2022a; Lu et al., 2022a). Confronted with the difficult degradation of various antibiotic contaminant, photocatalytic oxidation with semiconductor have attracted considerable attention due to their low toxicity, high stability and environmental-friendly (Zhang et al., 2020a; Guo et al., 2020; Guo et al., 2021). Among different photocatalysis materials. Bismuth vanadium oxide (BiVO4), as an n-type semiconductor with a narrow bandgap, has been considered as a promising visible light semiconductor catalyst due to its non-toxicity, low price, good chemical stability and response to visible light (Lu et al., 2018). Over the years, various efforts have been made to enhance the photocatalytic activity of BiVO4, such as morphology control (Zhang et al., 2018), noble metal modification (Zhu et al., 2020), element-doping (Regmi et al., 2017), forming heterojunction (Singh et al., 2016), and so on. Theoretically, the construction of heterojunction composite photocatalyst is the most convenient and effective method to improve the photocatalytic performance of photocatalytic materials. Compare with type II and p-n heterojunction, Z-scheme heterojunction has a unique advantage that can retain the prominent redox potential of each semiconductor photocatalyst(Lu et al., 2022b). Successful cases, ZnIn2S4/Ag6Si2O7 (Kong et al., 2021), NiFe2O4/Ag3VO4/Ag2VO2PO4 (Su et al., 2021), MoSe2@Bi2S3/CdS (Wang et al., 2021a), PCN-Sb2MoO6-Bi2O3 (Zhang et al., 2021a), Bi2WO6/GQDs/WO3 (Zhou et al., 2020), and so on. With the in-depth study of Z-scheme, S-scheme, which combines the advantages of Z-scheme, has become the research target of many researchers in recent years. S-scheme can accelerate the separation and transfer of interfacial charge, and give the photocatalysis better redox ability. Successful cases, Ni2P modified step-scheme SnNb2O6/CdS-diethylenetriamine (Hu et al, 2020), CdSe/SnNb2O6 S-scheme heterojunction (Ke et al, 2021), Bi2S3/BiVO4/Mn0.5Cd0.5 ternary S-scheme heterojunction (Zhao et al, 2022), TaON/Bi2MoO6 core–shell S-scheme heterojunction (Li et al, 2022b), Step-scheme g-C3N4/Zn0.2Cd0.8S-DETA composites (Mei et al, 2020), and so on.
ZnIn2S4 is a new type ternary sulfide photocatalyst with a narrow band gap, which has excellent photochemical conversion and stable physical chemical capabilities. More importantly, ZnIn2S4 has a negative valence band position can suit well to catalysts with positive conduction bands to form Z-scheme heterojunction, which makes it possible to effectively promote the separation of photogenerated charges and retain the prominent redox potential (Jo et al., 2016). Zhang et al. synthesis 2D/1D Z‑scheme CeO2/ZnIn2S4 by growing ZnIn2S4 nanosheets in situ on the surfaces of CeO2 nanorods (Zhang et al., 2020b), which could enhance the photocatalytic performance of aromatic alcohols and hydrogen evolution. Furthermore, a serious of ZnIn2S4-based Z-scheme heterojunction has been construct, such as Cu3P/ZnIn2S4 (Yang et al., 2020), ZnIn2S4/Nb2O5 (Wang et al., 2020a), ZnIn2S4/LaNiO3 (Wang et al., 2020b), and so on. In addition, there are many studies on ZnIn2S4/BiVO4, such as ZnIn2S4/BiVO4 for efficient CO2 photoreduction (Han et al., 2021), ZnIn2S4/BiVO4 for organics degradation (Yuan et al., 2020), and so on.
AgI is an outstanding photosensitive material, with a suitable forbidden band width (∼2.8 eV), which has potential performance in photocatalysis. However, the large particle size and poor stability also limits its practical application (Chen et al., 2013; Liang et al., 2015; Wang et al., 2016). Fortunately, after loading AgI particles on other photocatalyst can form heterojunction, such as AgI-Bi2WO6 (Xue et al., 2019), AgI/β-Ag2MoO4 (Zhang et al., 2017), AgI/BiVO4 (Chen et al., 2016), Ag3VO4/AgI (Zhang et al., 2018b), AgI/BiOCOOH (Li et al., 2018), which could enhance the stability and photocatalytic activity of pure AgI. Moreover, dual Z-scheme photocatalyst with a higher photocatalytic activity than normal Z-scheme photocatalyst. For example, Zhang et al. prepared ternary AgI/LaFeO3/g-C3N4 dual Z-scheme composite by ultrasound-assisted hydrothermal approach, and had an obviously enhanced photocatalytic degradation of norfloxacin (Zhang et al., 2020c). Therefore, Combine the band structure respective strengths of AgI, ZnIn2S4 and BiVO4, a ternary AgI/ZnIn2S4/BiVO4 dual Z-scheme composite photocatalyst was constructed to improve the migration and separation of charge for efficient photocatalytic performance with following dual Z-Scheme pathways.
Herein, the ternary AgI/ZnIn2S4/BiVO4 dual Z-scheme heterojunction was successfully synthesized by hydrothermal method and in-situ precipitation method. The structure and properties of AgI/ZnIn2S4/BiVO4 heterojunction were analyzed by a series of characterizations. Tetracycline (TC) was selected as the target organic pollutant to evaluate the photocatalytic activity of the as-synthesized photocatalyst under visible light irradiation. In addition, the stability of the composite photocatalyst was studied through cyclic experiments. Moreover, the possible degradation mechanism was discussed. Subsequently, this AgI/ZnIn2S4/BiVO4 photocatalyst has provided a novel route to fabricate dual Z-scheme heterojunctions for the practical application of effective environment remediation.
2 Materials and methods
2.1 Synthesis of ZnIn2S4, BiVO4 and ZnIn2S4/BiVO4 composites and AgI/ZnIn2S4/BiVO4 composite photocatalyst
All chemicals used in the present work were analytical grade and were used without any further modification. 0.378 mmol Zn(CH3COO)2·2H2O, 0.756 mmol In(NO3)3·4H2O and 3 mmol C3H7NO2S were dissolved in 50 mL of deionized water. After magnetically stirring for 30 min, the mixed solution was transferred to Teflon-lined stainless-steel vessel and heated at 180 °C for 12 h. After cooling to the ambient temperature, the products were washed with deionized water and absolute ethanol, and vacuum dried at 60 °C for 12 h. The obtained powder was collected and named as ZIS.
4.85 g Bi(NO3)3·5H2O was dissolved in 40 mL deionized water to form A solution, 1.17 g NH4VO3 was dissolved in 30 mL deionized water to form B solution. Stirring separately until completely dissolved, solution B was transferred to solution A. Then, 2 g SDS and a certain amount of ZIS were added into the above solutions, and the mixed solution was stirred for 30 min. Subsequently, the mixed solution was transferred to Teflon-lined stainless-steel vessel, then heated at 180 °C and kept for 24 h. The obtained products were washed several times with deionized water and ethanol. After drying at 60 °C, the ZnIn2S4/BiVO4 composite was obtained and marked as ZB. Pure BiVO4 was prepared without ZnIn2S4 and named as BVO.
5 mmol AgNO3 and KI was dissolved in 30 mL deionized water to form solution A and B, respectively. ZB was added into solution A at a molar ratio of Bi to Ag of 5:1, 2:1, 1:1, and 1:2, respectively. Stirring separately for 3 h until completely dissolved, and then solution B was transferred to solution A. Then, stirring in the dark for 3 h. Then the resulting precipitate was centrifuged, washed with deionized water and dried at 60 °C for 12 h in the oven. The molar ratios of Bi to Ag of 5:1, 2:1, 1:1, and 1:2 in composites were record the samples as AZB 5, AZB2, AZB1 and AZB0.5, respectively. Pure AI was prepared without ZB named as AI.
The schematic illustration of preparation procedure of AZB heterojunction photocatalyst was shown in Scheme 1.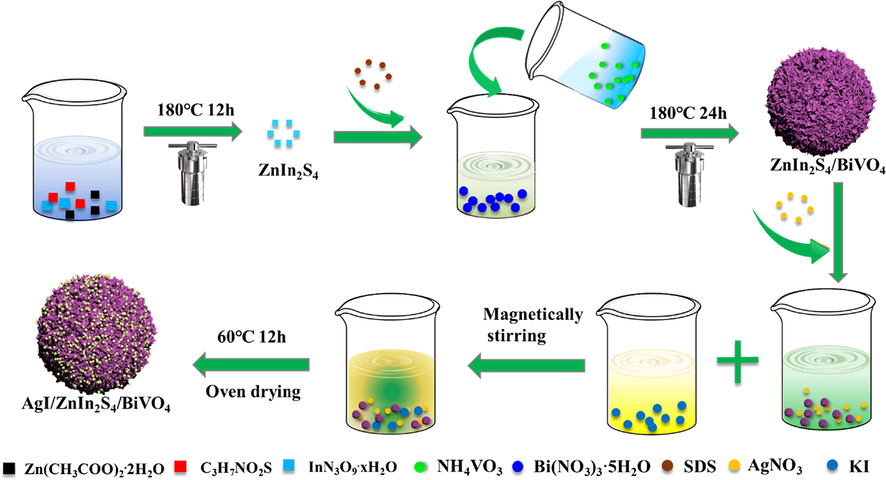
Schematic of AgI/ZnIn2S4/BiVO4 heterojunctions photocatalyst preparation.
2.2 Photocatalytic performance tests
The photocatalytic activity of the as-synthesized samples was evaluated by the degradation of TC under visible light. In a traditional draft, 30 mg photocatalyst was dispersed in 50 mL 20 mg/L TC solution. Then, the mixture has been executed by magnetically stirring for 30 min in the dark to achieve absorption–desorption equilibrium before 300 W Xenon lamp (λ > 420 nm) turned on. After the dark reaction, the Xenon lamp is turned on to start the photocatalytic reaction. (4) mL of the solution were collected every 15 min during the photoreaction. After centrifugation, the supernatant was collected. Then, the concentration of TC in the supernatant was analysed by an ultraviolet–visible spectrophotometer at λ = 356 nm. The degradation rate was calculated by the following formula:
Where C0 is the absorbance of the initial solution and Ci is the absorbance of the solution after the reaction.
2.3 Capturing experiment
Similar with the photocatalytic tests, 1 mmol IPA, 1 mmol EDTA-2Na, and 1 mmol BQ were used to conduct active species trapping tests to capture the hydroxyl radicals (•OH), photogenerated holes (h+) and superoxide anion radicals (•O2–), respectively. The detailed information about characterization is listed in Supporting Information.
3 Results and discussion
The XRD diffraction patterns of as-synthesized photocatalysts were displayed in Fig. 1. All the diffraction peaks of pure BVO, ZIS and AI were obviously assigned to the monoclinic phase BVO (JCPDS NO.14–0688) (Wang et al., 2021b), hexagonal phase ZIS (JCPDS NO. 65–2023) (Wang et al., 2022) and cubic phase AI (JCPDS NO.09–0399) (Guo et al., 2018), respectively. After the recombination of BVO and ZIS, the ZB materials maintains the monoclinic phase BVO, and the ZIS diffraction peak at 27.7° and 47.5° were close to the BVO diffraction peak at 28.9° and 47.3°, which led to the overlap of above peaks. Moreover, the intensity of these diffraction peaks gradually strengthened with the increasing mass ratio of AI. Similarly, as the AI composite ratio increases, the BVO diffraction peak in AZB samples become weaker than that of pure BVO. Furthermore, no other impurity peak was observed in AZB samples meant the well purity and crystallinity, indicting the successfully synthesis of AZB heterostructure.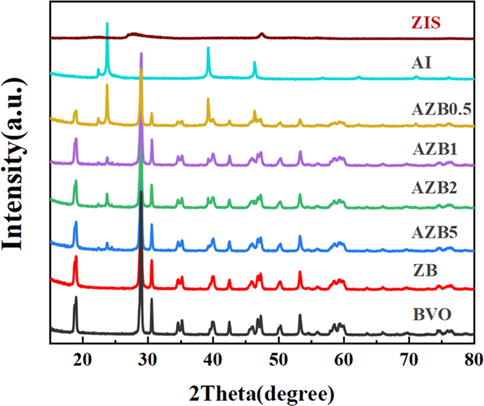
XRD patterns of as-prepared samples.
As displayed in Fig. 2, SEM was employed to investigate the morphology and structure of the as-synthesized photocatalysts. In Fig. 2a, pure BVO microspheres are formed by fast and tight combination of irregular nano-particles with an average radius of about 2 μm. As shown in Fig. 2b, the ZnIn2S4 possessed a micron flower with the radius of 1.5–2 μm, which was composed of numerous nanosheets. In Fig. 2c, AI possessed the fusiform with the radius of 300 nm. After combing ZIS with BVO, it can be clearly seen that ZIS was wrapped in microspheric BVO with a radius of 2 μm to form ZB. In Fig. 2d, the surface and voids of ZB sample were effectively wrapped by AI particles, making the surface of the AZB nanocomposites heterojunction rougher than that of pure BVO, ZIS, AI and ZB samples. Subsequently, the intricate crystal structures of AZB were investigated by TEM and HR-TEM. In Fig. 2e, and a large number of AI particles are wrapped on the surface of ZB samples with close contact between the components, which shows that ZIS were well attached to the AI.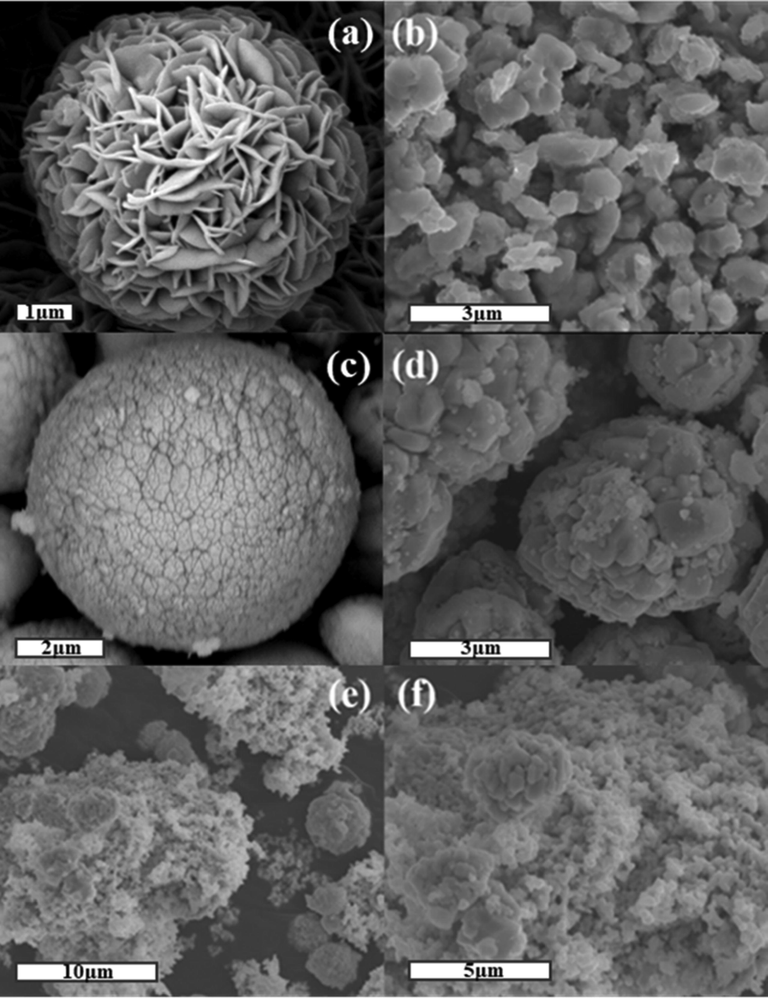
SEM images of (a)ZnIn2S4, (b) AgI, (c) (d)ZB, (e) (f) AZB1.
The microstructure of AZB nanocomposites was further studied by transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM). TEM images of AZB1 composite in Fig. 3a and 3b clearly show that AI nanoparticles are well dispersed on the surface of ZB microspheres. Fig. 3c is the high-resolution HRTEM image of AZB. Three types of lattice fringes with a lattice spacing of 0.230 nm, 0.308 nm and 0.322 nm can be observed, corresponding to the (2 0 0) crystal plane of AI (Chen et al., 2019), the (1 2 1) crystal plane of BVO (Pham et al., 2020) and (1 0 2) crystal plane of ZIS (Hussain et al., 2020), respectively. In addition, Fig. 3d shows the element plot of the AZB1 composite, showing a uniform spatial distribution of the Ag, I, Zn, In, S, Bi, V and O elements, consistent with the element in AZB composite, further indicating a successful synthesis of the AZB composite. In addition, Fig. S1 demonstrated the N2 adsorption–desorption isotherms and the corresponding pore size distribution of as-prepared AZB1 sample. The BET surface area, pore volume and average pore diameter of AZB1, were 2.0525 m2 g−1, 0.012170 cm3 g−1 and 22.2033 nm, which were also summarized in Fig. S1.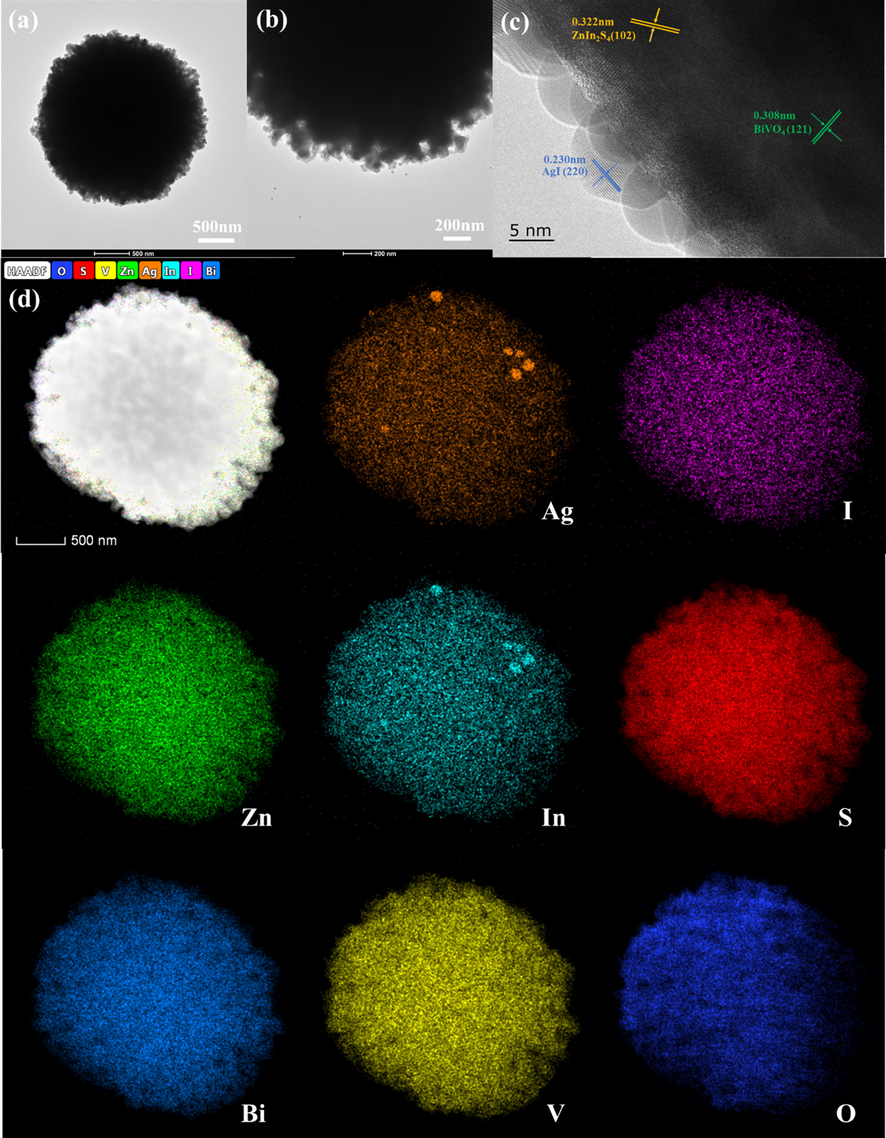
(a, b) TEM and (c) HRTEM images images of AZB1 sample. (d) HAADF and elemental mapping images of AZB1 sample.
XPS spectra were conducted to exhibit the composition and valence of chemical elements of the as-prepared AZB1, AI, ZIS and BVO samples. Fig. 4a and 4b, displayed the survey spectra of AZB1, AI, ZIS and BVO, which indicated the as-prepared samples consists of their own elements and no other elements existed. In Fig. 4c, the diffraction peaks of S 2p and Bi 4f was overlapped around the region of 160 eV. After analysis by 2j + 1 condition typical constraints of the spin–orbit splitting (Morales-Lun et al., 2017), Bi 4f can be divided into two diffraction peaks Bi 4f7/2 and Bi 4f5/2, respectively found at 159.4 eV and 164.1 eV. Meanwhile, it was also possible to deconvolve two diffraction peaks of S 2p3/2 and S 2p1/2 at 160.5 eV and 162.3 eV, confirm that the Bi element and S element were Bi3+ and S2- in the sample (Lu et al., 2019). Compared with ZIS and BVO, the XPS spectra peaks of Bi 4f and S2p had a slightly shift to positive position. Fig. 4d showed the V 2p XPS spectrum, the binding energy peaks of AZB1 at 517.0 eV, 524.2 eV and of BVO at 516.7 eV and 524.1 eV corresponded to V 2p3/2 and V 2p1/2, respectively, proving that the V element was existence of + 5 valence (Wang et al., 2021b). From Fig. 4e, the high-resolution spectrum of Zn 2p3/2 as well as Zn 2p1/2 at 1025.1 eV and 1022.0 eV indicated Zn2+. Similarly, it can be known that Zn2+ exist in ZIS and AZB1. In Fig. 4f, the band energy of In 3d at 441.9 eV, 452.6 eV and 442.0 eV, 452.7 eV were corresponded to In 3d5/2 and In 3d3/2, respectively, proving the presence of In3+ in ZnIn2S4 and AZB1. From Fig. 4g, two characteristic peaks of Ag 3d5/2 and Ag 3d3/2 were located at a binding energy of 368.4 eV, 374.3 eV and 368.6 eV, 374.5 eV, which proved the existence of Ag+. Fig. 4h showed the characteristic diffraction peaks at 619.3 eV and 630.7 eV, corresponded to I 3d5/2 and I 3d3/2, respectively. Same argument the characteristic diffraction peaks at 619.3 eV and 630.7 eV, corresponding to I 3d5/2 and I 3d3/2 in the AI. In Fig. 4i, the diffraction peak of ABZ1 at the binding energy of 530.7 eV is the characteristic peak of O 1 s, which was respectively attributed to lattice oxygen in Bi-O (529.8 eV), V-O (531.8 eV) and O—H (530.4 eV) adsorbed on the surface (Gao et al., 2017). For BVO, O 1 s spectrum peaks at 528.2 eV and 530.0 eV, corresponding to the lattice oxygen and surface-adsorbedoxygen species. In summary, it could be proved that BVO, ZIS and AI coexist in the AZB sample, which was consistent with the XRD results.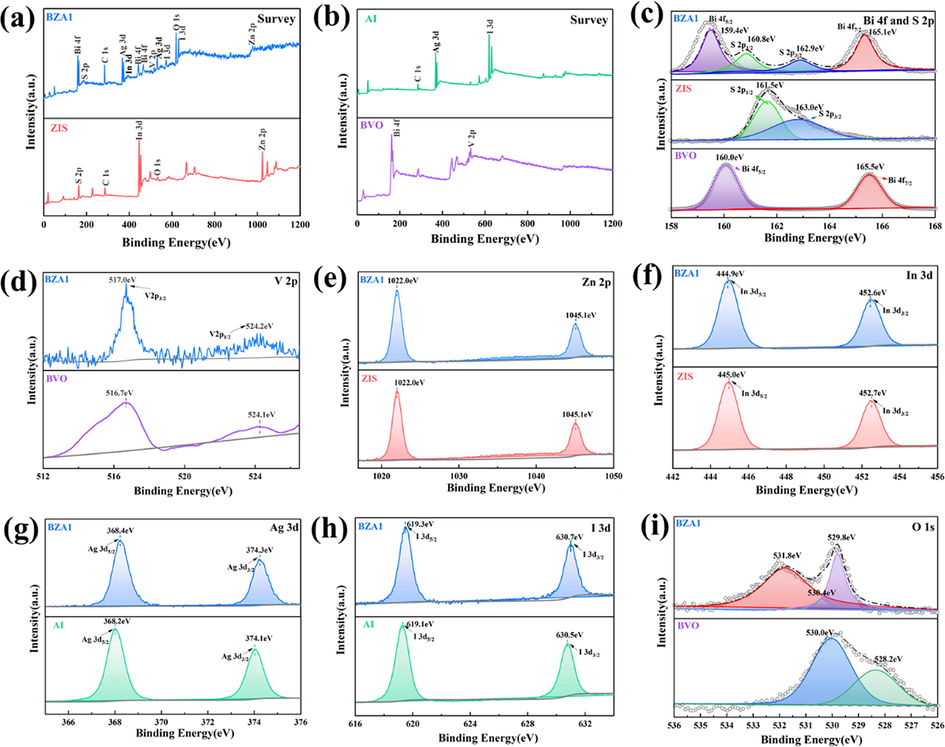
XPS spectrum of AZB1, AI, ZIS and BVO: (a) (b) full spectrum, (c)Bi 4f and S 2p, (d)V 2p, (e)Zn 2p, (f)In 3d, (g)Ag 3d, (h)I 3d, (i)O 1 s.
UV–vis were performed to investigate the light absorption performance of as-prepared photocatalysts. As can be seen in Fig. 5a, the adsorption edges of both pure AI and BVO were up to 466 nm and 570 nm in the visible light region, which meant they can utilize visible light. Since the ZIS was black powder and the absorption of light was strong, the ZB and AZB1 samples combined with ZIS could absorb more light in the visible region than pure AI and BVO, indicating that the composite photocatalytic system had a strong response-ability in the visible light region. The band gap energy of as-prepared samples was calculated by Tacu equation (Zhang et al., 2021b) and displayed in Fig. 5b. Since BiVO, AI and ZIS were direct bandgap semiconductors (n = 1 for direct transition), the calculated Eg of them were 2.40 eV, 2.70 eV and 2.20 eV, respectively. The positions of valence band were conducted by the positions of conduction band and valence band. In Fig. 5c, the EVB of BiVO, AI and ZIS were 2.76 V, 2.36 V and 1.66 V (vs NHE), respectively. Further, the conduction bands of BVO, AgI and ZIS could be calculated as 0.32 V, −0.34 V and −0.54 V, respectively.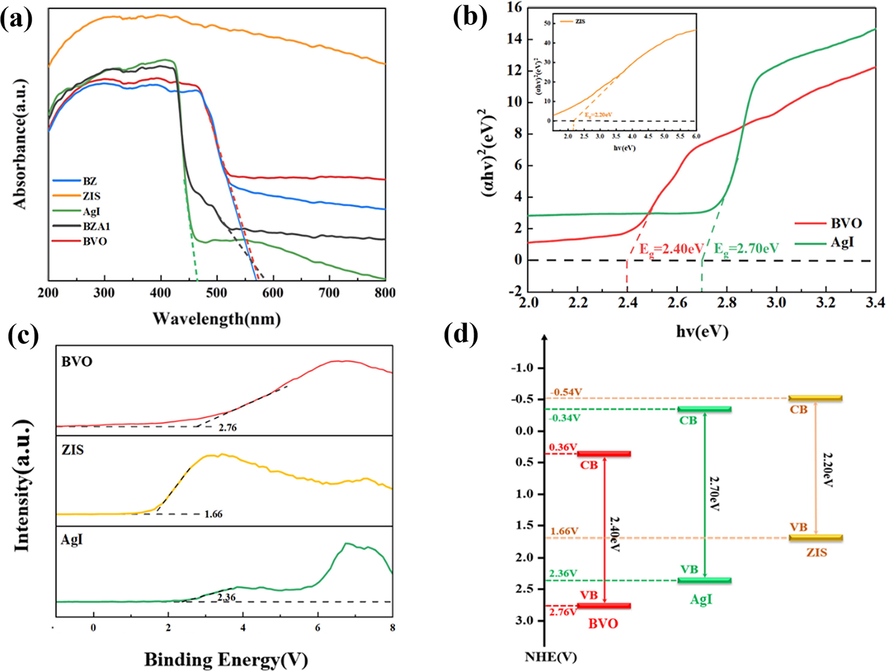
(a) UV–vis of as-prepared samples, (b) Plots of (αhv)2 against photon energy (hν), XPS valence band spectra and the calculated bandgap of AI、ZIS and BVO.
The photocatalytic activity of the as-prepared samples was evaluated by photodegradation of TC under visible light irradiation. In Fig. 6a, that TC wastewater was hard to degrade under light without photocatalyst, indicating TC had good stability under light irradiation. The TC removal efficiency of pure BiVO, AgI and ZIS only 42.11 %, 60.71 % and 54.19 % in 2 h, respectively. After combined with AgI and ZnIn2S4, the composite photocatalyst inhibited the recombination of photo-generated electrons and holes, and accelerated the conduction speed of the photo-generated carriers in the composite. The photocatalytic activity of AZB composite photocatalysts was higher than that of pure BVO, AgI and ZIS, indicating that the photocatalytic performance of the sample has been significantly improved. Furthermore, with the increasing ratio of AI, the degradation rate of TC wastewater for AZB composite photocatalysts increased firstly. Among them, AZB1 had the best photocatalytic activity for TC wastewater, with the degradation rate of TC up to 91.44 %. When the composite ratio of AI continued to increase, the decrease of photocatalytic activity can be attributed to the weakness of the synergistic effect among BiVO, AI and ZIS. Moreover, all photocatalytic reactions performed conform to the first-order reaction kinetic equation in Fig. 6b. The reaction rate constants for BVO, AI, ZIS, ZB, AZB5, AZB2, AZB1 and AZB0.5 were 0.00452, 0.00647, 0.00648, 0.00633, 0.00949, 0.01395, 0.02118 and,0.01329 min−1. The reaction rate of the AZB1 composite photocatalyst was 4.68, 3.27 and 3.27 times higher than that of pure BVO, AI, and ZIS, indicating that the synergistic effect among BVO, AI, and ZIS could improve the photocatalytic properties. In addition to photocatalytic efficiency, TOC and COD removal are also very important performance for photocatalyst. As shown in Fig. 6c, the TOC and COD removal efficiency of AZB1 samples were 57.85 % and 64.89 %, respectively, which were lower than photocatalytic properties. This is because TC in the solution does not completely degrade into inorganic substances, but existed as intermediate. Moreover, the stability and repetitiveness of photocatalysts were significant for practical application. As shown in Fig. 6d, AZB1 still remained high degradation rate (82.16 %) for TC wastewater after 10 cycles, which was only about 9 % lower than that of the first time. The above results prove that the formation of AZB composite heterojunction effectively inhibits the photo-corrosion phenomenon of the catalyst, and has excellent photocatalytic stability and recyclability.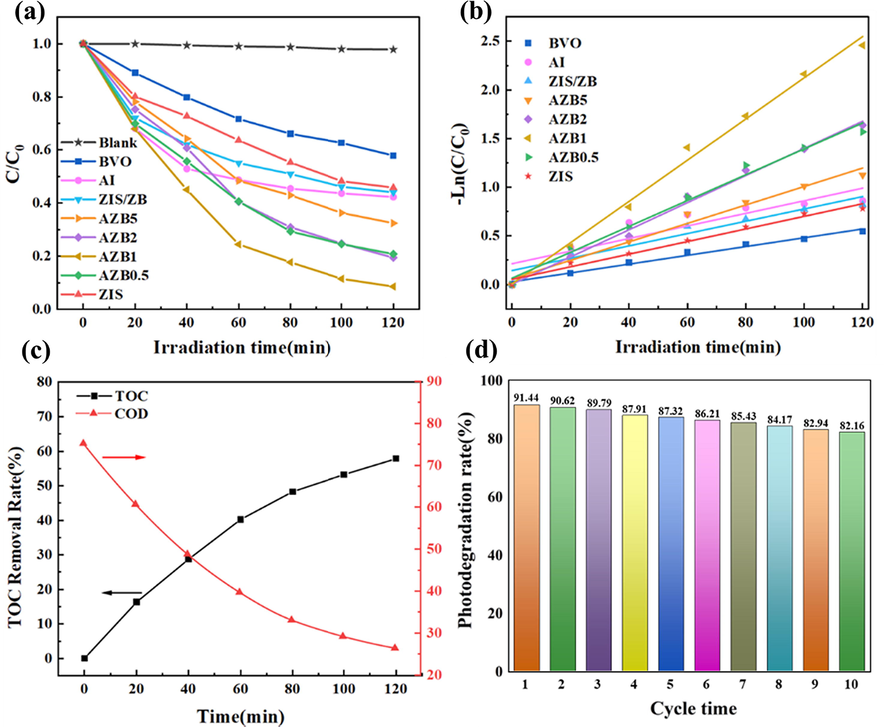
(a) The photocatalytic activity for TC of the as-prepared samples under visible light irradiation, (b) first-order reaction kinetics of photocatalytic degradation of TC, (c) The TOC and COD removal curve of AZB1 sample for TC degradation, (d) Ten cycles curve of AZB1 sample for TC degradation.
Transient photocurrent response and electrochemical impedance spectra were employed to analyze the generation and separation efficiency. As illustrated in Fig. 7a, the photocurrent response of AZB1 was much stronger than that of pure BVO, AI, and ZIS, indicating that more photo-generated carriers were generated (Zhou et al., 2021). Moreover, the EIS Nyquist plots were opposite to the photocurrent intensity which mean the AZB composite photocatalyst had the fastest photo-generated electron transfer efficiency. From the above results, it was drawn that the AZB could inhibit the recombination of photo-generated carriers and accelerate the migration rate of photo-generated electrons.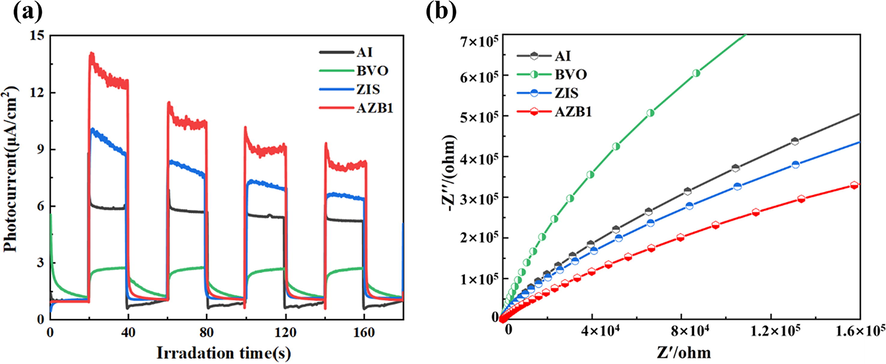
(a)Transient photocurrent responses and (b) EIS plots of BiVO、AgI、ZIS and AZB1 samples.
To verify photocatalytic mechanism, active radical capturing experiments and electron spin resonance (ESR) spectroscopy were employed to explore the role of •O2–, h+, and •OH in Fig. 8. BQ, EDTA-2Na and IPA were selected as trapping agents and added into the reaction system capture •O2–, h+, and •OH, respectively. It can be seen from Fig. 8(a) after adding BQ, the removal rate of TC was reduced to 41.76 %, indicated that •O2– also played a certain role in the degradation process. After adding trapping agent EDTA-2Na, the photodegradation efficiency was significantly inhibited (32.51 %), indicated that h+ played an important role in the photocatalytic degradation reaction. Moreover, the degradation effect of TC solution was decreased to 52.39 % after the addition of IPA capture agent, which indicated that •OH was also exist as the main active group. Since capturing h+ also inhibit the generation of •OH, the photodegradation efficiency of adding EDTA-2Na is slightly higher than adding IPA, indicating h+ directly oxidize a small amount of TC. The above results indicated that h+, •O2– and •OH were all involved in the reaction system for photocatalytic degradation of TC solution, and •O2– and •O2– were the main active species. To further verify the existence of free radicals, the ESR analysis was performed under visible light. As shown in Fig. 8b, no EPR signals regular of DMPO-•O2– and DMPO-•OH was observed under dark conditions. However, a series of obvious characteristic peaks was received under light conditions, indicating that •O2– and •OH free radicals were involved and played critical roles in photocatalytic reactions (Li et al., 2020).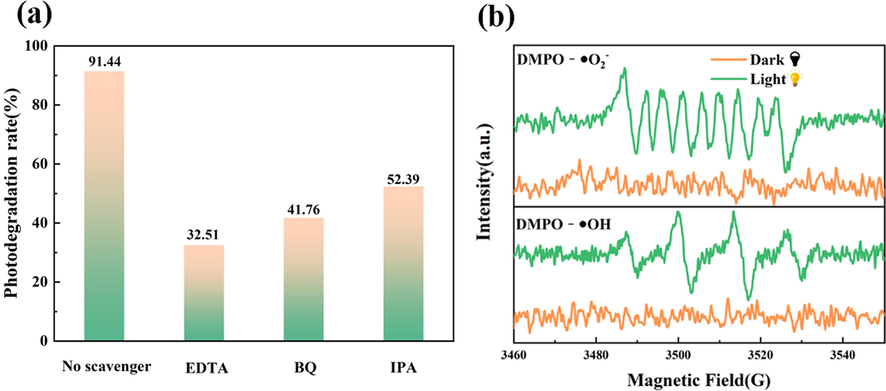
(a) DMPO spin-trapping ESR spectra over AZB1 samples under dark and visible light condition for DMPO−•O2− and DMPO−•OH, (b). The effect of different quenchers on the TC degradation by AZB1 photocatalysts.
According to the above results, the probable photocatalytic mechanism of AZB heterojunction was recommended in Fig. 9. If no interfacial effect was recognized after the construction of AZB heterojunction, the dual type-II photocatalytic mechanism was referred as shown in Fig. 9a. Under light illumination, the photoinduced electrons would shift from the CB of ZIS to the CB of AI then to CB of BVO. On the contrary, the photogenerated h+ will transfer from the VB of BVO to the VB of AI then to VB of ZIS. However, the CB potential of BVO (vs NHE, 0.32 V) is more positive than that of O2/•O2–(-0.33 V vs NHE) and the VB potential of ZIS is more negative than that of OH–/•OH (2.40 V vs NHE), indicating no •O2– and •OH was achieved, which is not consistent with ESR results (Shi et al., 2022). In Fig. 9b, if AZB heterojunction followed dual Z-scheme heterojunction, the photoelectrons on the CB position of AI and ZIS can move to the VB of ZIS and BVO via the Z-scheme route, respectively. Simultaneously, leaving the h+ on the VB of BVO (2.76 V vs NHE) and e- on the CB of ZIS (-0.54 V vs NHE), •O2– and •OH were generated in photocatalysis reaction. Therefore, •O2– and •OH should be the main active species, and h+ is also participating in the reaction and directly degrade TC, which was consistent with the result of active species trapping. This dual Z-scheme heterojunction not only effectively promote the separation of photogenerated charges, but also retain the prominent redox potential. Finally, the photocatalytic degradation process of TC by AZB was described as follows:
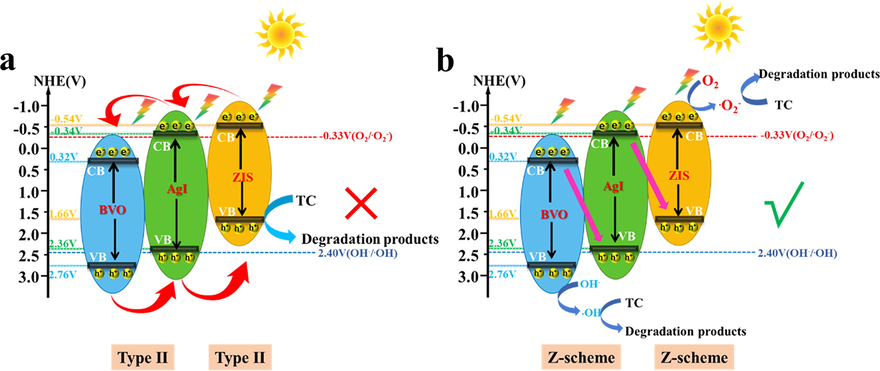
The probable the photocatalytic mechanism of TC over AZB heterojunction.
4 Conclusions
In summary, a novel ternary AgI/ZnIn2S4/BiVO4 dual Z-scheme heterojunction was successfully synthesized by hydrothermal method and in-situ precipitation method. Subsequently, the morphology, crystal structure, elemental composition analyses, composition and photoelectrochemical properties, as well as the charge carriers transfer mechanism of AgI/ZnIn2S4/BiVO4 dual Z-scheme heterojunction have been explored in detail. AgI/ZnIn2S4/BiVO4 heterojunction showed higher photocatalytic activity than that of pure AgI, ZnIn2S4 and BiVO4 towards degradation of TC. In addition, the most excellent photocatalytic activity was obtained by the molar ratio of Bi to Ag in the heterostructure is 1:1, which can be attributed to the formation of dual Z-scheme heterojunction effectively promotes the separation and transfer of photogenerated electron-hole pairs and greatly extends the life of carriers. Finally, a probable photocatalytic mechanism of AZB heterojunction with dual Z-scheme pathways were recommended by ESR and active species trapping experiments. The present work suggested a promising approach for the design of ternary dual Z-scheme heterojunction with multilevel electron transfer to present greater photo-absorption, charge separation, and photodegradation for environmental decontamination.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21906039 and 52000024, China), Shanghai Sailing Program (20YF1400300, China), Hebei Province 333 Talents Project (A202101020, China), Natural Science Foundation of Shaanxi Province (2019JM-429, China), Funding Project for Introduced Overseas Scholars of Hebei Province (C20190321, China), Science and Technology Project of Hebei Education Department (BJ2021010, China), Program for water resources research and promotion of Hebei Province (2019-55, China), Fundamental Research Funds for the Central Universities of Chang’an University (310829172002, 300102298104, 300102290501, China) and Donghua University, Basic Scientific Research Funds for Universities of Hebei Province and Student Science and Technology Foundation of Hebei GEO University (KAG202205, China), Doctoral research fund of Hebei Geo University (BQ2019041, China).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In-situ ion exchange synthesis of hierarchical AgI/BiOI microsphere photocatalyst with enhanced photocatalytic properties. Crystengcomm.. 2013;15:7556-7563.
- [CrossRef] [Google Scholar]
- Novel AgI/BiOBr/reduced graphene oxide Z-scheme photocatalytic system for efficient degradation of tetracycline. J. Alloy. Compd.. 2019;800:88-98.
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: mineralization efficiency and mechanism. ACS Appl. Mater. Interfaces. 2016;8:32887-32900.
- [CrossRef] [Google Scholar]
- Photocatalytic removal of tetrabromobisphenol A by magnetically separable flower-like BiOBr/BiOI/Fe3O4 hybrid nanocomposites under visible-light irradiation. J. Hazard. Mater.. 2017;331:1-12.
- [CrossRef] [Google Scholar]
- Study on highly enhanced photocatalytic tetracycline degradation of type Ⅱ AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater.. 2018;349:111-118.
- [CrossRef] [Google Scholar]
- Ternary Fe3O4/MoS2/BiVO4 nanocomposites: novel magnetically separable visible light-driven photocatalyst for efficiently degradation of antibiotic wastewater through p–n heterojunction. J. Mater. Sci. Mater. Electron.. 2020;31:16746-16758.
- [CrossRef] [Google Scholar]
- Preparation of a novel composite material LaCoO3/Bi2WO6 and its application in the treatment of tetracycline. J. Mater. Sci. Mater. Electron.. 2021;32:13813-13824.
- [CrossRef] [Google Scholar]
- Elegant construction of ZnIn2S4/BiVO4 hierarchical heterostructures as direct Z-Scheme photocatalysts for efficient CO2 photoreduction. ACS Appl. Mater. Interfaces. 2021;13(13):15092-15100.
- [CrossRef] [Google Scholar]
- Noble-metal-free Ni2P modified step-scheme SnNb2O6/CdS-diethylenetriamine for photocatalytic hydrogen production under broadband light irradiation. Appl. Catal. B-Environ.. 2020;269:118844
- [CrossRef] [Google Scholar]
- Synergetic effect of ZnIn2S4 nanosheets with metal-organic framework molding heterostructure for efficient visible- light driven photocatalytic reduction of Cr(VI) Arab. J. Chem.. 2020;13:5939-5948.
- [CrossRef] [Google Scholar]
- Fabrication of hierarchically structured novel redox-mediator-free ZnIn2S4 marigold flower/Bi2WO6 flower-like direct Z-scheme nanocomposite photocatalysts with superior visible light photocatalytic efficiency. Phys. Chem. Chem. Phys.. 2016;18:1000-1016.
- [CrossRef] [Google Scholar]
- Integrated S-scheme heterojunction of amine-functionalized 1D CdSe nanorods anchoring on ultrathin 2D SnNb2O6 nanosheets for robust solar-driven CO2 conversion. Solar RRL. 2021;5(4):2000805.
- [CrossRef] [Google Scholar]
- 0D/3D ZnIn2S4/Ag6Si2O7 nanocomposite with direct Z-scheme heterojunction for efficient photocatalytic H2 evolution under visible light. Int. J. Hydrog. Energy. 2021;46(55):28043-28052.
- [CrossRef] [Google Scholar]
- A novel 3D Z-scheme heterojunction photocatalyst: Ag6Si2O7 anchored on flower-like Bi2WO6 and its excellent photocatalytic performance for the degradation of toxic pharmaceutical antibiotics. Inorg. Chem. Front.. 2020;7:529-541.
- [CrossRef] [Google Scholar]
- Synthesis of flower-like AgI/BiOCOOH p-n heterojunctions with enhanced visible-light photocatalytic performance for the removal of toxic pollutants. Front. Chem.. 2018;2018(6):518.
- [CrossRef] [Google Scholar]
- Facile synthesis of Z-scheme NiO/α-MoO3 p-n heterojunction for improved photocatalytic activity towards degradation of methylene blue. Arab. J. Chem.. 2022;15:103513
- [CrossRef] [Google Scholar]
- Facile fabrication of TaON/Bi2MoO6 core–shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J.. 2022;428:131158
- [CrossRef] [Google Scholar]
- Bactericidal mechanism of BiOI/AgI under visible light irradiation. Chem. Eng. J.. 2015;279:277-285.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of doxycycline on ellipsoid-like BiVO4 synthesized by EDTA-assisted. Desalination Water Treat.. 2018;104:250-256.
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloy. Compd.. 2019;811:151976
- [CrossRef] [Google Scholar]
- Boosted tetracycline and Cr(VI) simultaneous cleanup over Z-Scheme BiPO4/CuBi2O4 p-n heterojunction with 0D/1D trepang-like structure under simulated sunlight irradiation. J. Alloys Compd.. 2022;919:165849
- [CrossRef] [Google Scholar]
- Facile construction of CoO/Bi2WO6 p-n heterojunction with following Z-Scheme pathways for simultaneous elimination of tetracycline and Cr(VI) under visible light irradiation. J. Alloys Compd.. 2022;904:164046
- [CrossRef] [Google Scholar]
- Mei, F. F., Li, Z., Dai, Kai, Zhang, J.F., Liang, C.H., 2020. Step-scheme porous g- g-C3N4/Zn0.2Cd0.8S-DETA composites for efficient and stable photocatalytic H2 production, Chinese J. Catal., 2020, 41, 41-49. https://doi.org/10.1016/S1872-2067(19)63389-9.
- The evolution of the Mo5+ oxidation state in the thermochromic effect of MoO3 thin films deposited by rf magnetron sputtering. J. Alloy Compd.. 2017;722:938-945.
- [CrossRef] [Google Scholar]
- Facile solvothermal synthesis of highly active monoclinic scheelite BiVO4 for photocatalytic degradation of methylene blue under white LED light irradiation. Arab. J. Chem.. 2020;13:8388-8394.
- [CrossRef] [Google Scholar]
- Mechanistic understanding of enhanced photocatalytic activity of N-doped BiVO4 towards degradation of ibuprofen: an experimental and theoretical approach. Mol. Catal.. 2017;432:220-231.
- [CrossRef] [Google Scholar]
- Magnetically retrievable CdS/reduced graphene oxide/ZnFe2O4 ternary nanocomposite for self-generated H2O2 towards photo-Fenton removal of tetracycline under visible light irradiation. Sep. Purif. Technol.. 2022;292:120987.
- [CrossRef] [Google Scholar]
- Band edge engineering in BiVO4/TiO2 heterostructure: enhanced photoelectrochemical performance through improved charge transfer. Acs Catal.. 2016;6:5311-5318.
- [CrossRef] [Google Scholar]
- Magnetically separable NiFe2O4/Ag3VO4/Ag2VO2PO4 direct Z-scheme heterostructure with enhanced visible-light photoactivity. J. Chem. Technol. Biotechnol.. 2021;96(10):2976-2985.
- [CrossRef] [Google Scholar]
- A Z-scheme ZnIn2S4/Nb2O5 nanocomposite: constructed and used as an efficient bifunctional photocatalyst for H2 evolution and oxidation of 5-hydroxymethylfurfural. Inorg. Chem. Front.. 2020;7:437-446.
- [CrossRef] [Google Scholar]
- Fabrication of novel p-n-p heterojunctions ternary WSe2/In2S3/ZnIn2S4 to enhance visible-light photocatalytic activity. Cryst. Growth Des.. 2022;10:108354.
- [CrossRef] [Google Scholar]
- Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity. Chem. Eng. J.. 2016;300:280-290.
- [CrossRef] [Google Scholar]
- Direct Z-scheme ZnIn2S4/LaNiO3 nanohybrid with enhanced photocatalytic performance for H2 evolution. Int. J. Hydrogen Energy. 2020;45:4113-4121.
- [CrossRef] [Google Scholar]
- Hollow MoSe2@Bi2S3/CdS core-shell nanostructure as dual Z-scheme heterojunctions with enhanced full spectrum photocatalytic-photothermal performance. Appl. Catal. B-Environ.. 2021;281:119482
- [CrossRef] [Google Scholar]
- Solvothermal synthesis of CoO/BiVO4 p-n heterojunction withmicro-nano spherical structure for enhanced visible light photocatalytic activity towards degradation of tetracycline. Mater. Res. Bull.. 2021;135:111161
- [CrossRef] [Google Scholar]
- In situ synthesis of visible-light-driven Z-scheme AgI/Bi2WO6 heterojunction photocatalysts with enhanced photocatalytic activity. Ceram. Int.. 2019;45:6340-6349.
- [CrossRef] [Google Scholar]
- Boosted photogenerated carriers separation in Z-scheme Cu3P/ZnIn2S4 heterojunction photocatalyst for highly efficient H2 evolution under visible light-ScienceDirect. Int. J. Hydrogen Energy. 2020;45:14334-14346.
- [CrossRef] [Google Scholar]
- All-solid-state BiVO4/ZnIn2S4 Z-scheme composite with efficient charge separations for improved visible light photocatalytic organics degradation. Chin. Chem. Lett.. 2020;31(2):547-550.
- [CrossRef] [Google Scholar]
- Tuning surface electronegativity of BiVO4 photoanodes toward high-performance water splitting. Appl. Catal. B-Environ.. 2020;162:118267
- [CrossRef] [Google Scholar]
- Superior visible light photocatalytic performance of reticular BiVO4 synthesized via a modified sol-gel method. RSC Adv.. 2018;8:10654-10664.
- [CrossRef] [Google Scholar]
- The band engineering of 2D-hybridized PCN-Sb2MoO6-Bi2O3 nanomaterials with dual Z-scheme heterojunction for enhanced photocatalytic water splitting without sacrificial agents. Sustain. Energ. Fuels. 2021;5:2325-2334.
- [CrossRef] [Google Scholar]
- AgI/β-Ag2MoO4 heterojunctions with enhanced visible-light-driven catalytic activity. J. Taiwan Inst. Chem. E. 2017;81:225-231.
- [CrossRef] [Google Scholar]
- Ag3VO4/AgI composites for photocatalytic degradation of dyes and tetracycline hydrochloride under visible light. Mater. Lett.. 2018;216:216-218.
- [CrossRef] [Google Scholar]
- Fabrication of ternary CoO/g-C3N4/Co3O4 nanocomposite with p-n-p type heterojunction for boosted visible-light. J. Chem. Technol. Biotechnol.. 2021;96:1854-1863.
- [CrossRef] [Google Scholar]
- 0D/2D CeO2/ZnIn2S4 Z-scheme heterojunction for visible-light-driven photocatalytic H2 evolution. Appl. Surf. Sci.. 2020;526:145749
- [CrossRef] [Google Scholar]
- Fabrication of a novel AgI/LaFeO3/g-C3N4 dual Z-scheme photocatalyst with enhanced photocatalytic performance. Mater. Lett.. 2020;262:127029
- [CrossRef] [Google Scholar]
- In-situ fabrication of Bi2S3/BiVO4/Mn0.5Cd0.5 S-DETA ternary S-scheme heterostructure with effective interface charge separation and CO2 reduction performance. J. Mater. Sci. Technol.. 2022;117:109-119.
- [CrossRef] [Google Scholar]
- Piezoelectric effect synergistically enhances the performance of Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction photocatalytic degradation of pollutants. Appl. Catal. B-Environ.. 2021;291:120019
- [CrossRef] [Google Scholar]
- Direct dual Z-scheme Bi2WO6/GQDs/WO3 inverse opals for enhanced photocatalytic activities under visible light. ACS Sustain. Chem. Eng.. 2020;8:7921-7927.
- [CrossRef] [Google Scholar]
- Novel nanoarchitechtonics olive-Like Pd/BiVO4 for the degradation of gaseous formaldehyde under visible light irradiation. J. Nanosci. Nanotechno.. 2020;20:2689-2697.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104159.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







