Translate this page into:
The biobleaching potential of laccase produced from mandarin peelings: Impetus for a circular bio-based economy in textile biofinishing
⁎Corresponding authors. junuofin@gmail.com (John O. Unuofin), zkhetsha@cut.ac.za (Zenzile P. Khetsha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The quest for circular bioeconomy has been on the rise in recent years, and it is anticipated to fulfil the environmental sustainability aspect of the sustainable development goals (SDG 2030). In this regard, our investigation attempted the biotechnological appraisal of an enzymatic derivative of bacterial (Pseudomonas sp. HRJ16) mandarin peelings (MP) fermentation as a vehicle for an environmentally benign and sustainable textile bioscouring. Production of the bacterial exudate (HRJ16 laccase) was optimized by response surface methodology (RSM), using the common low-cost agroindustrial waste (MP). HRJ16 laccase was further assessed for its advantageous biochemical and catalytic properties, and then applied in synthetic dye decolorization and denim bleaching. Results emphasized the extremotolerance of the exudate to temperature, pH, salts, cations and surfactants, when at least ca. 80 % residual activity was recollected after exposure to the different extreme operating conditions. The interesting capabilities of the HRJ16 in this study culminated in its successful bioscouring of denim fabric over 6 h and the spontaneous decolorization of the resultant effluent. This constitutive properties of HRJ16 might make it a crucial catalyst for achieving a circular bioeconomy in the textile industry.
Keywords
Circular bioeconomy
Denim bleaching
Dye decolorization
Environmental sustainability
Laccase
1 Introduction
The European Commission (2012) remarks bioeconomy as: production of renewable biological resources and the conversion of these resources and waste streams into value added products, such as food, feed, bio-based products and bioenergy. In recent times, this remark has been nuanced into several other perceptions that would altogether promote resource efficiency and sustainability. For example, circular bioeconomy although mostly used discussed from the viewpoint of biomass valorization may also be adopted as an invaluable concept in industrial settings. This is especially expedient when trying to ensure resource conservation, as it upholds the zero-waste policy. Water is not only an essential resource in the textile industry, but also requisite for biological life and socio-economic development (Unuofin, 2020a). The textile industry is the second largest polluter of fresh water sources after agriculture, and it consumes not less than 79 trillion litres for annual production (Niinimaki et al., 2020). Sadly, a lot of volumes represent runoffs of single-use operations, which are often toxic and lack aesthetic appeal. This venture practised by the textile industry has overstretched availability of freshwater withdrawals for human day-to-day livings. The world’s population has been on a phenomenal increase in recent decades and so has her enthusiasm for fashion. Regrettably, there has not been any corresponding increase in the availability of freshwater resources for sustenance of humankind. A major contributor to the runoff of dye-sullied wastewater is the aggressive abrasion and bleaching of fabrics with nature-inimical chemicals, such as salts, ions, and micro-particles to give a stone-washed appearance (Unuofin 2020b; Niinimaki et al., 2020). This is a symbol of contemporary popular culture; as a result, pollution of water bodies seems an inescapable crisis begging for intervention. Following the enactment of UN Sustainable Development Goal on clean water and sanitation, water resource conservation has been regarded a critical issue and must be effectively addressed through sustainable approaches. This would imply the reuse and repurpose of spent freshwater resources; hence cost-effective solutions are highly sought after to achieve this goal, worldwide. Recent literatures have highlighted the need for a circular bioeconomy of the fashion industry, through responsible consumer behaviour as well as proper waste management practices which are discussed from the upstream purview of fabric in order to mitigate increased fossil energy inputs and atmospheric pollution (Ikram, 2022; Papamichael et al., 2022). However, our viewpoint enunciates the possibility of enzyme-enabled responsible manufacture or biofinishing of denim fabrics and textile water resource recycling or repurposing. Therefore, amongst the prospects for greener methods of manufacture of popular culture fabrics and treatment of wastewater, laccase has gained much espousal due to its simplicity of operation and tenacity in the event of unfavourable operational conditions. Traditionally, laccase oxidizes its substrates using molecular atmospheric oxygen as a co-substrate, which is reduced to water as a by-product (Unuofin et al. 2019a). This property makes it advantageous over other ligninolytic enzymes, which require the exogenous support of other chemicals. Moreover, its broad substrate specificity can be further enabled by electron shuttles known as mediators, which improves its dexterity (Unuofin et al. 2019a). In addition, its synthesis from low-cost environmental wastes makes it an ideal biochemical that epitomizes a circular bio-based economy (Odeniyi et al., 2017; Unuofin et al., 2019b,c). However, its perception as a circular bioeconomy catalyst in the textile industry has not been overtly documented. Xeric environments have been classified as extreme environments, where surviving microbial inhabitants do so through physiological, biochemical and molecular mechanisms essential for biotechnology (Lebre et al. 2017). It has been surmised that laccase secretion could serve as an adaptational, survival and developmental strategy by microorganisms under such extreme, nutrient-limiting conditions (Unuofin et al. 2019a; Arregui et al. 2019). Hence it is not unreasonable to assume that the laccases secreted under such conditions might possess robust characteristics for application in environmental technology. To our knowledge there are few studies that reported the utilization of bacterial and fungi cellulases and oxidases in denim bleaching and bioscouring (Pazarlioglu et al., 2005; Maryan and Montazer, 2013; Panwar et al., 2020; Zhang et al., 2022), albeit bacterial laccases have been preferred over fungal laccases due to their polyextremotolerance. Pseudomonad laccases have been extensively studied, especially with regard to their application in bioremediation of wastewater pollutants (Chauhan and Jha, 2018; Haq et al., 2022). However, to our knowledge, there is limited information on the biochemical and biocatalytic behaviour of Pseudomonas species isolated from xeric environments and their subsequent application in denim bleaching. This therefore warrants the first investigation of a xeric environment-isolated Pseudomonad laccase and its ability to enable a closed-loop cycle in the bleaching of denim. In our study, we assessed the ability of HRJ16 strain to optimally produce laccase possessing robust biochemical properties as well as great biobleaching capabilities from inexpensive agro residues.
2 Materials and methods
2.1 The inoculum
A laccase-producing bacterial strain coded Hb28a, isolated from lithospheric microbiota under xeric conditions, i.e. rock scrapings from a semi-arid region of South Africa (32°78′59″S and 26°84′85″E), was employed in this study. It was selectively enriched by orbital incubation (140 rpm at 30 °C) for 7 days in physiological saline supplemented with 1g/L kraft lignin, 0.02g/L of potassium hydrogen phthalate (PHP) and vanillin. Thereafter, fivefold serial dilutions of 1 mL aliquots in physiological saline was performed under aseptic conditions, where 500 µL volumes were spread on a mineral salt medium comprising (g/L): agar; 13.0, NaNO3; 2.6, K2HPO4; 0.4, KH2PO4; 0.6, MgSO4·7H2O; 0.5, NaCl; 0.5, which was supplemented with 1 g/L kraft lignin, 0.02 g/L PHP and 0.02 g/L vanillin, and then incubated at 30 °C for 7 days. It was further screened for laccase activity as described by Unuofin et al. (2019d), using 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,6 dimethoxyphenol (DMP), guaiacol, 1-naphthol, syringaldazine and potassium ferrocyanoferrate (PFC). The bacterial strain was selected on the basis of its oxidation of the aforementioned chemical substrates and was further identified using 16S rRNA sequence analysis as the pseudomonad, Pseudomonas aeruginosa HRJ16 (used henceforth as HRJ16) with accession number MF073259 in GenBank, National Center for Biotechnology Information (NCBI). Appropriate portions of axenic culture were maintained as glycerol stock at −80 °C or as working stock at 4 °C. Prior to further use, working stock culture was gradually adapted to room temperature before it was passed to freshly prepared broth, where it was standardized to OD600 nm of 0.8 after 18 h of incubation.
2.2 Screening, process optimization and laccase production
A one variable at a time (OVAT) was used to screen for significant cultural and nutritional factors, whereas statistical optimization (response surface methodology) was adopted to optimize laccase production. In this manner, the pH, temperature, agitation, carbon sources, nitrogen sources, agroindustrial residues, and aromatic and inorganic inducers, independent of other factors in the experiment. One percent (1 % w/v) carbohydrates (glucose, trehalose, xylose, cellobiose, fructose, galactose), and an aliphatic alcohol (glycerol), were varied as carbon sources, while 0.2 % w/v nitrogenous sources (NaNO3, KNO3, NH4NO3, l-asparagine, yeast extract, tryptone), and 0.05 % w/v inducers (CoCl2, CuSO4, ferulic acid, acetaminophen, 4-nitrophenol, vanillic acid, 2,5-xylidine) were screened for their respective contributions to laccase production. The impacts of agro-industrial residues, such as, maize stover (MS), wheat bran (WB), grape stalks (GS) and mandarin peelings (MP) were assessed. For response surface methodology (RSM), four cardinal independent variables observed from OVAT were inputted into a central composite design (CCD) generated by Design-Expert (Stat-Ease, Inc., Minneapolis, USA, version 10.0.3). The 30 experimental (3-level-4-factorial) runs produced by this algorithm were reproduced empirically in 100 mL Schott bottles containing 20 mL mineral salt medium (MSM) (stated infra) and inoculated with 400 µL standardized culture (1.53 × 108 cfu/mL). Thereafter, results were fit to a quadratic model Eq (1), which was further explored to understand and optimize the interactions of different variables.
Y = Responses.
K = Total number of independent factors.
Β0 = Intercept.
βi, βii, and βij = Coefficient values for linear, quadratic and interaction effects respectively.and xj indicate coded levels for independent variables.
All experiments were conducted in triplicates and bottles were incubated at 30 °C for 96 h except otherwise stated. The aforementioned MSM comprised (g/L pH citrate): KH2PO4; 0.514, K2HPO4; 0.32, KNaC4H4O6·4H2O; 0.32, NaCl; 0.08, MnSO·H2O; 0.032, MgSO4·7H2O; 0.192, CaCl2·2H2O; 0.008, CuSO4·5H2O; 0.0008, FeCl3·7H2O; 0.0008, ZnSO4; 0.0008. (Sigma-Aldrich, South Africa). Bulk fermentation for laccase production entailed supplementing MSM with mandarin peelings; 11.25 g/L, NaNO3; 0.375 g/L, 0.05 % w/v acetaminophen and orbital conditions of 100 rpm at 30 °C based on RSM numerical optimization outcomes. Aqueous crude laccase extracts were separated by cold centrifugation (4 °C) at 20,124 × g for 12 min. Laccase activity was measured as the oxidation of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) in a reaction comprising 50 µL appropriately diluted laccase, phosphate buffered (100 mM, pH 6) 2 mM ABTS, and 40 µL of 20 % v/v trichloroacetic acid (TCA) to terminate a 10 min reaction at 30 °C. The colorimetric output of ABTS oxidation was visualized at 420 nm (ε = 36,000 M−1 cm−1) with the aid of a SynergyMX 96-well microtitre plate reader (BioTek Instruments). One unit of laccase was extrapolated as the concentration that successfully oxidized 1 µmol of ABTS per min.
2.3 Biochemical characterization of laccase and molecular signature
Certain biochemical properties of crude HRJ16 laccase were evaluated in buffered solutions, according to Unuofin (2020b). Temperature optima was assessed at ranges between 0 °C and 90 °C by incubation in phosphate buffer (pH 6, 100 mM) for 30 mins, whereas temperature stability measurements were taken over 1440 mins for 40 °C −90 °C. Optimal pH was evaluated at 30 °C for 30 mins, while incubated in buffer ranging from pH 3–11 (citrate, phosphate and carbonate-bicarbonate) and stability measurements were observed for the same buffers over 455 mins. Effect of different concentrations of some metal ions (Mn2+, Cu2+, Fe2+, Fe3+, Ba2+, Zn2+, Mg2+, Co2+), surfactants (SDS, Tween 20), Chelator (EDTA) solvent (DMSO), halides (F-, Cl-) were assessed after 30 min preincubation with crude laccase. Correspondingly, substrate specificity studies were conducted in replicates, as described by the aforesaid study. Laccase-catalyzed oxidations of ABTS, guaiacol, syringaldazine (SGZ), α-naphthol and PFC in a phosphate buffer (100 mM; pH6) were monitored and estimated at the respective wavelengths and substrate extinction coefficients: 420 nm (ε = 36,000 M−1 cm−1), 470 nm (ε = 26,600 M−1 cm−1), 530 nm (ε = 65,000 M−1 cm−1), 520 nm (ε = 57,490 M−1 cm−1) and 420 nm (ε = 1023 M−1 cm−1). Briefly, 2 mM substrates in pH 6 potassium phosphate buffer were incubated at room temperature with 100 μL crude laccase, except for ABTS which was reacted with 60 μL of appropriately diluted laccase at 30 °C, allowed to react for 10 min and thereafter stopped with 40 μL 20 % TCA. Genomic DNA of HRJ16 was extracted according to the method of Unuofin et al. (2019e). Pellets of axenic HRJ16 were vortexed in nuclease free water and then boiled in an AccuBlock Digital dry bath (TECHNE, LAsec SA) at 100 °C for 10 mins and then centrifuged at 15,155 × g for 5 mins. The resulting supernatant contained the genomic DNA and was aseptically withdrawn for PCR. The PCR conditions for DNA amplification, with primers used, and the procedures for gel electrophoresis and visualization of target bands have been previously described (Unuofin et al., 2019e). The PCR mixture contained 5 μL template DNA, 12.5 μL 2 × OneTaq PCR MasterMix (Biolabs, South Africa), 1 μL (10 μM) of each forward and reverse primer, and was adjusted to a total volume of 25 μL with nuclease-free sterile water (Life Science). PCR amplification was run on a thermal cycler (G-STORM, UK) and the amplicons were run on 1.7 % w/v agarose gel (Merck, SA) in a Submarine Electrophoresis System (Mupid-One, Takara, ADVANCE Co., ltd. Japan) for 45 mins at 100 V, and thereafter had their bands visualized with the aid of ethidium bromide stains (Sigma-Aldrich, South Africa) in a transilluminator (ALLIANCE 4.7, France) with the UVITEC Cambridge software.
2.4 Dye decolorization and denim bioscouring
Fifty milliliters (50 mL) phosphate buffered homogenates (50 mM, pH 6) comprising 100 mg synthetic dyes and their respective colour index numbers (CI): Azure B 52,010 (AB), Malachite Green 42,000 (MG), Reactive Blue 4 61,205 (RB), Methyl Orange 13,025 (MO), Congo Red 22,120 (CR), and Brilliant Blue G 42,655 (BB) were prepared and incubated with HRJ16 laccase. Control treatments contained the decolourization mixtures without the crude laccases. Two milliliters (2 mL) of reaction mixture comprised 50 µL of crude laccase (416.4 U/mL) and 1950 µL dye solution in hermetically sealed tubes. All the decolourizing reactions were carried out at 30 °C and absorbances were intermittently recorded for over 80 h with the aid of a Synergy MX Microplate reader (BioTekTM) at the respective wavelengths (Unuofin, 2020b). Thereafter, their decolorization efficiencies were calculated as follows:
Where, Ainitial is the initial absorbance, and Aobserved is the observed absorbance.
The biobleaching potential of HRJ16 laccase was assayed on an indigo-dyed denim garment. Square portions (4 cm × 4 cm) of the fabric were snipped out and thereafter exposed to two treatments which comprised: (i) crude laccase and (ii) crude laccase + 2 mM ABTS. However, a treatment comprising 2 mM ABTS only was adopted as control. The setups were incubated in phosphate buffer (pH6; 100 mM) at 30 °C, 120 rpm for a period of 6 h. Thereafter, each swatch was gently rinsed in sterile deionized water to minimize abrasion of fabric during dye wash-down. The rinsed pieces were thereafter air-dried and observed under the dissection microscope (×30) (KYOWA TOKYO No.751252). Furthermore, their absorbance coefficients were evaluated at 300 nm to 900 nm wavelength range with a spectrophotometer (VWR® UV-3000PC), from which % reflectance was extrapolated through the following equation:
2.5 Data analysis
Results of replicates were pooled and expressed as mean ± standard deviation (SD) using Microsoft Excel Spreadsheet. Data were subsequently subjected to one-way analysis of variance (ANOVA) and the least significant difference was carried out. Significance was identified at P ≤ 0.05.
3 Results and discussion
The idea of circular bio-based economy in the fashion industry was articulated in response to the need for its alignment with sustainable development goals, and indeed sustainability (Ikram, 2022; Papamichael et al., 2022). This entails the substitution of synthetic finite materials and chemicals as well as conventional processes with open-ended environmental footprints and feedback, with greener, natural, sustainable resources and processes that would promote the recycling, upcycling or downcycling of textile feedstock and process waters. Although not fully explored, textile biofinishing has a potential to derive beneficial application in recent fashion trends, due its ecofriendly procedures. In this study, a pseudomonad laccase was appraised for its potentials in industrial and environmental applications, particularly its ability to effect a cleaner hue enhancement of a denim. The laccase producing strain was isolated from a xeric environment (rock scraping) and has demonstrated its ability to secrete laccase using inorganic nitrogen and organic carbon sources (Figs. 1a and b). It had been isolated based on selective enrichment and its ability to oxidize some traditional laccase substrates during qualitative screening that has been described in previous studies (Unuofin et al. 2019d). In this regard, it was able to oxidize all five substrates, although we assumed the oxidation of at least three was the major criterion for selection. Its further identification as Pseudomonas confirms the ubiquity and adaptability of this bacteria genus to a decent range of extreme environments (Chiellini et al., 2019). However, this might be made possible by some adaptive mechanisms (Lebre et al. 2017). Amongst the respective nutrient sources, NaNO3 and glycerol gave an optimal laccase yield of ca. 29.92 U/mL (Fig. 1a) and ca. 6.6 U/mL (Fig. 1b), respectively. Glycerol, a polyol has been well reported to induce constitutive laccase production in white rot fungi and yeasts (Pezella et al., 2017). However, to our knowledge, this study remains among the rare cases of the facilitation of optimal bacterial laccase secretion by glycerol. Recent studies have highlighted the preference of the inorganic nitrate, NaNO3, for optimal production of laccase; albeit the secreting strains were isolated from organic nitrogen-rich environments (Unuofin et al., 2019b,c). Other optima for cultural and nutritional parameters assessed include pH 5 (ca. 3.8 U/mL), acetaminophen (ca. 0.15 U/mL), 100 rpm (ca. 20 U/mL) and mandarin peelings (ca. 30 U/mL). These optima portray the need for weakly acidic conditions and gentle agitation for constitutive laccase production. However, a major highlight was the phenomenal contribution of the agroindustrial residue, mandarin peelings (MP). A fourfold turnover in laccase production was observed for MP, when compared with glycerol, a three-carbon substrate. Kachlishvili et al. (2021) reported the enhanced production of laccase in response to increasing MP concentrations; whereas a shortened time course for laccase was observed using the same substrate, compared to other carbon sources (Elisashvili et al. 2018). However, the aforementioned investigations focused on laccase production from white-rot basidiomycetes (WRB), which have already been established as excellent laccase producers. In our study, this phenomenon might be indicative of our strain’s preference of complex carbon substrates (lignocellulose) for profuse laccase production. Similar outcomes have been observed by Unuofin et al. (2019b,c), and is of particular interest in the biotechnology for biofuels.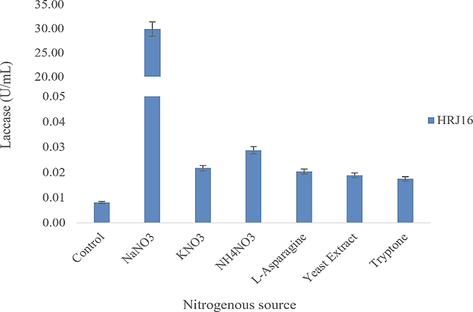
Effect of nitrogen sources on laccase production.
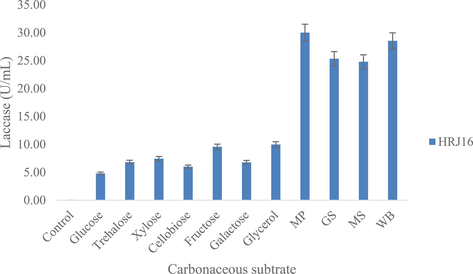
Effect of carbon sources on laccase production.
Trends observed from the preliminary screening identified pH 5, NaNO3, 100 rpm and MP as cardinal factors which should be further exploited to stimulate even better laccase turnover. In the regard, they were employed in the central composite design (CCD) RSM algorithm, which proposed a reduced cubic model (Table 1a). Here, the significant interactions amongst the selected variables were expressed at P < 0.05, where the interactive influences of pH-agitation (AC), mandarin peelings-agitation (BC) mandarin peelings-NaNO3 (BD), pH-mandarin peelings-agitation (ABC), and pH- mandarin peelings-NaNO3 (ABD) were presented as crucial to overall laccase production by the model. Correspondingly, the nature of their respective contribution was fully elucidated by eq. (7), where positive and negative coefficients implied positive impact and negative impacts, respectively.(See Table 1b).
R2 = 0.9387; Adj R2 = 0.8815; Pred R2 = 0.7361; Adeq Precision = 13.702.
Sum of
Mean
F
p-value
Source
Squares
df
Square
Value
Prob > F
Model
24.33
14
1.74
16.40
< 0.0001
significant
A-pH
10.08
1
10.08
95.12
< 0.0001
B-Mandarin Peel
1.64
1
1.64
15.50
0.0013
C-Agitation Speed
2.07
1
2.07
19.53
0.0005
D-NaNO3
0.32
1
0.32
3.01
0.1031
AB
0.10
1
0.10
0.99
0.3360
AC
2.30
1
2.30
21.68
0.0003
AD
0.17
1
0.17
1.64
0.2200
BC
0.87
1
0.87
8.17
0.0119
BD
0.61
1
0.61
5.78
0.0296
CD
0.35
1
0.35
3.30
0.0895
A2
0.33
1
0.33
3.11
0.0981
C2
0.50
1
0.50
4.72
0.0463
ABC
1.64
1
1.64
15.50
0.0013
ABD
1.22
1
1.22
11.50
0.0040
Residual
1.59
15
0.11
Lack of Fit
1.34
10
0.13
2.72
0.1402
not significant
Pure Error
0.25
5
0.049
Cor Total
25.91
29
Independent variables (X)
Dependent variables (ln Y) Laccase Activity (U/mL)
Run Order
pH
Mandarin peelings (g/200 mL)
Agitation speed (rpm)
NaNO3 (g/ 200 mL)
Actual value
Predicted value
1
3
2
50
0.1
1.77
1.69
2
5
2
100
0.1
4.36
4.19
3
4
1.5
75
0.15
2.12
1.68
4
4
1.5
75
0.15
1.69
1.68
5
3
1
100
0.1
1.73
1.78
6
4
1.5
50
0.15
2.13
2.40
7
4
1
75
0.15
1.31
1.37
8
3
1
50
0.1
1.18
1.23
9
5
1
50
0.1
3.70
3.62
10
3
2
50
0.2
1.91
2.09
11
3
2
100
0.1
1.72
1.89
12
3
2
100
0.2
1.72
1.69
13
3
1
50
0.2
1.25
1.31
14
5
1
100
0.1
1.52
1.37
15
5
1
100
0.2
1.57
1.55
16
5
1.5
75
0.15
1.92
2.73
17
5
2
100
0.2
2.49
2.48
18
4
1.5
75
0.15
1.60
1.68
19
4
2
75
0.15
1.86
1.98
20
5
1
50
0.2
4.52
4.38
21
4
1.5
75
0.1
1.55
1.81
22
4
1.5
100
0.15
1.66
1.72
23
4
1.5
75
0.15
1.66
1.68
24
3
1
100
0.2
1.16
1.27
25
5
2
50
0.2
3.26
3.10
26
4
1.5
75
0.15
1.66
1.68
27
5
2
50
0.1
4.30
4.22
28
4
1.5
75
0.15
2.03
1.68
29
3
1.5
75
0.15
1.73
1.24
30
4
1.5
75
0.2
1.57
1.54
In this regard, pH, agitation and carbon source were prominent influencers of laccase production, individually, and their corresponding inverse relationships, particularly the pH-agitation interaction translated into optimal laccase production (Fig. 2). This particular outcome coincides with an earlier study where a Citrobacter strain was employed (Unuofin et al., 2019c). Conversely, when these cardinal parameters were kept constant at respective maximum limits (pH: 5, agitation: 100 rpm), overall laccase production of the study (244.92 U/mL) was observed to be exerted by a high carbon–nitrogen ratio (30:1). This simply infers an inverse relationship between mandarin peelings and NaNO3. Interestingly, this outcome was forecasted by variables outside the defined limits of the design matrix in a confirmation experiment during numerical optimization of the model that predicted a value of 244.15 U/mL, and it connotes the algorithm’s insignificant lack-of-fit. The profuse secretion of laccase under this condition is not unreasonable to comprehend, since laccase has been well thought to be stimulated by nitrogen limiting conditions. However, care must be also taken to find a stoichiometric balance during large scale production as excess amounts of monosaccharides will instead favour the synthesis of exopolysaccharides than laccase. In this regard, it will be necessitous to take into consideration, the intrinsic properties of the lignocellulosic biomass being employed for laccase production, which might also inform the type of pre-treatment such biomass must undergo before adoption as feedstock.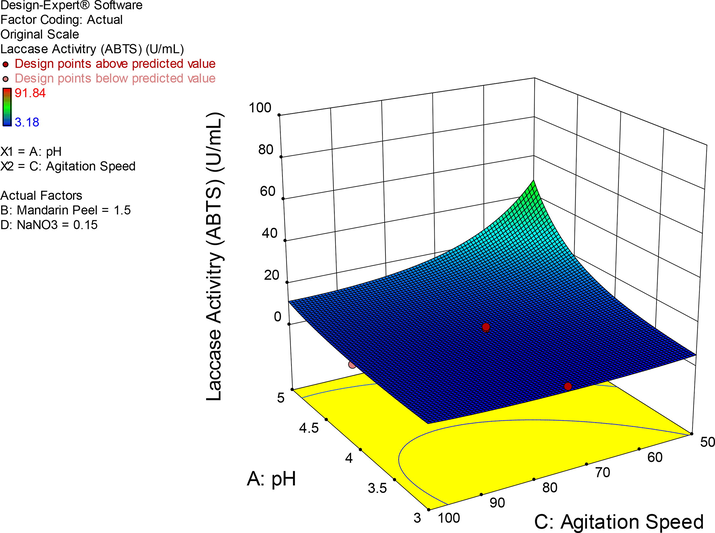
Response of the interaction of pH and agitation speed at constant concentrations of MP and NaNO3.
3.1 Biochemical and molecular features of HRJ16 laccase
Optimal reaction conditions, such as pH 8 and 70 °C were recorded for HRJ16 laccases (Fig. 3a and Fig. 3b). Correspondingly, the relative activity profile of the aforementioned parameters tested portrayed the enzyme as a thermophilic, neutral to alkaline-enabled protein. A spore laccase of Bacillus amyloliquefaciens had an optimum pH and temperature of 7.5 and 60 °C (El-Bendary et al., 2021); whereas Edoamodu and Nwodo (2021) recorded pH 6 and 80 °C as optimal regime for laccase activity in Enterobacter asburiae ES1. Our statistics for laccase activity were matched by an impressive stability record at pH 3–11 over 455 mins as well as 40 °C – 90 °C, over 400 mins (Fig. 4a and Fig. 4b); albeit incidental evaluations at 1140 and 1440 mins revealed detectable activities of at least ca. 60 % residual laccase. A similar trend was observed in an Achromobacter specie (Unuofin et al., 2019e); however, stability properties exhibited thereof were at least ca. 100 % for all pH regimes over 455 mins and at least ca. 92 % for temperatures spanning 40 °C and 80 °C, over 1440 mins, respectively. In comparison, Alcaligenes faecalis XF1 laccase achieved at least ca. 80 % stability at 60–80 °C over 120 min, while a 10-day stability of at least 80 % was observed at pH 6–8 (Mehandia et al., 2020). Essentially, pH and temperature extremes are what characterizes most industrial reaction conditions. Subsequently, these parameters are for the most part unchanged during toxic waste discharge up till certain distances downstream from initial contact point. Knowing these conditions hardly change, it is necessary to appropriately employ thermotolerant green catalysts as bacterial laccases to treat wastes, prior to discharge. On incubation with different concentrations of some cations, chelator and surfactant (Fig. 4c), HRJ16 laccase exhibited remarkable stability, presenting residual activities that insinuated inducement especially notable with Mn2+ (2.5 mM and 7 mM), Cu2+ (2.5 mM and 5 mM), and Co2+ (1 mM). In a study by Motamedi and co-authors (2021) on a metagenome derived laccase (PersiLac2), 10 mM concentrations of K+, Li2+, Mg2+, Fe2+, Zn2+, Al2+, Ni2+, Mn2+ elicited residual activities beyond 250 %, while 600 mM and 800 mM Cu2+ stimulated laccase activity beyond 200 %. However, the major highlight was the overexpression of activity due to the comparative stimulation of different concentrations of chelator and surfactant (Fig. 4c). These particular outcomes coincide with a previous study, where all concentrations of Mn2+, Fe3+, Ba2+, Cu2+, EDTA and SDS effected progression of residual activity beyond the control (Unuofin, 2021). However, this positive stimulation was not observed for Motamedi et al. (2021), who recorded noticeable inhibitory activities of some surfactants, albeit at higher concentrations (10 mM and 25 mM). Our results further demonstrate the capacity of HRJ16 laccase as an essential green chemical for commercial applications where heavy metals, surfactants or chelators would be a barrier. Particularly, the laccase was able to thrive in the presence of the chelator (EDTA), which will have otherwise impeded its catalytic proficiency. Other biochemical characteristics portrayed by HRJ16 laccase, which are worthy of mention include tolerance of high concentrations of NaCl, DMSO and Tween 20 (Fig. 4d) as well as its remarkable inducement by the most potent inhibitory halide NaF (Fig. 4e). Similarly, DMSO at 5 %, 10 % and 20 % v/v concentrations elicited a residual activity beyond 100 %, while 1 M and 2 M concentrations of NaCl correspondingly stimulated residual activities beyond 100 % (Motamedi et al., 2021). In another study, the non-ionic surfactant, Tween 20 recorded at least 80 % residual activity at 0.1 mM, 1.0 mM and 5.0 mM (Sondhi et al., 2021), while the inhibitory activity of NaF was expressed at 10 mM thereby permitting about 1 % residual activity (Sondhi et al., 2021). From the extrapolation of a kinetic plot (R2 = 0.982) (Fig S2), HRJ16 laccase was presumed to elicit a Km of 2.6 µM, Kcat of 1.85 × 104 S-1 and a specificity constant of 6.94 × 103 µM−1 s−1 (Table 2). This indicates a strong affinity HRJ16 laccase has for the ABTS assay substrate as well as the spontaneity and rapidity at which substrate molecules are being transformed to products or intermediates. A further look at Table 2 portrays its comparison to other laccases. This trend had been earlier detected during assessment of substrates for laccase catalytic compatibility (Fig. 5), where ABTS and PFC were more readily transformed than their organic confrére. Moreover, they may serve as excellent electron shuttles to initiate the catalytic oxidation of larger or unreactive substrates, hence affording laccase a wide range of unorthodox applications in environmental biotechnology.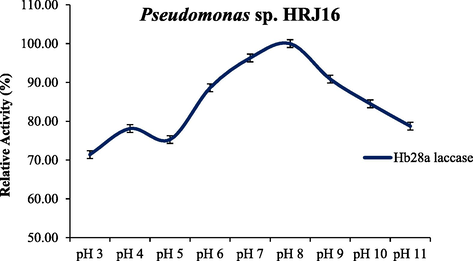
Effect of pH regime on HRJ16 laccase activity.
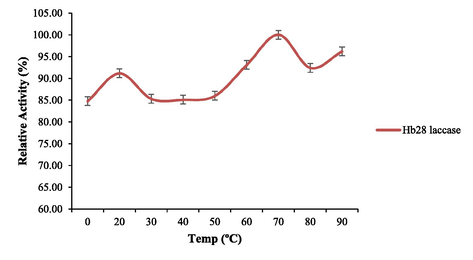
Effect of temperature profile on HRJ16 laccase activity.
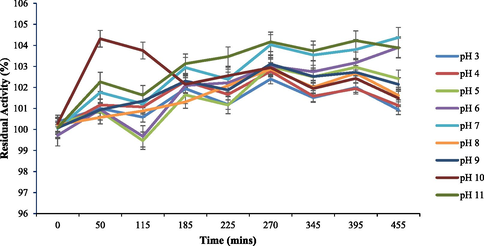
pH stability exhibited by HRJ16 laccase at 30 °C.
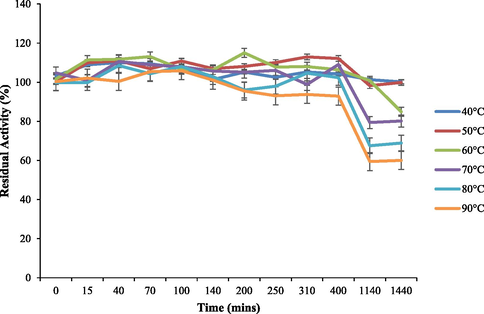
Thermostable behaviour of HRJ16 laccase at pH 6 (100 mM phosphate buffer).
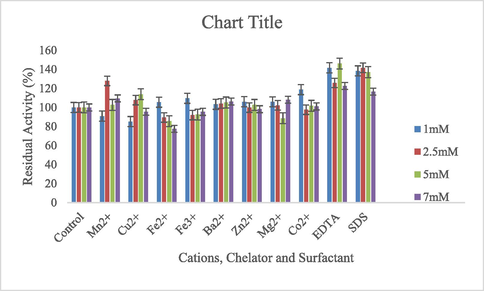
HRJ16 laccase response to varying concentrations of cations, chelator and surfactant.
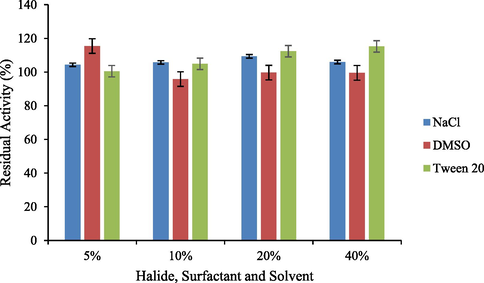
Response of HRJ16 laccase to halide (NaCl), surfactant (Tween 20) and solvent (DMSO).
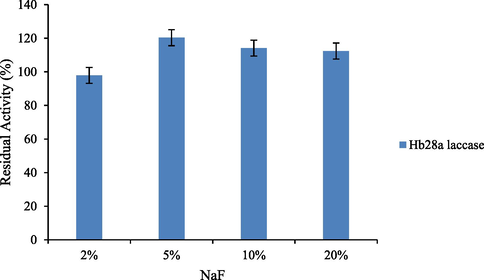
Effect of increasing NaF concentrations on HRJ16 laccase stability.
Strain
Substrate
pH
Temperature
Km (µM)
Kcat (s−1)
Kcat/Km (µM−1 s−1)
Reference
Pseudomonas sp. HRJ16 laccase
ABTS
6
30 °C
2.67
1.85 × 104
6.94 × 103
This study
Bacillus sp. MSK-01
ABTS
4.5
37 °C
1624
177.04
0.109
Sondhi et al. (2021)
Alcaligenes faecalis
2,6-DMP
8
80 °C
500
1243.75
2.4875
Mehandia et al. (2020)
Pp4816 (recombinant)
ABTS
3.4
24 °C
0.31
10.1
32.6
Olmeda et al. (2021)
Pa5930 (recombinant)
ABTS
3.4
24 °C
0.33
2.31
7.0
Olmeda et al. (2021)
rlac1338
ABTS
6.5
55 °C
210
34.39
0.16
Dai et al. (2021)
lac2-9
ABTS
6.5
55 °C
76
46.94
0.62
Dai et al. (2021)
Pseudomonas putida KT2440
ABTS
4
20 °C
488
2.40
4900 ± 90
Granja-Travez and Bugg 2018
Pseudomonas fluorescens Pf-5
ABTS
4
20 °C
214
2.2
10300 ± 160
Granja-Travez and Bugg 2018
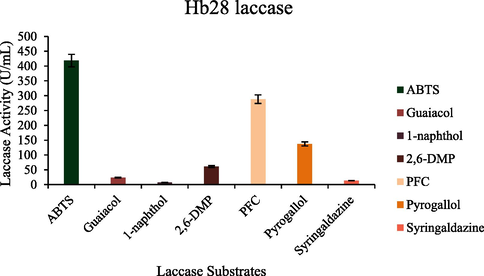
Substrate specificity of HRJ16 laccase.
The molecular snapshot survey of laccase producing HRJ16 used in this study revealed it to have multiple homologous genes (Fig. 6), which might be suggestive of their participation in diverse morpho-functional activities. In essence, such genes might comprise constitutive, which are regarded as housekeeping genes, and inducible loci, which are only expressed under certain environmental stresses just as was corroborated by Yan et al. (2019). Multiple laccase genes have been reported in plants (Arcuri et al., 2020; Liu et al., 2020), animals, especially herbivorous insects (Chen et al., 2020), fungi (Torres-Farrada et al., 2017) and bacteria (Feng et al., 2015), and they may comprise of constitutive and inducible genes. The benefit of having such retinue of gene loci would be especially derived from applications beyond the spectrum of constitutively expressed laccase. Here, an operon switch would permit the seamless secretion and catalytic transformation of recalcitrant toxic wastes.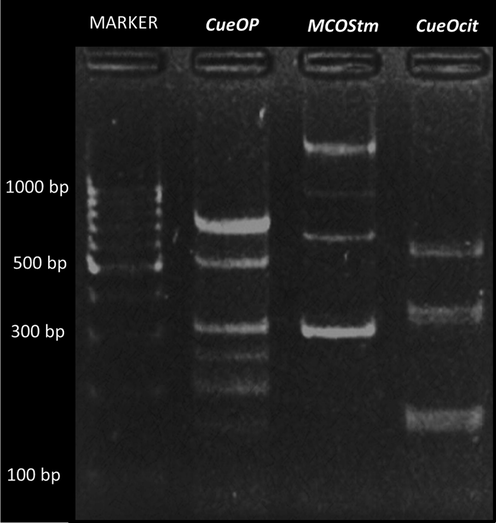
Gel representation (1.7 % w/v) of putative HRJ16 laccase genes amplified by PCR. Lane 1: molecular marker, lane 2: CueOP, lane 3: MCOStm, lane 4: CueOcit. The band sizes of laccase encoding genes can be observed with respect to the molecular marker and are as follows: CueOP; 156, 222, 261, 307, 559 and 745 bp; MCOStm; 303,653, 1025 and1483 bp; CueOcit;164, 339 and 542 bp.
3.2 Biodecolorization and bioscouring capability of HRJ16 laccase
Astoundingly high concentrations (0.2 % w v-1) of synthetic dyes of the heterocyclic cationic, triarylmethane, azo and anthraquinone groups were degraded over 80 h without the exogenous intervention of redox mediators (Fig. 7a). Specifically, AB was visibly decolorized from 16 h (ca. 49.3 %) of incubation up till terminal incubation period. This pattern differs markedly from the linear pattern it had portrayed in earlier studies (Unuofin, 2020b,c). Conversely, other synthetic dyes exhibited linear decolourization patterns, with marginal differences in decolorization occurring as incubation approached terminal stages. Navas et al. (2020), without the intervention of redox mediators, achieved increased decolorization of Methyl Orange at 60 °C over 24 h (ca. 50.95) under acid conditions (pH 5). Decolorization was minimal at neutral (pH 7: ca. 24.36) and alkaline conditions (pH 9: ca. 33.45); whereas only pH 5 was reported for effective decolorization of malachite green at 24 h (ca. 71.59). In another study, a 5 day treatment of Congo Red and Reactive Blue 4 at 37 °C elicited 16.43 % and 41.61 % decolorization, respectively (Liu et al. 2021); where a minimum decolourization was observed in BB, as only ca. 17.5 % decolourization was permitted. To our knowledge, the high substrate (dye) concentration might be responsible for the low levels of decolorization, which could have originated from a dead-end substrate-enzyme-intermediate complex. Moreover, steric hinderances that might be caused by the atomic orientation of the dyes could make laccase accessibility relatively unachievable. This, therefore, warrants further work, especially with regard to enzyme engineering, to enable the enzyme’s resilience and dexterity towards unorthodox substrates and their intermediates, as they may promote the degradation of heterogeneous toxic wastes. Reports regarding decolorization of high concentrations of dyes are gradually being documented, however, studies published by Bagewadi et al. (2017), Zhuo et al. (2017) and Kumari et al. (2018) are the closest coincidence to this present study.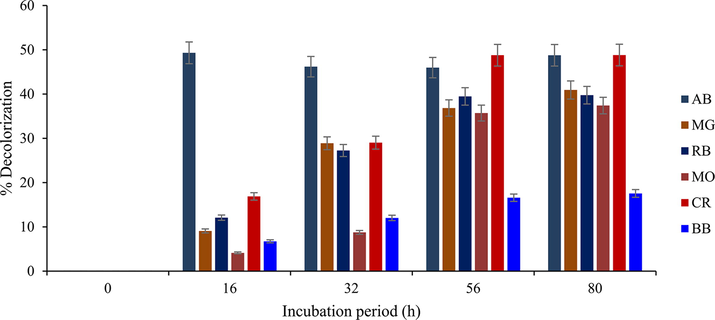
Decolourization pattern exhibited by HRJ16 laccase at 30 °C on 0.2 % synthetic dyes viz AB; azure B, MG; malachite green, RB; reactive blue, MO; methyl orange, CR; congo red, BB; brilliant blue. Phosphate buffer was used, with no mediator.
Experiential dye tainting of the submerging medium was not drastic when only HRJ16 laccase were added to indigo fabric. However, a heterogeneous mix comprising HRJ16 laccase and a mediator (ABTS) (E + M) was sufficient to stimulate a progression of the medium’s colour from colourless to green, then indigo over 6 h (Fig S3). This began immediately after exogenous supply of TCA – a component of the laccase assay, which is suggestive of laccase oxidation of ABTS. Furthermore, the resultant indigo colour of the medium, which is the original pigment of the fabric evinced the empirical biobleaching of denim. A parallel experiment which had all other components of the reaction mixture, except laccase, ascertained the non-interference of TCA, since it is a potent bleaching agent. It could therefore be surmised that laccase biobleaching activity is experientially enhanced by an oxidized mediator, as corroborated by Solís-Oba et al. (2008). The aforementioned investigators through colorimetric assessment showed that the oxidized mediator-treated fabric had the brightest hue. Zhang et al. (2018) reported that a reaction mix comprising manganese peroxidase in malonate buffer with Mn2+ and 1-hydroxybenzotriazol (HBT: mediator), but without H2O2, could produce little or no biobleaching effects. Moreover, experiential bleaching effects were triggered when laccase was added to the mix. This phenomenon not only consolidates laccase’s status as an effective biobleaching agent but also reveals its potential as a vehicle for generating H2O2. In our study, a brighter hue was observed in fabrics that had undergone zymo-modification, however, it was brightest, post-treatment, with oxidized mediator (Fig. 7b Inset). This phenomenon was noted through colorimetric assessment by Solís-Oba et al. (2008), albeit there was no striking difference between their untreated and enzyme-modified fabric. This implies that HRJ16 laccase only requires oxidized mediators for acceleration of bleaching. Other mediators successfully applied include violuric acid (Iracheta-Cárdenas et al., 2016) and HBT (Yavuz et al., 2014). Consequently, other oxygenases might possess the denim bioscouring ability; this has been confirmed in a recent study (Zhang et al., 2020), where the singular and synergistic actions of manganese peroxidase and laccase (Zhang et al. 2022) produced enhance denim biobleaching at an acidic pH (4.8). This outcome coincided with Panwar et al. (2020), who observed improved reflectance at pH 4, but marginal or no reflectance at pH 8. With regard to reflectance, the constrast between the control (untreated) and the experimental (treated) is comparable to what was observed by Iracheta-Cárdenas et al. (2016), where the treated fabric recorded a higher reflectance reading (Fig. 7b). According to Tian et al. (2013) and references therein, Indigo dye is transformed by laccase oxidization, and it changes from its deep blue colour, through light blue to yield isatin (indole-2, 3-dione), a red substance, and further decomposed to anthranilic acid (2-aminobenzoic acid), which is a colourless substance. However, the spontaneous stepwise decolorization of the denim leachate, hours after the initiation of bleaching (Unuofin, 2020c) portrays a different colour transformation, though it completely transformed to a colourless solution. Although the mechanisms that govern this interesting outcome have not been satisfactorily ratiocinated, efforts are being made to understand the mechanism of this dual reaction (denim bleaching and dye decolorization). This is hoped to create a seamless dyeing of denims and the concomitant cleaning of dye baths. Moreover, intermediates formed during decolorization might serve as valuable feedstock for various industrial applications and should be characterized in future studies. The decolorization of denim leachates might not fix all the puzzles that riddle the textile industry, but it is indeed a major step towards achieving a circular biobased economy therein.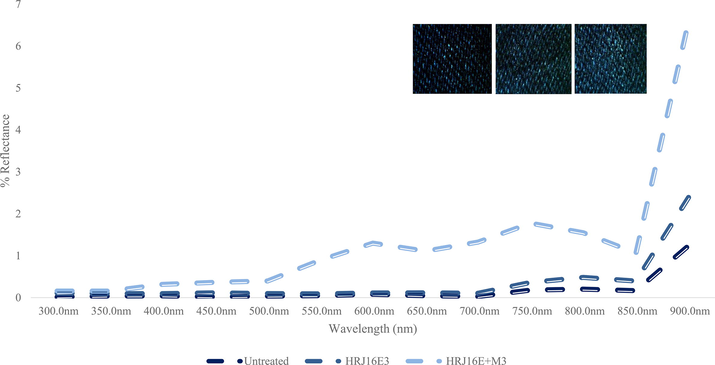
Spectrum plot of Pseudomonas sp. HRJ16 laccase treated denim. HRJ16E3 represents enzyme only treatment, whereas HRJ16E3 + M3 represents enzyme + mediation treatment. Inset: experiential bleaching of denim fabric showing, from left to right: Untreated, HRJ16 laccase and HRJ16 laccase + mediator.
4 Conclusion
In this study, we described a laboratory-scale attempt at biobleaching denim using a crude laccase from HRJ16, which was isolated from a xeric environment. Here, the bacterial strain, isolated by selective enrichment, was able to secrete copious amounts of HRJ16 laccase with an activity of 244.92 U/mL from an environmental waste (MP) through an optimized statistical design (RSM), which suggested a high carbon to nitrogen ratio (30:1) and improved the ergonomics of production. Interestingly, the enzyme produced (HRJ16 laccase) portrayed polyextremotolerance- an attribute we ascribe to the presence of multiple laccase-encoding genes, especially to some chemical agents and environmental conditions renowned as potential bottlenecks of real-life laccase applications. The robustness of this bacterial laccase was further verified by its ability to not only wash the indigo dye off the fabric, but also proceed to decolorize the resultant effluent in a 6 h trial; hence, providing a potential solution to water pollution by the textile industry. In this regard, it is pressingly necessary that further studies be carried out to recreate this phenomenon on a larger scale and with the fortification of omics and enzyme engineering, in order to enable its commercialization.
Acknowledgements
The authors gratefully acknowledge the financial support from the South Africa Medical Research Council (SAMRC), JOU was supported by the National Research Foundation [Grant No: 138445] and ZPK was enabled by Central University of Technology, Free State. Prof AI Okoh, UU Nwodo and Dr. Onele MN Gcilitshana are duly acknowledged for their support.
Author contributions
JOU conceptualized the study, performed experiments, analysed data and wrote first draft; KMM participated in writing and review; ZPK provided funds and reviewed the manuscript. All authors gave approval of the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Genome-wide identification of multifunctional laccase gene family in Eucalyptus grandis: Potential targets for lignin engineering and stress tolerance. Trees 2020:1-14.
- [Google Scholar]
- Arregui, L., Ayala, M., Gomex-Gil, X., Gutierrez-Soto, G., Hernandez-Luna, C.E., de los Santos, M.H., Levin, L., Rojo-Dominguez, A., Romero-Martinez, D., Saparrat, M.C.N., Trujillo-Roldan, M.A, and Valdez-Cruz, N.A. (2019). Laccases: structure, function and potential application in water bioremediation. Microb. Cell Fact. 18, 200.
- Purification and immobilization of laccase from Trichoderma harzianum strain HZN10 and its application in dye decolorization. J. Genet. Eng. Biotechnol.. 2017;15:139-150.
- [Google Scholar]
- Pilot scale production of extracellular thermos-alkali stable laccase from Pseudomonas sp S2 using agro waste and its application in organophosphorus pesticides degradation. J. Chem. Technol. Biotechnol.. 2018;93:1022-1030.
- [Google Scholar]
- A novel Laccase gene from Litopenaeus vannamei is involved in the immune responses to pathogen infection and oxidative stress. Develop. Comparat. Immunol.. 2020;105:103582
- [Google Scholar]
- Pseudomonas strains isolated from different environmental niches exhibit different antangonistic ability. Ethol. Ecol. Evol.. 2019;31(5):399-420.
- [Google Scholar]
- European Commission, (2012). Innovating for sustainable growth: A bioeconomy for Europe. Brussels 2012.
- Dai, S., Yao, Q., Yu, G., Liu, S., Yun, J., Xiao, X., Deng, Z., and li, H. (2021). Biochemical characterization of a novel laccase and improvement of its efficiency by directed evolution on dye degradation. Front. Microbiol. 12, 633004.
- Edoamodu, C.E., and Nwodo, U.U. (2021). Marine sediment derived bacteria Enterobacter asburiae ES1 and Enterobacter sp. Kamsi produce laccase with high dephenolisation potentials. Prep. Biochem. Biotechnol. DOI: 10.1080/10826068.2021.1992781.
- El-BeEndary, M.A., Ezzat, S.A., Ewais E.A., and Al-Zalama, M.A. (2021). Optimization of spore laccases production by Bacillus amyloliquefaciens isolated from wastewater and its potential in green biodecolorization of synthetic textiles dyes. Prep. Biochem. Biotechnol. 51(1), 16-27.
- Efficient production of lignin-modifying enzymes and phenolics removal in submerged fermentation of olive mill by-products by white-rot basidiomyctes. Int. Biodeteriorat. Biodegrad.. 2018;134:39-47.
- [Google Scholar]
- Laccase activity is proportional tothe abundance of bacterial laccase-like genes in soil from subtropical arable land. World J. Microbiol. Biotechnol.. 2015;31:2039-2045.
- [Google Scholar]
- Characterization of multicopper oxidase CopA from Pseudomonas putida KT2440 and Pseudomonas fluorescens Pf-5: Involvement in bacterial lignin oxidation. Arch. Biochem. Biophys.. 2018;660:97-107.
- [Google Scholar]
- Genotoxicity evaluation of paper industry wastewater prior and post-treatment with laccase producing Pseudomonas putida MTCC 7525. J. Clean Prod.. 2022;342:130981
- [Google Scholar]
- Transition toward green economy: Technological Innovation’s role in the fashion industry. Curr. Opin. Green Sust. Chem.. 2022;37:100657
- [Google Scholar]
- A Pycnoporus sanguineus laccase for denim bleaching and its comparison with an enzymatic commercial formulation. J. Environ. Manag.. 2016;177:93-100.
- [Google Scholar]
- Enhancement of laccase production by Cerrena unicolor through fungal interspecies interaction and optimum conditions determination. Arch. Microbiol.. 2021;203:3905-3917.
- [Google Scholar]
- Characterization of a mildly alkalophilic and thermostable recombinant Thermus thermophiles laccase with applications in decolourization of dyes. Biotechnol. Lett.. 2018;40:285-295.
- [Google Scholar]
- Xerotolerant bacteria: surviving through a dry spell. Nat. Rev. Microbiol.. 2017;15(5):285-296.
- [Google Scholar]
- Chemically induced oxidative stess improved bacterial laccase-ediated degradation and detoxification of the synthetic dyes. Ecotoxicol. Environ. Saf.. 2021;226:112823
- [Google Scholar]
- Evolutionary divergence of function and expression of laccase genes in plants. J. Genet.. 2020;99(1):1-16.
- [Google Scholar]
- A cleaner production of denim garment using one step treatment with amylase/cellulase/laccase. J. Clean. Prod.. 2013;57:320-326.
- [Google Scholar]
- Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnol. Rep.. 2020;25:e00413.
- [Google Scholar]
- Effcient removal of various textile dyes from wastewater by novel thermo-halotolerant laccase. Bioresour. Technol.. 2021;331:125468
- [Google Scholar]
- Fast decolorization of azo dyes in alkaline solutions by a thermostable metal-tolerant bacterial laccase and proposed degradation pathways. Extremophiles. 2020;24:705-719.
- [Google Scholar]
- The environmental price of fast fashion. Nat. Rev. Earth Environ.. 2020;1(4):189-200.
- [Google Scholar]
- Production characteristics, activity patterns and biodecolourisation applications of thermostable laccases from Corynebacterium efficiens and Enterobacter ludwigii. J. Sci. Ind. Res.. 2017;76:562-569.
- [Google Scholar]
- Structural analysis and biochemical properties of laccase enzymes from two Pediocioccus species. Microb. Biotechnol.. 2021;14(3):1026-1043.
- [Google Scholar]
- Sustainable denim bleaching by a novel thermostable bacterial laccase. Appl. Biochem. Biotechnol.. 2020;192:1238-1254.
- [Google Scholar]
- Building a new mind set in tomorrow fahion development through circular strategy models and framework of waste management. Curr. Opin. Green Sust. Chem.. 2022;36:100638
- [Google Scholar]
- Laccase: production by Trametes versicolor and application to denim washing. Process Biochem.. 2005;40:1673-1678.
- [Google Scholar]
- A step forward in laccase exploitation: recombinant production and evaluation of techno-economic feasibility of the process. J. Biotechnol.. 2017;259:175-181.
- [Google Scholar]
- Biotechnological treatment for colorless denim and textile wastewater treatment with laccase and ABTS. Rev. Int. Contam. Ambie.. 2008;24(1):5-11.
- [Google Scholar]
- Purification and characterization of a novel white highly thermo stable laccase from a novel Bacillus sp. MSK-01 having potential to be used as anticancer agent. Int. J. Biol. Macromol.. 2021;170:232-238.
- [Google Scholar]
- Decolorization of indigo dye and dye-containing textile effluent by Ganoderma weberianum. Afr. J. Microbiol. Res.. 2013;7(11):941-947.
- [Google Scholar]
- Diversity of ligninolytic enzymes and their genes in strains of the genus Ganoderma: applicable for biodegradation of xenobiotic compounds? Front. Microbiol.. 2017;8:898.
- [Google Scholar]
- Garbage in garbage out: the contribution of our industrial advancement to wastewater degeneration. Environ. Sci. Poll. Res.. 2020;27(18):22319-22335.
- [Google Scholar]
- Treasure from dross: application of agroindustrial wastes-derived thermo-halotolerant laccases in the simultaneous bioscouring of denim fabric and decolorization of dye bath effluents. Ind. Crop. Prod.. 2020;147:112251
- [Google Scholar]
- Sustainability potentials of novel laccase tinctures from Stenotrophomonas maltophilia BIJ16 and Bordetella bronchiseptica HSO16: From dye decolourization to denim bioscouring. Biotechnol. Rep.. 2020;25:e00409.
- [Google Scholar]
- The sustainable production of a novel laccase from wheat bran by Bordetella sp. JWO16: toward total environment. Catalysts. 2021;11(6):677.
- [Google Scholar]
- Recovery of laccase-producing gammaproteobacteria from wastewater. Biotechnol. Rep.. 2019;21:00320.
- [Google Scholar]
- Production of polyextremotolerant laccase by Achromobacter xylosoxidans HWN16 and Citrobacter freundii LLJ16. Biotechnol. Rep.. 2019;22:e00337.
- [Google Scholar]
- Aptitude of oxidative enzymes for treatment of wastewater pollutants: a laccase perspective. Molecules. 2019;24(11):2064.
- [Google Scholar]
- Utilization of agroindustrial wastes for the production of laccase by Achromobacter xylosoxidans HWN16 and Bordetella bronchiseptica HSO16. J. Environ. Manag.. 2019;231:222-231.
- [Google Scholar]
- Maize stover as a feedstock for enhanced laccase production by two gammaproteobacteria: a solution to agroindustrial waste stockpiling. Ind. Crop. Prod.. 2019;129:611-623.
- [Google Scholar]
- Expression profile of laccasegene family in White-Rot Basidiomycete Lentinula edodes under different environmental stresses. Genes. 2019;10(12):1045.
- [Google Scholar]
- Using Ceriporiopsis subvermispora CZ-3 laccase for indigo carmine decolourization and denim bleaching. Int. Biodeterior. Biodegrad.. 2014;88:199-205.
- [Google Scholar]
- A novel homodimer laccase from Cerrena unicolor BBP6: Purification, characterization, and potential in dye decolorization and denim bleaching. PLoS One. 2018;13(8):e0202440.
- [Google Scholar]
- Expression of a novel manganese peroxidase from Cerrena unicolor BBP6 in Pichia pastoris and its application in dye decolorization and PAH degradation. Biochem. Eng. J.. 2020;153:107402
- [Google Scholar]
- The synergism of manganese peroxidase and laccase from Cerrena unicolor BBP6 in denim dye decolorization and the construction of gene coexpression system in Pichia pastoris. Biochem. Eng. J.. 2022;177:108230
- [Google Scholar]
- Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem. Eng. J.. 2017;117:62-72.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104305.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







