Translate this page into:
Extract with high 1,1-diphenyl-2-picrylhydrazyl (DPPH) inhibitory capability from pericarp and seed of mangosteen (Garcinia mangostana L.) using microwave-assisted extraction (MAE) two-phase solvent technique
⁎Corresponding author at: Department of Chemical Engineering, Faculty of Industrial Technology, Sepuluh Nopember Institute of Technology, Keputih Sukolilo, Surabaya 60111, Indonesia. hakunmarta9105@gmail.com (Hakun Wirawasista Aparamarta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The human body needs compounds that are antioxidants to prevent oxidative stress. Some parts of the mangosteen fruit (Garcinia mangostana L.) have been known as sources of bioactive compounds that have antioxidant properties. The pericarp and seeds of mangosteen were extracted using the MAE method to produce the extract with the greatest antioxidant activity. There are two types of solvent mixtures used in the extraction process: single-phase and two-phase solvents. The solvents used were ethanol (EtOH), ethyl acetate (EtOAc), isopropyl alcohol (IPA), and water. First, utilizing dried mangosteen pericarp powder as the raw material, a study was undertaken to determine the ideal operating conditions for the MAE process. A one-factor-at-a-time approach was used to find the best operating conditions. A mixture of solvents with varied ratios (mL/mL), extraction temperature (°C), extraction time (min), and solid to solvent ratio (g/mL) were applied as independent variables. Then, dried mangosteen seed powder extraction was carried out based on the best-operating conditions previously achieved. The DPPH scavenging activity, total phenolic content (TPC) value, and α-mangostin content of the two extracts were compared. It was discovered that the mangosteen pericarp extract showed higher antioxidant activity (IC50 DPPH = 9.40 µg/mL) than the mangosteen seed extract (IC50 DPPH = 37.54 µg/mL), even slightly better than ascorbic acid (IC50 DPPH = 10.47 µg/mL). The best extract was produced from the bottom phase of two-phase solvent system (EtOAc:EtOH:Water 2:1:2), with an MAE temperature of 50 °C, a time of 4 min, and a solid-to-solvent ratio of 1:16. The TPC value of the best extract is 903.54 mgGAE/g extract, with a yield of 16.53 % and an α-mangostin concentration of 0.11 %.
Keywords
α-mangostin
DPPH scavenging activity
MAE
Mangosteen pericarp
Mangosteen seeds
And total phenolic content
1 Introduction
Oxidative processes in the body, such as energy maintenance, detoxification, and ongoing immune reactions, are responsible for the formation of various forms of active oxygen, Reactive Oxygen Species (ROS) (Škrovánková et al., 2012). The imbalance between the overproduction of ROS or the accretion of free radicals in the body and the potential of biological systems to detoxify reactive substances results in oxidative stress, which is a major factor in the development of several age-related degenerative and chronic diseases (Dhalaria et al., 2020). To stabilize free radicals, it needs antioxidants. Natural antioxidants are divided into two categories, namely enzymatic and non-enzymatic. Catalase, glutathione peroxidase, and superoxide dismutase (SOD) are enzyme-based antioxidants that work to prevent oxidative stress (OS), while ascorbic and lipoic acid, polyphenols, and carotenoids are non-enzymatic antioxidants that fight OS indirectly by binding to redox metals and chelating agents.(Gad and Sayd, 2015).
Various tissues of the mangosteen (Garcinia mangostana L.), such as pericarp, seeds, and leaves, are known to contain bioactive compounds, such as phenolics and flavonoids (Aizat et al., 2019). Extracts produced from mangosteen pericarp have been studied to act as an antioxidants (Ghasemzadeh et al., 2018; Mohammad et al., 2019; Zarena et al., 2012). Not only as an antioxidant, one of the most abundant compounds contained in mangosteen tissue is α-mangostin which is known to have many therapeutic roles, namely as antitumor or anticancer (Aukkanimart et al., 2017; Chae et al., 2016; Han et al., 2018; Irene and Claudio, 2020; Ma et al., 2019; Santhanakrishnan et al., 2020; Xu et al., 2017; Yokoyama et al., 2019; Zhang et al., 2018), anti-cholinesterase (Khaw et al., 2020), anti-inflammatory (Chae et al., 2017; Lotter et al., 2020; Tao et al., 2018; Tarasuk et al., 2017), anti-depressant (Lotter et al., 2020), anti-viral (Tarasuk et al., 2017) and anti-bacterial (Ahmad et al., 2019). It is important to isolate this substance from the mangosteen tissues due to its many health benefits. The low solubility of this molecule in water makes it difficult to extract, and as a result, its bioavailability in the body is likewise low (Lee et al., 2019). To obtain the extract with the optimum therapeutic advantages, in this study as an antioxidant, it is crucial to accurately choose the extraction method and the solvent to be utilized. Therefore, the method for isolate of α-mangostin is implied to get an optimal extract yield.
The previous study, mangosteen tissues has been extracted using a variety of techniques including supercritical fluid extraction (SFE) (Zarena and Udaya Sankar, 2011; Zarena et al., 2012; Lee et al., 2019), aqueous micellar biphasic system (AMBS) (Tan et al., 2017; Ng et al., 2018), maceration (Sage et al., 2018), ultrasound-assisted extraction (UAE) (Zhang et al., 2015) and MAE (Fang et al., 2011; Ghasemzadeh et al., 2018; Mohammad et al., 2019). The previous study's supercritical CO2 extraction method with EtOH as a co-solvent yielded an extract with an IC50 in DPPH of 14 g/ml, which was the extract with the best antioxidant activity (Zarena et al. 2012). However, it takes quite a while to complete this method, about 5 h. A mixture of 72.4:27.6 (v/v) EtOAc and EtOH can be used in the MAE method to create an extract with an IC50 in DPPH of 20.64 g/ml (Ghasemzadeh et al., 2018). Consequently, the MAE method was chosen for this investigation since it provides several benefits, including a short time and the use of less solvent (Aparamarta et al., 2020). When employing the MAE method for extraction, it is crucial to study the operating conditions of temperature, power, time, and solvent (Gill, 2020). However, no research has ever been done on how different solvents, particularly-two-phase solvents, affect the extraction of mangosteen tissues utilizing the MAE method.
The extraction procedure depends greatly on the solvent selection. High dielectric constant solvents can absorb microwaves more readily, leading to a superheating phenomenon that can more easily induce the plant matrix to lyse and speed up mass transfer (Boukhatem et al., 2020). Aparamarta et al. (2016) used extraction using a two-phase solvent to separate and purify triacylglycerols from Nyamplung oil. To ensure that extracts with chemicals have the purest ones, two-phase solvents can separate compounds based on their degree of polarity. The usage of single-phase and two-phase solvents was investigated in this study.
This study was focused on producing extracts with good antioxidant activity. Therefore, the DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was chosen to determine the free radical scavenging activity of the extracts obtained. DPPH is a free radical reagent that is relatively safe, inexpensive and works according to the mechanism of free radical stabilization in the body (Amarowicz and Pegg, 2019). The green solvents include aquades, EtOH, EtOAc, and IPA were investigated to get the optimal yield. According to research by Mamat et al (2020), the amount of α-mangostin in the late-ripe mangosteen fruit was also discovered to be more concentrated in the mangosteen seed and pericarp. This study claimed that the content of α-mangostin compounds in mangosteen seeds was higher than that of mangosteen pericarp. However, no one researcher has never been examined with DPPH scavenging activity. Therefore, this works were studied the yield extracts from mangosteen pericarp and seeds with DPPH scavenging activity.
2 Material and methods
2.1 Materials
This study used mangosteen fruit obtained from the West Java area through an online store. The pericarp, fruit, and seeds of the mangosteen are separated. Mangosteen pericarp and mangosteen seeds were dried under the sun until they obtained a constant weight, ± 40 h and ± 20 h, respectively. Then the two materials were each ground and sieved to get a dry powder measuring 100 mesh. The chemicals used are solvents in the form of EtOAc, EtOH 96 %, IPA, aquades, methanol, all technical grade, DPPH (Sigma Aldrich), standard α-mangostin (Sigma Aldrich), Thin Layer Chromatography (TLC) Plate Silica gel 60 F254 (Merck), formic acid (pro-analytical grade Merck), toluene (pro-analytical grade Merck), gallic acid (Sigma Aldrich), ascorbic acid (Sigma Aldrich) were obtained from CIMS (CV Chemical Indonesia Multi Sentosa). Folin-Ciolciteau (Merck) reagent, and sodium carbonate were obtained from CV Sumber Ilmiah Persada. The material used for the LC-MS/MS analysis is acetonitrile with gradient grade specifications for liquid chromatography LiChrosolv, methanol, formic acid obtained from Merck KGa, Darmstadt Germany, and H2O from processing with evaqua equipment. Microwave (Electrolux EMM2308X with modifications by CV Margi Rahayu Sejahtera) with digitally regulated temperature precision of ± 2 °C with a condenser running at 16 °C was used in this study. Other glass equipment such as three-neck flask, beaker glass, erlenmeyer, filter paper, condenser, dropper, vial, separator funnel, micropipette, and test equipment, namely UV (UVTEC Cambridge) and spectrophotometer (Cecil CE 1011).
2.2 Extraction of mangosteen pericarp
Extraction was carried out using the one factor at a time method. For the study of solvent selection, extraction was carried out using 10 g of dried mangosteen pericarp powder, which was put into a three-neck flask with the initial setting temperature of 60 °C, time of 6 min, solid to solvent ratio of 1:10 (w/v). The power in the microwave is set to fluctuate around 450 W so that the temperature can be kept constant. Then, after obtaining the best solvent, it was continued for studies with variations in temperature, time, and solid to solvent ratio, respectively. The extract obtained was filtered using fine filter paper. For two-phase solvents, the top phase and bottom phase extracts were separated using a separator funnel. The wet extract was used for TLC assay. After that, the extract was dried and placed in a closed container to avoid direct exposure to light. The dry extract was stored at 4 °C for further analysis.
2.3 Extraction of mangosteen seeds
After obtaining the solvent, temperature, time, and solid-to-solvent ratio, which resulted in an extract with the best DPPH scavenging activity, TPC value, and yield with dry mangosteen pericarp as raw material, these conditions were applied for extraction with 10 g of dry mangosteen seed as raw material. The wet extract was used for the TLC assay with the same method as the wet extract of mangosteen pericarp. Then, to avoid direct light exposure, dry and cover in a closed container. The dried extract of the mangosteen seed was stored at 4 °C for further analysis.
2.4 Thin Layer Chromatography (TLC) assay
The TLC assay was carried out using a mobile phase with toluene:ethyl acetate:formic acid content of 80:20:1 (v/v/v) (Chewchinda and Vongsak, 2019). This is used to determine whether the extract obtained contains α-mangostin or not. The wet extract was dripped onto the TLC plate. Then the plate is immersed in the mobile phase and carried out in a tightly closed bottle. After soaking, the TLC paper was then dried at room temperature. TLC plate readings were performed using a UV tool (UVITEC Cambridge) with UV lamps at 254 and 366 nm wavelengths.
2.5 DPPH scavenging activity assay
The dry extract was evaluated for DPPH scavenging activity by following the method of Ghasemzadeh et al. (2018) but with some adjustments. The extract was dissolved in methanol until a concentration of 20 µg/mL was obtained. Approximately 1 mL of the diluted extract sample was mixed with 3 mL of freshly prepared DPPH reagent (concentration 40 µg/mL), incubated, and shaken at 125 rpm for 30 min without direct light exposure. The absorbance was observed using a spectrophotometer at a wavelength of 517 nm. DPPH scavenging activity was measured as DPPH inhibition (%) as shown in Eq. (1) (
is blank;
is sample absorbance).
For IC50 value, extract samples and standard solutions (ascorbic acid and gallic acid) were made in various concentrations. Then, in the same way, the strength of the extract and the standard solution was measured in inhibiting DPPH. A graph of the concentration of extract and standard solution versus inhibition of DPPH was made to obtain the required concentration to inhibit DPPH by 50 %, or IC50.
2.6 Total phenolic content (TPC) assay
The TPC of the extract obtained was evaluated using the Folin-Ciocalteu method by Mohammad et al. (2019) with slight modifications. The 40 μg/mL of sample extract or gallic acid at a series of concentrations was dissolved in 96 % of EtOH. The resulting dilution of the extract (0.5 mL) or gallic acid was mixed with 0.5 mL of Folin-Ciocalteu reagent for about 2–3 min and gently shaken. Then they added sodium carbonate solution 7 % w/v (10 mL) and incubated for 30 min without exposure to light. The absorbance was analyzed using a spectrophotometer at a wavelength of 758 nm. Results are reported as milligrams of gallic acid equivalent per gram of extract (mg GAE/g extract).
The process for making standard solutions of gallic acid was used for the calibration curve. A standard solution of gallic acid was prepared with a concentration of 0; 5; 10; 50; and 100 µg/mL. The absorbance of the standard solution was measured, and a calibration curve was made.
2.7 Liquid Chromatography-Mass Spectrometry/Mass spectrometry (LC-MS/MS) analysis
The content of α-mangostin in the extract can be determined using LC-MS/MS. This analysis was carried out at the Instrumental Analysis Laboratory, Department of Chemical Engineering, State Polytechnic of Malang. A Mass Spectrophotometer (TSQ Quantum Access Max), Liquid Chromatography (LC, Accella 1250 made in the United States), ultrasonic cleaner, micropipette, analytical balance, and vials were used.
The standard material for the α-mangostin was made at a concentration of 1000 µg/mL with methanol as a solvent, then diluted to 4 µg/mL. The extract was weighed and dissolved in 20 mL of methanol. Sonication was carried out for 10 min and then centrifuged at 10000 rpm for 5 min. It was filtered with a 0.2 µ PTFE (Polytetrafluoroethylene) filter and put in a vial. The mobile phase was made with 0.1 % formic acid (A) in acetonitrile and 0.1 % formic acid in H2O (B).
The operating conditions of the Ultra High-Performance Liquid Chromatography (UPLC), which consists of a vacuum degasser, a quaternary pump, and a thermostatic autosampler, are controlled by a computer through the x-calibur 2.1 programs. The mobile phase was programmed at a speed of 300 L/min with a composition setting of 0.0–0.6 min 70 % B; 0.6–3.0 min 10 % B; 3.0–5.0 min 10 % B; 5.0–5.5 min 70 % B. Hypersil Gold specification column (50 mm 2.1 mm 1.9 µm). The injection volume on LC was 1 L. The column was controlled at 30 °C, and the autosampler compartment was set at 16 °C. The operating conditions of MS/MS Triple Q with an ESI (Electrospray Ionization) ionization source are controlled by TSQ Tune software, which is operated with positive ionization mode on the target compound. The spray voltage was set at 3.0 kV, the evaporation temperature was set at 275 °C, the capillary temperature was 300 °C, the nitrogen as the sheath gas pressure of 40 psi, and aux gas pressure of 10 psi.
2.8 Statistical analysis
This study used the one-factor-at-a-time method with single replication. The software used to conduct statistical analysis was Minitab 18. Meanwhile, a one-way analysis of variance (ANOVA) was used to check the significant difference between variables. The variable was considered significant when the P-value was < 0.05.
3 Results and discussion
3.1 Effect of various solvents
The MAE method is an extraction technique using microwaves as an energy transmitter. Using the MAE method, it will be more profitable to use solvents that have a high dielectric constant, for example, water. This is because solvents with a high dielectric constant will absorb microwaves more quickly and convert them into heat energy instantly (Boukhatem et al., 2020; Flórez et al., 2015). However, not all bioactive compounds can be extracted using water.
In a previous study conducted by Fang et al. (2011), the optimum temperature for mangosteen pericarp extraction using the MAE method was 70 °C with 95 % of EtOH as the solvent. Meanwhile, Zarena et al. (2012) obtained the optimum temperature of 50 °C using the SFE method with 95 % EtOH as co-solvent. So that the middle temperature was set, which was 60 °C in this study, then the effect of temperature was also studied after obtaining the best solvent. In addition, the temperature of 60 °C was chosen because there is a solvent consisting of a mixture of organic solvents and water. The dielectric constant of water is the highest, which is 80.1 (Poole, 2019), so it is hoped that the use of low temperatures can damage plant matrix cells and can increase the yield and antioxidant properties of the extract obtained. Temperatures that are too high can cause gelatinization of the starch contained in the mangosteen pericarp (Tjahjani et al., 2014). Continuous heating beyond the occurrence of peak viscosity provides continuous suction of water, with some starch dispersed into the solution and some swollen granular fragments remaining (BeMiller and Whistler, 2009). If gelatinization occurs, the filtering process will be more difficult because of the high viscosity.
The selection of organic solvents such as EtOAc, EtOH, and IPA was based on their ability to extract α-mangostin in mangosteen pericarp (Bundeesomchok et al., 2016; Ng et al., 2018). In addition, these solvents are also classified as green solvents, meanings that the use of these solvents will be safer for the environment and have low toxicity (Jesus and Filho, 2020). Although EtOAc has a low dielectric constant of 6.02, compared to EtOH and IPA, which have dielectric constant of 24.6 and 19.92, respectively, EtOAc has the role of forming two-phase solvents when mixed with water. This is because EtOAc is only partially soluble in water and vice versa (Poole, 2019). The use of two-phase solvents will separate the extracted compounds according to their polarity and hydrophilic or hydrophobic properties. The use of this two-phase solvent method has been studied by Aparamarta et al. (2016) and Zhao et al. (2021), where compounds with low polarity will be extracted in the non-polar solvent phase (top phase), while those with higher polarity will be extracted in the polar solvent phase (bottom phase). Extract results from various solvents can be seen in Table 1. *DPPH inhibition of α-mangostin (20 µg/mL) = 24.74 %.
No
Solvent
Solvent Ratio (ml/ml)
DPPH Inhibition (%)
TPC (mg GAE/g extract)
Yield (%)
Single-phase solvent
1
EtOAc
46.25
431.93
8.56
2
EtOH
53.73
577.64
14.74
3
IPA
53.38
559.43
12.29
4
EtOAc:IPA
1:3
51.46
512.59
12.10
5
EtOAc:IPA
1:1
51.84
515.20
11.93
6
EtOAc:IPA
3:1
53.59
569.84
10.15
7
EtOAc:EtOH
1:3
52.52
549.02
14.72
8
EtOAc:EtOH
1:1
52.64
554.23
14.03
9
EtOAc:EtOH
3:1
57.12
601.06
13.95
Two-phase solvent
10
EtOAc:IPA:water top phase
2:1:2
52.44
546.42
7.28
11
EtOAc:IPA:water bottom phase
2:1:2
56.21
585.45
14.34
12
EtOAc:EtOH:water top phase
2:1:2
57.44
606.27
14.67
13
EtOAc:EtOH:water bottom phase
2:1:2
50.80
543.82
9.39
18
EtOAc: water top phase
1:1
55.08
580.25
8.37
19
EtOAc:water bottom phase
1:1
48.40
470.96
7.73
It can be seen in Table 1 that the highest yield of mangosteen pericarp extract was obtained from extraction using EtOH solvent, that is 14.74 %, followed by mixed solvent EtOAc according to the ratio. The greater the proportion of EtOH in the single-phase solvent mixture, the higher the yield is obtained. The same is true for IPA solvents. This can happen because the dielectric constant of EtOH and IPA is higher than that of EtOAc. The same result was obtained by Ghasemzadeh et al. (2018), where the addition of EtOH as a solvent increased the yield of α-mangostin extract. Likewise, with the results obtained on the extraction of marmelosin from Aegle marmelos using the MAE method, where the extract yield obtained on 80 % EtOH is higher than the use of pure EtOH, this is because 80 % EtOH contains water which has a higher dielectric constant (Sonar and Rathod, 2020). Thus, the mixture of EtOAc:EtOH and EtOAc:IPA resulted in a higher extract yield than the use of pure EtOAc solvent.
In the use of a two-phase solvent, it can be seen that water that has a high dielectric constant can increase the yield value of the extract if the two extracts are combined. The extract yield obtained from the mixture of EtOAc:IPA:water and EtOAc:EtOH:water was much different. In the EtOAc:IPA:water mixture, the extract yield from the top phase was higher than the extract yield from the bottom phase, while in the EtOAc:EtOH:water mixture, it was the opposite. This is due to the higher solubility of IPA in EtOAc than in water, so the volume of the top phase is also higher than that of the bottom phase (Hong et al., 2002), which affects the extract yield. Meanwhile, EtOH has a higher solubility in water than EtOAc (Lin et al., 2005). Likewise, this phenomenon occurs in the EtOAc:water mixture, which has a higher extract yield in the bottom phase because the solubility of EtOAc to water is higher than the solubility of water to EtOAc (Poole, 2020).
In two-phase extraction conditions using a microwave with a mixture of water solvents, water will absorb microwave energy more quickly and will damage the plant cell matrix. Water molecules will go up to where the top (non-polar) phase is and distribute the extracted compounds (Zhao et al., 2021). EtOH in the mixture has an important role. It can extract the bioactive compounds contained in the mangosteen pericarp, which has a moderate level of polarity (Bundeesomchok et al., 2016). Likewise, the role of IPA, which is almost similar to EtOH, can be seen from the extract obtained from a mixture of only EtOAc:water solvents. Since EtOH and IPA have lower interfacial tension (22.39 and 21.79 mN/m) than water (72.8 mN/m), they facilitate mass transfer across the phase boundary so that the extracted compounds can be more abundant and well distributed (Poole, 2019).
However, a high yield value does not always have a high TPC or DPPH inhibition activity. Table 1 shows that a high TPC value is directly proportional to high DPPH inhibition. This result is the same as the research conducted by Mohammad et al. (2019), which showed that extracts that have a large composition of phenolic compounds will have high antioxidant activity as well. The TPC value and DPPH inhibition were higher in the use of two-phase solvents, namely the extract using a mixture of EtOAc:EtOH:water 2:1:2, which had a TPC value of 606.27 mgGAE/g extract and DPPH inhibition of 57.44 %. This is possible due to an increase in the purity of a compound that has high antioxidant activity and separates several other impurities. As is based on the nature of the solvent, “Like Dissolve Like”, where each compound has a tendency to dissolve into a solvent that has the same polarity. Because of this, the composition of the solvent becomes a very influential factor in the purification process of a compound (Seidel, 2006).
The TLC analysis was carried out qualitatively to identify the α-mangostin compound, which has been widely studied and has many benefits, including as an antioxidant in. The results of the TLC analysis of mangosteen pericarp extract with various variations of single-phase solvents can be seen in Fig. 1, and two-phase solvents in Fig. 2.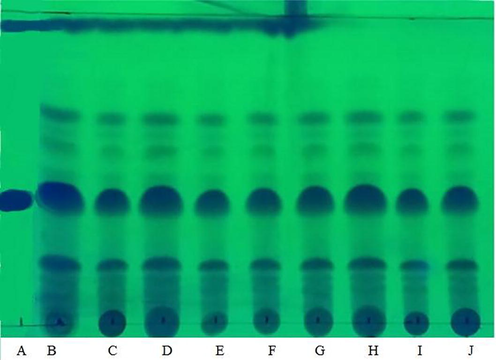
TLC analysis results of extracts from different single-phase solvent systems A = standard α-mangostin, B = EtOAc, C = EtOH, D = IPA, E = EtOAc:IPA 3:1, F = EtOAc:IPA 1:1, G = EtOAc:IPA 1:3, H = EtOAc:EtOH 3:1, I = EtOAc:EtOH 1:1, G = EtOAc:EtOH 1:3.
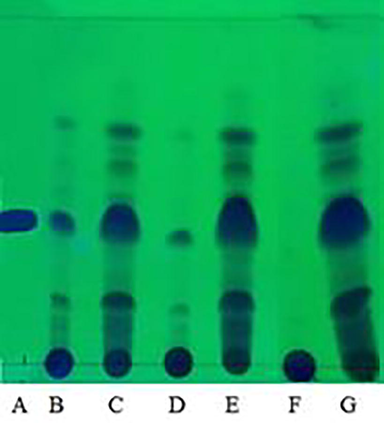
TLC analysis results of extracts from different two-phase solvent systems A = standard α-mangostin, B = EtOAc:IPA:Water 2:1:2 bottom phase, C = EtOAc:IPA:Water 2:1:2 top phase, D = EtOAc:EtOH:Water 2:1:2 bottom phase, E = EtOAc:IPA:Water 2:1:2 top phase, F = EtOAc:Water 1:1 bottom phase, G = EtOAc:Water 1:1 top phase.
From Fig. 1 and Fig. 2, it can be seen that the extract obtained at this stage contains the α-mangostin. This can be indicated by the α-mangostin standard spot used, where the standard spot is located parallel to or almost parallel to the spot originating from each extract test. In addition, it can be identified by the retention factor (Rf) value of α-mangostin extract, where the standard Rf value of α-mangostin is 0.37 (Chewchinda and Vongsak, 2019), and the Rf value of α-mangostin extract of this study is in the range of 0.37.
The thicker the spot stain on the TLC plate, the more compound content in the analyzed sample. The spot can be formed depending on what compounds you want to see and the mobile phase used (Cai, 2014). Spot TLC extract from EtOAc was the thickest than extracts from other solvents. However, it turns out that the thickness of the spot is not always directly proportional to the ability of an extract to inhibit DPPH. This is because not all bioactive compounds can synergize with each other to increase the effectiveness of treatment. Bioactive compounds can be positive or negative or both in their effects according to their concentration, which may depend on the type of component or substance, combination with other biological substances, cumulative dose, route of exposure, or bioavailability of the compound (Shrinet et al., 2021).
In addition, it can be seen in Fig. 1 and Fig. 2 that there are still many compounds left at the initial point of droplet extract. This indicates that there are other compounds that are not carried away by the mobile phase solvent from TLC so that they do not leave spot marks on the TLC plate. Possibly, the compounds that are not carried away also have a role as antioxidants, as seen from the results of the DPPH inhibition of extracts from the bottom phase EtOAc:EtOH:water which was much higher than the extract from pure EtOAc, which was only 46.25 %. Compounds with low log P values may be contained in the bottom phase extract of the solvent EtOAc:EtOH:water. Log P is the partition coefficient which is the ratio between the concentration of compounds distributed in the non-polar phase (Clipid) and the concentration of compounds distributed in the aqueous phase (Cwater) under conditions of thermodynamic equilibrium (Dima, 2020). The lower the log P-value, the more hydrophilic the compound. Several phenolic compounds contained in mangosteen pericarp that have antioxidant properties are rutin, (-) epicatechin, γ-mangostin, and quercetin (Fatmawati et al., 2015; Sungpud et al., 2019). These compounds have a low log P value of −2.02, 0.49, 5.744, and 1.48, respectively, so they are easily dissolved in water.
Based on the data above, the solvent EtOAc:EtOH:water 2:1:2 was chosen to obtain an extract with good antioxidant properties. This is because, although the yield obtained is still lower than the extract obtained with the EtOH solvent, the extract from the bottom phase EtOAc:EtOH:water solvent has a much higher DPPH inhibition value. The solvent EtOAc:EtOH 3:1 was not chosen even though it has almost the same DPPH inhibition value because the goal of this research in the future is to be used as a drug. For drugs that are taken orally, there are several provisions, including good bioaccessibility. Bioaccessibility itself is the fraction of ingested biocomponents that can be accessed for absorption through the epithelial lining of the digestive tract. One of the requirements for a component to have good bioaccessibility is to have a log P ≤ 5 (Dima, 2020).
3.2 Effect of extraction temperature
Extraction temperature greatly affects the extract yield, as can be seen in Table 2. The DPPH inhibition, TPC value, and yield of the extract increased from 40 °C to 50 °C and then decreased. The P-value also shows a number < 0.05, seen in Table 5 and Table 6. This shows that the effect of temperature on DPPH inhibition and TPC value of an extract is very significant. But it is not so significant for the yield because it has a P-value > 0.05.
No
Temperature (°C)
Times (min)
Solid to Solvent Ratio (g/mL)
Extract Phase
DPPH Inhibition (%)
TPC (mgGAE/g extract)
Yield (%)
1
40
6
1:10
Bottom
58.67
638.14
13.39
Top
47.68
442.99
8.98
2
50
Bottom
60.98
704.49
13.61
Top
47.98
466.41
9.24
3
60
Bottom
57.44
606.92
14.17
Top
47.62
456.65
9.29
4
70
Bottom
56.41
591.30
13.92
Top
46.40
419.57
9.26
No
Temperature (°C)
Times (min)
Solid to Solvent Ratio (g/mL)
Extract Phase
DPPH Inhibition (%)
TPC (mgGAE/g extract)
Yield (%)
1
50
2
1:10
Bottom
56.58
605.94
10.15
Top
46.03
428.35
7.12
2
4
Bottom
62.45
752.30
12.93
Top
50.55
544.47
8.54
3
6
Bottom
60.58
699.61
13.45
Top
48.64
469.34
9.16
4
8
Bottom
57.41
637.16
12.16
Top
47.41
450.80
8.36
No
Temperature (°C)
Times (min)
Solid to Solvent Ratio (g/mL)
Extract Phase
DPPH Inhibition (%)
TPC (mgGAE/g extract)
Yield (%)
1
50
4
1:8
Bottom
61.12
709.37
9.59
Top
46.11
421.52
7.53
2
1:10
Bottom
62.03
754.25
13.06
Top
47.41
451.77
8.92
3
1:12
Bottom
64.17
818.65
13.58
Top
49.15
469.34
9.37
4
1:14
Bottom
65.84
841.10
14.49
Top
49.97
491.78
10.58
5
1:16
Bottom
67.41
903.54
16.53
Top
51.68
542.52
11.15
6
1:18
Bottom
66.49
889.88
16.67
Top
51.26
542.52
10.10
Source
DF
Adj SS
Adj MS
F-Value
P-Value
Temperature (°C)
3
0.001799
0.000600
24.94
0.00001919
Times (min)
3
0.005838
0.001946
80.95
0.00000003
Solid to Solvent ratio (g/ml)
5
0.007085
0.001417
58.94
0.00000005
Error
12
0.000288
0.000024
Total
23
0.030970
Model Summary
S
R-sq
R-sq(adj)
R-sq(pred)
0.0049030
99.07 %
98.21 %
0.0049030
Source
DF
Adj SS
Adj MS
F-Value
P-Value
Temperature (°C)
3
13,051
4350.2
237.19
0.00000000006059
Times (min)
3
36,095
12031.8
656.01
0.00000000000014
Solid to Solvent ratio (g/ml)
5
66,315
13262.9
723.13
0.00000000000002
Error
12
220
18.3
Total
23
248,182
Model Summary
S
R-sq
R-sq(adj)
R-sq(pred)
4.28263
99.91 %
99.83 %
*
As previously explained, the use of the solvent EtOAc:EtOH:water has a powerful microwave absorbing power due to the presence of water. Thus, in a short time, the extraction temperature of 50 °C has obtained a high value of % DPPH and TPC inhibition. An increase in temperature can decrease viscosity, increase intermolecular interactions, decrease surface tension and increase pressure in the cell, which causes the movement of compounds in the cell to the solvent more easily. However, an excessive increase in temperature can reduce the yield of the extract because some of the desired compounds may be degraded (Fu et al., 2018; Lin et al., 2019; Martiny et al., 2021; Shang et al., 2021; Sonar and Rathod, 2020; Xie et al., 2017).
3.3 Effect of extraction time
The effect of extraction time was studied by adjusting the temperature to 50 °C. It can be seen from Table 5 and Table 6 that the time variable greatly affects DPPH inhibiton and TPC value because it has a P-value of < 0.05. While, less significant to the value of yield extract. The increase in DPPH inhibition, TPC value, and yield extract occurred from 2 to 4 min, then decreased thereafter, it can be seen in Table 3. This phenomenon is possible due to an equilibrium condition that has been reached between the concentration of the extract that is outside the cell wall (in solution) and that which is inside the cell wall. The more prolonged the extraction time, the more the solution becomes saturated, and the extracted power decreases (Desai et al., 2010).
During the irradiation process, heat accumulation occurs in the solvent used for extraction due to the absorption of microwave energy. The energy is channeled into the cell matrix by increasing intermolecular interactions, causing mass transfer from within the cell to the solution to be faster. However, the longer the extraction time, the more degradation of the desired analyte will occur, and other impurity compounds will also be extracted. So that it can reduce the yield of the extract as well as its purity (Aparamarta et al., 2020; Fu et al., 2018; Lin et al., 2019; Martiny et al., 2021; Sonar and Rathod, 2020; Xie et al., 2017). Thus, using a long time will not make the extract have better antioxidant properties.
3.4 Effect of solid to solvent ratio
The use of solvents affects the production process of an extraction product. The number of solvents used efficiently can increase the production effectiveness of an extract. It can be seen from Table 5, Table 6, and Table 7 that the ratio of solid to solvent has significance for DPPH inhibition, TPC value, and yield of extraction with P-value < 0.05. Table 4 shows that DPPH inhibition, TPC value, and yield of extraction continued to increase from 1:8 to 1:16 and then slightly decreased to a ratio of 1:18.
Source
DF
Adj SS
Adj MS
F-Value
P-Value
Temperature (°C)
3
0.6621
0.2207
0.24
0.869613
Times (min)
3
6.7060
2.2353
2.39
0.119780
Solid to Solvent ratio (g/ml)
5
69.9246
13.9849
14.95
0.000085
Error
12
11.2256
0.9355
Total
23
97.3630
Model Summary
S
R-sq
R-sq(adj)
R-sq(pred)
0.967195
88.47 %
77.90 %
*
The increase in extraction yield is influenced by the phenomenon of mass transfer that occurs in the process. An increase in the amount of solvent increases the concentration difference between the solvent and the solid, which acts as a driving force for mass transfer. As a result, compounds or analytes in mangosteen pericarp cells will tend to leave the cell matrix (Sonar and Rathod, 2020). In this study, the best solid to solvent ratio was 1:16. This result is in accordance with the results obtained by Fang et al. (2011), whose extract still increased at a ratio of 1:10 as the highest variable, and Mohammad et al. (2019), who got the best results at a ratio of 1:25 as the lowest variable.
3.5 Results of analysis of variance
Although the analysis of the yield variance of mangosteen pericarp extract showed the figure of 88.47 % for R2, this data can still be used because it is still above 80 % for the fit of the model with the experimental results (Zarena et al., 2012). When viewed from Table 5, Table 6, and Table 7, each variable has a significant effect on DPPH inhibition and TPC values. Meanwhile, yield does not have a significant effect. In Table 2 and Table 3, the DPPH inhibition and TPC values decreased at a temperature of 50 °C to 60 °C as well as from 4 min to 6 min. However, there was an increase in yield under these conditions. Although there was an increase in yield, the best conditions were selected from extracts that had DPPH inhibition and high TPC values. Because the yield significantly increased when assessed through the analysis of variance, a temperature of 50 °C and an extraction time of 4 min was chosen for extraction. Because the more time is used, the more energy is needed. This causes higher production costs.
Thus, the best MAE operating conditions were obtained using a solvent system of EtOAc:EtOH:water 2:1:2, a temperature of 50 °C, an extraction time of 4 min, and a solid to solvent ratio of 1:16. Then, these conditions are used for mangosteen seed extraction.
3.6 Mangosteen seed extract
Mangosteen seed extraction is useful for comparing which extract is better between mangosteen seed extract and mangosteen pericarp extract. MAE operating conditions are set according to the best previously obtained conditions. The results of the mangosteen seed extract can be seen in Table 8.
Temperature (°C)
Times (min)
Solid to Solvent Ratio (g/mL)
Extract Phase
DPPH Inhibition (%)
TPC (mgGAE/g extract)
Yield (%)
50
4
1:16
Bottom
39.43
338.59
15.72
Top
24.56
201.98
24.11
It can be seen from Table 8 that the extract with mangosteen seed as raw material did not have good activity, as seen from the DPPH inhibition, which was lower than the extract obtained with mangosteen pericarp as raw material, at only 39.43 %. This may be due to the fact that the antioxidant compounds present in the mangosteen seed are much lower in number when compared to the compounds found in the mangosteen pericarp. The TPC values in the mangosteen seed extract were also less, which was only 338.59 mgGAE/g extract for the bottom phase and 201.98 mgGAE/g extract for the top phase. However, the extract yield obtained was higher, especially in the top phase, which was 24.11 %. This may be due to the fact that mangosteen seeds contain more fat, which is 21.18 % (Ajayi et al., 2007). There are also differences in the results of the extract before and after drying. Dried mangosteen pericarp extract was in the form of a reddish-brown solid powder for the bottom phase and yellow for the top phase. However, the mangosteen seed extract was in the form of a thick liquid like oil for the top phase after the drying method. Mangosteen pericarp extract has a more concentrated color than mangosteen seed extract before the drying method.
3.7 DPPH scavenging activity of the best mangosteen pericarp extract and mangosteen seed extract
The antioxidant activity of the best extract obtained was compared with that of gallic acid and ascorbic acid. Comparisons were made by looking at the IC50 value in the graph of sample concentration versus DPPH inhibition.
It can be seen from Fig. 3 and Table 9, that the best mangosteen pericarp extract has an IC50 in DPPH radical scavenging activity of 9.4 µg/mL, 1.47 µg/mL lower than ascorbic acid. This means that the extract has slightly better antioxidant activity than ascorbic acid, which is a commonly consumed antioxidant.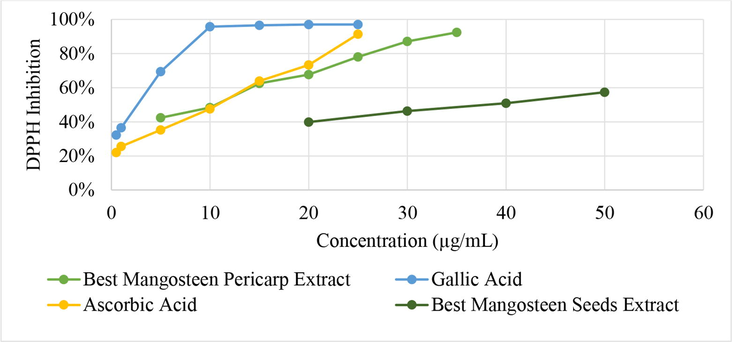
Graph of sample concentration versus DPPH inhibition.
No
Sample
IC50 (µg/mL)
1
Best Mangosteen Pericarp Extract
9.40
2
Best Mangosteen Seed Extract
37.54
3
Gallic Acid
2.28
4
Ascorbic Acid (Vit. C)
10.47
Antioxidants are defined as substances that neutralize free radicals. The imbalance between the formation of ROS (free radicals in the body) and antioxidant defense activity causes “oxidative stress“. Severe oxidative stress can cause mitochondrial and membrane mutations or disorders. It can also cause cell damage and tissue damage (Škrovánková et al., 2012).
Antioxidants can be classified as “primary antioxidants” or “secondary antioxidants”. Primary antioxidants actively inhibit oxidation reactions, while secondary antioxidants act indirectly, for example, in reactions with pro-oxidants (Amarowicz and Pegg, 2019). The antioxidant components of medicinal plants include antioxidants, especially phenolics such as phenolic acids, flavonoids, terpenes, tocopherols, vitamin C (ascorbic acid), and carotenoid groups (Škrovánková et al., 2012).
Phenolic compounds, as primary antioxidants, work according to two mechanisms: hydrogen atom transfer (HAT) or single electron transfer (SET) (Amarowicz and Pegg, 2019). The antioxidants that are most effective in stopping free radical chain reactions usually contain an aromatic ring capable of donating H• to free radicals formed during lipid oxidation. The antioxidant radicals thus formed are stabilized by the delocalization of unpaired electrons around the phenol ring to form stable resonance hybrids (Gad and Sayd, 2015). In the study of the structural activity of phenolic acids and their derivatives, the presence of a propanoic side chain and conjugated double bonds in the side chain have an intense effect on phenoxyl radicals through resonance, thereby increasing antioxidant power (Dhalaria et al., 2020).
In this study, ascorbic acid was used as a comparison antioxidant compound with the best extract obtained. Ascorbic acid has the ability to stabilize DPPH radicals by transferring hydrogen atoms and electrons they have converted to dehydroascorbate (de Menezes et al., 2021). The reaction can be seen in Fig. 4. When viewed from the stoichiometry of the reaction, it takes ± 0.25 mol of ascorbic acid to inhibit 50 % of 1 mol of DPPH radical. In this study, 10.47 µg/mL of ascorbic acid was required for 40 µg/mL of DPPH. In other words, it takes 0.20 mol of ascorbic acid to inhibit by 50 % the DPPH radical. This result is quite close to stoichiometry, so this research can be said to be quite relevant to the existing theory.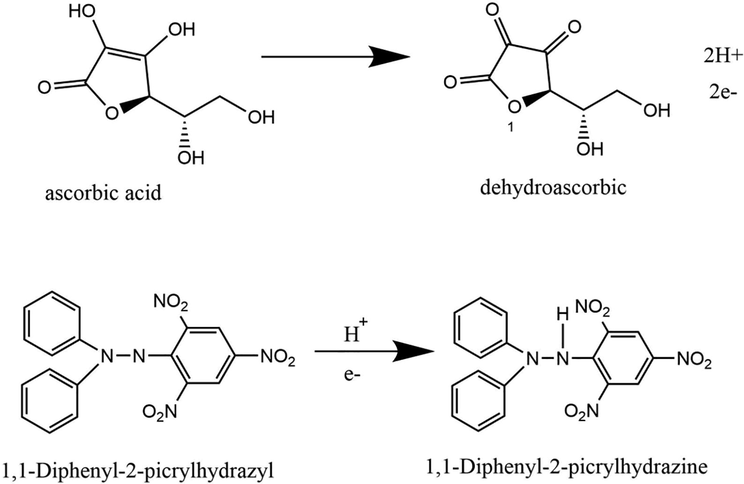
The reaction of the DPPH radical scavenging by ascorbic acid. Modified from de Menezes et al. (2021).
The antioxidant components may act independently or more effectively in combination when synergism is manifested (together, they have a stronger effect) by various mechanisms (Škrovánková et al., 2012). Thus, with a high TPC value, it is possible for the extract to have better or worse antioxidant activity. Some of the phenolic compounds found in mangosteen pericarp extract include α-mangostin, γ-mangostin, rutin, vanillic acid, (+) epicatechin (Sungpud et al., 2019), gartanin, garcinone C, garcinone D, and garcinone E (Chaivisuthangkura et al., 2009). The chemical structure of these phenolic compounds can be seen in Fig. 5. When viewed from the chemical structure, these compounds have an aromatic ring capable of donating H• to free radicals. Thus, antioxidant radicals are formed, which are then stabilized by the delocalization of unpaired electrons around the phenol ring to form stable resonance hybrids.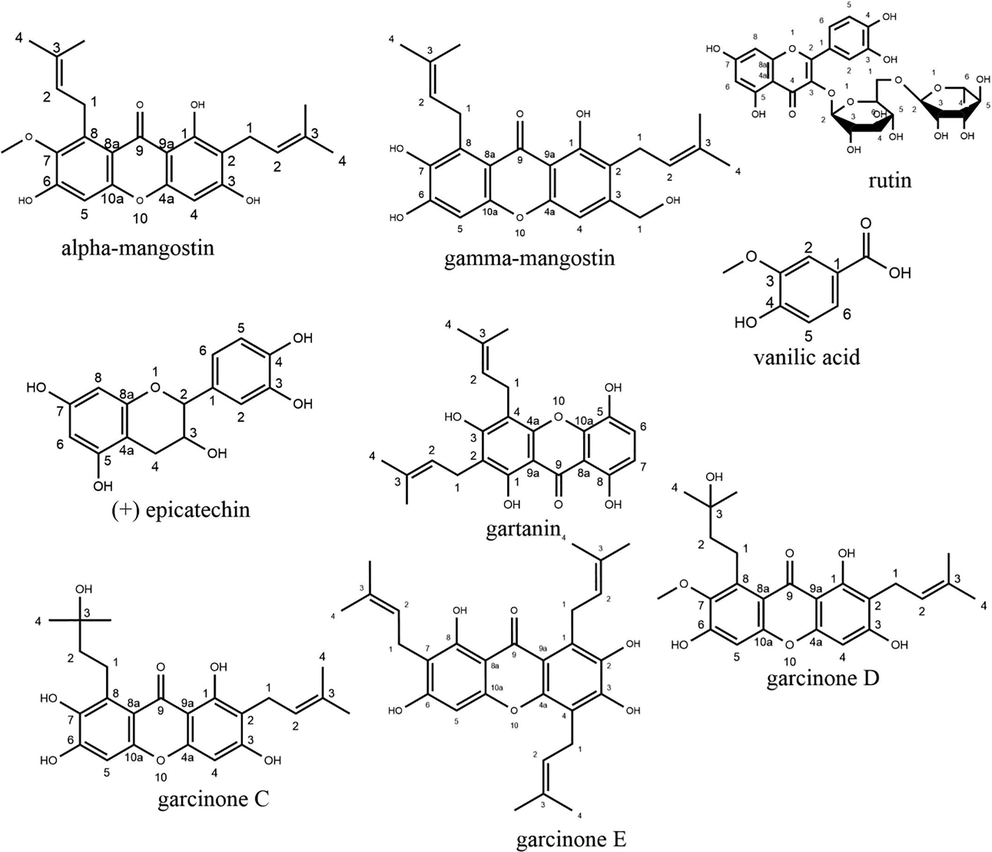
The structure of phenolic compounds contained in Garcinia mangostana L.
3.8 LC-MS/MS analysis results of the extract with the best DPPH inhibition
LC-MS/MS analysis was used to determine what compounds were contained in the extract obtained with the ability of separation and mass spectroscopy. The concentration of the compounds can also be known. The compounds in the extract detected using LC-MS/MS analysis can be seen in Table 10 and Fig. 6.
No
Compound Name
m/z
[M + H]+
(m/z from MS2)
1
α-mangostin
354.5–356.5
411.00
2
Gartanin
340.5–341.5
397.00
3
γ-mangostin
340.5–341.5
397.00
4
β-mangostin
368.5–369.5
425.00
5
Garcinone C
396.5–397.5
415.00
6
Garcinone D
354.5–355.5
429.00
7
Garcinone E
464.5–465.5
465.00
8
9-Hydroxycalabaxanthone
352.5–353.5
409.00
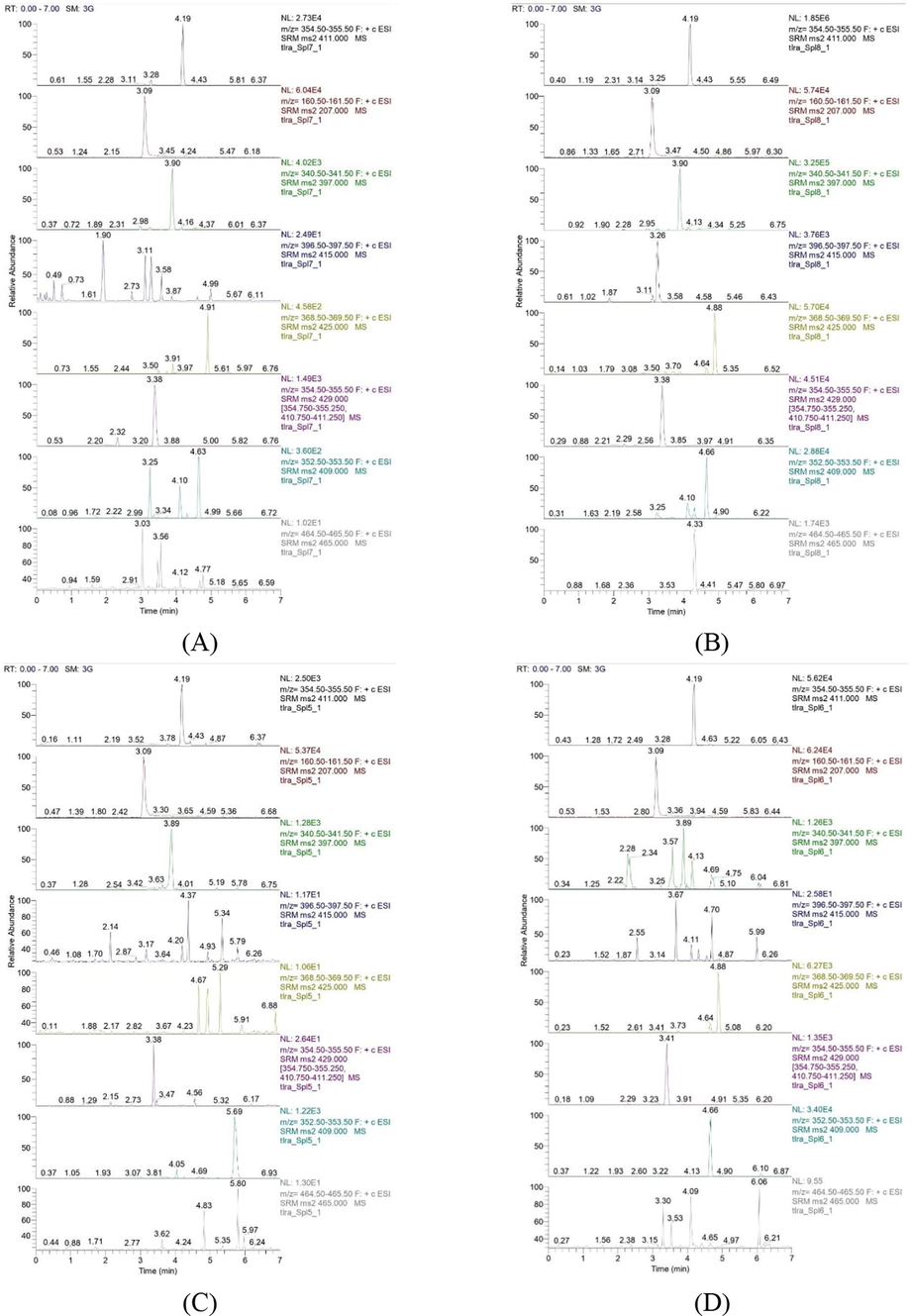
LC-MS/MS analysis results of the bottom phase of mangosteen pericarp extract (A), the top phase of mangosteen pericarp extract (B), the bottom phase of mangosteen seed extract (C), the top phase of mangosteen seed extract (D) using the best MAE operating conditions.
From Table 11, it can be seen that the levels of α-mangostin contained in the bottom phase of the mangosteen pericarp extract were much lower than the top phase but had a stronger DPPH inhibitory power, as shown in Table 5. Likewise, for the extract from the mangosteen seed. This may occur because in the bottom phase, this may occur because there are other bioactive compounds in the bottom phase that are more polar and have higher antioxidant power than α-mangostin. This result is almost the same as the research conducted by Chhouk et al. (2016) and Sungpud et al. (2019), where the extract obtained also has low levels of α-mangostin compounds but still has antioxidant properties. A comparison of the DPPH inhibition ability of this study with other studies can be seen in Table 12.
No
Raw Material
Extract Phase
α-mangostin content
ppm
(%)
1
Pericarp
Bottom
6.97
0.11
2
Phase
964.89
18.56
3
Seed
Bottom
0.02
0.00
4
Phase
0.32
0.00
No.
Method
Yield (% w/w)
α-mangostin (mg/g mangosteen pericarp)
α-mangostin (% w/w extract)
DPPH assay
Reference
1.
Ultrasound-Assisted Extraction (UAE)
–
37.34
–
DPPH Inhibition 43.043 %
Suvarnakuta et al. (2011)
2.
SFE
13.21
–
51.09
IC50 14.0 µg/mL
Zarena et al., (2012)
3.
SFE-mediated hydrothermal extraction
39.9
–
0.203
IC50 73.2 µg/mL
Chhouk et al., (2016)
4.
MAE
–
120.68
–
IC50 20.64 µg/mL
Ghasemzadeh et al. (2018)
5.
MAE
–
–
10.048
IC50 176.9 µg/mL
Mohammad et al. (2019)
6.
Maceration
–
–
0.34
IC50 1820 µg/mL
Sungpud et al. (2019)
7.
MAE
16.531
–
0.11
IC50 9.40 µg/mL
This Research
4 Conclusions
Mangosteen extract with high DPPH inhibition ability was studied in this study using the MAE method with a one factor at a time approach. As a result, the solvent variation affected both DPPH inhibition, TPC value, and yield extract. Solvents having a high dielectric constant have good extraction capabilities. The use of a two-phase solvent resulted in two types of extracts being in the top phase (non-polar) and the bottom phase (polar). The bottom phase extract had a more extraordinary DPPH inhibition ability than the top phase extract. The extraction temperature and time did not significantly affect the yield of the extraction but significantly affected the results of DPPH inhibition and TPC value. Meanwhile, the solid to solvent ratio significantly affected both DPPH inhibition, TPC value, and yield extract. The best extract was obtained with an IC50 DPPH value of 9.4 µg/mL, a TPC value of 903.54 mgGAE/g extract, a yield of 16.53 %, and α-mangostin content of 0.11 % from the use of the solvent EtOAc:EtOH:water 2:1:2 bottom phase, using the MAE method with a temperature of 50 °C, a time of 4 min, and a solid to solvent ratio of 1:16. Mangosteen pericarp is better used as raw material for antioxidant extraction because it has a better DPPH scavenging activity, TPC value, and yield of extraction than extracts made with mangosteen seed as raw material.
Acknowledgment
This study was supported by the Institute of Research and Public Service (LPPM) of Sepuluh Nopember Institute of Technology (ITS) and partially supported by the Massachusetts Institute of Technology (MIT)-Indonesia Research Collaboration managed by Bandung Institute of Technology (ITB).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- tetracycline, erythromycin, and clindamycin against acne involved bacteria. Chin. Herbal Med.. 2019;11:412-416.
- [Google Scholar]
- Valorization of mangosteen, “The Queen of Fruits”, and new advances in postharvest and in food and engineering applications: A review. J. Adv. Res.. 2019;20:61-70.
- [CrossRef] [Google Scholar]
- Chemical analysis and preliminary toxicological evaluation of Garcinia mangostana seeds and seed oil. Food Chem.. 2007;101:999-1004.
- [CrossRef] [Google Scholar]
- Natural antioxidants of plant origin. In: Advances in Food and Nutrition Research (1st ed.). Elsevier Inc.; 2019.
- [Google Scholar]
- Aparamarta, H.W., Saputra, T., Claratika, A., Ju, Y., Gunawan, S., 2016. Separation and Purification of Triacylglycerols from Nyamplung (Calophyllum inophyllum) Oil by Batchwise Solvent Extraction. https://doi.org/10.1021/acs.iecr.5b04877.
- The effect of high oleic and linoleic fatty acid composition for quality and economical of biodiesel from crude Calophyllum inophyllum oil (CCIO) with microwave-assisted extraction (MAE), batchwise solvent extraction (BSE), and combination of MAE–BSE meth. Energy Reports. 2020;6:3240-3248.
- [CrossRef] [Google Scholar]
- In vitro and in vivo inhibitory effects of α-mangostin on cholangiocarcinoma cells and allografts. Asian Pacific J. Cancer Prev.. 2017;18:707-713.
- [Google Scholar]
- Starch Chemistry and Technology (Third edit. ed.). Elsevier Inc.; 2009.
- Understanding the phenomena of extraction of essential oils by the microwave accelerated distillation process: case of the Washington Navel variety. Eur. J. Biol. Res.. 2020;10:167-181.
- [CrossRef] [Google Scholar]
- Extraction of α-mangostin from Garcinia mangostana L. using alternative solvents: Computational predictive and experimental studies. LWT - Food Sci. Technol.. 2016;65:297-303.
- [CrossRef] [Google Scholar]
- Cai, L., 2014. Thin layer chromatography. Curr. Protoc. Essent. Lab. Tech. 2014, 6.3.1-6.3.18. https://doi.org/10.1002/9780470089941.et0603s08.
- Xanthones with pancreatic lipase inhibitory activity from the pericarps of Garcinia mangostana L. (Guttiferae) Eur. J. Lipid Sci. Technol.. 2016;118:1416-1421.
- [CrossRef] [Google Scholar]
- Mangosteen extract prevents dextran sulfate sodium-induced colitis in mice by suppressing NF-κB activation and inflammation. J. Med. Food. 2017;20:727-733.
- [CrossRef] [Google Scholar]
- Prenylated xanthone composition of Garcinia mangostana (Mangosteen) fruit hull. Chromatographia. 2009;69:315-318.
- [CrossRef] [Google Scholar]
- Development and validation of a high-performance thin layer chromatography method for the simultaneous quantitation of α- and γ-mangostins in Thai stingless bee propolis. Rev. Bras. Farmacogn.. 2019;29:333-338.
- [CrossRef] [Google Scholar]
- Supercritical carbon dioxide-mediated hydrothermal extraction of bioactive compounds from Garcinia Mangostana pericarp. J. Supercrit. Fluids. 2016;110:167-175.
- [CrossRef] [Google Scholar]
- A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal. Chim. Acta. 2021;1157:338398
- [CrossRef] [Google Scholar]
- Extraction of natural products using microwaves as a heat source. Sep. Purif. Rev.. 2010;39:1-32.
- [CrossRef] [Google Scholar]
- Bioactive compounds of edible fruits with their anti-aging properties: A comprehensive review to prolong human life. Antioxidants. 2020;9:1-38.
- [CrossRef] [Google Scholar]
- Dima, C., 2020. Bioavailability and bioaccessibility of food bioactive compounds ; overview and assessment by in vitro methods 1–23. https://doi.org/10.1111/1541-4337.12623.
- Combined microwave-assisted extraction and high-speed counter-current chromatography for separation and purification of xanthones from Garcinia mangostana. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.. 2011;879:3023-3027.
- [CrossRef] [Google Scholar]
- The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine. 2015;22:49-51.
- [CrossRef] [Google Scholar]
- Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol.. 2015;90:590-607.
- [CrossRef] [Google Scholar]
- Microwave and enzyme co-assisted aqueous two-phase extraction of polyphenol and lutein from marigold (Tagetes erecta L.) flower. Ind. Crops Prod.. 2018;123:296-302.
- [CrossRef] [Google Scholar]
- Antioxidant Properties of Rosemary and Its Potential Uses as Natural Antioxidant in Dairy Products—A Review. Food Nutr. Sci.. 2015;06:179-193.
- [CrossRef] [Google Scholar]
- Alpha-mangostin-rich extracts from mangosteen pericarp: Optimization of green extraction protocol and evaluation of biological activity. Molecules. 2018;23:1-16.
- [CrossRef] [Google Scholar]
- Gill, B.S., 2020. Chapter 1 - Technologies for extraction and production of bioactive compounds.
- Bioactivity evaluation of natural product α-mangostin as a novel xanthone-based lysine-specific demethylase 1 inhibitor to against tumor metastasis. Bioorg. Chem.. 2018;76:415-419.
- [CrossRef] [Google Scholar]
- Liquid-liquid equilibria of ternary mixtures of water + 2-propanol with ethyl acetate, isopropyl acetate, or ethyl caproate. Fluid Phase Equilib.. 2002;202:239-252.
- [CrossRef] [Google Scholar]
- Doxorubicin and α-Mangostin oppositely affect luminal breast cancer cell stemness evaluated by a new retinaldehyde-dependent ALDH assay in MCF-7 tumor spheroids. Biomed. Pharmacother.. 2020;124
- [CrossRef] [Google Scholar]
- Jesus, S. De, Filho, R.M., 2020. Recent advances in lipid extraction using green solvents 133. https://doi.org/10.1016/j.rser.2020.110289.
- Probing simple structural modification of α-mangostin on its cholinesterase inhibition and cytotoxicity. Arch. Pharm. (Weinheim). 2020;353:1-9.
- [CrossRef] [Google Scholar]
- Supercritical carbon dioxide extraction of α-mangostin from mangosteen pericarp with virgin coconut oil as co-extractant and in-vitro bio-accessibility measurement. Process Biochem.. 2019;87:213-220.
- [CrossRef] [Google Scholar]
- Enhancement of liquid phase splitting of water + ethanol + ethyl acetate mixtures in the presence of a hydrophilic agent or an electrolyte substance. Fluid Phase Equilib.. 2005;237:21-30.
- [CrossRef] [Google Scholar]
- Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol.. 2019;136:305-315.
- [CrossRef] [Google Scholar]
- Studies on Haloperidol and Adjunctive α-Mangostin or Raw Garcinia mangostana Linn Pericarp on Bio-Behavioral Markers in an Immune-Inflammatory Model of Schizophrenia in Male Rats. Front. Psychiatry. 2020;11:1-15.
- [CrossRef] [Google Scholar]
- Inhibition of pancreatic cancer stem cell characteristics by α-Mangostin: Molecular mechanisms involving Sonic hedgehog and Nanog. J. Cell. Mol. Med.. 2019;23:2719-2730.
- [CrossRef] [Google Scholar]
- Optimization of green extraction for the recovery of bioactive compounds from Brazilian olive crops and evaluation of its potential as a natural preservative. J. Environ. Chem. Eng.. 2021;9:105130
- [CrossRef] [Google Scholar]
- Optimization of the antioxidant-rich xanthone extract from mangosteen (Garcinia mangostana L.) pericarp via microwave-assisted extraction. Heliyon. 2019;5
- [CrossRef] [Google Scholar]
- Direct recovery of mangostins from Garcinia mangostana pericarps using cellulase-assisted aqueous micellar biphasic system with recyclable surfactant. J. Biosci. Bioeng.. 2018;126:507-513.
- [CrossRef] [Google Scholar]
- Poole, C.F., 2019. Solvent selection for liquid-phase extraction, in: Liquid-Phase Extraction. Elsevier, pp. 45–89. https://doi.org/10.1016/B978-0-12-816911-7.00002-5.
- Poole, C.F., 2020. Chapter 2, in: Poole, C.F. (Ed.), Handbooks in Separation Science. Elsevier Inc., pp. 45–89. https://doi.org/https://doi.org/10.1016/B978-0-12-816911-7.00002-5.
- From the Front or Back Door? Quantitative analysis of direct and indirect extractions of á- mangostin from mangosteen (Garcinia mangostana) PLoS ONE. 2018;13(10):1-12.
- [CrossRef] [Google Scholar]
- Studies on the invitro anticancer activity of mangostin and acetylated mangostin against MCF-7 cell lines. Chem. Data Collect.. 2020;28
- [CrossRef] [Google Scholar]
- Shang, X. chao, Chu, D., Zhang, J. xu, Zheng, Y. fen, Li, Y., 2021. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 259. https://doi.org/10.1016/j.seppur.2020.118169.
- Initial and Bulk Extraction. In: Natural Products Isolation Vol 20. (2nd). New Jersey: Humana Press Inc; 2006. p. :27-46.
- [Google Scholar]
- Bioactive compounds and their future therapeutic applications. Nat. Bioact. Compd.. 2021;337–362
- [CrossRef] [Google Scholar]
- Antioxidant Activity and Protecting Health Effects of Common Medicinal Plants. Advances in Food and Nutrition Research 2012
- [CrossRef] [Google Scholar]
- Microwave assisted extraction (MAE) used as a tool for rapid extraction of Marmelosin from Aegle marmelos and evaluations of total phenolic and flavonoids content, antioxidant and anti-inflammatory activity. Chem. Data Collect.. 2020;30
- [CrossRef] [Google Scholar]
- Tuning of virgin coconut oil and propylene glycol ratios for maximizing the polyphenol recovery and in vitro bioactivities of mangosteen (Garcinia mangostana L.) pericarp. Process Biochem.. 2019;87:179-186.
- [CrossRef] [Google Scholar]
- Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem.. 2011;125:240-247.
- [CrossRef] [Google Scholar]
- Recovery of mangostins from Garcinia mangostana peels with an aqueous micellar biphasic system. Food and Bioproducts Processing. 2017;102(1):233-240.
- [CrossRef] [Google Scholar]
- α -Mangostin alleviated lipopolysaccharide induced acute lung injury in rats by suppressing NAMPT/NAD controlled inflammatory reactions. Evidence-based Complement. Altern. Med.. 2018;2018
- [CrossRef] [Google Scholar]
- Tarasuk, M., Songprakhon, P., Chimma, P., Sratongno, P., Na-Bangchang, K., Yenchitsomanus, P. thai, 2017. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 240, 180–189. https://doi.org/10.1016/j.virusres.2017.08.011.
- Antioxidant Properties of Garcinia Mangostana L (Mangosteen) Rind. Procedia Chem.. 2014;13:198-203.
- [CrossRef] [Google Scholar]
- Microwave-assisted aqueous two-phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of four flavonoids in Crotalaria sessiliflora L. Ind. Crops Prod.. 2017;95:632-642.
- [CrossRef] [Google Scholar]
- Development and in vivo evaluation of self-microemulsion as delivery system for α-mangostin. Kaohsiung J. Med. Sci.. 2017;33:116-123.
- [CrossRef] [Google Scholar]
- Discovery of a new class of MTH1 inhibitor by X-ray crystallographic screening. Eur. J. Med. Chem.. 2019;167:153-160.
- [CrossRef] [Google Scholar]
- Optimisation of ethanol modified supercritical carbon dioxide on the extract yield and antioxidant activity from Garcinia mangostana L. Food Chem.. 2012;130:203-208.
- [CrossRef] [Google Scholar]
- Xanthones enriched extracts from mangosteen pericarp obtained by supercritical carbon dioxide process. Separation and Purification Technology. 2011;80(1):172-178.
- [CrossRef] [Google Scholar]
- Dynamic ultrasonic-assisted extraction coupled with paralleled counter-current chromatography for continuous extraction and online isolation of xanthenones from Garcinia mangostana. Separation and Purification Technology. 2015;144:215-222.
- [CrossRef] [Google Scholar]
- The naturally occurring xanthone α-mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J. Biol. Sci.. 2018;25:1090-1095.
- [CrossRef] [Google Scholar]
- One-pot process for simultaneously obtaining oil and sinigrin from field pennycress (Thlaspi arvense) seeds using microwave-assisted biphasic extraction. Ind. Crops Prod.. 2021;166
- [CrossRef] [Google Scholar]







