Translate this page into:
The use of untargeted and widely targeted metabolomics to distinguish between asphyxia and sudden cardiac death as the cause of death in rats: A preliminary study
⁎Corresponding authors. wzy218@xjtu.edu.cn (Zhenyuan Wang), huangp@ssfjd.cn (Ping Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
It is important to determine the cause of death in the case of asphyxia. However, it is difficult to conclude death by asphyxia, especially when the deceased has underlying heart disease, because there are often no specific and representative corpse signs for both asphyxia and sudden cardiac death (SCD). The aim of the present work was to investigate the potential of metabolomics to discriminate asphyxia from SCD as the cause of death. A total of thirty male Sprague–Dawley rats were used to construct models of asphyxia, SCD (interfering cause of death), and cervical dislocation (control). Untargeted and widely targeted metabolomics approaches were used to obtain rat pulmonary metabolic profiles in this study. First, the metabolic alterations resulting from asphyxia were explored. There were significant changes found in carbohydrate metabolism, the endocrine system, and the sensory system. Second, we screened potential biomarkers and built classification models to determine the cause of death. Moreover, some biomarkers remained differentiated at 24 h and 48 h postmortem, so the cause of death could still be determined after death. This study showed the application potential of metabolomics to investigate the metabolic changes occurring in the process of death, as well as to determine the cause of death on the basis of metabolic differences even after death.
Keywords
Widely targeted metabolomics
Cause of death
Asphyxia
Sudden cardiac death
After death
1 Introduction
In forensic pathology practice, determining the cause of death is among the most remarkable tasks. Asphyxia is a frequent violent cause of death exhibiting high rates of occurrence and death, while sudden cardiac death (SCD) is also a major public health issue worldwide that results in many deaths (Ma et al., 2016, Azmak, 2006, Kong et al., 2011, Feng et al., 2018). The signs that suggest death from asphyxia include petechial hemorrhage, ligature groove, throttling marks, and neck muscle hemorrhage. However, petechial hemorrhage is nonspecific and the other signs might be inapparent in cases of manual strangulation, smothering, or positional asphyxia (Mosek et al., 2020, Fracasso et al., 2011, Püschel et al., 2004). Heart organic changes, such as coronary heart disease (CHD), cardiac infarction, and cardiomyopathy, suggest the occurrence of SCD. However, these diseases can only prove that the deceased had a basis for SCD and do not necessarily mean that SCD actually occurred. In addition, the possibility of SCD varies with the degree of disease. For example, the severity of coronary artery stenosis before death occurs in CHD patients is debatable (Saukko and Knight, 2015). Therefore, asphyxia and SCD are both determined by excluding other causes of death, according to histological and toxicological assessments and scene situations, if there are no witnesses, video surveillance, or clinical records.
However, it is very difficult and controversial to determine the cause of death if the deceased suffered from intermediate heart disease and is suspected to have died from asphyxia (especially if the asphyxiation signs are inapparent). Asphyxia is most likely homicide or suicide, while SCD is accidental death due to a disease attack. Therefore, it is of great forensic significance and social value to study the differential diagnosis between asphyxia and SCD.
At present, most studies on the determination of asphyxia use molecular biological techniques to find differentially expressed mRNAs, miRNAs or proteins, such as DUSP1/KCNJ2 (Zeng et al., 2018), miRNA-122 (Zeng et al., 2016), miRNA-3185 (Han et al., 2020; Han et al., 2021), SP-A (Zhu et al., 2000), HIF-1α (Cecchi et al., 2014), GLUT1/VAGF (Zhao et al., 2008), AQP-5 (Wang et al., 2012), cytochrome C/AIF (Zhang et al., 2019) and MRP-8/-14 (Gutjahr and Madea, 2019). The same is true for SCD (Sabatasso et al., 2016, Chen et al., 2012, Campobasso et al., 2008, Carvajal-Zarrabal et al., 2017). However, these diagnostic biomarkers cannot be applied in practical work because their sensitivity and specificity need further investigation, as well as the effect of postmortem degradation. Metabolism is fundamental to all biological activities, and the degradation products of proteins and RNAs are also metabolites. Therefore, we suppose that looking for diagnostic biomarkers from a metabolic perspective may obtain better results. Mass spectrometry (MS)-based metabolomics is a high-throughput omics technique that can profile the endogenous metabolic status in a biological system by investigating low-molecular-weight metabolites (Patti et al., 2012, Nicholson et al., 1999). Metabolic status can reveal alterations or conditions that are correlated with diseases or health problems. Metabolomics has served as a powerful tool to uncover potential diagnostic and prognostic biomarkers in recent years (Wang et al., 2016, Khamis et al., 2017, Griffiths et al., 2010, van Ravenzwaay et al., 2007) and has also played a role in forensic science (Locci et al., 2020a, Szeremeta et al., 2021). For example, many studies have focused on disturbed metabolic pathways and possible diagnostic and prognostic biologic signatures in perinatal asphyxia (Denihan et al., 2015, Fattuoni et al., 2015, Sachse et al., 2016, Denihan et al., 2017, Locci et al., 2018, Locci et al., 2020b, Debuf et al., 2021). Chighine et al. studied the urine of the methadone intoxicated infants and perinatal asphyxia newborns and showed that metabolomics can offer useful additional information regarding the cause of death (Chighine et al., 2022). In addition, the metabolic changes and biomarkers of some complex causes of death, such as fatal anaphylactic shock, hypothermia, and acute myocardial ischemia, have also been preliminarily investigated (Hu et al., 2012, Rousseau et al., 2019, Wang et al., 2017).
Two articles from another research team were found through searches of Google Scholar, using the search terms “death from asphyxia” and “metabolomics”. Locci and his collaborators investigated dynamic metabolic alterations in asphyxia and ventricular fibrillation swine plasma by 1H nuclear magnetic resonance spectroscopy and liquid chromatography (LC)–tandem mass spectrometry (MS/MS) in 2017 (Varvarousis et al., 2017) and recently studied the potential of metabolomics to provide additional evidence for the diagnosis of mechanical asphyxia in swine models (Locci et al., 2021). These two studies focused on the metabolic changes occurring within minutes of primitive cardiac arrest (CA) and CA secondary to asphyxia, and proved that there were metabolic differences in experimental animals with different death mechanisms. However, the efficiency of the metabolic difference in the differential diagnosis of cause of death after death needs further study. Therefore, our research team employed gas chromatography (GC)–MS–based metabolomics to preliminarily find that metabolic differences could be used to distinguish causes of death (Zhang et al., 2021). To obtain a greater range of metabolite detection and to carry out research at a longer postmortem interval, widely targeted metabolomics (Chen et al., 2013) combined with untargeted techniques were employed in this study to obtain more metabolic data by use of LC–MS/MS, to investigate potential diagnostic biomarkers and attempt to discriminate asphyxia from SCD as the cause of death at 48 h postmortem in rat models.
2 Material and methods
2.1 Animal experiments
A total of thirty male Sprague–Dawley rats (weighting 230–270 g) were commercially provided (randomly chosen) by the Animal Center of Xi’an Jiaotong University. The experimental animals were housed in a temperature-controlled (Ta, 25 ± 3 °C) environment with a 12–h light/dark cycle and were provided with food and water ad libitum before the modeling. They were randomized into three different groups depending on the cause of death. Urethane (20 %, 1.0–1.2 g/kg) was intraperitoneally injected to guarantee sufficient anesthesia in each rat to ensure that they experienced minimal pain along with discomfort prior to being sacrificed. The animal procedures in this research work were granted approval by the Committee of the Ethics of Animal Experiments of Xi’an Jiaotong University and were carried out as per the recommendations in the Guide for the Care and Use of Laboratory Animals Committee of Xi’an Jiaotong University.
In the asphyxia group, ligature strangulation was used to construct the model. We placed a noose derived from cotton thread around the neck of each rat, and we inserted a small stick into the noose beginning from the back of the neck. After that, tightening of the noose was performed via rotation of the stick to asphyxiate each rat in the presence of constant pressure until the rat died (between 3.8 and 5.3 min, mean value: 4.4 min, standard deviation: 0.4 min). In the SCD group, coronary ligation was used to cause acute myocardial ischemia, which is a very common cause of SCD. We cut the thorax and pericardium of each rat to expose the heart first. Then, the left anterior descending coronary artery was ligatured so that the rat died within 6.7 and 10.2 min (mean value: 8.5 min, standard deviation: 1.0 min). Hemostatic operations were performed at this time. In the control group, we set brainstem injury as the control cause of death because the rats died quickly while experiencing the least amount of biochemical stress. Thus, the rats were sacrificed by cervical dislocation, as this method best exhibits normal levels of metabolism of a living organism. Part of the lung tissue of each rat was collected and subsequently kept at − 80 °C until use. The rat cadavers were also kept for further processing.
2.2 Sample preparation
The lung tissues were thawed on ice. For each sample, we weighed 1 g of tissue and then homogenized it using a frozen homogenization equipment (Jingxin, Shanghai, China) via cold stainless-steel grinding balls at 65 Hz for two minutes. Subsequently, we weighed an estimated 50 mg of the tissue homogenate and then plunged it into 1 mL of 70 % methanol in water containing internal standards. The internal standards included l-phenylalanine-2-13C (99 %), hippuric acid-d5, l-carnitine-d3 chloride, kynurenic acid-d5, phenoxy-d5-acetic acid, and 4-fluoro-L-2-phenylglycine, and their concentrations were all 1 μg/mL (these internal standards were used to monitor the MS/MS detection and correct the signal shift, including m/z, RT, and peak intensity). Homogenization of the mixture was performed again for five minutes, and samples were then spun at 12,000 rpm for ten minutes at 4 °C. The resultant supernatant was transferred into a new EP tube. In addition, quality control (QC) samples were prepared by combining 10 μL of supernatant of each sample (including decayed samples mentioned below). All samples were kept in a −20 °C freezer overnight. After centrifugation at 12,000 rpm for three minutes at 4 °C, 200 μL of the supernatant was placed in the injection bottle for on-board analysis.
2.3 Acquisition of metabolomics data
The extracted metabolites were separated by a Shim-pack UFLC SHIMADZU CBM30A ultrahigh-performance liquid chromatography (UHPLC) platform (https://www.shimadzu.com/). Both untargeted and widely targeted metabolomics techniques were utilized in this study. A quadrupole-time of flight (QTOF) mass spectrometer (Triple TOF 6600, AB SCIEX) was first utilized to obtain MS/MS spectra in information-dependent acquisition (IDA) mode. The QTOF mass data were matched with public databases (including the Metlin, HMDB, and KEGG databases) to identify metabolites by the MetDNA algorithm (Shen et al., 2019). The precursor ion (Q1)–characteristic fragment ion (Q3) pairs of metabolites whose match scores were greater than 0.6 were selected and added to the list of multiple reaction monitoring (MRM) transitions in the following widely targeted detection.
Widely targeted metabolomics was performed using a triple quadrupole-linear ion trap mass spectrometer (QTRAP, QTRAP® 6500+ system, AB SCIEX) in MRM mode under the same UHPLC conditions. The scheduled MRM detection was used according to the list of MRM transitions and retention times, which was created by earlier QTOF detection and the self-built database MWDB (Metware Biotechnology Co., Ltd., Wuhan, China). The metabolites were relatively quantified using the MRM mode of QTRAP, as previously described (Tan et al., 2020; Xiao et al., 2021). The detailed methods of widely targeted metabolomics have been reported in previous articles (Chen et al., 2013; Xiao et al., 2022). During the instrumental detection, one QC sample was inserted after every ten samples to monitor the UHPLC–MS/MS system. The UHPLC conditions, experimental parameters for the QTOF MS/MS and QTRAP MS/MS scans are available in the Supplementary Information_1.docx. Detailed information on the well-matched metabolites in QTOF-MS/MS detection and metabolites in QTRAP-MS/MS detection are shown in the Supplementary Information_2.xlsx.
2.4 Data processing and multivariate analysis
We adopted Analyst v1.6.3 software to acquire and preprocess the mass spectrometry data. MultiQuant 3.0.2 software was employed to integrate and calibrate the chromatographic peaks to obtain the relative contents of the corresponding metabolites. First, the intrinsic variation of the dataset was analyzed and visualized using principal component analysis (PCA), which is a classic unsupervised pattern recognition approach. Second, three criteria were used to discover the metabolic alteration between the asphyxia and controls and to screen the differential metabolites between the rats that died from asphyxia and from SCD. The three criteria included the VIP values in the orthogonal partial least squares discriminant analysis (OPLS-DA) model, fold change (FC) values, and P values in the Mann–Whitney U test. The metabolic features with variable importance in projection (VIP) values greater than 1.0, FC values greater than 2.0 or <0.5 (FC cutoff of 2.0), and P values < 0.05 were statistically significant (Kind et al., 2009; Storey and Tibshirani, 2003). Tenfold cross validations and 200 permutation tests were performed to examine the issue of overfitting when building the OPLS-DA models. Next, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways associated with the differential metabolites were enriched using the KEGG database.
Finally, a random forest (RF) model was built to distinguish between the two causes of death using their differential metabolites. The classification ability was assessed by calculating the area under the curve (AUC), which is a parameter in the receiver operating characteristic (ROC) curve analysis. The Monte Carlo cross validation procedure was employed 100 times to generate the ROC curves.
2.5 Exploring the effects after death
The rat cadavers mentioned above were degraded in a closed chamber under a moderate ambient temperature (Ta, 25 ± 1 °C) along with relative humidity (RH, 50 ± 5 %). The remaining decayed lung tissues were obtained at 24 h and 48 h postmortem. The lung tissues were prepared, and widely targeted metabolomics data were obtained and processed as mentioned above. The relative contents of the differential metabolites screened between the asphyxia and SCD groups were extracted. Then, the Wilcoxon test was performed to compare the relative content changes in these differential metabolites as the PMI increased. In addition, we established RF models to explore the classification abilities of the metabolites that remained different after death.
3 Results
3.1 Metabolomics profiling
A total of 848 metabolites from the lung tissues of rats in the asphyxia, SCD, and control groups were qualitatively detected and relatively quantified by the UHPLC–MS/MS detection platform in the MRM mode. Prior to the analysis of the metabolomics profiles, representative total ion chromatograms (TICs) for the QC samples were plotted, as shown in Fig. S1 in the Supplementary Information_1.docx. An obvious overlay with minimal drift in the retention times and similar intensities of all peaks were observed. In addition, all the QC samples grouped together, as well as did not exhibit any separation pattern according to the PCA score plot in Fig. S2. Therefore, these two results demonstrated the high stability of the instruments and the high reproducibility of data acquisition and preprocessing. The overall variances of the three groups (without QC samples) were then explored by PCA, as shown in Fig. 1. A clear distinction between the asphyxia group and the other two groups was found along PC1, which accounted for 27.22% of the overall variance.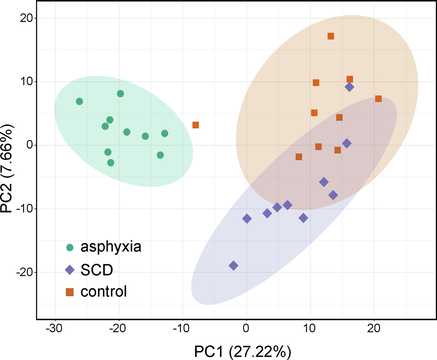
A plot of the PCA scores illustrating the rat pulmonary metabolomics data obtained from the asphyxia, SCD, as well as controls. SCD: sudden cardiac death.
3.2 Metabolic changes linked to death originating from asphyxia compared to the control group and metabolic differences between death from asphyxia and SCD
First, to elucidate the metabolic alterations linked to death originating from asphyxia, OPLS-DA, FC analysis, as well as Mann–Whitney U tests were undertaken between the asphyxia and controls. The plot of the OPLS-DA scores is shown in Fig. 2a, in which remarkable separation of the asphyxia group from the controls was observed. Evaluation of the model was performed with 200 permutation tests, which exhibited an R2Y of 0.990 and a Q2 of 0.943 (p < 0.005), as depicted in Fig. S3a. The VIP value and FC value are displayed as a volcano plot in Fig. 2b. A total of 112 differential metabolites were discovered, with 65 upregulated metabolites and 47 downregulated metabolites. Although the tissue types are different, it can be found that some of these metabolites are similar to the results of previous literature (Locci et al., 2021, Varvarousis et al., 2017), such as succinate (metabolic intermediate in the tricarboxylic acid cycle), glutamate, tyrosine (amino acid), and carnitine(coenzyme). KEGG pathway analysis was further used to enrich and analyze all of the differential metabolites, as displayed in Fig. 3a. We found that the differential metabolites were enriched in numerous pathways, including carbohydrate metabolism, the endocrine system, and the sensory system.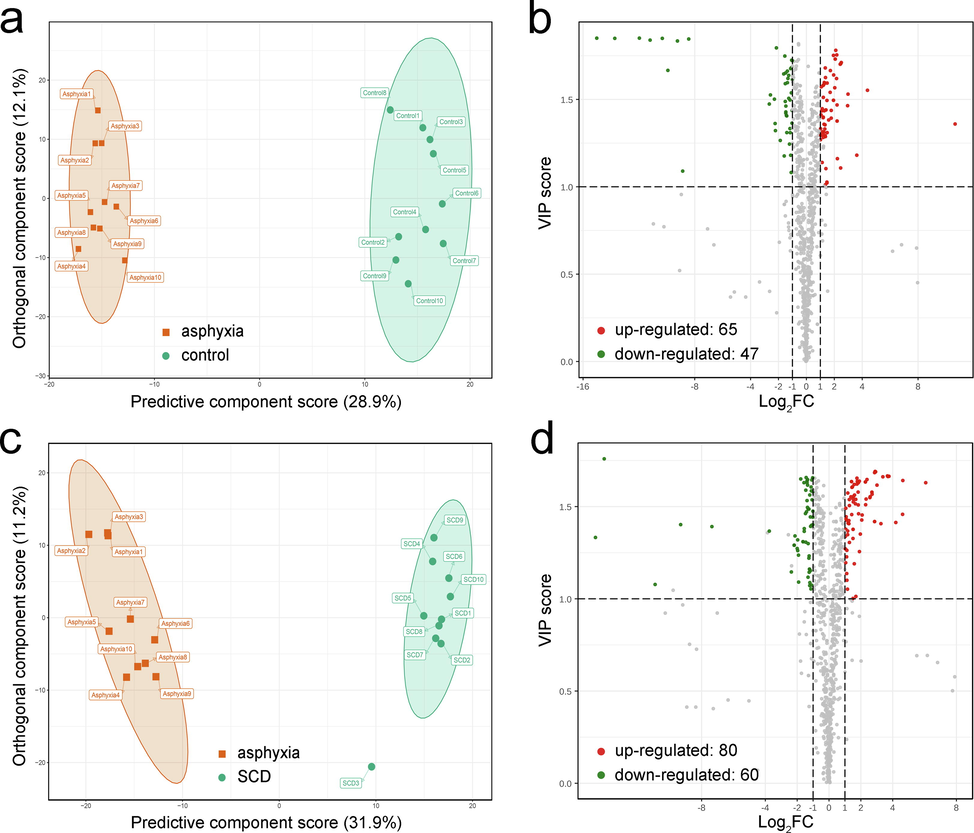
Screening of differential metabolites. The score plot of the OPLS-DA model for the asphyxia group (red) and control group (green) (a). Volcano plot for the asphyxia and control groups, where there were 65 upregulated (red) and 47 downregulated (green) differential metabolites (b). The score plot of the OPLS-DA model for the asphyxia group (red) and SCD group (green) (c). Volcano plot for the asphyxia and SCD groups, where there were 80 upregulated (red) and 60 downregulated (green) differential metabolites (d). In the volcano plots, the X-axis represents the logarithm of the FC value, and the greater the absolute value on the X-axis, the greater is the quantitative difference between the two groups (the FC cutoff was 2.0). The Y-axis represents the VIP values, and the greater the value on the Y-axis, the more important the metabolite is in the discrimination of the two groups (the VIP cutoff was 1.0). SCD: sudden cardiac death.
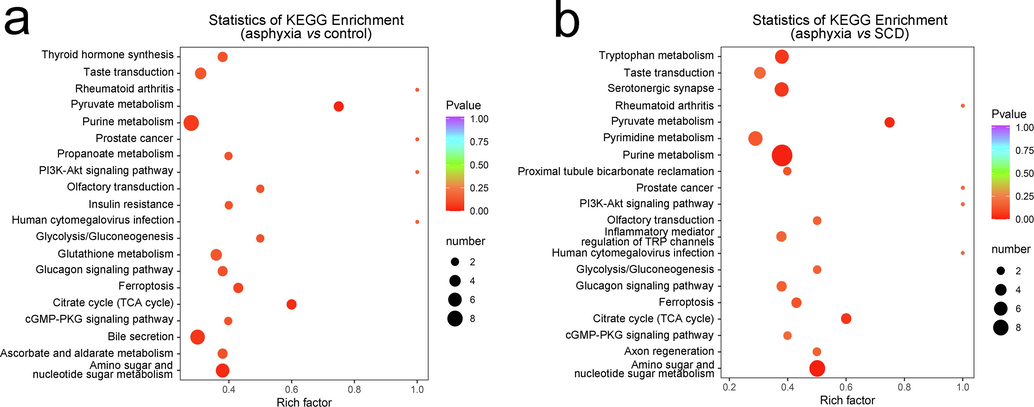
KEGG enrichment scatter plot of the top 20 KEGG pathways of significantly differential metabolites between the asphyxia and control groups (a) and between the asphyxia and SCD groups (b). The X-axis represents the rich factor, which refers to the ratio of the number of differential metabolites located in this pathway. The point size represents the number of significantly differential metabolites that are enriched in this pathway. The color gradation represents the P value. KEGG: Kyoto Encyclopedia of Genes and Genomes.
Subsequently, the same analyses were conducted between the asphyxia and SCD groups to investigate the metabolic differences for the two causes of death. The score plot of the OPLS-DA model is shown in Fig. 2c with 200 permutation tests (R2Y = 0.981 and Q2 = 0.928, p < 0.005) in Fig. S3b. The volcano plot is shown in Fig. 2d. We found 140 differential metabolites, with 80 upregulated metabolites and 60 downregulated metabolites. The 140 differential metabolites were also subjected to KEGG pathway enrichment analysis, as shown in Fig. 3b. The metabolic differences between the two causes of death were mainly concentrated in carbohydrate metabolism, the endocrine system, nucleotide metabolism, amino acid metabolism, and the sensory system. These differential metabolites were plotted with a heatmap to show a better overview of the biochemical differences between the different causes of death, as shown in Fig. S4 and Fig. S5. Each column represents a sample, and each line represents a metabolite with its index number. The details of these metabolites are listed in the Supplementary Information_2.xlsx.
3.3 Biomarkers for postmortem diagnosis
The 140 metabolites that were found to be different in the asphyxia group in contrast with the SCD group could be regarded as prospective biomarkers to distinguish asphyxia from SCD as the cause of death in rat models. An RF classification model with 10-fold cross-validation was established using these biomarkers, which resulted in an AUC of 0.99 with a 95 % confidence interval (CI) of 0.97–1.00. The ROC curve is plotted in Fig. 4a.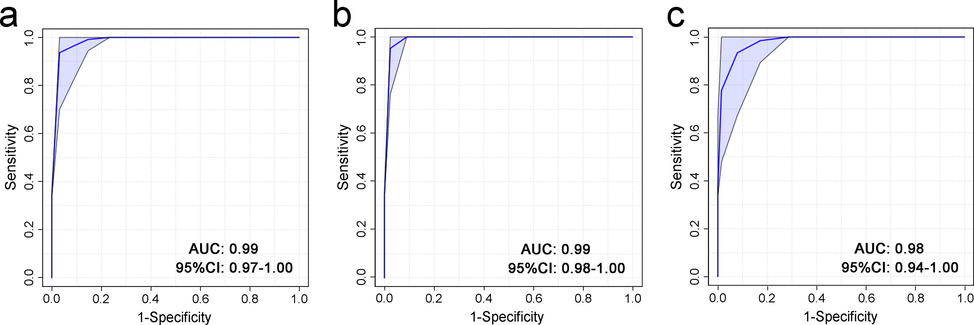
ROC curves of the RF classification models that were established using the 140 differential metabolites in fresh tissues (a), 30 differential metabolites in 24-h decomposed tissues (b), and 10 differential metabolites in 48-h decomposed tissues (c). Tenfold cross-validation was employed to evaluate the models. AUC: area under the curve. CI: confidence interval.
Although an excellent presentation of biomarkers was found to distinguish asphyxia from SCD as the cause of death, there was a concern regarding the stability along with the robustness of these metabolites after death. Hence, the relative contents of the 140 metabolites in decayed lung tissues (at 24 h and 48 h postmortem) were also collected in MRM mode. These metabolites were again evaluated by FC analysis and Wilcoxon tests to determine which metabolites were still different between the groups.
Of the 140 differential metabolites between the asphyxia and SCD groups in the fresh samples, 30 metabolites remained differential at 24 h postmortem, and 10 of these 30 metabolites remained differential at 48 h postmortem. The 10 metabolites and the change trend of their relative contents are displayed in Fig. 5. Some increased and some decreased with the postmortem interval (PMI). Another two RF models were built based on the 30 differential metabolites at 24 h postmortem and 10 differential metabolites at 48 h postmortem, as shown in Fig. 4b and Fig. 4c, respectively. These models both showed good classification abilities, with AUCs of 0.99 and 0.98 (95 % CI: 0.98–1.00 and 0.94–1.00), respectively.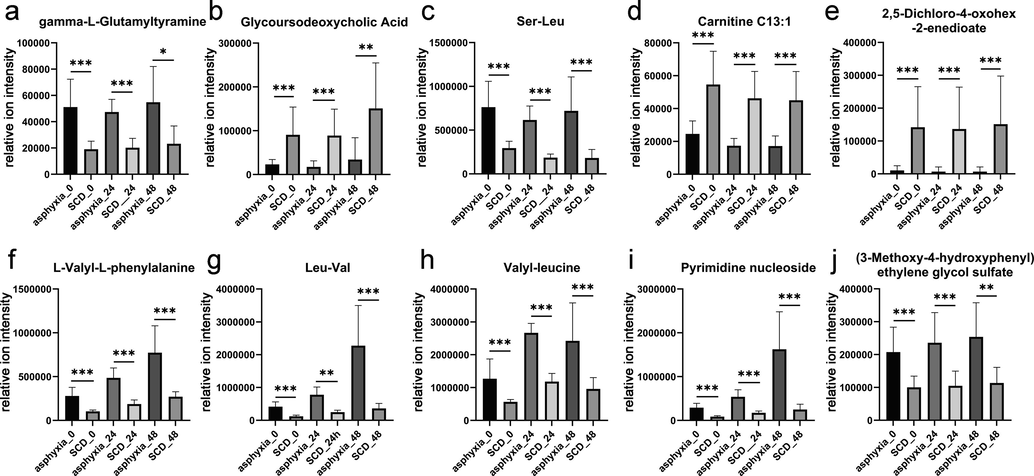
The change in the relative contents of the 10 metabolites that remained significantly different in fresh, 24 h-decomposed, and 48 h-decomposed lung tissues. *: statistically significant differences were observed (***P < 0.001; **P < 0.01; and *P < 0.05).
4 Discussion
A widely targeted metabolomics strategy was used in this study combined with QTOF-MS/MS-based untargeted data collection to further improve the metabolite detection range. A very large amount of metabolomics data was obtained and relatively quantified by use of the MRM technique.
Brainstem injury served as the control in this study, which represents a rapid mode of death with the lowest amount of biochemical stress, best reflecting the normal level of metabolism in live rats. Therefore, we explored the metabolic alterations after death from asphyxia via comparing the asphyxia group with the controls. The unsupervised PCA results showed that there were clear metabolic alterations in the lung tissues of the rats that died from asphyxia, and the metabolic alterations were different from those of the rats that died from SCD.
4.1 Metabolic changes linked to death from asphyxia in rats
Asphyxia is characterized by the inability of cells in the body to either receive or utilize oxygen and produces great external stress on the body (Sauvageau and Boghossian, 2010). With regard to asphyxia, the first effects observed should be related to the aerobic oxidation process of energy-supplying substances. The KEGG enrichment analysis results were consistent with this notion. Among carbohydrate metabolism pathways, pyruvate metabolism, glycolysis/gluconeogenesis, and the citrate cycle (tricarboxylic acid cycle, TCA cycle) are all related to the energy supply process. Glycolysis refers to the process in which glucose or glycogen is converted into lactic acid, and a small amount of ATP is produced through a series of reactions. This pathway is an important way to quickly provide energy in times of hypoxia. During glycolysis, the formation of pyruvate from glucose is known as the glycolysis pathway or the Embden-Meyerhof-Parnas (EMP) pathway, which is a common step in both the aerobic and anaerobic oxidation of glucose. As the final product of the EMP pathway, pyruvate can be reduced in the cytoplasm to produce lactic acid for energy, or it can be oxidized in the mitochondria to produce acetyl-CoA, which then participates in the TCA cycle. The TCA cycle is the final step in the aerobic oxidation of three nutrients (sugars, lipids, and amino acids) and is also the hub of the metabolic connection between sugars, lipids, and amino acids (Lowenstein, 1969, Weitzman, 1987). Asphyxia leads to hypoxic conditions in the body. As a result, glycolysis activity increases to produce ATP, and metabolic disorders of the TCA cycle might occur.
In addition to carbohydrate metabolism, there were also large changes in the endocrine system. Thyroid hormones can act on nearly every cell in the body to promote the oxidation of sugars and fats. Glucagon, which is generated by alpha cells in the pancreas, works by raising the content of glucose along with fatty acids in the bloodstream. Its effects oppose those of insulin, which is generated by beta cells in the pancreas and can lower extracellular glucose. Therefore, we inferred that the body tended to produce more thyroid hormones and glucagon and less insulin to increase the energy supply in the bloodstream and enhance oxidation in response to the lack of energy and the great stress caused by hypoxia.
It is strange to find that there are possible metabolic changes related to taste and olfactory transduction in the lung when asphyxia occurs according to KEGG pathway analysis. A 2018 study found that the human olfactory receptors in the olfactory knob become less sensitive under hypoxic conditions (Bigdaj et al., 2018). In addition, Garg et al. considered the lung to be a sensory organ due to the existence of pulmonary neuroendocrine cells (Garg et al., 2019), and there is another interesting article in which the researchers found that there are taste receptors in the seminiferous tubules and epididymis (the reason is currently unknown)(Mosinger et al., 2013). According to these reports, our result could be possible, and we suppose that the presence of relevant metabolic changes does not mean that the lung can transmit the sensation of smell and taste. However, the possible reasons for the results are unclear.
In addition, the PI3K-Akt signaling pathway and cGMP-PKG signaling pathway are very common and important metabolic pathways. Our results indicated that asphyxia affects biological functions through these two pathways, and there have been numerous previous studies supporting this result (Mahneva et al., 2019, Wang et al., 2013, Dawson-Scully et al., 2010, Gorbe et al., 2010, Zhang et al., 2018, Zou et al., 2015, Xie et al., 2019). For example, the promotion of glycolysis mentioned above may be regulated through the PI3K-Akt pathway (Xie et al., 2019).Ferroptosis is an iron-dependent modulated cell death process resulting from unrestricted lipid peroxidation coupled with subsequent membrane damage (Tang and Kroemer, 2020). Its classic induction is related to the tripeptide glutathione (GSH). GSH is often referred to as the body’s primary antioxidant. It is pivotal for the activity of glutathione peroxidase 4 (GPX4), the selenoenzyme that works as a core ferroptosis repressor. GSH depletion or inhibition of its synthesis indirectly inactivates GPX4, leading to the accumulation of toxic free radical reactive oxygen species (ROS), which promote membrane lipid peroxidation and induce ferroptosis (Tang and Kroemer, 2020, Cao and Dixon, 2016). Studies have shown a link between hypoxia and ferroptosis (An et al., 2020, Chen et al., 2020, Fuhrmann et al., 2020). Our results also indicated that the ferroptosis process might be altered by regulating GSH metabolism in the body after asphyxia.
4.2 Postmortem differential diagnosis for death from asphyxia in rats
According to the PCA score plot, we found that the SCD group was more similar to the controls than was the asphyxia group (according to the distances between groups), indicating that fewer metabolic alterations occurred after SCD than after asphyxia. We found that the enriched pathways based on the differential metabolites from asphyxia in the SCD group and from asphyxia in the control group were mostly the same. Therefore, we supposed that the metabolic differences between SCD and asphyxia were mainly derived from asphyxia as the cause of death. However, it is also possible that both causes of death affected the same pathways but to different degrees. Regardless, we were excited to find substantial metabolic differences in the lung tissues of rats that died from asphyxia, as well as SCD based on our metabolomics strategy. Excellent classification performance was obtained using the RF model based on metabolic differences, which demonstrated that the metabolomics strategy has great potential to distinguish complex causes of death.
More importantly, it was found that some of the metabolic differences were maintained even after death. Although a decreasing number of metabolites remained significantly different as the PMI increased, the classification models based on these metabolites could still robustly discriminate asphyxia from SCD after death. Although we investigated only the differential metabolites up to 48 h after death, this preliminary study is still meaningful because a large proportion of corpses are found and examined within two days in forensic practice. This study proposes a metabolomics approach to identify complex causes of death, which is also applicable after death.
4.3 Limitations and outlook
Although the current idea has worked well in preliminary animal experiments, it is still a long way away from practical application. There are three main limitations in this study. The first one is the human individual differences in metabolic status during life. Age, sex, and health conditions could be potential factors that lead to baseline differences in the metabolome. Compared to standardized animals, it is difficult to screen differential metabolites that are related to only the cause of death in the general population. The second one is factors after death, such as PMI, exogenous bacteria, and insects. We only investigated the differential metabolites up to 48 h postmortem, so the applicability of this approach to a longer PMI range is uncertain. The animal bodies were placed in a controlled and closed environment, which prevented the potential effects of temperature, humidity, exogenous bacteria, and insects. The third one is the translational issue from experimental animals to humans. Undoubtedly, the status of metabolism must be different in humans and rats. The results of this study cannot be applied directly to humans. However, this study provides a practicable path to study the determination of complex causes of death. In the future, an extensive collection of human samples is needed, as Chighine et al. proposed (Chighine et al., 2021). Based on the population samples, absolute quantitative detection, confirmatory experiments, and in-depth research on various influencing factors should be performed.
Author contributions
KZ, PH designed the study, KZ, RL, HY performed the measurements and analyzed the data, PH, PX provided the instruments, KZ, YT wrote the manuscript, ZW, PH supervised the projects. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81730056, 81722027, 82072115), the National Key R&D Program of China (2022YFC3302002), and the Science and Technology Committee of Shanghai Municipality (21DZ2270800 and 19DZ2292700).
Acknowledgements
The authors are thankful to Wuhan Metware Biotechnology Company for cooperation in data collection and analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hypoxia-augmented and photothermally-enhanced ferroptotic therapy with high specificity and efficiency. J. Mater. Chem. B. 2020;8:78-87.
- [Google Scholar]
- Asphyxial Deaths: A Retrospective Study and Review of the Literature. Am. J. Forensic Med. Pathol.. 2006;27:134-144.
- [Google Scholar]
- The Effects of Hypoxic Hypoxia on Olfactory Sensitivity in Humans. Biophysics (Oxf).. 2018;63:463-468.
- [CrossRef] [Google Scholar]
- Sudden Cardiac Death and Myocardial Ischemia Indicators: A Comparative Study of Four Immunohistochemical Markers. Am. J. Forensic Med. Pathol. 2008:29.
- [Google Scholar]
- Use of Cardiac Injury Markers in the Postmortem Diagnosis of Sudden Cardiac Death. J. Forensic Sci.. 2017;62:1332-1335.
- [CrossRef] [Google Scholar]
- Markers of mechanical asphyxia: immunohistochemical study on autoptic lung tissues. Int. J. Legal Med.. 2014;128:117-125.
- [CrossRef] [Google Scholar]
- A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant. 2013;6:1769-1780.
- [CrossRef] [Google Scholar]
- Pathophysiology of sudden cardiac death as demonstrated by molecular pathology of natriuretic peptides in the myocardium. Forensic Sci. Int.. 2012;223:342-348.
- [CrossRef] [Google Scholar]
- The role of ferroptosis in chronic intermittent hypoxia-induced liver injury in rats. Sleep Breath.. 2020;24:1767-1773.
- [CrossRef] [Google Scholar]
- Looking for Post-Mortem Metabolomic Standardization: Waiting for Godot—The Importance of Post-Mortem Interval in Forensic Metabolomics. Chem. Res. Toxicol.. 2021;34:1946-1947.
- [CrossRef] [Google Scholar]
- Infant urinary metabolomic profile in a fatal acute methadone intoxication. Int. J. Legal Med.. 2022;136:569-575.
- [CrossRef] [Google Scholar]
- Controlling anoxic tolerance in adult Drosophila via the cGMP–PKG pathway. J. Exp. Biol.. 2010;213:2410-2416.
- [CrossRef] [Google Scholar]
- A Metabolomic Approach in Search of Neurobiomarkers of Perinatal Asphyxia: A Review of the Current Literature. Front. Pediatr. 2021
- [Google Scholar]
- Metabolomic Profiling in Perinatal Asphyxia: A Promising New Field. Biomed. Res. Int.. 2015;2015:254076.
- [CrossRef] [Google Scholar]
- Untargeted metabolomic analysis and pathway discovery in perinatal asphyxia and hypoxic-ischaemic encephalopathy. J. Cereb. Blood Flow Metab.. 2017;39:147-162.
- [CrossRef] [Google Scholar]
- Perinatal Asphyxia: A Review from a Metabolomics Perspective. Molecules. 2015;20:7000-7016.
- [CrossRef] [Google Scholar]
- Sudden Cardiac Death in Mainland China. Circ. Arrhythmia Electrophysiol.. 2018;11:e006684.
- [Google Scholar]
- Petechial bleedings in sudden infant death. Int. J. Legal Med.. 2011;125:205-210.
- [CrossRef] [Google Scholar]
- Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol.. 2020;36:101670.
- [Google Scholar]
- Garg, A., Sui, P., Verheyden, J.M., Young, L.R., Sun, X., 2019. Chapter Three - Consider the lung as a sensory organ: A tip from pulmonary neuroendocrine cells. In: Wellik, D.M.B.T.-C.T. in D.B. (Ed.), Organ Development. Academic Press, pp. 67–89. https://doi.org/10.1016/bs.ctdb.2018.12.002.
- Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic Res. Cardiol.. 2010;105:643-650.
- [Google Scholar]
- Targeted Metabolomics for Biomarker Discovery. Angew. Chemie Int. Ed.. 2010;49:5426-5445.
- [CrossRef] [Google Scholar]
- Inflammatory reaction patterns of the lung as a response to alveolar hypoxia and their significance for the diagnosis of asphyxiation. Forensic Sci. Int.. 2019;297:315-325.
- [CrossRef] [Google Scholar]
- Systematic Review of the Incidence of Sudden Cardiac Death in the United States. J. Am. Coll. Cardiol.. 2011;57:794-801.
- [CrossRef] [Google Scholar]
- Identification of the miRNA-3185/CYP4A11 axis in cardiac tissue as a biomarker for mechanical asphyxia. Forensic Sci. Int.. 2020;311:110293
- [CrossRef] [Google Scholar]
- Model for the prediction of mechanical asphyxia as the cause of death based on four biological indexes in human cardiac tissue. Sci. Justice. 2021;61:221-226.
- [CrossRef] [Google Scholar]
- GC–MS-based metabolic profiling reveals metabolic changes in anaphylaxis animal models. Anal. Bioanal. Chem.. 2012;404:887-893.
- [CrossRef] [Google Scholar]
- Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev.. 2017;36:115-134.
- [CrossRef] [Google Scholar]
- FiehnLib: Mass Spectral and Retention Index Libraries for Metabolomics Based on Quadrupole and Time-of-Flight Gas Chromatography/Mass Spectrometry. Anal. Chem.. 2009;81:10038-10048.
- [CrossRef] [Google Scholar]
- A longitudinal 1H-NMR metabolomics analysis of urine from newborns with hypoxic-ischemic encephalopathy undergoing hypothermia therapy. Clinical and medical legal insights. PLoS ONE. 2018;13:e0194267.
- [Google Scholar]
- Forensic NMR metabolomics: one more arrow in the quiver. Metabolomics. 2020;16:118.
- [CrossRef] [Google Scholar]
- Metabolomics improves the histopathological diagnosis of asphyxial deaths: an animal proof-of-concept model. Sci. Rep.. 2021;11:10102.
- [CrossRef] [Google Scholar]
- Citric Acid Cycle. Elsevier; 1969.
- Retrospective analysis of 319 hanging and strangulation cases between 2001 and 2014 in Shanghai. J. Forensic Leg. Med.. 2016;42:19-24.
- [CrossRef] [Google Scholar]
- NO/cGMP/PKG activation protects Drosophila cells subjected to hypoxic stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol.. 2019;223:106-114.
- [Google Scholar]
- Cases of asphyxia in children and adolescents: a retrospective analysis of fatal accidents, suicides, and homicides from 1998 to 2017 in Hamburg, Germany. Int. J. Legal Med. 2020:1-9.
- [Google Scholar]
- Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc. Natl. Acad. Sci.. 2013;110:12319-12324.
- [CrossRef] [Google Scholar]
- “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181-1189.
- [CrossRef] [Google Scholar]
- Metabolomics: the apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol.. 2012;13:263-269.
- [CrossRef] [Google Scholar]
- A serum metabolomics signature of hypothermia fatalities involving arginase activity, tryptophan content, and phosphatidylcholine saturation. Int. J. Legal Med.. 2019;133:889-898.
- [CrossRef] [Google Scholar]
- Early markers for myocardial ischemia and sudden cardiac death. Int. J. Legal Med.. 2016;130:1265-1280.
- [CrossRef] [Google Scholar]
- The Role of Plasma and Urine Metabolomics in Identifying New Biomarkers in Severe Newborn Asphyxia: A Study of Asphyxiated Newborn Pigs following Cardiopulmonary Resuscitation. PLoS ONE. 2016;11:e0161123.
- [Google Scholar]
- Knight’s Forensic Pathology. CRC Press; 2015.
- Classification of Asphyxia: The Need for Standardization. J. Forensic Sci.. 2010;55:1259-1267.
- [CrossRef] [Google Scholar]
- Metabolic reaction network-based recursive metabolite annotation for untargeted metabolomics. Nat. Commun.. 2019;10:1516.
- [CrossRef] [Google Scholar]
- Statistical significance for genomewide studies. Proc. Natl. Acad. Sci.. 2003;100:9440-9445.
- [CrossRef] [Google Scholar]
- Applications of Metabolomics in Forensic Toxicology and Forensic Medicine. Int. J. Mol. Sci. 2021
- [CrossRef] [Google Scholar]
- Comprehensive ESI-Q TRAP-MS/MS based characterization of metabolome of two mango (Mangifera indica L) cultivars from China. Sci. Rep.. 2020;10:20017.
- [CrossRef] [Google Scholar]
- The use of metabolomics for the discovery of new biomarkers of effect. Toxicol. Lett.. 2007;172:21-28.
- [CrossRef] [Google Scholar]
- Metabolomics profiling reveals different patterns in an animal model of asphyxial and dysrhythmic cardiac arrest. Sci. Rep.. 2017;7:16575.
- [CrossRef] [Google Scholar]
- Intrapulmonary aquaporin-5 expression as a possible biomarker for discriminating smothering and choking from sudden cardiac death: A pilot study. Forensic Sci. Int.. 2012;220:154-157.
- [CrossRef] [Google Scholar]
- Metabolic risk factors associated with sudden cardiac death (SCD) during acute myocardial ischemia. Forensic Sci. Res.. 2017;2:126-131.
- [CrossRef] [Google Scholar]
- Sildenafil Inhibits Hypoxia-Induced Transient Receptor Potential Canonical Protein Expression in Pulmonary Arterial Smooth Muscle via cGMP-PKG-PPARγ Axis. Am. J. Respir. Cell Mol. Biol.. 2013;49:231-240.
- [CrossRef] [Google Scholar]
- Weitzman, J.K.-P., 1987. Krebs citric acid cycle: half a century and still turning, in: Biochem. Soc. Symp., pp. 1–198.
- Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int.. 2021;141:110128.
- [CrossRef] [Google Scholar]
- UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem.. 2022;378:131999.
- [CrossRef] [Google Scholar]
- PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol. Med. Rep.. 2019;19:783-791.
- [CrossRef] [Google Scholar]
- G6PC3, ALDOA and CS induction accompanies mir-122 down-regulation in the mechanical asphyxia and can serve as hypoxia biomarkers. Oncotarget. 2016;7:74526-74536.
- [Google Scholar]
- DUSP1 and KCNJ2 mRNA upregulation can serve as a biomarker of mechanical asphyxia-induced death in cardiac tissue. Int. J. Legal Med.. 2018;132:655-665.
- [CrossRef] [Google Scholar]
- Cytoplasmic upregulation of Cyto c and AIF serve as biomarkers of mechanical asphyxia death. Am. J. Transl. Res.. 2019;11:4568-4583.
- [Google Scholar]
- Exploring metabolic alterations associated with death from asphyxia and the differentiation of asphyxia from sudden cardiac death by GC-HRMS-based untargeted metabolomics. J. Chromatogr. B. 2021;1171:122638.
- [CrossRef] [Google Scholar]
- PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review) Mol. Med. Rep.. 2018;18:3547-3554.
- [CrossRef] [Google Scholar]
- Tissue-specific differences in mRNA quantification of glucose transporter 1 and vascular endothelial growth factor with special regard to death investigations of fatal injuries. Forensic Sci. Int.. 2008;177:176-183.
- [CrossRef] [Google Scholar]
- Immunohistochemical investigation of a pulmonary surfactant in fatal mechanical asphyxia. Int. J. Legal Med.. 2000;113:268-271.
- [CrossRef] [Google Scholar]
- Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J. Bone Miner. Metab.. 2015;33:615-624.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104322.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







