Translate this page into:
Extraction of Pb (II) and Co (II) using N,N-dioctylsuccinamate based room temperature ionic liquids containing aliphatic and aromatic cations
⁎Corresponding author. mudassir.iqbal@sns.nust.edu.pk (Mudassir Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Synthesis and Characterization of six novel N,N-dioctylsuccinamate based room temperature ionic liquids (RTILs) bearing imidazolium, pyridinium, ester imidazolium, and quaternary ammonium cations is reported. Extraction of Pb(II) and Co(II) by these RTILs has been investigated. Ionic liquids (ILs) synthesized were [C4mim][N88SA], [C8mim][N88SA], [C4Py][N88SA], [C8Py][N88SA], [α-mim-ester][N88SA] and [N2244][N88SA] termed as L1, L2, L3, L4, L5 and L6 respectively. Liquid-liquid extraction was performed and all the six systems showed excellent extractability results for both Pb(II) and Co(II). During the process of extraction several factors i.e., nature of cation, pH of the aqueous phase, equilibration time, and initial metal ion concentration were investigated. The extraction efficiency of above 98 % for all types of extractants was observed. The nature of cation its concentration, equilibration time, and pH of the aqueous phase significantly influenced the extraction efficiency. Maximum extraction was observed at pH values between 4 and 8 and optimum contact time was observed to be 40–45 min. Increasing the metal ion concentration decreased the extraction efficiency. The extraction efficiency of both metal ions decreased in the order [N88SA][C8mim] (L2) > [α-mim-Ester][N88SA] (L5) > [N88SA][C4mim] (L1). This is evident from the order of extraction behaviour that increasing the bulkiness of cation, results in stronger complexation, hence increasing extraction.
Keywords
RTILs
Imidazolium
Pyridinium
Quarternary Ammonium
N
N-dioctylsuccinamate
Pb(II)/Co(II) Extraction
1 Introduction
The industrial effluent is continuously contaminating natural resources of water with heavy metals and metalloids with high density in the range of 3.5–7 g cm−3 (Masindi and Muedi, 2018). Apart from industrial effluent, other natural sources of heavy metals are volcanic activity, soil erosion, and geological weathering. Heavy metal pollution has an imminent surge because of anthropogenic activity such as industrial and agricultural work (Briffa et al., 2020). Heavy metals contaminate soil, air, and water. Contamination of the aquatic environment by heavy metals results in serious consequences because of toxicity to the aquatic life and aquatic habitat (Kumar, 2019). Heavy metals when introduced even in trace amounts to water are eventually toxic to humans. In the food chain, these heavy metals keep on accumulating and because humans are at the apex of the food chain so they are affected the most (Sonone, 2020). Water is the necessity of all life forms; safe and clean water is also crucial for human exitance. Heavy metal toxicity affects the human central nervous system, mental activities, and body fluid composition, affects lung’s function, damages kidney, liver, and other vitals. Long periods of exposure have resulted in muscular dystrophy, Alzheimer’s disease, sclerosis, and carcinogenesis (Tchounwou, 2012; Engwa, 2019).
Recent reports are showing an alarming situation of these heavy metals in the aquatic system (Ouyang, 2018; Huseen and Mohammed, 2019; Vardhan et al., 2019; Foong et al., 2020). Analysed data in these reports conclude that average content of some heavy metals such as chromium (Cr), manganese (Mn), cobalt (Co), nickel (Ni), arsenic (As), and cadmium (Cd) has exceeded the permissible limit of world health organization and the United States Environmental Protection Agency USEPA (Kumar, 2019; Saha and Paul, 2019). Cobalt is Lustrous, Silvery blue colored heavy metal with a density: 8.86 g/cm3. Cobalt has extensive application in industry such as paint, pottery, electroplating, electronics, metallurgy, food preservation, and cancer treatment (Manohar et al., 2006). Nuclear power plant waste contains a significant amount of cobalt. The permissible limit of cobalt in the livestock wastewater and irrigation water is between 0.05 and 1 mg per litter (Bhatnagar et al., 2010). Cobalt poisoning causes edema, congestion, and lung bleeding in people who have been exposed to the metal. Chronic exposure to cobalt affects the skin (allergic dermatitis), visceral organs (leading to nausea, vomiting, and diarrhea), respiratory issues (irritation, asthma, pneumonia, and fibrosis), cardiac problems (heart failure, cardiomyopathy), liver and kidney dysfunction, genetic mutations, and cancer (Waheed, 2021; Rengaraj and Moon, 2002). Lead is another silver-grey colored heavy metal with a density of 11.3 g/cm3. In ancient times, it has been used as hair dye, pottery glaze, and insecticide (Tayyaba, 2017). Lead is used in a variety of products, including vehicle batteries, computer screen protectors, ammunition and projectiles, cable sheeting, corrosive substances canisters, roofing, glass windows, and lead plumbing. Exposure to lead leads to reproductive health problems like, sperm damage, premature or stillbirth, and miscarriages. Other health issues such as cognitive impairment, nerve damage, brain injury, iron deficiency, renal failure and abdominal pain have also been reported.
Different water treatment methods to remove heavy metals with different success rates can be adopted such as chemical precipitation, membrane filtration, carbon adsorption, co-precipitation, and ion exchange. Most of these treatment systems have flaws such as the formation of secondary waste, expensive operating, and maintenance costs, and so on. Solvent extraction is not only easy but economical, eco-friendly, and simple to operate (Waheed, 2021; Iqbal, 2020).
Green Chemistry is the most plausible and widely accepted solution to cope with environmental hazards. In recent research, the use of Ionic liquids (IL) seems to be vital, creative, and fertile approach for the removal of heavy metals from the environment (Sengupta, 2013; Mohapatra, 2013; Sengupta, 2015; Ilyas, 2022). The ionic liquids are molten salts that have melting point below 100 and are characterized by high thermal stability, high ionic conductivity, low vapour pressure, and miscibility in many solvents (Lawal, 2019; Li, 2021; Dai, 2021). ILs have been extensively used for the efficient extraction of heavy metals (Waheed, 2021; Gujar, 2021; Tran and Lee, 2020; Mohapatra, 2016; Sengupta, 2015; Hu, 2021), extraction of aromatics from aliphatics (Yu, 2021), extraction of phenols (Skoronski, 2020)and enhanced oil recovery (Bin-Dahbag, 2014).
ILs often act as an ion exchanger between water and organic phase leading to loss of IL part. To modify the ILs properties such as hydrophilicity, hydrophobicity and miscibility, alkyl chains can be substituted on ILs components. ILs having modified cationic and anionic components leading to unique properties are named as task-specific ionic liquids (TSILs) (Tran, 2020; Wu, 2022). The extraction of metal ions using ionic liquids have different types of mechanisms depending on the type of ionic liquids used. These are basic types are solvation, cation exchange mechanism, anion exchange mechanism, ion association mechanism etc. (Nayak and Devi, 2017).
For the development of extractants two competing classes of compounds are organophosphorus and amides. Organophosphorus Compounds are not so environmentally friendly due to their incomplete incinerability. Over past years, amide based extractants viz. diglycolamides (Costa et al., 2016), and succinamides (Yan, 2019) have been extensively used. Amides are environmentally benign as they produce only gases which can be separated from non-incinerable impurities (Cui, 2015). Very little is reported regarding the use of N,N-dioctylsuccinamate as extractants Table 1 (Yoshizuka, 1990; Tran, 2020). Literature survey revealed that this method of liquid liquid extraction has not been exploited against two major heavy metals such as Pb (II) and Co (II). Thus, we envisioned that the introduction of succinimide based ILs will emerge as a green and efficient method of heavy metals extraction. Moreover, extraction by using imidazole (Yan, 2021; Budnyak, 2018), pyridinium (Hu, 2022: Basaiahgari and Gardas, 2021) and ammonium (Foltova, 2019; Wang, 2019) based ionic liquids have been widely accepted for metal extraction.
Imidazolium based IL’s
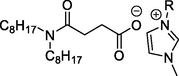
L1 has R = C4H9, [C4mim][N88SA]
L2 has R = C8H17, [C8mim] [N88SA]
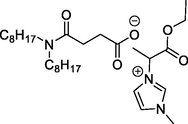
L5 has [α-mim-ester][N88SA]
Pyridinium based IL’s
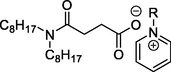
L3 has R = C4H9, [C4Py][N88SA]
L4 has R = C8H17, [C8Py][N88SA]
Quaternary Ammonium based IL’s
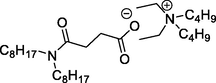
L6 has [N2244][N88SA]
In this work, we report for the first time, six different novel succinamide anion based task specific ionic liquids (TSILs), by metathesis reaction (Wang, 2019; Mohapatra, 2013). Succinamide based cations were prepared by a convenient, mild, metal free reaction method using precursor N,N-dioctylsuccinamate. Cationic component of these TSILs were prepared by changing the alkyl substitution in imidazole, pyridine and diethylamine. Two different alkyl chains, butyl and octyl were substituted to obtain new imidazolium, pyridinium and tertiary amine cations. Further, these TSILs were employed for the extraction of Pb(II) and Co(II) metals from their aqueous solutions. The extraction efficiency of all the TSILs was determined and compared at different shaking times, pH of the aqueous phase and for target metal ion concentration in the aqueous phase.
2 Materials and methods
All chemicals for the synthesis of ILs and extraction of metals viz. Succinic anhydride, dioctylamine, 1-methylimidazole, 1-bromooctane, 1-bromobutane, ethyl-2-bromopropanoate, pyridine, sodium hydride, benzophenone, sodium metal, lead nitrate, cobalt nitrate, and buffers solutions were reagent grade and purchased from Sigma Aldrich were used without further purification. All solvents used in synthesis procedures viz. tetrahydrofuran, methanol, chloroform, toluene, dichloromethane, n-hexane, and ethyl acetate were dried using standard procedures and stored over molecular sieves.
2.1 Synthesis of ionic liquids
2.1.1 Synthesis of cationic parts
Six different types of cationic parts were synthesized as described below and shown in Figs. 1 and 2. For the synthesis procedure please see supplementary data section S 2.1.1.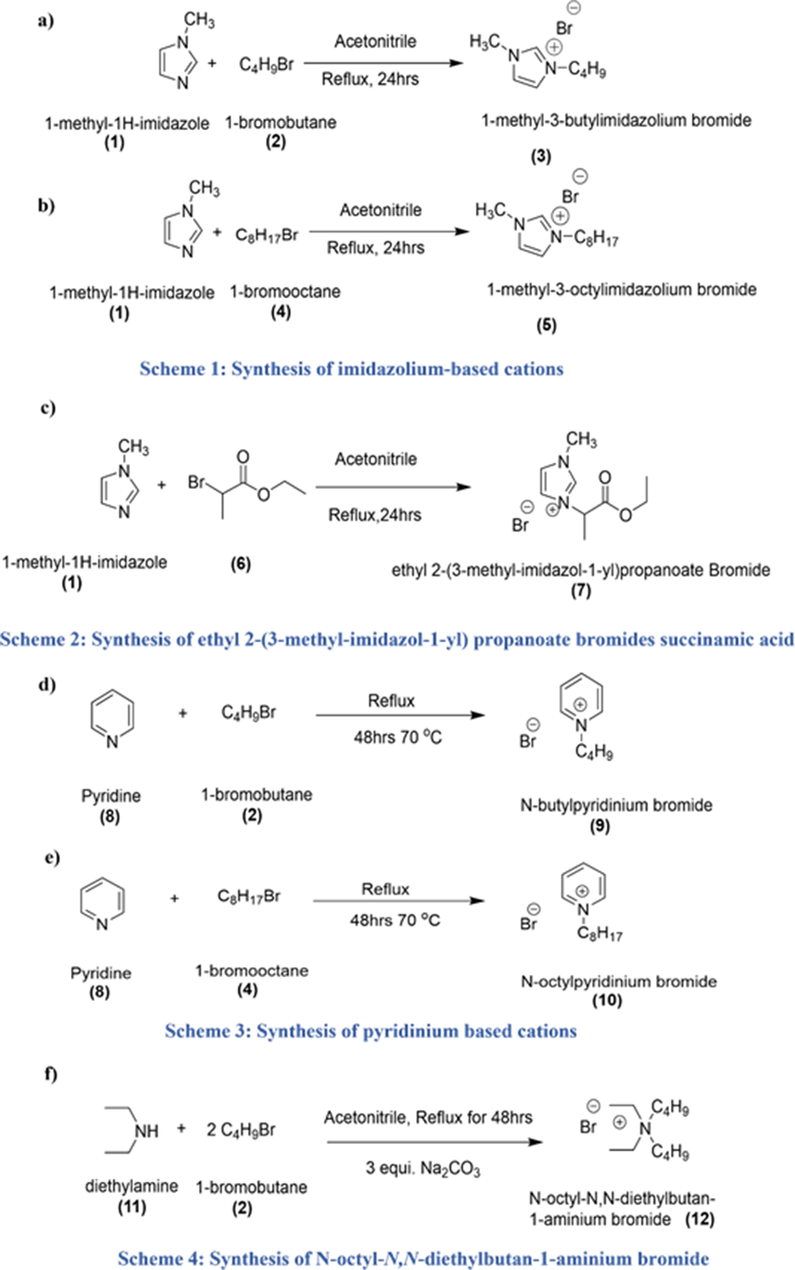
Scheme1, 2, 3, and 4 represent synthesis of Cations.
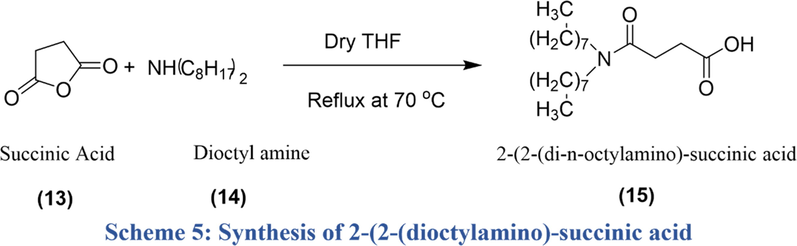
Scheme5 represent synthesis of Anion.
2.1.2 Synthesis of anionic part (N88SA)
For synthesis procedure please see supplementary data section 2.1.2.
2.1.3 Synthesis of TSILs
Six new N,N-dioctylsuccinamate based ionic liquids TSIL’s were prepared as shown in Fig. 3. For the synthesis procedure please see supplementary data section 2.1.3.1.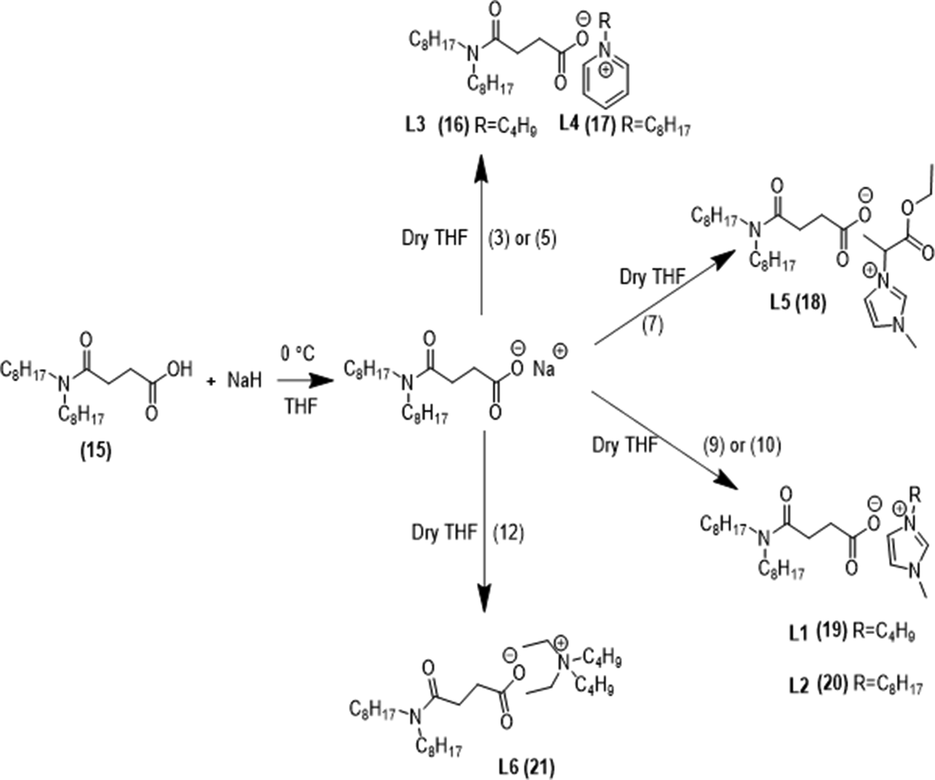
Synthesis of TSIL’s.
2.2 Characterization
FTIR spectra of all the synthetic products were recorded on Bruker PLATINUM ATR and vertex 70 FT-IR spectrophotometer in the range of 4000–500 cm−1.1H NMR and 13C NMR spectra were recorded on Bruker (300 MHz) and (75 MHz) spectrometer. Chemical shift values (δ ppm) are reported in using the residual solvent signal as an internal standard. Analytical TLC was performed using Merck prepared plates (silica gel 60F-254 on aluminium) to monitor the progress of the reaction during synthesis. Purity of the synthesized ionic liquids were checked with thin layer chromatography and NMR data, no impurity was found in the prepared samples.
1-methyl-3-butylimidazolium bromide (3): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H sp2);2900 cm−1(CH2 sp3)carbon;1629 cm−1 imidazole (C⚌N), 1572 cm−1 (C⚌C)aromatic ring; 1460 cm−1 (CH2,CH3). At 1166 cm−1 (C—N) imidazole ring; 755 cm−1 (CH2 butyl chain).1H NMR: (CDCl3, ppm): 0.85–0.9 (t, 3H; CH3),1.35 (m, 2H;CH2), 1.8 (m, 2H; CH2), 3.0 (t, 2H; CH2), 4.06 (s, 3H; NCH3), 4.2(t, 2H; NCH2), 7.4(d, 1H; NCH), 7.6(d, 1H; NCH),10 (s,1H; NCHN).
1-methyl-3-octylimidazolium bromide (5): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H sp2); 2900 cm−1(CH2 sp3) carbon;1629 cm−1 imidazole (C⚌N); 1572 cm−1 (C⚌C) aromatic ring; 1460 cm−1 (CH2, CH3). At 1166 cm−1 (C—N) imidazole ring; 755 cm−1 (CH2 octyl chain).1H NMR: (CDCl3, ppm): 0.4 (t, 3H; CH3),0.86 (m, 2H; CH2), 1.47 (m, 10H; CH2), 3.9 (s, 3H; NCH3), 4.2(t, 2H; NCH2), 7.2 (d, 1H; NCH), 7.3 (d, 1H; NCH), 9.5 (s,1H; NCHN).
Ethyl 2-(3-methyl-imidazol-1-yl propanoate Bromide (7): FTIR: 3400 cm−1 (OH); 2925 cm−1 (C—H) sp2 aromatic carbons; 2900 cm−1 (CH2)sp3 carbon atoms; 1741 cm−1(C⚌O) ester band;1629 cm−1 (C⚌N)aromatic imidazole; 1572 cm−1(C⚌C) aromatic ring; 1460 cm−1 (CH2, CH3);1173 cm−1 (C—N)imidazole ring; 755 cm−1 (CH2) butyl chain.1H NMR: (CDCl3, ppm): 0.8 (t, 3H; CH3),1.4 (d, 3H;CH-CH3), 3.6 (m, 2H; OCH2), 3.7(q,1H;HCC = O) 3.5 (s, 3H; NCH3), 5.5(d, 1H; CH = CH), 7.3(d, 1H; CH = CH),9.5 (s,1H; NCHN).
Synthesis of N-Butyl pyridinium bromide (9): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H sp2); 2900 cm−1(CH2 sp3) carbon;1635 cm−1 (C⚌N)pyridine; 1572 cm−1 (C⚌C) aromatic ring; 1460 cm−1 (CH2,CH3); 1173 cm−1 (C—N) pyridine ring; 755 cm−1 (CH2 butyl chain). 1H NMR: (CDCl3, ppm): 0.9 (t, 3H; CH3), 1.37 (m, 2H; CH2), 1.9 (m, 2H; CH2), 4.9 (t, 2H; CH2), 8.1(t, 2H; CH), 8.5(t, 2H; CH), 9.4 (d, 1H; NCH).
N-octyl pyridinium bromide (10): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H sp2); 2900 cm−1(CH2 sp3)carbon; 1635 cm−1 (C⚌N)pyridine; 1572 cm−1 (C⚌C)aromatic ring; 1460 cm−1 (CH2,CH3); 1173 cm−1 (C—N) pyridine ring; 755 cm−1 (CH2 octyl chain). 1H NMR: aromatic ring; 1460 cm−1 (CH2,CH3); 1173 cm−1 (C—N) pyridine ring; 755 cm−1 (CH2 butyl chain). (CDCl3, ppm): 0.9 (t, 3H; CH3), 1.37 (m, 2H; CH2), 1.9 (m, 2H; CH2), 4.9(t, 2H; CH2), 8.1(t, 2H; CH), 8.5(t, 2H; CH), 9.4 (d, 1H; NCH).
Synthesis of diethyl dibutyl ammonium [N2244] bromide (12): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H) sp2 aromatic carbons; 2900 cm−1 (CH2) sp3 carbon atoms;1460 cm−1 (CH2, CH3);1246 cm−1 (C—N) amines; 755 cm−1 (CH2) butyl chain. 1H NMR: 0.85 (t, 6H, CH3), 1.1 (t,6H, CH3), 1.33(m,4H, CH2), 1.4 (t, 4H, CH2), 2.4 (t, 4H, CH2), 2.47 (s, 4H, CH2).
Synthesis of 2-(2-(di-n-octylamino)-succinic acid (15): FTIR: 2932 cm−1 (CH2 str), 1713 cm−1 (C⚌O of acid), 1607 cm−1 (C⚌O of amide), 1463 cm−1 (CH2 bend); at 2930 cm−1 (sp2 aromatic C—H); at 2900 cm−1 sp3 CH3; 1120 cm−1 (C—O linkage). 1H NMR ppm: (CDCl3); 0.88–0.92 (m, 3H), 1.28–1.32 (m, 20H), 1.57 (t, 4H), 2.52 (t, 2H), 2.76 (t, 2H). 13C NMR ppm (CDCl3); 14.8, 23.2, 26.4, 29.2,30.1, 32.0, 48.3, 167.2, 172.4.
Synthesis of 1-Methyl-3-butylimidazolium N,N-dioctylsuccinamate [C4mim][N88SA] (L1): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H str of sp2); 2900 cm−1 (CH2 str of sp3 C); 1629 cm−1 (C⚌N); 1615 cm−1 (C⚌O of carboxylate);1607 cm−1 (C⚌O of amide);1569 cm−1 (C⚌C); 1166 cm−1 (C—N); 755 cm−1 (CH2 groups of the butyl chain).1H NMR: (CDCl3,ppm):0.8(t, 3H; CH3),1.2–1.4(m, 20H;CH2), 2.5–2.6 (t,2H;COCH2), 3.2(t,4H;H2CNCO), 4.1 (s, 3H; NCH3), 4.3(t, 2H; NCH2), 7.4(d, 1H; CH = CH), 7.5(d, 1H; CH = CH),10.3 (s,1H; NCHN). 13C NMR ppm (CDCl3); 14.8, 20.8, 23.2, 25.2, 26.4, 29.2, 30.1, 32.8, 34.3, 44.1, 48.3, 118.6, 119.5, 121.4, 167.2, 172.4.
Synthesis of 1-Methyl-3-octylimidazolium N,N-dioctylsuccinamate [C8mim][N88SA] (L2): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H str of sp2); 2900 cm−1 (CH2 str); 1630 cm−1 (C⚌N); 1615 cm−1 (C⚌O of carboxylate);1607 cm−1 (C⚌O of amide); 1569 cm−1 (C⚌C); 1460 cm−1 (CH2 and CH3); 1166 cm−1 (C—N); 755 cm−1 (of the butyl chain). 1H NMR: (CDCl3, ppm):0.8(t, 3H; CH3),1.2–1.4(m, 30H;CH2), 2.5–2.6 (t,2H;COCH2), 3.2(t,4H;H2CNCO), 4.1 (s, 3H; NCH3), 4.3(t, 2H; NCH2), 7.4(d, 1H; CH = CH), 7.5(d, 1H; CH = CH),10.3 (s,1H; NCHN). 13C NMR ppm (CDCl3); 14.8, 20.8, 23.2, 25.2, 26.4, 29.2, 30.1,32, 32.8, 34.3, 44.1, 48.3, 52.1 118.6, 119.5, 121.4, 167.2, 172.4.
Synthesis of N-butylpyridinium N,N-dioctylsuccinamate [C4Py][N88SA] (L3): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H str of sp2); 2900 cm−1 (CH2 str of sp3 C); 1569 cm−1 (C⚌C); 1460 cm−1 (CH2 and CH3);1620 cm−1 (C⚌O carboxylate),1605 cm−1 (C⚌O amide), 1571 cm−1 (C⚌C); 1409 cm−1 (C—O); 1166 cm−1 (C—N) pyridine ring 755 cm−1 (of the butyl chain). 1H NMR: (CDCl3,ppm): 0.8(t,3H;CH3),1.30 (m,2H;CH2CH2CH3), 2.01(t,2H;NCH2), 8.7–8.8 (t,4H;N = CH2), 8.2–8.3 (m,4H;CH2,meta), 8.7 (m,2H;CH2,para), 2.5–2.6 (t,2H;COCH2), 2.68 (t,2H;CH2CON), 3.2 (t,4H;H2CNCO). 13C NMR ppm (CDCl3); 13.9, 14.8, 20.8, 23.2, 26.4, 29.2, 30.1, 31.8, 32.0, 48.3, 70.6, 126.2, 141.8, 142.2, 167.2, 172.4.
Synthesis of N-octylpyridinium N,N-dioctylsuccinamate [C8Py][N88SA] (L4): FTIR: 3414 cm−1 (OH); 2925 cm−1 (C—H str of sp2); 2900 cm−1 (CH2 str of sp3 C); 1569 cm−1 (C⚌C); 1460 cm−1 (CH2 and CH3);1620 cm−1 (C⚌O carboxylate), 1605 cm−1 (C⚌O amide);1571 cm−1 (C⚌C); 1409 cm−1 (C—O); 1166 cm−1 (C—N) pyridine ring; 755 cm−1 (of the butyl chain).1H NMR:(CDCl3,ppm):0.8(t,3H;CH3), 1.27–1.28 (m,30H;CH2), 2.01 (t,2H;NCH2), 8.7–8.8 (t,4H;N = CH2), 8.2–8.3 (m,4H;CH2,meta), 8.7(m,2H;CH2,para), 2.5–2.6 (t,2H;COCH2), 2.68 (t,2H;CH2CON), 3.2 (t,4H;H2CNCO). 13C NMR ppm (CDCl3); 13.9, 14.8, 20.8, 23.2, 26.4, 29.2, 30.1, 31.8, 32.0, 50.2, 48.3, 70.6, 126.2, 141.8, 142.2, 167.2, 172.4.
Synthesis of 1-Methyl-3-ester-imidazolium N,N-dioctylsuccinamate [α-mim-ester][N88SA] (L5): FTIR: 3414 cm−1 (OH); 3405 (C⚌N); 2925 cm−1 (C—H str of sp2); 2900 cm−1 (CH2 str of sp3 C); 1741 cm−1 (ester carbonyl); 1569 cm−1 (C⚌C); 1460 cm−1 (CH2 and CH3); 1166 cm−1 (C—N) pyridine ring;1620 cm−1 (C⚌O carboxylate);1605 cm−1 (C⚌O amide);1571 cm−1 (C⚌C); 1409 cm−1(C—O);1166 cm−1(C—N)imidazole ring; 755 cm−1 (of the butyl chain). 1H NMR: (CDCl3, ppm): 0.8 (t, 3H; CH3) 0.92 (m, 3H), 1.28–1.32 (m, 16H), 1.4 (d, 3H;CH-CH3), 4.2 (m, 2H; OCH2), 4.7 (q,1H;HCC = O) 3.5 (s, 3H; NCH3), 5.5 (d, 1H; CH = CH), 7.3 (d, 1H; CH = CH),9.5 (s,1H; NCHN), 1.28–1.32 (m, 16H), 1.57 (t, 4H), 2.52 (t, 2H), 2.8 (t, 2H). 13C NMR ppm (CDCl3); 14.8, 18.5, 23.2, 26.4, 29.2, 30.1, 32.0, 48.3,59, 63.2, 122.9, 141.5, 169.2, 172,178.4.
Synthesis of diethyl dibutyl ammonium N,N-dioctylsuccinamate [N2244][N88SA] (L6): FTIR: 3414 cm−1 (OH); 3405 (C⚌N); 2924 cm−1 (C—H str of sp2); 2900 cm−1 (CH2 str of sp3 C); 1569 cm−1 (C⚌C); 1460 cm−1 (CH2 and CH3); 1620 cm−1 (C⚌O carboxylate);1605 cm−1 (C⚌O amide);1571 cm−1 (C⚌C); 1409 cm−1 (C—O); 1246 cm−1 (C—N) amines; 755 cm−1 (of the butyl chain).1H NMR:(CDCl3,ppm): 0.8 (t, 3H; CH3), 1.2–1.35 (m, 18H;CH2), 2.3–2.5 (m,4H;CH2), 2.5–2.6 (t,2H;COCH2), 3.2 t,4H;H2CNCO), 4.3(t, 8H; NCH2).O); 1166 cm−1 (C—N) imidazole ring; 75 5 cm−1 (of the butyl chain). 13C NMR ppm (CDCl3); 11.5, 14.8, 20.0, 23.2, 26.4, 29.2, 30.1, 32.0, 48.3, 59.2, 65.1, 169.2, 175.4.
2.3 Metal extraction and its determination
The concentration of Lead Pb (II) and Co (II) was determined by Atomic Absorption Spectroscopy before and after the metal extraction, the percent extraction of the metal was calculated by using following equation.
Ci represents the concentration of metal ions in the aqueous phase before extraction. Cf is the concentration of the metal ions in the aqueous phase after extraction.
Extraction procedures were carried out as follows. The aqueous solution in different concentrations i.e., 50, 100, 150, 200, and 250 ppm of Pb(II) or Co(II) were prepared by using lead nitrate [(Pb(NO3)2] and cobalt nitrate [Co(NO3)2]. Buffer solutions were used to maintain the pH 2, 4, 6, 8, and 10 of the solution. 0.1 M solution of a given ionic liquid was prepared in toluene. The extraction experiments were carried out by mixing equal volumes of both organic and aqueous phases in a screwed cap and were shaken for 15 to 75 min in a shaking incubator at ambient temperature (25C ± 1 °C). Organic and aqueous phases were shaken together at different parameters were changed such as pH values, metal ion concentrations, and shaking duration. Then the shaken solutions were allowed to stand in a separatory glass funnel to separate the phases. The concentration of lead and cobalt in the aqueous phase before and after the experiment was determined using atomic absorption spectroscopy.
3 Results and discussion
Six different succinimide-based TISL’s, were prepared by metathesis method using 2-(2-(di-n-acetylamino)-succinic acid as an anion. Imidazolium based, pyridinium based, and ammonium-based cations were prepared by decorating functional groups such as butyl, octyl and esters groups on imidazolium cations. Butyl and octyl chains were attached to obtain pyridinium based cations. Butyl chains were tied to get ammonium-based cations All samples were characterized spectroscopically and their Pb(II) and Co(II) metal extraction potential was carried out. Three variables were used to understand the extraction efficacy. In general, pH was varied from 2 to 10, metal ion concentration between 50 and 250 ppm, and ligand metal contact time was varied from 15 to 75 mins.
3.1 Synthesis of TSIL’s
Synthesis of novel succinimide-based TSIL’s was started from succinamic acid preparation, by replacing characteristics carbonyl (C⚌O) band of succinic acid with dioctylamino moiety. In the FTIR spectra, succinamic acid of the anhydride band of succinic acid at 1800 cm−1 disappeared and carbonyl appeared at 1720 cm−1. At 1630 cm−1 stretching vibrations of C⚌O of amide is present, strong band at 2930 cm−1 and 2900 cm−1 are due to symmetric stretching of sp2 aromatic C—H bond and sp3 CH2, respectively. At 1430 cm−1 a band shows the bending vibration of CH2, at 1120 cm-1C-O linkage is indicated, near 750 cm−1 rocking vibrations of CH2 that are in the long chain of octyl group (Mathew, 2016).
In case of all the six Ligands, the appearance of the broadband at 1629 cm−1 confirmed the exchange reaction between succinamic acid and different cations, suggesting the successful reaction progress. The band of C⚌O at 1720 cm−1 present in the spectra of succinamic acid disappeared and shifted to 1629 cm−1 as a broadband resulting from the merging of C⚌O of carboxylate ion and C⚌O of amide (Fu, 2018). A broad bell-shaped band around 3414 cm−1 was found in all TSILs, as this band is found in the OH group it reveals hygroscopic nature of the ionic liquid. Moreover, the bands around 2925 cm−1 are related to the C—H stretching of sp2 aromatic carbons, while at 2900 cm−1 correspond to the CH2 stretching of sp3 carbon atoms. Band for C⚌C stretching of an aromatic ring appeared at 1565–1570 cm−1, bending vibrations of CH2 and CH3 groups have appeared approximately at 1460 cm−1 and 1395 cm −1. A strong band at 755–770 cm−1 is due to rocking bending vibrations of CH2 and CH3 groups of the butyl chain and octyl chain respectively (Lopes, et al., 2019).
For the TSILs (16) and (17) succinamic acid was treated with imidazolium-based cations (3) and (5), respectively. Apart from the above bands C⚌N band of the imidazole ring emerged at 1629 cm−1 and the C—N stretch appeared at 1166 cm-1 (Mehmood et al., 2021). Synthesis of [α-mim-ester][N88SA] (18) was further characterized by ester carbonyl popping up at 1741 cm−1 and the appearance of C⚌N at 1615 cm−1. For the preparation of (19) and (20), succinamic acid was treated with pyridinium-based cations (9) and (10), respectively. Along with the characteristics, bands of the interaction of pyridine-based cations with succinamic acid, C⚌N band of pyridine ring is emerged at 1635 cm−1 and C—N around 1166 cm−1. Synthesis of succinimaic acid based quaternary ammonium salt (21) was identified by new band for (C—N stretch) around 1160 cm -1.
3.2 Extraction of Pb(II) and Co(II) by TSILs
Extraction of the Pb(II) and Co(II) was measured by changing different parameters such as equilibration time, pH values, and metal ion concentration as discussed in following sections.
For metal extraction by ligands five ligands were used ligand 3 [C4Py][N88SA] (L3) was not found suitable for any of the extraction because organic and aqueous phases could not be separated.
3.2.1 Effect of equilibration time on metal extraction
In order to investigate the effect of different equilibration times, on the extraction of Pb(II) and Co(II), pH and TSIL’s concentration was kept constant at 7 and 0.1 M, respectively. Equilibration of organic to aqueous phases was done in 1:1 vol ratio for 15 to 75 min in constant shaking. Extraction profiles of Pb(II) and Co(II) different Ligands in respect to time are shown in Fig. 4. For Pb(II) and Co(II), L1 demonstrated maximum extraction at 60 min. The extraction rate was increased exponentially up to 30 min, after which the increase in extraction was not significantly higher, and maximum extraction was reached at 60 min. Shaking speed and the complex formation constant governs the extraction efficiency. Although L2 demonstrated maximum extraction at 60 min, it was only raised by 2 % from 45 min. So, if we increase the speed of shaking, the surface area will grow significantly, and more molecules will interact and form complexes in a short period of time. Metal extraction in L4 reaches its peak at 60 min. After 30 min, there is a significant increase in % extraction, as seen in the Fig. 4 c) after 30 min extraction is raised negligible. In the case of L5, the maximum extraction time is 60 min. The same pattern may be found here as well. If the shaking pace is raised, the maximum extraction time lie in between 30 and 45 min. Extraction is caused by the formation of stable complexes between [N88SA]- and cations. The higher the extraction efficiency, the more stable the complexes are. L6 extracted maximum metal ions in 45 min contact time. The results showed that the extraction experiment's kinetics were fast, with maximum extraction occurring at roughly around 45 min at shaking speed of 300 rpm after which the increase in % extraction was not significant. We believed that doubling the shaking speed to 600 rpm would bring the maximum extraction time around 30 min. Changing the shaking speed is found to be a tuning factor for reducing the equilibration time for maximum extraction (Rout, 2013; Anthony et al., 2001). The extraction results of TSIL’s for Pb(II) and Co(II) with such fast extraction kinetics make it appropriate for industrial applications because the contact time is a limiting factor for metal recovery feasibility. For first 30 mins the extraction of Co(II) was leading as compared to the Pb (II). Around 40 to 45 mins a major shift in extraction time was observed where Pb(II) was succeeding the extraction as compared to Co(II). The extraction efficiency of both metal ions decreased in the order [N88SA][C8mim] (L2) > [α-mim-Ester][N88SA] (L5) > [N88SA][C4mim] (L1). This is evident from the order of extraction behaviour that increasing the bulkiness of cation, results in stronger complexation, hence increasing extraction. This suggest that the time of contact is dependent on the size of the metal. Size of the metal affects the charge density affecting the hydration. After 45 mins L2, and L4 having octyl chains have selectivity for Pb (II) as compared to Co (II). For L6 after 45 mins there was selectivity for Pb(II) having more than 98 % extraction.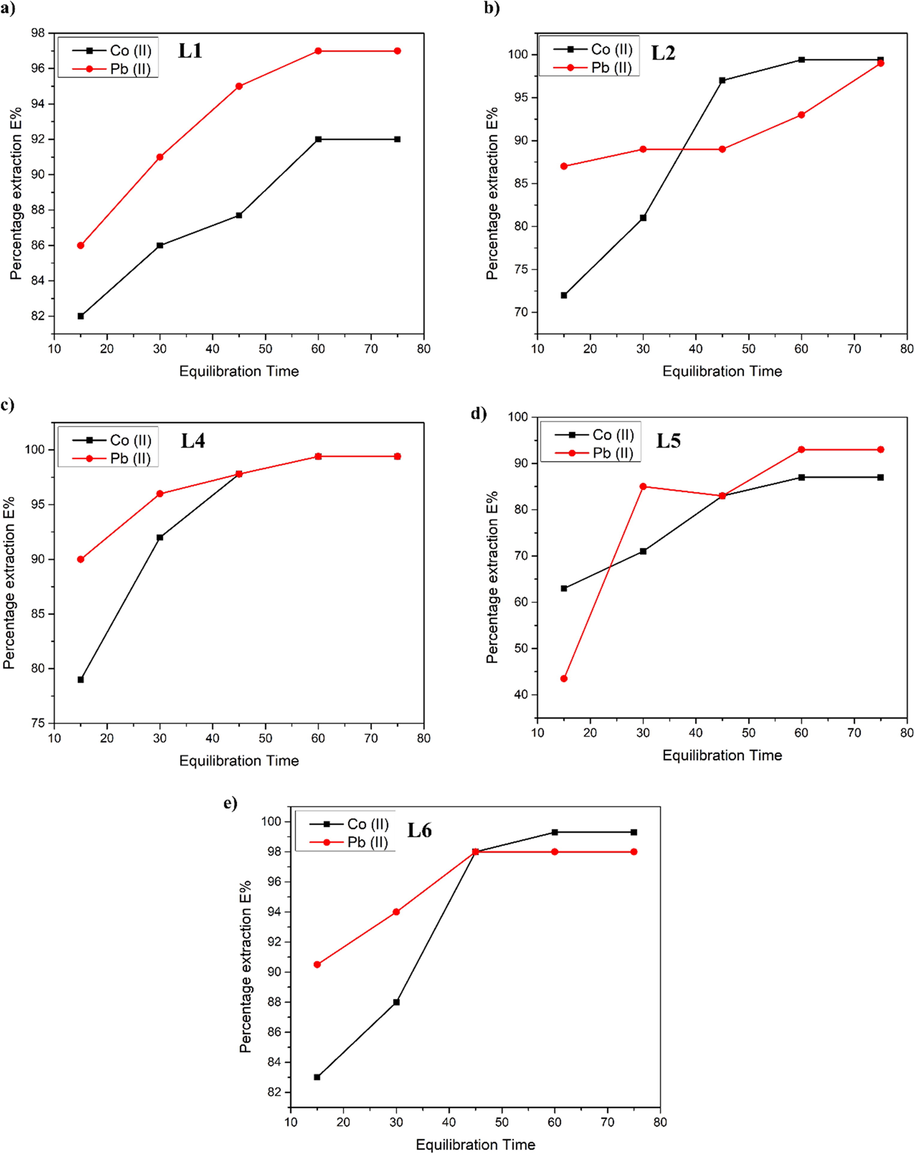
Percentage extraction by L1-L6 as a function of equilibration time.
3.2.2 Effect of pH of aqueous phase on extraction
Extraction profile of Co(II) and Pb(II) was monitored on a range of pH values (2, 4, 6, 8, and 10) of the aqueous phase. Extraction experiments were carried out using 100 ppm aqueous solutions of different metal ions and contact time of 75 min. It was revealed that the extraction and separation can be tailored by the pH value of the aqueous phase. The extraction profile on different pH values has been shown in Fig. 5.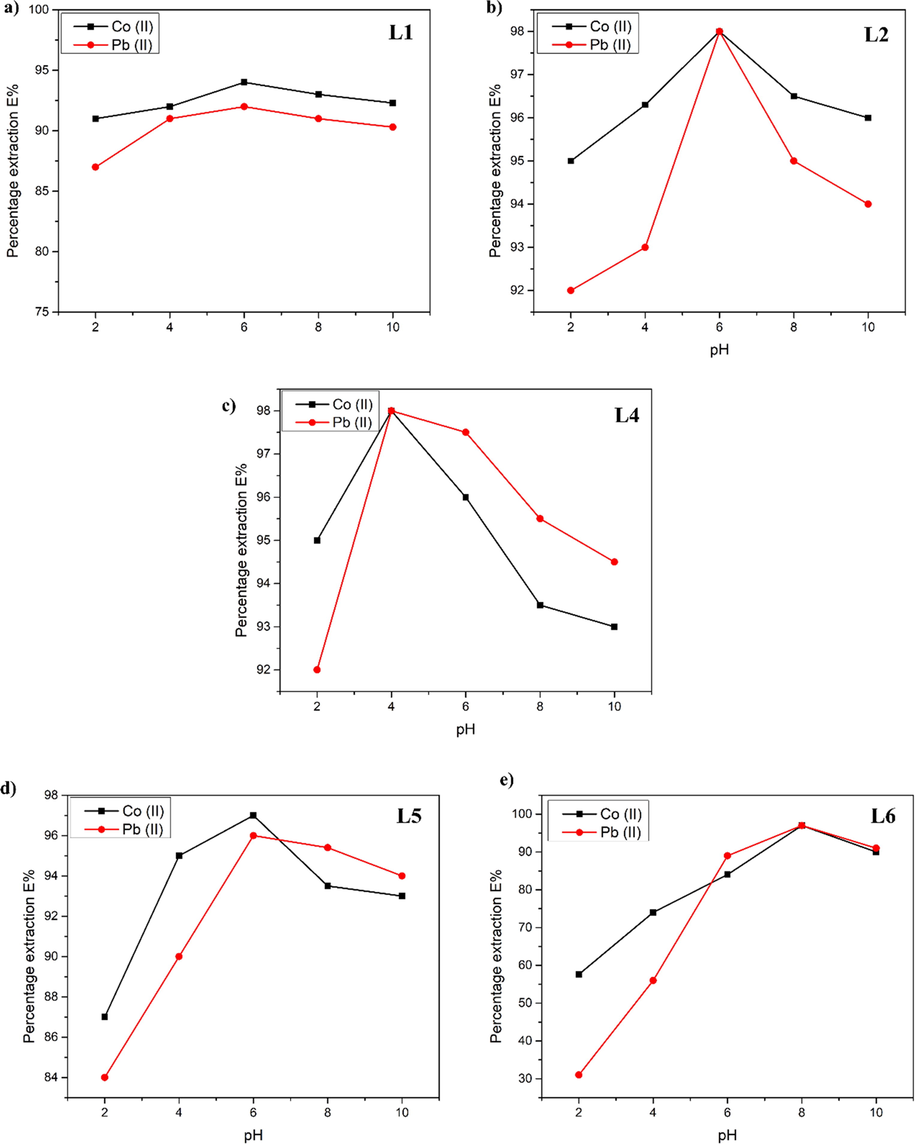
Percentage extraction of TSLI’s as a function of pH. The extraction percentage of Co(II) and Pb(II) in chloride solution at various pH by different ligands. The concentration of extractants were fixed 0.01 M.
All the ligands showed maximum extraction at different pH values of the aqueous phase. The interaction between TSIL anions and metal cations could be responsible for the TSILs' ability to remove metal ions (Tran et al., 2020).The extraction efficiency was improved by raising the pH level. At a pH of 6, imidazolium-based ionic liquids with various chain lengths and ester groups demonstrated the highest distribution ratios. The extraction efficiency of the metal ions Co(II) and Pb(II) showed declining trend as follows. [N88SA]-[C8mim]+ (L2) > ester imidazole based(L5) > [N88SA]-[C4mim]+ (L1),
Ion exchange of imidazolium base ligands was found relatively easy as compared to pyridinium ligands. These findings suggest that increasing the bulkiness of the cation improves Pb(II) and Co(II) ion extraction. In the case of both Pb(II) and Co(II), the distribution ratio of metal ions was greater at higher pH values (6–10) than at pH 2, where extraction efficiency was lowered. The maximum extraction of Pb(II) and Co(II) by L2 was seen at pH 6. For both Pb(II) and Co(II), the best distribution ratio was obtained at pH 4 in the case of L4. The extraction was reduced at pH 2 and increased dramatically at pH 4. Extraction was reduced at pH 6, 8, and 10, however the reduction was less than that seen at pH 2. We believe that the extraction decreases as the pH rises due to metal ion hydrolysis. For Pb(II) and Co(II), the L5 had the best distribution ratio at pH 6. In the case of L1 and L2, the pattern of decreasing extraction by altering pH was the same. At pH 2, the extraction was between 84 and 86 %. At pH 10, the % extraction ranges from 90 to 92 %.
The distribution ratios of Pb(II) and Co(II) increase with increasing pH values, as shown in Fig. 5 e) and are significantly removed by L6 at pH 8. Ionic solutions based on quaternary amines also extract well at higher pH levels. In case of all ligands, pH values of aqueous phase revealed increase in pH values after extraction trials as found in case of other reported literature (Moon, 2020; Tran, 2019). We considered, that in case of all the TSIL’s, at low pH levels, the competing interaction between metal and H+ ions is very strong, however at higher pH values, the competition between the H+ ions and the metal ions becomes less dominating, increasing the extraction efficiency of the metal ions. Octyl chain containing imidazole and pyridinium cations showed decrease in efficiency for both metals at specific pH of 6 and 4 respectively. Imidazole based cations favoured the extraction of Co(II) at all pH values with butyl chain while in ester based imidazole after pH 6 Pb(II) has selectively higher metal extraction. In case of ammonium based ILs both metals have competing metal extraction after pH 6. These results suggest that Ph can be very helpful in selective removal of either metal from any aqueous samples.
3.2.3 Effect of metal ion concentration on the extraction efficiency
Effect of metal ion concentration on the extraction efficiency of Co(II) and Pb(II) was observed by keeping pH and shaking time constant as shown in Fig. 6. Concentration was varied from 50 ppm, 150 ppm, 200 ppm and 250 ppm. The shaking period was set 45 min, during which most of the ligands demonstrated the best extraction in previous section. For keeping pH constant, the nature of the cation was the deciding factor. pH values showing maximum extraction efficiency for each ligand were used. Maximum extraction was seen in L2 and L5 at 6 pH, L4 at 4 pH, and L6 at 8 pH.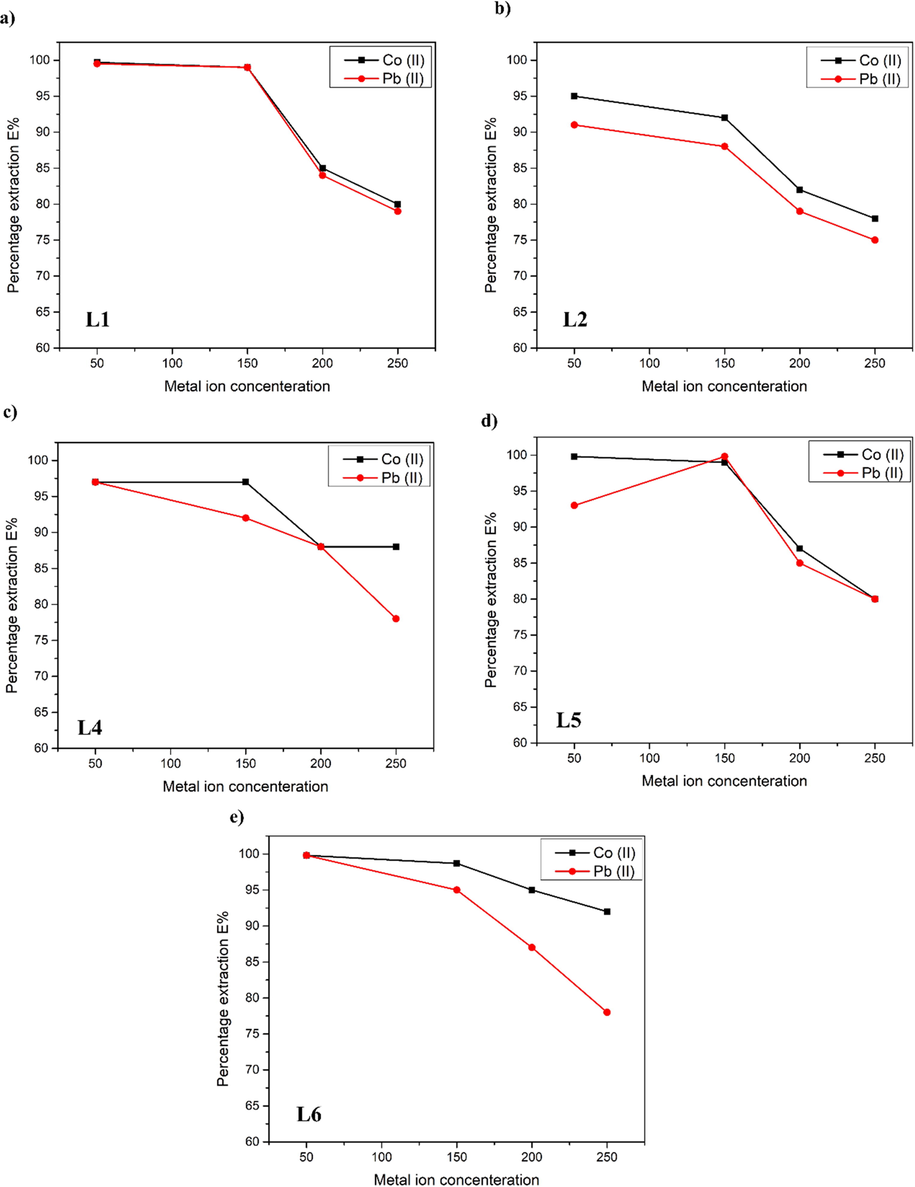
Percentage extraction by TSLI’s as a function of metal ion concentration.
Co(II) and Pb(II) exist as an octahedral and square planar configuration in aqueous solution (e.g [Co(H2O)6]2+, and [Pb(H2O)4]2+). The solvent extraction of Co(II) and Pb(II) by these TSILs may be represented as:
Where M2+ represents metal ions. A- represents [N88SA]- (anion), while B+ represent cation (Tran, 2020). Here the mechanism of the extraction is the cation exchange. The extraction of the metal occurs after the cation exchange mechanism followed by the extraction (Ooi and Ng, 2018). The cationic part of ILs is replaced by the metal, the reaction is reversible and at equilibrium extraction is achieved. It is worth mentioning that the concentration has a major role in the extraction efficacy. The extraction profiles of both metals Co(II) and Pb(II) has almost identical trend on changing the metal ion concentration. The extraction efficiency of L2 decreases as the metal ion concentration rises. The L4 has a maximum distribution of 50 ppm and a minimum dispersion of 200 ppm. While the L5 showed maximal extraction at 50 ppm, with a slight drop at 150 ppm, but a considerable decrease at 200 ppm, which continued to decrease until 250 ppm. When number of metal ions present increases at higher concentrations, extraction efficiency reduces. Similar extraction profile was found with L6, which demonstrated maximum extraction at 50 ppm but dropped below 80 % after 200 ppm. The concentration of ligand was kept constant while the concentration of metal ions was raised, resulting in a decrease in extraction. This led to the assumption that if the ligand concentration was increased in tandem with the metal ion concentration, extraction would increase. Limpidity of the aqueous phase was decreased when pyridine-based ligands L3 and L4 were used for extraction, so the exact extraction efficiency was not really observed (Neves, 2009; Li, 2015). Moreover, limpidity was found dependant on the carbon chain. It increased when chain length was small i.e., butyl chain (L3) as compared to when chain length was octyl (L4).
3.2.4 Suggested extraction mechanism
In view of above information, extraction of Pb(II) and Co(II) by synthesized ionic liquids can be considered as ion exchange mechanism. The decrease in metal ion concentration with increase in acidity leads to Cation exchange mechanism. Cation exchange mechanism can be represented by the equation below:
A strong influence on Pb(II) and Co(II) extraction by H + ions have been investigated as most of the ionic liquids showed comparatively less extraction values at low pH like 2 and 4 except pyridine based ligand which showed maximum extraction at pH 4.
H+ interrupted in extraction mechanism by inducing a competing equilibrium reaction demonstrated in the following equation.
This formation of adducts of succiniamide anion with that of proton could be attributed to a sharp decrease in Pb(II) and Co(II) extractions as the acidity is increased.
3.2.5 Comparison with literature and discussion
In a report, using the reactive ILs (RILs) based on the imidazole derivatives for heavy metals separation such as Cd(II), Cu(II), Pb(II), and Zn(II) ions was found selective towards Cd and not efficient against Pb(II) (Szczepański, et al., 2021). For pyridinium based ILs extraction of the Pb(II) was possible above pH 3 (Wieszczycka, 2022). Another report using pyridinium based ILs Hexadecyl pyridinium chloride was prepared having a long alkyl chain, concluded the selective Pd (II) extraction from a mixture of metals (Cu(II), Co(II), Ni(II), Fe(III), Al(III) and Sn(IV)) in hydrochloric acid media (Tong, 2014). Functionalized silica based pyridinium cation IL revealed 15 to 45 mins equilibrium time at 4–6 pH was optimum for extraction of Pb(II), Cd(II) and Zn(II) (Wieszczycka, 2021). We are first time reporting the ammonium based ILs for Pb(II) and Co(II). In a previous report, using mono and di ILs using diglycoamide and imidazole cations showed extraction at lower pH values for a contact time of 75 mins for 99 % metal extraction of Co(II) and Pb(II) (Waheed, et al., 2021). Co (II) extraction from the weak acidic solution using the succinimide based ionic liquid showed that the Imidazole based ILs are more efficient than the pyridinium based ILs (Tran, 2020). Ammonium based ionic liquids showed the extraction efficacy for cobalt in range of 40–60 % at neutral Ph (Vergara, 2014).
In case of equilibration time the generally higher the equilibration time higher metal extraction was achieved. Butyl chain and ester based imidazole were selective for the Pb(II) at different equiliberation times. Using octyl based imidazole IL can be used at lower equiliberation times for the separation of Pb(II) with around 90 % efficiency till 30mins time. For pyridinium and ammonium based ILs the optimum time for the extraction were 40 and 45mins respectively with 98 % efficacy for both metal ions.
Effect of the pH on the extraction efficacy of succinimide based TSILs revealed that the octyl chain containing imidazole based ILs are severely affected by the pH values as compared to the butyl based imidazole ILs. Where the pH 6 was found optimum for both metal ion Co(II) and Pb(II) upto 100 %. pH below or higher than 6 made a drop in the IL efficacy. Extraction this shows that the neutral pH increased the efficacy of the ILs. In case of butyl chain both ions have maximum extraction around 6 pH with 90–95 % extraction efficiency. In case of the imidazole ester same pH 6 was found the most efficient with selectivity for Co(II) ion at this pH while for the Pb(II) higher pH values have increasing extraction. For pyridinium based ILs the optimum pH shifted to 4 that is weak acidic environment supported the extraction of both metals, Higher pH rendered toxic for the extraction process. For Ammonium based ILs the pH 8 was found the optimum values for both Co(II) and Pb(II).
Effect of metal ion concentration with succinimide based TSILs have very interesting behaviours. Imidazole based ILs showed that the octyl chain was selective for the Co(II) extraction at all concentrations with 90 % efficacy at lowest concentration of 50 ppm. Imidazole ester was showing 100 % extraction for both ions at 150 ppm concentrations. Pyridinium based ILs are selective for Co (II) at 150 ppm with 98 % extraction efficacy. Ammonium based ionic liquid have the highest efficacy with all the metal ion concentration with selectivity for Co(II) over Pb(II).
Considering all these results we conclude that our new succinimide based ILs are novel, unique, efficient, and new substitute, with better optimization in terms of time, metal ion concentration and pH for Pb(II) and Co(II) extraction.
4 Conclusion
Six new N,N-dioctylsuccinamate based ionic liquids were synthesized by metathesis procedure. These ligands acted as TSILs for the extraction of two heavy metal Pb(II) and Co(II) ions from the aqueous solutions. Spectroscopic techniques such as FTIR, 13C NMR and 1H NMR were employed to affirm their structures. Further, extraction behaviour was monitored by changing equilibration time, initial pH of the aqueous phase, and metal ion concentration. The maximum extraction time for different ligands was found in range of 45 to 60 mins. Taking pH as a variable concluded that pH values 6–8 were useful for efficeient extraction of metal ions from aqueous phase.. Another major factor was found to be metal ion concentration, at higher metal ion concentration efficiency was decreased probably due to availability of less binding sites for the metals. It is believed that the mechanism involved for metals were extraction was ion exchange, and therefore, low metal extraction at lower pH values is attributed to the competition of metal ions with protons in acidic/aqueous media. Succinimide ILs can be a used for metal extraction in weak acidic solutions. Comparison of the different cationic species of ILs showed that the extraction by imidazolium based ionic liquids were slightly higher than pyridinium based ionic liquids. It seems that the length of chains aids in separation of organic and aqueous phases, as the longer chain length separated easily, while the smaller chain lengths (butyl) increased the limpidity of water and thus not separated completely, which resulted in low extraction efficiency. Considering all these results we conclude that new succinimide based ILs are novel, and efficient with better optimization in terms of time, metal ion concentration and pH for Pb(II) and Co(II) extraction.
CRediT authorship contribution statement
Nizakat Azra: Conceptualization, Methodology, Software, Writing – original draft. Farzana Nazir: Writing – original draft, Software. Mah Roosh: Writing – review & editing. Muhammad Awais Khalid: Writing – review & editing. Muhammad Adil Mansoor: Writing – review & editing. Sher Bahadar Khan: . Mudassir Iqbal: Supervision, Writing – review & editing.
Acknowledgement
Authors acknowledge the support from School of Natural Sciences, National University of Sciences and Technology (NUST), H-12 Islamabad,) Pakistan. This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (D-321-130-1441). The authors therefore gratefully acknowledge the DSR technical and financial support.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Solution thermodynamics of imidazolium-based ionic liquids and water. J. Phys. Chem. B. 2001;105(44):10942-10949.
- [Google Scholar]
- Ionic liquid–based aqueous biphasic systems as sustainable extraction and separation techniques. Curr. Opin. Green Sustain. Chem.. 2021;27
- [Google Scholar]
- Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J.. 2010;48(2):181-186.
- [Google Scholar]
- Experimental study of use of ionic liquids in enhanced oil recovery.. 2014;4(165):1-7.
- Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9):e04691
- [Google Scholar]
- Imidazole-2yl-phosphonic acid derivative grafted onto mesoporous silica surface as a novel highly effective sorbent for uranium (VI) ion extraction.. 2018;10(7):6681-6693.
- [Google Scholar]
- N, N′-tetrasubstituted succinamides as new molecules for liquid–liquid extraction of Pt (IV) from chloride media. Separ. Purif. Technol.. 2016;158:409-416.
- [Google Scholar]
- Effect of diluents on the extraction and separation of Fe (III) and Cu (II) from hydrochloric acid solutions using N. N, N′, N′-tetrabutyl succinamide.. 2015;152:1-6.
- [Google Scholar]
- Extraction mechanism analysis and energy saving enhancement of extraction separation of methyl tert-butyl ether and methanol by ionic liquid based on molecular dynamics simulation.. 2021;279:119717
- Mechanism and health effects of heavy metal toxicity in humans. Poisoning in the modern world-new tricks for an old dog. 2019;10
- Samarium/cobalt separation by solvent extraction with undiluted quaternary ammonium ionic liquids.. 2019;210:209-218.
- Foong, C.Y., Wirzal, M.D.H., Bustam, M.A., 2020. A review on nanofibers membrane with amino-based ionic liquid for heavy metal removal 297: p. 111793.
- A novel room temperature POSS ionic liquid-based solid polymer electrolyte.. 2018;53(11):8420-8435.
- Selective uptake of thorium (IV) from nitric acid medium using two extraction chromatographic resins based on diglycolamide-calix [4] arenes: Application to thorium-uranyl separation in an actual sample. J Chromatogr A. 2021;1653:462401
- [Google Scholar]
- Efficient selective separation of yttrium from holmium and erbium using carboxyl functionalized ionic liquids. 2021;269:118774
- Removal of aluminum to obtain high purity gadolinium with pyridinium-based ionic liquids. 2022:105930
- Heavy metals causing toxicity in fishes. In: Journal of Physics: Conference Series. IOP Publishing; 2019.
- [Google Scholar]
- Separation of platinum group metals from model chloride solution using phosphonium-based ionic liquid.. 2022;278:119577
- An overview of molecular extractants in room temperature ionic liquids and task specific ionic liquids for the partitioning of actinides/lanthanides. J. Radioanal. Nucl. Chem.. 2020;325(1)
- [Google Scholar]
- Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere. 2019;236:124364
- [Google Scholar]
- Brief bibliometric analysis of “ionic liquid” applications and its review as a substitute for common adsorbent modifier for the adsorption of organic pollutants. Environ. Res.. 2019;175:34-51.
- [Google Scholar]
- An inexpensive N-methyl-2-pyrrolidone-based ionic liquid as efficient extractant and catalyst for desulfurization of dibenzothiophene. 2015;274:192-199.
- Extractive distillation using ionic liquids-based mixed solvents combined with dividing wall column. 2021;269:118713
- Lopes, M.M., et al., 2019. Molecular Interactions in Ionic Liquids: The NMR Contribution towards Tailored Solvents. In: Nuclear Magnetic Resonance, IntechOpen.
- Adsorption performance of Al-pillared bentonite clay for the removal of cobalt (II) from aqueous phase. Appl. Clay Sci.. 2006;31(3–4):194-206.
- [Google Scholar]
- Rhodamine 6G assisted adsorption of metanil yellow over succinamic acid functionalized MCM-41.. 2016;131:177-185.
- FTIR fingerprints discriminate ionic liquids’ antibacterial activity.. 2021;208:104200
- A novel CMPO-functionalized task specific ionic liquid: synthesis, extraction and spectroscopic investigations of actinide and lanthanide complexes.. 2013;42(13):4343-4347.
- Highly efficient diglycolamide-based task-specific ionic liquids: synthesis, unusual extraction behaviour, irradiation. and fluorescence studies. 2013;19(9):3230-3238.
- [Google Scholar]
- Separation of carrier-free 90Y from 90Sr using flat sheet supported liquid membranes containing multiple diglycolamide-functionalized calix [4] arenes. Supramol. Chem.. 2016;28(5–6):360-366.
- [Google Scholar]
- Solvent Extraction Separation of Co (II) and Ni (II) from Weak Hydrochloric Acid Solution with Ionic Liquids Synthesized from. Organophosphorus Acids. 2020;29(5):55-63.
- [Google Scholar]
- Studies on extraction of gallium (III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud. Hydrometallurgy. 2017;171:191-197.
- [Google Scholar]
- Evaluation of cation influence on the formation and extraction capability of ionic-liquid-based aqueous biphasic systems. 2009;113(15):5194-5199.
- Ooi, E., Ng, Y.S., 2018. Removal of Lead from Water by Liquid-Liquid Extraction using trihexyltetradecylphosphonium Chloride in Different Diluents. In: E3S Web of Conferences. EDP Sciences.
- Heavy metal loss from agricultural watershed to aquatic system: A scientometrics review.. 2018;637:208-220.
- Kinetics of adsorption of Co (II) removal from water and wastewater by ion exchange resins. Water Res.. 2002;36(7):1783-1793.
- [Google Scholar]
- Liquid–liquid extraction of neodymium (III) by dialkylphosphate ionic liquids from acidic medium: the importance of the ionic liquid cation.. 2013;15(39):16533-16541.
- Assessment of heavy metal toxicity related with human health risk in the surface water of an industrialized area by a novel technique. Human Ecol. Risk Assess.: Int. J.. 2019;25(4):966-987.
- [Google Scholar]
- A diglycolamide-functionalized task specific ionic liquid (TSIL) for actinide extraction: solvent extraction, thermodynamics and radiolytic stability studies.. 2013;118:264-270.
- Extracted species of Np (IV) complex with diglycolamide functionalized task specific ionic liquid: diffusion, kinetics and thermodynamics by cyclic voltammetry. J. Radioanal. Nucl. Chem.. 2015;304(2):563-570.
- [Google Scholar]
- An insight into the complexation of UO22+ with diglycolamide-functionalized task specific ionic liquid: Kinetic, cyclic voltammetric, extraction and spectroscopic investigations.. 2015;102:549-555.
- Use of phosphonium ionic liquids for highly efficient extraction of phenolic compounds from water. 2020;248:117069
- Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Lett. Appl. NanoBioSci.. 2020;10(2):2148-2166.
- [Google Scholar]
- Szczepański, P., et al., New reactive ionic liquids as carriers in polymer inclusion membranes for transport and separation of Cd (II), Cu (II), Pb (II), and Zn (II) ions from chloride aqueous solutions. 2021. 638: p. 119674.
- Ultra-Fine Purification of Scrap Lead by Electrolysis. Pakistan J. Eng. Appl. Sci. 2017
- [Google Scholar]
- Heavy metal toxicity and the environment. Mol., Clin. Environ. Toxicol. 2012:133-164.
- [Google Scholar]
- Extraction mechanism, behavior and stripping of Pd (II) by pyridinium-based ionic liquid from hydrochloric acid medium.. 2014;147:164-169.
- Comparison of the extraction and stripping behavior of iron (III) from weak acidic solution between ionic liquids and commercial extractants.. 2019;57(12):787-794.
- Synthesis of succinimide based ionic liquids and comparison of extraction behavior of Co (II) and Ni (II) with bi-functional ionic liquids synthesized by Aliquat336 and organophosphorus acids.. 2020;238:116496
- Tran, T.T., Lee, M.S., 2020. Separation of Mo (VI), V (V), Ni (II), Al (III) from synthetic hydrochloric acidic leaching solution of spent catalysts by solvent extraction with ionic liquid 247: 117005.
- Separation of Mo (VI), V (V), Ni (II), Al (III) from synthetic hydrochloric acidic leaching solution of spent catalysts by solvent extraction with ionic liquid. Separ. Purif. Technol.. 2020;247:117005
- [Google Scholar]
- A review on heavy metal pollution, toxicity and remedial measures. Curr. Trends Future Perspect.. 2019;290
- [Google Scholar]
- The removal of heavy metal cations from an aqueous solution using ionic liquids.. 2014;92(11):1875-1881.
- Diglycolamide Based Mono and Di-Ionic Liquids Having Imidazolium Cation for Effective Extraction and Separation of Pb (II) and Co (II) Russian J. Inorg. Chem.. 2021;66(7):1040-1046.
- [Google Scholar]
- Waheed, K., et al., 2021. Diglycolamide based mono and di-ionic liquids having imidazolium cation for effective extraction and separation of Pb (II) and Co (II) 66(7): 1040–1046.
- Recent advances in metal extraction improvement: Mixture systems consisting of ionic liquid and molecular extractant.. 2019;210:292-303.
- Determination of bisphenolic pollutants in raw bovine milks and their derivative products using an in-situ metathesis reaction microextraction based on dicationic imidazolium-based ionic liquids. Microchem. J.. 2019;149:104028
- [Google Scholar]
- Novel highly efficient ionic liquid-functionalized silica for toxic metals removal.. 2021;265:118483
- Novel Mesoporous Organosilicas with Task Ionic Liquids: Properties and High Adsorption Performance for Pb (II).. 2022;27(4):1405.
- A novel one-step strategy for extraction and solidification of Th (IV) based on self-assembly driven by malonamide-based [DC18DMA]+ ionic liquids.. 2022;430:132717
- Synthesis of tailored bis-succinamides and comparison of their extractability for U (VI) Th (IV) and Eu (III). 2019;213:322-327.
- [Google Scholar]
- Bio-inspired hydroxylation imidazole linked covalent organic polymers for uranium extraction from aqueous phases.. 2021;420:129658
- Solvent extraction of palladium (II) and platinum (IV) with N. N-dioctylsuccinamic acid from aqueous chloride media.. 1990;63(1):221-226.
- [Google Scholar]
- Prediction of the Liquid-Liquid Extraction Properties of Imidazolium-Based Ionic Liquids for the Extraction of Aromatics from Aliphatics.. 2021;61(7):3376-3385.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104099.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







