Translate this page into:
Novel aromatic carboxamides from dehydroabietylamine as potential fungicides: Design, synthesis and antifungal evaluation
⁎Corresponding author. njguwen@163.com (Wen Gu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
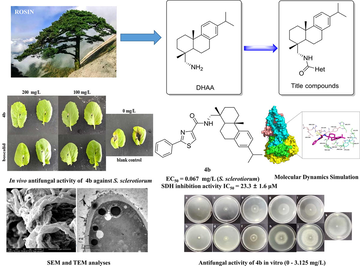
The present study was carried out to design and synthesize a number of novel aromatic carboxamide derivatives of dehydroabietylamine. The preliminary antifungal assay indicated that most of title compounds displayed moderate to good antifungal activity toward the six fungal strains in vitro. Compounds 3i, 3q, 4b and 4d showed significant antifungal activity against Sclerotinia sclerotiorum, with EC50 values ranging from 0.067 ∼ 0.393 mg/L. Compounds 3i, 4b and 4d also showed pronounced mycelial growth inhibition activities against B. cinerea and A. solani. Furthermore, in the in vivo assay, compound 4b exhibited brilliant protective activity against S. sclerotiorum-infected rape leaves. Meanwhile, the in vivo bioassay on tomato plants infected by B. cinerea showed that compound 3i and 4d displayed excellent protective activity at 200 mg/L, which were near to boscalid. Primary mechanistic study revealed that 4b could inhibit sclerotia formation as well as reduce the exopolysaccharide level. SEM and TEM analysis indicated that 4b possessed a strong ability to destroy the surface morphology of mycelia, cell structure and seriously interfere with the growth of the fungal pathogen. In addition, 4b exhibited good inhibitory activity (IC50 = 23.3 ± 1.6 μM) toward succinate dehydrogenase (SDH). Molecular modeling study confirmed the binding modes between compound 4b and SDH. The above antifungal results and fungicidal mechanism study revealed that this class of dehydroabietylamine derivatives could be potential SDH inhibitors and lead compounds for novel fungicides development.
Keywords
Dehydroabietylamine
Aromatic derivatives
Carboxamide
Antifungal activity
Succinate dehydrogenase inhibitor
Molecular modeling
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- HRMS
-
high-resolution mass spectrometry
- EC50 value
-
median effective concentration
- SD
-
standard deviation
- SAR
-
structure-activity relationship
- SDHI
-
succinate dehydrogenase inhibitor
- SEM
-
scanning electron microscopy
- TEM
-
transmission electron microscopy
- MDS study
-
Molecular dynamics simulation study
- RMSD
-
root-mean-square deviation
- RMSD
-
root mean square fluctuations
Abbreviations
1 Introduction
Fungicides, which are one of the most important types of pesticides, could control plant diseases caused by various plant pathogenic fungi. In fact, the application of fungicides has become one of the most economical and effective ways to reduce the huge loss caused by fungal pathogens in agriculture (Fisher et al., 2012; Pennisi, 2010; Brown, 2002). However, the widespread application and misuse of fungicides caused huge threat to the fragile ecosystem and human’s health. Moreover, the increasing resistances of fungal pathogens have emerged to be the main problem that would enhance the risk to global food safety (Liu et al., 2016; Yoon et al., 2013; Mitani et al., 2001; Wang et al., 2013). Thereby to discover more effective and environmental-friendly antifungal agrochemicals has strikingly drawn researcher’s concern.
SDH has been recognized as the key component of respiratory enzyme complexes in the pivotal biochemical process. Its physiological function is to catalyze succinate oxidation coupling with electron transfers from succinate to ubiquinone (Yao et al., 2017a, 2017b; Sun et al., 2005; Jin et al., 2017; Liang et al., 2021; Yang et al., 2019). Over the past sixty years, continuing studies have confirmed that inhibiting the normal physiological function of SDH within phytopathogenic fungi could be an effective strategy to control the fungal infections in grains, vegetables and fruits, and the SDH enzyme was also the key target site for novel fungicide design (Yan et al., 2019; Liu et al., 2020; Liu et al., 2020). To date, twenty-four fungicides based on SDHIs have been commercialized. However, the unlimited use of such fungicides also led to the resistance of pathogenic fungi. Therefore, there are still urgent needs for novel structures of SDHIs to ensure the high yield and quality of crop production.
Natural products have been regarded as a rich and promising source for the candidates in the discovery of novel small molecule pesticides. Among them, some naturally occurring abietane-type diterpenoids and semisynthetic derivatives have displayed notable antifungal activity. For example, several abietane type diterpenoids isolated from Taxodium distichum Rich. were evaluated for antifungal activity against two wood rot fungi Trametes versicolorand and Fomitopsis palustris. Two diterpenoids taxodione and 14-deoxycoleon U showed significant antifungal activities against two fungal strains (Kusumoto et al., 2010). Ferruginol, an abietance diterpene carnosic acid found in Labiatae herb, sage and rosemary, has profound antifungal activity against wood rot fungi. Mechanism study indicated that ferruginol could cause cell dysfunction of T. versicolor including microtubule disruption, reduction in self-defensive capability, inhibition of DNA repair and up-regulation of autophage-related protein (Chen et al., 2015). Recently, some 1,3,4-thiadiazole-thiazolidinone derivatives of dehydroabietic acid were reported to show significant antifungal activity toward Gibberella zeae (Chen et al., 2016). Dehydroabietylamine (DHAAm), as an abietane-type diterpenoid derivative, was an important modified product of dehydroabietic acid obtained from Pinus rosin (Cai et al., 2022). Some synthetic derivatives of DHAAm exhibited significant and diverse biological activities such as antibacterial, antioxidant, antiviral, antileishmanial, antimalarial and anticancer activities (Salehi et al., 2014; Roa-Linares et al., 2016; Dea-Ayuela et al., 2016; Sadashiva et al., 2015; Liu et al., 2013). Meanwhile, only a few literatures have reported the synthesis of DHAAm derivatives exhibiting pronounced antifungal activity to plant pathogenic fungi. Tao et al. has synthesized dehydroabietyl-based amide derivatives with thiophene group, and some derivatives displayed significant antifungal activity toward B. cinerea in vitro and in vivo, which was stronger than the positive control penthiopyrad (Tao et al., 2020). Mao et al. designed and synthesized rosin-based 1,3,4-thiadiazole derivatives (Mao et al., 2021). The in vivo antifungal results demonstrated excellent protective effect on cucumber plants. Gao et al. has synthesized rosin-based acyl-thiourea compounds, which displayed moderate in vitro antifungal activity against T. cucumeris (Gao et al., 2018).
Heterocyclic compounds have received extensive attentions in the research of fungicides due to their noticeable antifungal activities (Zhang et al., 2018a, 2018b; Zhang et al., 2019). Specifically, pyridine, furan, thiazole and pyrazole rings were all important pharmacophores frequently used in SDHI fungicide design (Yao et al., 2017a, 2017b; Wu et al., 2021; Yu et al., 2020). For example, thifluzamide, which was invented by Dow Agroscience, showed excellent antifungal activity against Rhizoctonia solani causing sheath blight in rice paddy (Guo et al., 2019). Boscalid, fenfuram and bixafen with pyridine, furan, and pyrazole heterocycles, respectively, displayed excellent antifungal activities against a variety of plant pathogenic fungi (Fishel and Dewdney, 2015; Syngenta, 2020). Moreover, most reported SDH inhibitors contain the common carboxamide moiety as the key active group (Liu et al., 2019). Therefore, incorporating different types of heterocycles into the scaffold of DHAAm through the carboxamide linker may probably produce novel dehydroabietyl-based antifungal derivatives (Fig. 1). According to this assumption, a series of novel dehydroabietylamine derivatives containing carboxamide group were designed and synthesized. The antifungal potentials against six plant pathogenic fungi were also evaluated. Moreover, the in vivo antifungal assays on rape leaves and tomato plants was conducted to further evaluate the antifungal activity of some active compounds. SEM and TEM analysis were performed to observe the morphological features of fungal pathogens. Mechanism studies such as SDH enzyme inhibition activity and molecular dynamics simulation of the active compounds were also presented in this work.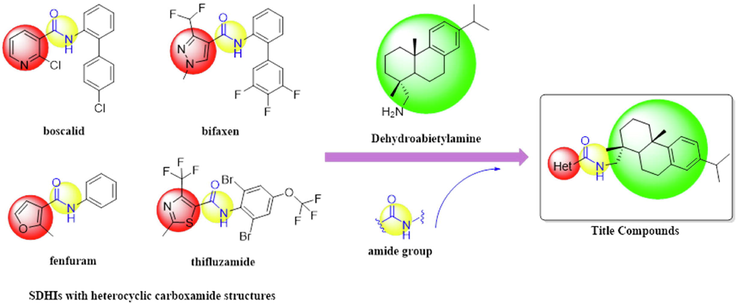
Design strategy of the title compounds.
2 Materials and methods
2.1 General
All reagents and solvents were analytical grade. Thin-layer chromatography (TLC) and column chromatography was conducted on silica gel (200 ∼ 300 mesh), which were both purchased from Qingdao Marine Chemical Factory, China. 1H NMR and 13C NMR spectra were generated from Bruker AV-400 and AV-600 spectrometers (Bruker Co. ltd, Switzerland). Other instruments were utilized similar with our previous report. (Yang et al., 2021).
2.2 Synthetic procedure
The synthesis methods of intermediate 1 and dehydroabietylamide (2) were referenced by previously reported method (Gao et al., 2018; Nian et al., 2018). The reaction mixture of aromatic acid (1.20 mmol) and dehydroabietylamine (2, 1.0 mmol) were dissolved in 10 mL of CH2Cl2. 1-Ethyl-3(3-dimethylpropylamine)carbodiimide (EDCI, 1.20 mmol) and 4-dimethylaminopyridine (DMAP, 0.10 mmol) were added into the solution. The mixture was stirred at 25 ℃ and monitored by TLC. When the reaction completed, the organic phase was washed with water (3 × 10 mL) and brine (3 × 10 mL), dried and concentrated in vacuo. The crude products were further purified by Silica gel column chromatography to give the title compounds 3a-3z, 4a-4f and 5a-5e. The data and spectra of title compounds could be seen in supporting information.
2.3 Antifungal activity of title compounds in vitro
Six plant pathogenic fungal strains, Rhizoctonia solani (R. solani), Fusarium oxysporum (F. oxysporum), Sclerotinia sclerotiorum (S. sclerotiorum), Alternaria solani (A. solani), Gibberella zeae (G. zeae) and Botrytis cinerea (B. cinerea) were obtained by Agricultural Culture Collection of China (ACCC). The antifungal activities of all newly synthesized carboxamide derivatives 3a-3z, 4a-4f and 5a-5e were evaluated in vitro against above crop pathogenic fungi by the mycelium growth rate method based on the previous report. (Zhang et al., 2018a, 2018b). The stock solutions of the title compounds were prepared by dissolving each compound in DMSO at a concentration of 10 g/L. Then the stock solution were added and mixed to potato dextrose agar (PDA) medium to obtain the primary concentration (50 mg/L). Boscalid was assayed as a positive control and DMSO mixed with PDA was used as the blank control. The mycelial disks (5.0 mm) of each phytopathogenic fungus were inoculated on PDA plates at 25 °C. After an incubation period (3–7 days), the diameters (mm) of inhibition zones were measured by the cross-bracketing method. Each compound was measured in triplicate, and EC50 values of active compounds were calculated with the concentration-dependent curves by using SPSS v.22.0 software.
2.4 In vivo bioassay on rape leaves infected by S. Sclerotiorum
The detailed experimental procedures of in vivo bioassay of compound 4b were performed by previously reported methods (Yang et al., 2021). The susceptible rape leaves were collected from planting base of Nanjing Forest University. Healthy rape leaves were sprayed with compounds 4b and boscalid (positive control) at the dosages of 100 or 200 mg/L, and subsequently cultivated for 24 h before inoculation with S. sclerotiorum respectively. Results were observed as diameters of symptoms after cultivation for 5 days at 25 °C. There were nine replicates for each treatment. The protective activity rate (%) was calculated as the following formula: (diameter of lesion in negative control − diameter of lesion in the treatment)/diameter of lesion in the negative control.
2.5 In vivo bioassay on tomato plants infected by B. Cinerea
The tomato plants were purchased from Suojincun Agricultural Market in Nanjing City. The detailed experimental procedures of in vivo testing were performed by previously reported methods (Wang et al., 2017). The tomato plants were also used to evaluate in vivo antifungal activity. Conidia of B. cinerea obtained from 4-week-old cultures on PDA plates were suspended in 0.5 % (v/v) Tween-80 solution to ensure 1 × 106 spores per mL. When tomato plants grew to about 30 leaves, the solutions of the tested compounds (3i, 4b and 4d) and positive controls (boscalid) at the concentration of 200 mg/L were sprayed evenly onto tomato leaves. After dried naturally, these tomato plants were sprayed with prepared spore suspension, the tomato plants were maintained at 24 ± 1 °C and above 80 % relative humidity in greenhouse. The disease index was investigated when the untreated tomato plants developed symptoms, and then the control efficacy was calculated.
2.6 Sclerotia formation inhibition assay
The mycelial disks of S. sclerotiorum were inoculated on PDA mediums containing three concentrations (0.2, 0.1 and 0.05 mg/L) of 4b and boscalid. The PDA mediums were cultured at 25 °C in the dark for 2 weeks. All sclerotia were collected and dried at 60 °C for 1 day. Then the weight of sclerotia and sclerotia formation inhibition were calculated.
2.7 Effect of compound 4b on exopolysaccharide of S. Sclerotiorum strains
S. sclerotiorum strain was cultured in PDB medium for logarithmic growth phase, and then the fungal suspension was added into 25 mL PDB medium with compound 4b at dosages of 0.2, 0.1 and 0.05 mg/L, which was incubated for 3 days in a rotary shaker (180 rpm, 28 °C). Then the precipitated mycelium was removed by centrifugation (12000 rpm, 4 °C for 30 min), and 75 mL ethanol was added into the liquidator for precipitation overnight. Further, the precipitation was collected by centrifugation (12000 rpm, 4 °C for 30 min) to get extracellular polysaccharide (EPS) crude product. Then all the treated samples were dried to constant weight at 70 °C. Each treatment was tested for three replicates. The mass of exopolysaccharide for each treatment was measured.
2.8 SEM observations
SEM observations were carried out to study the effects of 4b on S. sclerotiorum microstructure. After treating with DMSO (control), compound 4b (3.125 mg/L, 0.781 mg/L and 0.195 mg/L) and positive control boscalid (3.125 mg/L, 0.781 mg/L and 0.195 mg/L) for 72 h, mycelia blocks (5.0 mm × 4.0 mm) were sheared from PDA plates. All the samples were treated by 4 % glutaraldehyde for 4 h. Each sample was then dehydrated with graded ethanol series (20, 50, 80, and 90 %) for 10 min. Then, all samples were dried, observed and photographed by using scanning electron microscope with 20 μm resolution ratio (Wang et al., 2021).
2.9 TEM observations
After treatment with compound 4b (0.067 mg/L) for three days, the mycelial blocks of S. sclerotiorum on PDA plates were harvested, dehydrated and embedded in resin at 70 °C for 1 day, and then cut into thin sections. After the samples were double-stained with uranyl acetate and lead citrate, the morphology and ultrastructure of hyphae cells were observed by a transmission electron microscope (JEM-1400, JEOL, ltd., Tokyo, Japan).
2.10 Cell cytotoxicity
The cytotoxicity of compounds 4b, 4d and boscalid in A549 cells (human lung adenocarcinoma cell line) were determined by following the MTT assay methods (Fei et al., 2021). The results were expressed in terms of cell viability and IC50 for each compound.
2.11 In vitro SDH inhibition assay
The procedure of SDH inhibition assay in vitro was carried out by consulting some previous reports (Cheng et al., 2021; Lu et al., 2021; Zhang et al., 2019).
2.12 Molecular modeling
2.12.1 Homology modelling
The NCBI protein database (https://www.ncbi.nlm.nih.gov/protein) was used to search the SDH amino acid sequence of S. sclerotiorum. The employed hypothetical protein sequences were XP_001591238, XP_001594577, XP_001597467, and XP_001593251, reported by Birren. We applied succinate dehydrogenase from porcine heart (PDB ID: 1ZOY) as the template, and the three-dimensional (3D) structure of the SDH was obtain by SWISS-MODEL, a fully automated protein structure homology-modelling server (https://swissmodel.expasy.org/).
Molecular docking study was performed to investigate the binding mode between the compound and the S. sclerotiorum SDH using Autodock vina 1.1.2 (Trott and Olson, 2010). The 3D structure of the compound was built by ChemBioDraw Ultra 14.0 and ChemBio3D Ultra 14.0 softwares. The AutoDockTools 1.5.6 package (Sanner, 1999; Morris et al., 2009) was employed to generate the docking input PDBQT files. The gridbox of the SDH was set as center_x: 86.459, center_y: 65.6, and center_z: 85.537 with dimensions size_x: 15, size_y: 15, and size_z: 15. The value of exhaustiveness was set to 20. The default parameters were used if it was not mentioned. Then an MD study was performed to revise the docking result.
2.12.2 Molecular dynamics simulation
The Amber 14 (Pierce et al., 2012; Götz et al., 2012; Salomon-Ferrer et al., 2013) and AmberTools 15 programs were used for MD simulations of the selected docked pose. The ligand was first prepared by ACPYPE (Da Silva and Vranken, 2012), a tool based on ANTECHAMBER (Wang et al., 2004; Wang et al., 2006) for generating automatic topologies and parameters in different formats for different molecular mechanics programs. Subsequently, the produced file was introduced to tleap program where Amber-ff14SB force fields implemented in AmberTools 15 were recruited to generate Amber topology and initial coordinates files. The solvated complex was submitted to a short energy minimization process including 2500 steps of steepest descent and 2500 steps of conjugate gradient followed by a 1000 ps heating step from 0 K to 300 K, and 500 ps of density equilibration with weak restraints using the GPU accelerated PMEMD (Particle Mesh Ewald Molecular Dynamics) module. The final production of dynamic simulation was performed for 30 ns under periodic boundary condition where no constraint was applied to the protein.
Binding energies (ΔGbind in kcal/mol) were calculated for ligand-receptor complexes using the Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) method. Moreover, to identify the key protein residues responsible for the ligands binding process, the binding free energy was decomposed on a per-residue basis. All the molecular dynamics were performed on a Linux-based (Ubuntu 14.04) GPU work station.
3 Results and discussion
3.1 Chemistry
The synthetic route for title compounds was shown in Scheme 1. Dehydroabietic acid (DHAA) was used as the starting materials, and key intermediate dehydroabietylamide (2) was synthetized via two step (amide reaction and reduction reaction). Then, intermediate 2 was reacted with different aromatic acid to give title compounds 3a-3z, 4a-4f and 5a-5e via amide reaction under mild condition. The structures were confirmed by their NMR and HRMS spectroscopic data.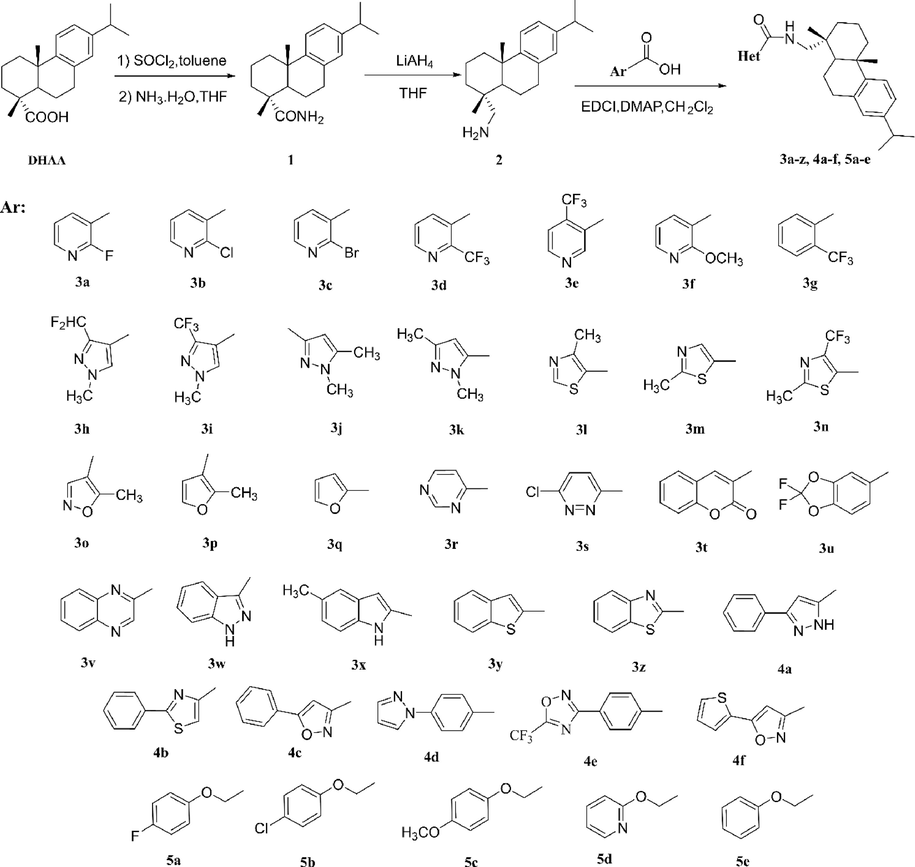
Synthetic route for the title compounds 3a-3z, 4a-4f and 5a-5e.
3.2 In vitro antifungal activity
Comparing with the commercial fungicide boscalid, all compounds were screened for their in vitro antifungal activity toward six plant pathogenic fungi at 50 mg/L. In vitro antifungal activities of compounds 3a-3z, 4a-4f and 5a-5e were listed in Table 1, which displayed the mycelial growth inhibitory rates of compounds 3a-3z against six representative fungi at 50 mg/L. Some compounds effectively inhibited the in vitro mycelium growth of S. sclerotiorum. Several of them demonstrated fair to excellent mycelial growth inhibition activities against S. sclerotiorum, A. solani and B. cinerea. Notably, compound 3i and 3q exhibited more than 95 % inhibitory rates toward S. sclerotiorum, which were approached with boscalid (100 %). Compounds 3e, 3o, 3 l and 3 m also showed considerable anti-S. sclerotiorum effects with inhibition rates ranging from 80 % to 95 %. For A. solani, compounds 3i and 3o showed the same level inhibitions with boscalid (91.8 %). For B. cinerea, only compound 3i displayed more than 90 % inhibitiory rate, which was superior over boscalid (75.3 %). For G. zeae, five compounds (3e, 3i, 3 l, 3o, 3 s) presented more than 80 % inhibitory rate. Overall, a majority of compounds 3a-3z showed mild or poor mycelial growth inhibition activities against R. solani and F. oxysporum with 0 ∼ 70 % inhibition, except that compounds 3i and 3q, which exhibited more than 70 % inhibition rates against R. solani. On the whole, compounds 3i and 3q showed broad-spectrum antifungal activities to all test strains.
Compd
Inhibition rate (%)a
R. solani
B. cinerea
F. oxysporum
S. sclerotiorum
A. solani
G. zeae
DHAA
40.8 ± 1.0
53.9 ± 2.2
42.0 ± 1.0
52.9 ± 0.8
56.9 ± 1.2
39.0 ± 1.8
2
32.5 ± 1.0
46.6 ± 0.8
23.6 ± 2.0
32.9 ± 0.8
72.2 ± 1.2
51.9 ± 1.6
3a
15.3 ± 1.1
20.0 ± 0.1
28.5 ± 1.7
4.78 ± 1.7
11.1 ± 5.5
21.3 ± 0.8
3b
26.2 ± 1.3
16.9 ± 0.1
20.3 ± 2.8
21.5 ± 1.2
64.3 ± 0.1
27.9 ± 8.0
3c
29.0 ± 5.3
0
13.6 ± 0.1
28.2 ± 2.9
20.6 ± 3.6
42.3 ± 1.8
3d
26.5 ± 2.2
19.0 ± 3.6
31.2 ± 3.6
40.4 ± 3.3
7.9 ± 2.7
31.4 ± 0.4
3e
61.7 ± 1.0
24.1 ± 1.8
1.7 ± 1.5
91.6 ± 0.6
69.8 ± 1.4
83.2 ± 1.2
3f
6.5 ± 0.1
9.7 ± 3.5
17.3 ± 0.1
17.0 ± 4.5
52.3 ± 0.1
39.7 ± 6.9
3 g
0.3 ± 0.1
0
3.2 ± 1.0
3.18 ± 0.9
46.8 ± 5.9
10.8 ± 1.7
3 h
33.7 ± 1.3
29.3 ± 2.1
42.9 ± 0.8
25.5 ± 4.0
17.5 ± 1.3
41.2 ± 1.6
3i
74.1 ± 2.3
93.8 ± 0
51.4 ± 0.1
98.1 ± 0.5
97.8 ± 0.1
85.3 ± 1.3
3j
33.3 ± 1.1
0
16.0 ± 1.1
6.2 ± 5.1
4.8 ± 0.1
33 ± 5.8
3 k
35.2 ± 2.1
53.8 ± 5.3
16.0 ± 1.1
37.8 ± 1.6
26.7 ± 1.8
59.1 ± 3.6
3 l
45.1 ± 2.1
84.6 ± 1.9
19.1 ± 1.9
86.6 ± 3.3
90.6 ± 1.3
90.1 ± 0.4
3 m
27.7 ± 4.3
0
19.1 ± 0.1
94.9 ± 0.5
63.5 ± 1.4
74.2 ± 1.9
3n
63.2 ± 1.1
40.0 ± 2.7
0.2 ± 0.1
73.3 ± 4.1
85.9 ± 1.0
73.6 ± 1.5
3o
62.6 ± 1.9
82.1 ± 2.1
50.5 ± 3.2
84.5 ± 1.2
95.0 ± 1.3
83.6 ± 0.8·
3p
5.6 ± 4.8
15.4 ± 0
5.7 ± 2.8
7.8 ± 0.8
40.5 ± 0.1
14.9 ± 2.9
3q
70.4 ± 1.4
55.9 ± 1.8
17.9 ± 1.0
98.1 ± 1.4
88.9 ± 0.1
66.7 ± 0.9
3r
34.0 ± 1.9
4.1 ± 3.2
14.8 ± 2.8
30.1 ± 1.7
52.3 ± 0.1
36.2 ± 1.0
3 s
54.5 ± 2.1
53.8 ± 0.1
42.4 ± 1.0
93.8 ± 3.7
81.1 ± 1.3
84.3 ± 0.8
3 t
0.3 ± 0.1
78.0 ± 2.3
0
36.6 ± 2.9
64.3 ± 0.1
11.6 ± 0.9
3u
20.9 ± 4.3
11.8 ± 1.7
12.4 ± 0.1
83.9 ± 0.4
59.4 ± 2.5
69.1 ± 0.9
3v
1.3 ± 0.1
0
0.2 ± 0.1
15.0 ± 2.2
33.3 ± 4.8
6.2 ± 2.6
3w
17 ± 2.7
23.1 ± 0
0
28.1 ± 1.8
61.9 ± 0.1
18.1 ± 4.0
3x
34 ± 0.1
25.2 ± 1.8
8.1 ± 0.1
45.4 ± 2.4
49.3 ± 3.3
8 ± 3.7
3y
0 ± 0
16.9 ± 1.6
1.3 ± 0.1
16.5 ± 4.7
9.5 ± 4.8
10.4 ± 4.2
3z
1.3 ± 0.1
0
0
0
39.7 ± 1.4
1.6 ± 1.4
4a
22.7 ± 4.2
22.4 ± 4.0
9.3 ± 1.0
18.9 ± 1.1
2.2 ± 0.1
2.5 ± 0.1
4b
33.4 ± 1.1
92.9 ± 2.1
16.1 ± 2.1
98.4 ± 0.8
97.1 ± 1.2
78.5 ± 3.9
4c
7.8 ± 2.1
40.3 ± 2.8
1.5 ± 2.5
36.0 ± 8.1
23.9 ± 0.1
12.6 ± 3.9
4d
34.6 ± 0.1
92.9 ± 2.9
0
99.2 ± 0.8
93.5 ± 0.1
82.2 ± 1.0
4e
0
55.4 ± 1.6
1.7 ± 1.5
92.1 ± 0.9
82.9 ± 1.0
81.0 ± 0.1
4f
15.9 ± 0.1
15.4 ± 4.1
0
15.0 ± 0.1
34.8 ± 0.1
6.1 ± 2.6
5a
40.9 ± 1.2
87.8 ± 2.9
16.0 ± 1.1
93.7 ± 0.1
89.1 ± 0.1
76.1 ± 0.1
5b
27.7 ± 2.9
52.9 ± 1.3
0
29.1 ± 0.1
23.9 ± 0.1
40.5 ± 4.2
5c
27.7 ± 4.3
61.5 ± 2.0
6.9 ± 2.1
29.6 ± 0.9
29.7 ± 5.5
1.0 ± 0.2
5d
41.1 ± 1.3
60.2 ± 2.3
27.7 ± 2.7
86.8 ± 0.3
79 ± 1.2
75.5 ± 1.1
5e
20.6 ± 3.9
40.3 ± 2.8
0
38.9 ± 6.7
52.3 ± 0.1
1.8 ± 1.0
boscalid
44.2 ± 1.6
75.3 ± 2.0
49.4 ± 3.2
100.0 ± 0.0
91.8 ± 1.3
91.7 ± 1.4
Among compounds 4a-4f and 5a-5e (Table 1), some of them exhibited good mycelial growth inhibitory activities toward A. solani and S. sclerotiorum. On the other hand, most of them demonstrated moderate activities against B. cinerea and G. zeae. However, a majority of them displayed inferior inhibition against F. oxysporum and R. solani than boscalid, which were similar with compounds 3a-3z. In detail, two compounds (4b and 4d) exhibited impressive anti-S. sclerotiorum effects with the corresponding inhibition rates of 98.4 % and 99.2 %, which approached to boscalid (100 %). Besides, 4e and 5a showed good antifungal activity against S. sclerotiorum. For B. cinerea, compound 4b and 4d also exhibited excellent antifungal activity, which were significantly better than boscalid.
To assess the fungicidal potency, the EC50 values of active compounds with inhibitions over greater than 98 % against S. sclerotiorum were examined. The results in Table 2 suggested that compounds 3i, 3q, 4b and 4d obviously inhibited S. sclerotiorum with EC50 values of 0.067 ∼ 0.393 mg/L. Remarkably, compound 4b exhibited the promising antifungal activity (EC50 = 0.067 mg/L), which were superior over boscalid (EC50 = 0.118 mg/L). 4d also displayed good mycelial growth inhibition (EC50 = 0.092 mg/L), which was comparable to boscalid. However, compounds 3i and 3q showed lower inhibitory activity against S. sclerotiorum than boscalid (EC50 = 0.393 and 0.128 mg/L, respectively).
fungi
compd
EC50 (mg/L)
regression equation
R2
Confidence Interval 95 %
S. sclerotiorum
3i
0.393
y = 1.77 × + 0.718
0.933
0.145–1.34
3q
0.128
y = 1.63 × + 1.45
0.981
0.021–0.548
4b
0.067
y = 1.30 × + 1.53
0.984
0.044–0.096
4d
0.092
y = 2.19 × + 2.27
0.953
0.071–0.118
boscalid
0.118
y = 1.92 × + 1.79
0.978
0.089–0.154
G. zeae
3i
1.10
y = 1.31 × − 0.054
0.924
0.742–1.81
3 l
1.55
y = 1.16 × − 0.219
0.969
0.97–2.91
boscalid
0.431
y = 1.98 × + 0.723
0.942
0.331–0.569
B. cinerea
3i
0.199
y = 1.70 × + 1.19
0.986
0.148–0.267
4b
2.18
y = 1.44x − 0.486
0.943
1.43–3.84
4d
1.63
y = 1.31 × − 0.276
0.948
1.06–2.85
boscalid
1.41
y = 2.52 × − 0.377
0.988
0.811–3.10
A. solani
3i
0.154
y = 1.92 × − 1.55
0.998
0.117–0.202
3o
0.248
y = 2.03 × − 1.23
0.943
0.089–0.737
4b
0.323
y = 1.93 × + 0.947
0.938
0.138–0.837
boscalid
0.117
y = 1.90 × + 1.77
0.991
0.089–0.154
R. solani
3i
6.54
y = 1.72 × − 1.40
0.979
2.34–11.8
3q
4.41
y = 1.10 × − 0.709
0.957
3.84–15.3
boscalid
greater than50
/
/
/
For G. zeae, compounds 3i and 3 l exhibited good antifungal activity with the EC50 value of 1.10 and 1.55 mg/L, respectively. For B. cinerea, compounds 3i, 4b and 4d (greater than90 % mycelial growth inhibition) were examined for EC50 values. In result, 3i displayed excellent antifungal activity (EC50 = 0.199 mg/L), which was much stronger than boscalid (EC50 = 1.41 mg/L). Compounds 4b and 4d also displayed good inhibition to B. cinerea, which was approached to boscalid. As for A. solani, compounds 3i and 4b showed good activity (EC50 = 0.154 mg/L and 0.323 mg/L, respectively), which were equipotent to boscalid (EC50 = 0.117 mg/L). As for R. solani, only compounds 3q and 3i showed better antifungal inhibition than boscalid, with EC50 = 0.154 mg/L and 0.323 mg/L, respectively. In general, compounds 3i and 4b displayed broad spectrum of antifungal activity toward the test fungi.
In addition, to visualize the inhibitory effect, the mycelium growth of S. sclerotiorum on PDA medium plates containing five concentrations of compound 4b was presented in Fig. 2. In result, the hyphae growth and extension of S. sclerotiorum were dramatically restricted in a concentration-dependent manner. In particular at 0.049 mg/L, compound 4b displayed 42.8 % inhibition to mycelia growth, which was superior over boscalid (35.2 % inhibition). Even under the lower dosage (0.012 mg/L), compound 4b could also moderately inhibit the mycelium growth (32.2 % inhibition), whereas boscalid displayed only 10.0 % inhibition. The results indicated that the compounds were endowed with perceptible antifungal competency.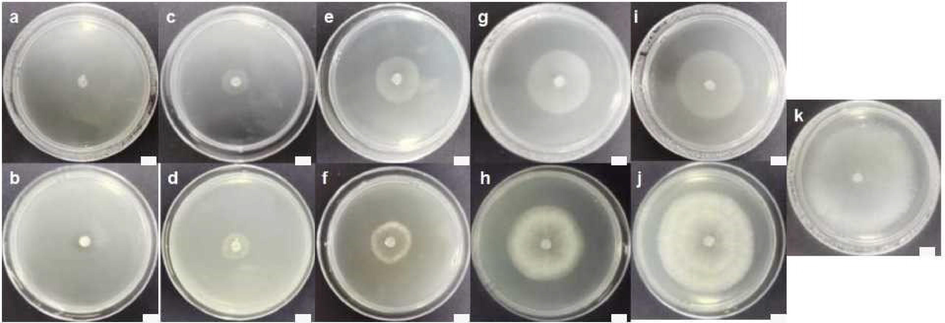
Mycelium growth and extension of S. sclerotiorum after treatment with different concentrations of compound 4b on agar medium: (a) 3.125; (c) 0.781; (e) 0.195; (g) 0.049; (i) 0.012 and (k) 0 mg/L; Boscalid on agar medium: (b) 3.125; (d) 0.781; (f) 0.195; (h) 0.049; and (j) 0.012 mg/L (25 ℃ for 2 d). The scale bars in all graphs were 1.0 cm.
3.3 SAR discussion
As illustrated in Table 1, among series 3a-3z, compounds could be divided as derivatives with single heterocycles (3a-3 s) and derivatives with benzo-heterocycles (3 t-3z). Notably, compounds with pyrazole (3i), thiazole (3 l and 3 m), isoxazole (3o) and furan (3q) group demonstrated good antifungal activities against one or more fungal strains, which were better than most compounds with six-member rings (pyridine and phenyl ring) (i.e. 3a-3d, 3f and 3 g). For instance, compound 3i displayed more than 90 % inhibition rates against B. cinerea, S. sclerotiorum, and A. solani, which were comparable to boscalid. In contrast, other compounds with benzo-heterocycles fragments (3 t-3z) exhibited weak to moderate antifungal activity against several tested fungi, which were quite inferior to the derivatives with single heterocycles. In additions, the other type of compounds, which contained the benzene-heterocyclic fragments (4a-4f), displayed different extent antifungal activities against six test fungi. In detail, compound 4b with 2-phenyl-thiazole moiety exhibited prominent antifungal activity toward S. sclerotiorum, B. cinerea and A. solani, which were much stronger than compounds with 3-phenylpyrazole and 3-phenylisoxazole moieties (4a and 4c). Meanwhile, compounds 4d and 4e containing pyrazole-phenyl and 1,2,4-oxadiazole-phenyl moieties also displayed strong activities to several fungal strains, while 4f with thiophene-isoxazole moiety only showed very weak activity. These results indicated that different combinations of two aromatic rings had significant effect on antifungal activity.
Moreover, the substituents on aromatic rings could exert substantial effect the antifungal activity. For example, as for series 5, compound 5a with electron withdrawing 4-fluoro group on 2-phenoxyacetamide moiety showed stronger anti-S. sclerotiorum activity and anti-A. solani activity than compounds 5b-5e. For other series, the derivatives (3e, 3i, 3n and 4e) with electron withdrawing group such as CF3 generally displayed much better antifungal activities than the derivatives (3f and 5c) with electron donating OCH3 group. In addition, compound 3e with 4-CF3-pyridine exhibited stronger activity than 2-CF3-pyridine derivative 3d, suggesting that the position of substituents could also affect the antifungal activity to some extent.
In brief, it could make the conclusion that among these aromatic carboxamides from dehydroabietylamine, compounds contained with five member-rings, 2-phenyl-thiazole or pyrazole-phenyl moieties displayed more potent antifungal activities than compounds with six member-rings, phenylpyrazole or 3-phenylisoxazole moieties. The electron-withdrawing substituents on heterocycles were generally more beneficial to the antifungal activity than the electron-donating ones.
3.4 In vivo bioassay on rape leaves infected by S. sclerotiorum.
The active compound 4b was further evaluate for the protective effects against S. sclerotiorum infected-rape leaves. The efficacy of the treatment was presented in Fig. 3 and Table 3. Compound 4b displayed strong protective effects at 200 mg/L, which was active at the same level with boscalid (100 %). Even under low dosage (100 mg/L), 4b still demonstrated good protective effects toward S. sclerotiorum. These observations indicated that compound 4b exhibited prominent protective effects in vivo with broad chance of structure modification and optimization for further development.
In vivo protective effects of compound 4b on rape leaves infected by S. sclerotioruma.
Compd.
Treatment
(mg/L)Diameter lesion
length (mm)Protective activitya
(%)
4b
200
5.0 ± 0.06
100
100
5.0 ± 0.07
100
Boscalid
200
5.0 ± 0.05
100
100
5.0 ± 0.1
100
Negative Control
0
27.5 ± 0.398
/
3.5 In vivo bioassay on tomato plants infected by B. cinerea.
The in vivo anti-B. cinerea activity of three compounds 3i, 4b and 4d on tomato plants were further evaluated in greenhouse experiments. The results was presented in Fig. 4 and Table 4. Compound 3i and 4d displayed pronounced inhibitory effect toward B. cinerea, with the control efficacies of 86.0 % and 89.0 %, respectively, which were approached to boscalid (95.0 %). In addition, compound 4b also afforded notable effectiveness of 78.0 % against B. cinerea. In summary, the compound 3i and 4d could effectively control the fungal infection on tomato plants. The letters a–b denoted the results of difference significance analysis. Means followed by the same letter within the same column are not significantly different (p greater than 0.05, Fisher’s LSD multiple comparison test).
In vivo antifungal activity of compounds 3i, 4b and 4d to B. cinerea-infected tomato plants in greenhouse experiment.
Compd.
Disease index
Control efficacy (%)
3i
4.0 ± 0.60 a
86.0
4b
6.0 ± 1.91 a
78.0
4d
3.0 ± 0.98 a
89.0
boscalid
1.0 ± 0.04a
95.0
Negative Control
29 ± 7.89b
/
3.6 Sclerotia formation inhibition Assay.
Sclerotia, which served as the initial inoculum in the S. sclerotiorum infection process, could survive for several years in soil under adverse environmental conditions. Therefore, inhibition of sclerotia formation plays a key role in controlling S. sclerotiorum. As could be seen in Fig. 5B, compound 4b exhibited a good inhibition effect on the sclerotia formation (95.2, 60.6, and 15.1 % inhibition) comparable to that of boscalid (Fig. 5C, 100, 50.6, and 28.1 % inhibition) at 0.2, 0.1, and 0.05 mg/L, respectively.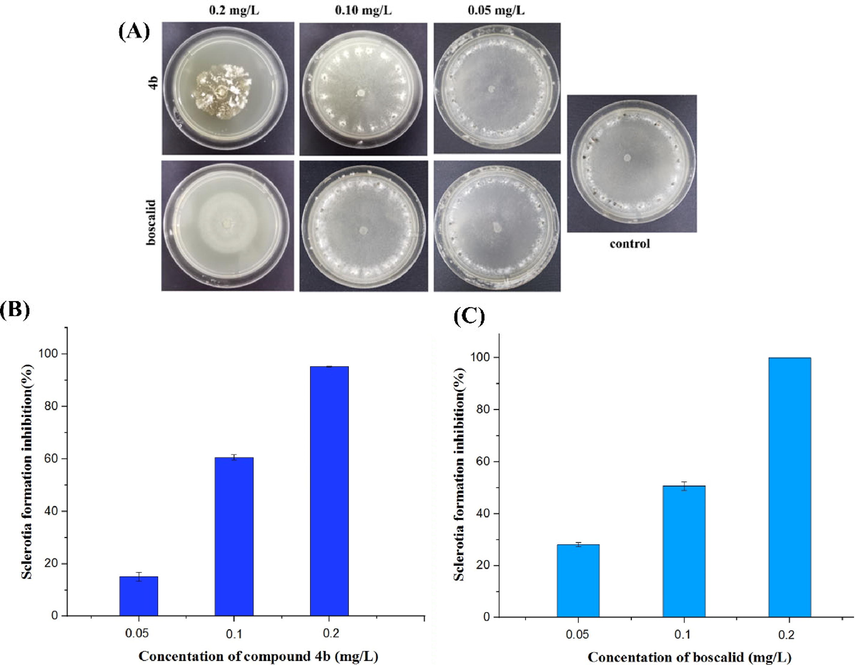
Effect of compound 4b (A, B) and boscalid (A, C) on the sclerotia formation.
3.7 Effect of compound 4b on exopolysaccharide of S. Sclerotiorum strains
Exopolysaccharide is a major component for the growth of fungal cells. The exopolysaccharide mass of the mycelia in the blank control was 0.685 mg/mL (Fig. 6). After treatment with compound 4b, the exopolysaccharide content was reduced, especially at 0.2 mg/L group (exopolysaccharide content 0.125 mg/mL). These results indicated that 4b could concentration-dependently reduce the formation of exopolysaccharide of S. sclerotiorum.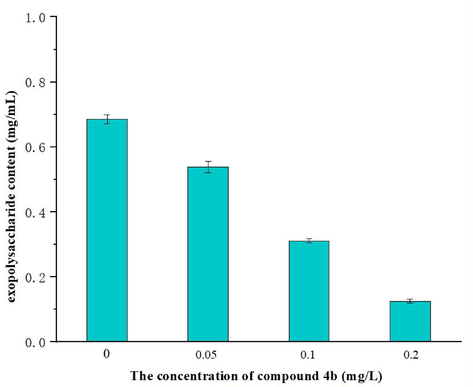
Effect of compound 4b on exopolysaccharide of S. sclerotiorum strains.
3.8 SEM analysis
Compound 4b with the best EC50 (0.067 mg/L) was selected for SEM analysis. The mycelia of S. sclerotiorum were treated with 4b and boscalid at three concentrations of 0.195 mg/L (Fig. 7e, f), 0.781 mg/L (Fig. 7c, d) and 3.125 mg/L (Fig. 7a, b). The structural changes of mycelia were observed through SEM. As a result, the mycelia of the sample without treatment of 4b were regular and smooth in surface, and present an intact structure (Fig. 7g). In contrast, the structure of mycelia treated with 4b were shrunk at 0.195 and 0.781 mg/L (Fig. 7e, c). Moreover, drastic changes in mycelial morphology were observed. And the mycelia presented noticeable shrinkage and different extent of distortion after treating with 4b at 3.125 mg/L (Fig. 7a). However, when treated with boscalid at 0.195 and 0.781 mg/L, the mycelia were not notably crumpled and shriveled. Only at 3.125 mg/L (Fig. 7b), the mycelial morphology was seriously injured, which was comparable to the effect of 4b at the same dosage. This result revealed that 4b had a strong capacity to damage the surface morphology of mycelia and seriously interfere with the growth and reproduction of S. sclerotiorum strain.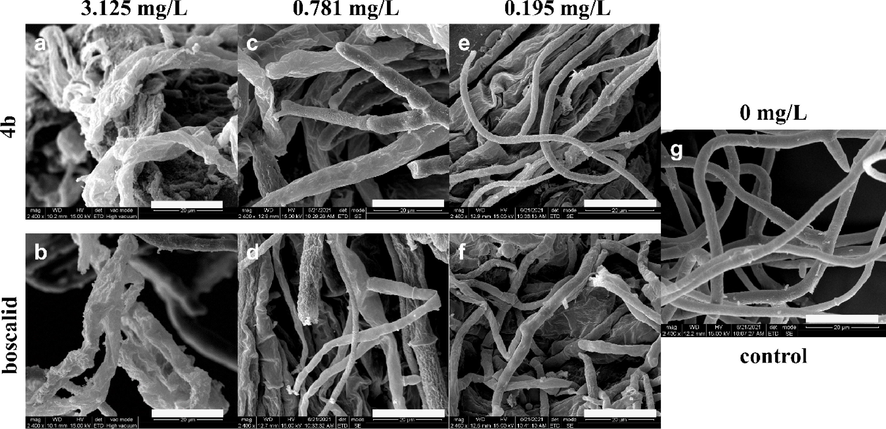
Scanning electron micrographs of S. sclerotiorum hyphae in the untreated control (g) and (a) 3.125 mg/L, (c) 0.781 mg/L, (e) 0.195 mg/L of compound 4b; (b) 3.125 mg/L, (d) 0.781 mg/L, (f) 0.195 mg/L of positive control boscalid. The scale bars of graphs a-g were 20 μm.
3.9 TEM analysis
As for the TEM observation, the mycelium cells in the blank control group (Fig. 8A) had complete structure, with uniform cytoplasm, the cell wall (CW), plasma membrane (PM), mitochondria and other organelles are clearly visible. After treatment with compound 4n, the structure of the cells were damaged, the cell wall became thickened and the mitochondria (M) even disappeared (Fig. 8B). These results indicated that compound 4b had an excellent ability to destory the ultrastructure of hyphae cells.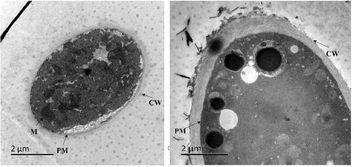
TEM observation of the cellular structure of S. sclerotiorum (A) DMSO control and (B) compound 4b at 0.067 mg/L, the scale bar of graphs was 2 μm.
3.10 Cell cytotoxicity assay
Two selected compounds with high antifungal activity and SDH inhibitory activity were evaluated on their cytotoxicity to A549 cells. As illustrated in Table 5, the viability of A549 cells treated by compounds 4b and 4d at 10 μM were comparable to boscalid. In addition, compounds 4b and 4d exhibited substantially low cytotoxicity (IC50 = 91.1 μM and 86.3 μM, respectively), which were also comparable to boscalid (IC50 = 86.7 μM). On the whole, by comparing with boscalid, compounds 4b and 4d displayed sufficient safety and exhibited the potential for the development of fungicides.
compd
A549 cell
Cell viability (%)
IC50 (μM)
4b
89.6
91.1
4d
88.2
86.3
boscalid
92.5
86.7
3.11 In vitro SDH inhibition assay
Compounds 3i, 3q, 4b and 4d exhibited moderate to good SDH inhibition with IC50 values ranging in 20 ∼ 50 μM (Table 6). Among them, compound 4b exhibited the highest inhibitory activity with IC50 value of 23.3 ± 1.6 μM. In addition, compounds 4d, 3i and 3q also exhibited moderate to good inhibitory activities. These results suggested that the inhibition of SDH could be potential mechanism for the title compounds as fungicides.
compd
IC50 (μM)
regression equation
r2
3i
46.5 ± 0.5
y = 2.11 x-3.51
0.933
3q
49.1 ± 0.9
y = 2.47 x-4.17
0.989
4b
23.3 ± 1.6
y = 2.68 x-3.66
0.979
4d
28.9 ± 3.7
y = 1.79 x-2.62
0.992
boscalid
6.14 ± 0.8
y = 1.56 x-1.23
0.910
3.12 MDS study
According to the SDH inhibition results, the binding mechanism of SDH with 4b and boscalid were further explored. First, the MDS study was carried out. Besides, the RMSD values of the protein backbone were calculated and plotted for investigating the dynamic stability of SDH-4b complex and SDH-boscalid complex. It was observed that the protein structures of the two systems were stabilized during the 30-ns simulation (Fig. 9).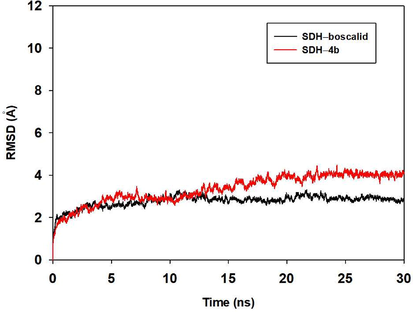
The root-mean-square deviations (RMSDs) of all the atoms of SDH-4b complex and SDH-boscalid complex during the 30 ns simulation.
Subsequently, RMSF for residues of protein in both SDH-4b and SDH-boscalid complex were also calculated (Fig. 10), which described various flexibilities in the binding pocket of SDH when 4b and boscalid were present. Furthermore, in the binding pocket of SDH, most of the amino acid residues interacting with 4b and boscalid also exhibited small flexibility with RMSF < 2 Å, indicating that residues would be more rigid as a result of binding with 4b and boscalid.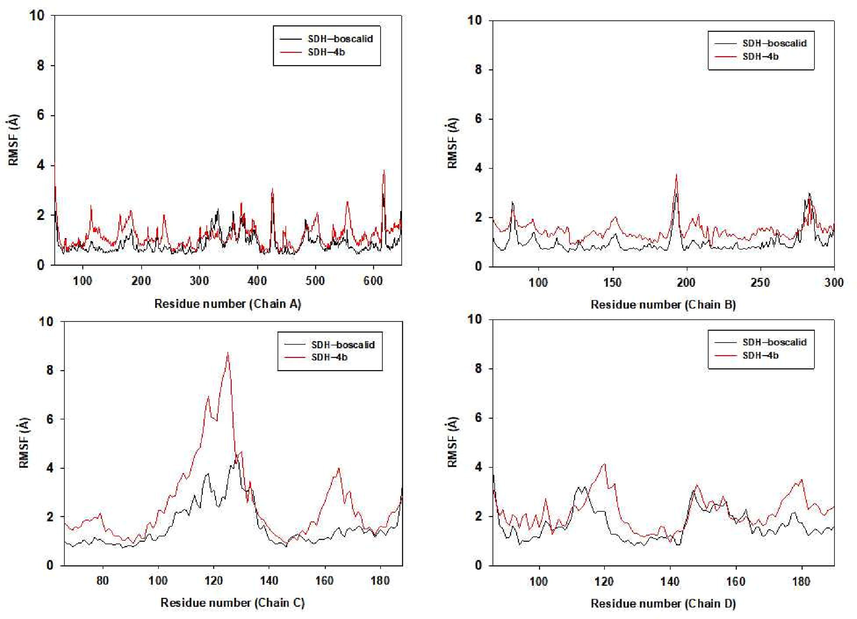
The RMSFs of residues of the whole protein in complexes SDH-4b and SDH-boscalid during the 30 ns simulation.
The per residue interaction free energies contained four parts, including Van der Waals (ΔEvdw), solvation (ΔEsol), electrostatic (ΔEele) and total contribution (ΔEtotal) (Fig. 11). In the SDH-4b complex, the D/Arg-154 residue has an excellent electrostatic (ΔEele) contribution (Fig. 11a). Detailed data analysis displayed that the D/Arg-154 residue was approached to 4b via two hydrogen bond interactions (bond lengths: 3.2 Å and 3.5 Å) (Fig. 12a). Besides, the B/Trp-230 and D/Tyr-144 residues (Fig. 11a) have an appreciable Van der Waals (VDW) interactions with 4b (Fig. 12a). In additions, decomposed energy interaction originated from VDW interactions, apparently through hydrophobic interactions. On the other hand, the D/Asp-143 residue has moderate electrostatic (ΔEele) contribution, with the ΔEele of < − 2.5 kcal/mol (Fig. 12b) in SDH-boscalid complex. This analysis revealed that the D/Asp-143 residue was adjacent to phenyl group of boscalid, forming the anion-π interaction (Fig. 12b). Besides, the residue B/Pro-226 (ΔEvdw of < − 2.0 kcal/mol), have an appreciable VDW interactions with the boscalid because of the close proximity between the residue and the boscalid (Fig. 12b). With the exception of the B/Pro-226 and D/Asp-143 residues, the decomposed energy interaction originated from VDW interactions, obviously through hydrophobic interactions (i.e. B/Trp-229, B/Trp-230, B/Ile-275, C/Leu-72, C/Trp-81, C/Ile-82, C/Leu-86 and C/Phe-87). Moreover, total binding free energy of two complexes were calculated according to the MMGBSA approach, and the ΔGbind values were found for 4b (-42.6 kcal/mol) and boscalid (–32.1 kcal/mol), which demonstrated that 4b could form a tight interaction with SDH.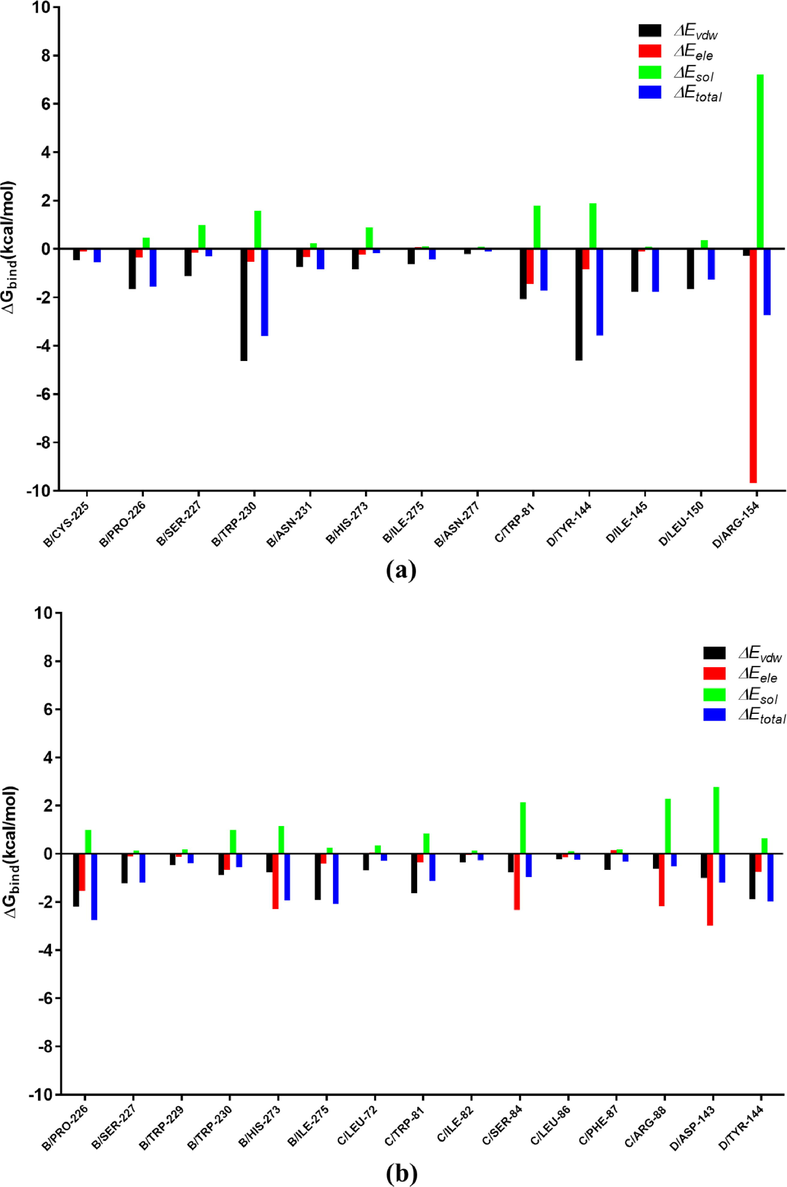
Decomposition of the binding energy on a per-residue basis in complex SDH-4b (a) and complex SDH-boscalid (b).
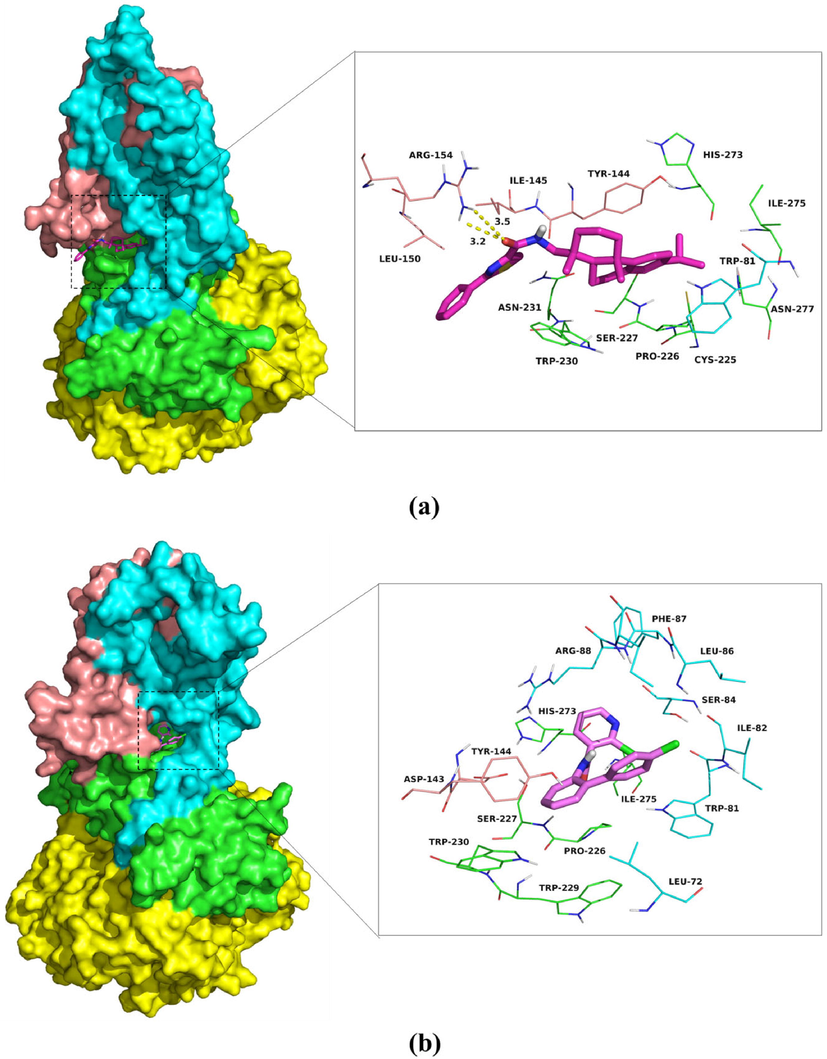
The predicted binding modes of compound 4b (a) and boscalid (b) in SDH binding pockets obtained from molecular dynamics simulation (chains A, B, C and D of SDH were colored by yellow, cyan, green and salmon, respectively; compound 4b and boscalid were shown in rose red and pink sticks, respectively).
In brief, MDS study offered effective clues and reasonable analysis of binding mechanism of SDH with 4b and boscalid, which could give the preliminary conduction for the discovery of novel fungicides.
4 Conclusions
In this work, 37 heterocyclic carboxamide derivatives of dehydroabietylamine were synthesized and characterized. In vitro test displayed that most of the synthesized compounds demonstrated moderate to excellent antifungal activities toward six phytopathogenic fungi. Compounds 3i, 3q, 4b and 4d showed significant antifungal activity toward S. sclerotiorum, and compounds 3i, 4b and 4d also displayed prominent antifungal activity toward B. cinerea with EC50 values comparable to boscalid. In the in vivo antifungal assay, compound 4b exhibited brilliant protective activity against S. sclerotiorum-infected rape leaves. The in vivo antifungal bioassay on tomato plants demonstrated that compound 3i and 4d displayed excellent anti-B. cinerea efficacy at 200 mg/L, which were comparable to boscalid. In the antifungal mechanism study, compound 4b could dose-dependently inhibit the sclerotia formation and reduce the exopolysaccharide level of S. sclerotiorum. The SEM and TEM analyses revealed that 4b could injure the morphological integrity and the cell ultrastructure of S. sclerotiorum mycelia. Furthermore, compound 4b and 4d presented good inhibitory activity against SDH. Molecular modeling suggested that compound 4b could bind well with the active site of SDH. These works indicated that this type of compounds could be considered as effective candidates for the new fungicides development.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31770616) and the Natural Science Foundation for Colleges and Universities in Jiangsu Province of China (No. 17KJA220002).
Declaration of Competing Interest
The authors declare that they have no known compering interest financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science. 2002;297:537-541.
- [Google Scholar]
- Construction of two rosin-based BioAIEgens with distinct fluorescence and mechanochromic propertites for rewritable paper. Dyes Pigm.. 2022;204:110454
- [Google Scholar]
- Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers.. 2016;20:897-905.
- [Google Scholar]
- Proteomics investigation reveals cell death-associated proteins of Basidiomycete Fungus Trametes versicolor treated with ferruginol. J. Agric. Food Chem.. 2015;63:85-91.
- [Google Scholar]
- Design, synthesis and inhibitory activity of novel 2,3-dihydroquinolin-4(1H)-one derivatives as potential succinate dehydrogenase inhibitors. Eur. J. Med. Chem.. 2021;214:113246
- [Google Scholar]
- Synthesis and antileishmanial activity of C7-and C12-functionalized dehydroabietylamine derivatives. Eur. J. Med. Chem.. 2016;121:445-450.
- [Google Scholar]
- Copper(II) and iron(III) complexes of chiral dehydroabietic acid derived from natural rosin: metal effect on structure and cytotoxicity. Metallomics. 2021;13:mfab014.
- [Google Scholar]
- Fungicide Resistance Action Committee's (FRAC) Classification Scheme of Fungicides According to Mode of Action. Florida: Miami-Dade County; 2015.
- Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186-194.
- [Google Scholar]
- Improved application of natural forest product terpene for discovery of potential botanical fungicide. Ind. Crop. Prod.. 2018;126:103-112.
- [Google Scholar]
- Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized Born. J. Chem. Theory Comput.. 2012;8:1542-1555.
- [Google Scholar]
- Discovery of novel thiazole carboxamides as antifungal succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2019;67:1647-1655.
- [Google Scholar]
- Synthesis of novel fenfuram-diarylether hybrids as potent succinate dehydrogenase inhibitors. Bioorg. Chem.. 2017;73:76-82.
- [Google Scholar]
- Antifungal abietane-type diterpenes from the cones of Taxodium distichum Rich. J. Chem. Ecol.. 2010;36:1381-1386.
- [Google Scholar]
- Design, synthesis biological activity, and docking of novel fluopyram derivatives containing guanidine group. Bioorg. Med. Chem. 2021:115846.
- [Google Scholar]
- Antitumor and scavenging radicals activities of some polyphenols related to dehydroabietylamine derivatives. J. Asian Nat. Prod. Res.. 2013;15:819-827.
- [Google Scholar]
- Synthesis and antifungal activities of novel strobilurin derivatives containing quinolin-2(1H)-one moiety. Chem. Res. Chin. Univ.. 2016;32:600-606.
- [Google Scholar]
- Novel 4-pyrazole Carboxamide Derivatives Containing Flexible Chain Motif: Design, Synthesis and fungicidal Activity. Pest. Manage. Sci.. 2019;75:2892-2900.
- [Google Scholar]
- Bioactivity-oriented modification strategy for SDH inhibitors with superior activity against fungal strains. Pestic. Biochem. Physiol.. 2020;163:271-279.
- [Google Scholar]
- Design, Synthesis, Biological Evaluation, and Molecular Modeling of Novel 4H-Chromene Analogs as Potential Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem.. 2021;69:10709-10721.
- [Google Scholar]
- Pine rosin as a valuable natural resources in the synthesis of fungicides candidates for controlling Fusaium Oxyspourum on cucumber. J. Agric. Food Chem.. 2021;69:6475-6484.
- [Google Scholar]
- Antifungal activity of the novel fungicide cyazofamid against phytophthora infestans and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol.. 2001;70:92-99.
- [Google Scholar]
- AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30:2785-2791.
- [Google Scholar]
- Synthesis and biological evaluation of N-(5-(2,5-dimethyl-phenoxy)-2,2-dimethylpentyl)-benzamide derivatives as novel farnesoid X receptor (FXR) antagonist. Lett. Drug Des. Discovery.. 2018;15:923-936.
- [Google Scholar]
- Routine access to millisecond time scale events with accelerated molecular dynamics. J. Chem. Theory Comput.. 2012;8:2997-3002.
- [Google Scholar]
- Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem.. 2016;108:79-88.
- [Google Scholar]
- A non-cytotoxic N-dehydroabietylamine derivative with potent antimalarial activity. Exp. Parasito.. 2015;155:68-73.
- [Google Scholar]
- Synthesis, antibacterial and antioxidant activity of novel 2,3-dihydroquinazolin-4(1H)-one derivatives of dehydroabietylamine diterpene. J. Iran Chem. Soc.. 2014;11:607-613.
- [Google Scholar]
- Routine microsecond molecular dynamics simulations with Amber on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput.. 2013;9:3878-3888.
- [Google Scholar]
- Python: a programming language for software integration and development. J. Mol. Graph. Model.. 1999;17:57-61.
- [Google Scholar]
- Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043-1057.
- [Google Scholar]
- Syngenta Crop Protection AG, Research and Development, Switzerland, 2020. FRAC classification on mode of action. Mode of action of fungicides. http://www.frac.info/ (accessed on 2020).
- Antifungal application of rosin derivatives from renewable pine resin in crop protection. J. Agric. Food Chem.. 2020;68:4144-4154.
- [Google Scholar]
- AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput Chem.. 2010;31:455-461.
- [Google Scholar]
- Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopathogenic fungi. J. Agric. Food Chem.. 2013;61:8632-8640.
- [Google Scholar]
- Design, synthesis and fungicidal activity of 2-substituted phenyl-2-oxo-, 2-hydroxy and 2-acyloxyethylsulfonamides. Molecules. 2017;22:738.
- [Google Scholar]
- Development and testing of a general amber force field. J. Comput Chem.. 2004;25:1157-1174.
- [Google Scholar]
- Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol Graph. Model.. 2006;25:247-260.
- [Google Scholar]
- Bioactivity-guided synthesis accelerates the discovery of 3–(iso)quinolinyl-4-chromenones as potent fungicide candidates. J. Agric. Food. Chem.. 2021;69:491-500.
- [Google Scholar]
- Yang, S., Synthesis, Biological Evaluation, and 3D-QSAR Studies of N-(Substituted pyridine-4-yl)-1-(substituted phenyl)-5-trifluoromethyl-1H-pyrazole-4-carboxamide Derivatives as Potential Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem.. 2021;69:1214-1223.
- [Google Scholar]
- Design, synthesis, and antifungal activity of carboxamide derivatives possessing 1,2,3-triazole as potential succinate dehydrogenase inhibitors. Pestic. Biochem. Physiol.. 2019;156:160-169.
- [Google Scholar]
- Design, Synthesis, and Antifungal Activity of Novel Thiophene/Furan-1,3,4-Oxadiazole Carboxamides as Potent Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem.. 2021;69:13373-13385.
- [Google Scholar]
- Synthesis and biological activity of novel succinate dehydrogenase inhibitor derivatives as potent fungicide candidates. J. Agric. Food Chem.. 2019;67:13185-13194.
- [Google Scholar]
- Discovery of novel succinate dehydrogenase inhibitors by the integration of in silico library design and pharmacophore mapping. J. Agric. Food Chem.. 2017;65:3204-3211.
- [Google Scholar]
- Design, synthesis, and fungicidal evaluation of novel pyrazole-furan and pyrazole-pyrrole carboxamide as succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2017;65:5397-5403.
- [Google Scholar]
- Recent trends in studies on botanical fungicides in agriculture. Plant. Pathol.. 2013;29:1-9.
- [Google Scholar]
- Design, Synthesis, and Evaluation of the Antifungal Activity of Novel Pyrazole-Thiazole Carboxamides as Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem.. 2020;68:7093-7102.
- [Google Scholar]
- Design and discovery of novel chiral antifungal amides with 2-(2-oxazolinyl)aniline as a promising pharmacophore. J. Agric. Food Chem.. 2018;66:8957-8965.
- [Google Scholar]
- Design, synthesis and antifungal evaluation of novel pyrazole carboxamides with diarylamines scaffold as potent succinate dehydrogenase inhibitors. Bioorg. Med. Chem. Lett.. 2018;28:3042-3045.
- [Google Scholar]
- Design, synthesis, and antifungal activities of novel aromatic carboxamides containing a diphenylamine scaffold. J. Agric. Food Chem.. 2019;67:5008-5016.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104330.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







