Translate this page into:
Role of rhodium doping into lanthanum cobalt oxide (LaCoO3) perovskite and the induced bifunctional activity of oxygen evolution and reduction reactions in alkaline medium
⁎Corresponding authors. mghanem@ksu.edu.sa (Mohamed A. Ghanem)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Rhodium doping into the lanthanum cobalt oxide (LaCo1-xRhxO3) has induced significant bifunctional electrochemical activity towards the OER and ORR in alkaline solution with a substantially lower OER/ORR onset potential of 1.36/0.73 V vs HRE, smaller Tafel slope (116/90 mV/dec), low charge-transfer resistance, and an OER and ORR potential gap of only 0.65 V.

Abstract
Transition metal oxides, especially perovskites, have been considered effective electrocatalysts for the oxygen evolution (OER) and oxygen reduction (ORR) reactions in an alkaline solution. Here, a series of lanthanum cobalt rhodium oxide perovskites with the chemical formula LaCo1-xRhxO3 (LCRO, 0.1 ≤ x ≤ 0.70) were prepared through the approach of solid-phase synthesis and their bifunctional electrocatalytic activity was assessed for both the OER and ORR. The crystallinity, morphology, surface, and electrocatalytic features of the LCRO were significantly correlated with the rhodium content. The LaCo0.7Rh0.3O3 electrocatalysts with x = 0.3 showed enhanced electrocatalytic bifunctional performance with a substantially lower OER/ORR onset potential of 1.38/0.73 V vs HRE, smaller Tafel slope (116/90 mV/dec), and low charge-transfer resistance, which is the most efficient catalyst among the other studied ratios and superior to the pristine lanthanum cobalt oxide benchmark electrocatalysts. The LaCo0.7Rh0.3O3 electrode exhibit good bifunctional electrocatalytic behavior and long-term durability with an OER and ORR onset potential gap (ΔE = EOER − EORR) of only 0.65 V, which could be credited to the enriched oxygen vacancies, lattice expansion and the improved electrical conductivity upon the doping of larger size of Rh ions. The LaCo1-xRhxO3 catalysts are obtained from abundant materials that have the potential of highly-active bifunctional OER and ORR electrocatalysts.
Keywords
Lanthanum cobalt oxide
Perovskites
Rhodium
Oxygen evolution and reduction reactions
1 Introduction
During the past years, electrochemical water-splitting has been an active research area for the production of hydrogen fuel for cleaner energy (Lee et al., 2022; Marini et al., 2012). Alkaline water electrolysis involves the reaction of oxygen evolution (OER, 4OH− → O2 + 2H2O + 4e−), which is a kinetically sluggish reaction owing to the proton-coupled multi-electron transfer process and the requirement for a large overpotential (Wang et al., 2016; Debe, 2012). Further, the reaction of oxygen reduction (ORR, O2 + 2H2O + 4e− → 4OH−) is another electrochemical process for energy storage devices and fuel cells (Feng et al., 2018; Shahid et al., 2017). The development of economic, highly active catalysts for oxygen redox reactions for application in direct solar and electrochemical water electrolysis and metal-air batteries is considered the most technical task in energy technology (Xia et al., 2016). Therefore, there has been growing interest in developing and preparing non-precious metal catalytic materials that are highly efficient to replace the precious- and rare-metal-based electrocatalysts (Cheng et al., 2013; Chen et al., 2011; Wu et al., 2013)and to improve the overall activity and kinetics of the ORR and OER (Dou et al. 2020).
Perovskite-based oxides (ABO3) are of considerable interest for creating next-generation catalytic materials, providing enriched bifunctional (OER) and (ORR) (Suntivich et al., 2011; Dias et al., 2020) electrocatalytic activity, and possessing benefits over precious-metal-based catalysts in terms of production cost and high durability at high temperature (Matsumoto et al., 1977; Bockris and Otagawa,1984; Kobussen and Broers, 1981; Matsumoto and Sato, 1980). Moreover, their rich composition variant, crystal structure, and morphology allow further tuning of their electronic and electrocatalytic activities for enhanced catalytic performance for the OER and ORR (Tanaka and Misono, 2001; Harris, 1970; Cui et al., 2021). Among the available catalysts, lanthanum-based perovskites, such as LaCoO3, LaNiO3, LaCrO3, LaMnO3, and LaFeO3, have been extensively investigated for various electrochemical energy reactions owing to their structural stability, high electronic and ionic conductivities, and the capacity to fully or partially replace the cation of A-, B-site elements as well as the anion at the O-site to engineer the structural, physical, and electronic properties (Dias et al., 2020, Zhu et al., 2015; Celorrio et al., 2015; Petrie et al., 2016; Mefford et al., 2016). The full or partial substitution of ABO3 perovskites leads to the weakening of the metal–oxygen bond and improves the B—O bond covalency, oxygen defects, and redox surface-active oxygen species, thus enhancing the electronic and ionic conductivity as well as the ORR and OER electrocatalytic activity (Dong et al., 2021; Xu et al., 2020; Zhang et al., 2020; Zhu et al., 2016). Within this context, the iron (Kim, et al., 2019) or nickel (Liu et al., 2019) doping in cobalt-based perovskites improves the ORR electrochemical activity in solid oxide fuel cell reaction. Moreover, the partial exchanging of the O-sites in the structure of ABO3 perovskite with anions of Br–, Cl– and F– (Ghanem et al., 2021, 2016; Zhang et al., 2020), N3– or S2– (Liu et al., 2019) significantly weakens the metal–oxygen bond, and consequently promotes the lattice oxygen activity and diffusion (Zhang et al., 2020). The oxygen vacancy concentration on the perovskite structure is found to have a pronounced effect on oxygen mobility as well as catalytic activity and selectivity. For instance, the exchange of Sr for La at the A site and the substitution of Co for Fe at the B site in ABO3 perovskites have shown dramatic increases in the oxygen diffusion rate, which improves the overall activity of the water oxidation reaction (Chang et al., 2020). Furthermore, the Ab initio and DFT modeling have shown that the alkaline water electrolysis activity of LaCoO3 can be enhanced by tuning the Co—O bond covalency and the oxygen vacancy concentration via partial Sr2+ substitution (Mefford et al., 2016). The results demonstrated that in alkaline media the OER was significantly enhanced due to the increase of oxygen vacancies, which modulated the Fermi energy of the overlapped transition metal-3d and oxygen-2p orbitals. In addition, the eg filling factor of the B-metal in the ABO3 perovskite has been shown to control the intermediate species binding energy, and consequently, significant perovskite water oxidation activity can be gained (Suntivich et al., 2011). The recent works have reported significant enhancement of the bifunctional OER and ORR performances of strontium iron and cobalt perovskites by partial doping with halide anions, with the activity increasing in the order Br < Cl < F (Liu et al., 2019; Ghanem et al., 2021, 2016). Herein and for the first time, we report the intrinsic bifunctional OER and ORR activities of a series of lanthanum cobalt oxides partially substituted by rhodium, LaCo1-xRhxO3 (LCRO, x = 0.1, 0.15, 0.20, 0.30, 0.40, 0.60, and 0.70). The effect of rhodium doping on the lanthanum cobalt oxide crystal structure, surface morphology, and oxygen vacancy concentration was investigated by diffractive X-ray, scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) mapping, and X-ray photoelectron spectroscopy (XPS). The OER and ORR bifunctional electrochemical activity of LCRO in alkaline solution were significantly correlated with the Rh content and the LaCo0.7Rh0.3O3 electrode materials with x = 0.3 showed enhanced electrocatalytic performance with a significant negative shift (170 mV) in the OER onset potential, Tafel slope of 116 mV/dec, ultra-low charge-transfer resistance, and current density of 104.2 mA cm−2 at 1.6 VRHE, which is a more active catalyst than other investigated Rh-doped ratios. Based on the electrochemical measurements, the LaCo0.7Rh0.3O3 electrocatalyst was very stable during the prolonged OER and ORR process in an alkaline medium. This work explores the controlled chemical modifications/doping of a new set of 3d-4d transition metals of Co and Rh within the LCRO perovskite while maintaining the crystal structure and enabling monitoring of the effects of the covalency and oxygen vacancy concentration on the bifunctional OER and ORR activity.
2 Experimental
2.1 Materials preparation and synthesis
Lanthanum cobalt rhodium perovskite oxides (LCRO) with nominal composition of LaCo1-xRhxO3 (x = 0.1, 0.15, 0.20, 0.30, 0.40, 0.60, and 0.7) were prepared using a standard high-temperature solid-state reaction method. Mixtures of La2O3 (99.95 %, Sigma-Aldrich), Co3O4 (99.9 %, Sigma-Aldrich), and Rh2O3 (99.99 %, Johnson-Matthey) with the various target product stoichiometric molar ratios were ground together in an agate pestle and mortar, placed in a platinum crucible, and heated to 1150 °C in air. The partly reacted product was reground, pressed into a pellet, and sintered for two further periods of 48 h at 1150 °C in air. The catalysts then were left to cool at room temperature, ground, and stored in a desiccator for further characterization.
2.2 Catalysts characterizations
The phase structures of the obtained samples were investigated using X-ray diffraction (XRD, Rigaku Miniflex 600, Rigaku Corporation, Tokyo, Japan). The XRD results were obtained within the 2Θ range of 5–80° with 0.02° step and a scan speed of 10° min−1 and operated voltage and current of 40 kV and 15 mA respectively. The Rietveld refinement method was used to obtain the space group and the crystalline parameters from the XRD data. The catalyst morphologies and elemental compositions of the synthesized catalysts were examined by SEM (S-4800, Hitachi) fitted with an EDX spectrometer (EX350, Horiba). X-ray photoelectron spectroscopic analysis of the produced materials was achieved using the spectrometer of Escalab 250 XPS (Thermo Fisher).
2.3 Catalyst electrodes synthesis and electrochemical measurements
Electrochemical characterizations were evaluated at room temperature using a three-electrode cell connected to an electrochemical Bio-Logic two-channel potentiostat (VMP3). The catalyst ink was physically cast onto an electrode of carbon paper (CP, SIGRACET® GDL-24BC, area of 1.0 × 1.0 cm2), which was employed as the working electrode, and a high-purity graphite sheet (1.0 × 1.0 × 0.3 cm3) electrode and the saturated calomel electrode (SCE) were employed as the auxiliary and reference electrodes, respectively. The electrode potential was normalized to the hydrogen reference electrode (RHE) using the equation of E(RHE) = E(SCE) + 0.243 + 0.059pH. The CP electrode was pre-activated in hot concentrated HNO3 for 40 sec followed by rinsing with deionized water and drying before catalyst casting. The ink of the LaCo1-xRhxO3 catalysts was prepared by dispersing 10 mg of catalyst powder in 1.0 ml of water: isopropanol solution (volume ratio of 0.5:0.5), and then 10 µl of Nafion solution was added. The mixture was sonicated for 30 min to generate a homogeneous electrocatalyst ink. Finally, 60 µl of as-prepared catalyst ink was coated on the surface of the CP electrode, resulting in an approximate catalyst loading of 0.6 mg/cm2. The OER and ORR were investigated in an aqueous solution of 1.0 M KOH (pH 13.8) and before each experiment, N2 gas was purged through the KOH electrolyte for 30 min to remove the dissolved oxygen. The electrochemical measurement of the ORR activity was performed in a 0.1 M KOH aqueous solution using a rotating ring-disk electrode (RRDE, make, and supply) comprising a glassy carbon disk and a platinum ring electrode. To prepare the catalyst dispersion, 10.0 mg of the investigated catalyst was dispersed in 1.0 ml of water: isopropanol solution (volume ratio of 0.5:0.5), and then 10 µl of Nafion solution was added. The H2O2 yield and the number of transferred electrons (n) during the ORR over the oxyhalide catalyst were calculated using equations (1) and (2), respectively.
3 Results and discussion
3.1 Characterizations of the LaCo1-xRhxO3 catalysts
the XRD patterns of the LaCo1-xRhxO3 catalysts doped with different ratios of Rh are shown in Fig. 1. The obtained catalysts are highly crystalline with the main diffraction peaks at 2θ of 23.10, 32.74, 40.32, 47.06, 53.01, 58.35, 68.7, and 78.22 consistent with the (0 1 2), (1 1 0), (2 0 2), (0 2 4), (1 1 6), (3 0 0), (2 0 8), and (1 2 8) diffraction planes, respectively (PDF card no: 01–074-9491). Moreover, the cubic perovskite structure adopted by the majority of these LaCo1-xRhxO3 catalysts is shown in Fig. 1. The LaCo1-xRhxO3 catalysts with x = 0.1, 0.20, 0.30, 0.40, and 0.60 have a single phase of cubic perovskite, as evidenced by the single uniform diffraction peak of the (1 1 0) crystal plane at 2θ = 32.74, shown in Scheme 1. For x = 0.15, i.e., LaCo0.85Rh0.15O3, a perovskite structure type was identified through the breadth of the reflections, as shown in Fig. 1b (red line), which indicates a degree of phase separation for this composition or distortion of the perovskite structure to a lower symmetry (GdFeO3 structure type). However, for x = 0.7, i.e., LaCo0.3Rh0.7O3, a single perovskite phase material was not formed, and the PXD pattern indicated phase separation into two perovskites with compositions LaRhO3 (GdFeO3 structure type) and LaCo0.85Rh0.15O3. The shift of the reflections to the lower 2θ as the level of rhodium increases represents the expansion of the unit cell as Rh3+ replaces Co3+ with the lattice parameter expanding from 3.876(2) Å (LaCo0.9Rh0.1O3) to 3.914(2) Å (LaCo0.4Rh0.6O3) indicating on the weakening of the B—O bond upon the Rh doping.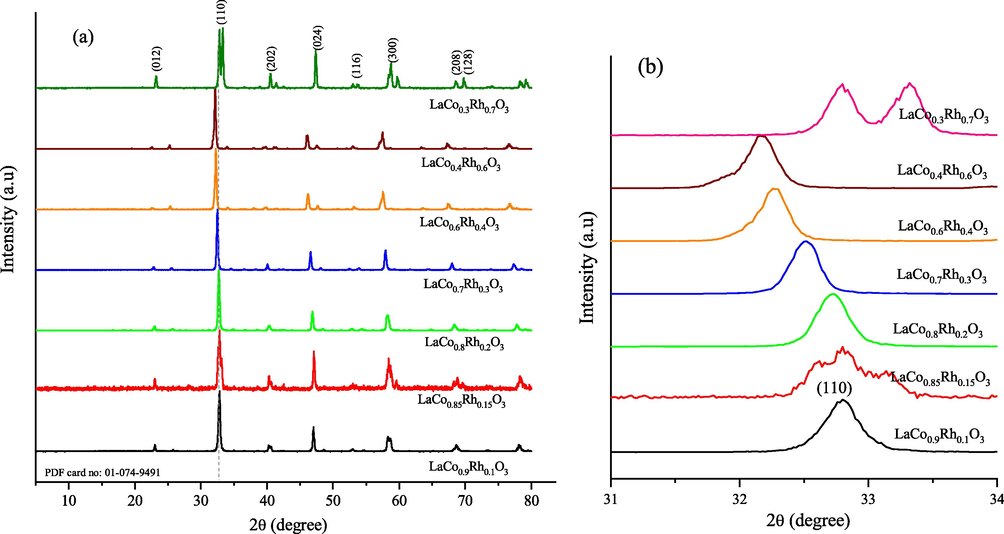
(a) XRD patterns of as-prepared LaCo1-xRhxO3 perovskite catalysts with different doped Rh ratios prepared by solid-state reactions are indexed using reference peak (PDF # 01-074-9491), (b) the corresponding enlarged view of the (1 1 0) diffraction plane.
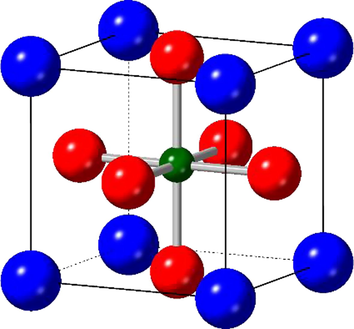
The perovskite structure of LaCo1-xRhxO3 is adopted for the majority of × values. La blue, oxygen red, and Co/Rh dark green.
The surface morphological nature of the LaCo1-xRhxO3 catalysts with x = 0.1, 0.2, 0.3, and 0.4 was examined by SEM, as displayed in Fig. 2. The SEM micrographs in Fig. 2a and 2b show the LCRO catalyst at Co/Rh ratios of 0.1 and 0.2, respectively, have large, coarse, irregular particles with sizes of several micrometers. However, as shown in Fig. 2c and 2d, at Rh content of 0.3 and 0.4, the catalyst particles become more uniform and have a smoother surface with smaller particle sizes within the range of 1.0 to 3.0 µm. The dense oxide particles indicate that the catalysts are probably crystalline and free from pores, which is consistent with XRD results. However, the SEM microstructure characterization images show the presence of interstitial macropores between microparticles, which could facilitate the mass transport of the reactants and products during the electrochemical reactions.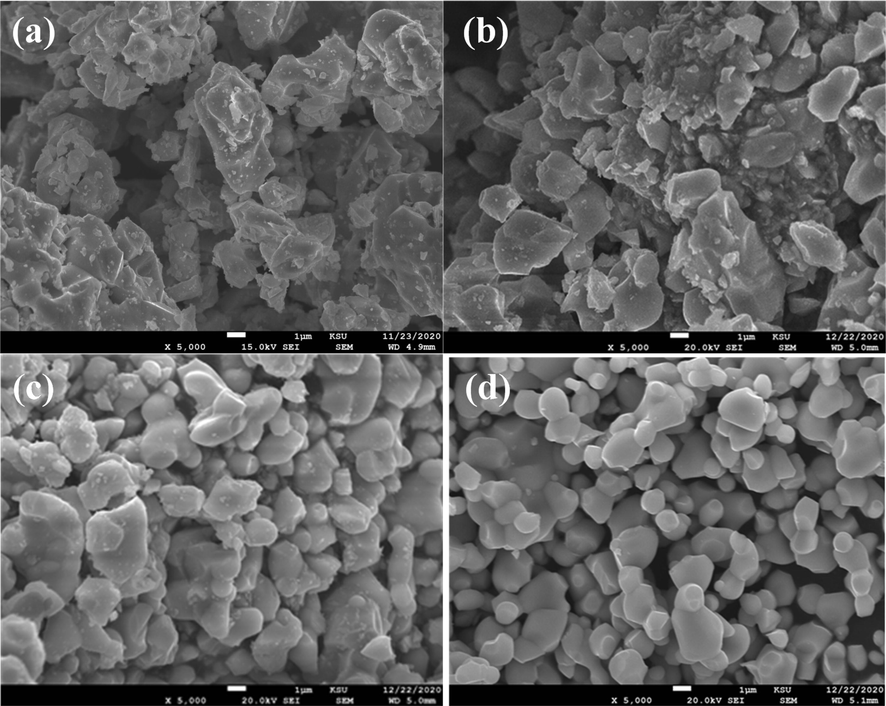
Morphological SEM characterization of LaCo1-xRhxO3 catalysts prepared with different Rh content of (a) x = 0.1, (b) x = 0.2, (c) x = 0.3 and (d) x = 0.4.
The EDX composition mapping and elemental analysis of the LaCo0.7Rh0.3O3 catalysts are shown in Fig. 3. In addition, Table 1 reports the EDX elemental composition analysis and the corresponding mole ratios for some samples of the LaCo1-xRhxO3 catalysts. The EDX mapping of the LaCo0.7Rh0.3O3 catalyst discloses the existence of La, Co, Rh, and O components uniformly distributed throughout the sample (Fig. 3). Particularly, the EDX analysis of the LaCo0.7Rh0.3O3 catalyst reveals the element wt.% of 55.48, 17.05, 10.58, and 16.89 for La, Co, Rh, and O elements, respectively, which corresponds to the chemical composition of LaCo0.72Rh0.31O4.40, which is very close to the intended composition of the LaCo0.7Rh0.3O3 catalyst. Moreover, Table S1 shows that the mole ratios of La, Co, Rh, and O obtained from EDX element composition analysis are in good agreement for the LaCo1-xRhxO3 catalysts with x = 0.1, 0.2, 0.3 and 0.7. From the EDX composition mapping and elemental analysis, we can conclude that La, Co, Rh, and O elements are homogeneously distributed throughout the LaCo1-xRhxO3 catalysts and their composition mole ratios match the intended stoichiometric compositions of the catalysts.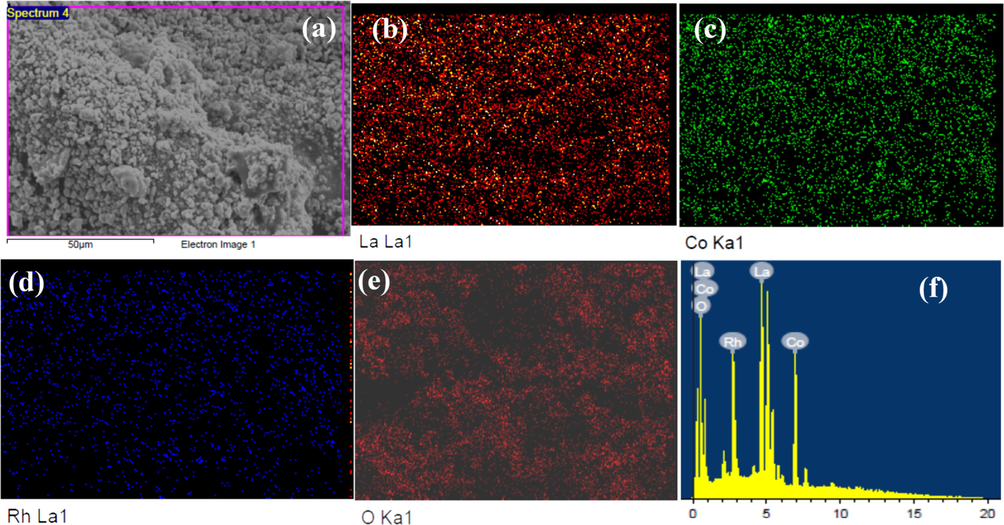
EDX elemental maps and composition analysis of LaCo0.7Rh0.3O3 sample, (a) SEM image, (b) La, (c) Co, (d) Rh, (e) O, and (f) EDX histogram of La, Co, Rh, and O elements.
Catalyst intended composition
La wt. % (mole ratio)
Co wt. % (mole ratio)
Rh wt. % (mole ratio)
O wt. % (mole ratio)
LaCo0.9Rh0.1O3
50.06 (1.0)
20.17 (0.93)
4.07 (0.10)
25.17 (4.32)
LaCo0.8Rh0.2O3
51.34 (1.0)
17.35 (0.79)
8.14 (0.21)
23.17 (3.92)
LaCo0.7Rh0.3O3
49.01 (1.0)
14.17 (0.72)
11.23 (0.31)
24.86 (4.40)
LaCo0.3Rh0.7O3
50.36 (1.0)
6.49 (0.3)
22.23 (0.685)
20.93 (3.55)
The effect of partial replacement of cobalt by rhodium on the surface functionality and oxygen vacancies of the LaCo1-xRhxO3 catalysts was examined by XPS. The wide survey and the narrow scan XPS spectra of the LaCo0.9Rh0.1O3, LaCo0.7Rh0.3O3, and LaCo0.3Rh0.7O3 catalysts are shown in Fig. 4. The comparative XPS wide spectra of the LaCo1-xRhxO3 catalysts with x = 0.1, 0.3, and 0.7, as illustrated in Fig. 4a reveal the presence of La 3d, Co 2p, Rh 3d, and O 1s peaks. Moreover, a C 1s peak due to carbon surface contamination is regularly present. Fig. 4(b–d) shows the O 1s deconvoluted core spectra of LaCo0.9Rh0.1O3, LaCo0.7Rh0.3O3, and LaCo0.3Rh0.7O3 catalysts, whereas Table 2 tabulates the binding energies (BE) of O 1s deconvoluted peaks and oxygen vacancy ratios for the LaCo1-xRhxO3 catalysts with x = 0.1, 0.3 and 0.7. In the asymmetrical O 1s, the core-level spectrum of the LaCo0.9Rh0.1O3 catalyst (Fig. 4b) can be deconvoluted and assigned into two peaks of lattice O2− anion (1st peak) and O1−/Ochem (2nd peak), presumably because the quantity of chemisorbed oxygen species is too little. However, the asymmetrical O1s core-level spectra in Fig. 4c–d disclose the existence of a peak (3rd peak) at lower BE that can be assigned to the lattice oxygen of O2− anion of, while the peak in the middle is related to the surface O1− ions (2nd peak), and the peak 3 in at higher energy is related to the oxygen surface chemisorbed species (Ochem) (Dupin et al., 2000; Lemoine et al., 2019).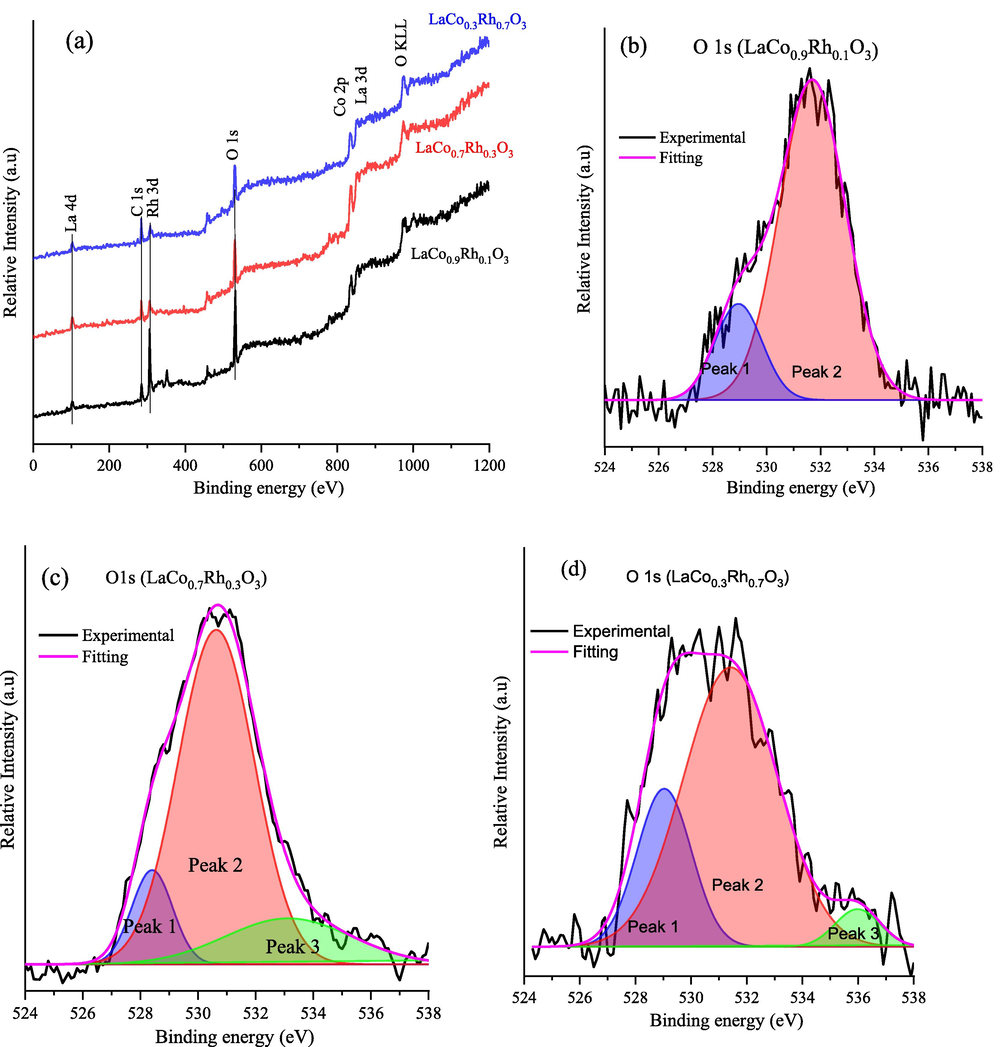
Surface and chemical composition of LaCo1-xRhxO3 catalysts. (a) Comparative XPS survey spectra of LaCo1-xRhxO3 catalysts at x = 0.1, 0.3, and 0.7 and the core-level of O 1s spectra of (b) LaCo0.9Rh0.1O3, (c) LaCo0.7Rh0.3O3, and (d) LaCo0.3Rh0.7O3 catalysts.
Catalyst
Peak BE (O2−)/eV
Peak 1 area %
Peak 2 (O1−)/eV
Peak area %
Peak 3 (Ochem)/eV
Peak 3 area %
[O1−/ O2−]
[O1−+ Ochem/ O2−]
LaCo0.9Rh0.1O3
528.96
17.78
531.70
82.22
–
–
4.62
3.25
LaCo0.7Rh0.3O3
528.4
10.9
530.64
72.60
533.13
16.45
6.66
8.17
LaCo0.3Rh0.7O3
529.0
23.50
531.42
71.91
535.98
4.58
3.06
3.25
From the data in Table 2 and with an increasing ratio of rhodium, the overall peak BE of the LaCo0.7Rh0.3O3 catalyst is shifted to lower BE compared to LaCo0.9Rh0.1O3, which indicates weaker Co—O bonding because the withdrawal of the electron density away from the Co–atom, consequently enhancing the reactivity of lattice oxygen (O2−) and decreasing the energy required for O2− activation.32,33 Besides, the O 1s deconvoluted peak area ratios of [O1−/O2−] as well as [(O1− + Ochem)/O2−]/O2−] were much higher in the LaCo0.7Rh0.3O3 catalyst (6.66, 8.17) than for the LaCo0.3Rh0.7O3 (4.62, 3.25) and the LaCo0.9Rh0.1O3 (6.66, 8.17) catalysts.
Finally, the doping of Rh ions into LaCo1-xRhxO3 at x = 0.3 enriched the O1− and Ochem site concentration, which is directly associated with the surface oxygen vacancies and defects (Dupin et al., 2000; Lemoine et al., 2019). It has been widely reported that creating more oxygen vacancy concentration within the perovskite structure via doping/modifying the B site significantly enhances the catalytic activity by increasing the Co 3d/O 2p bands overlapping, B—O bond covalency, and rate of lattice oxygen diffusion which contributes to the surface (Pan et al., 2020; Sun et al., 2021). In addition, the deconvoluted XPS core spectra of rhodium (Rh 3d) shown in Fig. S1a revealed the presence of Rh 3d5/2 and Rh 3d3/2 levels peaks located at binding energies of 306.26 and 310.24 eV, respectively. Fig. S1b shows the deconvoluted core XPS spectra of the Co 2p zone which compromised multi-peaks that related to the Co 2p3/2 and Co 2p1/2 levels and extended over the 740 to 800 eV binding energy values. The XPS spectra of Co 2p are complex presumably due to the presence of satellite peaks and different cobalt oxidation states (Co2+ and Co3+) that coordinate to a different number of oxygen atoms.
3.2 Electrochemical performance of LaCo1-xRhxO3 catalysts towards the OER
To investigate the role of Rh doping and generated oxygen vacancies on the electrochemical activity of the LaCo1-xRhxO3 catalysts towards the electrochemical OER, CV surveys were performed in an N2-saturated 1.0 M KOH solution within the OER potential range. Fig. 5a shows the comparative linear sweep voltammograms (LSV) at 50 mV s−1 while Fig. 5b shows a magnified LSV at a current of 10 mA/cm2 for the LaCo1-xRhxO3 catalysts with different Rh content in 1.0 M KOH solution. Fig. 5c shows a plot of Rh content (x values) and the onset potential (ηonset, extracted at 1.0 mA/cm2) and the overpotential needed to achieve 10 mA/cm2 (η10 mA/cm2) for the LaCo1-xRhxO3 catalysts while Fig. 5d shows the corresponding OER Tafel slopes of the studied LaCo1-xRhxO3 catalysts as obtained from LSV data in Fig. 5a. Moreover, Table 3 shows the overall characteristic catalyst electrochemical parameters of the onset potential ((ηonset), the overpotential at the current density of 10 mA/cm2 (η10 mA/cm2), and the Tafel slope of the LaCo1-xRhxO3 catalysts. The results revealed that the LaCo1-xRhxO3 catalyst electrodes exhibit the typical electrochemical features of OER of water electrolysis. Among the studied electrocatalysts, the LaCo0.7Rh0.3O3 electrode with x = 0.3 was the most active electrocatalyst. Fig. 5c shows an inverted volcanic plot where the OER onset potential (ηonset) and similarly the (η10 mA/cm2) gradually decrease as the Rh content increases, reaching minimum values of 1.38 and 1.53 V vs HRE, respectively, at Rhx = 0.3, indicating the superior intrinsic catalytic activity of the LaCo0.7Rh0.3O3 catalyst towards the OER. The results in Fig. 5 and Table 2 prove that the OER electrocatalytic activity of the LaCo1-xRhxO3 electrodes mainly depends on the co-doping level of Rh atoms and the LaCo0.7Rh0.3O3 electrode (x = 0.3) is the most active electrode with an oxygen evolution catalyst mass activity of 290.8 A/cm2 g as measured from the polarization curves in Fig. 5a, which is much higher than those for the other studied electrodes (Table 3).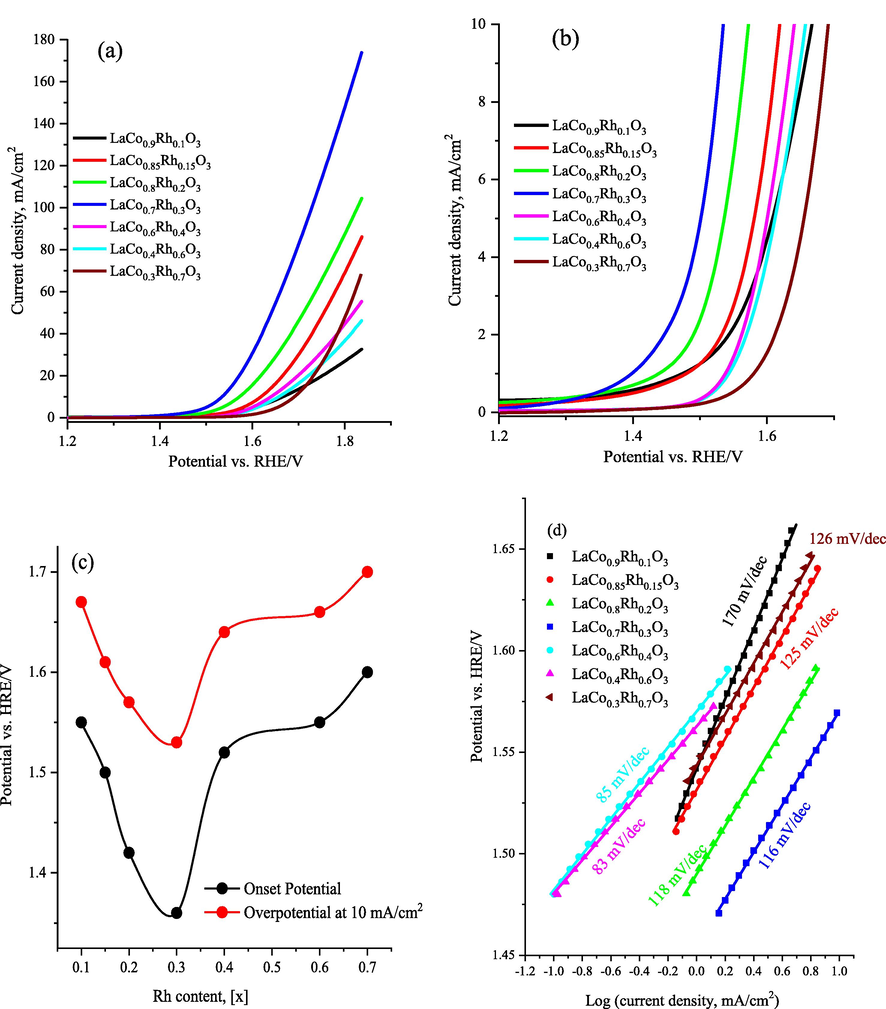
(a) linear sweep voltammetry (LSV) within the potential range of the OER at 5 mV/s and in 1.0 M KOH of the Rh substitution LaCo1-xRhxO3 catalysts (x = 0.1, 0.15, 0.2, 0.3, 0.4, 0.6, 0.7) loaded carbon paper substrate, (b) magnification of the LSV (a) showing the OER onset potential and the overpotential at 10 mA/cm2, (c) the plot of Rh content (x values) and the onset potential (extracted at 1.0 mA/cm2) and the overpotential required to achieve a current density of 10 mA/cm2 of the LaCo1-xRhxO3 catalysts, (d) Tafel plots obtained by fitting the LSV data to the Tafel equation of the Rh substitution LaCo1-xRhxO3 catalysts.
Rh ratio (x)
OER onset potential/ V
η OER @10 mA/cm2
OER Tafel slope mV/dec
Mass activity @1.6 V (A/cm2 g)
Ref.
0.1
1.55
1.67
170
14.50
This work
0.15
1.50
1.67
125
25.55
This work
0.2
1.42
1.57
118
52.30
This work
0.3
1.38
1.53
116
104.20
This work
0.4
1.52
1.64
85
17.60
This work
0.6
1.54
1.66
83
14.53
This work
0.7
1.59
1.70
126
5.60
This work
LaCo0.7Rh0.3O3 (0.1 M KOH)
1.50
1.58
150
33.50
This work
LaCoO3 (microparticles)
1.58
1.70
92
∼35.0
(Shen et al., 2022)
LaCoO3 (dense, porous, hollow particles)
(1.58, 1.54, 1.52)
(1.66, 1.61, 1.58)
(76, 74, 72)
(∼10.0, ∼37.5, ∼67.5)
(Kim et al., 2017)
The water oxidation kinetics of the LaCo1-xRhxO3 electrodes were observed by Tafel plots obtained by fitting the LSV data to the Tafel equation (Tüysüz et al., 2013). Fig. 5d shows the Tafel slope of the LaCo1-xRhxO3 electrodes during OER their values are also reported in Table 3.We can note that as the Rh content increases, the Tafel slope shifts towards lower values and reach ∼ 116 mV/dec for LaCo0.7Rh0.3O3 electrode which indicates which is indicative of the facile surface kinetics and enhanced charge-transfer rate for the OER due to the increased oxygen vacancy content. Moreover, the mass activities of the LaCo1-xRhxO3 catalysts at 1.8 V vs HRE presented in Table 3 reveal significant enhancement from 14.50 to 104.20 mA/cm2 g upon the increase of the Rh content (x) from 0.1 to 0.3 respectively. It is worth noting that the OER mass activity and onset potential achieved by the LaCo0.7Rh0.3O3 electrode are higher than those previously reported lanthanum-based perovskite catalysts (Shen et al., 2022; Lu et al., 2019; Dias et al., 2020; Kim et al., 2017).
The charge carrier transfer resistance during the OER for the Rh-substituted LaCo1-xRhxO3 catalysts was analyzed by electrochemical impedance spectroscopy (EIS). Fig. 6a shows typical Nyquist plots for LaCo1-xRhxO3 supported on a CP electrode in 1.0 M KOH electrolyte at 1.65 V vs HRE. Moreover, the corresponding fitted equivalent circuit is provided in Fig. 6a (inset) which matches the solution resistance (Rs), double-layer capacitance (Q1), and charge-transfer resistance (R1). Notably, the results in Fig. 6a revealed the radii of the arc in the impedance plots of LaCo0.7Rh0.3O3 are lower than those of the other electrodes with different Rh contents. Furthermore, the result indicates that the LaCo0.7Rh0.3O3 electrode exhibits faster charge-transfer kinetics and highly effective electrocatalyst for OER. Table 4 reports the impedance parameters of the fitted equivalent circuit and LaCo0.7Rh0.3O3 shows the lowest R1 value of 12.32 O, signifying lower resistance for charge-transfer and, thereby, fast electrode kinetics, agreeing with its superior intrinsic activity toward OER.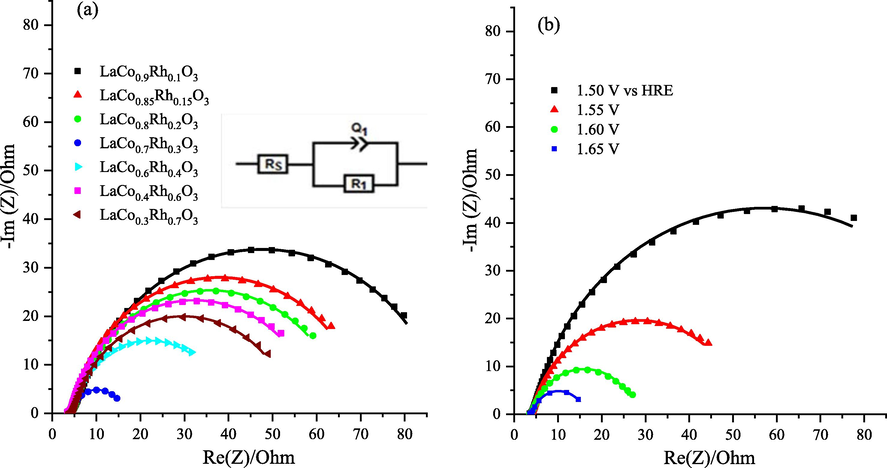
(a) EIS Nyquist diagram and equivalent circuit of the LaCo1-xRhxO3 catalysts in 1.0 M KOH electrolyte measured at 1.65 V vs HREand within the frequency region of 0.01 to 105 Hz with a 20 mV amplitude. The insets are the fitted equivalent circuit (b) EIS Nyquist plots and equivalent circuit for the LaCo0.7Rh0.3O3 at the different applied potential within the frequency region of 0.01 to 105 Hz and a 20 mV amplitude in 1.0 M KOH. The solid lines are the fitted curves, and the symbols represent the experimental data.
Catalysts
Rs(ohm)
Q1 mF.s(a−1)
R1 (ohm)
LaCo0.9Rh0.1O3
3.93
8.21
83.10
LaCo0.85Rh0.15O3
3.36
9.28
66.38
LaCo0.8Rh0.2O3
4.48
6.08
61.50
LaCo0.7Rh0.3O3
3.58
2.34
12.32
LaCo0.6Rh0.4O3
3.93
6.02
34.96
LaCo0.3Rh0.7O3
3.42
1.26
56.34
In addition, the resistance for charge transfer of the most active catalyst LaCo0.7Rh0.3O3 was examined at different applied voltages by EIS analysis. Typically, Fig. 6b shows the Nyquist plots acquired for the LaCo0.7Rh0.3O3 catalyst at 20 mV amplitude and from 0.01 to 105 Hz frequency range. The radii of the arc for the LaCo0.7Rh0.3O3 electrode decrease with increasing applied potential from 1.50 to 1.65, demonstrating that the resistance for the charge transfer of the LaCo0.7Rh0.3O3 catalyst decreases with increasing applied potential, showing superior OER features.
Moreover, Table 5 tabulates the EIS parameters of solution resistance (Rs), double-layer capacitance (Q1), and charge-transfer resistance (R1) resulting from the equivalent fitted circuit (Fig. 6a inset) at various applied potentials of the LaCo0.3Rh0.7O3 electrode. The results indicated that R1 considerably declined from 105.7 to 12.32 O by increasing the applied potential from 1.51 to 1.64 V, approving the enrichment of the charge transfer and faster OER kinetics at the LaCo0.7Rh0.3O3 catalyst.
Potential (V vs HRE)
Rs(ohm)
Q1 mF.s(a−1)
R1 (ohm)
1.50
3.51
2.45
105.7
1.55
3.57
2.40
51.65
1.60
3.54
4.56
23.70
1.65
3.58
2.34
12.32
The electrocatalytic features of the LaCo0.7Rh0.3O3 electrode during the OER in 1.0 M KOH were further improved by studying the electrolyte concentration, catalyst loading effect, and long-term durability at a predefined current density or constant potential. Fig. S2a (Supporting Information) displays the LSV at 50 mV s−1 for LaCo0.3Rh0.7O3/CP electrodes with different catalyst loadings. Moreover, Fig. S2b shows the plot of the relationship of catalyst loading against the potential at 10 mA/cm2 (black line) in addition to the current density attained at 1.8 V vs HRE (red line) for the OER. Apparently, LaCo0.3Rh0.7O3 loading of around 0.3 mg/cm2 was found to give superior OER activity (Fig. S2b) where the minimum potential at 10 mA/cm2 and the highest current density at 1.8 V vs RHE were achieved. Further, Fig. S2c elucidates the LSV profiles at 50 mV/s of 0.3 mg/cm2 LaCo0.7Rh0.3O3 catalyst upon increasing KOH concentration (0.1 – 5.0 M), which revealed a decrease in the onset potential as well as an enhancement of the OER current density at more positive potential. This behavior could be attributed to the Nernst effect and OH− ion concentration increment at the catalyst–electrolyte interface respectively which signifies the OER enhancement at LaCo0.3Rh0.7O3 in concentrated alkaline media.
The results in Fig. 7 display the long-term chronoamperometry (current–time) and chronopotentiometry (potential-time) profiles at a predefined applied voltage and current density through the continued OER using the LaCo0.7Rh0.3O3 electrode in 1.0 M KOH solution. The LaCo0.7Rh0.3O3 electrode shows long-term electrochemical durability with a reasonably stable current and potential during the electrochemical water oxidation process (Fig. 7a). Furthermore, the capability of the LaCo0.3Rh0.7O3 electrode to operate at different current densities was investigated (Fig. 7b). The electrodes exhibited a potential of 1.54 V vs RHE to attain a current density of 10 mA/cm2 required for 10 % solar to hydrogen production efficiency, outperforming the benchmarked catalysts prepared by other approaches.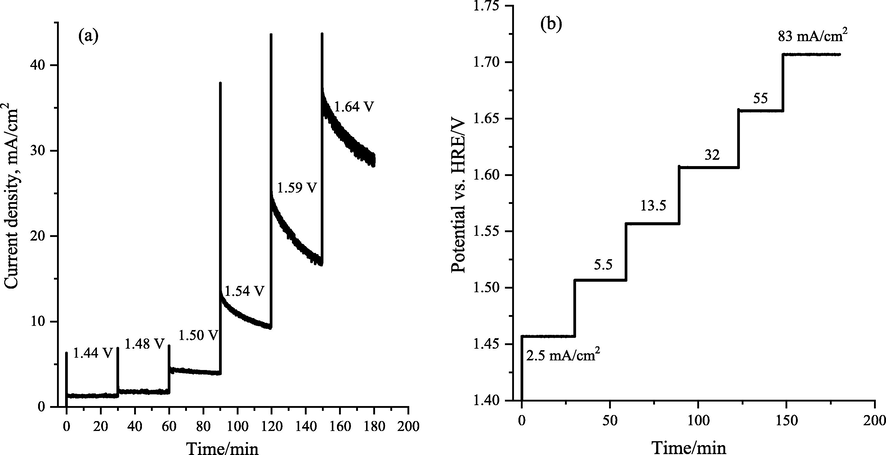
Electrochemical durability tests of the LaCo0.7Rh0.3O3 catalyst (loading 0.3 mg/cm2) during OER in 1.0 M KOH, (a) Chronoamperometric response at different applied voltage steps, and (b) chronopotentiometry response of the catalyst at a different applied current density during continued oxygen evolution reaction.
3.3 Electrochemical performance of the LaCo1-xRhxO3 catalysts for the ORR
The ORR electrocatalytic features of the LaCo1-xRhxO3 catalysts were determined via CV in a 1.0 M KOH solution. Fig. 8a shows the CV for the LaCo0.7Rh0.3O3 (0.3 mg/cm2) catalyst examined in N2– and O2-saturated 1.0 M KOH solutions using a glassy carbon electrode (GC, 3.0 mm diameter). As shown in Fig. 8a, the CV curves display a characteristic ORR peak in the O2-saturated medium, which completely vanishes in the N2-saturated KOH solution. Besides, the onset potential of oxygen reduction is at 0.76 V vs HRE, and a peak current of −0.2 mA/cm2 was obtained, which proves the enhancement of ORR at LaCo0.7Rh0.3O3 catalyst in alkaline solution. In addition, Fig. 8b presents comparative LSV curves for ORR activity acquired using a CP electrode loaded with the LaCo1-xRhxO3 catalysts with different Rh contents in a 1.0 M KOH solution. In addition, Fig. 8d and Table 6 summarize the overall characteristic catalyst electrochemical parameters of the ORR onset potential, the overpotential at a current density of 10 mA/cm2, Tafel slope, and ΔE (the potential gap between the OER and ORR onset potentials) of the LaCo1-xRhxO3 catalysts. The results show that the LaCo0.7Rh0.3O3 electrode with x = 0.3 is the most effective electrocatalyst for the ORR. The onset potential of ORR, as well as the potential at 10 mA/cm2 ((10 mA/cm2), have shifted to more positive values of 0.73 and 0.64 V vs HRE, respectively, at Rh (x) = 0.3, indicating the superior catalytic activity of the LaCo0.7Rh0.3O3 catalyst towards the ORR. Moreover, the OER/ORR overpotential gap (ΔE) of the LaCo0.7Rh0.3O3 catalyst is about 0.65 V, which is considerably lower than that of the other investigated Rh-doped LaCo1-xRhxO3 catalysts. as well as that of the reference analog hybrids.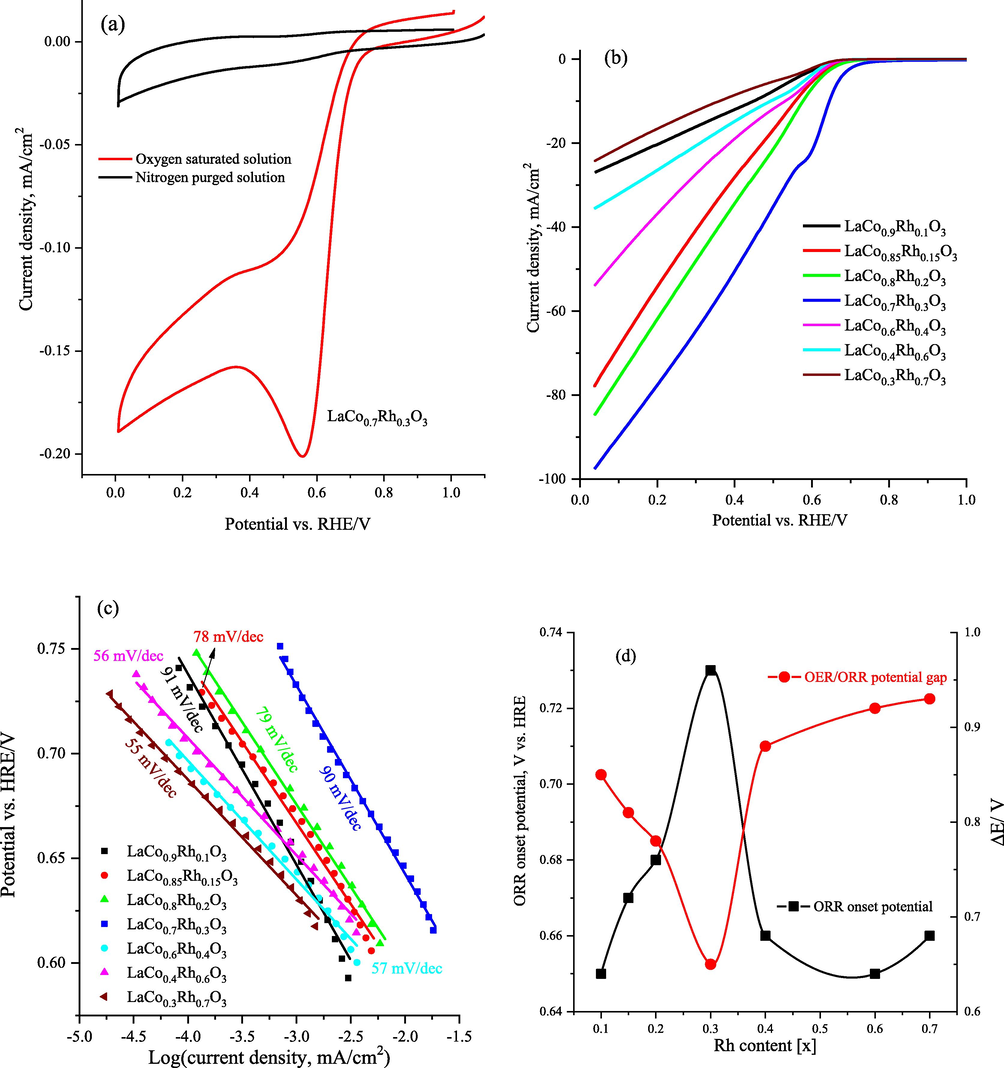
(a) cyclic voltammetry curves at a scan rate of 10 mV/s of LaCo0.7Rh0.3O3 catalyst loaded on glassy carbon electrode in 1.0 M KOH solution saturated with N2 (black line) or oxygen (red line) at a scan rate of 10 mV/s,(b) cathodic-going linear sweep voltammograms within the ORR potential window at 10 mV/s of LaCo1-xRhxO3 catalysts loaded on carbon paper electrode in 1.0 M KOH solution, (c) the corresponding Tafel plots obtained by fitting the LSV data to the Tafel equation of the Rh doped LaCo1-xRhxO3 catalysts, (d) plot of the relationship of the Rh content and ORR onset potential and OER/ORR potential gap.
Rh ratio (x)
ORR onset potential/ V
η ORR at 10 mA/cm2
ORR Tafel slope mV/dec
ΔE (OER-ORR)/V
0.1
0.65
–
91
0.85
0.15
0.67
0.56
78
0.81
0.2
0.68
0.57
79
0.78
0.3
0.73
0.64
90
0.65
0.4
0.66
0.53
57
0.88
0.6
0.65
0.49
56
0.92
0.7
0.66
–
55
0.93
To verify the ORR catalytic pathways for the LaCo0.7Rh0.3O3 catalyst, we adopted an RRDE technique to screen the formation of hydrogen peroxide species (H2O2) during the ORR process. Fig. 9a displays the cathodic LSV plots logged at 2000 rpm in O2-saturated electrolyte at LaCo0.7Rh0.3O3 catalyst using the disk (lower part) and ring electrodes (upper part). For both the disk and ring electrodes, the ORR onset potentials commence at 0.7 V vs HRE, and the wave of 0.2 and 0.025 mA cm−2 is achieved at 0.54 V vs HRE, revealing the oxygen reduction at disc LaCo0.7Rh0.3O3 and peroxide species oxidation at platinum ring electrodes respectively.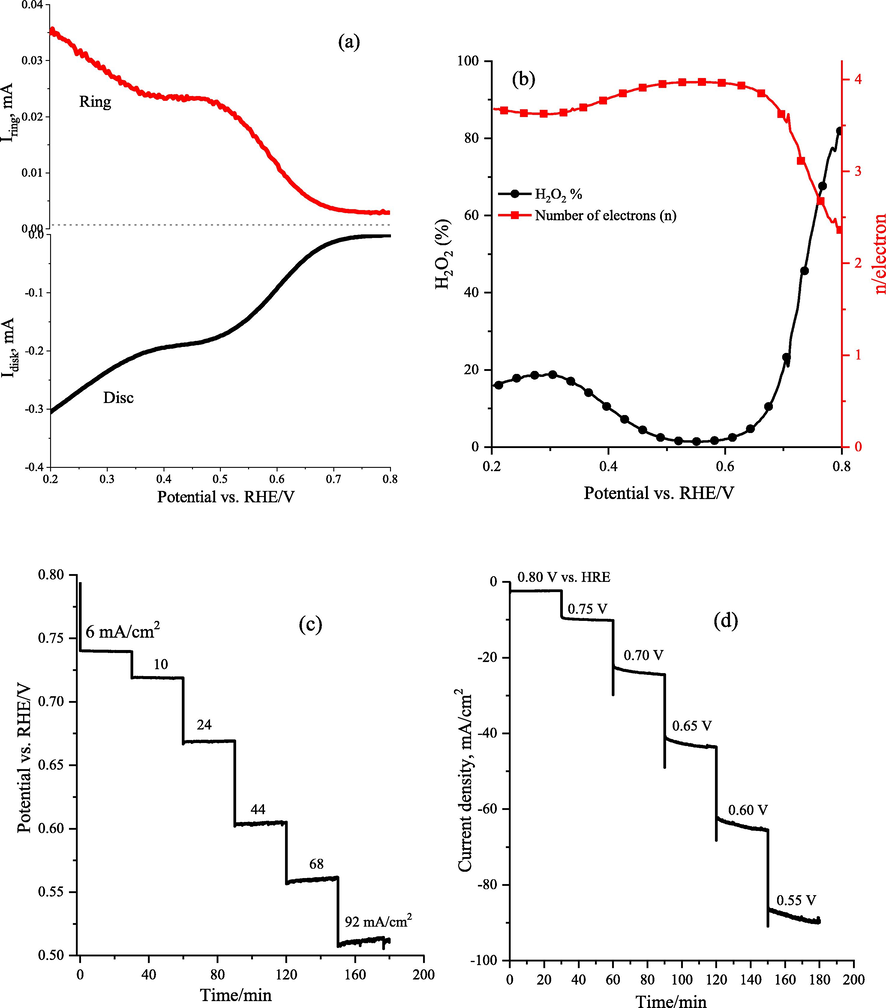
(a) Rotating ring-disc electrode (RRDE) polarization curves of LaCo0.7Rh0.3O3 electrode at 2000 rpm in O2 saturated medium with the ring current (upper region), disc current (lower region), and current corresponding to hydrogen peroxide obtained from the ring current, (b) the corresponding average electron transfer number (n) and H2O2 production rate as a function of the applied potential,(c) chronopotentiometry at different applied current densities, and (d) chronoamperometry at the different applied potential for theLaCo0.7Rh0.3O3 electrode in 1.0 M KOH electrolyte.
Through calculation, Fig. 9b displays the average electron transfer number (n) for the LaCo0.7Rh0.3O3 electrode in the potential range from 0.7 to 0.2 V HRE, which was 3.9, indicating that the ORR involves a four-electron pathway for the LaCo0.7Rh0.3O3 electrode. Moreover, the H2O2 production rate calculated from the ring current at the LaCo0.7Rh0.3O3 electrode was around 5–10 % in the potential range of 0.7–0.2 VRHE (Fig. 9b), which indicates the minimum production rate of hydrogen peroxide and confirms that a high selective 4e– ORR process was achieved.
Finally, Fig. 9c and 9d show the long-term stability of the LaCo0.7Rh0.3O3 electrode towards the ORR as evaluated through chronoamperometric/chronopotentiometry measurements in O2-saturated 1.0 M KOH solution. The LaCo0.3Rh0.7O3 catalyst exhibited stable behavior during the ORR, with no substantial change of the measured potential or current density throughout the measurement for up to 3 h, as shown in Fig. 9c and 9d. The highest current density of 92 mA/cm2 was achieved for LaCo0.7Rh0.3O3 with 0.30 mg/cm2 loading at 0.4 V vs HRE.
4 Conclusions
We have validated the fabrication of highly efficient bifunctional OER/ORR catalysts of rhodium-doped LaCo1-xRhxO3 (LCRO, 0.1 ≤ x ≤ 0.70) perovskite-based oxides via solid-state reactions. A range of characterization methods revealed the effect of the Rh doping level on the LaCo1-xRhxO3 surface morphology, oxygen deficiencies, and electrocatalytic activities of the studied LaCo1-xRhxO3 catalysts towards the OER and ORR in alkaline solution. It was established that the LaCo0.7Rh0.3O3 hybrid with x ∼ 0.3 doping is one of the rare and highly active bifunctional electrocatalysts for OER/ORR. The LaCo0.7Rh0.3O3 catalysts with x ∼ 0.3 doping revealed exceptional OER/ORR bifunctional electrocatalytic activities, low onset potentials (1.38 V OER and 0.73 V ORR), an efficient four-electron ORR pathway, and a small OER/ORR onset potential gap (ΔE) of 0.65 V, which is considerably superior to the reported lanthanum-based perovskite catalysts. The enhanced electrochemical OER and ORR activities of the LaCo0.7Rh0.3O3 catalyst over the other Rh doping ratios can be rationalized due to the lattice expansion, higher oxygen vacancies, and enhanced B—O bond covalency, which increases the active sites and the electrical conductivity of the LaCo1-xRhxO3 catalysts.
Acknowledgments
This project was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdelaziz City for Science and Technology (KACST), Kingdom of Saudi Arabia, Award Number 13-ENE-1227-02.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The electrocatalysis of oxygen evolution on perovskites. J. Electrochem. Soc.. 1984;131(2):290-302.
- [CrossRef] [Google Scholar]
- Oxygen reduction at carbon-supported lanthanides: the role of the B-site. ChemElectroChem. 2015;3:283-291.
- [Google Scholar]
- Effects of oxygen mobility in la–Fe-based perovskites on the catalytic activity and selectivity of methane oxidation. ACS Catal.. 2020;10(6):3707-3719.
- [CrossRef] [Google Scholar]
- A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci.. 2011;4(9):3167-3192.
- [CrossRef] [Google Scholar]
- Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem.. 2013;125(9):2534-2537.
- [CrossRef] [Google Scholar]
- Waste to wealth: Defect-rich Ni-incorporated spent LiFePO4 for efficient oxygen evolution reaction. Sci China Mater. 2021;64(11):2710-2718.
- [Google Scholar]
- Electrocatalyst approaches and challenges for automotive fuel cells. Nature. 2012;486(7401):43-51.
- [CrossRef] [Google Scholar]
- Lanthanum-based perovskites for catalytic oxygen evolution reaction. ChemElectroChem. 2020;7(15):3173-3192.
- [CrossRef] [Google Scholar]
- Materials engineering in perovskite for optimized oxygen evolution electrocatalysis in alkaline conditions. Small. 2021;17(2):e2006638.
- [Google Scholar]
- [High-Temperature Shock Enabled Nanomanufacturing for Energy-Related Applications. Adv. Energy Mater.. 2020;10:2001331.
- [Google Scholar]
- Systematic XPS studies of metal oxides, hydroxides, and peroxides. PCCP. 2000;2(6):1319-1324.
- [CrossRef] [Google Scholar]
- Cobalt-based hydroxide nanoparticles@N-doping carbonic frameworks core-shell structures as highly efficient bifunctional electrocatalysts for oxygen evolution and oxygen reduction reactions. Nano Res.. 2018;11(3):1482-1489.
- [CrossRef] [Google Scholar]
- Efficient bi-functional electrocatalysts of strontium iron oxy-halides for oxygen evolution and reduction reactions in alkaline media. J. Electrochem. Soc.. 2016;163(6):H450-H458.
- [CrossRef] [Google Scholar]
- Halide-doping effect of strontium cobalt oxide electrocatalyst and the induced activity for oxygen evolution in an alkaline solution. Catalysts. 2021;11(11)
- [CrossRef] [Google Scholar]
- Porous Perovskite-Type Lanthanum Cobaltite as Electrocatalysts toward Oxygen Evolution Reaction. ACS Sustainable Chem. Eng.. 2017;5:10910-10917.
- [CrossRef] [Google Scholar]
- Functional role of Fe-doping in co-based perovskite oxide catalysts for oxygen evolution reaction. J. American Chem. Soc.. 2019;141(13):5231-5240.
- [CrossRef] [Google Scholar]
- The oxygen evolution on La0.5Ba0.5CoO3. J. Electroanal. Chem. Interfacial Electrochem.. 1981;126(1–3):221-240.
- [CrossRef] [Google Scholar]
- Mini review on H2 production from electrochemical water splitting according to special nanostructured morphology of electrocatalysts. Fuel. 2022;308:122048
- [CrossRef] [Google Scholar]
- Investigation of mixed-metal (oxy)fluorides as a new class of water oxidation electrocatalysts. Chem. Sci.. 2019;10(40):9209-9218.
- [CrossRef] [Google Scholar]
- Recent advances in anion-doped metal oxides for catalytic applications. J. Mater. Chem. A. 2019;7(13):7280-7300.
- [CrossRef] [Google Scholar]
- Engineering oxygen vacancies into LaCoO3 perovskite for efficient electrocatalytic oxygen evolution. ACS Sustainable Chem. Eng.. 2019;7(3):2906-2910.
- [CrossRef] [Google Scholar]
- Advanced alkaline water electrolysis. Electrochim. Acta. 2012;82:384-391.
- [CrossRef] [Google Scholar]
- Electrochemical properties of the single crystal La0.7Pb0.3MnO3 electrode. Electrochim. Acta. 1980;25(5):585-589.
- [CrossRef] [Google Scholar]
- Catalytic activity for electrochemical reduction of oxygen of lanthanum nickel oxide and related oxides. J. Electroanal. Chem. Interfacial Electrochem.. 1977;79(2):319-326.
- [CrossRef] [Google Scholar]
- Water electrolysis on La(1–x)Sr(x)CoO(3-δ) perovskite electrocatalysts. Nat. Commun.. 2016;7:11053.
- [CrossRef] [Google Scholar]
- Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun.. 2020;11(1):2002.
- [CrossRef] [Google Scholar]
- Enhanced bifunctional oxygen catalysis in strained LaNiO3 perovskites. J. Am. Chem. Soc.. 2016;138(8):2488-2491.
- [CrossRef] [Google Scholar]
- Cobalt oxide nanocubes interleaved reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction in an alkaline medium. Electrochim. Acta. 2017;237:61-68.
- [CrossRef] [Google Scholar]
- Correlating the electronic structure of perovskite La1−xSrxCoO3 with activity for the oxygen evolution reaction: The critical role of Co 3-d hole state. J. Energy Chem.. 2022;65:637-645.
- [CrossRef] [Google Scholar]
- A bifunctional perovskite oxide catalyst: The triggered oxygen reduction/evolution electrocatalysis by moderated Mn-Ni co-doping. J. Energy Chem.. 2021;54:217-224.
- [CrossRef] [Google Scholar]
- A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science. 2011;334(6061):1383-1385.
- [CrossRef] [Google Scholar]
- Advances in designing perovskite catalysts. Curr. Opin. Solid State Mater. Sci.. 2001;5(5):381-387.
- [CrossRef] [Google Scholar]
- Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res.. 2013;6(1):47-54.
- [CrossRef] [Google Scholar]
- Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater.. 2016;28(2):215-230.
- [CrossRef] [Google Scholar]
- A carbon-nanotube-supported graphene-rich non-precious metal oxygen reduction catalyst with enhanced performance durability. Chem. Commun.. 2013;49(32):3291-3293.
- [CrossRef] [Google Scholar]
- Earth-abundant nanomaterials for oxygen reduction. Angew. Chem.. 2016;55(8):2650-2676.
- [CrossRef] [Google Scholar]
- Ruddlesden-Popper perovskites in electrocatalysis. Mater. Horizons. 2020;7(10):2519-2565.
- [CrossRef] [Google Scholar]
- Attenuating a metal-oxygen bond of a double perovskite oxide: Via anion doping to enhance its catalytic activity for the oxygen reduction reaction. J. Mater. Chem. A. 2020;8(28):14091-14098.
- [CrossRef] [Google Scholar]
- Recent Advances of lanthanum-based perovskite oxides for catalysis. ACS Catal.. 2015;5(11):6370-6385.
- [CrossRef] [Google Scholar]
- Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem. Mater.. 2016;28(6):1691-1697.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104256.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







