Translate this page into:
Nucleophilic modification of flavonoids for enhanced solubility and photostability towards uniform colouration, bio-activation and ultraviolet-proof finishing of silk fabric
⁎Corresponding author at: College of Textile and Clothing Engineering, Soochow University, China. yuyangzhou@suda.edu.cn (Yuyang Zhou) zhouyuyang_suda@hotmail.com (Yuyang Zhou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study targets on the molecular-decoration of flavonoids (FLs) with a reactive ultraviolet absorber (UV-ABS) to enhance their low-solubility and photo-sensitivity, further serving for uniform and ultraviolet (UV)-proof treatment of silk. Quercetin (QUE) and rutin (RUT) showing structural discrepancy in disaccharide moiety are compared. The chemical structures of modified flavonoids (MOD-FLs), adsorption behaviour/mechanism of MOD-FLs on silk, and the colour features and functionalities of treated silk were explored. Results reveal that the absorbance of FLs in water significantly increases after modification indicating the enhanced solubility, which is reconfirmed by the water/n-butanol phase separation experiment. Two intense adsorption bands of MOD-FLs in UVA/UVB range imply their potential use as UV-proof finisher for textile. The exhaustion of MOD-FLs on silk gradually descends as pH increases. MOD-FLs display a closer trend to Psuedo 2nd-order kinetic model than FLs indicating the dominance of chemical adsorption due to the ionic nature of UV-ABS part. MOD-QUE show superior building-up property than MOD-RUT on silk, which is beneficial to achieving diverse intensity of colour and functionality on silk. Fewer aggregates of MOD-FLs are observed on silk surface than FLs. After modification, the colour fading of QUE and RUT treated silk after 8 h light exposure significantly reduce by 71.96% and 42.91%, respectively. MOD-FLs treated silk disinfect over 80 % of E. coli and S. aureus. However, a significant antioxidant decrease of FLs occurs after modification. MOD-FLs (5% owf) imparts silk with high UPF values (50+). In general, the strategy for enhancing the water solubility and photostability of FLs is developed to better serve as bio-based modifiers for textile which is also transferable to the modification of other natural molecules.
Keywords
Flavonoid
Natural extract
Nucleophilic reaction
Eco-textile
UV protective
Silk
1 Introduction
The growing concern on the health, hygienic and protective aspects of textile among customers, is driving textile manufactures to explore the ‘green’ alternatives to synthetic dyes and functional reagents. Bio-based molecules primarily originated from plants are receiving increasing attention among various sectors including cosmetics, food, solar cells, etc.(Shahid et al., 2013, Kasiri and Safapour 2014) For textile processing, bio-based molecules are becoming preferable over synthetic chemicals, not only due to their environmental compatibility and less-to-none toxicity, but also related to the integration of the coloration and functionalization of textiles into one-bath process.(Shahid-ul-Islam et al., 2013, Zhou et al., 2015, Rather et al., 2020, Li et al., 2021, Zhang et al., 2022) And as such, the energy, water and time consumptions during conventional stepwise textile process are reduced towards a sustainable textile processing, and also in alignment with the aim for global carbon neutralization.
Silk is a significant protein-based natural polymer with a well-earned reputation as the “queen of textiles” owing to its smooth and lustrous appearance, wearing comfort, hygroscopicity, etc. Silk has also been discovered to be an appropriate material for medical usage because of its desirable mechanical property, biocompatibility, and controllable degradability.(Li et al., 2012) Nevertheless, silk textile suffers from inherent high ultraviolet (UV) transmittance, and also being a favourable media for microbial growth and propagation, leaving it inadequate for the protection of the wearers or even inducing skin diseases. Although conventional UV absorbers and antimicrobials are readily incorporated to silk textiles, recent studies confirm their shortcomings such as toxicity, poor activity, and weak washing fastness,(Alebeid and Zhao 2017, Zhang et al., 2018) falling out of the scope of eco-friendly textiles.
Flavonoids (FLs) take up the majority of plants extracts, and are famous for their defense against microbial attack. Quercetin (QUE) and rutin (RUT) are two typical flavonoid compounds (Fig. 1a), widely found in various fruits and vegetable tissues. Their unique difference is the presence of a disaccharide group located in the C-ring of RUT, whereas which is absent in QUE. Unfortunately, RUT and QUE are sparingly soluble in water (i.e. < 0.01 and 0.12 g/L at 20 °C, respectively).(Chebil et al., 2007) From the perspective of the dyeing and finishing of textiles, such defect probably results in the inhomogeneous colouraion and functionalization rising from the uncontrollable aggregation and deposition of FLs molecules on fibre surface or between fibres. Moreover, the pleiotropic functions of QUE and RUT on fibre could not be fully expressed because of their low solubility and bioavailability. To date, several modification methods such as o-methylation, glycosylation, sulfation, and acylation are able to manipulate the solubility and bioactivity of FLs,(Plaza et al., 2014, Tran et al., 2019, Yu and Bulone 2021) whereas most research are carried out within the fields of food, medicine and agriculture. It is also reported that QUE treated textiles suffer from colour degradation after periods of exposure to the xenon arc lamp.(Smith et al., 2000) Similar observation also showed that the QUE treated silk without mordant display poor light fastness. Thus apart from low solubility, how to improve the photostabilty of FLs on fibres is another pending issue.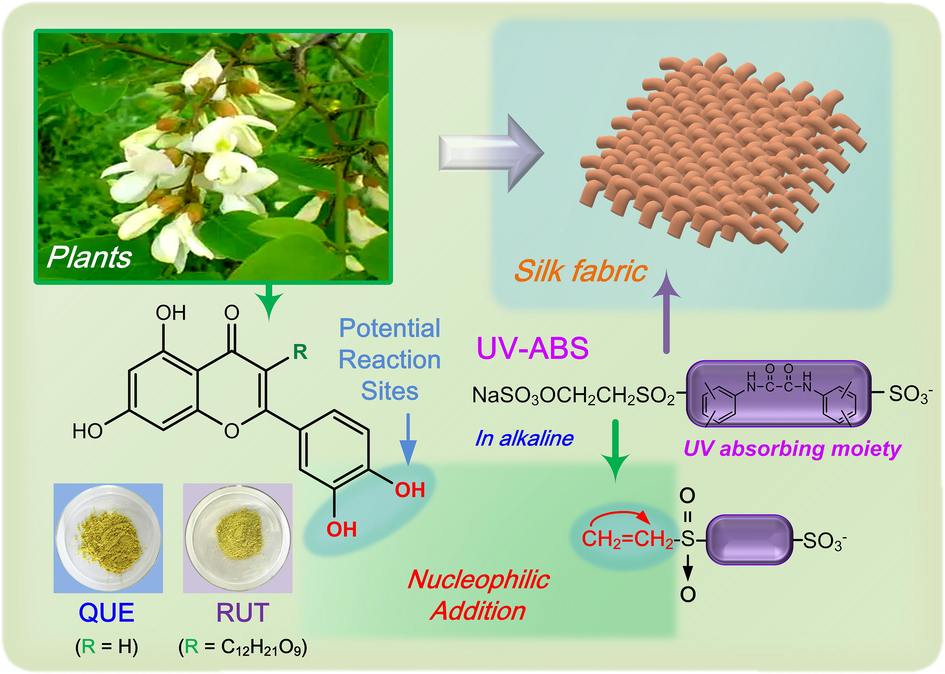
Modification of QUE and RUT with UV-ABS for silk finishing.
Several hydroxyl groups are present in the structures of QUE and RUT, especially those in B-ring, making them possible for nucleophilic modification.(Torres et al., 2012) In addition, vinylsulfone reactive dyes are able to form covalent bond with cellulosic fibre through nucleophilic addition of vinylsulfone group to hydroxyl group in alkaline medium.(El-Shishtawy et al., 2014, Youssef et al., 2014) Inspired by such mechanism, two water-soluble FLs are designed and synthesized using a commercial reactive UV absorber (UV-ABS) with vinylsulfone group (Fig. 1b) for the functionalisation of silk in this study. To date, the research related to this UV-ABS is mainly focused on the direct functionalisation of fibres.(Ibrahim et al., 2015, Kotb 2017) Less emphasis has been placed on the modification of bio-molecules for textile eco-dyeing and finishing. Our previous study confirmed the feasibility of modification of curcumin with keto-enol tautomeric structure.(Zhou and Tang 2016) However, FLs take the dominant part in the family of bio-based molecules, which makes their modification more important and in urgent needs. The chemical modification of FLs and further use for textile processing is proposed in Fig. 1. The solubility and photostability of FLs as well as the uniformity of their distribution on silk are expected to be improved simultaneously. The structures of modified quercetin (MOD-QUE) and modified rutin (MOD-RUT) were examined. The adsorption behaviours of modified flavonoids (MOD-FLs) on silk, the colour feature, bioactivity and UV shielding property of treated silk were also investigated and compared.
2 Materials and methods
2.1 Materials
Silk fabric with code of 12103 was obtained from Suzhou Jiaduoli Silk Apparel Co. ltd., China. QUE and RUT (Purity of both are 95 %) were purchased from Xi'an Qing Yue Biotechnology Co. ltd., China. An anionic UV-ABS (UV-Sun® CEL LIQ) with oxalanilide and vinylsulfone structures(Kotb 2017, Abo El-Ola and Kotb 2021) was obtained from Huntsman International LLC., USA. All the chemicals such as sodium carbonate, broth, agar, were analytical reagents.
2.2 FLs modification
QUE (1 g) and RUT (1 g) were mixed with sodium carbonate at pH of 9.6 in a boiling flask for 15 min. Then, 2.9 g and 1.4 g of UV-ABS were added dropwise to the two mixtures mentioned above to 50 mL, respectively. The mixture was subject to vigorous stirring at 50 °C for another 2 h. After reaction, a diluted HCl solution was added towards a final pH of 3 to precipitate the unreacted FLs. Then, the mixture was filtered through filter paper three times and stored as stock solution in the dark. The yields for QUE and RUT are 79 % and 73 %, respectively.
2.3 Silk treatment
In general, the silk treatment was carried out by immersing silk fabrics in the conical flasks which contains FLs and MOD-FLs, and buffer solutions. Then the conical flasks were placed in a thermal controllable shaker for customised periods. Finally, the residual solutions were subjected to evaluating the exhaustion rate. The resultant fabrics were thoroughly washed and dried in the open air for further test.
The adsorption kinetic study was processed in a homothermal shaker at constant temperature of 85 ◦C using 5 % owf (weight of chemical to fabric) of FLs and MOD-FLs. The pH was adjusted by McIlvaine buffer at 3.8. The quantities of FLs and MOD-FLs in the treatment bath were continuously monitored from beginning to 120 min. To investigate the pH impact and building up property, conventional elevating temperature process (Starting from 30 °C to 85 °C by 2 °C/min increasing rate and preserving at 85 °C for 1 h) was carried out in an oscillating dyeing machine to simulate the mass production procedure. The pH values and concentrations of FLs and MOD-FLs were as follows, (i) pH impact: pH range 2.7–6.8, concentration 5 % owf; (ii) building up property: pH 4.2, concentration range 0.5 %-11 % owf.
2.4 Measurements
2.4.1 UV–Vis spectroscopy, FTIR and HPLC-MS
The absorbance and absorption spectrum of the solution were examined by a Shimadzu UV-1800 UV–Vis spectrophotometer (Shimadzu Co. ltd., Japan). The quantities of FLs and MOD-FLs in solution were calculated from their calibration curves based on Lambert-Beer’s law. The exhaustion rate was determined using Eq. (1),
Based on previous studies(Kuramochi et al., 1998, ROTHWELL et al., 2005), an n-butanol/water phase separation experiment was also carried out to demonstrate the solubility enhancement of FLs after modification (Figure S1). 0.32 mM FLs or MOD-FLs were added to a capped glass tube holding 100 mL n-butanol/water mixture (v:v = 1:1). The mixture was then subject to vigorous shaking and sonication, and finally stood for 1 h.
The FTIR spectrum was obtained using a Nicolet 5700 FTIR spectrometer (Thermo Fisher Scientific Inc., USA) with an accumulation of 32 scans and resolution of 4 cm−1. The MOD-FLs solutions were further purified by centrifugation at 4000 r/min for 30 min. The resultant clear supernatants were used for HPLC-MS analysis under a negative-ion mode. An electrospray ionization-mass spectrometry (ESI-MS) was adopted for mass spectra using a Thermo Scientific TSQ Quantum Access Max (Thermo Fisher Scientific Inc., USA). Chromatographic separation was achieved on C18 column (250 × 2.l mm, 5 μm). The mobile phase consisted of 0.1 % formic acid (A) and 100 % ethanol (B) with a composition of 50 % A and 50 % B. The flow rate was 100 μL/min with an injection volume of 4.0 μL. The molecular weights of MOD-QUE and MOD-RUT are confirmed as m/z 484.8 (double charged ion) and m/z 638.8 (double charged ion) by HPLC-MS, respectively. (Figure S2).
2.4.2 Surface zeta potential
The fabric's surface electric potential was obtained on a SurPASS electrokinetic analyzer (Anton Paar GmbH, Austria). A couple of squared fabric (10 × 20 mm2) was stabilized in the cell chamber leaving a flowing channel in between. The current was measured using Ag/AgCl electrodes. The pH was changed in the range of 3–7 with HCl/NaOH.
2.4.3 Colour feature, uniformity and fastness
The a*/b* colour coordinates of fabric samples were evaluated using a Datacolor 600 (Datacolor Technology Co., USA). Each sample was folded twice for measurement. The colour uniformity was quantified by the standard deviation (STDEV, See Eq. (2)) of a*/b* value inspired by previous study.(Li et al., 2022) Thus, the lower value of STDEV (a*/b*) reveals the better colour uniformity.
The fastness evaluation including washing, rubbing and light exposure were carried out according to our previous studies.(Zhou et al., 2015, Zhou et al., 2020).
2.4.4 Morphology
The surface morphology of fabric was observed by a Hitachi S-4800 SEM (Hitachi High-Technologies Co., USA). Before test, the fabric was sputter coated with Au at a current of 30 mA for 30 s.
2.4.5 Functionality
The sample obtained in building up study of 2.3. Silk treatment was used. The antioxidant of the fabric sample was evaluated by ABTS+ decolorization assessment. More details were present in our previous work.(Zhou et al., 2015, Zhou et al., 2016) The antibacterial assessment was carried out according to our previously reported method.(Zhou et al., 2015, Zhou et al., 2016) The ultraviolet protection factor (UPF) and the UV transmittance of the fabrics were assessed on a Labsphere UV-1000F ultraviolet transmittance analyzer (Labsphere Inc., USA).
3 Results and discussion
3.1 Chemical structural identification
3.1.1 UV–Vis absorption spectroscopy
To explore the photo-adsorption property and solubility of FLs, MOD-FLs and UV-ABS, their UV–Vis absorption spectra both in aqueous and 80 % ethanol solutions are examined. Both QUE and RUT show extremely low absorbance (See the dashed lines in Fig. 2a) in water, indicating their quite low concentration in water solution. Undissolved FLs molecules form visible aggregates to the bottom of container (See the inserted photos). Those results demonstrate the poor solubility of QUE and RUT in water rising from the large hydrophobic phenyl rings in their chemical structures(Codorniu-Hernández et al., 2003) (See Fig. 1a). It is observed that the absorbance of RUT is slightly higher than that of QUE in water within the UV region. This is due to the presence of hydrophilic disaccharide moiety in RUT which makes it showing better solubility than QUE.(Samsonowicz and Regulska 2017) In all, the poor solubility of FLs probably leads to their inhomogeneous distribution on the fibre surface or within the fibre, thus failing in uniformly furnishing functionality and colour on final textile products.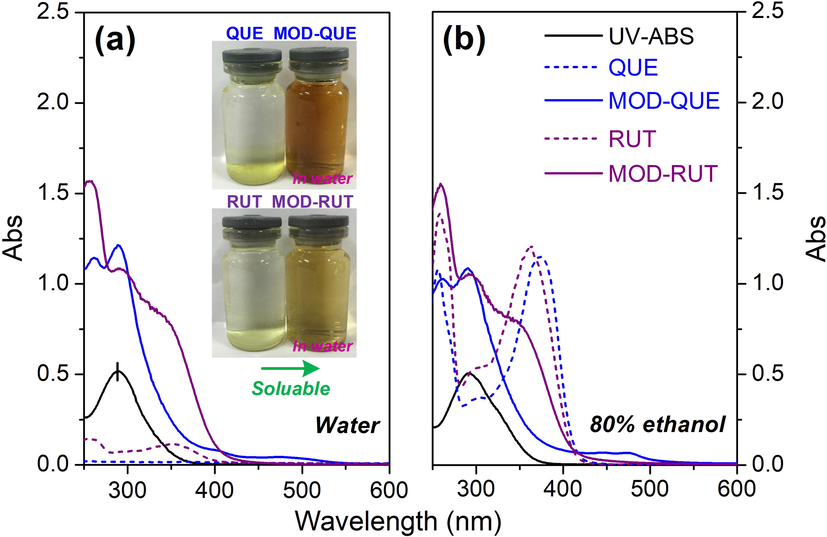
UV–Vis absorption spectra of FLs, MOD-FLs and UV-ABS: (a) in water at pH 3 and (b) in 80 % ethanol solution. (Note: The concentrations of FLs, MOD-FLs and UV-ABS are 0.05 g/L).
As seen in Fig. 2b, both QUE and RUT show much higher solubility in ethanol solution than in pure water due to the polarity of solvent.(Do et al., 2014) The spectra of FLs in 80 % ethanol display-two obvious characteristic absorption bands in the UV–Vis regions: band I (300–500 nm) and band II (200–280 nm), which are assigned to cinnamoyl and benzoyl systems, respectively.(Singh et al., 2017) The UV-ABS is well dissolved in water owing to its ionic sulfonic group and also shows a unique UV absorption peak at 290 nm. UV-ABS has no adsorption in the visible light region (400 – 800 nm), which is beneficial for the modification of FLs without undesirable colour change. Apparently, MOD-FLs in water display broad and intense peaks in the wavelength range from 250 to 400 nm. This phenomenon is attributed to the superposed peaks of UV-ABS and FLs sections of the MOD-FLs. The solutions of MOD-FLs were homogeneous and transparent with no precipitates found in the container. A phase separation experiment of MOD-FLs and FLs was also carried out to illustrate the solubility enhancement in water. As seen in Figure S1, a darker shade in water phase whereas a lighter shade in n-butanol phase, is observed which reconfirms the improvement of solubility after modification. Moreover, MOD-FLs possess two broad bands in UVA (320–380 nm) and UVB (280–320 nm) ranges, revealing their potential for UV-proof finishing silk textile. In addition, the distinctive spectra of MOD-FLs against the respective spectra of UV-ABS and FLs, indicates occurrence of chemical reaction between UV-ABS and FLs rather than their physical mixtures.
3.1.2 FTIR
QUE and RUT display similar FTIR spectra as they both belong to the FLs family (Fig. 3), however with several differences. The peak at 2938 cm-1 is the consequence of C—H stretching in the saccharide moiety of RUT. Stretching peaks at 912 and 807 cm-1 are attributed to the β-glycosidic linkages between the sugars of RUT.(Song et al., 2009, Qian et al., 2017) The peaks at 1369, 1141, 1041 and 1001 cm-1 of UV-ABS are identified as the characteristic peaks of sulfate and sulfonic groups. The peak at 1226 cm-1 is attributed to sulfone group.(Youssef et al., 2014) The bands at 1614 and 1563 cm-1 were due to the carbonyl stretching of amide I, and the N—H bending of amide II (Šijaković-Vujičić et al., 2007, Souza et al., 2012) from the oxalamide group of UV-ABS. The above observations verify the basic structure of the UV-ABS comprises of oxalanilide moiety, and sulfatoethylsulfone and sulfonic groups. The MOD-FLs show distinctive FTIR spectra compared with FLs. The intensity of the peak at 3412 cm-1 turns weaker after modification due to the decreasing number of free hydroxyl groups. The peaks at 1249 and 1049 cm-1 represent Ar–O and C—O stretching vibrations, respectively, which can be taken as an evidence of the formation of ether bond between FLs and UV-ABS.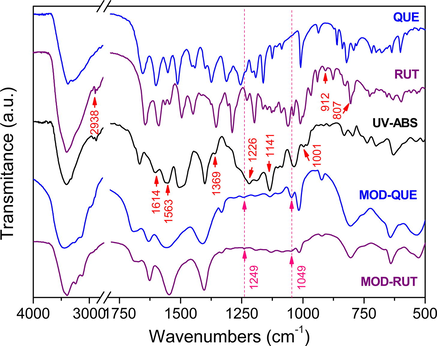
FTIR spectra of MOD-FLs, FLs and UV-ABS.
3.2 Adsorption behavior
During adsorption, pH value exerts great impact on the ionization degree of FLs and the surface charge intensity of silk(Zhou et al., 2016), which directly affects their affinity and the final adsorption quantity of FLs, thus is explored in priority. Obviously, QUE and MOD-QUE displayed much lower exhaustion rate than RUT and MOD-RUT (Fig. 4a). This phenomenon is attributable to the hydrophilic saccharide moiety in RUT molecules, which not only increases their steric hindrance against the adsorption onto the fibre surface and the diffusion into the fibre interior, but also increases their desorbing propensity from silk compared with QUE. The exhaustion of FLs to silk marginally changes at the pH range from 3 to 5, but decreases sharply over pH 5. Distinctively, a gradual descending trend for the exhaustion of MOD-FLs on silk along with the pH increase is observed. It is preliminarily estimated that the sulfonic moiety of the MOD-FLs enhances the ion − ion interaction between MOD-FLs and silk (verified in following Adsorption kinetic study). Moreover, MOD-FLs displayed lower exhaustion on silk than FLs. This is due to the enhanced solubility of FLs after modification, which reduces the quantity of unfixed precipitates on silk surface (verified by SEM imaging), and also facilitates their desorption from silk compared with the case of FLs. Considering the adsorption quantity and the protection of silk fibre, pH 4 is used for the following study.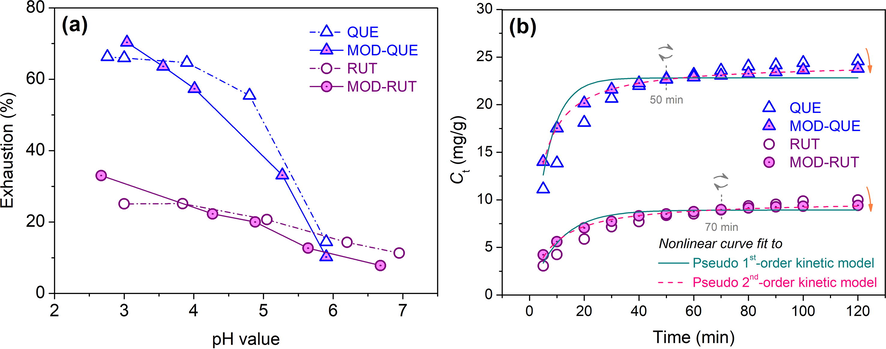
Adsorption of modified flavonoids to silk: (a) pH impact, (b) adsorption kinetics, and (c) building up property.
To further verify the interaction mode between MOD-FLs and silk, a batch of adsorption kinetic curves describing the adsorption quantity against time under 70 °C were created for further analysis. Pseudo 1st and 2nd order kinetic models (Table S1) are introduced for the nonlinear regression analysis of the experimental data. Pseudo 1st and 2nd order models are important tools to explore the adsorption kinetics, which are obtained through the integration of the general equation Eq. (3)((Chairat et al., 2005, Moussout et al., 2018).
The normalized deviations (ND) is adopted to explain the closeness to the models.(Sun and Tang 2011) As seen in Fig. 4b, the adsorption behaviour of FLs and MOD-FLs to silk is a rate-controlling process in general, indicating a chemisorption mechanism. Fast adsorption occurs in the first 40 min. After 90 min, adsorption approaches to an equilibrium level. Table 1 shows that Pseudo 2nd order kinetic model display higher R2 and lower NDs than Pseudo 1st order kinetic model. And also with respect to the fitted line plots in the Figure, the experimental points are located close to the Pseudo 2nd order kinetic model (dash line). Thus, Pseudo 2nd order kinetic model was selected to interpret the adsorption of FLs and MOD-FLs to silk. MOD-FLs show higher R2 and lower NDs than FLs, which indicates the higher dominants of ion-ion interaction after modification during adsorption. The zeta potential is the potential difference across phase boundaries between fibres and liquids. The larger decrease of isoelectric potential (IEP) of silk after the adsorption of MOD-FLs than of FLs reconfirms the ion-ion interaction (Figure S3). Briefly, the sulfonic groups on MOD-FLs takes up a greater number of ammonia sites on silk than in the case of FLs. Thanks to the enhanced solubility, MOD-FLs also own higher k2 and lower t1/2 than FLs due to their easier adsorption to the fibre surface or penetrate into the internal of fibre. Another benefit from the enhanced solubility is that the lower temperature is sufficient for the adsorption of well dispersed MOD-FLs to silk, rather than the case of FLs rely on the expansion of fibre and dispersity at higher temperature. It is also worth noted that MOD-FLs show a faster initial adsorption (See Fig. 4b and also indicated by hi) to silk than FLs. However, adsorption quantity of QUE and RUT turn higher after 50 min and 70 min, respectively. This is due to the higher solubility and stronger charge density of FLs after modification that induces more favorable adsorption than FLs to silk. As the adsorption proceeds, more hydrophobic FLs in the solution are aggregates to silk surface (See SEM images in Morphological study), finally resulting in a higher adsorption quantity at equilibrium.
Parameter
QUE
MOD-QUE
RUT
MOD-RUT
Pseudo 1st-order kinetic model
k1 (/min)
0.0884
0.1610
0.0497
0.0945
Ce (mg/g)
23.56
22.81
9.49
8.93
R2
0.9259
0.8958
0.9523
0.9115
ND (%)
2.71
1.11
3.54
2.28
Pseudo 2nd-order kinetic model
k2 (g/[mg⋅min])
0.0047
0.0106
0.0053
0.0133
Ce (mg/g)
26.27
24.40
11.24
9.93
hi (mg/[g⋅min])
3.24
6.31
0.67
1.31
t1/2 (min)
8.10
3.87
16.79
7.57
R2
0.9895
0.9977
0.9902
0.9929
ND (%)
1.07
0.18
1.77
0.74
The building-up property is highly related to the utilization, colour yield and adsorption quantity of bio-extracts on textiles from a practical perspective. In other words, the better building-up property is, the larger ranges of colour depth and functionality on final textile product can be achieved cater to the diverse demands of consumers. As depicted in Fig. 4c, MOD-QUE show superior building-up property than MOD-RUT on silk, which indicates a wider spectrum of absorption quantity can be achieved for diverse colour depth and functionality. Those are further explored in the following sections.
3.3 Fabric characterization
3.3.1 Colour feature, uniformity and fastness
As depicted in Fig. 5a, the a*/b* values of FLs treated silk fabrics gradually increases with the increasing pH, towards the yellowish-reddish region of colour space, which is mainly attributed to the deprotonation of the hydroxyls on FLs.(Zhou and Tang 2017) The silk fabrics treated with QUE has higher b* values than those treated with RUT, indicating their stronger yellow hue due to the higher adsorption rate. Conversely, the a*/b* plots of the silk treated with MOD-FLs at higher pH shift towards the origin of colour space, revealing a decrease in the colour saturation mainly due to the lower exhaustion of MOD-FLs (verified in Fig. 4a). It is also found the colour change of the MOD-FLs treated silk along with pH variation, is milder than that of FLs treated silk. This is due to the fact that the hydroxyls on FLs were occupied or affected by the introduction of UV-ABS after modification, thus reducing the pH impact on the protonation phenomenon. The inserted photograph of the silk fabrics treated at pH 4 can visibly detect the colour difference between the FLs and MOD-FLs treated silk. To increase the concentration of MOD-FLs further intensifies the colour shade and saturation of treated silk (Figure S4).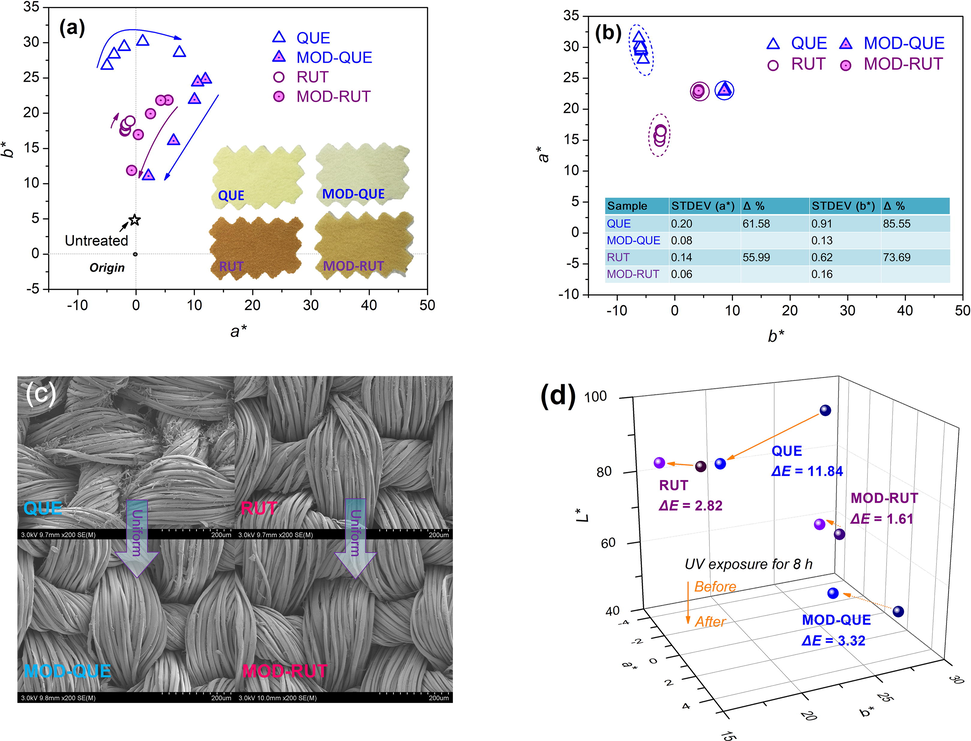
(a) Colour feature of silk treated at different pH values, (b) Colour uniformity of treated silk, (c) SEM images of treated silk and (d) Colour change of silk after exposed to UV light for 8 h.
The colour uniformity is fundamental to the appearance quality of the final products. Fig. 5b displays the a*/b* plots of 10 different positions from the centre to edge of the fabric sample. Obviously, the a*/b* plots of MOD-FLs treated silk are more centralised than those of FLs treated silk, indicating their good colour uniformity. Such amelioration is contributed by the enhanced solubility of flavonoids after modification, enables easier diffusion and migration of MOD-FLs within fibre. The colour uniformity is further quantified by comparing standard deviation (STDEV) of the a*/b* values of MOD-FLs and FLs treated silk (See the inserted table). The modification of FLs with UV absorber significantly enhanced the colour uniformity of treated silk. From the percentage variations (Δ%), more obvious uniformity enhancement is occurred on QUE than on RUT after modification, due to its greater improved solubility. In order to further explore the uniformity, the surface morphologies of FLs and MOD-FLs treated silk were examined by SEM. As shown in Fig. 5c, large quantities of aggregated FLs precipitates (verified in the individual SEM images of QUE and RUT in Figure S5) are observed on silk surface and between the silk fibres, especially in the case of QUE. Much fewer particles can be seen on the MOD-FLs treated silk, thanks to the enhancement of solubility. Integrating the results of adsorption quantity in 3.2. Adsorption behaviour, it is estimated that most MOD-FLs are diffused into the interior of silk fibres.
During usage, it is unavoidable for textile to subject to repeated washing. Thus, the colour fastness of MOD-FLs treated silk fabrics is an important parameter influencing the quality of the final products. As the data listed in the Table 2, MOD-FLs treated silk has much higher rubbing fastness than FLs treated silk, rising from the enhanced solubility that facilitates a better disperse in the solution and better diffusion into the fibre rather than remain on the surface. Owing to the introduction of UV absorber, the MOD-FLs treated silk show better light fastness than FLs treated silk. This enables us to further investigate the colour variations (ΔE) of the treated silk after 8 h light exposure. As shown in the 3-dimentional colour space (Fig. 5d), the ΔE values of silk treated with QUE and RUT are 11.84 and 2.82, respectively, which further reduces to 3.32 (71.96 %) and 1.61 (42.91 %), respectively. This manifests that the UV absorber part of the MOD-FLs can efficiently absorb the UV light which reduces the radiation impact of the UV on the structures of FLs.
Samples
Color change
Stain
Rub fastness
Light fastness
Silk
Cotton
Dry
Wet
QUE
3
4–5
4–5
3
3–4
3
RUT
4
4–5
5
4–5
4–5
4
MOD-QUE
3
5
5
5
5
4
MOD-RUT
4
5
5
5
5
4–5
3.3.2 Bioactive properties
The bacterial growth often occurs on silk products due to their hygroscopic property and protein nature that leads to the deterioration, staining and foul odor, and finally adversely impact human health through the transmission of infections and diseases. The antibacterial mode of FLs is due to cell lysis and disruption of the cytoplasmic membrane by action upon the membrane permeability leading to leakage of cellular components and eventually cell death.(Tagousop et al., 2018) FLs treated textile have been confirmed to be effective in the inhibition of bacterial growth, however, the antibacterial activity of the MOD-FLs treated silk requires further investigation. As seen in Fig. 6a, untreated silk has very low bacterial reduction rate (<30 %). The UV-ABS provides silk with a certain degree of antibacterial property due to its oxaline structure. 5 % owf FLs and MOD-FLs treated silk showed antibacterial activities over 80 % against E. coli (Gram-) and S. aureus (Gram+ ). QUE and MOD-QUE due to their higher adsorption quantities on silk (verified in 3.2. Adsorption behaviour), impart better antibacterial performance than RUT and MOD-RUT. Previous studies confirm that the antibacterial activity of FLs is highly dependent on the hydroxyl groups with special positions (Cushnie and Lamb 2005, Xie et al., 2015). However, there is only a minor decrease of antibacterial rate occurred on the silk treated with MOD-FLs compared with those treated with FLs. Such phenomenon is an accumulative effect of the reduction in the number of bioactive hydroxyls on FLs after modification, and the introduction of UV-ABS which also shows bacterial killing effect. Further, we quantified the bioactive efficiency of FLs and MOD-FLs based on the adsorption quantity obtained in 3.2. Adsorption behaviour. Interestingly, the antibacterial efficiencies (orange dots in the figure) of RUT and MOD-RUT on silk are higher than those of QUE and MOD-QUE. It is preliminarily speculated that the higher water solubility of RUT and MOD-RUT than QUE and MOD-QUE enables fast interaction with bacterial cells membranes or penetration into the internal of cells.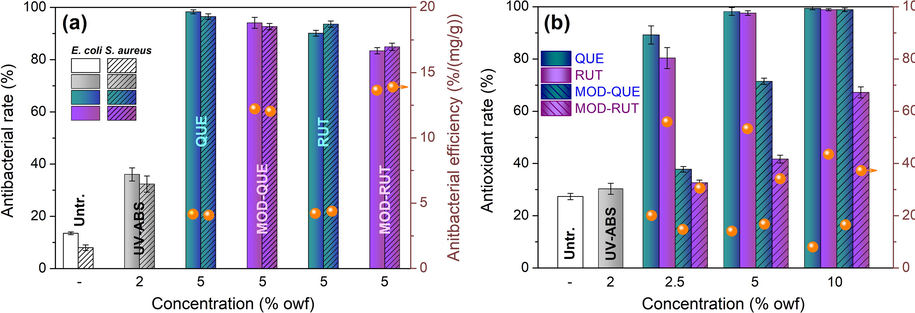
(a) Antibacterial and (b) antioxidant properties of Untr., UV-ABS treated, FLs and MOD-FLs treated silk.
The antioxidant activity of textiles materials due to their direct contact with human skin is gradually being paid attention in recent years. Previous studies confirmed antioxidant activity of FLs on textiles, which stimulates us to further explore the antioxidant activity of the MOD-FLs on silk. The catechol moiety in the B-ring, the 3-hydroxyl group in the C-ring, and the hydroxyl groups in the Ar-ing contribute to the antioxidant action of FLs.(Lemańska et al., 2001) As seen in Fig. 6b, original silk and UV-ABS treated silk showed poor antioxidant activity. The antioxidant rate of treated silk increases at higher quantity of FLs and MOD-FLs. A significant decrease of antioxidant rate of FLs is occurred after modification, which is mainly due to the reduced number of hydroxyls. It is also found that MOD-QUE provides silk with higher antioxidant rate than MOD-RUT at the same concentration. Similar to the antibacterial activity above, RUT and MOD-RUT show higher antioxidant efficiency than than that of QUE and MOD-QUE. In general, the MOD-FLs are able to provide ideal antibacterial property and medium-to-high level of antioxidant activity, and those properties can be manipulated by controlling the adsorption quantity during silk treatment.
3.3.3 UV protective property
Increasing attention has been paid to the human skin protection from the near-earth UV radiation due to the gradually aggravated spoilage of ozone layer. As a significant out layer covering the human skin, textiles have been highly expected to possess the UV protection performance. As the MOD-FLs display intense and broad peak within the wavelength of UVA (320–380 nm) and UVB (280–320 nm) regions (Fig. 2), it is supposed that the silk fabrics treated with MOD-FLs have the capacity of UV protection. As depicted in Fig. 7, untreated silk has a high UV transmittance with an extremely low UPF (<5), falling in UV protection. The UV-ABS treated silk fabric is very effective in blocking the transmittance of the lights in UVA and UVB ranges with a UPF of 27.6 (‘Very good’ Level). Both FLs and MOD-FLs treated silk show much lower UV transmittance than untreated and UV-ABS along treated silk especially in the wavelength range of 280–360 nm which contributes by the combining UV adsorption effects of FLs and UV-ABS. Moreover, the silk fabrics treated with 5 % owf MOD-FLs show higher UPF values (50+ ) than those treated with 5 % owf FLs, which are rated as the ‘Excellent’ level according to AS/NZS 4399.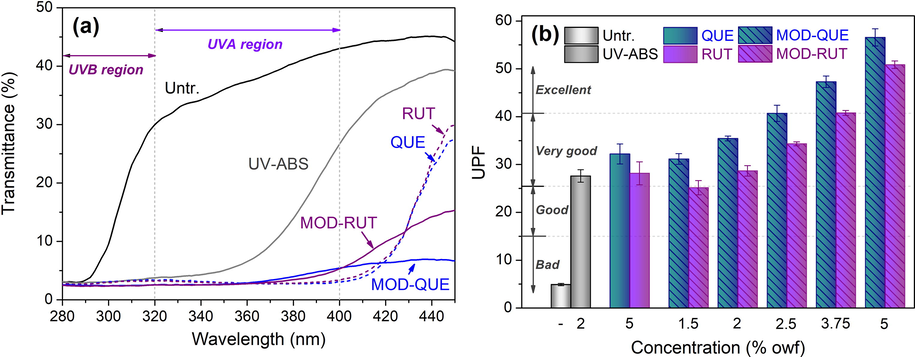
UV transmittance (a) and UPF (b) of the untreated, UV absorber treated, flavonoids treated and modified flavonoids treated silk fabrics.
4 Conclusions
This work presents a facile method to simultaneously enhance the solubility and photo-stability of FLs with a reactive UV-ABS for uniform and ultraviolet-stable colouration/functionalization of silk. The chemical structures and solution properties of MOD-FLs, and the colour features and functionalities of the treated silk were also evaluated. MOD-FLs have much better solubility in water and higher uptake rates on silk at lower pH value than FLs. MOD-QUE has better building up property on silk than MOD-QUE. Obvious enhancement of colour uniformity and light fastness of FLs treated silk after modification are observed and quantified. MOD-FLs treated silk exhibited good antibacterial activity, moderate antioxidant activity and desirable UV protective performance. In general, this study introduces a modification method for enhancing the water solubility and photostability of FLs to better serve for the eco-dyeing and finishing of textiles. Further research is expected to develop bio-based reactive and UV protective modifier based on the mechanism from this work to further gain the sustainability of textiles and the related processing.
Acknowledgements
This study was funded by Opening Project of Key Laboratory of Clean Dyeing and Finishing Technology of Zhejiang Province, China (QJRZ2106), Soochow University Start-up Fund, China (NH11500422), and the Research Innovation Program for College Graduates of Jiangsu Province, China (KYLX16_0137). We also thank the support from China National Textile and Apparel Council Key Laboratory of Natural Dyes, Soochow University.
References
- Abo El-Ola, S. M. and Kotb, R. M. 2021. Functional versatility of hybrid composite finishing of chitosan-titania NPs-organic UV-absorber for polyacrylonitrile fabric. Journal of Engineered Fibers and Fabrics. 16, 155892502110648. https://doi.org/10.1177/15589250211064845.
- Review on: developing UV protection for cotton fabric. J. Text. Inst.. 2017;108:2027-2039.
- [CrossRef] [Google Scholar]
- An adsorption and kinetic study of lac dyeing on silk. Dyes Pigm.. 2005;64:231-241.
- [CrossRef] [Google Scholar]
- Solubility of flavonoids in organic solvents. J. Chem. Eng. Data. 2007;52:1552-1556.
- [CrossRef] [Google Scholar]
- Theoretical study of flavonoids and proline interactions. aqueous and gas phases. J. Mol. Struct. (Thoechem.). 2003;623:63-73.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343-356.
- [CrossRef] [Google Scholar]
- Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal.. 2014;22:296-302.
- [CrossRef] [Google Scholar]
- Nucleophilic addition of reactive dyes on amidoximated acrylic fabrics. Scient. World J.. 2014;2014:305930
- [CrossRef] [Google Scholar]
- A cleaner production of ultra-violet shielding wool prints. J. Clean. Prod.. 2015;92:187-195.
- [CrossRef] [Google Scholar]
- Natural dyes and antimicrobials for green treatment of textiles. Environ. Chem. Lett.. 2014;12:1-13.
- [Google Scholar]
- Innovative multi-protection treatments and free-salt dyeing of cotton and silk fabrics. J. Eng. Fibers Fabr.. 2017;12:60-71.
- [Google Scholar]
- Application of UNIFAC models to partition coefficients of biochemicals between water and n-octanol or n-butanol. Fluid Phase Equilib.. 1998;144:87-95.
- [Google Scholar]
- The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radical Biol. Med.. 2001;31:869-881.
- [Google Scholar]
- Surface modification and functionalization of silk fibroin fibers/fabric toward high performance applications. Mater. Sci. Eng., C. 2012;32:627-636.
- [CrossRef] [Google Scholar]
- One-pot high efficiency low temperature ultrasonic-assisted strategy for fully bio-based coloristic, anti-pilling, antistatic, bioactive and reinforced cashmere using grape seed proanthocyanidins. J. Clean. Prod.. 2021;315:128148
- [CrossRef] [Google Scholar]
- Establishing an energy-saving scouring/bleaching one-step process for cotton/spandex fabric using UVA-assisted irradiation. RSC Adv.. 2022;12:9404-9415.
- [CrossRef] [Google Scholar]
- Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci.. 2018;4:244-254.
- [CrossRef] [Google Scholar]
- Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food. Chem.. 2014;62:3321-3333.
- [CrossRef] [Google Scholar]
- Effect of complexes of cyanidin-3-diglucoside-5-glucoside with rutin and metal ions on their antioxidant activities. Food Chem.. 2017;232:545-551.
- [CrossRef] [Google Scholar]
- Instrumental characterization of merino wool fibers dyed with Cinnamomum camphora waste/fallen leaves extract: an efficient waste management alternative. J. Clean. Prod.. 2020;273:123021
- [CrossRef] [Google Scholar]
- Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem.. 2005;53:4355-4360.
- [Google Scholar]
- Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A, Mol. Biomol. Spectros.. 2017;173:757-771.
- [CrossRef] [Google Scholar]
- Shahid, M., Shahid-ul-Islam, and Mohammad, F., 2013. Recent advancements in natural dye applications: a review. Journal of Cleaner Production. 53, 310-331. https://doi.org/10.1016/j.jclepro.2013.03.031
- Shahid-ul-Islam, Shahid, M., and Mohammad, F., 2013. Perspectives for natural product based agents derived from industrial plants in textile applications – a review. Journal of Cleaner Production. 57, 2-18. https://doi.org/10.1016/j.jclepro.2013.06.004
- Transcription of gel assemblies of bola type bis(oxalamide)-dicarboxylic acid and -diester gelators into silica nanotubes and ribbons under catalyzed and non-catalyzed conditions. Croat. Chem. Acta. 2007;80:591-598.
- [Google Scholar]
- Flavonoid-surfactant interactions: a detailed physicochemical study. Spectrochim. Acta Part A, Mol. Biomol. Spectrosc.. 2017;170:77-88.
- [CrossRef] [Google Scholar]
- The photostabilities of naturally occurring 5-hydroxyflavones, flavonols, their glycosides and their aluminium complexes. J. Photochem. Photobiol. A: Chem.. 2000;136:87-91.
- [CrossRef] [Google Scholar]
- Quercetin molecularly imprinted polymers: preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta. 2009;80:694-702.
- [CrossRef] [Google Scholar]
- Structural characterization of a new dioxamic acid derivative by experimental (FT-IR, NMR, and X-ray) analyses and theoretical (HF and DFT) investigations. J. Mol. Struct.. 2012;1016:13-21.
- [CrossRef] [Google Scholar]
- Adsorption and UV protection properties of the extract from honeysuckle onto wool. Ind. Eng. Chem. Res.. 2011;50:4217-4224.
- [CrossRef] [Google Scholar]
- Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Alternat. Med.. 2018;18:252.
- [CrossRef] [Google Scholar]
- Enzymatic modification of chitosan with quercetin and its application as antioxidant edible films. Appl. Biochem. Microbiol.. 2012;48:151-158.
- [CrossRef] [Google Scholar]
- Engineering faster transglycosidases and their acceptor specificity. Green Chem.. 2019;21:2823-2836.
- [CrossRef] [Google Scholar]
- Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr. Med. Chem.. 2015;22:132-149.
- [CrossRef] [Google Scholar]
- Synthesis and application of disazo reactive dyes derived from sulfatoethylsulfone pyrazolo[1,5-a]pyrimidine derivatives. J. Saudi Chem. Soc.. 2014;18:220-226.
- [CrossRef] [Google Scholar]
- De-glycosylation and enhanced bioactivity of flavonoids from apple pomace during extraction with deep eutectic solvents. Green Chem.. 2021;23:7199-7209.
- [CrossRef] [Google Scholar]
- Zhang, Y., Shahid-ul-Islam, Rather, L. J. et al., 2022. Recent advances in the surface modification strategies to improve functional finishing of cotton with natural colourants - A review. Journal of Cleaner Production. 335, 130313. https://doi.org/10.1016/j.jclepro.2021.130313
- New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem. Eng. J.. 2018;336:123-132.
- [CrossRef] [Google Scholar]
- Modification of curcumin with a reactive UV absorber and its dyeing and functional properties for silk. Dyes Pigm.. 2016;134:203-211.
- [CrossRef] [Google Scholar]
- Natural flavonoid-functionalized silk fiber presenting antibacterial, antioxidant, and UV protection performance. ACS Sustain. Chem. Eng.. 2017;5:10518-10526.
- [CrossRef] [Google Scholar]
- Simultaneous dyeing and functionalization of silk with three natural yellow dyes. Ind. Crops Prod.. 2015;64:224-232.
- [CrossRef] [Google Scholar]
- Bioactive and UV protective silk materials containing baicalin - the multifunctional plant extract from Scutellaria baicalensis Georgi. Mater. Sci. Eng. C, Mater. Biol. App.. 2016;67:336-344.
- [CrossRef] [Google Scholar]
- Facile and green preparation of bioactive and UV protective silk materials using the extract from red radish (Raphanus sativus L.) through adsorption technique. Arabian J. Chem.. 2020;13:3276-3285.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104343.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







