Translate this page into:
Nickel-catalyzed esterification of mandelic acids with alcohols
⁎Corresponding author. ablimit1970@126.com (Abdukader Ablimit)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mandelates and their derivatives are widely used in organic synthesis, drug discovery, biodegradable polymers and other related fields. Therefore, the effective and simple synthesis of these compounds has attracted much attention. In this paper, a nickel(II)-catalyzed esterification of mandelic acids with different alcohols was realized for the synthesis of mandelic acid esters. This transformation was conducted under mild reaction conditions with yields up to 95%, and was successfully utilized in the gram-scale synthesis of medicine cyclandelate.
Keywords
Mandelate
Nickel(II)-catalyzed
Esterification
Alcohols
1 Introduction
Esterification is a common and important transformation in organic synthesis, which reaching almost one-quarter of the bulk reactions in the manufacture of pharmaceuticals and drugs (Otera, 2003; Dugger et al., 2005). Great efforts have been made for the development of efficient protocols for esterification under mild reaction conditions in recent years (Zheng et al., 2021; Padala et al., 2015; Lu et al., 2017; Xiong et al., 2017; Vandamme et al., 2016; Yu et al., 2014; Lozano et al. 2017; Liu et al., 2011; Houston et al., 2004; Maki et al., 2005; Maki et al., 2007). Besides, the direct C—H oxyacylation of functionalized alkanes has also provided feasible approaches towards esters form carboxyl and carbonyl compounds (Li et al., 2016; Huang et al., 2017; Wu et al., 2016; Zhu et al. 2016). For example, Yu and co-workers developed the α-oxyacylation of ketones with benzylic alcohols or acyl peroxides in the presence of Bu4NI under metal-free conditions (Guo et al., 2014; Zhou et al., 2015); Recently, Zhang group reported the 1,2-dibromoethane- and KI-mediated α-acyloxylation of ketones with carboxylic acids without the use of strong oxidants (Wang et al., 2020). Despite of many achievements witnessed the direct synthesis of functionalized esters still need to be further developed.Scheme 1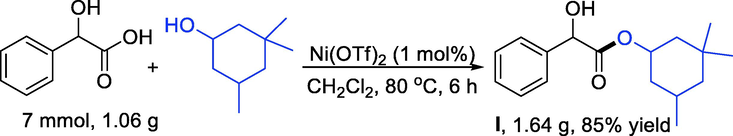
Gram-scale synthesis of cyclandelate (I).
Mandelic acid ester containing molecules are valuable biologically active compounds with high therapeutic importance and economic impact. Among them, cyclandelate (I) is a kind of clinical used vasodilator (Bast, et al., 1987; White, et al. 1990). MA-6-APA (II) is a kind of modified penicillin with distinctive bioactivities (Fulenmeier et al., 1976). Homatropine (III) (Glushkov et al., 1977), and oxybutynin (Su et al., 2003) are used as anti-cholinergic medications (Fig. 1). Besides, mandelate derivatives can be readily transformed into other functionalized molecules such as 1,2-diols β-amino alcohols and α-amino acid derivatives, and can be used as key intermediates for the synthesis of various medicine such as Plavix (clopidogrel) (Meijden et al., 2009) and Duloxetine (Majer et al 2009); Traditionally, mandelate derivatives can be synthesized via two routes, hydrolysis of cyanohydrins (Poechlauer, et al., 2004) and Friedel-Crafts arylation of glyoxalate derivatives (Majer, et al., 2011; Majer, et al., 2008; Dong et al. 2007; Kwiatkowski, et al. 2006; Li, et al., 2006). However, the former route requires the use of toxic cyano reagents such as HCN, and the arenes in the latter route are limited to electron-rich ones. In 2017, Yamamoto reported the synthesis of mandelate derivatives from arylboronic acids and glyoxylate hemiacetals catalyzed by palladium (Sugaya, et al. 2017). Xu and Poterała developed the direct esterification of mandelic acids under the catalysis of zirconocene (Tang, et al., 2017) or in the presence of SOCl2 (Poterała, et al. 2017), respectively. Although significant achievements were realized, more general and convenient approaches towards mandelates are highly demanded.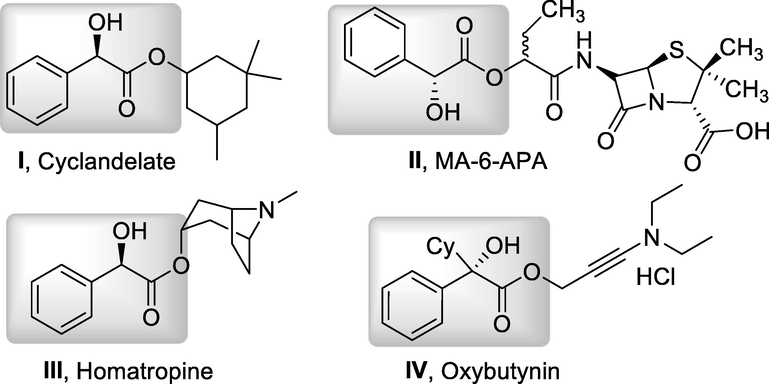
Some biologically active compounds bearing mandelic acid ester motifs.
2 Experimental
Unless otherwise stated, all reagents and solvents were purchased from commercial suppliers and used without further purification. 1H NMR and 13C NMR spectra were recorded at Vaian Inova-400 MHz NMR spectrometer using CDCl3 as solvent and TMS as an internal standard.
2.1 Typical procedure for synthesis of mandelate 3aa
A 10 mL Schlenk tube equipped with a stir bar was charged with mandelic acid 1a (0.5 mmol), methanol 2a (0.5 mL), and Ni(OTf)2 (1 mol%) at 80 °C for 6 h. After removing of volatile materials from the reaction mixture under vacuum, the resulted residue was purified by flash column chromatography on silica gel to give the methyl 2-hydroxy-2-phenylacetate 3aa.
2.2 Characterization of products
2.2.1 methyl 2-hydroxy-2-phenylacetate (3aa)
Known compound (Tang, et al. 2017). Yellow liquid, yield 93 %; 1H NMR (400 MHz, CDCl3) δ 7.37 (d, J = 7.4 Hz, 2H), 7.29 (dd, J = 14.9, 7.2 Hz, 3H), 5.14 (s, 1H), 3.88 (s, 1H), 3.67 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 174.09, 138.37, 128.63, 128.50, 126.67, 72.99, 52.91.
2.2.2 ethyl 2-hydroxy-2-phenylacetate (3ab)
Known compound (Yao, et al. 2008). Yellow liquid, yield 92 %; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 7.3 Hz, 2H), 7.35 (d, J = 6.9 Hz, 3H), 5.15 (d, J = 5.6 Hz, 1H), 4.25~4.12 (m, 2H), 3.63 (s, 1H), 1.23~1.19 (m, 3H); 13C NMR (101 MHz, CDCl3) δ 173.67, 138.47, 128.56, 128.39, 126.55, 72.92, 62.20, 14.02.
2.2.3 propyl 2-hydroxy-2-phenylacetate (3ac)
Known compound (Tang, et al. 2017). Yellow liquid, yield 94 %; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 7.4 Hz, 2H), 7.37~7.31 (m, 3H), 5.17 (d, J = 4.3 Hz, 1H), 4.14~4.07(m, 2H), 3.59 (d, J = 5.1 Hz, 1H), 1.60 (dt, J = 14.1, 7.1 Hz, 2H), 0.84 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.80, 138.50, 128.53, 128.38, 126.51, 72.87, 67.70, 21.82, 10.11.
2.2.4 butyl 2-hydroxy-2-phenylacetate (3ad)
Known compound (Keshavarz, et al. 2019). Yellow liquid, yield 95 %; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 7.5 Hz, 2H), 7.37~7.28 (m, 3H), 5.16 (d, J = 5.5 Hz, 1H), 4.20~4.09 (m, 2H), 3.74 (d, J = 5.7 Hz, 1H), 1.55 (m, J = 14.5, 2H), 1.23 (m, J = 14.7 Hz, 2H), 0.84 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.78, 138.53, 128.51, 128.36, 126.53, 72.92, 65.95, 30.40, 18.85, 13.53.
2.2.5 hexyl 2-hydroxy-2-phenylacetate (3ae)
Known compound (Tang, et al. 2017). Yellow liquid, yield 86 %; 1H NMR (400 MHz, CDCl3) δ 7.36 (d, J = 7.6 Hz, 2H), 7.32~7.27 (m, 3H), 5.11 (d, J = 5.7 Hz, 1H), 4.10 (t, J = 6.6 Hz, 2H), 3.57 (d, J = 5.7 Hz, 1H), 1.53~1.50 (m, 2H), 1.15 (d, J = 15.1 Hz, 6H), 0.80 (t, J = 6.6 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.80, 138.52, 128.52, 128.37, 126.52, 72.89, 66.27, 31.22, 28.35, 25.25, 22.45, 13.93.
2.2.6 octyl 2-hydroxy-2-phenylacetate (3af)
Known compound (Das et al., 2017). Yellow Liquid, yield 81 %; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 6.9 Hz, 2H), 7.37~7.30 (m, 3H), 5.17 (d, J = 5.3 Hz, 1H), 4.16 (t, J = 6.6 Hz, 2H), 3.60 (d, J = 5.6 Hz, 1H), 1.59~1.55 (m, 2H), 1.33~1.14 (m, 10H), 0.89 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.75, 138.59, 128.50, 128.34, 126.52, 72.91, 66.21, 60.39, 31.70, 28.83, 25.59, 22.61, 21.00, 14.12.
2.2.7 isopropyl 2-hydroxy-2-phenylacetate (3ag)
Known compound (Hong, et al. 2019). White solid, yield 85 %, m.p. 38~39 °C; 1H NMR (400 MHz, CDCl3) δ 7.37 (dt, J = 17.7, 8.6 Hz, 5H), 5.13 (s, 1H), 5.07 (dt, J = 12.4, 6.2 Hz, 1H), 3.96 (s, 1H), 1.28 (d, J = 6.2 Hz, 3H), 1.11 (d, J = 6.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.97, 138.72, 128.34, 128.11, 126.39, 72.92, 69.74, 60.25, 21.57, 21.27, 20.83, 14.06.
2.2.8 isopentyl 2-hydroxy-2-phenylacetate (3ah)
Known compound (San et al., 2018). Yellow liquid, yield 75 %; 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 4.4 Hz, 2H), 7.37~7.32 (m, 3H), 5.18 (s, 1H), 4.23~4.16 (m, 2H), 3.87 (s, 1H), 1.56~1.51(m, 1H), 1.49~1.47 (m, 2H), 0.87~0.81 (m, 6H); 13C NMR (101 MHz, CDCl3), Ketone form: δ 173.83, 138.56, 128.58, 128.43, 126.58, 72.99, 64.89, 37.11, 24.96, 22.39, 16.20. Enol form: δ 173.91, 138.63, 128.55, 126.60, 72.96, 70.63, 34.14, 25.80, 22.33, 11.13.
2.2.9 allyl 2-hydroxy-2-phenylacetate (3ai)
Known compound (Yin, et al. 2009). White solid, yield 78 %, m.p. 41~42 oC; 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 7.9, 1.4 Hz, 2H), 7.40~7.29 (m, 3H), 5.83~5.78 (m, 1H), 5.23~5.19 (m, 2H), 5.16 (dd, J = 4.8, 1.3 Hz, 1H), 4.65~4.60 (m, 2H), 3.60 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 173.40, 138.33, 131.22, 128.64, 128.53, 126.64, 118.77, 72.99, 66.48.
2.2.10 prop-2-yn-1-yl 2-hydroxy-2-phenylacetate(3aj)
Known compound (Yin, et al. 2009). Yellow Liquid, yield 83 %; 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 7.5, 2H), 7.38~7.32 (m, 3H) 5.22 (s, 1H), 4.70 (dd, J = 15.5, 2.5 Hz, 2H), 3.81 (s, 1H), 2.49 (t, J = 2.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 172.76, 137.76, 128.65, 128.62, 126.67, 76.75, 75.79, 75.76, 72.93, 53.26.
2.2.11 methyl 2-hydroxy-2-(4-propoxyphenyl)acetate (3ba)
Yellow liquid, yield 78 %; 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 5.2 Hz, 2H), 6.90 (d, J = 5.2 Hz, 2H), 5.13 (s, 1H), 3.92 (t, J = 4.4 Hz, 2H), 3.75 (s, 3H), 3.62 (s, 1H), 1.83~1.80 (dd, J = 4.8 Hz, 9.2 Hz, 2H), 1.05 (t, J = 4.8, 3H); 13C NMR (101 MHz, CDCl3) δ 174.36, 159.37, 130.33, 127.93, 114.62, 72.59, 69.55, 52.87, 22.57, 10.53. HRMS (ESI-TOF): m/z [M + NH4]+ calcd for C12H20NO4: 242.1387; found: 242.1389.
2.2.12 propyl 2-hydroxy-2-(4-propoxyphenyl)acetate (3bc)
Yellow liquid, yield 76 %; 1H NMR (400 MHz, CDCl3) δ 7.30 (d, J = 8.8 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 5.10 (s, 1H), 4.12~4.04 (m, 2H), 3.88 (t, J = 6.6 Hz, 2H), 3.64 (s, 1H), 1.81~1.73 (m, 2H), 1.63~1.54 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H), 0.82 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) Ketone form: δ 174.10, 159.28, 130.61, 127.82, 114.56, 72.61, 69.58, 64.72, 37.12, 24.95, 22.60, 11.14. Enol form: δ 174.03, 159.26, 130.56, 127.80, 114.54, 72.57, 70.47, 34.12, 25.83, 22.35, 16.16, 10.55. HRMS (ESI-TOF): m/z [M + NH4]+ calcd for C14H24NO4: 270.1700; found: 270.1693.
2.2.13 butyl 2-hydroxy-2-(4-propoxyphenyl)acetate (3bd)
Yellow liquid, yield 74 %; 1H NMR (400 MHz, CDCl3) δ 7.32~7.28 (m, 2H), 6.86 (q, J = 4.9 Hz, 2H), 5.09 (s, 1H), 4.13 (qt, J = 10.8, 6.7 Hz, 2H), 3.89 (t, J = 6.6 Hz, 2H), 3.52 (s, 1H), 1.83~1.74 (m, 2H), 1.61~1.49 (m, 2H), 1.31~1.18 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H), 0.85 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.94, 159.17, 130.50, 127.72, 114.46, 72.48, 69.47, 65.76, 30.39, 22.51, 18.83, 13.51, 10.45. HRMS (ESI-TOF): m/z [M + NH4]+ calcd for C15H26NO4: 284.1856; found: 284.1848.
2.2.14 isopropyl 2-hydroxy-2-(4-propoxyphenyl)acetate (3bg)
White solid, yield 70 %, m.p. 63~64 oC; 1H NMR (400 MHz, CDCl3) δ 7.32~7.28 (m, 2H), 6.88~6.84 (m, 1H), 5.08~5.02 (m, 2H), 3.90 (t, J = 6.6 Hz, 1H), 3.50 (s, 1H), 1.84~1.75 (m, 1H), 1.26 (d, J = 6.3 Hz, 2H), 1.10 (d, J = 6.3 Hz, 1H), 1.02 (t, J = 7.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 173.39, 159.10, 130.57, 127.66, 114.41, 72.51, 69.84, 69.45, 22.52, 21.52, 10.47. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C14H20NaO4: 275.1254; found: 275.1266.
2.2.15 isopentyl 2-hydroxy-2-(4-propoxyphenyl)acetate (3bh)
Yellow liquid, yield 72 %; 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 5.08 (s, 1H), 4.13 (pd, J = 10.9, 6.2 Hz, 2H), 3.87 (t, J = 6.6 Hz, 2H), 3.64 (s, 1H), 1.83~1.74 (m, 2H), 1.56~1.42 (m, 2H), 1.01 (t, J = 7.4 Hz, 3H), 0.89 (dd, J = 6.6, 1.0 Hz, 1H), 0.83 (dd, J = 7.8, 6.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) Ketone form: δ 174.10, 159.28, 130.61, 127.83, 114.56, 72.61, 69.58, 64.72, 37.12, 34.12, 24.95, 22.39, 16.21, 11.37. Enol form: 174.03, 159.26, 130.56, 127.80, 114.54, 72.57, 70.47, 34.07, 25.83, 22.60, 22.35, 16.16, 11.12, 10.55. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C16H24NaO4: 303.1567; found: 303.1567.
2.2.16 methyl 2-hydroxy-2-(4-(trifluoromethyl)phenyl)acetate (3ca)
Known compound (Sugaya, et al. 2017). White solid, yield 90 %, m.p. 40~41 oC; 1H NMR (400 MHz, CDCl3) 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 13.5, 4H), 5.25 (s, 1H), 4.22 (s, 1H), 3.74 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 173.38, 141.93, 131.59 (q, JC-F = 33.33 Hz), 126.89, 125.42 (q, JC-F = 4.04 Hz), 123.94 (q, JC-F = 270.1 Hz), 72.29, 53.19.
2.2.17 ethyl 2-hydroxy-2-(4-(trifluoromethyl)phenyl)acetate (3cb)
White solid, yield 89 %, m.p. 87~88 oC; 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 8.3 Hz, 4H), 5.22 (s, 1H), 4.21 (dd, J = 25.0, 6.9 Hz, 2H), 3.89 (s, 1H), 1.21 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 172.90, 142.20, 130.13 (q, JC-F = 32.32 Hz), 126.81, 125.34 (q, JC-F = 2.02 Hz), 121.27 (q, JC-F = 273.71 Hz),72.29, 62.53, 13.84. HRMS (+ESI-TOF): m/z [M + NH4]+ calcd for C11H15F3NO3: 266.0999; found: 266.1005.
2.2.18 propyl 2-hydroxy-2-(4-(trifluoromethyl)phenyl)acetate (3cc)
White solid, yield 90 %, m.p. 73~74 oC; 1H NMR (400 MHz, CDCl3) δ 7.59 (dd, J = 14.5, 7.8 Hz, 4H), 5.24 (s, 1H), 4.11 (d, J = 7.0 Hz, 2H), 3.90 (s, 1H), 1.59 (dd, J = 13.9, 6.9 Hz, 2H), 0.81 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.00, 142.27, 130.78 (q, JC-F = 32.32 Hz), 126.80, 125.34 (q, JC-F = 4.04 Hz), 121.26 (q, JC-F = 272.7 Hz), 72.26, 67.98, 21.70, 9.94. HRMS (ESI-TOF): m/z [M + NH4]+ calcd for C12H17F3NO3: 280.1155; found: 280.1163.
2.2.19 benzyl 2-hydroxy-2-phenylacetate (3ak)
Known compound (Tang, et al. 2017). Yellow liquid, yield 84 %; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.1 Hz, 2H), 7.38~7.30 (m, 6H), 7.20 (d, J = 2.8 Hz, 2H), 5.23 (t, J = 8.2 Hz, 2H), 5.14 (d, J = 12.3 Hz, 1H), 3.44 (d, J = 5.8 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 173.49, 138.17, 134.98, 128.62, 128.50, 128.53, 128.47, 127.96, 126.60, 72.98, 67.70.
2.2.20 phenethyl 2-hydroxy-2-phenylacetate (3al)
Known compound (Tang, et al. 2017). White solid, yield 85 %, m.p. 60~62 oC; 1H NMR (400 MHz, CDCl3) δ 7.35~7.31 (m, 5H), 7.21 (d, J = 5.4 Hz, 3H), 7.02 (d, J = 3.2 Hz, 2H), 5.11 (d, J = 5.6 Hz, 1H), 4.40~ 4.28 (m, 2H), 3.57 (d, J = 5.6 Hz, 1H), 2.90~2.78 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 173.63, 138.30, 137.25, 128.88, 128.63, 128.56, 128.46, 126.67, 126.64, 72.93, 66.56, 34.90.
2.2.21 3-phenylpropyl 2-hydroxy-2-phenylacetate (3am)
Known compound (Tang, et al. 2017). Yellow Liquid, yield 83 %; 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 4.8 Hz, 2H), 7.48 ∼ 7.40 (m, 3H), 7.34 (t, J = 4.0 Hz, 2H), 7.27 (t, J = 4.8 Hz, 1H), 7.10 (d, J = 4.8 Hz, 2H), 5.28 (s, 1H), 4.22 (t, J = 6.3 Hz, 2H), 3.91 (s, 1H), 2.58 (t, J = 4.8 Hz, 2H), 2.01 ∼ 1.94 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 173.75, 140.77, 138.57, 128.65, 128.52, 128.48, 128.42, 126.62, 126.11, 72.94, 65.24, 31.77, 30.02.
2.2.22 4-nitrobenzyl 2-hydroxy-2-phenylacetate (3an)
Known compound (Sugaya, et al. 2017). White solid, yield 81 %, m.p. 141~142 oC; 1H NMR (400 MHz, DMSO‑d6) δ 8.16 (d, J = 8.7 Hz, 2H), 7.46~7.37 (m, 4H), 7.33 (dt, J = 20.1, 7.0 Hz, 3H), 6.23 (d, J = 5.2 Hz, 1H), 5.30 (d, J = 5.1 Hz, 1H), 5.28 (s, 2H); 13C NMR (101 MHz, DMSO‑d6) δ 172.92, 142.50, 138.23, 128.60, 127.86, 126.63, 123.64, 73.09, 73.06, 65.47.
2.2.23 2-hydroxy-2-(4-(trifluoromethyl)phenyl)acetate (3ao)
White solid, yield 77 %, m.p. 63~64 oC; 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 7.8 Hz, 1H), 7.40 (dt, J = 12.2, 5.5 Hz, 7H), 7.11 (d, J = 7.5 Hz, 1H), 5.65 (d, J = 15.1 Hz, 1H), 5.52 (d, J = 15.1 Hz, 1H), 5.30 (s, 1H), 3.70 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 172.54, 147.48, 141.76, 133.91, 130.96, 129.37, 128.80, 127.17, 125.79 (q, J = 3.7 Hz), 125.36, 72.57, 64.73.
2.2.24 4-chlorobenzyl 2-hydroxy-2-phenylacetate (3ap)
Known compound (Gao, et al. 2019). White solid, yield 80 %, m.p. 136~137 oC; 1H NMR (400 MHz, DMSO‑d6) δ 7.43 (d, J = 6.8 Hz, 2H), 7.35 (d, J = 7.6 Hz, 5H), 7.29 (d, J = 8.4 Hz, 2H), 6.14 (d, J = 5.3 Hz, 1H), 5.23 (d, J = 5.3 Hz, 1H), 5.11 (d, J = 2.9 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 172.73, 138.69, 133.94, 133.68, 129.05, 128.39, 128.29, 128.11, 126.57, 72.97, 65.73.
2.2.25 2-chlorobenzyl 2-hydroxy-2-phenylacetate (3aq)
White solid, yield 82 %, m.p. 87~88 oC; 1H NMR (400 MHz, CDCl3) δ 7.45 (dd, J = 7.8, 1.8 Hz, 2H), 7.38~7.34 (m, 4H), 7.24 (td, J = 7.5, 1.9 Hz, 1H), 7.23~7.13 (m, 2H), 5.30 (d, J = 3.8 Hz, 2H), 5.27 (s, 1H), 3.63 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 173.37, 138.12, 133.55, 132.77, 129.74, 129.61, 129.50, 128.67, 128.60, 126.91, 126.67, 73.03, 64.99. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C15H13ClNaO3: 299.0445; found: 299.0448.
2.2.26 2-nitrobenzyl 2-hydroxy-2-(4-(trifluoromethyl)phenyl)acetate (3co)
White solid, yield 78 %, m.p. 123~124 oC; 1H NMR (400 MHz, CDCl3) δ 8.08 (dd, J = 7.7, 1.7 Hz, 1H), 7.66 (d, J = 8.3 Hz, 4H), 7.49~7.54 (m, 2H), 7.16~7.13 (m, 1H), 5.61~5.49 (m, 2H), 5.36 (s, 1H), 3.60 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 172.54, 147.48, 141.76, 133.92, 131.20, 130.96, 129.37, 128.80, 127.17, 125.79 (q, J = 3.7 Hz), 125.36, 122.71, 72.57, 64.73. HRMS (ESI-TOF): m/z [M−H]- calcd for C16H11F3NO5: 373.0589; found: 373.0585.
2.2.27 2-chlorobenzyl 2-hydroxy-2-(4-(trifluoromethyl)phenyl)acetate (3cq)
White solid, yield 75 %, m.p. 73~74 oC; 1H NMR (400 MHz, DMSO‑d6) δ 7.69 (q, J = 8.5 Hz, 4H), 7.44 (d, J = 8.0 Hz, 1H), 7.37~7.30 (m, 2H), 7.30~7.24 (m, 1H), 6.45 (s, 1H), 5.40 (s, 1H), 5.19 (d, J = 2.9 Hz, 2H); 13C NMR (101 MHz, DMSO‑d6) δ172.04, 144.32, 133.45, 133.06, 130.48, 130.44, 129.74, 127.90, 127.59, 125.59, 125.55, 125.52, 125.48, 72.29, 64.02, 63.99, 63.95, 40.56, 40.35, 40.14, 39.93, 39.72, 39.51, 39.30. HRMS (ESI-TOF): m/z [M−H]- calcd for C16H11ClF3O3: 343.0349; found: 343.0341.
2.2.28 3,3,5-trimethylcyclohexyl 2-hydroxy-2-phenylacetate
Known compound (Tang, et al. 2017). White solid, yield 85 %, m.p. 52~53 oC (Reference value:50~53 °C); 1H NMR (400 MHz, CDCl3) δ 7.42 (dt, J = 7.9, 1.6 Hz, 2H), 7.38~7.28 (m, 3H), 5.12 (d, J = 1.6, 1H), 5.00~4.90 (m, 1H), 3.76 (s, 1H), 2.05~1.12 (m, 4H), 1.02~0.84 (m, 10H), 0.79~0.67(m, 2H); 13C NMR (101 MHz, CDCl3) δ 173.38, 138.58, 28.49, 128.27 126.42, 47.36, 47.33, 43.77, 43.32, 40.19, 39.72, 32.94, 32.87, 32.31, 32.24, 27.06, 26.98, 25.48, 25.41, 22.21, 22.13.
3 Results and discussion
Bearing this idea in mind, we started our investigation by reaction conditions optimization employing the reaction of mandelic acid (1a) in methanol (2a) as the model reaction, as shown in Table 1. Firstly, common Lewis acid catalysts such as Yb(OTf)3, Ni(OTf)2, Zn(OTf)2, Sc(OTf)3, La(OTf)3, Bi(OTf)3, Sn(OTf)2, and TfOH were analyzed, and Ni(OTf)2 was found a suitable catalyst to give methyl mandelate 3aa in 68 % yield (Entries 1–9, Table 1). Next, the loading of catalyst was optimized via using 0.5 mol% and 1.5 mol% of Ni(OTf)2. Lower catalyst loading led to decreased yield, while increase the loading of catalyst to 1.5 mol% did not gave a higher yield (Entries 10–11, Table 1). Interestingly, an isolated yield of 86 % was obtained if the reaction was conducted for 6 h (Entries 12–14, Table 1). Further investigation indicated that 80 °C was an optimal reaction temperature (Entries 15–16, Table 1). We finally tested the reaction in different solvents, such as CH3CN, DMSO, CH2Cl2, THF, and DMF (Entries 17–21, Table 1). The reaction in CH2Cl2 gave a yield of 84 %. Thus, CH2Cl2 was chosen as the best solvent for this transformation.
Entry
Catalyst (mol%)
Solvent
Temp. (oC)
t (h)
Yield (%)
1
Yb(OTf)3 (1)
–
80
12
37
2
Ni(OTf)2 (1)
80
12
68
3
Yb(OTf)2·H2O (1)
80
12
50
4
Zn(OTf)2 (1)
80
12
20
5
Sc(OTf)3 (1)
80
12
62
6
La(OTf)3 (1)
80
12
55
7
Bi(OTf)3 (1)
80
12
24
8
Sn(OTf)2 (1)
80
12
31
9
TfOH (1)
80
12
–
10
Ni(OTf)2 (0.5)
80
12
55
11
Ni(OTf)2 (1.5)
80
12
64
12
Ni(OTf)2 (1)
80
4
83
13
Ni(OTf)2 (1)
80
6
86
14
Ni(OTf)2 (1)
80
8
81
15
Ni(OTf)2 (1)
50
6
57
16
Ni(OTf)2 (1)
100
6
71
17b
Ni(OTf)2 (1)
CH3CN
80
6
–
18b
Ni(OTf)2 (1)
DMSO
80
6
–
19b
Ni(OTf)2 (1)
DCM
80
6
84
20b
Ni(OTf)2 (1)
THF
80
6
–
21b
Ni(OTf)2 (1)
DMF
80
6
–
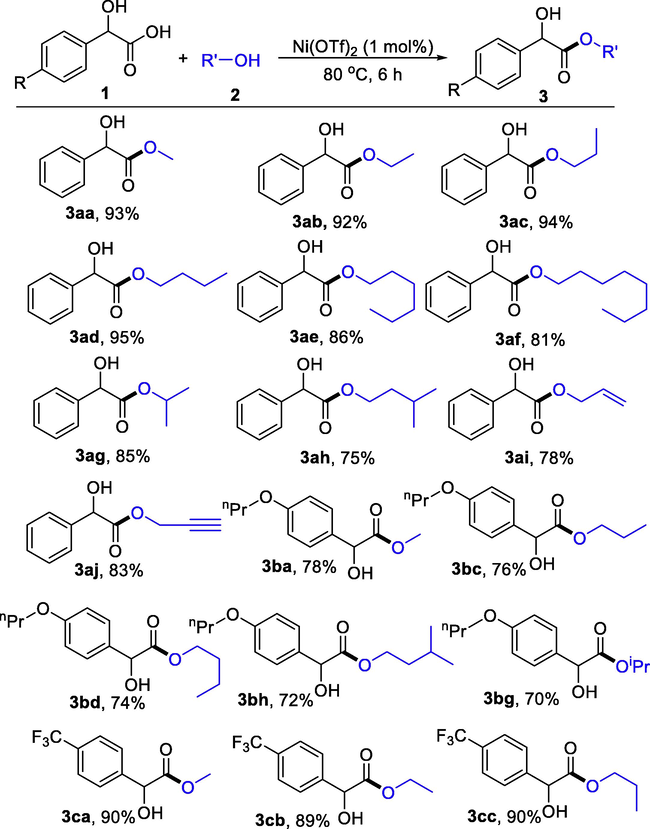
With the optimized reaction conditions established, we investigated the substrate scope of simple alcohol reacted with mandelic acid as in Table 2. As expected, alcohols such as ethanol, propanol, butanol, hexanol and octanol were all suitable reaction partners to give corresponding mandelic acid esters in 81–95 % yields (3ab-3af). Isopropanol and isoamyl alcohol reacted with mandelic acid to deliver products 3ag and 3ah in 85 % and 75 % yields, respectively. Reactive allyl alcohol and propargyl alcohol led to products 3ai and 3aj in yields of 78 % and 83 %. Substituted mandelic acids were also used as substrates. For example, electron-donating alkoxy and electron-withdrawing trifluoromethyl substituted mandelic acids reacted with variously substituted alcohols to give products 3ba-3 cc in 70–90 % yields. Aliphatic α-hydroxycarboxylic acid compounds have also been tried, unfortunately, no corresponding ester compounds have been obtained.
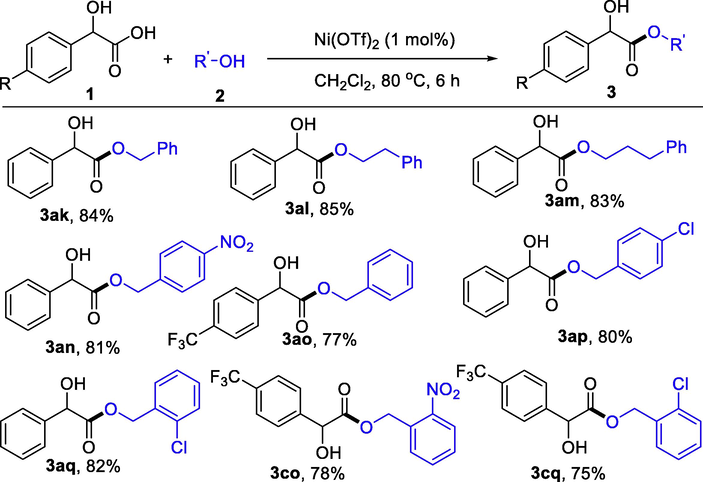
To expand the substrate to aromatic alcohols with high boiling points, we utilized CH2Cl2 as the solvent. As in Table 3, phenylmethanol, 2-phenylethanol, and 3-phenylpropan-1-ol reacted smoothly with mandelic acid to generate esters 3ak-3am in 83–85 % yields. Nitro- and chloro-substituted phenylmethanol underwent the esterification to produce 3an-3aq in 77–82 % yields.
To investigate the practicality of this esterification procedure, a gram-scale reaction between mandelic acid and 3,3,5-trimethylcyclohexanol was conducted to produce medicine cyclandelate (I) in 85 % yield (1.64 g). The result indicated that current catalyst system may be suitable to industrial productions (Scheme 1).
A mechanism for this transformation was proposed and listed in Scheme 2. The coordination of Ni(OTf)2 to mandelic acid 1a gives intermediate A, which was attacked by methanol 2a to form intermediate B. The subsequent dehydration of B leads to intermediate C. Finally, product 3a is generated after de-coordination.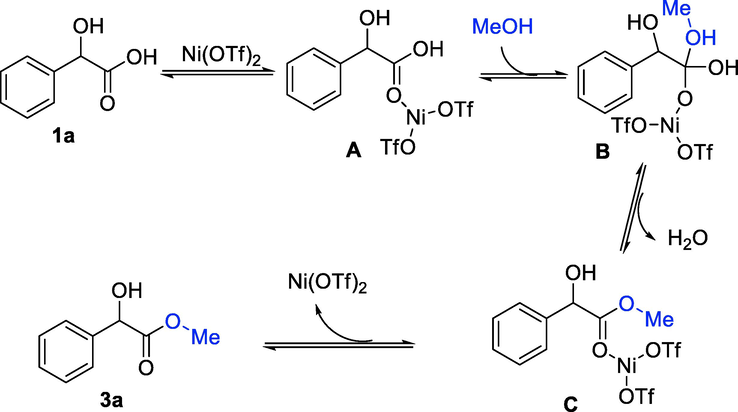
Proposed mechanism.
4 Conclusions
In summary, we have developed a nickel (II)-catalyzed esterification of mandelic acid with various alcohols for the synthesis of mandelic acid ester derivatives. Nickel trifluoromethanesulfonate was used as efficient catalyst to deliver the α-hydroxy esters in yields up to 95 %. The features of the reaction lie in the mild reaction conditions, low catalyst loading and broad substrates scope. Moreover, gram-scale synthesis of medicine cyclandelate was realized in 85 % yield.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 22061040 and 21562039) and the Natural Science Foundation of Xinjiang (No. 2020D01C024).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cyclandelate as a calcium modulating agent in rat cerebral cortex. Drugs. 1987;33:67-74.
- [Google Scholar]
- Asymmetric Friedel-Crafts Alkylations of Indoles with Ethyl Glyoxylate Catalyzed by (S)-BINOL-Titanium(IV) Complex: Direct Access to Enantiomerically Enriched 3-Indolyl(hydroxy)acetates. Adv. Synth. Catal.. 2007;349:1597-1603.
- [Google Scholar]
- Survey of GMP bulk reactions run in a research facility between 1985 and 2002. Org. Proc. Res. Dev.. 2005;9:253-258.
- [Google Scholar]
- Cobalt-Catalyzed transfer hydrogenation of α-Ketoesters and N-Cyclicsulfonylimides using H2O as hydrogen Source. Adv. Synth. Catal.. 2019;361:3991-3997.
- [Google Scholar]
- Synthesis and pharmacological activity of tropine phenylglyoxylate and its derivatives: A new method for the preparation of homatropine. Pharm. Chem. J.. 1977;11:905-909.
- [Google Scholar]
- The Bu4NI-catalyzed alfa-acyloxylation of ketones with benzylic alcohols. Chem. Commun.. 2014;50:6240-6242.
- [Google Scholar]
- Chiral-at-Iron catalyst: expanding the chemical space for asymmetric earth-abundant metal catalysis. J. Am. Chem. Soc.. 2019;141:4569-4572.
- [Google Scholar]
- Boric acid catalyzed chemoselective esterification of α-Hydroxycarboxylic scids. Org. Lett.. 2004;6:679-681.
- [Google Scholar]
- Aerobic copper catalyzed α-oxyacylation of ketones with carboxylic acids. Org. Chem. Front.. 2017;4:163-169.
- [Google Scholar]
- Novel SO3H-functionalized polyoxometalate-based ionic liquids as highly efficient catalysts for esterification reaction. J. Mol. Struct.. 2019;1189:272-278.
- [Google Scholar]
- Highly diastereoselective Friedel−Crafts reaction of furans with 8-Phenylmenthyl Glyoxylate. Org. Lett.. 2006;8:5045-5048.
- [Google Scholar]
- Bu4NI-Catalyzed α-Oxyacylation of carbonyl compounds with toluene derivatives. Org. Lett.. 2016;18:1916-1919.
- [Google Scholar]
- Enantioselective Friedel−Crafts reaction of indoles with carbonyl compounds catalyzed by bifunctional cinchona alkaloids. Org. Lett.. 2006;8:4063-4065.
- [Google Scholar]
- Palladium-catalyzed aerobic oxidative direct esterification of alcohols. Angew. Chem.. 2011;123:5250-5254.
- [Google Scholar]
- Highly selective biocatalytic synthesis of monoacylglycerides in sponge-like ionic liquids. Green Chem.. 2017;19:390-396.
- [Google Scholar]
- Esterification of the primary benzylic C-H bonds with carboxylic acids catalyzed by Ionic Iron(III) complexes containing an imidazolinium cation. Org. Lett.. 2017;19:1132-1135.
- [Google Scholar]
- Highly Enantioselective Synthesis of 2-Furanyl-hydroxyacetates from Furans via the Friedel−Crafts Reaction. Org. Lett.. 2008;10:2955-2958.
- [Google Scholar]
- Highly Enantioselective Friedel−Crafts Reaction of Thiophenes with Glyoxylates: Formal Synthesis of Duloxetine. Org. Lett.. 2009;11:4636-4639.
- [Google Scholar]
- Enantioselective Friedel-Crafts reaction of acylpyrroles with glyoxylates catalyzed by BINOL–Ti(IV) complexes. Org. Lett.. 2011;13:5944-5947.
- [Google Scholar]
- Maki, T.; Ishihara, K.; Yamamoto, H. N-Alkyl-4-boronopyridinium Halides versus Boric Acid as Catalysts for the Esterification of α-Hydroxycarboxylic Acids. Org. Lett. 7, 5047-5050.
- New boron(III)-catalyzed amide and ester condensation reactions. Tetrahedron. 2007;63:8645-8657.
- [Google Scholar]
- Esterification: Methods, Reactions and Applications. Weinheim, Germany: Wiley-VCH; 2003.
- Efficient and practical approach to esters from acids/2-oxoacids/2-oxoaldehydes & /2-oxoesters. Tetrahedron. 2015;71:9388-19295.
- [Google Scholar]
- Asymmetric Catalysis on Industrial Scale. Weinheim: Wiley-VCH; 2004. p. :149-164.
- Chemoenzymatic preparation of enantiomerically enriched (R)-(–)-Mandelic acid derivatives: application in the synthesis of the active agent pemoline. Eur. J. Org. Chem.. 2017;2017:2290-2304.
- [Google Scholar]
- A large-scale asymmetric synthesis of (S)-cyclohexylphenyl glycolic acid. Tetrahedron: Asymmetry. 2003;14:3593-3600.
- [Google Scholar]
- Palladium catalyzed synthesis of mandelate derivatives from arylboronic acids and glyoxylate hemiacetals. Tetrahedron Lett.. 2017;58:2495-2497.
- [Google Scholar]
- Zirconocene-catalyzed direct (trans)esterification of acyl acids (esters) and alcohols in a strict 1 : 1 ratio under solvent-free conditions. Green Chem.. 2017;19:5396.
- [Google Scholar]
- Attrition-Enhanced deracemization in the synthesis of Clopidogrel-A practical application of a new discovery. Org Process Res Dev.. 2009;13:1195-1198.
- [Google Scholar]
- Direct esterification of carboxylic acids with perfluorinated alcohols mediated by XtalFluor-E. Org. Lett.. 2016;18:6468-6471.
- [Google Scholar]
- 1,2-Dibromoethane and KI mediated α-acyloxylation of ketones with carboxylic acids. Chin. Chem. Lett.. 2020;31:711-714.
- [Google Scholar]
- Chemical synthesis of dual-radiolabelled cyclandelate and its metabolism in rat hepatocytes and mouse J774 cells. Xenobiotica. 1990;20:71-79.
- [Google Scholar]
- KI-catalyzed α-acyloxylation of acetone with carboxylic acids. Org. Biomol. Chem.. 2016;14:5936-5939.
- [Google Scholar]
- CDI-promoted direct esterification of P(O)-OH compounds with phenols. Tetrahedron Lett.. 2017;58:2482-2486.
- [Google Scholar]
- Enzyme-Catalyzed enantioselective hydrolysis of ethyl mandelate in ionic liquids. J. Mol. Catal.. 2008;22:341-345.
- [Google Scholar]
- A rapid and green approach to chiral α-hydroxy esters: asymmetric transfer hydrogenation (ATH) of α-keto esters in water by use of surfactants. Tetrahedron: Asymm.. 2009;20:2033-2037.
- [Google Scholar]
- Highly site-selective direct C-H Bond functionalization of phenols with α-Aryl-α-diazoacetates and Diazooxindoles via Gold Catalysis. J. Am. Chem. Soc.. 2014;136:6904-6907.
- [Google Scholar]
- Green esterification of carboxylic acids promoted by tert-Butyl Nitrite. Eur. J. Org. Chem.. 2021;18:2713-12271.
- [Google Scholar]
- Bu4NI-catalyzed direct α-oxyacylation of diarylethanones with acyl peroxides. Org. Biomol. Chem.. 2015;13:9751-9754.
- [Google Scholar]
- NBS/DBU mediated one-pot synthesis of α-acyloxyketones from benzylic secondary alcohols and carboxylic acids. Org. Biomol. Chem.. 2016;14:10998-11001.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104407.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1