Translate this page into:
Hierarchical TiO2 microspheres supported ultrasmall palladium Nanocrystals: A highly efficient catalyst for Suzuki reaction
⁎Corresponding author at:at: School of Chemistry, Tiangong University, No.399 BinShuiXi Road, XiQing District, Tianjin 300387, PR China. pan@tiangong.edu.cn (Shiguang Pan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
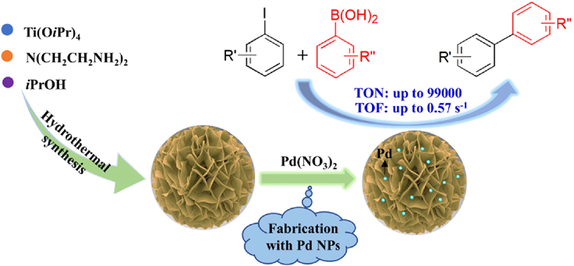
Hierarchical TiO2 microspheres supported ultrasmall palladium nanocrystals were developed. High catalytic efficiency for Suzuki reaction was achieved with an ultra-low loading of Pd species. The catalyst could be recovered by centrifugation and reused 3 times without loss of catalytic activity. The TON and TOF of the catalyst reached as high as 99,000 and 0.57 s−1, respectively.
Abstract
A hierarchical titanium dioxide microspheres-supported palladium catalyst (Pd/TiO2-350) was prepared and characterized using BET, XRD, XPS, SEM, EDX, and TEM analyses. An ICP-OES analysis of Pd/TiO2-350 further confirmed the successful Pd immobilization on TiO2 with a palladium loading of 0.1 mmol g−1. Pd/TiO2-350 efficiently catalyzed the Suzuki-Miyaura reaction of aryl iodides with arylboronic acids to give the corresponding biaryl derivatives in good to excellent yields. After the reaction, the catalyst was recovered by centrifugation and reused three times without significant loss of its catalytic activity. Moreover, the loading of palladium species further decreased to 0.001 mol%, and the total turnover number and turnover frequency of the catalyst reached as high as 99 000 and 0.57 s−1, respectively.
Keywords
Palladium
Titanium Dioxide
Biaryls
Suzuki-Miyaura Reaction
Heterogeneous Catalysis
1 Introduction
Cross-coupling reactions, represented by carbon–carbon bond formation, are an important class of organic reactions that enable key steps in the construction of complex bioactive molecules, drugs and pesticides, organic optoelectronic materials, and other functional molecules (Meijere and Diederich, 2004). Among them, palladium-catalyzed Suzuki-Miyaura coupling reactions of aryl halides with arylboronic acids are one of the most effective methods for carbon–carbon bond formation and the most widely studied cross-coupling reactions (Miyaura and Suzuki, 1995; Hassan et al., 2002; Yin and Liebscher, 2007; Fihri et al., 2011; Mpungose et al., 2018). Various Pd-based homogeneous catalytic systems have been developed for the Suzuki reaction in the past years. The catalytic system shows advantages in simple preparation, high catalytic activity and good selectivity, and an in-depth study of the catalytic mechanism could also be performed (Marion et al., 2006; Marion and Nolan, 2008; Meconi et al., 2017; Li et al., 2018; Zhou et al., 2020; Yang et al., 2021; D’Alterio et al., 2021). However, the high cost due to limited Pd resources and the ease-of-aggregation behavior of palladium limit its large-scale applications (Greenwood and Earnshaw, 2012).
To overcome these limitations, heterogeneous catalysts with high stability and reusability have attracted extensive attention in recent years (Vásquez-Céspedes et al., 2021). In this century, various heterogeneous palladium catalysts have been developed and widely applied in the Suzuki reaction (Miyaura and Suzuki, 1995; Hassan et al., 2002; Yin and Liebscher, 2007; Fihri et al., 2011; Mpungose et al., 2018). Palladium complexes and nanoparticles have been immobilized on various solid supporting materials, such as metal oxides, (Borkowski et al., 2014; Nasrollahzadeh et al., 2015; Hu et al., 2017; Halligudra et al., 2021) silica, (Kim et al., 2012; Kotadia et al., 2014) metal–organic frameworks, (Pascanu et al., 2015; Augustyniak et al., 2016; Veisi et al., 2021; Cartagenova et al., 2022; Lin et al., 2022; Wu et al., 2022) polymers, (Ichikawa et al., 2017; Shokouhimehr et al., 2018; Guo et al., 2019; Ohno et al., 2020; Lin et al., 2021) natural materials (Mallikarjuna et al., 2017; Mallikarjuna et al., 2019; Bathula et al., 2020) and so on, and high performances of these catalysts were achieved. However, some issues remain with these systems, particularly the need for high Pd loading, often accompanied by severe aggregation of Pd nanoparticles. Although homogeneous catalytic systems with ultra-low loading of palladium species have been established, (Roy and Uozumi, 2018) there are few examples of heterogeneous catalytic systems with high recyclability (Yamada et al., 2012; Handa et al., 2015; Ding et al., 2020). Thus, developing highly active and reusable heterogeneous catalysts are demanded to realize green-sustainable organic transformation.
On the other hand, titania (TiO2) has been among the most widely studied metal oxide supports over the past years due to its various functional applications (Parrono and Palmisano, 2021). Different TiO2-based heterogeneous catalytic systems have been investigated for the Suzuki reaction (Sreedhar et al., 2011; Liu et al., 2014; Nasrollahzadeh and Mohammad Sajadi, 2016; Han et al., 2017; Mondal et al., 2017; Rohani et al., 2019; Wang et al., 2019; Veisi et al., 2019; Eskandari et al., 2019; Das et al., 2020; Tovar et al., 2021). Due to the equal ionic radii and strong interaction of Ti(IV) (0.605 Å) and Pd(IV) (0.615 Å)/Pd(II) (0.640 Å), highly dispersed ultrasmall Pd nanoparticles could be achieved using TiO2 as a support, because Pd species diffusely adhered to lattice interstices of TiO2, and then highly dispersed seeds of Pd precipitate can be obtained by the precipitant (Zhao et al., 2021). In previous work, the catalyst of TiO2 microspheres-supported palladium nanoparticles was developed for CO oxidation (Zhai et al., 2018). The results suggested that a Pd catalyst supported on TiO2 microspheres showed higher activity and stability. The special nanosheet structure in the TiO2 sphere induced close contact between TiO2 and Pd nanoparticles during the calcined procedure, which positively affects Pd's dispersion and stability. This study provided a common and efficient method for highly dispersed and stabilized noble metals. In this work, a hierarchical-titania-microspheres-supported palladium nanocatalyst was prepared according to the previous method with a minor modification and characterized by different technologies including N2 adsorption–desorption isotherm, XRD, XPS, SEM, EDX, TEM, and ICP-OES analyses. The highly dispersed ultrasmall Pd nanoparticles (1.9–3.9 nm) immobilized on TiO2 showed an outstanding catalytic performance for the coupling reaction of aryl iodides with arylboronic acids at ultra-low amount loading of palladium species (0.001–0.02 mol %), and the total turnover number and turnover frequency of the catalyst reached as high as 99,000 and 0.57 s−1, respectively. Furthermore, the catalyst could be recycled three times without significant loss of catalytic activity.
2 Results and discussion
The catalyst of hierarchical TiO2 microspheres-supported palladium nanoparticles (Pd/TiO2-350) was prepared according to our previous method with a minor modification, (Zhai et al., 2018) as shown in Scheme 1. A mixture of titanium tetraisopropoxide; diethylenetriamine and isopropanol was stirred at room temperature for 3 min and then transferred into a Teflon autoclave, heated to 200 °C in an oven for 24 h, followed by wet-impregnated with Pd(NO3)2, and finally calcined in a muffle furnace at 350 °C (2 °C min−1) for 4 h to give the desired catalyst, which was denoted as Pd/TiO2-350. Palladium nanoparticles immobilized on Degussa P25, and commercial anatase (CA) were prepared following the same incipient wetness impregnation method and calcinated at the same temperature, denoted as Pd/TiO2-P25 and Pd/TiO2-CA, respectively.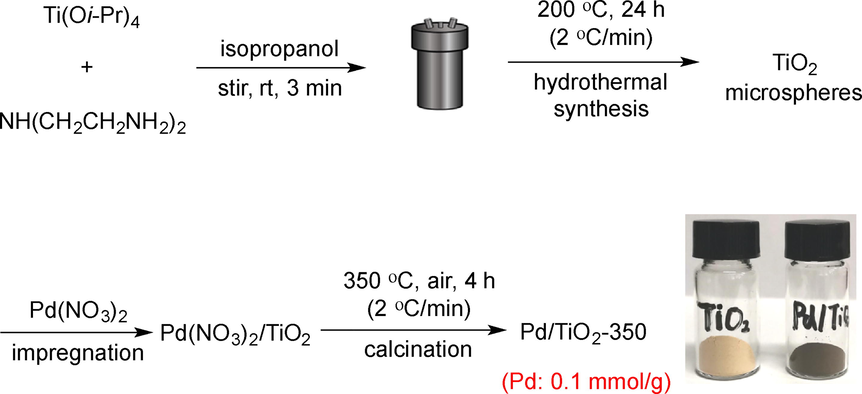
The synthesis procedure of the Pd/TiO2-350 catalyst.
Various analytical methods characterized the as-synthetized TiO2 microspheres and the catalyst of Pd/TiO2-350. Field-emission scanning electron microscopy (FE-SEM) images of as-synthetized TiO2 microspheres and Pd/TiO2-350 catalyst were shown in Fig. 1a and 1b. The as-synthetized TiO2 microsphere was formed by the self-assembly of TiO2 nanoplates, and Pd/TiO2-350 also showed similar spherical structures; however, the surface morphology of the TiO2 microspheres slightly changed after loading Pd species. Energy-dispersive X-ray (EDX) analysis of Pd/TiO2-350 showed the presence of palladium in the catalyst (Fig. 1c), clearly confirming the immobilization of palladium species onto TiO2 microspheres. An ICP-OES analysis of Pd/TiO2-350 further confirmed the successful Pd immobilization on TiO2 with a mass loading of 10.7 mg g−1, which means the palladium loading was 0.1 mmol g−1.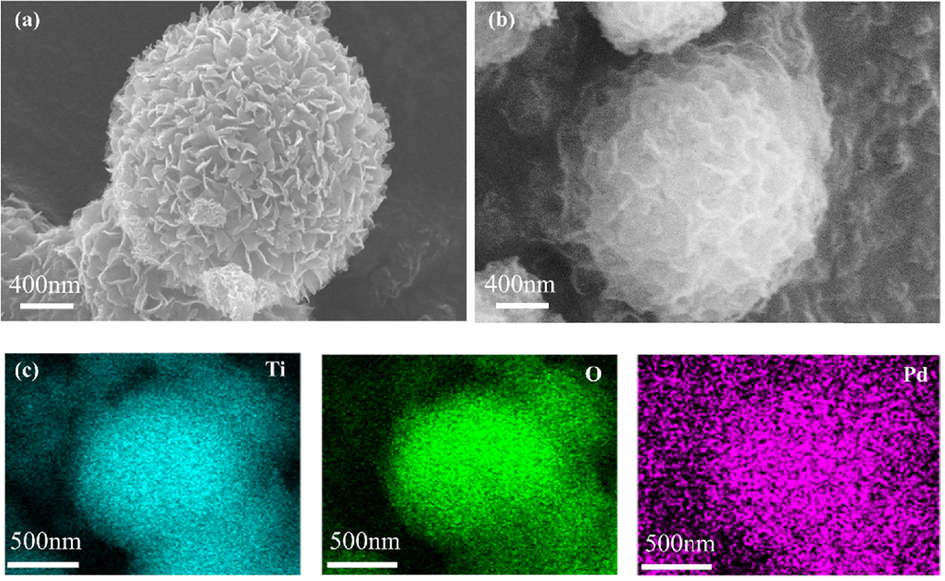
SEM images of (a) TiO2 microspheres, (b) Pd/TiO2-350 catalyst; and (c) EDX mappings of Pd/TiO2-350 catalyst.
The TEM image revealed that palladium nanoparticles were generated and dispersed inside the TiO2 matrix (Fig. 2a). The HR-TEM spectra of the Pd/TiO2-350 confirmed the size of Pd nanoparticles (Fig. 2b) in the range of 1.9–3.9 nm (average: 3.1 nm). Lattice distances of 0.224 nm in the HR-TEM spectra of the Pd/TiO2-350 catalyst are ascribed to the (1 1 1) planes of the Pd(0) species. To further characterize the palladium state of the Pd/TiO2-350 catalyst, an X-ray photoelectron spectroscopy (XPS) analysis was conducted. The XPS spectra of Pd 3d revealed the presence of Pd(0) and Pd(II) species in the Pd/TiO2-350 catalyst (Fig. 3b). XRD patterns of the as-synthetized TiO2 microspheres and the supported palladium catalyst are shown in Fig. 4. The as-synthetized TiO2 microspheres displayed the typical peak of the crystalline anatase phase of TiO2 (JCPDS 84–1285) at 25.3°, 36.9°, 37.8°, 38.6°, etc. However, no information of the Pd(0) and Pd(II) phases was detected in the XRD patterns of the prepared catalyst, probably due to the high dispersion of Pd nanocrystals.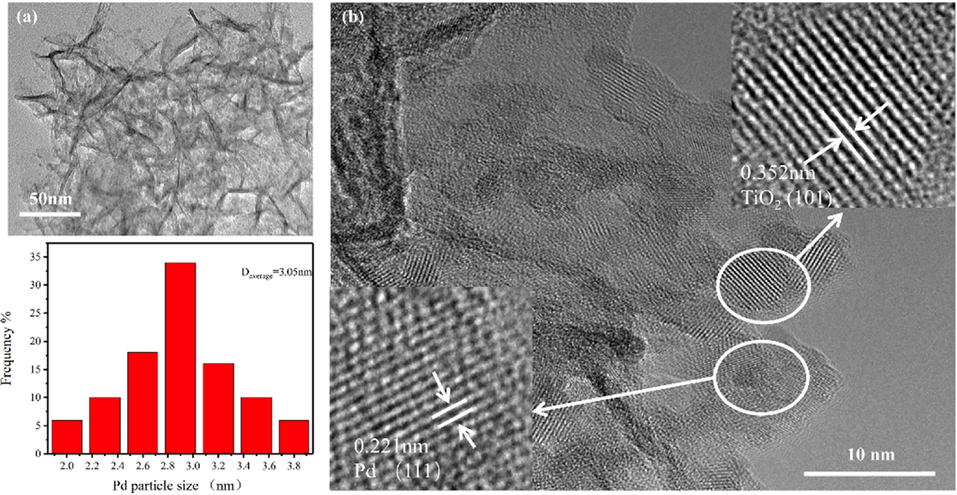
TEM images of Pd/TiO2-350 catalyst.
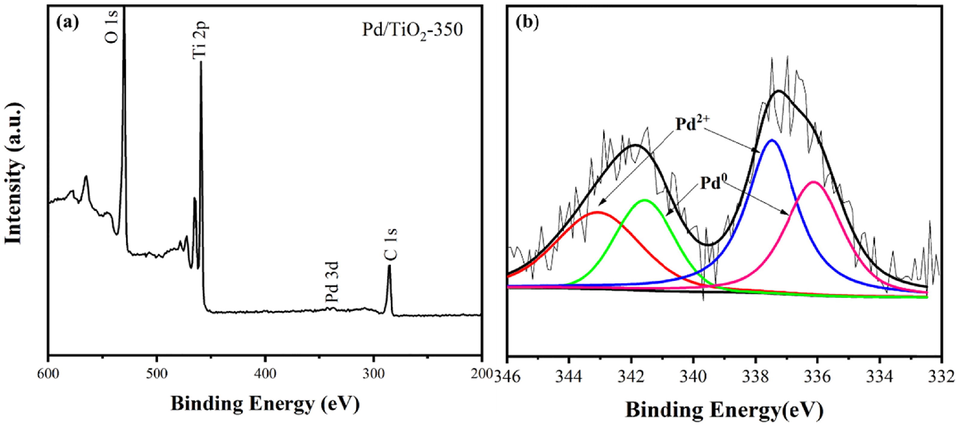
(a) Full range XPS spectrum, (b) Pd 3d XPS spectrum of the Pd/TiO2-350 catalyst.
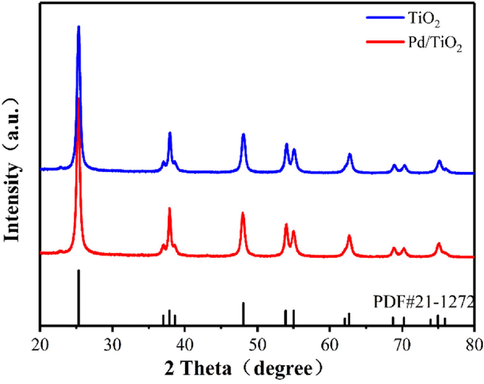
XRD patterns of TiO2 microspheres and the prepared Pd/TiO2-350.
With the hierarchical TiO2 microspheres-supported palladium catalyst (Pd/TiO2-350) in hand, we examined the reaction conditions for the Suzuki-Miyaura reaction (Table 1). The reaction of iodobenzene with phenylboronic acid performed in the presence of Pd/TiO2-350 (0.02 mol% active metal) in EtOH/H2O (v/v = 1:1) under ambient conditions. Firstly, several inorganic and organic bases were examined for this transformation (entries 1–5). The use of K2CO3 gave the desired biphenyl (3a) in 99 % GC yield, and the highest turnover frequency (TOF) reached 1650 h−1. The Pd/TiO2 catalysts that calcinated at different temperatures (300 °C, 400 °C, and 450 °C) were also submitted to the same reaction (Supporting information, Table S1). The results showed that the Pd/TiO2 with a calcination temperature of 350 °C has an excellent catalytic activity, and the highest yield of 3a was achieved. No desired product was detected when the reaction was carried out without a base or a palladium catalyst (entries 6 and 7). The as-synthetized TiO2 microspheres were used only as a catalyst in this transformation. However, none of 3a was formed, clearly confirming that an ultra-low amount of palladium species (0.02 mol% active metal) promoted this transformation (entry 8). Palladium nanoparticles immobilized on commercially available titania (Degussa P25 and anatase) were prepared under the same conditions as Pd/TiO2-350 and applied to this transformation. However, the yields of these catalysts (80 % and 59 %, respectively) didn’t exceed that of Pd/TiO2-350 under the same conditions (Entries 9 and 10). These results clearly confirmed that the synthesized hierarchical TiO2 microsphere was superior to commercial titania as the support. When bromobenzene was used as a substrate, which replaced iodobenzene in this reaction, the yield only reached 69 % even at a prolonged reaction time (Entry 11). We chose the conditions in entry 2 for further examinations.
Entry
Pd cat.
Base
Yield of 3a [%]b
TOF [h−1]
1
Pd/TiO2-350
Na2CO3
<5
<83
2
Pd/TiO2-350
K2CO3
99(93)c
1650
3
Pd/TiO2-350
Cs2CO3
82
1367
4
Pd/TiO2-350
NEt3
35
583
5
Pd/TiO2-350
Pyridine
0
0
6
Pd/TiO2-350
–
0
0
7
–
K2CO3
0
0
8
-/TiO2-350
K2CO3
0
0
9
Pd/TiO2-P25
K2CO3
80
1333
10
Pd/TiO2-CA
K2CO3
59
983
11d
Pd/TiO2-350
K2CO3
69
48
Furthermore, the physical properties of titania (TiO2) and the prepared catalysts were analyzed by BET analysis. As shown in Table 2, the as-synthetized TiO2 microspheres have a large surface area (235.8 m2 g−1), while commercially available titania (Degussa P25 and anatase) has a smaller surface area. After the immobilization of palladium species and calcination, the surface area of Pd/TiO2-350 decreased slightly, but the pore volume and average pore diameter increased obviously. Although the average pore diameter of Pd/TiO2-P25 reached 20.25 nm, the surface area and pore volume were smaller than that of Pd/TiO2-350. The physical property of Pd/TiO2-CA did not change significantly after loading palladium species. The results were in line with the catalytic activities of the catalysts, indicating that the large surface area of the as-synthetized TiO2 microspheres facilitates high dispersion of Pd.
Samples
SBET
[m2 g−1]Pore volume [cm3 g−1]
Average pore diameter [nm]
TiO2 microspheres
235.8
0.499
8.46
TiO2-P25
53.8
0.336
23.48
TiO2-CA
9.7
0.037
15.30
Pd/TiO2-350
210.3
0.586
11.14
Pd/TiO2-P25
39.7
0.201
20.25
Pd/TiO2-CA
9.0
0.038
16.91
With the optimal reaction in hand, we next examined the substrate scope in the presence of Pd/TiO2-350 (0.02 mol% active metal) in EtOH/H2O (v/v = 1:1). As shown in Table 3, the reactions of iodobenzene with different arylboronic acids gave the desired products (3b-3f) in good to excellent yields (Entries 1–5). Similarly, it was found that iodobenzene with both an electron-donating group and an electron-withdrawing group reacted with phenylboronic acid to form the corresponding biphenyl derivatives in high yields (Entries 6–18). Functional groups such as methyl, methoxy, chloro, cyano and ketone groups can be tolerated in this transformation, and excellent yields were achieved. Furthermore, no dechlorination occurred in the case of chloro-substituted phenylboronic acids, providing an opportunity for further functionalization of the products (Entries 3, 8, 14 and 17).
Entry
1 [R = ]
2 [R’ =]
Yield of 3 [%]b
1
H
OCH3
3b, 99
2
H
CH3
3c, 85
3
H
Cl
3d, 99
4
H
CN
3e, 99
5
H
COCH3
3f, 99
6
OCH3
CH3
3g, 99
7
OCH3
H
3b, 95
8
OCH3
Cl
3h, 96
9
OCH3
CN
3i, 91
10
CH3
CH3
3j, 90
11
CH3
CN
3k, 99
12
CH3
COCH3
3l, 96
13
CN
CH3
3k, 90
14
CN
Cl
3m, 86
15
CN
COCH3
3n, 89
16
COCH3
H
3f, 99
17
COCH3
Cl
3o, 99
18
COCH3
CN
3n, 92
To demonstrate the generality of this method, we further decreased the palladium loading in this transformation. The reaction of iodobenzene (1a) with phenylboronic acid (1b) was carried out in a 10.0 mmol scale in the presence of 0.001 mol% palladium species, and the desired biphenyl 3a was obtained in 99 % yield at a prolonged reaction time (Scheme 2a). The product of 3b was also obtained in an excellent yield under similar reaction conditions (Scheme 2b). The total turnover number and turnover frequency of the catalyst reached as high as 99 000 and 0.57 s−1, respectively. These results clearly demonstrated that the Suzuki-Miyaura reaction using Pd/TiO2-350 as a catalyst is a practical method for synthesizing biphenyl derivatives.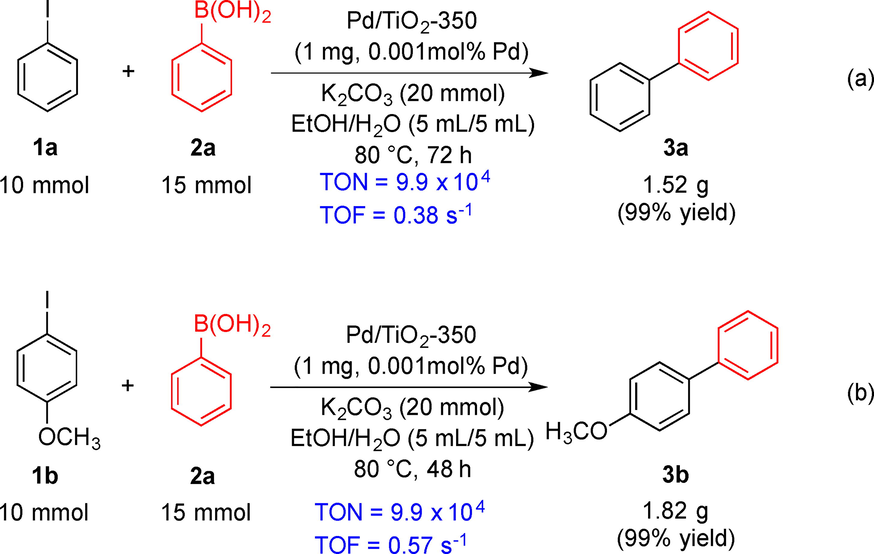
Gram-scale synthesis of biaryl derivatives.
Catalyst recycling is important in relation to both industrial applications and green-sustainable transformation. A recycling experiment with Pd/TiO2-350 catalyst was performed in the reaction of iodobenzene with phenylboronic acid. After completion of the reaction, the catalyst was separated from the reaction mixture by centrifugation, washed with ethyl acetate, and air-dried carefully for the next cycle. ICP analysis showed that 2.3 % of palladium species were leached to the solution. The catalyst could be reused three times without significant loss of its catalytic activity (Fig. 5).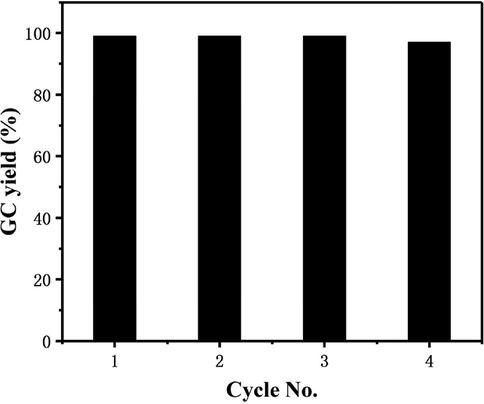
Recycling experiments of Pd/TiO2-350.
A comparison of the efficiency of various titania-supported palladium catalysts with Pd/TiO2-350 in the Suzuki coupling reactions in the literature is listed in Table 4. This comparison clearly showed that the catalyst Pd/TiO2-350 showed a high turnover number (TON) and turnover frequency (TOF) under less-toxic reaction conditions. The weakness of this system is its low activity towards bromobenzene and inactivity in relation to chlorobenzene.
Entry
Catalyst
(mol% Pd)Conditions
Recycle
(times)Ref.
1
TiO2-Pd(0) (0.8)
EtOH/H2O, K2CO3, rt
7
Tovar et al., 2021
2
Pd//TiO2 (0.7)
NMP/H2O, Na2CO3, 120 °C
5
Veisi et al., 2019
3
Au-Pd//TiO2 (1)
EtOH/H2O, K2CO3, rt, blue LED
5
Veisi et al., 2021
4
Pd/MTiO2 (0.96)
H2O, K2CO3, TBAB, 85 °C
6
Wang et al., 2019
5
TiO2/IL-Pd (0.2)
EtOH/H2O, K2CO3, 25 °C
6
Yang et al., 2021
6
LIS-Pd/TiO2 (0.3)
EtOH/H2O, K2CO3, 60 °C (MWI)
4
Zhai et al., 2018
7
Pd/TiO2-350
(0.001–0.02)EtOH/H2O, Cs2CO3, 80 °C
4
this work
A possible catalytic reaction cycle for the Suzuki reaction catalyzed by Pd/TiO2-350 was shown in Scheme 3. At first, over the active Pd-sites present in TiO2-supported heterogeneous catalyst, oxidative addition occurs, followed by transmetallation of arylboronic acid and reductive elimination of the final product.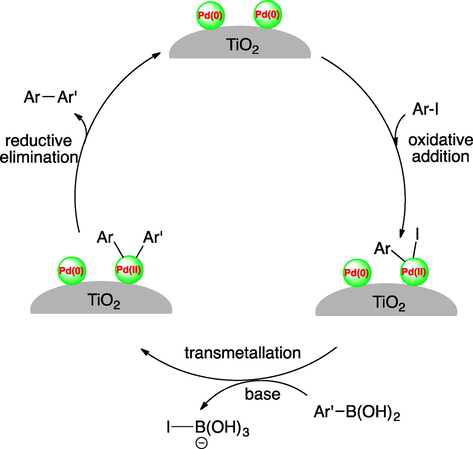
A possible mechanism for the Suzuki reaction catalyzed by Pd/TiO2-350.
In summary, a hierarchical titanium dioxide microspheres-supported palladium catalyst (Pd/TiO2-350) was prepared and applied to the Suzuki-Miyaura coupling of aryl iodides with arylboronic acids. The highly dispersed ultrasmall Pd nanoparticles (1.9–3.9 nm) immobilized on TiO2 showed an outstanding catalytic performance, and the loading of palladium species could be decreased to 0.001 mol%. After the reaction, the catalyst could be reused 3 times without significant loss of catalytic activity. Efforts to extend the range of applications for the supported palladium catalysts to other transformations are currently ongoing in our laboratory.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China,China (No. 21908164), Tianjin Science and Technology Program (No. 21ZYJDJC00100), Tianjin Innovative Research Team in Universities (Grant No. TD13-5031), and Tianjin 131 Research Team of Innovative Talents. S.P. also acknowledges financial support from Tianjin Municipal Education Commission (No. 2019KJ007).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dalton Trans.. 2016;45:13525-13531.
- [CrossRef]
- Colloids Surf. B Biointerfaces. 2020;192:111026
- [CrossRef]
- J. Catal.. 2014;319:87-94.
- [CrossRef]
- Catal. Sci. Technol.. 2022;12:954-961.
- [CrossRef]
- Chem.-Eur. J.. 2021;27:13481-13493.
- [CrossRef]
- ACS Appl. Mater. Interfaces. 2020;12:23844-23852.
- [CrossRef]
- Commun. Chem.. 2020;3:43.
- [CrossRef]
- Appl. Organomet. Chem.. 2019;33:e5093.
- Chem. Soc. Rev.. 2011;40:5181-5203.
- [CrossRef]
- Chemistry of the Elements (second ed.). Oxford: Elsevier; 2012.
- ChemSusChem. 2019;12:1421-1427.
- [CrossRef]
- ACS Omega. 2021;6:34416-34428.
- [CrossRef]
- Nanoscale. 2017;9:6026-6032.
- [CrossRef]
- Science. 2015;349:1087-1091.
- [CrossRef]
- Chem. Rev.. 2002;102:1359-1470.
- [CrossRef]
- RSC Adv.. 2017;7:7964-7972.
- [CrossRef]
- Adv. Synth. Catal.. 2017;359:2269-2279.
- [CrossRef]
- Langmuir. 2012;28:6441-6447.
- [CrossRef]
- RSC Adv. 2014;4:32826-32833.
- [CrossRef]
- ChemCatChem. 2018;10:3096-3106.
- [CrossRef]
- . Technol.. 2021;11:3676-3680.
- [CrossRef]
- Catal. Lett. 2022
- [CrossRef]
- Chem. Commun.. 2014;50:12356-12359.
- [CrossRef]
- Mater. Lett.. 2017;205:138-141.
- [CrossRef]
- Int. J. Biol. Macromol.. 2019;126:352-358.
- [CrossRef]
- J. Am. Chem. Soc.. 2006;128:4101-4111.
- [CrossRef]
- Acc. Chem. Res.. 2008;41:1440-1449.
- [CrossRef]
- Organometallics. 2017;36:2088-2095.
- [CrossRef]
- Meijere A., de Diederich F., eds. Metal-Catalyzed Cross-Coupling Reactions. Wiley; 2004.
- Chem. Rev.. 1995;95:2457-2483.
- [CrossRef]
- J. Colloid Interface Sci.. 2017;508:378-386.
- [CrossRef]
- Molecules. 2018;23:1676.
- [CrossRef]
- J. Mol. Catal. Chem.. 2015;396:31-39.
- [CrossRef]
- J. Colloid Interface Sci.. 2016;465:121-127.
- [CrossRef]
- Adv. Synth. Catal.. 2020;362:4687-4698.
- [CrossRef]
- Titanium Dioxide (TiO₂) and Its Applications. Elsevier; 2021.
- ChemSusChem. 2015;8:123-130.
- [CrossRef]
- Catal. Sci. Technol.. 2019;9:3820-3827.
- [CrossRef]
- Adv. Synth. Catal.. 2018;360:602-625.
- [CrossRef]
- Green Chem.. 2018;20:3809-3817.
- [CrossRef]
- Adv. Synth. Catal.. 2011;353:2823-2836.
- [CrossRef]
- J. Catal.. 2021;398:133-147.
- [CrossRef]
- Org. Process Res. Dev.. 2021;25:740-753.
- [CrossRef]
- Appl. Organomet. Chem.. 2019;33:e4909.
- Sci. Rep.. 2021;11:21883.
- [CrossRef]
- Colloids Surf. Physicochem. Eng. Asp.. 2019;572:283-289.
- [CrossRef]
- New J. Chem.. 2022;46:8575-8582.
- [CrossRef]
- J. Am. Chem. Soc.. 2012;134:3190-3198.
- [CrossRef]
- Catal. Sci. Technol.. 2021;11:3189-3197.
- [CrossRef]
- Chem. Rev.. 2007;107:133-173.
- [CrossRef]
- New J. Chem.. 2018;42:18066-18076.
- [CrossRef]
- ACS Appl. Nano. Mater.. 2021;8:159-166.
- [CrossRef]
- IScience. 2020;23:101377
- [CrossRef]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104410.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







