Translate this page into:
Synthesis, anticancer evaluation, and molecular modeling study of new 2-(phenylamino)pyrazolo[1,5-a]pyrimidine analogues
⁎Corresponding author. N_elmetwaly00@yahoo.comnmm (Nashwa M. El-Metwaly) nmmohamed@uqu.edu.sa (Nashwa M. El-Metwaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The reaction of 3-amino-5-phenylaminopyrazoles 2 with 3-(dimethylamino) acrylonitrile derivatives resulted in a series of substituted pyrazolopyrimidine analogues 4 and 6. The DFT studies of the isolated compounds showed that the frontier molecular orbitals energy gap was close and in the 2.65–2.81 eV range where the derivative 6b has the lowest and both of 4a and 4c have the highest values. Meanwhile, the anticancer activity of the newly synthesized pyrazolopyrimidine analogues have been tested against several different cell lines (MCF-7, PC3, Hep-2 and WI38). The investigated pyrazolopyrimidines showed remarkable cytotoxicity activity against the MCF-7 and Hep-2 cell lines. In comparison to the effects of 5-fluorouracil, IC50 = 10.19 ± 0.42 and 7.19 ± 0.47, compounds 6a-c demonstrated potential anticancer activity with IC50 values for MCF-7 (10.80 ± 0.36–19.84 ± 0.49 μM) and Hep-2 (8.85 ± 0.24–12.76 ± 0.16 μM). Important details regarding the protein's binding sites were disclosed when the produced analogues docked with the crystal structure of the KDM5A protein, which was located in the protein data library.
Keywords
3-aminopyrazoles
Pyrazolopyrimidines
Molecular modelling
MCF-7
Molecular docking
1 Introduction
A significant heterocyclic moiety, the pyrimidine moiety exhibits a wide range of biological and pharmacological activity (Sathisha, Gopala et al. 2016, Jubeen, Iqbal et al. 2018, Nerkar 2021). Nucleotides, nucleic acids, vitamins, coenzymes, purines, pterins, and uric acids are examples of naturally occurring molecules that contain this six-membered 1,3-diazine ring with nitrogen at the 1 and 3 positions (Johnson and Khan 2018). The numerous medicinal uses of pyrimidine may be explained by the fact that it is a component of DNA and RNA (Joshi, Nayyar et al. 2016, Taylor, Houlihan et al. 2019). The first analogues to be examined for biological action were 5-halogenated analogues of pyrimidine moiety (Alcolea Palafox 2014, Matyugina, Logashenko et al. 2015). This heterocyclic moiety is part of several drugs like a fused, stiff, and planar N-heterocyclic system that contains both of pyrazole and pyrimidine moieties is known as the pyrazolo[1,5-a]pyrimidine (PP) (Arias-Gómez, Godoy et al. 2021, Mahapatra, Prasad et al. 2021). Due to its exceptional synthetic flexibility, this fused pyrazole is a preferred scaffold for combinatorial library design and drug discovery (Mackman, Sangi et al. 2015, Shirvani, Fassihi et al. 2019). This elasticity enables structural modifications all the way around its border. After the chief critical evaluation involving this interesting scaffold (Jismy, Tikad et al. 2020), several reviews regarding to the achievement and subsequent derivatization procedures have been designated in the literature (El Sayed, Hussein et al. 2018, Pinheiro et al. 2020, Kumar, Das et al. 2021). In spite of these discoveries, the synthetic alterations covering this theme continue to be an exploration in terms of the effectiveness of the development, the influence on the setting, and the investigation of its various uses. The methods that aim to shorten synthesis routes, use affordable reagents, and create waste-prevention or -reduction techniques should be covered in these studies. Typically, the preparation of PP analogues includes the formation of pyrimidine rings by reaction with various 1,3-biselectrophilic reagents with aminopyrazoles (Castillo and Portilla 2018, Al-Azmi 2019, Salem, Helal et al. 2019). Pyrazolo[1,5-a]pyrimidine analogues is considered an important fragment for bioactive materials through their unique properties corresponding to selectivity as a protein inhibitor (Asano, Yamazaki et al. 2012), anticancer (Zhao, Ren et al. 2016), between others outstanding qualities (Lunagariya, Thakor et al. 2018, Ali, Ibrahim et al. 2019, Almehmadi, Alsaedi et al. 2021). Additionally, the pyrazolo[1,5-a]pyrimidine biocompatibility and lower toxicity resulted in a wide range derivatives to be employed as bioactive materials under commercial names like “Zaleplon, Indiplon, Lorediplon, Reversan, Dorsomorphin, Pyrazophos, Anagliptin, and Presatovir” (Fig. 1) (Eftekhari-Sis and Zirak 2015). Recently, this molecular component has been the subject of research for potential new uses in the field of materials sciences, because of its outstanding photophysical characteristics as an emerging fluorophore (Castillo, Rosero et al. 2017, Tigreros, Aranzazu et al. 2020, Tigreros, Macias et al. 2021, Tigreros, Zapata-Rivera et al. 2021). Similarly, the propensity of pyrazolo[1,5-a]pyrimidine analogues to take the crystalline shapes with prominent conformations and supramolecular behaviors (Secrieru, O’Neill et al. 2019, Tigreros, Macías et al. 2022) could magnify their uses against the solid-state. From the previous literatures as substituted PPs presented good bioactivity, it encouraged us to synthesis another PP substituted with different groups such as substituted amide, substituted amino, and nitrile groups besides study their anticancer activities toward different cell lines in addition to study their theoretical activity through modeling and docking stimulation toward cancer protein.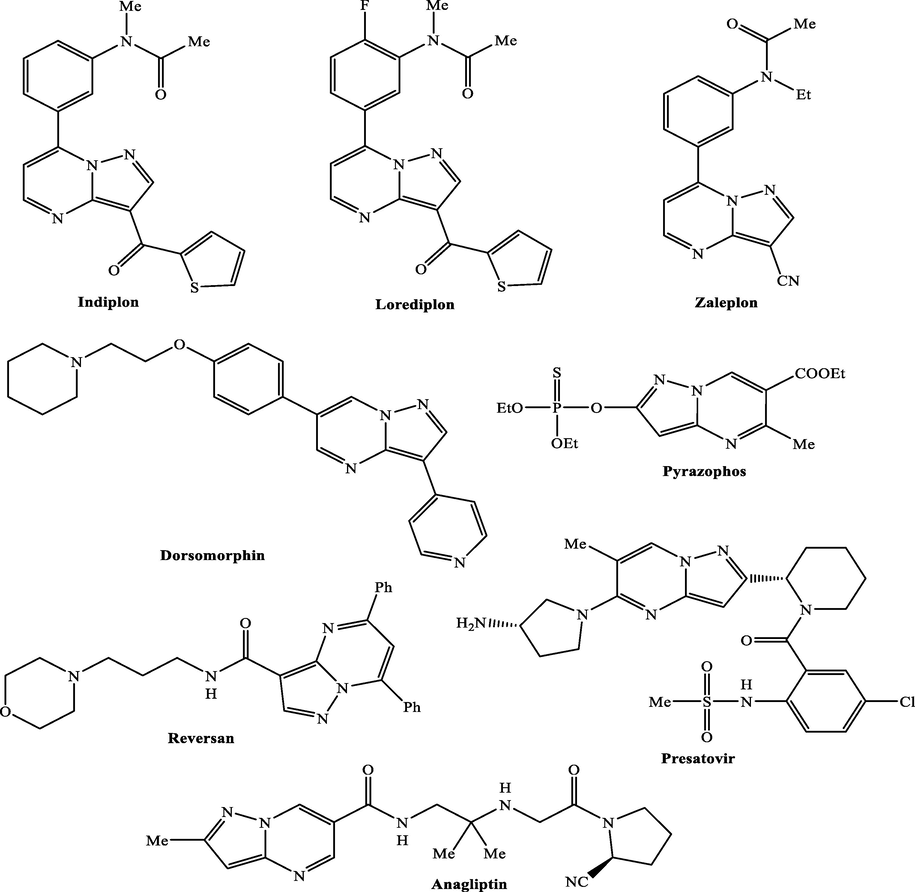
Chemical structures of some derivatives bearing pyrazolopyrimidine motif.
2 Experimental
2.1 General remarks
Melting point measurements are performed using the Gallenkamp electric apparatus. The IR spectra were captured using a Thermo Scientific Nicolet iS10 FTIR spectrometer (KBr discs). The NMR spectra were obtained in DMSO‑d6 using a JEOL NMR spectrometer at 500 MHz (1H NMR) and 125 MHz (13C NMR). The mass analyses were carried out on a Thermo Scientific Focus/DSQII Quadrupole GC/MS (70 eV). The C, H, and N elemental analyses were carried out using a Perkin-Elmer 2400 analyzer.
2.2 Preparation of 3-amino-N-aryl-5-(phenylamino)-1H-pyrazole-4-carboxamide analogues 2a-c
Hydrazine hydrate 80 % (1.00 ml, 20 mmol) was added to each solution of the ketene S,N-acetal derivative 1a, 1b, or 1c (15 mmol) in 40 ml of ethanol. The mixture in each case was heated under reflux for 6 h and the precipitated solid was filtered to pick up the targeting 3-amino-5-phenylamino-pyrazole derivatives 2a, 2b, and 2c, respectively (Elgemeie, Elghandour et al. 2004, Elgemeie and Jones 2004, Mukhtar, Hassan et al. 2021).
2.3 Preparation of 7-amino-N-aryl-6-cyano-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4a-c
To a solution of 3-amino-5-phenylamino-pyrazole derivative 2a, 2b, or 2c (5 mmol) in 40 ml dioxane and 0.2 ml triethylamine, 2-((dimethylamino)methylene)-malononitrile (3) (0.60 g, 5 mmol) was added. The solution was heated under reflux for 3–4 h, the progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to 30 °C. Crystallization of the obtained solid from a dioxane yielded the conforming pyrazolo[1,5-a]pyrimidine analogues 4a, 4b, and 4c, respectively.
7-Amino-6-cyano-2-(phenylamino)-N-(p-tolyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (4a):
Yellow crystals, yield = 71 %, m.p. = 222–223 °C, Rf = 0.43 (hexane:EtOAc = 3:1). IR (ν/cm−1): 3343, 3305, 3267, and 3190 (NH2 and 2 N—H), 3056 (C—H, aromatic), 2941 (C—H, aliphatic), 2210 (C≡N), 1661 (C⚌O). 1H NMR (δ/ppm): 2.29 (s, 3H, –CH3), 6.87 (s, 2H, exchangeable by D2O, NH2), 7.14 (t, J = 7.50 Hz, 1H), 7.31 (d, J = 8.50 Hz, 2H), 7.40 (t, J = 8.50 Hz, 2H), 7.46 (d, J = 8.50 Hz, 2H), 7.58 (d, J = 8.50 Hz, 2H), 8.58 (s, 1H, pyrimidine-H), 10.25 (s, 1H, exchangeable by D2O, N—H), 11.68 (s, 1H, exchangeable by D2O, N—H). 13C NMR (δ/ppm): 21.31 (–CH3), 91.08 (C-6), 98.14 (C-3), 116.82 (C≡N), 118.64 (2 Ph-C), 121.11 (2 Ar-C), 123.54 (Ph-C), 129.35 (2 Ar-C), 129.98 (2 Ph-C), 134.67 (Ar-C), 136.70 (Ar-C), 139.96 (Ph-C), 151.38 (fused-C), 155.19 (C-2), 160.47 (C-5), 162.86 (C⚌O), 166.23 (C-7). MS m/z (%): 383 (M+, 27.80). Analysis for C21H17N7O (383.15): Calcd.: C, 65.79; H, 4.47; N, 25.57 %. Found: C, 65.91; H, 4.41; N, 25.65 %.
7-Amino- N-(p-anisyl)-6-cyano-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (4b):
Yellow crystals, yield = 65 %, m.p. = 230–231 °C, Rf = 0.54 (hexane:EtOAc = 3:1). IR (ν/cm−1): 3332, 3294, 3245, and 3192 (NH2 and 2 N—H), 3067 (C—H, aromatic), 2951 (C—H, aliphatic), 2211 (C≡N), 1658 (C⚌O). 1H NMR (δ/ppm): 3.79 (s, 3H, –OCH3), 6.91 (s, 2H, exchangeable by D2O, NH2), 7.04 (d, J = 9.00 Hz, 2H), 7.19 (t, J = 7.50 Hz, 1H), 7.46 (t, J = 7.50 Hz, 2H), 7.56 (d, J = 9.00 Hz, 2H), 7.75 (d, J = 9.00 Hz, 2H), 8.62 (s, 1H, pyrimidine-H), 10.43 (s, 1H, exchangeable by D2O, N—H), 11.56 (s, 1H, exchangeable by D2O, N—H). 13C NMR (δ/ppm): 56.04 (–OCH3), 90.87 (C-6), 97.44 (C-3), 114.91 (2 Ar-C), 116.57 (C≡N), 118.69 (2 Ph-C), 122.42 (2 Ar-C), 123.58 (Ph-C), 130.04 (2 Ph-C), 132.63 (Ar-C), 140.18 (Ph-C), 151.75 (fused-C), 154.80 (C-2), 159.05 (Ar-C), 161.17 (C-5), 163.24 (C⚌O), 165.89 (C-7). MS m/z (%): 399 (M+, 43.29). Analysis for C21H17N7O2 (399.14): Calcd.: C, 63.15; H, 4.29; N, 24.55 %. Found: C, 63.01; H, 4.22; N, 24.44 %.
7-Amino-N-(p-chlorophenyl)-6-cyano-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide (4c):
Yellowish brown crystals, yield = 68 %, m.p. = 243–244 °C, Rf = 0.46 (hexane:EtOAc = 3:2). IR (ν/cm−1): 3343, 3276, 3202 (NH2 and 2 N—H), 3083 (C—H, aromatic), 2214 (C≡N), 1665 (C⚌O). 1H NMR (δ/ppm): 6.94 (s, 2H, exchangeable by D2O, NH2), 7.13 (t, J = 7.50 Hz, 1H), 7.34 (t, J = 7.50 Hz, 2H), 7.48 (d, J = 9.00 Hz, 2H), 7.56 (d, J = 9.00 Hz, 2H), 7.70 (d, J = 7.50 Hz, 2H), 8.60 (s, 1H, pyrimidine-H), 10.41 (s, 1H, exchangeable by D2O, N—H), 10.94 (s, 1H, exchangeable by D2O, N—H). 13C NMR (δ/ppm): 90.38 (C-6), 98.87 (C-3), 116.43 (C≡N), 118.55 (2 Ph-C), 121.69 (2 Ar-C), 123.30 (Ph-C), 128.85 (2 Ar-C), 129.81 (2 Ph-C), 133.17 (Ar-C), 135.93 (Ar-C), 139.49 (Ph-C), 152.07 (fused-C), 155.66 (C-2), 161.89 (C-5), 162.52 (C⚌O), 166.14 (C-7). MS m/z (%): 403 (M+, 36.54). Analysis for C20H14ClN7O (403.09): Calcd.: C, 59.49; H, 3.49; N, 24.28 %. Found: C, 59.67; H, 3.44; N, 24.15 %.
2.4 Synthesis of 7-amino-N3-aryl-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide analogues 6a-c
A mixture of each 3-aminopyrazole compound 2a, 2b, or 2c (5 mmol) 2-cyano-3-(dimethylamino)acrylamide (5) (0.69 g, 5 mmol) in 30 ml dioxane and 0.2 ml triethylamine was heated under reflux for 3–4 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the solid that obtained upon cooling to 30 °C was filtered and crystallized EtOH/DMF mixture (2:1) to give the corresponding pyrazolo[1,5-a]pyrimidine analogues 6a, 6b, and 6c, respectively.
7-Amino-2-(phenylamino)–N3-(p-tolyl)pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide (6a):
Orange crystals, yield = 62 %, m.p. = 265–266 °C, Rf = 0.33 (hexane:EtOAc = 1:1). IR (ν/cm−1): 3369, 3311, 3260, 3194 (NH2 and 2 N—H), 3062 (C—H, aromatic), 2938 (C—H, aliphatic), 1658 (C⚌O). 1H NMR (δ/ppm): 2.32 (s, 3H, –CH3), 6.70 (s, 2H, exchangeable by D2O, NH2), 7.07 (t, J = 7.50 Hz, 1H), 7.28–7.35 (m, 4H), 7.44 (d, J = 8.50 Hz, 2H), 7.57 (d, J = 8.50 Hz, 2H), 7.88 (s, 2H, exchangeable by D2O, NH2), 8.18 (s, 1H, pyrimidine-H), 10.52 (s, 1H, exchangeable by D2O, N—H), 11.37 (s, 1H, exchangeable by D2O, N—H). 13C NMR (δ/ppm): 21.33 (–CH3), 98.51 (C-3), 104.29 (C-6), 118.78 (2 Ph-C), 120.93 (2 Ar-C), 123.47 (Ph-C), 129.27 (2 Ar-C), 129.97 (2 Ph-C), 135.48 (Ar-C), 136.17 (Ar-C), 140.36 (Ph-C), 143.62 (fused-C), 155.39 (C-2), 158.96 (C-5), 163.21 (C⚌O), 165.56 (C-7), 167.09 (C⚌O). MS m/z (%): 401 (M+, 67.11). Analysis for C21H19N7O2 (401.16): Calcd.: C, 62.83; H, 4.77; N, 24.42 %. Found: C, 62.94; H, 4.69; N, 24.50 %.
7-Amino-N3–(p-anisyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide (6b):
Brown powder, yield = 60 %, m.p. = 271–272 °C, Rf = 0.41 (hexane:EtOAc = 1:2). IR (ν/cm−1): 3337, 3282, 3229 (NH2 and 2 N—H), 3078 (C—H, aromatic), 2954 (C—H, aliphatic), 1655 (C⚌O). 1H NMR (δ/ppm): 3.81 (s, 3H, –OCH3), 7.01 (d, J = 9.00 Hz, 2H), 7.07 (s, 2H, exchangeable by D2O, NH2), 7.17 (t, J = 7.50 Hz, 1H), 7.38 (d, J = 7.50 Hz, 2H), 7.56 (t, J = 7.50 Hz, 2H), 7.72 (d, J = 9.00 Hz, 2H), 7.88 (s, 2H, exchangeable by D2O, NH2), 8.21 (s, 1H, pyrimidine-H), 10.26 (s, 1H, exchangeable by D2O, N—H), 11.11 (s, 1H, exchangeable by D2O, N—H). 13C NMR (δ/ppm): 56.05 (–OCH3), 97.85 (C-3), 103.02 (C-6), 114.56 (2 Ar-C), 119.07 (2 Ph-C), 122.44 (2 Ar-C), 123.54 (Ph-C), 129.81 (2 Ph-C), 130.69 (Ar-C), 139.86 (Ph-C), 143.73 (fused-C), 154.98 (C-2), 158.46 (Ar-C), 159.13 (C-5), 163.05 (C⚌O), 164.82 (C-7), 166.95 (C⚌O). MS m/z (%): 417 (M+, 55.08). Analysis for C21H19N7O3 (417.15): Calcd.: C, 60.42; H, 4.59; N, 23.49 %. Found: C, 60.21; H, 4.48; N, 23.63 %.
7-Amino-N3–(p-chlorophenyl)-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide (6c):
Brown powder, yield = 58 %, m.p. = 293–294 °C, Rf = 0.45 (hexane:EtOAc = 1:2). IR (ν/cm−1): 3351, 3307, 3266, 3208 (NH2 and 2 N—H), 3087 (C—H, aromatic), 1657 (C⚌O). 1H NMR (δ/ppm): 6.79 (s, 2H, exchangeable by D2O, NH2), 7.09 (t, J = 7.50 Hz, 1H), 7.28 (d, J = 8.50 Hz, 2H), 7.33–7.38 (m, 4H), 7.63 (d, J = 8.50 Hz, 2H), 7.67 (s, 2H, exchangeable by D2O, NH2), 8.24 (s, 1H, pyrimidine-H), 10.58 (s, 1H, exchangeable by D2O, N—H), 11.46 (s, 1H, exchangeable by D2O, N—H). 13C NMR (δ/ppm): 98.77 (C-3), 105.43 (C-6), 118.81 (2 Ph-C), 121.89 (2 Ar-C), 123.63 (Ph-C), 129.18 (2 Ar-C), 129.78 (2 Ph-C), 133.05 (Ar-C), 135.92 (Ar-C), 140.43 (Ph-C), 144.07 (fused-C), 156.70 (C-2), 158.56 (C-5), 162.91 (C⚌O), 165.12 (C-7), 167.25 (C⚌O). MS m/z (%): 423 (M+ + 2, 16.94), 421 (M+, 48.32). Analysis for C20H16ClN7O2 (421.11): Calcd.: C, 56.95; H, 3.82; N, 23.24 %. Found: C, 57.13; H, 3.89; N, 23.36 %.
2.5 Computational studies
The produced compounds were geometrically optimized using Gaussian 09 W program (Frisch, Trucks et al. 2009) at DFT/B3LYP level and 6–311++G basis set (Lee, Yang et al. 1988, Perdew and Wang 1992, Becke 1993). Positive frequencies obtained for all derivatives were taken as evidence for the optimized geometries stability. The Materials Studio package DMol3 module (BIOVIA 2017) was employed for estimating the Fukui indices by applying the GGA and B3LYP functional methods with DNP basis set (version 3.5) (Delley 2006).
2.6 MTT assay
The produced pyrazolo[1,5-a]pyrimidine derivatives were tested for their ability to suppress the growth of the subsequent cancer cell lines: liver carcinoma (HepG2), laryngeal carcinoma (Hep-2), breast cancer (MCF-7), and prostate cancer PC3. Normal fibroblast cells were also used in the MTT test (WI38). In this test, a colorimetric method is used to change the yellow hue of “tetrazolium bromide” (MTT) into a purple formazan by utilizing mitochondrial succinate dehydrogenase. Cell lines were bred in the meantime using RPMI-1640 media with 10 % bovine serum at 37 °C with 5 % CO2. Next incubation for 24 hrs., cells were uncovered to various concentrations of the produced hybrids. After this, the cells were brooded for a further 4 h before receiving 5 mg/ml of MTT thaw, Liquefaction of the produced purple formazan.
2.7 Molecular docking study
The estimation of molecular docking was carried out using the molecular docking application (MOE 2015.10). We obtained the PDB for the KDM5A structure from (https://www.rcsb.org/PDB codes- 5IVE). The following instructions were followed for arranging the produced protein. i) The structure of the enzyme is cleaned up of objective substances. ii) The MOE method allowed hydrogen atoms to be added to protein while minimizing process, with RMS values of 0.01 kcal.mol−1 and an RMS range of 0.1. iii) The ligands were produced using the MOE builder interface.
3 Results and discussion
3.1 Preparation of 2-(phenylamino)pyrazolo[1,5-a]pyrimidine analogues 4 and 6
In light of the previous literature (Elgemeie, Elghandour et al. 2004, Elgemeie and Jones 2004, Mukhtar, Hassan et al. 2021), the building block 3-aminopyrazole derivatives 2a-c were prepared by reacting the ketene S,N-acetal compounds 1a-c with hydrazine hydrate in boiling ethanol for 6 h (Scheme 1).
Synthesis of 3-aminopyrazole scaffolds 2a-c.
The aminopyrazole compounds 2a-c are used as starting materials for the synthesis of various substituted pyrazolo[1,5-a]pyrimidine analogues. They reacted with 2-((dimethylamino)methylene)-malononitrile (3) to form the 7-amino-N-aryl-6-cyano-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4a-c (Scheme 2). The reaction was carried out by refluxing the reactants for 4 h in dioxane and triethylamine. The intermediate (A) is thought to be formed by the nucleophilic addition of an amino group (from pyrazole) to the β-carbon of acrylonitrile derivative 3. This intermediate (A) undergoes dimethylamine molecule elimination, and the resulting intermediate (B) cyclizes to the pyrimidine ring system via intramolecular addition of an internal N—H group (pyrazole) on the nitrile function. The structures of 7-aminopyrazolo[1,5-a]pyrimidine compounds 4a-c were established by their compatible spectral data. The IR spectrum of 4a (as an example) showed absorptions at 3343, 3305, 3267, and 3190 cm−1 referring to the NH2 and 2 N—H stretching frequencies. The absorptions that observed 2210 and 1661 cm−1 were attributed to the nitrile (-C≡N), and amidic carbonyl (C⚌O) functions. 1H NMR spectrum revealed singlet signals at δ 2.29 and 6.87 ppm for the protons of methyl and amino functions, respectively. The aromatic protons are resonated as triplet and doublet signals at in the region δ 7.14–7.58 ppm. The signal signals at δ 8.58, 10.25 and 11.68 ppm are attributed to the protons of pyrimidine-C and two N—H groups, respectively. The 13C NMR spectrum revealed seventeen signals for twenty-one carbon atoms. The characteristic signals of pyrimidine-CH and amide carbonyl are observed at δ 160.47 and 162.86 ppm. The mass analysis showed the molecular ion peak at m/z = 383 for the formula C21H17N7O.![Synthesis of 2-(phenylamino)pyrazolo[1,5-a]pyrimidine analogues 4a-c.](/content/184/2023/16/1/img/10.1016_j.arabjc.2022.104437-fig3.png)
Synthesis of 2-(phenylamino)pyrazolo[1,5-a]pyrimidine analogues 4a-c.
The chemical reactivity of 3-aminopyrazoles 2a-c with 2-cyano-3-(dimethylamino)acrylamide (5) was also studied. To obtain the targeting 2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide compounds 6a-c, the reaction was carried out in boiling dioxane and triethylamine (Scheme 3). The designed structures of compounds 6a-c were confirmed by their compatible spectral data. The IR spectrum of 6a showed absorptions at 3369, 3311, 3260, and 3194 cm1 to indicate the stretching vibrations of NH2 and two N—H groups. The amide carbonyl group is identified by the broad absorption near 1658 cm−1. The 1H NMR spectrum revealed singlet signals at δ 2.32 and 6.70 ppm for the protons of methyl and amino groups, respectively. The aromatic protons are observed as triplet, multiplet, and doublet signals at δ 7.07–7.57 ppm. The protons of amino group (CONH2) are resonated as singlet at δ 7.88 ppm. The singlet signals that are resonated at δ 8.18, 10.52, and 11.37 ppm identified the pyrimidine-H and two N—H functions. The mass analysis showed the molecular ion peak at m/z = 401 for the formula C21H19N7O2.![Synthesis of 2-(phenylamino)pyrazolo[1,5-a]pyrimidine analogues 6a-c.](/content/184/2023/16/1/img/10.1016_j.arabjc.2022.104437-fig4.png)
Synthesis of 2-(phenylamino)pyrazolo[1,5-a]pyrimidine analogues 6a-c.
3.2 DFT molecular modeling study
The studied compounds optimized structures revealed that the 2-phenylamino pyrazolopyrimidine moiety have almost planar configuration where the nitrogen atom of the phenylamino groups were slightly shifted out plane, NH(Pham)–C2(Pp)-N1(Pp)-N8(Pp) = 178.0° and N1(Pp)–C2(Pp)–NH(Pham)-C1(Pham) = 2.6°. Likewise, the amino and cyano substituents, in 4a-c derivatives, were coplanar with the pyrazolopyrimidine, e.g., NH2(Pp)-C7(Pp)-C6(Pp)-C5(Pp) = -179.4°, C5(Pp)-C6(Pp)–CN(Pp)–NC(Pp) = 179.3° and NH2(Pp)-C7(Pp)-C6(Pp)–CN(Pp) = 0.4°. Although, the carboxamide carbonyl carbon atoms were out the pyrazolopyrimidine plane, e.g., N4(Pp)-C3a(Pp)–C3(Pp)–CO(carb) = 3.3°, whereas its oxygen and nitrogen atoms were strongly shifted out, i.e., C2(Pp)–C3(Pp)–CO(carb)–OC(carb) and C2(Pp)–C3(Pp)–CO(carb)–NH(carb) were −142.0° and 36.2°, respectively. In 6a-c derivatives, the carboxamide group, at position 6, were slightly shifted out the pyrazolopyrimidine plane, e.g., N8(Pp)-C7(Pp)-C6(Pp)–CO(carb1) = -179.1°, C7(Pp)-C6(Pp)–CO(carb1)–OC(carb1) = -2.0° and C5(Pp)-C6(Pp)–CO(carb1)–NH2(carb1) = -1.9°. (Fig. 2) (Table S1).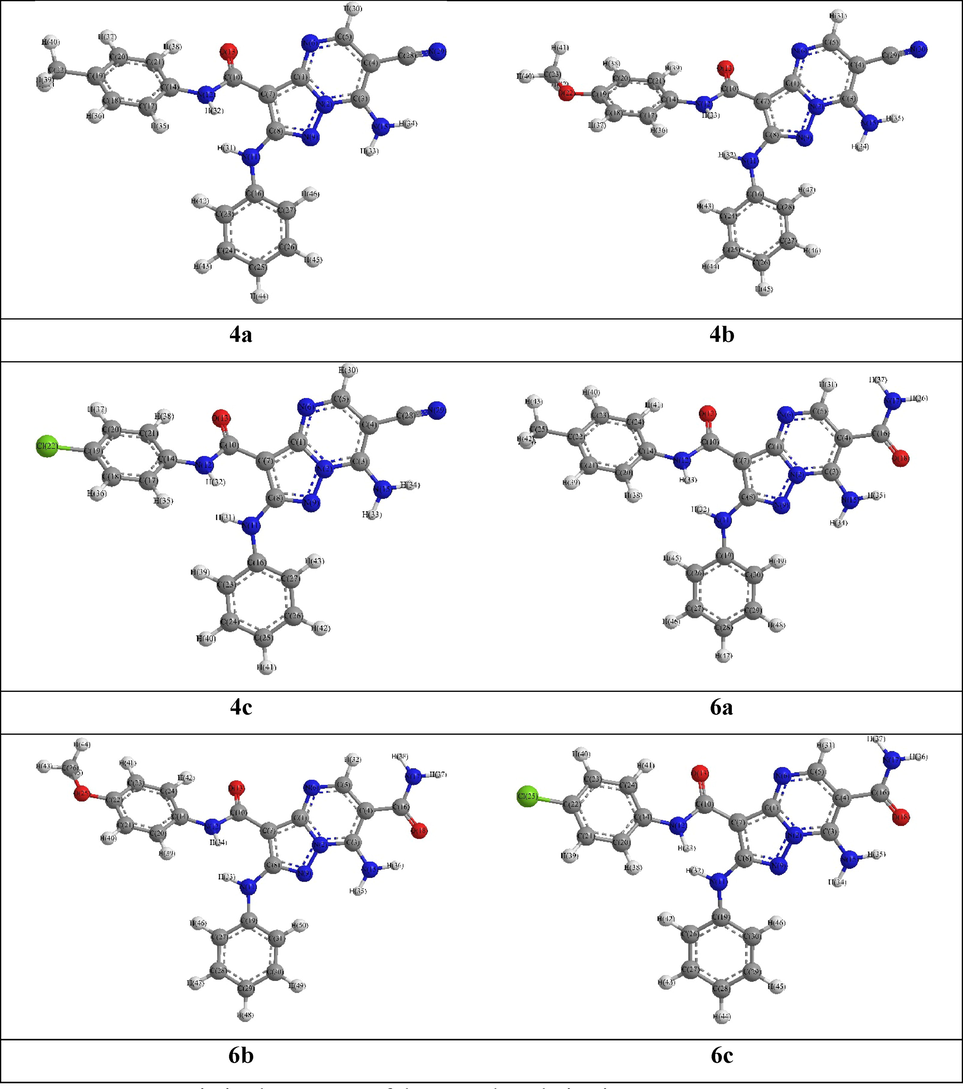
DFT Optimized structures of the 4a and 6a derivatives.
Additionally, the DFT calculated bond length and angle were almost match with those measured in comparable compounds single crystal X-ray (Liu and Liao 2006, Xiaobao, Li et al. 2008, Burnett, Johnston et al. 2015), i.e., lengths exhibited 0.00–0.13 Å difference and 0.048–0.059 Å RMSD while the angles were different by 0.0–11.5° with 2.7–5.2° RMSD, which were attributed to the absence intermolecular columbic interactions in the quantum chemical calculations, but the practical gained for molecules in solid crystal lattice (Sajan, Joseph et al. 2011) (Tables S2-S3).
3.2.1 Frontier molecular orbitals
The HOMO-LUMO configuration and energy explain the electron donating or receiving ability of the molecule (Bulat, Chamorro et al. 2004) where lesser energy gap leads to more ease intramolecular charge transfer (Xavier, Periandy et al. 2015, Makhlouf, Radwan et al. 2018) that may affect in the molecule’s biological activity (Bouchoucha, Zaater et al. 2018). The FMO 3D graph showed that the HOMO of all derivatives was localized mainly on the phenyl rings as well as the heteroatoms lone pair of electrons whilst the LUMO was principally constructed from the π*-orbitals of the 6-cyano and 6-carboxamide 3-carboxamide pyrazolopyrimidine moiety, in 4a-c and 6a-c, respectively (Fig. 3).(See Fig. 4).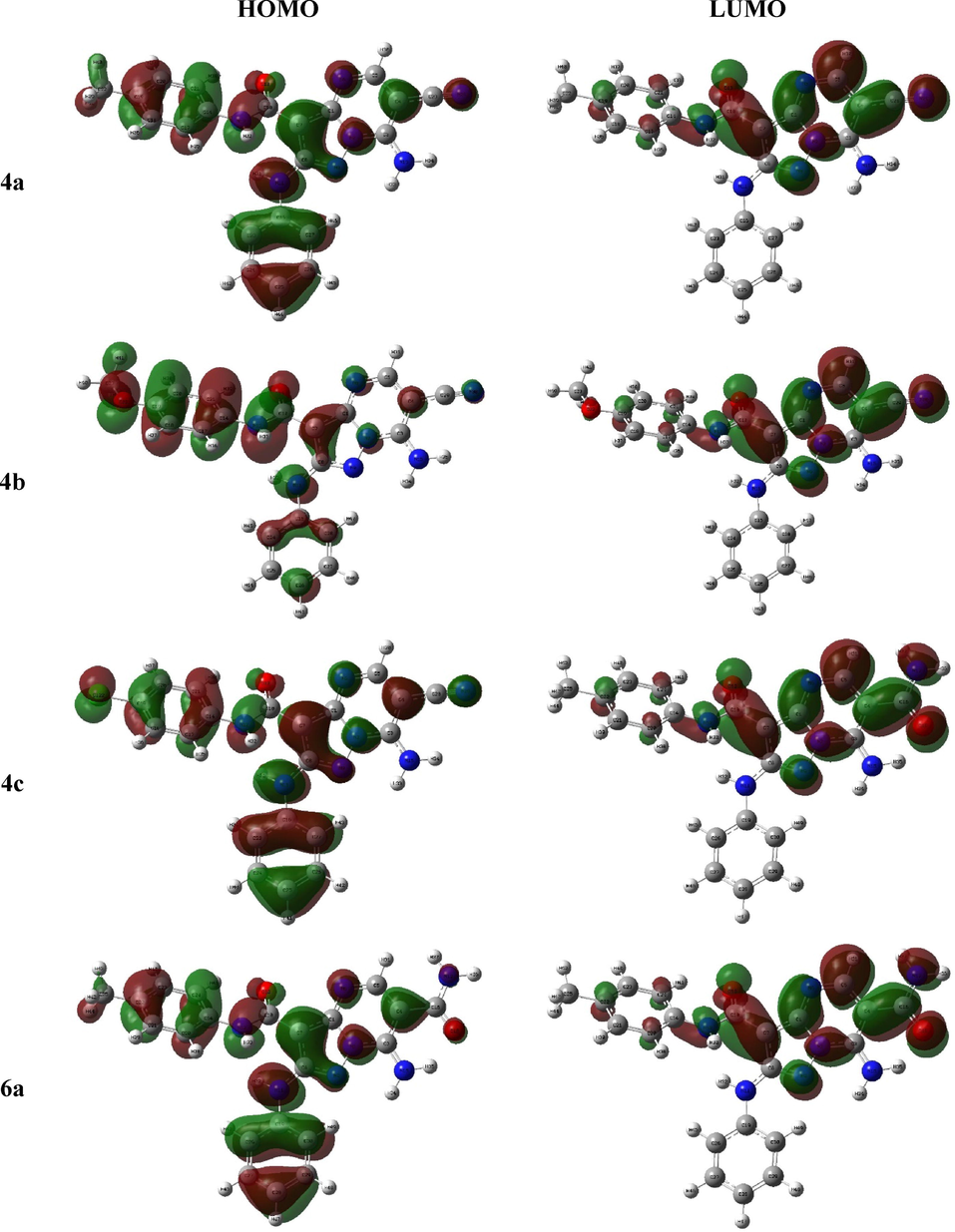
The frontier molecular orbital of the investigated compounds.
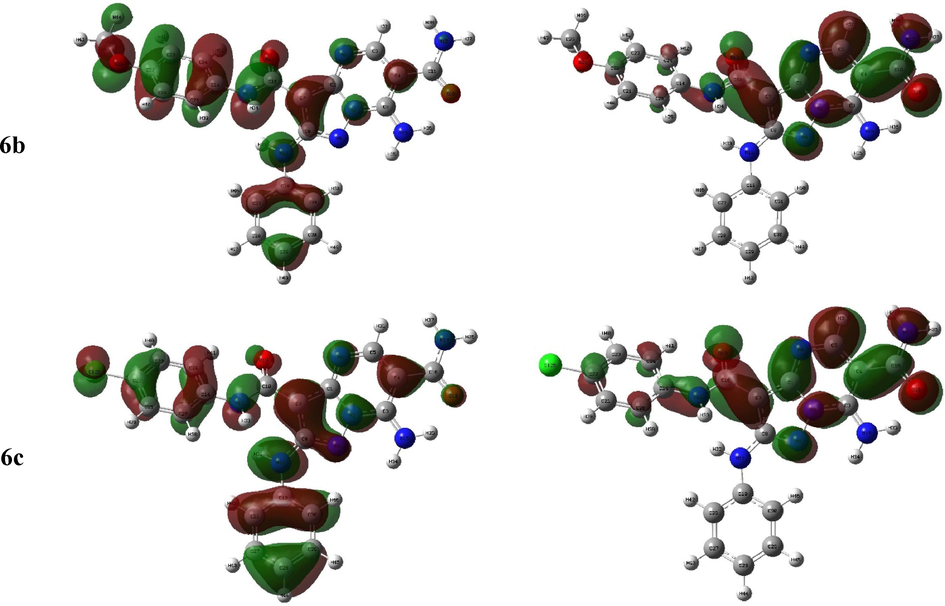
The frontier molecular orbital of the investigated compounds.
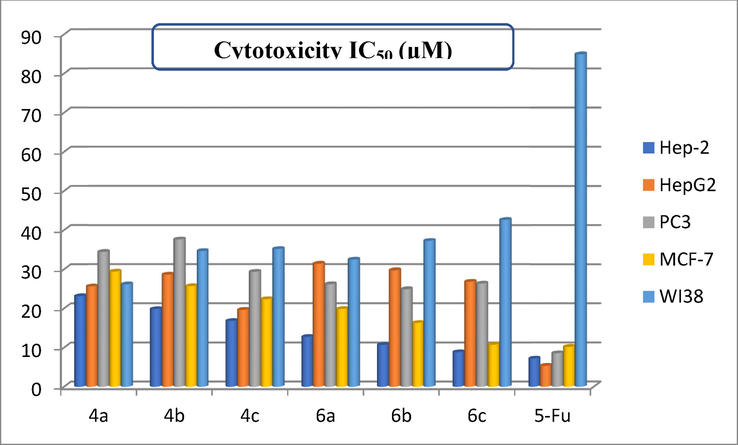
IC50 of the cytotoxic activity for the examined derivatives against human tumor cell lines.
The abovementioned facts affected in the HOMO-LUMO energies, EH and EL. So, the data indicated that the EH and EL of all derivatives have close values of the, range −5.65 - −6.18 and −3.06 - −3.37 eV, respectively, where 4c has highest EH and EL. In addition, the derivatives 6b and both of 4a and 4c exhibited the minimum and maximum ΔEH-L gap, 2.59–2.81 eV, the compounds may be ordered according to their energy gap as 4a = 4c > 6c ≈ 6a > 4b > 6b (Table 1).
Compound
EH
EL
ΔEH-L
χ
η
δ
ω
Dipole
4a
−6.04
−3.24
2.81
4.64
1.40
0.71
7.67
6.27
4b
−5.82
−3.20
2.62
4.51
1.31
0.76
7.76
5.49
4c
−6.18
−3.37
2.81
4.77
1.40
0.71
8.11
6.17
6a
−5.83
−3.10
2.73
4.46
1.36
0.73
7.30
1.77
6b
−5.65
−3.06
2.59
4.36
1.30
0.77
7.32
0.86
6c
−5.96
−3.22
2.74
4.59
1.37
0.73
7.68
3.93
Furthermore, chemical reactivity descriptors, namely, electronegativity (χ), global hardness (η), softness (δ) and electrophilicity (ω) were determined utilizing the values of the EH and EL using the subsequent expressions (Xavier, Periandy et al. 2015).
As shown in Table 1, from the electronegativity (χ) and global softness (δ) values, the derivative 6b has minimum the Lewis’s acid character and the maximum charge transfer ability, respectively. Thus, the examined derivatives were sorted, according to softness, as 6b > 4b > 6a = 6c > 4a = 4c. Furthermore, the molecule dipole moment (μ) accounts the intermolecular interactions where the higher the dipole moment, the stronger the intermolecular interactions will be. The dipole moments of the studied compounds were ranged from 0.86 to 8.17 D for compound 6b and 4c, respectively.
3.2.2 Atomic Mulliken’s charges and Fukui’s indices
The Mulliken’s atomic charges shade light on the charge transfer and electronegativity properties of the molecule (Bhagyasree, Varghese et al. 2013). In all compounds, the pyrazolopyrimidine nitrogen atom N4(Pp) has positive charge, 0.077–0.084. In contrast, the N1(Pp) and N8(Pp) have positive and negative charges, 0.045–0.098 and −0.031 - −0.082, respectively, in 4a-c derivatives, while they possessed the opposite charges in 6a-c derivatives. Noteworthy, the pyrazolopyrimidine carbon atom C7(Pp) in the cyano derivatives, 4a-c, have negative charge, −0.332 - −0.361, while in carboxamide derivatives, 6a-c, it is positively charged, 0.161–0,266. Thus, this behavior may be attributed to the electron withdrawing effect of the cyano group in comparison to the carboxamide group. In contrary, the nitrogen atoms of the cyano and amine substituents in addition to the oxygen atoms of the carboxamide groups were negatively charged whereas the nitrogen atoms of both phenylamine and carboxamide in all compounds were acquired positive charge (Table 2).
Atom
4a
4b
4c
6a
6b
6c
N1(Pp)
0.082
0.098
0.045
−0.020
−0.019
−0.029
C2(Pp)
−0.538
−0.544
−0.515
−0.544
−0.535
−0.522
C3(Pp)
0.124
0.068
0.112
0.166
0.086
0.133
C3a(Pp)
−0.484
−0.444
−0.498
−0.581
−0.539
−0.599
N4(Pp)
0.079
0.077
0.079
0.080
0.083
0.084
C5(Pp)
−0.111
−0.111
−0.114
−0.099
−0.069
−0.102
C6(Pp)
0.701
0.719
0.724
0.626
0.714
0.620
C7(Pp)
−0.344
−0.332
−0.361
0.254
0.161
0.266
N8(Pp)
−0.062
−0.082
−0.031
0.015
0.019
0.022
NH(Pham)
0.138
0.145
0.139
0.124
0.136
0.129
C1(Pham)
0.038
0.017
0.059
0.130
0.078
0.158
C4(Pham)
−0.256
−0.255
−0.264
−0.263
−0.488
−0.272
CO(carb)
−0.360
−0.326
−0.385
−0.417
−0.345
−0.403
OC(carb)
−0.203
−0.203
−0.199
−0.207
−0.208
−0.205
NH(carb)
0.143
0.133
0.119
0.128
0.127
0.115
C1(Phcarb)
−0.336
−0.538
−0.589
−0.267
−0.487
−0.545
C4(Phcarb)
0.673
−0.737
0.413
0.685
−0.718
0.406
NH2(Pp)
−0.297
−0.297
−0.294
−0.426
−0.426
−0.425
CN(Pp)
−0.895
−0.915
−0.907
NC(Pp)
−0.163
−0.162
−0.160
CO(carb1)
−0.435
−0.492
−0.450
OC(carb1)
−0.316
−0.318
−0.315
NH2(carb1)
−0.382
−0.387
−0.386
Me(Phcarb)
−0.702
−0.527
−0.690
OMe(Phcarb)
−0.030
−0.032
Cl(Phcarb)
0.536
0.533
Moreover, the Fukui’s indices (
), as a measure of the atomic reactivity toward nucleophilic attack (El Adnani, Mcharfi et al. 2013, Mi, Xiao et al. 2015, Olasunkanmi, Obot et al. 2016, Messali, Larouj et al. 2018) (Table S4), revealed similar patterns, e.g., the derivatives 4a-c showed that cyano nitrogen, NC(Pp), followed by the pyrazolopyrimidine carbon at positions 5 and 3a, C5(Pp) and C3a(Pp), while in 6a-c, the carbon of the fused ring at position 5, C5(Pp), occupied the top site followed by oxygen and carbon of the carboxamide group, OC(carb1) and CO(carb1), respectively (Table 3). Alternatively, the electrophilic attack Fukui’s indices (
) presented close patterns for the highly susceptible atoms, e.g., in derivatives 4a and 6a, the carbon 4 and nitrogen atoms of the phenylamine group, C4(ph) and NH(Pham), were occupied the second and third positions after cyano nitrogen atom, NC(Pp), in derivative 4a while both were on the top in in derivative 6a, respectively. Whereas, the methoxy derivatives 4b and 6b showed that the methoxy oxygen, OMe(ph), occupied the first place while the C1(ph) and C4(ph) atoms became the second and third susceptible sites, respectively. Lastly, the indices of radical attack (
) offered varied patterns, where in 4a-b, the cyano nitrogen, NC(Pp), was on the top followed by the carbon 5 of the pyrazolopyrimidine, C5(Pp), whereas, it occupied the first place in 6a-b before the carboxamide oxygen, OC(carb1) (Table 3).(See Table 4). 5-Fluorouracil (5-Fu) is the known drug for antitumor assessments.
4a
4b
4c
6a
6b
6c
atom
atom
atom
atom
atom
atom
NC(Pp)
0.058
OMe(Phcarb)
0.064
Cl(Phcarb)
0.077
C4(Pham)
0.058
OMe(Phcarb)
0.057
Cl(Phcarb)
0.069
C4(Pham)
0.055
C1(Phcarb)
0.050
C4(Pham)
0.058
NH(Pham)
0.056
C4(Phcarb)
0.046
C4(Pham)
0.061
NH(Pham)
0.051
C4(Phcarb)
0.050
NC(Pp)
0.058
C3(Pp)
0.037
C1(Phcarb)
0.042
NH(Pham)
0.059
C4(Phcarb)
0.040
NC(Pp)
0.046
NH(Pham)
0.054
C4(Phcarb)
0.036
C3(Phcarb)
0.040
C3(Pp)
0.038
C3(Pp)
0.033
C3(Pham)
0.045
C3(Pp)
0.035
C2(Pham)
0.033
C2(Phcarb)
0.037
C2(Pham)
0.035
atom
atom
atom
atom
atom
atom
NC(Pp)
0.110
NC(Pp)
0.112
NC(Pp)
0.106
C5(Pp)
0.085
C5(Pp)
0.085
C5(Pp)
0.084
C5(Pp)
0.098
C5(Pp)
0.099
C5(Pp)
0.097
OC(carb1)
0.075
OC(carb1)
0.076
OC(carb1)
0.071
C3a(Pp)
0.051
C3a(Pp)
0.052
C3a(Pp)
0.050
CO(carb1)
0.059
CO(carb1)
0.060
CO(carb1)
0.055
CN(Pp)
0.045
CN(Pp)
0.047
OC(carb)
0.046
C3a(Pp)
0.046
C3a(Pp)
0.046
C3a(Pp)
0.046
N4(Pp)
0.044
N4(Pp)
0.045
Cl(Phcarb)
0.045
N4(Pp)
0.043
N4(Pp)
0.045
Cl(Phcarb)
0.043
atom
atom
atom
atom
atom
atom
NC(Pp)
0.084
NC(Pp)
0.079
NC(Pp)
0.082
C5(Pp)
0.054
C5(Pp)
0.052
Cl(Phcarb)
0.056
C5(Pp)
0.061
C5(Pp)
0.059
Cl(Phcarb)
0.061
OC(carb1)
0.051
OC(carb1)
0.049
C5(Pp)
0.054
C4(Pham)
0.039
OMe(Phcarb)
0.040
C5(Pp)
0.060
C4(Pham)
0.041
CO(carb1)
0.035
OC(carb1)
0.049
OC(carb)
0.038
OC(carb)
0.039
C4(Pham)
0.041
C3(Pp)
0.037
OC(carb)
0.035
C4(Pham)
0.042
C3(Pp)
0.037
C4(Phcarb)
0.034
OC(carb)
0.038
CO(carb1)
0.035
OMe(Phcarb)
0.035
C3(Pp)
0.037
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
C5(Pp)
4.08
C5(Pp)
5.50
CO(carb)
4.57
CO(carb1)
4.92
CO(carb1)
6.00
CO(carb1)
4.58
C3a(Pp)
3.64
C3a(Pp)
4.73
C5(Pp)
4.04
C5(Pp)
3.70
C5(Pp)
4.72
CO(carb)
4.14
CO(carb)
3.38
C2(Pp)
4.40
C3a(Pp)
3.57
CO(carb)
3.57
C3a(Pp)
3.83
C5(Pp)
3.65
CN(Pp)
3.00
CN(Pp)
4.27
CN(Pp)
2.80
C3a(Pp)
3.29
C2(Pp)
3.67
C3a(Pp)
3.29
N1(Pp)
2.50
N4(Pp)
4.09
N1(Pp)
2.38
OC(carb1)
2.78
N4(Pp)
3.46
OC(carb1)
2.73
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
C1(Phcarb)
9.50
C1(Phcarb)
12.50
C1(Phcarb)
15.00
C2(Pham)
11.00
C1(Phcarb)
10.50
C2(Pham)
11.67
C2(Pham)
7.75
C2(Phcarb)
5.13
C2(Pham)
8.25
C1(Pham)
7.67
C2(Pham)
6.67
C1(Phcarb)
11.00
C1(Pham)
7.00
OMe(Phcarb)
4.27
C1(Pham)
5.75
C1(Phcarb)
7.00
C2(Phcarb)
4.63
C1(Pham)
8.33
NH(Pham)
4.25
C2(Pham)
4.25
NH(Pham)
4.91
NH(Pham)
5.09
OMe(Phcarb)
4.07
NH(Pham)
5.90
C2(Phcarb)
3.00
C6(Phcarb)
3.89
C6(Pham)
3.20
C6(Pham)
3.30
C6(Phcarb)
3.88
C6(Pham)
3.50
Compound
Cytotoxicity IC50 (μM)
Hep-2
HepG2
PC3
MCF-7
WI38
4a
23.12 ± 0.45
25.61 ± 0.21
34.44 ± 0.53
29.42 ± 0.23
26.15 ± 0.02
4b
19.82 ± 0.17
28.65 ± 0.43
37.64 ± 0.16
25.68 ± 0.16
34.64 ± 0.39
4c
16.78 ± 0.28
19.62 ± 0.13
29.36 ± 0.29
22.36 ± 0.23
35.18 ± 0.26
6a
12.76 ± 0.16
31.41 ± 0.05
26.19 ± 0.34
19.84 ± 0.49
32.47 ± 0.31
6b
10.71 ± 0.39
29.73 ± 0.66
24.90 ± 0.64
16.28 ± 0.11
37.26 ± 0.43
6c
8.85 ± 0.24
26.81 ± 0.26
26.37 ± 0.29
10.80 ± 0.36
42.64 ± 0.53
5-Fu
7.19 ± 0.47
5.33 ± 0.36
8.54 ± 0.23
10.19 ± 0.42
84.93 ± 0.28
As the Fukui’s indices may be inaccurately predicted the active site for nucleophilic and electrophilic attack, the relative electrophilicity and nucleophilicity descriptors, and , respectively, were calculated (Roy, de Proft et al. 1998, Roy, Krishnamurti et al. 1998, Roy, Pal et al. 1999), where , and δ is global softness (Table S4). The relative nucleophilicity descriptors data, , presented resemble patterns, i.e., the 4a-c derivatives exhibited the C5(Pp) at the top position except 4c in which the CO(carb) was the first. While, the 6a-c derivatives displayed the CO(carb1) as the most active site followed by the C5(Pp) (Table 3). As well, the relative electrophilicity descriptors, , displayed that the phenyl carboxamide carbon C1(Phcarb) atom was the most active site in all derivatives except 6a and 6c in which the phenylamine carbon C2(Pham) was on the top. The second position was occupied by difference atoms, i.e., the phenylamine carbon C2(Pham) presented in 4a, 4c and 6b, while the 4b, 6a and 6c have the C2(Phcarb), C1(Pham) and C1(Phcarb), respectively (Table 3).
3.3 Biological evaluation
3.3.1 In vitro anticancer activity
In comparison to 5-fluorouracil (5-Fu), the cytotoxicity of six pyrazolo[1,5-a]pyrimidine compounds was examined across four cancer cell lines HepG2, Hep-2, PC3, MCF-7, and normal fibroblast cells (WI38) (Abd El-Meguid, Awad et al. 2019). Fig. 5 reflected the variations of cytotoxicity in expression of IC50. Inspired the structural lineaments associated to anticancer activity are shown by the results of the investigated pyrazolopyrimidine compounds. The synthesized pyrazolo[1,5-a]pyrimidines revealed as a whole respectable cytotoxic effectiveness with distinct reactivity to hinder the growing of MCF-7 rather than Hep-2. Initially, 7-amino-N-aryl-6-cyano-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4a-c were exhibited sensible inhibition activities toward MCF-7 (IC50 lies between 22.36 ± 0.23–29.42 ± 0.23 μM) than Hep-2 (IC50 lies between 16.78 ± 0.28–23.12 ± 0.45 μM), HepG2 (IC50 lies between 19.62 ± 0.13–25.61 ± 0.21 μM), and PC3 (IC50 lies between 29.36 ± 0.29–34.44 ± 0.53 μM) cell lines. Meanwhile, 7-Amino-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamides 6a-c were displayed respectable cytotoxic effectiveness toward MCF-7 (IC50 lies between 10.80 ± 0.36–19.84 ± 0.49 μM) than Hep-2 (IC50 lies between 8.85 ± 0.24–12.76 ± 0.16 μM), HepG2 (IC50 lies between 26.81 ± 0.26–31.41 ± 0.05 μM), and PC3 (IC50 lies between 24.90 ± 0.29–26.90 ± 0.34 μM) cell lines.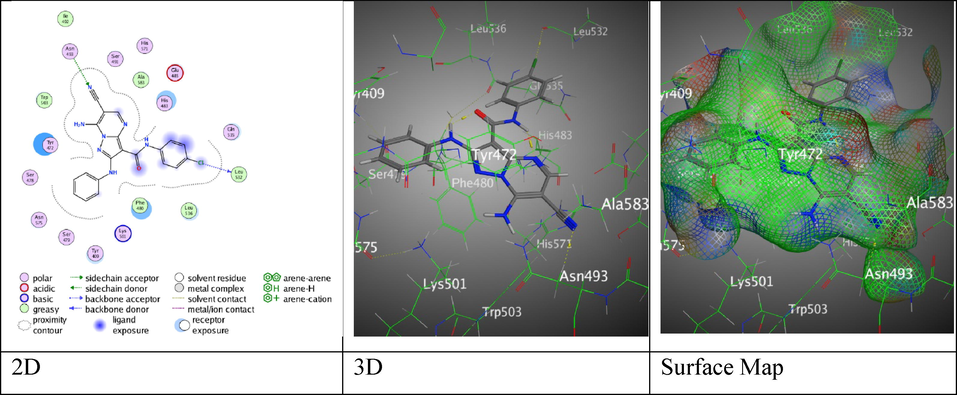
The interaction between hybrid 4c with (PDB ID: 5IVE).
3.3.2 Structural activity relationship
Rendering to cytotoxic outcomes of the prepared 7-amino-N-aryl-6-cyano-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4a-c, it was perceived that 2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-6-carbonitrile hybrids presented high grade of reactivity towards adenocarcinoma cell line (MCF-7) (Rahmouni, Souiei et al. 2016). However, hybrid 4c (Ar = 4-ClC6H4) showed reasonable cytotoxic effectiveness with IC50 = 22.36 ± 0.23 μM toward against MCF-7 cell line, more than compound 4b (Ar = 4-MeOC6H4) which demonstrated workable inhibition with IC50 = 25.68 ± 0.16 μM. However, the hybrid 4a (Ar = 4-MeC6H4) revealed a little inhibition across MCF-7 with IC50 = 29.42 ± 0.23 μM. Meanwhile, the previous survey offered that 2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamides displayed substantial effectiveness over (MCF-7) (Arias-Gómez, Godoy et al. 2021). Moreover, 7-Amino-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide hybrid 6c (Ar = 4-ClC6H4) demonstrated eminent effectiveness across MCF-7 with IC50 = 10.80 ± 0.36 μM. Furthermore, hybrid 6b (Ar = 4-MeOC6H4) released pretty effectiveness with IC50 = 16.28 ± 0.11 μM, then hybrid 6a (Ar = 4-MeC6H4) showed acceptable effectiveness across MCF-7 with IC50 = 19.84 ± 0.49 μM. In accordance to the literature survey, the order of anti-tumor effectiveness follows the arranging of hybrids to these findings: 4-OCH3 > 4-Cl > 4-CH3 (Abbas and Abd El-Karim 2019, Al‐Anazi, Mahmoud et al. 2019). Additional examination of the prepared pyrazolo[1,5-a]pyrimidine hybrids was accomplished to realize their cytotoxic effectiveness on the normal cell fibroblast cells (WI38).
3.3.3 Molecular docking study
According to the scientific literature survey, new pyrazolo[1,5-a]pyrimidines have shown promise as KDM5A “histone lysine demethylase” inhibitors applied to treat breast cancer cells (Gehling, Bellon et al. 2016). These pyrazolo[1,5-a]pyrimidines possess structure like to ligand active site 5IVE. The finest structure of little tested compounds and the conformation of the KDM5A protein, that was recognized from (PDB- ID: 5IVE), were docked together (Metwally, Mohamed et al. 2019). The technique of docking progression was passed out to normalize the compound's preferred style of interrelating with the enzyme's active site. Meanwhile, the binding score (S, Kcal/mol) was utilized to compute the binding similarity of ligand over the enzyme active site; a low score of energy shows a good affinity. Through several prepared derivatives as a ligand and proteins as a target in a molecular docking simulation, the target-ligand interaction was located. The molecular docking study that was presented in Table 5, 7-amino-N-aryl-6-cyano-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4a-c displayed adequate binding score S = -7.4318, −7.5101, and −7.9031 Kcal/mol, respectively (Table 5). Hybrids 4a and 4b exhibited the same H- acceptor bonds between Asn 493 with N-atom of nitrile moiety over intermolecular distances (3.63 and 3.39 Å) (Figures S1 and S2). Meanwhile, hybrid 4c represented two H-bonds (H-donor and H-acceptor), Cl- atom with Leu 532 through (3.41 Å), N- atom of nitrile moiety with Asn 493 through (3.62 Å) (Fig. 5).
Code
S (Kcal/mol)
RMSD
Interaction with ligand
Types of Interactions
Distance (Å)
4a
−7.4318
1.0057
N- atom of nitrile moiety with Asn 493
H-acceptor
3.63
4b
−7.5101
0.9794
N- atom of nitrile moiety with Asn 493
H-acceptor
3.39
4c
−7.9031
0.9711
Cl- atom with Leu 532
N- atom of nitrile moiety with Asn 493H-donor
H- acceptor3.41
3.62
6a
−7.0158
1.3333
O- atom of amide group with Asn 493
H-acceptor
2.99
6b
−7.6607
1.2059
N- atom of amide moiety with Ser 491
O- atom of amide moiety with Asn 493H-donor
H-acceptor2.91
3.04
6c
−8.0107
1.0928
N- atom of amide moiety with Ser 491
O- atom of amide moiety with Asn 493
Cl- atom with Leu 532H-donor
H-donor
H-acceptor2.89
3.30
3.00
5-Fu
−4.2410
0.9567
O-atom of carbonyl moiety with Lys 501
Pyrimidine ring with Phe 480H-acceptor
π-π3.07
3.70
Meantime, 7-Amino-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide derivatives 6a- 6c revealed decent binding score S = -7.0158, −7.6607, and −8.0107 Kcal/mol, respectively. Hybrid 6a displayed H- acceptor amongst O- atom of amide group with Asn 493 through (2.99 Å) (Figure S3). Meanwhile, compound 6b displayed two H-bonds, H-donor among N-atom of amino moiety with Ser 491 through (2.91 Å), and H-acceptor between O-atom of amide substituent with Asn 493 through (3.04 Å) (Figure S4). Moreover, compound 6c revealed two H-donors among O-atom of amide substituent with Asn 493 through (3.30 Å), N- atom of amide moiety with Ser 491through (2.89 Å), and one H-acceptor between Cl- atom with Leu 532 through (3.00 Å) (Fig. 6).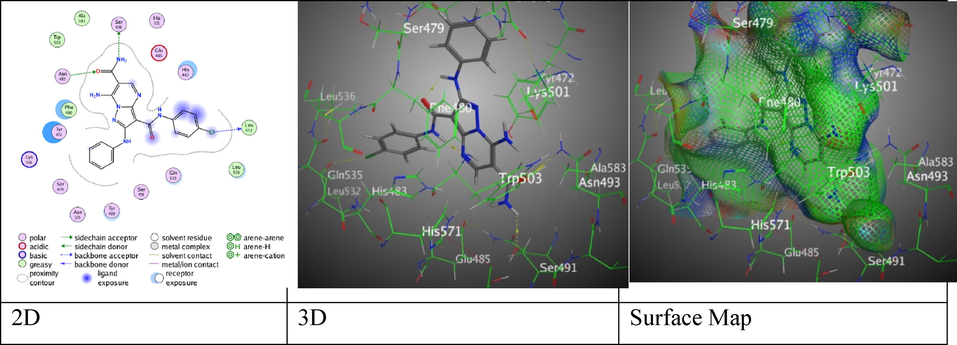
The interaction between hybrid 6c and (PDB ID: 5IVE).
Furthermore, 5-Fu presented H-acceptor among O-atom of carbonyl moiety with Lys 501 through (3.07 Å), and π-π attraction between pyrimidine ring with Phe 480 through (3.70 Å) through RMSD = 0.9567 with low score S = -4.2410 Kcal/mol (Figure S5).
Finally, the outcomes of molecular docking were presented the subsequent summaries: 1) the docking scores of the synthesized hybrids gave acceptable values ranged between −7.0158 to −8.0107 Kcal/mol. 2) 7-Amino-2-(phenylamino)- pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide hybrids 6a-c were unveiled the furthermost many types of bindings like H-donor, H-acceptor, and π-π that offered superior confirmation for the best binding with the diverse amino-acids of 5IVE. 3) The synthesized hybrids were attached with the same amino-acids of protein over dissimilar polar and non-polar amino-acids like “Asn 493, and Leu 532” which presented good evidence for binding processes. 4) The resulted pictures of 2D, 3D, and surface map were reflected clear images of the close relationship between the synthesized ligands and the different types of 5IVE amino-acids.
4 Conclusion
A series of 7-amino-N-aryl-2-(phenylamino)pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4 and 6 were obtained through cyclocondensation of 3-aminopyrzoles 2a-c with 2-((dimethylamino)methylene)-malononitrile and/or 2-cyano-3-(dimethylamino)acrylamide. The DFT optimized structure of the investigated compounds indicated that all have almost planar configuration. All derivatives have similar HOMO and LUMO shapes and thus close ΔEH-L gap was displayed, 2.59–2.81 eV, obeying the order 4a = 4c > 6c ≈ 6a > 4b > 6b. As well, the prepared 2-(phenylamino)-pyrazolo[1,5-a]pyrimidine derivatives demonstrated appropriate cytotoxic effectiveness with dissimilar activities to inhibit the growth of MCF-7 and Hep-2 cancer cell lines. Mainly, 7-amino-N-aryl-6-cyano-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3-carboxamide analogues 4a-c were revealed respectable cytotoxic effectiveness toward MCF-7 rather than the rest of cell lines through IC50 values (22.36 ± 0.23–29.42 ± 0.23 μM). Meanwhile, 7-Amino-2-(phenylamino)-pyrazolo[1,5-a]pyrimidine-3,6-dicarboxamide hybrids 6a-c were revealed attentive inhibition over IC50 values against MCF-7 (10.80 ± 0.36–19.84 ± 0.49 μM) and Hep-2 (24.90 ± 0.29–26.90 ± 0.34 μM). All of the synthesized 2-(phenylamino)-pyrazolo[1,5-a]pyrimidine analogues were examined toward standard drug (5-Fu) with (IC50 = 7.19 ± 0.47 μM) for Hep-2 and (IC50 = 10.19 ± 0.42 μM) for MCF-7 in diminish to (WI38) normal cell. Moreover, the hypothetical outcomes of the molecular docking delivered acceptable approve over the findings of cytotoxic activity through the nominated (PDB Code-5IVE).
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Small Groups. Projects under grant number (RGP.1/102/43).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis and anticervical cancer activity of new benzofuran–pyrazol-hydrazono-thiazolidin-4-one hybrids as potential EGFR inhibitors and apoptosis inducing agents. Bioorg. Chem.. 2019;89:103035
- [Google Scholar]
- Synthesis of new 1,3,4-oxadiazole-benzimidazole derivatives as potential antioxidants and breast cancer inhibitors with apoptosis inducing activity. Russ. J. Gen. Chem.. 2019;89(348):356.
- [Google Scholar]
- 2-Amino-5-arylazothiazole-based derivatives. in vitro cytotoxicity, antioxidant properties, and bleomycin-dependent DNA damage. ChemistrySelect. 2019;4:5570-5576.
- [Google Scholar]
- Pyrazolo[1,5-a]pyrimidines: a close look into their synthesis and applications. Curr Org. Chem.. 2019;23(721):743.
- [Google Scholar]
- Anticancer drug IUdR and other 5-halogen derivatives of 2′-deoxyuridine: conformers, hydrates, and structure–activity relationships. Struct. Chem.. 2014;25(53):69.
- [Google Scholar]
- Design, synthesis and biological evaluation of certain CDK2 inhibitors based on pyrazole and pyrazolo[1,5-a]pyrimidine scaffold with apoptotic activity. Bioorg. Chem.. 2019;86(1):14.
- [Google Scholar]
- Synthesis of a new series of pyrazolo[1,5-a]pyrimidines as CDK2 inhibitors and anti-leukemia. Bioorg. Chem.. 2021;117:105431
- [Google Scholar]
- Functional pyrazolo[1,5-a]pyrimidines: current approaches in synthetic transformations and uses as an antitumor scaffold. Molecules. 2021;26:2708.
- [Google Scholar]
- Identification, synthesis, and biological evaluation of 6-[(6R)-2-(4-Fluorophenyl)-6-(hydroxymethyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidin-3-yl]-2-(2-methylphenyl) pyridazin-3(2 H)-one (AS1940477), a Potent p38 MAP kinase inhibitor. J. Med. Chem.. 2012;55(7772):7785.
- [Google Scholar]
- Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys.. 1993;98:5648-5652.
- [Google Scholar]
- Vibrational spectroscopic (FT-IR, FT-Raman, (1)H NMR and UV) investigations and computational study of 5-nitro-2-(4-nitrobenzyl) benzoxazole. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2013;102(99):113.
- [Google Scholar]
- Materials Studio. San Diego: Dassault Systèmes; 2017.
- Synthesis and characterization of new complexes of nickel (II), palladium (II) and platinum(II) with derived sulfonamide ligand: structure, DFT study, antibacterial and cytotoxicity activities. J. Mole. Struct.. 2018;1161(345):355.
- [Google Scholar]
- Condensation of frontier molecular orbital Fukui functions. J. Phys. Chem. A. 2004;108(342):349.
- [Google Scholar]
- Structural characterization of the aquaporin inhibitor 2-nicotinamido-1, 3, 4-thiadiazole. Acta Crystallogr. Sect. C: Struct. Chem.. 2015;71(1074):1079.
- [Google Scholar]
- Recent advances in the synthesis of new pyrazole derivatives. Targets Heterocycl. Syst.. 2018;22(194):223.
- [Google Scholar]
- Simple access toward 3-halo-and 3-nitro-pyrazolo[1, 5-a]pyrimidines through a one-pot sequence. RSC Adv.. 2017;7(28483):28488.
- [Google Scholar]
- Ground-state enthalpies: evaluation of electronic structure approaches with emphasis on the density functional method. J. Phys. Chem. A. 2006;110(13632):13639.
- [Google Scholar]
- Chemistry of α-oxoesters: a powerful tool for the synthesis of heterocycles. Chem. Rev.. 2015;115(151):264.
- [Google Scholar]
- DFT theoretical study of 7-R-3methylquinoxalin-2 (1H)-thiones (RH;CH3;Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci.. 2013;68(223):230.
- [Google Scholar]
- Tyrosine kinase inhibition effects of novel Pyrazolo[1,5-a]pyrimidines and Pyrido[2,3-d]pyrimidines ligand: Synthesis, biological screening and molecular modeling studies. Bioorg. Chem.. 2018;78(312):323.
- [Google Scholar]
- Potassium 2-Cyanoethylene-1-thiolate derivatives: a new preparative route to 2-Cyanoketene S, N-acetals and pyrazole derivatives. Synth. Commun.. 2004;34(3281):3291.
- [Google Scholar]
- 5-Amino-3-anilino-N-(chlorophenyl)-1H-pyrazole-4-carboxamide ethanol solvate. Acta Crystallogr. Sect. E: Struct. Rep.. 2004;60
- [Google Scholar]
- Frisch, M., Trucks, G., Schlegel, H., et al, 2009. Gaussian 09, Revision A. 1. Wallingford, CT, USA: Gaussian. .
- Identification of potent, selective KDM5 inhibitors. Bioorg. Med. Chem. Lett.. 2016;26(4350):4354.
- [Google Scholar]
- Efficient access to 3, 5-disubstituted 7-(Trifluoromethyl) pyrazolo[1,5-a]pyrimidines involving SN Ar and Suzuki cross-coupling reactions. Molecules. 2020;25:2062.
- [Google Scholar]
- Biosynthetic pathways frequently targeted by pharmaceutical intervention. Fundam. Med. Chem. Drug Metab.. 2018;1(281):323.
- [Google Scholar]
- Pyrimidine-fused derivatives: synthetic strategies and medicinal attributes. Curr. Top. Med. Chem.. 2016;16(3175):3210.
- [Google Scholar]
- Eco-friendly synthesis of pyrimidines and its derivatives: a review on broad spectrum bioactive moiety with huge therapeutic profile. Synth. Commun.. 2018;48(601):625.
- [Google Scholar]
- Efficient green protocols for the preparation of pyrazolopyrimidines. ChemistrySelect. 2021;6(5807):5837.
- [Google Scholar]
- Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37(785):789.
- [Google Scholar]
- Liu, M.G., Liao, Q.B., 2006. Ethyl 5-(triphenylphosphoranylideneamino)-1H-1,2, 3-triazole-4-carboxylate. Acta Crystallograph. Section E: Struct. Rep. Online 62, o2214 o2215.
- Design, synthesis, pharmacological evaluation and DNA interaction studies of binuclear Pt (II) complexes with pyrazolo [1,5-a] pyrimidine scaffold. Appl. Organomet. Chem.. 2018;32:e4222.
- [Google Scholar]
- Discovery of an oral respiratory syncytial virus (RSV) fusion inhibitor (GS-5806) and clinical proof of concept in a human RSV challenge study. ACS Publications J. Med. Chem.. 2015;58(1630):1643.
- [Google Scholar]
- Pyrimidine: A review on anticancer activity with key emphasis on SAR. Future J. Pharm. Sci.. 2021;7(1):38.
- [Google Scholar]
- Experimental and DFT insights into molecular structure and optical properties of new chalcones as promising photosensitizers towards solar cell applications. Appl. Surf. Sci.. 2018;452(337):351.
- [Google Scholar]
- 5′-Norcarbocyclic analogues of furano [2,3-d] pyrimidine nucleosides. Heterocycl. Commun.. 2015;21(259):262.
- [Google Scholar]
- A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: experimental and computer simulations studies. J. Mole. Struct.. 2018;1168:39-48.
- [Google Scholar]
- Design, synthesis, anticancer evaluation, molecular docking and cell cycle analysis of 3-methyl-4,7-dihydropyrazolo[1,5-a]pyrimidine derivatives as potent histone lysine demethylases (KDM) inhibitors and apoptosis inducers. Bioorg. Chem.. 2019;88:102929
- [Google Scholar]
- Theoretical evaluation of corrosion inhibition performance of three antipyrine compounds. Comput. Theor. Chem.. 2015;1072(7):14.
- [Google Scholar]
- Design, synthesis, molecular prediction and biological evaluation of pyrazole-azomethine conjugates as antimicrobial agents. Synth. Commun.. 2021;51(1564):1580.
- [Google Scholar]
- Use of pyrimidine and its derivative in pharmaceuticals: a review. J. Adv. Chem. Sci.. 2021;7(729):732.
- [Google Scholar]
- Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-3-yl]phenyl}methane sulfonamides on mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv.. 2016;6(86782):86797.
- [Google Scholar]
- Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev. B. 1992;46(12947):12954.
- [Google Scholar]
- Biological activities of [1,2,4]triazolo[1,5-a]pyrimidines and analogs. Med. Chem. Res.. 2020;29(1751):1776.
- [Google Scholar]
- Synthesis and biological evaluation of novel pyrazolopyrimidines derivatives as anticancer and anti-5-lipoxygenase agents. Bioorg. Chem.. 2016;66(160):168.
- [Google Scholar]
- Roy, R., De Proft, F.d., Geerlings, P., 1998. Site of protonation in aniline and substituted anilines in the gas phase: a study via the local hard and soft acids and bases concept. J. Physi. Chem. A 102, 7035 7040.
- Local softness and hardness based reactivity descriptors for predicting intra-and intermolecular reactivity sequences: carbonyl compounds. J. Phys. Chem. A. 1998;102(3746):3755.
- [Google Scholar]
- Natural bond orbital analysis, electronic structure, non-linear properties and vibrational spectral analysis of L-histidinium bromide monohydrate: a density functional theory. Spectrochim. Acta Part A: Mole. Biomole. Spectrosc.. 2011;81:85-98.
- [Google Scholar]
- Recent synthetic methodologies for pyrazolo[1,5-a]pyrimidine. Synth. Commun.. 2019;49(1750):1776.
- [Google Scholar]
- Biological activities of synthetic pyrimidine derivatives. World J. Pharm. Res.. 2016;5(1467):1491.
- [Google Scholar]
- Revisiting the structure and chemistry of 3 (5)-substituted pyrazoles. Molecules. 2019;25:42.
- [Google Scholar]
- Recent advances in the design and development of non-nucleoside reverse transcriptase inhibitor scaffolds. Chem. Med. Chem. 2019;14(52):77.
- [Google Scholar]
- Beyond DNA and RNA: the expanding toolbox of synthetic genetics. Cold Spring Harb. Perspect. Biol.. 2019;11:a032490
- [Google Scholar]
- Pyrazolo[1,5-a]pyrimidines-based fluorophores: a comprehensive theoretical-experimental study. RSC Adv.. 2020;10(39542):39552.
- [Google Scholar]
- Photophysical and crystallographic study of three integrated pyrazolo[1, 5-a]pyrimidine–triphenylamine systems. Dyes Pigm.. 2021;184:108730
- [Google Scholar]
- Pyrazolo[1,5-a]pyrimidinium salts for cyanide sensing: a performance and sustainability study of the probes. ACS Sustain. Chem. Eng.. 2021;9(12058):12069.
- [Google Scholar]
- Expeditious ethanol quantification present in hydrocarbons and distilled spirits: Extending photophysical usages of the pyrazolo[1,5-a]pyrimidines. Dyes Pigm.. 2022;202:110299
- [Google Scholar]
- NBO, conformational, NLO, HOMO–LUMO, NMR and electronic spectral study on 1-phenyl-1-propanol by quantum computational methods. Spectrochim. Acta Part A: Mole. Biomole. Spectrosc.. 2015;137:306-320.
- [Google Scholar]
- Synthesis and crystal structure of N-(1,3,4-Thiadiazol-2-yl)-1-[1-(6-chloropyridin-3-yl)methy]-5-methyl-1H-[1,2,3]triazol-4-carboxamide. Chinese J. Struct. Chem.. 2008;27(1389):1392.
- [Google Scholar]
- Design and synthesis of novel pyrazolo[1,5-a]pyrimidine derivatives bearing nitrogen mustard moiety and evaluation of their antitumor activity in vitro and in vivo. Eur. J. Med. Chem.. 2016;119(183):196.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104437.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







